Li3PO4-Coated Graphite Anode for Thermo-Electrochemically Stable Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
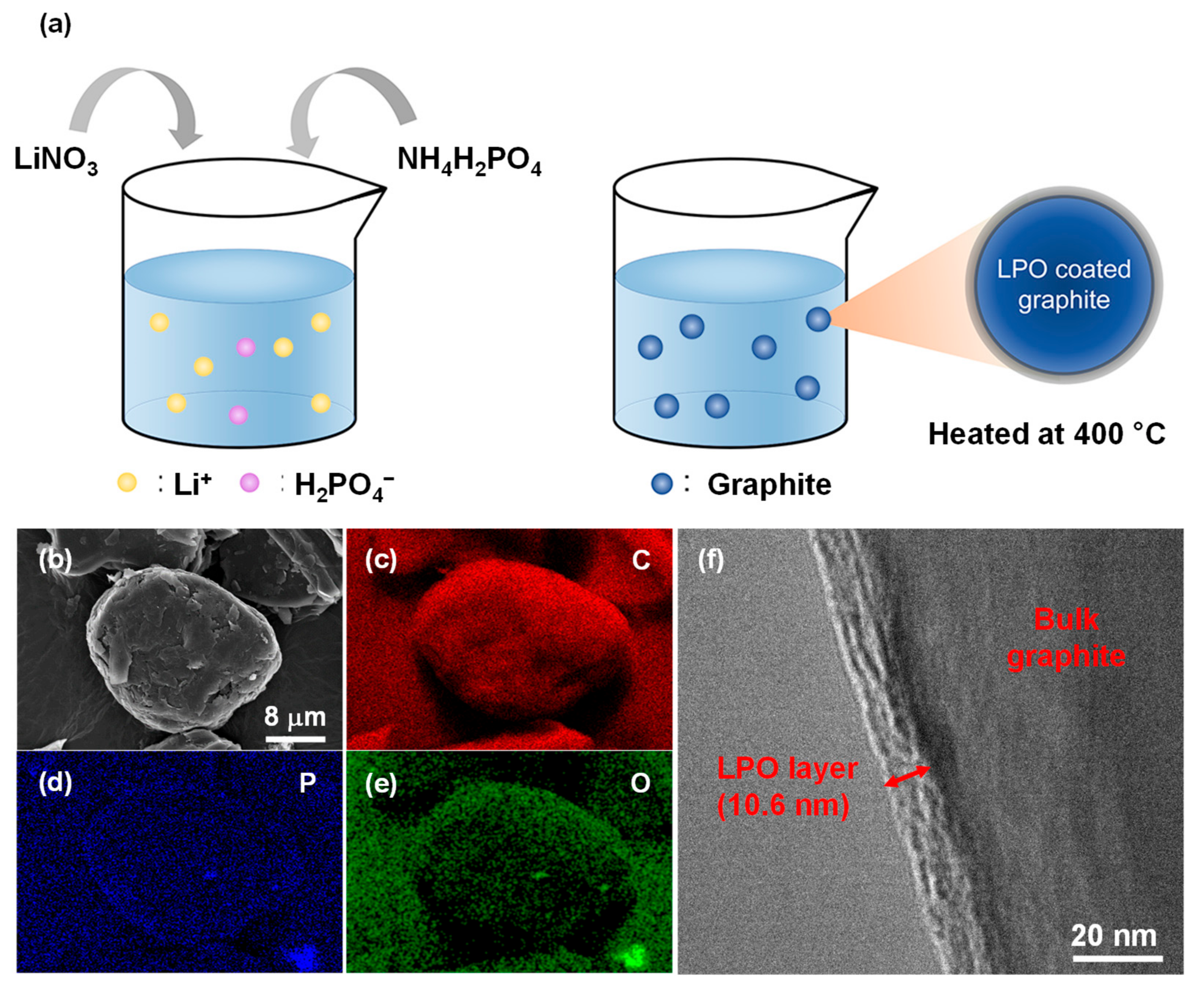
2.1. LPO Coating on the Particle of Graphite
2.2. Preparation of Electrode and Coin Cell Assembly
2.3. Analysis and Electrochemical Characterizations
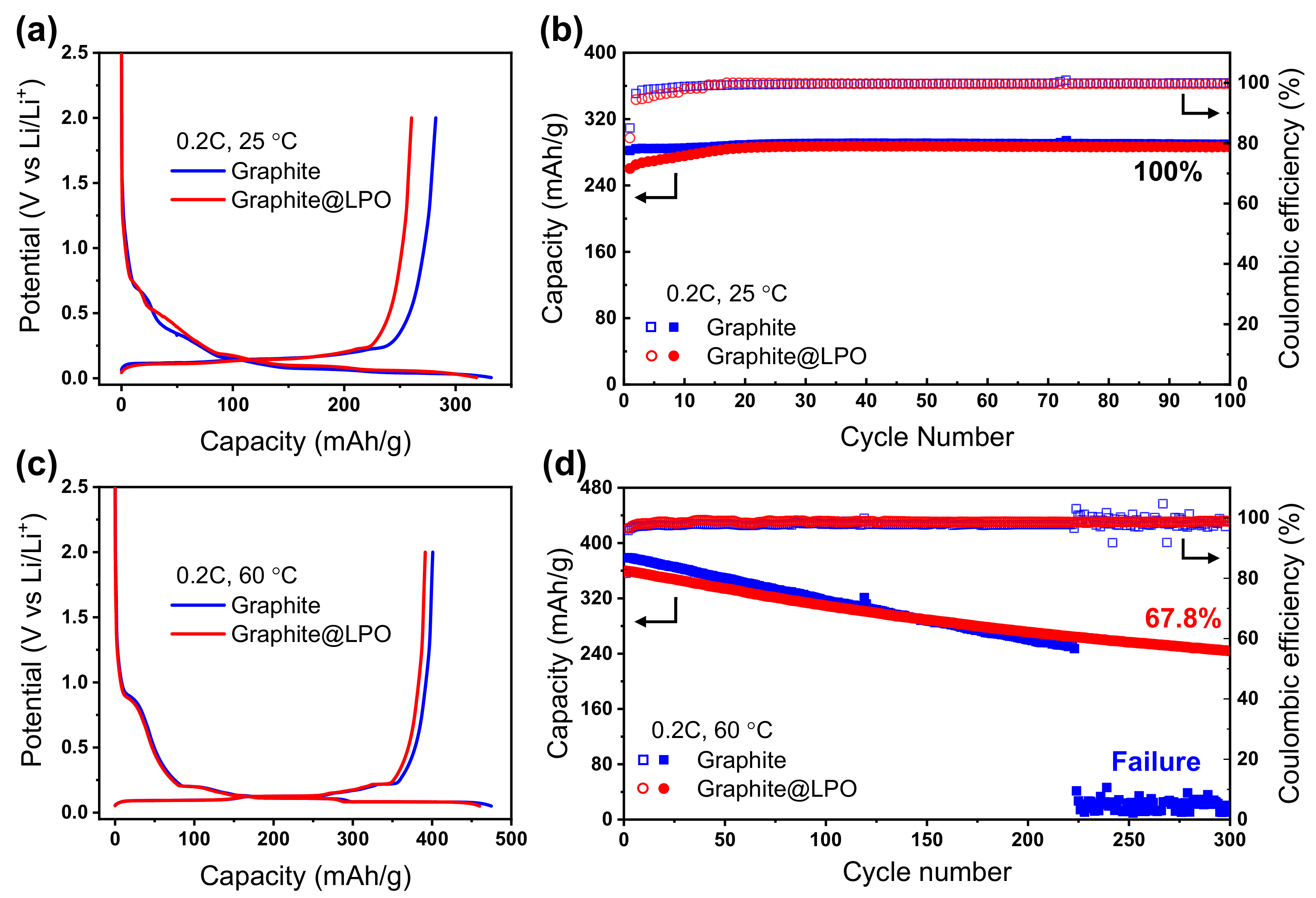
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Meng, Y.S.; Bréger, J.; Grey, C.P.; Ceder, G. Electrodes with High Power and High Capacity for Rechargeable Lithium Batteries. Science 2006, 311, 977. [Google Scholar] [CrossRef] [PubMed]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Gratz, E.; Apelian, D.; Wang, Y. A novel method to recycle mixed cathode materials for lithium ion batteries. Green Chem. 2013, 15, 1183–1191. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochim. Acta 2010, 55, 3909–3914. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Mohanty, D.; Dahlberg, K.; King, D.M.; David, L.A.; Sefat, A.S.; Wood, D.L.; Daniel, C.; Dhar, S.; Mahajan, V.; Lee, M.; et al. Modification of Ni-Rich FCG NMC and NCA Cathodes by Atomic Layer Deposition: Preventing Surface Phase Transitions for High-Voltage Lithium-Ion Batteries. Sci. Rep. 2016, 6, 26532. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.L.; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Yuan, T.; Cai, R.; Wang, K.; Ran, R.; Liu, S.; Shao, Z. Combustion synthesis of high-performance Li4Ti5O12 for secondary Li-ion battery. Ceram. Int. 2009, 35, 1757–1768. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Van Mierlo, J. Cost Projection of State of the Art Lithium-Ion Batteries for Electric Vehicles Up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Kurzweil, P.; Brandt, K. Chapter 3—Overview of Rechargeable Lithium Battery Systems. In Electrochemical Power Sources: Fundamentals, Systems, and Applications; Garche, J., Brandt, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–82. [Google Scholar]
- Xu, L.; Thompson, C.V. Mechanisms of the cyclic (de)lithiation of RuO2. J. Mater. Chem. A 2020, 8, 21872–21881. [Google Scholar] [CrossRef]
- Xu, L.; Thompson, C.V. Electrochemically controlled reversible formation of organized channel arrays in nanoscale-thick RuO2 films: Implications for mechanically stable thin films and microfluidic devices. ACS Appl. Nano Mater. 2021, 4, 13700–13707. [Google Scholar] [CrossRef]
- Xu, L.; Chon, M.J.; Mills, B.; Thompson, C.V. Mechanical stress and morphology evolution in RuO2 thin film electrodes during lithiation and delithiation. J. Power Sources 2022, 552, 232260. [Google Scholar] [CrossRef]
- Eom, J.-Y.; Cho, Y.-H.; Kim, S.-I.; Han, D.; Sohn, D. Improvements in the electrochemical performance of Li4Ti5O12-coated graphite anode materials for lithium-ion batteries by simple ball-milling. J. Alloys Compd. 2017, 723, 456–461. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Zhang, F.; Cai, L.; Zhang, L.; Wen, Z. Carbon-coated isotropic natural graphite spheres as anode material for lithium-ion batteries. Ceram. Int. 2017, 43, 9458–9464. [Google Scholar] [CrossRef]
- Glazier, S.L.; Li, J.; Louli, A.J.; Allen, J.P.; Dahn, J.R. An Analysis of Artificial and Natural Graphite in Lithium Ion Pouch Cells Using Ultra-High Precision Coulometry, Isothermal Microcalorimetry, Gas Evolution, Long Term Cycling and Pressure Measurements. J. Electrochem. Soc. 2017, 164, A3545–A3555. [Google Scholar] [CrossRef]
- Park, J.K. Principles and Applications of Lithium Secondary Batteries; Wiley: Weinheim, Germany, 2012. [Google Scholar]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Choi, J.; Ryou, M.-H.; Son, B.; Song, J.; Park, J.-K.; Cho, K.Y.; Lee, Y.M. Improved high-temperature performance of lithium-ion batteries through use of a thermally stable co-polyimide-based cathode binder. J. Power Sources 2014, 252, 138–143. [Google Scholar] [CrossRef]
- Robertson, A.D.; West, A.R.; Ritchie, A.G. Review of crystalline lithium-ion conductors suitable for high temperature battery applications. Solid State Ion. 1997, 104, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Li, S.; Tong, Z.; Wang, D.; Zhuang, H.L.; Wang, X.; Lu, Y. Lithium-Aluminum-Phosphate coating enables stable 4.6 V cycling performance of LiCoO2 at room temperature and beyond. Energy Storage Mater. 2021, 37, 67–76. [Google Scholar] [CrossRef]
- Kalluri, S.; Yoon, M.; Jo, M.; Park, S.; Myeong, S.; Kim, J.; Dou, S.X.; Guo, Z.; Cho, J. Surface Engineering Strategies of Layered LiCoO2 Cathode Material to Realize High-Energy and High-Voltage Li-Ion Cells. Adv. Energy Mater. 2017, 7, 1601507. [Google Scholar] [CrossRef]
- Yuan, H.; Song, W.; Wang, M.; Gu, Y.; Chen, Y. Lithium-ion conductive coating layer on nickel rich layered oxide cathode material with improved electrochemical properties for Li-ion battery. J. Alloys Compd. 2019, 784, 1311–1322. [Google Scholar] [CrossRef]
- Hasan, F.; Kim, J.; Song, H.; Lee, S.H.; Sung, J.H.; Kim, J.; Yoo, H.D. Effect of Particle Size and Doping on the Electrochemical Characteristics of Ca-doped LiCoO2 Cathodes. J. Electrochem. Sci. Tech. 2020, 11, 352–360. [Google Scholar]
- Zhang, J.-N.; Li, Q.; Ouyang, C.; Yu, X.; Ge, M.; Huang, X.; Hu, E.; Ma, C.; Li, S.; Xiao, R.; et al. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat. Energy 2019, 4, 594–603. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X.; Lei, D.; Qin, Y.; Wen, J.; Guo, F.; Wu, Y.A.; Rong, Y.; Kou, R.; Xiao, X. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y.W.; Kim, B.; Lee, J.G.; Park, B. A breakthrough in the safety of lithium secondary batteries by coating the cathode material with AlPO4 nanoparticles. Angew. Chem. Int. Ed. 2003, 42, 1618–1621. [Google Scholar] [CrossRef]
- Ma, X.; Wang, C.; Han, X.; Sun, J. Effect of AlPO4 coating on the electrochemical properties of LiNi0.8Co0.2O2 cathode material. J. Alloys Compd. 2008, 453, 352–355. [Google Scholar] [CrossRef]
- Qi, R.; Shi, J.-L.; Zhang, X.-D.; Zeng, X.-X.; Yin, Y.-X.; Xu, J.; Chen, L.; Fu, W.-G.; Guo, Y.-G.; Wan, L.-J. Improving the stability of LiNi0.80Co0.15Al0.05O2 by AlPO4 nanocoating for lithium-ion batteries. Sci. China Chem. 2017, 60, 1230–1235. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, X.; Zhao, T.; Li, L.; Xie, M.; Chen, R. Multifunctional AlPO4 coating for improving electrochemical properties of low-cost Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 cathode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 3773–3781. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-L.; You, L.-Q.; Cao, X.-Y.; Zhang, C.-F.; Song, D.-W.; Qu, L.-B. Co3(PO4)2-coated LiV3O8 as positive materials for rechargeable lithium batteries. Electron. Mater. Lett. 2012, 8, 411–415. [Google Scholar] [CrossRef]
- Hu, G.-R.; Deng, X.-R.; Peng, Z.-D.; Du, K. Comparison of AlPO4-and Co3(PO4)2-coated LiNi0.8Co0.2O2 cathode materials for Li-ion battery. Electrochim. Acta 2008, 53, 2567–2573. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Yoo, E.; Ishida, M.; Zhou, H. Fabrication of FePO4 layer coated LiNi1/3Co1/3Mn1/3O2: Towards high-performance cathode materials for lithium ion batteries. Electrochim. Acta 2012, 83, 253–258. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, H.-Q.; Yin, Y.-P.; Sun, X.-Y.; Bai, X.-T.; Shen, X.-L.; Zhuang, W.-D.; Lu, S.-G. FePO4-coated Li[Li0.2Ni0.13Co0.13Mn0.54]O2 with improved cycling performance as cathode material for Li-ion batteries. Rare Metals 2017, 36, 899–904. [Google Scholar] [CrossRef]
- Kim, K.C.; Jegal, J.-P.; Bak, S.-M.; Roh, K.C.; Kim, K.-B. Improved high-voltage performance of FePO4-coated LiCoO2 by microwave-assisted hydrothermal method. Electrochem. Commun. 2014, 43, 113–116. [Google Scholar] [CrossRef]
- Guan, P.; Zhou, L.; Yu, Z.; Sun, Y.; Liu, Y.; Wu, F.; Jiang, Y.; Chu, D. Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries. J. Energy Chem. 2020, 43, 220–235. [Google Scholar] [CrossRef]
- Zuo, D.; Tian, G.; Li, X.; Chen, D.; Shu, K. Recent progress in surface coating of cathode materials for lithium ion secondary batteries. J. Alloys Compd. 2017, 706, 24–40. [Google Scholar] [CrossRef]
- Sung, J.H.; Kim, T.W.; Kang, H.-K.; Choi, S.Y.; Hasan, F.; Mohanty, S.K.; Kim, J.; Srinivasa, M.K.; Shin, H.-C.; Yoo, H.D. Superior high voltage LiNi0.6Co0.2Mn0.2O2 cathode using Li3PO4 coating for lithium-ion batteries. Korean J. Chem. Eng. 2021, 38, 1059–1065. [Google Scholar] [CrossRef]
- Song, H.G.; Kim, J.Y.; Kim, K.T.; Park, Y.J. Enhanced electrochemical properties of Li(Ni0.4Co0.3Mn0.3)O2 cathode by surface modification using Li3PO4-based materials. J. Power Sources 2011, 196, 6847–6855. [Google Scholar] [CrossRef]
- Sung, J.H.; Hasan, F.; Yoo, H.D. Accelerated Formation of Surface Films on the Degradation of LiCoO2 Cathode at High Temperature. J. Korean Electrochem. Soc. 2020, 23, 57–65. [Google Scholar]
- Chong, J.; Xun, S.; Zhang, J.; Song, X.; Xie, H.; Battaglia, V.; Wang, R. Li3PO4-Coated LiNi0.5Mn1.5O4: A stable high-voltage cathode material for lithium-ion batteries. Eur. J. Chem. 2014, 20, 7479–7485. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Kim, M.-S.; Jeong, J.H.; Kim, D.-H.; Chung, K.Y.; Roh, K.C.; Kim, K.-B. Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: Improved thermal stability and high-voltage performance. J. Power Sources 2017, 360, 206–214. [Google Scholar] [CrossRef]
- Li, X.; Yang, R.; Cheng, B.; Hao, Q.; Xu, H.; Yang, J.; Qian, Y. Enhanced electrochemical properties of nano-Li3PO4 coated on the LiMn2O4 cathode material for lithium ion battery at 55 °C. Mater. Lett. 2012, 66, 168–171. [Google Scholar] [CrossRef]
- Bian, X.; Fu, Q.; Bie, X.; Yang, P.; Qiu, H.; Pang, Q.; Chen, G.; Du, F.; Wei, Y. Improved electrochemical performance and thermal stability of Li-excess Li1.18Co0.15Ni0.15Mn0.52O2 cathode material by Li3PO4 surface coating. Electrochim. Acta 2015, 174, 875–884. [Google Scholar] [CrossRef]
- Yan, W.; Yang, S.; Huang, Y.; Yang, Y.; Yuan, G. A review on doping/coating of nickel-rich cathode materials for lithium-ion batteries. J. Alloys Compd. 2020, 819, 153048. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, F.; Li, L.; Chen, M.; Zhong, X.; Li, W.; Lu, L. Effect of Li3PO4 coating of layered lithium-rich oxide on electrochemical performance. J. Power Sources 2017, 341, 147–155. [Google Scholar] [CrossRef]
- Liu, H.; Chen, C.; Du, C.; He, X.; Yin, G.; Song, B.; Zuo, P.; Cheng, X.; Ma, Y.; Gao, Y. Lithium-rich Li1.2Ni0.13Co0.13Mn0.54O2 oxide coated by Li3PO4 and carbon nanocomposite layers as high performance cathode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2634–2641. [Google Scholar] [CrossRef]
- Schweidler, S.; de Biasi, L.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Volume changes of graphite anodes revisited: A combined operando X-ray diffraction and in situ pressure analysis study. J. Phys. Chem. C 2018, 122, 8829–8835. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, H.J.; Lee, J.; Jeong, Y.K.; Choi, J.W.; Lee, H. Mussel-Inspired Polydopamine Coating for Enhanced Thermal Stability and Rate Performance of Graphite Anodes in Li-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 13973–13981. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-J.; Park, K.; Park, S.-H.; Lee, H. Unraveling the role of LiFSI electrolyte in the superior performance of graphite anodes for Li-ion batteries. Electrochim. Acta 2018, 259, 949–954. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, L.; Xu, Y.; Cao, X.; Engelhard, M.H.; Matthews, B.E.; Zhong, L.; Wu, H.; Jia, H.; Ren, X. Advanced electrolytes for fast-charging high-voltage lithium-ion batteries in wide-temperature range. Adv. Energy Mater. 2020, 10, 2000368. [Google Scholar] [CrossRef]
- Tao, T.; Chen, C.; Yao, Y.; Liang, B.; Lu, S.; Chen, Y. Enhanced electrochemical performance of ZrO2 modified LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries. Ceram. Int. 2017, 43, 15173–15178. [Google Scholar] [CrossRef]
- Lee, S.-H.; You, H.-G.; Han, K.-S.; Kim, J.; Jung, I.-H.; Song, J.-H. A new approach to surface properties of solid electrolyte interphase on a graphite negative electrode. J. Power Sources 2014, 247, 307–313. [Google Scholar] [CrossRef]
- Yamada, Y.; Iriyama, Y.; Abe, T.; Ogumi, Z. Kinetics of Lithium Ion Transfer at the Interface between Graphite and Liquid Electrolytes: Effects of Solvent and Surface Film. Langmuir 2009, 25, 12766–12770. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, J.H.; Kim, T.; Kim, S.; Hasan, F.; Mohanty, S.K.; Srinivasa, M.K.; Reddy, S.C.; Yoo, H.D. Li3PO4-Coated Graphite Anode for Thermo-Electrochemically Stable Lithium-Ion Batteries. Energies 2023, 16, 6141. https://doi.org/10.3390/en16176141
Sung JH, Kim T, Kim S, Hasan F, Mohanty SK, Srinivasa MK, Reddy SC, Yoo HD. Li3PO4-Coated Graphite Anode for Thermo-Electrochemically Stable Lithium-Ion Batteries. Energies. 2023; 16(17):6141. https://doi.org/10.3390/en16176141
Chicago/Turabian StyleSung, Jong Hun, Taewan Kim, Soljin Kim, Fuead Hasan, Sangram Keshari Mohanty, Madhusudana Koratikere Srinivasa, Sri Charan Reddy, and Hyun Deog Yoo. 2023. "Li3PO4-Coated Graphite Anode for Thermo-Electrochemically Stable Lithium-Ion Batteries" Energies 16, no. 17: 6141. https://doi.org/10.3390/en16176141
APA StyleSung, J. H., Kim, T., Kim, S., Hasan, F., Mohanty, S. K., Srinivasa, M. K., Reddy, S. C., & Yoo, H. D. (2023). Li3PO4-Coated Graphite Anode for Thermo-Electrochemically Stable Lithium-Ion Batteries. Energies, 16(17), 6141. https://doi.org/10.3390/en16176141







