Abstract
This research investigated the suitability of air-to-water generator (AWG) technology to address one of the main concerns in green hydrogen production, namely water supply. This study specifically addresses water quality and energy sustainability issues, which are crucial research questions when AWG technology is intended for electrolysis. To this scope, a reasoned summary of the main findings related to atmospheric water quality has been provided. Moreover, several experimental chemical analyses specifically focused on meeting electrolysis process requirements, on water produced using a real integrated AWG system equipped with certified materials for food contact, were discussed. To assess the energy sustainability of AWGs in green hydrogen production, a case study was presented regarding an electrolyzer plant intended to serve as energy storage for a 2 MW photovoltaic field on Iriomote Island. The integrated AWG, used for the water quality analyses, was studied in order to determine its performance in the specific island climate conditions. The production exceeded the needs of the electrolyzer; thus, the overproduction was considered for the panels cleaning due to the high purity of the water. Due to such an operation, the efficiency recovery was more than enough to cover the AWG energy consumption. This paper, on the basis of the quantity results, provides the first answers to the said research questions concerning water quality and energy consumption, establishing the potential of AWG as a viable solution for addressing water scarcity, and enhancing the sustainability of electrolysis processes in green hydrogen production.
1. Introduction
The energy transition from fossil fuels to renewable sources is needed to counteract the effects of global warming; improving the presence of renewable, or low emission, sources in the energy mix is a recognized mean to reduce the greenhouse gas emissions [1]. In this perspective, several countries agreed to establish carbon neutrality targets. In particular, the European Union (EU) has set the year to achieve the net-zero greenhouse gas emissions at 2050 [2].
Green hydrogen is one of the promising assets in the energy transition process [3]. As already well known, the adjective “green” is given to the hydrogen produced using the power derived from renewable sources [4]. Indeed, many countries all over the world are focusing on the green hydrogen chain development to address the energy transition targets [5]. Among them, Japan can be defined as the world leader in this field due to its energy policy being strongly based on the union between the hydrogen and renewables sources [3]. Also, the EU has identified hydrogen as one strategic tile in the complex mosaic comprising the green deal solution [6]. This is not only due to the hydrogen suitability for integration [7], or to its flexibility as an energy vector, but also to its possibility to be used as an energy storage and to its role in relation to energy security strategies [8]. In particular, hydrogen can play a strategic function as a long-term storage, which can be one of the main needs in the context of the renewable energy development [9]. Actually, photovoltaic and wind energies are expected to be the main contributors in the decarbonized energy mix [10]; thus, in order to cover the residual energy load [9], and to avoid the risk of electrical grid imbalances related to their non-programmability, long-term energy storage development is of the utmost importance [11]. The Fukushima Hydrogen Energy Research Field (FH2R), located in Namie city (Japan) [12], represents an interesting example of green hydrogen generation. This system is composed of a photovoltaic field with a 20 MW peak of power and an electrolysis unit of 10 MW. The intent of this installation was to balance the supply–demand curve of the power grid by means of hydrogen, avoiding other forms of energy storage. Moreover, in this case, the use of hydrogen was intended for vehicle feeding.
To produce green hydrogen, in addition to electricity, coming from renewable sources, the other required “raw material” is distilled water. Today, there is a huge concern about fresh water availability, which is the main source of distilled water. The current data about water scarcity and droughts are alarming. The World Health Organization (WHO) calculated that over 2 billion individuals, a number that has been destined to increase due to climatic change, suffer from water scarcity [13]. In Europe, during the summer of 2022, the combined drought indicator (CDI) results pointed out that 64% of this territory was under a state of warning or alert conditions [14]. In 2023, the protracted rainfall scarcity is giving a huge concern about the water availability in the next months [15]. In order to address this water issue, in addition to maintaining a better water management strategy, new sustainable water sources are required [16], especially considering the potential future new demands, such as that related to electrolysis. Actually, one of the first concerns, and an open research question, about green hydrogen, precisely regards the water source, as the countries where there is the highest potential of renewable energy are also areas where the extent of water scarcity is increasing [17].
To provide a first answer to such a concern is the main scope of the current paper, which proposed a study about an integrated air-to-water generation (AWG) system devoted to produce water for electrolysis. It must be remembered that atmospheric water harvesting is considered a mean to increase the resilience against the extent of water scarcity [18]. It can be interesting to observe that when the hydrogen is used to recover the stored energy, reacting into a fuel cell or burning in a thermic engine, one of the by-products is water vapor. Thus, the employment of a system that extracts water from the air, in the hydrogen chain, can close the process circle, as summarized in Figure 1, addressing the above mentioned research questions, which have also been underlined by the authors of [19].
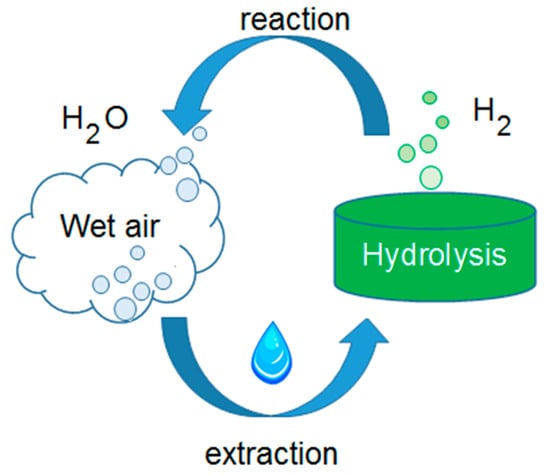
Figure 1.
Water–hydrogen closed cycle, by means of water extraction from the air.
A first study about the possible use of atmospheric water to feed the electrolysis process was presented by the authors of [20]; this study analyzed how to directly employ the vapor contained in the air to produce hydrogen by means of a proof-of-concept atmospheric water electrolyzer, which was purposely developed for such a scope. Their study underlined atmospheric water potentiality as a source for green hydrogen production and the related possibility to avoid water transportation. While this novel concept electrolyzer is still in the laboratory stage and has some drawbacks that need to be addressed in the future, it is worth noting that water extracted from the air can already be used in existing electrolyzers, as their technology is sufficiently mature. Such an option opens at least two research questions: the energy sustainability of the water extraction process applied to electrolysis, and the suitability of the produced water for such an employment.
This article provides the initial answers to the aforementioned two questions, in addition to proposing a solution within the context of water scarcity, which, as said before, is one of the main concerns underlying the green hydrogen production field. These answers were provided through a discussion on the current literature findings about water quality and on the water test results, which were purposely carried out for the current research on samples that were derived from an advanced, real-world air-to-water generation (AWG) machine. Moreover, they are supported by a study concerning the potential installation of such a kind of an AWG, whose behavior was determined by means of a physically based model, in a green hydrogen generation system on an island, powered by a photovoltaic field. These responses constitute a quantitative study aimed at assessing the practical applicability of AWG technology within the hydrogen chain, representing a novelty in AWG research.
This study was organized as follows: methodology description, some hints about electrolysis water requirements, in terms of quantity and quality, a short excursus on water extraction solutions, focusing on the integrated AWGs, with a brief discussion about the current water quality main issues. After these sections, the water test results are presented and analyzed, followed by the study case description and results comments. Finally, future perspectives and developments are presented.
2. Methodology
This study was developed with two aims: firstly, to enhance the knowledge about the quality of the water obtained from the air, oriented to the electrolysis use, and secondly, to obtain the first answers about the energy sustainability concerning the employment of an integrated AWG in the field of green hydrogen generation. In order to address such targets, the following steps were developed:
- A focus on the water quality required for electrolysis and a literature research regarding the AWG water quality findings, in order to better understand the main issues related to the intended use. After that, experimental tests, on specific parameters, were carried out on water that was derived from a real integrated AWG machine, similar to that used by the authors in [21], in the analytical chemistry labs at the University of Pavia.
Results were collected and discussed in order to better understand at which extent the water produced by an integrated AWG machine can be deemed as directly suitable for the intended use, or which treatments could be required to obtain the wanted purity level.
- A case study analysis was performed concerning an integration between the AWG machine and a system composed of a hydrolyser and a photovoltaic field, serving it. This section was carried out using a part of the methodology proposed, by the authors, in [21]; in particular, the following points were developed:
- ○
- Data collection about the required water quality and quantity, not only for the electrolyzer use, but also to maintain clean photovoltaic panels.
- ○
- Weather conditions data collection, in terms of temperatures and relative humidity of the installation site, taking into account the best sampling frequency that is suitable for the considered climate, in compliance with the findings reported by the authors in [22].
- ○
- Integrated AWG machine behavior analyses, carried out by means of the simulation tool described, by the authors, in [23].
- ○
- Covered needs evaluation, in particular, considering the water used for panel cleaning, applying the DIrt method [24], and the water required for the electrolysis process.
- ○
- Energy sustainability evaluation, intended as the possibility to cover the energy requirements of the system by means of an energy source that does not inflict long-term damage to the environment and can be replenished within the human lifetime [25]. In particular, in this case, it was analyzed as the possibility to cover the energy requirements by means of a renewable source.
3. Water Requirements for Electrolysis
Electrolysis is currently seen as the most available mean for green hydrogen production, due to its maturity in comparison to other water splitting technologies, such as photolysis and photoelectrolysis, thermochemical processes, biomass gasification, biomass reforming, dark fermentation, and photofermentation [26].
Ideally, the decomposition of water to hydrogen and oxygen is a straightforward process. In reality, there are overpotentials to produce hydrogen and oxygen gas, leading to an increase in the energy wasted as heat. Different setups for electrolysis are present in the literature, according to their technology readiness (Table 1) [27].

Table 1.
Different electrolysis setups in function of their technology maturity.
Membranes are used to separate the anodic and cathodic compartments. The efficiency of the hydrogen evolution can be increased using noble metals catalysts, like platinum. For obvious reasons, there is a lot of interest in developing catalysts and photocatalysts using earth-abundant materials [28].
Nickel metal was found to be useful in alkaline electrolysis, even if the overall process is less efficient compared to acidic electrolysis [29].
Different technologies might request different water purities, specified by the manufacturer (as for the current research). At any rate, usually, the minimum requirement is ASTM type II deionized water having a resistivity >1 MΩ cm, with a concentration of sodium and chloride ions <5 μg L−1 and total organic carbon <50 ppb; however, in many applications, a deionized water >10 MΩ cm is preferred [30]. Electrolytes should not contain anions, like sulphates, chlorides, and nitrates, as they can poison the catalyst or compete with oxidation/reductions reactions involved in hydrogen evolution [31].
When metal catalysts are used, carbon monoxide, generated, for example, from carbon contained in the oxygen evolving electrode, can have detrimental effects too.
For similar reasons, metal ions lead to deposition on the surface of the electrodes and should also be avoided.
As for the required water quantities, the stoichiometric balance requires 9 kg of water to produce 1 kg of hydrogen [32]. With current techniques, the water demand range is between 10 kg and 15 kg per kg of hydrogen [32].
4. Water Extraction from the Air: A Brief Summary of the Current Techniques
The total water content in the atmosphere has been estimated as 12,900 km3 [33]. Such an amount is almost equal to that of all the rivers and swamps of the planet, and it is justified with the consideration that the atmosphere is the first receiver and storage in the water cycle [34]. The atmospheric water has an average residence time of 8–10 days [35]. However, due to global warming, this period is increasing, indicating a rise in the water content present in the air. Today, there are several techniques for water harvesting from the air. Some of them are already mature and can be found on the market [36], while others are still in the developing stage. An exhaustive review of the field state-of-the-art techniques can be found in many recent works, such as the work published by the authors of [37], which deeply analyzes the current techniques development, or that published by the authors of [38], which underlines the importance of the development of atmospheric water harvesting in the future, or that published by the authors of [39], which particularly focused on the water extraction from air analyses in remote areas of the planet.
The current section aim was to provide a very short summary of the main diffused techniques and, in particular, to describe the integrated AWG machine approach, which is still a novelty and is recommended for further study [40]. One of the most diffused classifications for air harvesting water (AHW) techniques divides them into active and passive systems. Those that use an external source of electricity or other high-grade power are called “active”, while those that only employ solar heating and natural thermal gradients are called “passive” [41]. Even if the energy consumption is one of the main issues concerning the atmospheric water use, nevertheless, the distinction between the passive and active solutions is not always so definite. This is due to the fact that even if the water harvesting can be carried out in a completely passive mode, thus only using, at maximum, the solar heating, it is not said that the gathered liquid can be used as it is, avoiding any further energy-consuming treatment. The water quality issue will be discussed in deeper detail in the next section. It can be anticipated that the liquid collected from passive systems may require more intensive treatments, in comparison to that gathered via the active ones, in order to achieve the desired quality. This could require an extra energy to transform the condensate into a usable form.
At any rate, the said distinction classifies the following methods as passive:
- Fog nets: systems composed of a metallic mesh causing the fog or mist droplets coalescence. Various geometries and materials have been studied in order to enhance their yield. Such a solution can only be effective in those places where there are fogs [42].
- Radiative panels: surfaces exposed to the external environment, which exploit the natural temperature gradients and the radiation to the heaven vault to obtain the dew condensation. In order to enhance the panel performances, different bioinspired coating have been recently studied, including, in particular, solutions that alternate between hydrophilic and hydrophobic surfaces. They require large areas to collect meaningful water quantities and particular climates [18].
- Sorption-based AHW: systems composed of one or more layers of desiccants which adsorb the vapor from the air, releasing it, by means of solar heating, in a closed air volume to enhance the dew point. After that, through exploiting the natural outside temperature gradients, part of the vapor condensates. Such a kind of system produces typical yields of few liters per day [43]. An interesting development in this field is represented by moisture-adsorbent gels, which demonstrated the ability to directly release liquid water, with a slight heating at low temperature (40 °C) [44].
The following techniques can be classified as active:
- Compressor-based AWGs: these machines cool down a flow of air under its dew point, obtaining vapor condensation, by means of a thermodynamic reverse cycle, employing a refrigerant worked using a compressor. Such a kind of approach is the most diffused among the commercially available machines, as it provides water productions ranging from tens to thousands liters per day [36].
- Thermo-electric cooler (TEC)-based AWGs: systems that operate the cooling, on a flux of air, driving it under its dew point, by means of Peltier cells. The advantage, in comparison to the previous technique, is that the refrigerant and the compressor are avoided, thus the only moving parts are the fans. The disadvantage is that they can only work under a very narrow range of temperature and relative humidity, and their cooling efficiency is far less in comparison to that of a compressor reverse cycle [45].
- Hybrid sorption-based AWGs: machines that employ desiccants inside the compressor-based AWGs. These systems are able to enhance the water content in the air by means of desiccants and use the concept of the compressor-based machines to obtain absorb–desorb cycles. In current times, such solutions are still undergoing an experimental stage [46].
- Integrated AWGs: advanced compressor-based AWGs that are studied to permit the exploitation of all the useful effects of the thermodynamic reverse cycle [47]. Such an approach can be the mean to bypass the energy and economic issues related to water harvesting from the air and obtain sustainable solutions [48]. Using a compressor-based technique, there are two by-products of this water extraction procedure: a flux of cooled and dry air and a heat flux, at low temperatures, coming from the cooling process of the said air flow. The first one is suitable as the primary air into an air-conditioned area, such as that described, by the authors, in [23]. The second one is suitable for domestic water heating, similar to the example reported, by the authors, in [21]. A scheme of an integrated machine working cycle is reported in Figure 2. The use of these by-products inside a building can help the existing heating and cooling plants, providing energy savings. The lower the existing plants efficiency, the higher the energy and the economic savings will be.
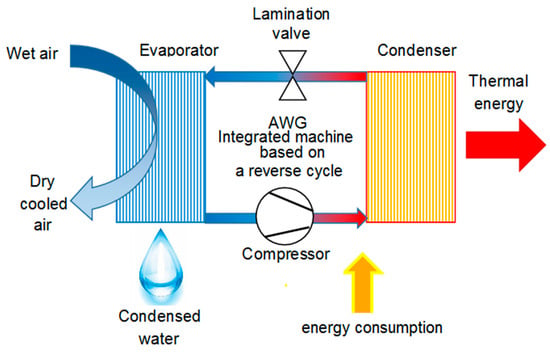 Figure 2. Integrated AWG working scheme. Image adapted from that published by the authors in [47].
Figure 2. Integrated AWG working scheme. Image adapted from that published by the authors in [47].
In general, it can be said that passive system yields are lower in comparison to those of the active ones and are less adaptable to the different weather conditions [41].
5. Water from Air Quality, and Some Hints and Comments about the Current Findings
Water quality is of the utmost importance, regardless of its intended use. Nevertheless, the attention on such a topic, where water obtained from the air is concerned, is not always taken into account. The authors underlined the importance of such an aspect from the beginning of their studies, designing an ad hoc water treatment [21]. It was tested with good results by the authors of [49]; however, in many cases, studies on water harvesting methods were only concentrated on the energy issue, and on the possibility to enhance the absorbent capacity of particular materials. For example, the authors of [43] highlighted the impossibility of conducting a systematic analysis of water quality when radiative cooling and vapor sorption techniques are employed due to a lack of relevant studies, while the authors of [50] stated a similar absence in the AWG field.
At any rate, in the last five years, the attention on the atmospheric water quality issue has increased. Thus, it is possible to find some papers considering, or focusing, on such a topic.
On the basis of the most updated literature and of the authors’ experience, the following findings and observations can thus be summarized:
- When the water source is the external air, previously mechanically filtered, the main chemical pollution, even under heavily air polluted environments, seems to only be composed of nitrites and ammonia [50]. Ammonia levels frequently exceed the limit of 0.5 mg/L and tend to increase when the relative humidity decreases [51]. Mechanical filtration can be found in active systems, while, normally, it is absent in the passive ones as it requires energy;
- Passive systems, that do not comprise any air filtration nor anti-intrusion protections, are affected by macroscopic pollutants and pollution vectors, such as dust particles, animals, insects, and so on [52], and the gathered water is more polluted in comparison to that collected by means of active systems (that are equipped with filters and protections) [50];
- The materials employed in the condenser surface and into the water collection systems can be the means of a further pollution, as some of the composing elements can be released into the water, as underlined by the authors of [53]. In this perspective, desiccants, liquid or solid, must also be deeply analyzed. In order to avoid any compounds being released into water, it is advisable to employ materials certified as “food-degree” quality;
- Viruses, bacteria, molds, and fungi pollution can affect the collected water; such an issue can be almost negligible at the beginning of the AWG use, but it can become very challenging with time [54];
- If indoor air, of inhabited buildings, is used for water extraction, it is possible to find more pollution in the condensate, due to the pollutants released by humans [53]. The biological contamination hazard could also increase for the same reason. If water harvesting is carried out in greenhouses, it may possible to find more salts [53];
- On the contrary, water obtained from external air is generally poor in mineral content, therefore showing a low conductivity [50]; such a residual conductivity is maybe due to the presence of ammonia and nitrites. Such a characteristic make such a kind of water particularly suitable for photovoltaic panel washing [24] and for various other industrial uses. In addition, a potential suitability for hydrogen generation is mentioned by the authors of [43];
- By analyzing the results reported by the authors of [50] and [53], it seems that the water extracted from the air could be, generally, less polluted in comparison to rain water and other surface waters.
From all the above statements, it can be derived that in terms of the functions of the intended uses of water, both inlet air filtration and water treatment could be strongly required. In particular, if the liquid is intended for human use, an air filtration and a water treatment should be always be provided to avoid and/or remove macro pollutants, pollution vectors, biological contamination, and chemical (nitrite and ammonia) pollution. Moreover, if human consumption is considered, a mineralization stage should be added. On the opposite, if the produced water is intended for hydrogen generation, the lower its mineral content, the most suitable such a liquid will be. Moreover, it is important to ascertain that no electrode poisoning compounds are present in harmful concentrations. It is also advisable to comprise a sterilization stage, for example using ultra-violet (UV) lamps, in order to prevent any biological proliferations.
In the next section, some test results, carried out on a water sample produced by a real integrated AWG system, are presented and discussed in the perspective of the use of water in an electrolysis process.
6. Integrated Machine Water Quality Analysis
For the current research, a real integrated AWG machine, characterized by a frame similar to that described by the authors in [21,23], was run to produce water to be tested. This system, based on a reverse thermodynamic system worked using a compressor, is composed of the following components:
- An inlet air section equipped with a double filtration layer, comprising pre-filtration and pocket filtration;
- An open screw compressor, with a cooling power of 100 kW and a heating power of 120 kW;
- Evaporative and heat recovery coils, built with materials that have a food-degree quality, and where vapor condensation occurs;
- Centrifugal evaporator fans, which are speed controlled and able to process a variable airflow;
- An outlet air section suitable for external air duct connection, for the cool and dry air recovery and use;
- Two condenser coils with alternative uses: a plate coil, suitable for the heat recovery, and a fin and tube coil, for the heat dissipation into the environment, in case the domestic water heating is not required;
- An expansion valve, ruled using a proportional integrative derivative (PID) controller, located between the coolant liquid receiver tank and the evaporator;
- A stainless steel water storage connected using a pump to the stainless steel water collection basin. Such a storage component is equipped with a faucet that allows users to completely drain the storage and to collect water samples before entering the water treatment unit;
- A 500 L capacity stainless steel water storage tank;
- A water treatment system that can be customized in function of the required final water use. For the purpose of the current study, water was directly taken before the treatment, in order to understand whether it was already suitable for the scope, or which treatments were required to achieve the required quality.
In order to provide water on which to carry out the chemical tests, the outlined machine, located in Riva San Vitale, Switzerland, in a productive area (both industrial and agricultural), was continuously operated for 8 h. In such a way, the on board water storage was fully filled. The drain faucet was opened and approximately 10 L of water were drawn off, partially used to rinse the water sample container, and then discharged. After that, 500 g of water were collected, stored, and delivered to the Pavia University for chemical analyses. Since only the chemical analyses were scheduled, no sterilization of the faucet, nor of the sample bottle, was required and thus performed.
The water collected was analyzed using an inductively coupled plasma mass spectrophotometer (ThermoFisher ICAP 7000 series, Thermo Fischer Scientific Inc., Waltham, MA, USA), following the experimental conditions described by the manufacturer. For all the metal ions listed in Table 2, the concentrations found (in μg/L) were all below the detectable threshold. For this reason, the assessed purity is in agreement with the absence of ions needed for water electrolysis, confirming the hypothesis of suitability for hydrogen production, as indicated by the authors of [43].

Table 2.
Metal ion concentrations in the water sample (conductivity = 16 μS/cm at 20 °C).
Even if the water does not contain the metal ions indicated in Table 2, a check should still be performed taking into account the actual electrolysis system requirements, in order to define whether filtration is needed. In particular, the conductivity of 16 μS/cm at 20 °C could require attention. Such an issue will be addressed in Section 7.2.
7. Case Study
In the current section, a study of the energy sustainability of an integrated machine, serving a photovoltaic field and an electrolysis system, has been described. Specifications regarding the annual yield of the field, as well as the electrolysis requirements, were provided from a company operating in Japan. A hybrid project, similar to that of Namie [12], but in a far smaller scale, was thought for the Japanese Island of Iriomote (the Island location is displayed in Figure 3). In the current case, a 2 MW peak photovoltaic field was calculated to provide electrical energy, in part, to directly supply the local grid and, in another part, to produce hydrogen for energy storage to balance the supply–demand curve of the grid. In particular, 1130 MWh/year of the produced electricity was intended for hydrogen generation. The energy consumption for the electrolysis was declared equal to 50 kWh/kg, in compliance with the findings made by the authors of [55]. This photovoltaic field was not equipped with batteries for energy storage.
Figure 3.
Iriomote Island. Picture taken from Google Earth.
The plant cannot employ sea water, not only because it is challenging to achieve the required water quality, but also because the brine disposal into the sea must be avoided. Furthermore, underground and surface water cannot be used either, as, in Iriomote Island, traditional sources must be preserved; thus, no water belonging to the island freshwater sources can be available for the plant.
The specifications, concerning water, are the following:
- A total of 10 kg of water is needed to produce 1 kg of hydrogen;
- The hydrogen will only be produced from May to September; in such a period, the hydrolyser requires 1520 L/day;
- An annual amount of 226,000 L of water is required to produce the intended hydrogen quantity;
- The water should be deionized. In particular, the concentrations of aluminum, iron, manganese, nickel, chromium, and zinc should be under 10 μg/L, and the allowed maximum conductivity is 5 μS/cm;
- The photovoltaic field requires about 6400 L of demineralized water to be entirely cleaned, taking into account the value of 3.2 L/kW found by the authors of [56]. Under the hypothesis of “clean plant”, the field is expected to provide 2713 MWh/year of electricity, with an average daily production of 7.43 MWh.
The integrated AWG machine considered for the current study is the same as the one that was previously described in Section 6 and was used to produce the water on which the analyses were carried out. In order to determine the machine behavior, in terms of its water production and energy consumption, in Iriomote Island, it was employed the physically based calculation model, which was used also for the case study in Villahermosa, Mexico [21], whose climate is very similar to that of this island. The general equations and approach characterizing the model were described, by the authors, in [23]; nevertheless, here, it can be useful to summarize its main features. The machine model is a physically based one, employing the object-oriented approach. It is composed of a configurable network of interlinked nodes, with each one of them representing the main components of the machine, such as the compressor, fans, heat exchangers, expansion valve, etc. Such nodes implement energy and mass conservation equations that are numerically resolved. The model uses, as its input, the environmental data, in terms of the total pressure, relative humidity, and temperature, which are the main physical parameters ruling the water extraction from the air process, as outlined by the authors in [23]. This model was implemented in a Java-written 1.0e software 1.0e, which allows the user to arrange the network of these nodes in order to reproduce the wanted machine configuration. Moreover, there are several parameters, typical of the main machine components, which can be modified in compliance with the manufacturer’s data, or experimental data, to fine tune the model. For the heat exchangers, a proprietary semi-empiric equation, including the enthalpy difference as a forcing parameter, was developed, in compliance with the findings reported by the authors of [57]. It is worth noting that the machine model presented by the authors in [21] and used for the current analysis was fine tuned on the machine employed for the water production reported in Section 6, obtaining an average error between the empirical results and the calculated ones of less than 5%. The accuracy of the tuned model was, thus, considered more than acceptable.
7.1. Weather and Data Set Choice
Taking into account the Koppen climate classification, the subtype climate of Iriomote is “Af” (tropical rainforest). In Table 3, the dry bulb temperature, T, and the relative humidity, RH, are reported as monthly averages. These values were calculated on the basis of five years of hourly data (2018–2022) provided by the weather station at New Ishigaki Airport, placed at only 37 km from Iriomote.

Table 3.
Average weather conditions at New Ishigaki Airport in terms of the temperature and the relative humidity.
Due to the climate characteristics of Iriomote, the machine behavior could be studied using, as data input, the monthly averages that means one average value of temperature and one of relative humidity for each month, with a negligible error, in compliance with what was described by the authors in [22]. At any rate, in order to be sure that the use of these averages did not introduce meaningful errors, in the first instance, a study of the machine behavior was carried out using the hourly data of the entire year. After that, the evaluation of its behavior was made using the monthly averages (one value for temperature and one of relative humidity, for each month). Comparing the two results, it was found that the difference in terms of production and consumption was negligible (less than 1%). Furthermore, these results confirmed that, as expected, the weather is stable enough to permit an analysis on the basis of the averages. It was then decided to use the average monthly day, meaning 24 values of temperature and 24 of relative humidity for each month. This choice was due to the necessity of obtaining a data set that was comparable with the outputs of the photovoltaic field in terms of its yield, which was given, in fact, in terms of the monthly average days. At any rate, such a kind of average was still determined on the basis of the hourly data set coming from the New Ishigaki Airport records. The total air pressure was assumed to be equal to 101,325 Pa, as the installation is expected to be placed a few meters above the sea level.
7.2. Water Treatment
The analyses carried out on the produced water and reported in Section 6 confirmed what was underlined in Section 5: the water obtained via the integrated AWG machine, which embodies a double air filtration layer and materials certified safe for the food and beverage contact, is poor in salt content and has a low conductivity.
Nevertheless, as the required conductivity is 5 μS/cm, and the found value during the test was 16 μS/cm, mainly due to ammonia and nitrites, some level of filtration should still be provided. The designed water treatment was composed of: a UV lamp, in order to avoid any biological growth and thus the presence of organic carbon, a mechanical filtration with a mesh of 1 μm, a mixed bed of ionic and cationic resins to block ammonia and nitrites, and a reverse osmosis to guarantee the required limits. The energy needed to run the entire treatment system can be evaluated in 0.01 kWh for each produced liter of water [23]. It is worth noting that, as underlined in Section 5, when AWGs are provided with air filters and are built with certificated materials for the water contact, if the air processed comes from the external environment, it seems that the water quality is scarcely affected from the contaminants that can be found in the air. In particular, for the considered AWG, tests carried out in the United Arab Emirates gave optimal results for the water quality, as reported by the authors in [49], even if the site was a productive one. Considering that Iriomote is a natural park, where human activities and emissions are minimized, it is reasonable to assume that the water quality will not meaningfully differ from that obtained in Switzerland (in an industrial and agricultural area).
7.3. Photovoltaic Field Production and Integrated Machine Behaviour
The case study was carried out under the restrictive hypothesis that the only electrical energy allowed is the energy directly derived from the photovoltaic field, without any compensation from the local grid. Such a hypothesis can be seen as the worst case as the system cannot work when the photovoltaic field does not produce enough energy.
In Table 4, it is reported the photovoltaic field expected power production during the average monthly day and hour by hour (determined on the basis of an entire year of hourly data). The green cells highlight the hours when the power production is enough to make the machine work, given that the AWG has an absorption range that goes from 23.7 kW (in January) to 30.3 kW (in July).

Table 4.
Photovoltaic power yield during the average monthly day in the Iriomote area. The green cells highlight the hours when the power production is enough to make the machine work.
Taking into account the photovoltaic yield reported above, it was possible to determine when the machine can actually work, during the average day, and to evaluate its yield in terms of the produced water using the weather data referred to the same period of the average day. In other words, the behavior of the machine during the very hours of power production by the photovoltaic field was studied.
The graph of Figure 4 summarizes the results concerning the integrated machine yield, in terms of the produced water, and regarding the number of daily “hours of light”, which is the period when the power production is deemed to be at least enough for the machine requirements.
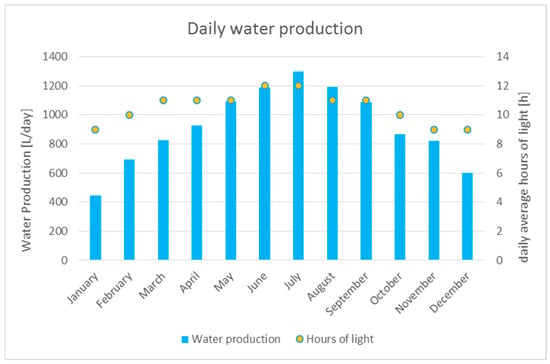
Figure 4.
Daily water production, in the hours of photovoltaic field work, of the integrated AWG. The columns represent the L/day provided by the machine, while the dots represent the average numbers of productive hours (when the light is enough to have the required energy to run the machine).
Analyzing the above results, it was determined that the machine in Iriomote can produce 335,816 L/year, exceeding the whole quantity required by the electrolysis system, which is 226,000 L/year. The water excess was determined to be equal to 109,816 L.
The efficiency of the water extraction from air process can be evaluated by means of the water energy transformation (WET) indicator, as studied and proposed by the authors in [58]. Its formulation, as reported in Equation (1), expresses the ratio between the water production, transformed into the equivalent energy, and the energy required to produce it. This indicator has the same approach of the coefficient of performance (COP) and energy efficiency ratio (EER) indicators, its formulation (Equation (1)) is outlined as follows:
where Qc = condensation latent heat per mass unit [kJ/kg], m = water mass [kg], and E = energy consumption [kJ].
In this study, a density of 1 kg/L was assumed for the produced liquid water.
The average daily energy consumption of the integrated AWG machine as well as its efficiency, which have precisely been illustrated in terms of the WET values, are reported in Figure 5.
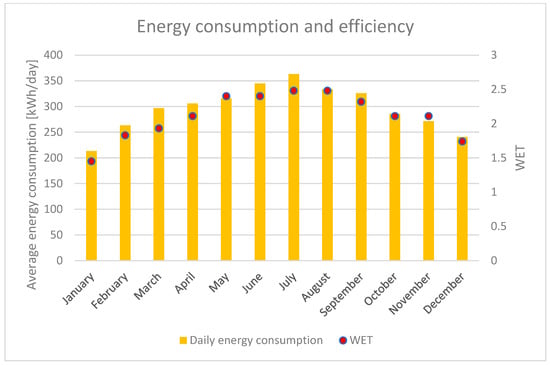
Figure 5.
Energy consumption and efficiency of water production of the integrated AWG, evaluated using the WET indicator. The columns represent the daily energy consumption, while the dots represent the WET values.
On the basis of the above results, it can be calculated that the total amount of energy required for annual whole water production is 108.3 MWh. Such an amount is equivalent to 9.6% of the whole energy consumed by the electrolyzer (1130 MWh/year, as reported above). Actually, the energy strictly needed to produce the water for the hydrogen generation is equal to 6.4% of the said amount. Nevertheless, instead of limiting the production to the pure necessity for the electrolysis process, it would be useful to produce as much water as possible in order to employ it into photovoltaic panel cleaning, as demineralized water is considered one of the best means for panel maintenance [59]. In the next sub paragraph, such a topic will be addressed to determine whether this operation is advisable or evaluating its energy efficiency.
7.4. Photovoltaic Panel Washing and Energy Recovery
Airborne particles, pollution deposition, and salty aerosol coalescence, on the surface of photovoltaic panels, cause efficiency reduction and, consequently, energy production losses. In general, it is advisable to conduct periodical cleaning to recover the efficiency, in particular, using demineralized water [59]. As said above, there is no available water for plant uses coming from island sources nor for hydrogen production, and much less for panel cleaning. The only possible available water is that coming from the AWG integrated machine, whose quality, however, is already in compliance with the panel cleaning requirements [24]. In order to better understand how much the cleaning operations are effective, it is important to evaluate the efficiency loss due to dirt deposition on the panels. To address such a purpose, in the current case study, the semi-empiric “DIrt simplified model” [24] was employed. This method provides a function that, over time, asymptomatically achieves the maximum efficiency degradation due to dirt. Such a function, which in its complete formulation is exponential, in first approximation, can be considered linear, and written as reported below:
where ηloss is the efficiency loss due to soiling, DIr is a constant, which summarizes all the deposition phenomena, and t is the time period, expressed in days, passed after the cleaning.
The DIr constant can be calculated for a specific photovoltaic field, directly measuring the efficiency loss in a given time period or evaluating it on the basis of results coming from similar cases. As data about the ηloss due to soiling in Iriomote Island were not available, results in the literature were considered. In particular, for the Canary Islands, an ηloss equal to 20% was found, due to soiling over a five-month period without any cleaning activity [60]. Such a location is characterized by the proximity to the ocean. Thus, dust deposition and the presence of a highly corrosive environment, due to salty aerosol coalescence, are the main causes of the decline in photovoltaic performances.
A photovoltaic field, located on Iriomote Island, can be affected by the same highly corrosive environment. In Iriomote, rainfalls are more regular and abundant than in the Canary Islands; nevertheless, during periods with no rain, the efficiency can face a strong decrease, as it occurs in the Kathmandu environment (humid subtropical climate, in the Koppen classification), where after 133 days the efficiency loss arose to about 30% [61]. At any rate, in order to take into account the rain contribution on cleaning and also the issue concerning the salty aerosol and the periods without rain, the ηloss value was reduced to a whole 7% over a six-month interval without any maintenance cleaning activity.
Considering the simplified DIrt model, after the said six-month interval, the ηloss can be considered constant, as described by the authors in [24].
Applying Equation (2) the DIr value for Iriomote can be evaluated as follows:
Once the DIr value is known, it is possible to calculate the entity of the efficiency losses due to soiling, ηloss(t), at any instant, t. The daily energy loss can be calculated by multiplying the “clean panel” energy yield of 7.43 MWh/day by the day-by-day efficiency loss.
Water overproduction permits the scheduling of a complete cleaning operation, taking into account the 6400 L required for the entire field, once every 22 days. In Figure 6, two energy loss behaviors are compared. The first one, in red, was calculated under the hypothesis of no maintenance cleaning, while the other, in green, was obtained taking into account the said cleaning interval of 22 days.
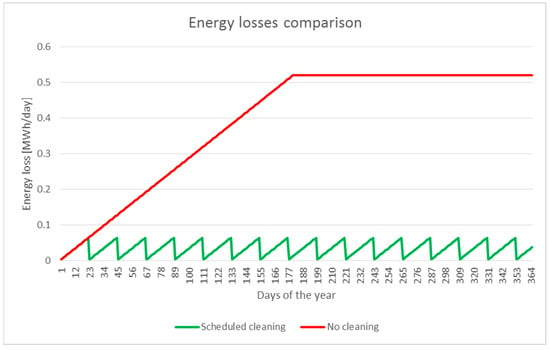
Figure 6.
Comparison between the energy efficiency loss curves. The curve in red is related to no cleaning actions, while the green one concerns the regular cleaning procedure.
By integrating the functions represented above, over the period of 1 year, it is possible to determine that the “no cleaning” scenario gives an energy loss of 143.4 MWh/year, while the “cleaning” one gives an energy loss of only 12.4 MWh/year. The difference between these two values can not only cover the energy required by the integrated machine, 108.3 MWh/year, but also the energy required by the automatic cleaning system, evaluated using data derived from a commercial cleaning robot datasheet [62] in 10 kWh for each entire solar field washing operation, thus equivalating to 0.34 MWh/year.
7.5. Results Analysis
The integrated AWG machine yield in the Iriomote climate can cover all the requirements of the electrolyzer plant, even if the machine can only work during the period when the sun radiance is enough to produce at least about 30 kW. It must be underlined that, during the night, the machine behavior would be better, as part of the sensible heat is reduced through the natural temperature excursion. It is clear that, if a grid exchange would be possible, the machine yield would be about two-thirds more of the reported one, and that the energy efficiency would be improved. At any rate, the daily production would not be constant during the whole year, even in the case of 24 h work, but it would be always more than enough to cover the daily electrolyzer requests. Perhaps, it would be enough to only work the machine during the period that goes from May to September, when the electrolyzer will actually be turned on. Instead, in the examined configuration, a water storage should be provided, to accumulate the production all year long, that should also be intended for panel cleaning. It is important to underline that the AWG machine energy consumption, in the worst case, is less than the 10% of that required by the electrolyzer, considering, in such a calculation, also the overproduction for the photovoltaic field washing. The energy recovery due to cleaning, taking into account a reasonable energy loss caused by the dirt, is more than enough to cover the entire energy consumption of the integrated AWG machine. The result is that the plant equipped with such a kind of machine can gain water independence without any aggravation of the energy consumption and, thus, it can be said that the solution is energy sustainable. Such a consideration is very important, as it once again underlines the energy sustainability of the integration approach in the plant design. To be independent in hydrogen production from the local aqueduct, or from the local suppliers, is of the utmost importance, in particular, taking into account the strategic field from which the hydrogen belongs, that is the energy supply chain. This independency becomes far more interesting if it can be achieved without further energy consumption and only with a smart integration of an AWG machine and a more efficient maintenance of the photovoltaic field, as demonstrated in the current case.
8. Future Developments
As described in Section 4, the integrated AWG machine, alongside water production, has been designed to allow users to employ the other two useful side effects of the thermodynamic reverse cycle. The first one is the flux of cooled and dry air, coming from the water extraction process. This flux is employable, in this case, into the inverter containers and/or into ancillary buildings, such as maintainers’ office and/or dwellings. It would also be interesting to investigate the possible efficiency improvement using the said air as a cooling mean of the electrolysis process. The second one is a low-temperature heat flux, which can be used as a mean to heat domestic water serving, for example, the ancillary buildings. The current case differs from the one described by the authors in [21], which concerned a medium-sized hotel, and the one reported by the authors in [23], which described a worker village. In these two cases, the integrated machine scope was to serve the dwellings accommodating more than one hundred people and its potentialities were fully exploited. The current case, instead, concerns an industrial plant, where the main scope was to serve the electrolyzer and the photovoltaic field. The ancillary buildings are expected to house a few number of employees; thus, it is reasonable to suppose that the by-side effects will not be fully employed, particularly the domestic water heating. In the current case, the energy recovery from the panel washing was more than enough to cover the energy consumption of the integrated AWG machine; thus, the energy sustainability was already reached without other efforts. Nevertheless, it would be interesting to understand the entity of the collectable energy savings using the integrated machine instead of other plants, such as air conditioning systems, for the inverter shelters and for the offices. Another development will concern the economic sustainability of the solution. The cost of the integrated AWG machine is a small percentage of the electrolyzer and its energy consumption is completely covered by the energy recovery due to cleaning. Moreover, given the island constraints described in Section 7, the only alternative to provide demineralized water for hydrogen production is to ship it from the main Japanese island. It is clear that the costs related to such an operation are not negligible. Nevertheless, a detailed cost analysis will be interesting, in particular, if extended to other study cases and placed in other parts of the world with different plant configurations and climates.
9. Conclusions
The current research studied the suitability of the AWG technology in providing an answer to one of the main concerns underlying green hydrogen production, which is the water supply. In particular, the water quality and the energy sustainability issues were addressed, which are open research questions if such a technology is considered for the electrolysis process. On the basis of the authors’ experience and of the current literature, the main water quality findings related to atmospheric water were summarized and discussed. After that, chemical analyses, focused on the electrolysis process requirements, were carried out on the water that was produced using a real integrated AWG system equipped with air filters and made with materials certified for food contact. It was found that the water purity was in agreement with the needed absence of metal ions, aluminum, iron, manganese, nickel, chromium, and zinc, when the water is intended for electrolysis, thereby giving a first answer to the research questions mentioned above. Moreover, the low conductivity found in the literature was confirmed. To provide a first answer to the energy sustainability of the AWG employment in green hydrogen production, a study case was analyzed concerning an electrolyzer plant, used as an energy storage for a 2 MW photovoltaic field, to be placed in Iriomote Island. In such an island, there is no allowable freshwater for the plant (neither for the hydrogen production and nor for the photovoltaic panel cleaning). The electrolysis process requires 226,000 L of water annually that should almost be pure from the mentioned metal ions and with a maximum conductivity of 5 μS/cm. The behavior of the integrated AWG machine, used for the quality analysis, was studied for the Iriomote climate. It was found that its yield was more than enough to cover the entire water demand, even if the machine can only work for a number of hours per day, that is when the photovoltaic field produces, at least, enough power to cover the amount that the AWG consumes. The extent of water overproduction, totaling more than 100,000 L/year, due to its pureness characteristics, was thought to be employed into photovoltaic panel washing. The entire energy consumption of the integrated AWG machine (also considering the amount required by the overproduction) is less than 10% of the whole electrolysis process, and it can be entirely covered through the energy recovery coming from the panel washing activity. Thus, the integrated approach confirms its energy sustainability, providing the second answer to these addressed research questions.
Author Contributions
Conceptualization, L.C. and A.M. methodology, L.C., P.C. and D.D.; software, P.C. and R.F.; validation, L.C. and A.M.; formal analysis, L.C. and R.F.; investigation, L.C.; resources, A.M.; data curation, L.C., R.F., D.D. and D.V.; writing—original draft preparation, L.C., R.F., D.D. and D.V.; writing—review and editing, L.C., A.M., P.C. and D.D.; visualization, L.C.; supervision, A.M.; project administration, L.C.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Water sample, electrolysis, and Iriomote data were provided by the Societè de l’Eau Aerienne Suisse (SEAS). D.D. and D.V. acknowledge support from the Ministero dell’Università e della Ricerca (MUR) and the University of Pavia through the program “Dipartimenti di Eccellenza 2023–2027”.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| Acronyms | |
| AWG | Air-to-water generator |
| AWH | Air water harvesting |
| CDI | Combined drought indicator |
| COP | Coefficient of performance |
| EER | Energy efficiency ratio |
| EU | European Union |
| FH2R | Hydrogen energy research field |
| HVAC | Heating ventilation air conditioning |
| PID | Proportional integrative derivative |
| PLC | Programmable logic controller |
| TEC | Thermo-electric cooler |
| WET | Water energy transformation |
| WHO | World Health Organization |
| UV | Ultra violet |
| Symbols | |
| DIr | Constant, which summarizes all deposition phenomena [1/h] |
| en | Energy [kWh] or [kJ] |
| ηloss | Efficiency loss due to soiling [-] |
| m | Water mass [kg] |
| Qc | Condensation energy [kJ] |
| R.H. | Relative humidity [%] |
| T | Dry bulb temperature [°C] |
| t | Time period passed after the cleaning (day) |
References
- United Nations Climate Change. Maintaining a Clear Intention to Keep 1.5 °C within Reach. Available online: https://unfccc.int/maintaining-a-clear-intention-to-keep-15degc-within-reach (accessed on 10 March 2023).
- European Commission. 2050 Long-Term Strategy. Available online: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2050-long-term-strategy_en (accessed on 10 March 2023).
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Green Hydrogen Definition. Available online: https://www.sciencedirect.com/topics/engineering/green-hydrogen (accessed on 22 June 2021).
- The Future of Hydrogen. IEA, Paris. 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 17 March 2023).
- European Commission. Hydrogen European Strategy. Available online: https://energy.ec.europa.eu/topics/energy-systems-integration/hydrogen_en#eu-hydrogen-strategy (accessed on 10 March 2023).
- European Commission. EU Strategy on Energy System Integration. Available online: https://energy.ec.europa.eu/topics/energy-systems-integration/eu-strategy-energy-system-integration_en (accessed on 10 March 2023).
- Bairrão, D.; Soares, J.; Almeida, J.; Franco, J.F.; Vale, Z. Green Hydrogen and Energy Transition: Current State and Prospects in Portugal. Energies 2023, 16, 551. [Google Scholar] [CrossRef]
- Schill, W.-P. Electricity Storage and the Renewable Energy Transition. Joule 2020, 4, 2059–2064. [Google Scholar] [CrossRef]
- Taseska, T.; Yu, W.; Wilsey, M.K.; Cox, C.P.; Meng, Z.; Ngarnim, S.S.; Müller, A.M. Analysis of the Scale of Global Human Needs and Opportunities for Sustainable Catalytic Technologies. Top. Catal. 2023, 66, 338–374. [Google Scholar] [CrossRef]
- Lamsal, D.; Sreeram, V.; Mishra, Y.; Kumar, D. Output power smoothing control approaches for wind and photovoltaic generation systems: A review. Renew. Sustain. Energy Rev. 2019, 113, 109245. [Google Scholar] [CrossRef]
- The World’s Largest-Class Hydrogen Production, Fukushima Hydrogen Energy Research Field (FH2R) Now is Completed at Namie Town in Fukushima. Available online: https://www.global.toshiba/ww/news/energy/2020/03/news-20200307-01.html (accessed on 1 May 2023).
- World Health Organization. Drinking Water. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 22 March 2023).
- Toreti, A.; Bavera, D.; Acosta Navarro, J.; De Jager, A.; Di Ciollo, C.; Maetens, W.; Magni, D.; Masante, D.; Mazzeschi, M.; Spinoni, J.; et al. Drought in Europe: August 2022: GDO Analytical Report; Publications Office of the European Union: Luxembourg, 2022. [CrossRef]
- Toreti, A.; Bavera, D.; Acosta Navarro, J.; Arias-Muñoz, C.; Avanzi, F.; Marinho Ferreira Barbosa, P.; De Jager, A.; Di Ciollo, C.; Ferraris, L.; Fioravanti, G.; et al. Drought in Europe March 2023; Publications Office of the European Union: Luxembourg, 2023. [CrossRef]
- Karimidastenaei, Z.; Avellán, T.; Sadegh, M.; Kløve, B.; Haghighi, A.T. Unconventional water resources: Global opportunities and challenges. Sci. Total Environ. 2022, 827, 154429. [Google Scholar] [CrossRef]
- Woods, P.; Bustamante, H.; Aguey-Zinsou, K.-F. The hydrogen economy—Where is the water? Energy Nexus 2022, 7, 100123. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, R.; Li, Y. Diversifying Water Sources with Atmospheric Water Harvesting to Enhance Water Supply Resilience. Sustainability 2022, 14, 7783. [Google Scholar] [CrossRef]
- Kabir, M.M.; Akter, M.M.; Huang, Z.; Tijing, L.; Shon, H.K. Hydrogen production from water industries for a circular economy. Desalination 2023, 554, 116448. [Google Scholar] [CrossRef]
- Thimmappa, R.; Gautam, M.; Bhat, Z.M.; Thodika, A.R.A.; Devendrachari, M.C.; Mukhopadhyay, S.; Dargily, N.C.; Thotiyl, M.O. An atmospheric water electrolyzer for decentralized green hydrogen production. Cell Rep. Phys. Sci. 2021, 2, 100627. [Google Scholar] [CrossRef]
- Cattani, L.; Magrini, A.; Cattani, P. Water Extraction from Air by Refrigeration—Experimental Results from an Integrated System Application. Appl. Sci. 2018, 8, 2262. [Google Scholar] [CrossRef]
- Cattani, L.; Magrini, A.; Leoni, V. Energy Performance of Water Generators from Gaseous Mixtures by Condensation: Climatic Datasets Choice. Energies 2022, 15, 7581. [Google Scholar] [CrossRef]
- Cattani, L.; Cattani, P.; Magrini, A. Air to Water Generator Integrated System Real Application: A Study Case in a Worker Village in United Arab Emirates. Appl. Sci. 2023, 13, 3094. [Google Scholar] [CrossRef]
- Cattani, L.; Cattani, P.; Magrini, A. Photovoltaic Cleaning Optimization: A Simplified Theoretical Approach for Air to Water Generator (AWG) System Employment. Energies 2021, 14, 4271. [Google Scholar] [CrossRef]
- Hollaway, L. Sustainable energy production: Key material requirements. In Woodhead Publishing Series in Civil and Structural Engineering, Advanced Fiber-Reinforced Polymer (FRP) Composites for Structural Applications, 2nd ed.; Bai, J., Ed.; Woodhead Publishing: Soston, UK, 2013; pp. 675–701. ISBN 9780128203460. [Google Scholar] [CrossRef]
- Lagioia, G.; Spinelli, M.P.; Amicarelli, V. Blue and green hydrogen energy to meet European Union decarbonisation objectives. An overview of perspectives and the current state of affairs. Int. J. Hydrogen Energy 2023, 48, 1304–1322. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Vadivel, D.; Sturini, M.; Speltini, A.; Dondi, D. Tungsten Catalysts for Visible Light Driven Ofloxacin Photocatalytic Degradation and Hydrogen Production. Catalysts 2022, 12, 310. [Google Scholar] [CrossRef]
- Gong, M.; Wang, D.-Y.; Chen, C.-C.; Hwang, B.-J.; Dai, H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016, 9, 28–46. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Attas, T.; Roy, S.; Rahman, M.M.; Ghaffour, N.; Thangadurai, V.; Larter, S.; Hu, J.; Ajayan, P.M.; Kibria, G. Seawater electrolysis for hydrogen production: A solution looking for a problem? Energy Environ. Sci. 2021, 14, 4831–4839. [Google Scholar] [CrossRef]
- Rosca, V.; Duca, M.; de Groot, M.T.; Koper, M.T.M. Nitrogen Cycle Electrocatalysis. Chem. Rev. 2009, 109, 2209–2244. [Google Scholar] [CrossRef]
- Shi, X.; Liao, X.; Li, Y. Quantification of fresh water consumption and scarcity footprints of hydrogen from water electrolysis: A methodology framework. Renew. Energy 2020, 154, 786–796. [Google Scholar] [CrossRef]
- Gleick, P.H. Water Resources. In Encyclopedia of Climate and Weather; Schneider, S.H., Ed.; Oxford University Press: New York, NY, USA, 2011; Volume 2, pp. 817–823. ISBN 978-019-531-386-4. [Google Scholar]
- Graham, S.; Parkinson, C.; Chahine, M. The Water Cycle. Available online: https://earthobservatory.nasa.gov/features/Water (accessed on 7 April 2023).
- Gimeno, L.; Eiras-Barca, J.; Durán-Quesada, A.M.; Dominguez, F.; van der Ent, R.; Sodemann, H.; Sánchez-Murillo, R.; Nieto, R.; Kirchner, J.W. The residence time of water vapour in the atmosphere. Nat. Rev. Earth Environ. 2021, 2, 558–569. [Google Scholar] [CrossRef]
- Atmospheric Research. Atmospheric Water Generator or Water-from-Air Machine Suppliers Links. Available online: http://www.atmoswater.com/manufacturers-and-suppliers-of-atmospheric-water-generators--water-from-air-machines.html (accessed on 6 April 2023).
- Tashtoush, B.; Alshoubaki, A. Atmospheric water harvesting: A review of techniques, performance, renewable energy solutions, and feasibility. Energy 2023, 280, 128186. [Google Scholar] [CrossRef]
- Raveesh, G.; Goyal, R.; Tyagi, S. Advances in atmospheric water generation technologies. Energy Convers. Manag. 2021, 239, 114226. [Google Scholar] [CrossRef]
- Ahrestani, Z.; Sadeghzadeh, S.; Emrooz, H.B.M. An overview of atmospheric water harvesting methods, the inevitable path of the future in water supply. RSC Adv. R. Soc. Chem. 2023, 13, 10273–10307. [Google Scholar] [CrossRef] [PubMed]
- Thavalengal, M.S.; Jamil, M.A.; Mehroz, M.; Bin Xu, B.; Yaqoob, H.; Sultan, M.; Imtiaz, N.; Shahzad, M.W. Progress and Prospects of Air Water Harvesting System for Remote Areas: A Comprehensive Review. Energies 2023, 16, 2686. [Google Scholar] [CrossRef]
- Nikkhah, H.; Azmi, W.M.B.W.; Nikkhah, A.; Najafi, A.M.; Babaei, M.M.; Fen, C.S.; Nouri, A.; Mohammad, A.W.; Lun, A.W.; Yong, N.L.; et al. A comprehensive review on atmospheric water harvesting technologies: From thermodynamic concepts to mechanism and process development. J. Water Process Eng. 2023, 53, 103728. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, R.; Zhang, Y.; Wang, J. Progress and Expectation of Atmospheric Water Harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Ansari, E.; Ferber, N.L.; Milošević, T.; Barron, J.; Karanikolos, G.N.; AlMarzooqi, F.; Dumée, L.F.; Calvet, N. Atmospheric water generation in arid regions—A perspective on deployment challenges for the Middle East. J. Water Process Eng. 2022, 49, 103163. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Liu, Y.; Shi, Y.; Dai, Y.; Yu, G. Super Moisture-Absorbent Gels for All-Weather Atmospheric Water Harvesting. Adv. Mater. 2019, 31, e1806446. [Google Scholar] [CrossRef]
- Asham, A.D.; Abdelaal, W.G.A.; Mamdouh, W.M. A Novel Ambiently Adaptive Atmospheric Water Generator Using Maximum Production Tracking Algorithm. Teh. Vjesn. Tech. Gaz. 2022, 29, 797–805. [Google Scholar] [CrossRef]
- Shemelin, V.; Pokorny, N.; Novotny, J. Experimental investigation of silica gel and zeolite coated fin-tube heat exchangers under arid climatic conditions. Energy Rep. 2022, 8, 331–341. [Google Scholar] [CrossRef]
- Cattani, L.; Cattani, P.; Magrini, A. Air to Water Generator Integrated Systems: The Proposal of a Global Evaluation Index—GEI Formulation and Application Examples. Energies 2021, 14, 8528. [Google Scholar] [CrossRef]
- Moghimi, F.; Ghoddusi, H.; Asiabanpour, B.; Behroozikhah, M. Is atmospheric water generation an economically viable solution? Clean Technol. Environ. Policy 2021, 23, 1045–1062. [Google Scholar] [CrossRef]
- Mandal, C.S.; Agarwal, M.; Reddy, V.; Kudapa, V.K. Water from air—A sustainable source of water. Mater. Today Proc. 2021, 46, 3352–3357. [Google Scholar] [CrossRef]
- Kaplan, A.; Ronen-Eliraz, G.; Ratner, S.; Aviv, Y.; Wolanov, Y.; Avisar, D. Impact of industrial air pollution on the quality of atmospheric water production. Environ. Pollut. 2023, 325, 121447. [Google Scholar] [CrossRef]
- Inbar, O.; Chudnovsky, A.; Ohneiser, K.; Ansmann, A.; Ratner, S.; Sirota, R.; Aviv, Y.; Avisar, D. Air-water interactions: The signature of meteorological and air-quality parameters on the chemical characteristics of water produced from the atmosphere. Sci. Total. Environ. 2021, 790, 147940. [Google Scholar] [CrossRef]
- Algarni, S. Assessment of fog collection as a sustainable water resource in the southwest of the Kingdom of Saudi Arabia. Water Environ. J. 2018, 32, 301–309. [Google Scholar] [CrossRef]
- Jurga, A.; Pacak, A.; Pandelidis, D.; Kaźmierczak, B. Condensate as a water source in terrestrial and extra-terrestrial conditions. Water Resour. Ind. 2023, 29, 100196. [Google Scholar] [CrossRef]
- Jahne, M.; Pfaller, S.; Garland, J.; Impellitteri, C. Evaluation of Atmospheric Water Generation Technology: Microbial Water Quality; U.S. Environmental Protection Agency: Washington, DC, USA, 2018; EPA/600/R-18/379.
- Grigoriev, S.; Fateev, V.; Bessarabov, D.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Jones, R.K.; Baras, A.; Al Saeeri, A.; Al Qahtani, A.; Al Amoudi, A.O.; Al Shaya, Y.; Alodan, M.; Al-Hsaien, S.A. Optimized Cleaning Cost and Schedule Based on Observed Soiling Conditions for Photovoltaic Plants in Central Saudi Arabia. IEEE J. Photovolt. 2016, 6, 730–738. [Google Scholar] [CrossRef]
- Gaspar, P.D. Handbook of Research on Advances and Applications in Refrigeration Systems and Technologies; Gaspar, P.D., Dihno da Silva, P., Eds.; IGI Global: Hershey, PA, USA, 2015; ISBN 978-146-668-398-3. [Google Scholar]
- Cattani, L.; Magrini, A.; Cattani, P. Water Extraction from Air: A Proposal for a New Indicator to Compare Air Water Generators Efficiency. Energies 2021, 14, 224. [Google Scholar] [CrossRef]
- Appels, R.; Lefevre, B.; Herteleer, B.; Goverde, H.; Beerten, A.; Paesen, R.; De Medts, K.; Driesen, J.; Poortmans, J. Effect of soiling on photovoltaic modules. Sol. Energy 2013, 96, 283–291. [Google Scholar] [CrossRef]
- Schill, C.; Brachmann, S.; Koehl, M. Impact of soiling on IV-curves and efficiency of PV-modules. Sol. Energy 2015, 112, 259–262. [Google Scholar] [CrossRef]
- Paudyal, B.R.; Shakya, S.R. Dust accumulation effects on efficiency of solar PV modules for off grid purpose: A case study of Kathmandu. Sol. Energy 2016, 135, 103–110. [Google Scholar] [CrossRef]
- Commercial Cleaning Robot Vendor Site. Available online: http://www.washpanel.com/index.php (accessed on 20 June 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).