Investigation of the Proton Exchange Membrane Fuel Cell System Cathode Exhaust Gas Composition Based on Test Bed Measurements
Abstract
:1. Introduction
2. Materials and Methods
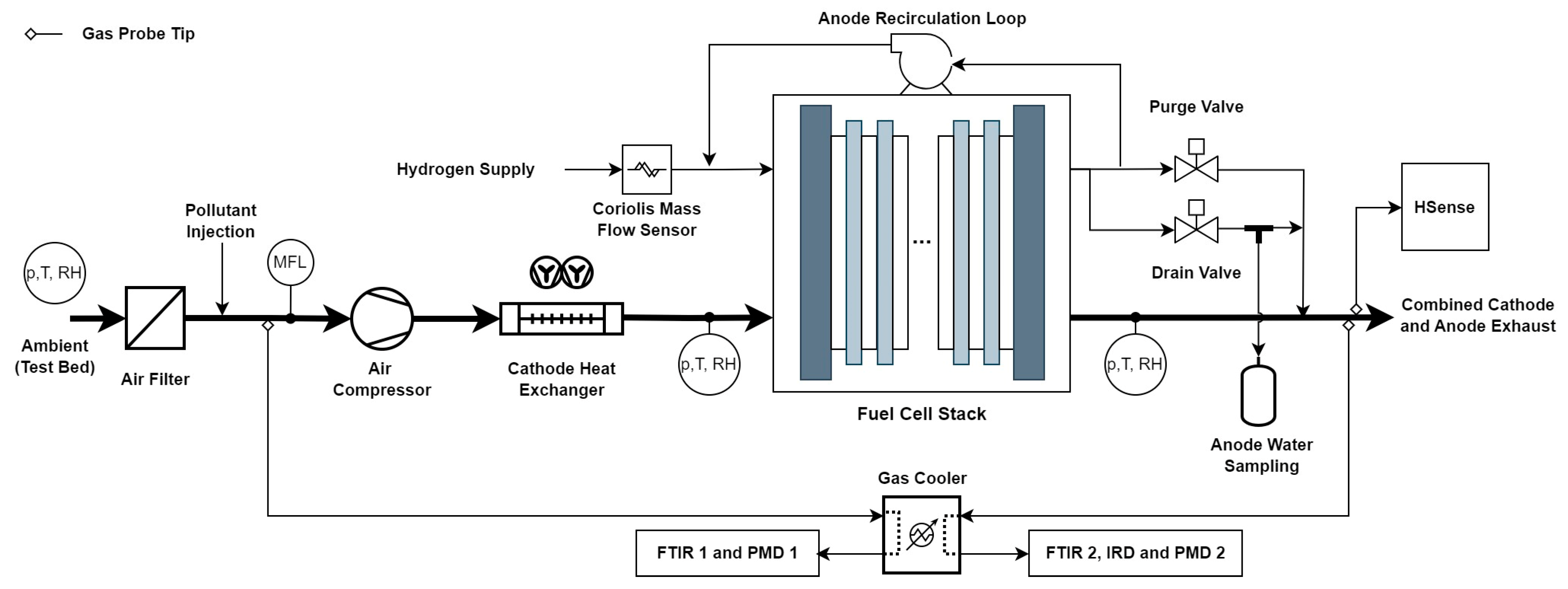
2.1. Test Bed Setup
2.2. Gas Measurement
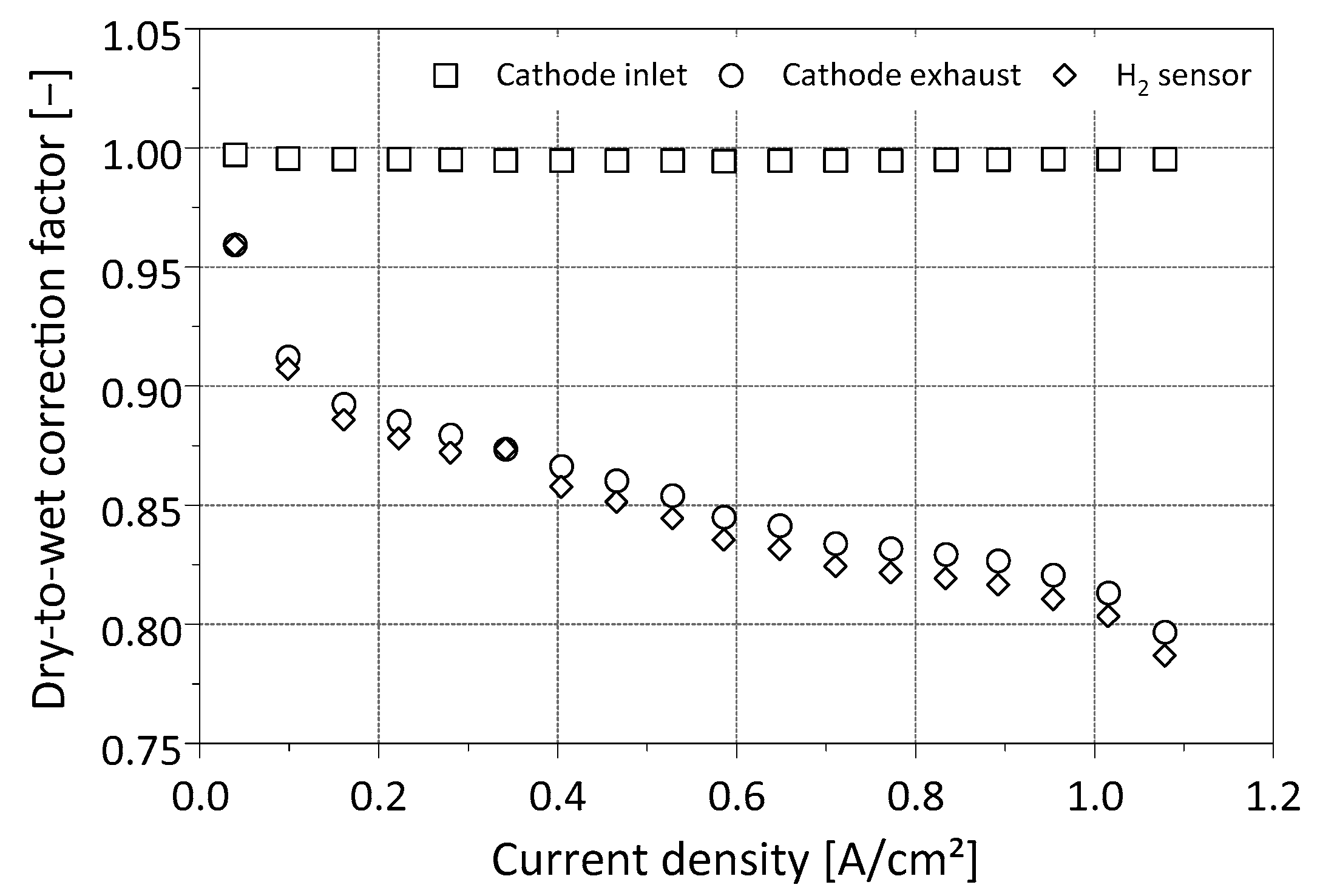
2.2.1. Compensation of Water Condensation in the Gas Cooler
2.2.2. Gas Mass Calculation
2.2.3. Gas Analyzer Comparison
2.3. Mathematical Models
- All gases are thermodynamically ideal gases.
- The cathode inlet air molar mass is based on synthetic air (consisting of N2 and O2 only) and water vapor.
- The water formed as a product is formed in a liquid state at the reaction site and contacts the cathode gas, enabling water vapor transfer. Therefore, a fully hydrated membrane was assumed, and the possibility of water transport through the membrane to the anode was excluded.
- All water formed as a product is susceptible to gas absorption and has the same temperature as the cathode exhaust gas.
- No liquid or gas diffusion through the membrane is considered.
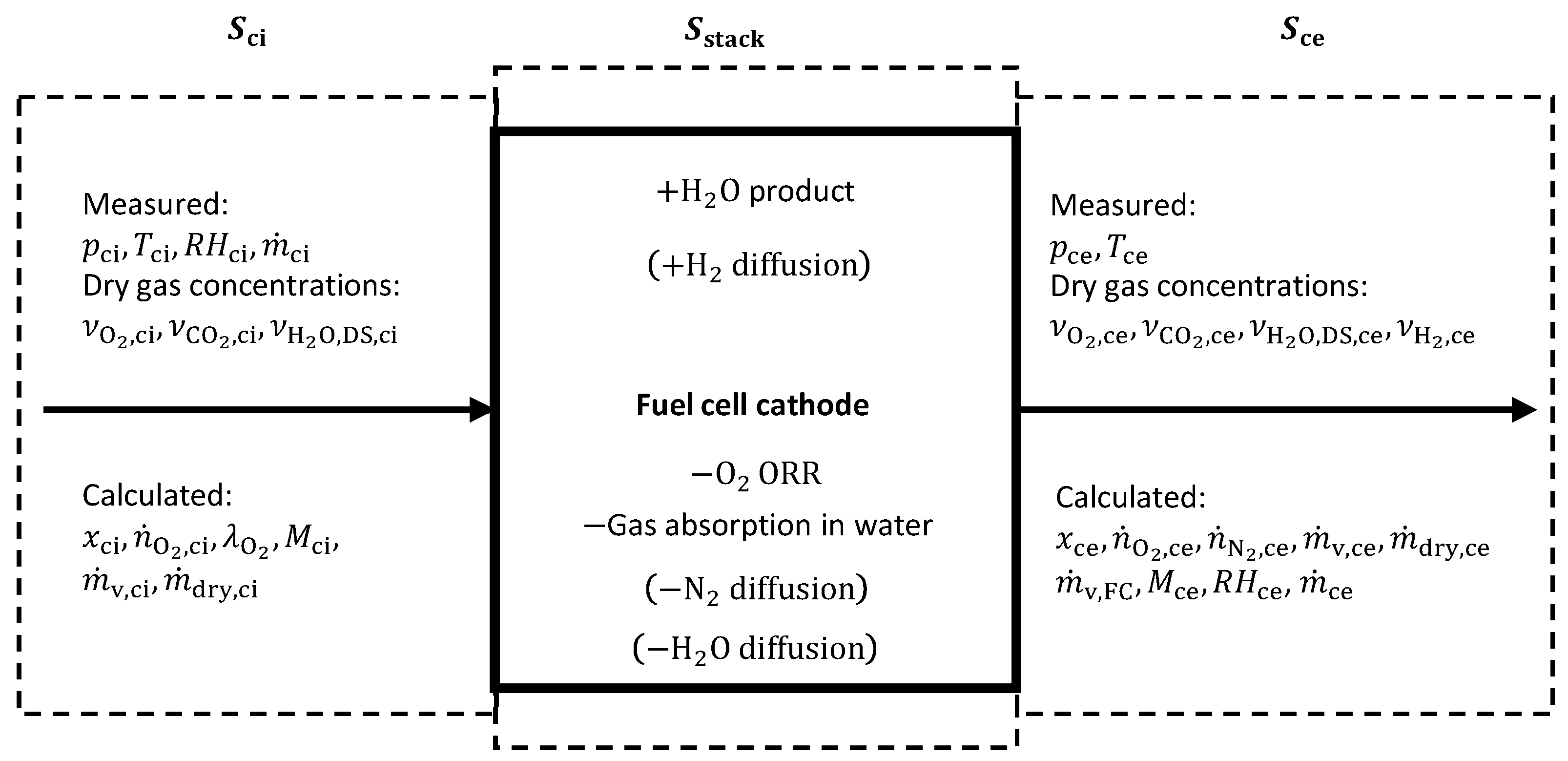
2.3.1. Cathode Inlet Calculations
2.3.2. Cathode Exhaust Gas Calculations: Humid Air—Model-A
- Case 1: : The fuel cell exhaust gas is not saturated, and all the water produced is transported out of the fuel cell as water vapor. In this case, the exhaust relative humidity is calculated for every time step; .
- Case 2: : The fuel cell exhaust gas is considered to be saturated, with possible presence of liquid water and water vapor. In this case, the exhaust relative humidity is set to one. Values above one are fictitious, because when saturated, the maximum value that can be reached is one; , calculated based on Equation (9) with the parameters of the cathode exhaust.
2.3.3. Cathode Exhaust Gas Calculations: Humid Air—Model-B, including Exhaust Hydrogen
2.3.4. Cathode Exhaust Gas Calculations: Humid Air—Model-C, including Membrane Water Diffusion
2.3.5. Cathode Exhaust Gas Calculations: Humid Air—Model-D, including Nitrogen Diffusion
2.3.6. Gas Absorption in the Fuel Cell Product Water
3. Results and Discussion
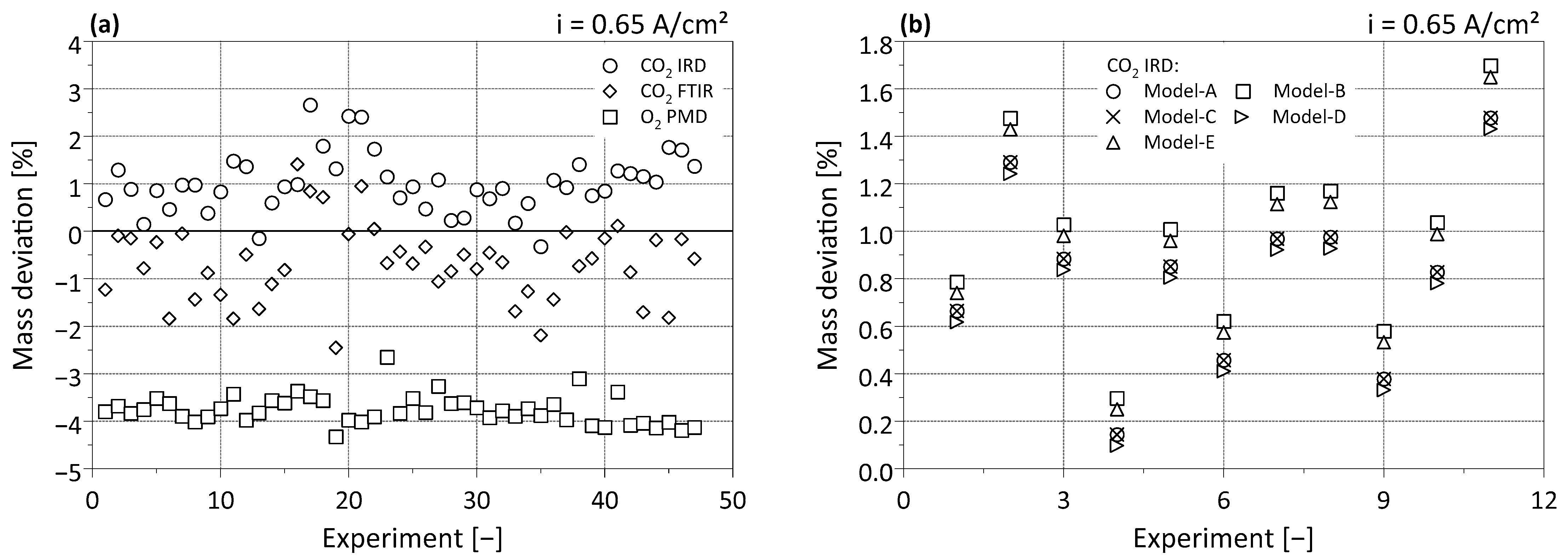
3.1. Model Evaluation
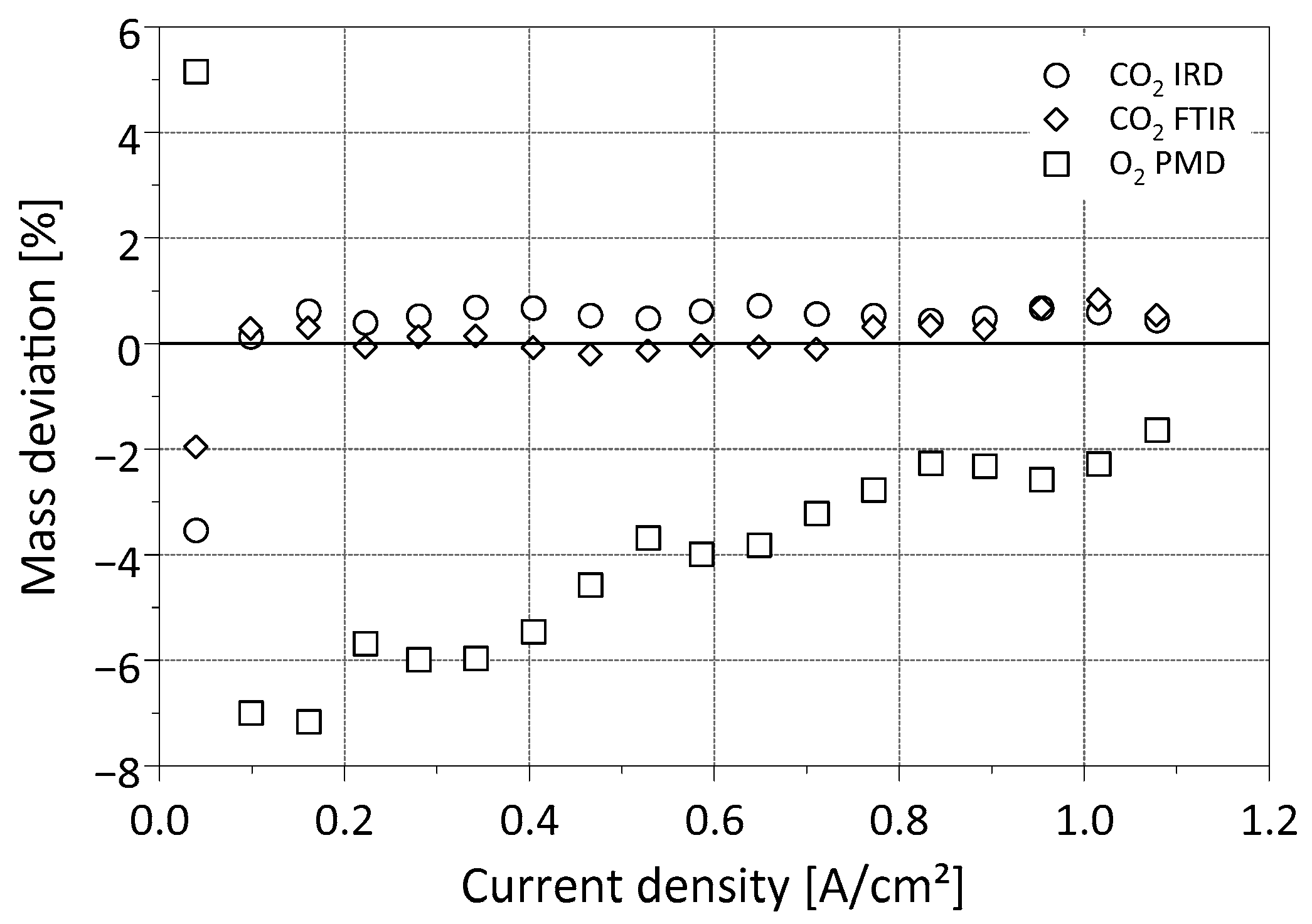
3.1.1. Steady-State Fuel Cell Operation
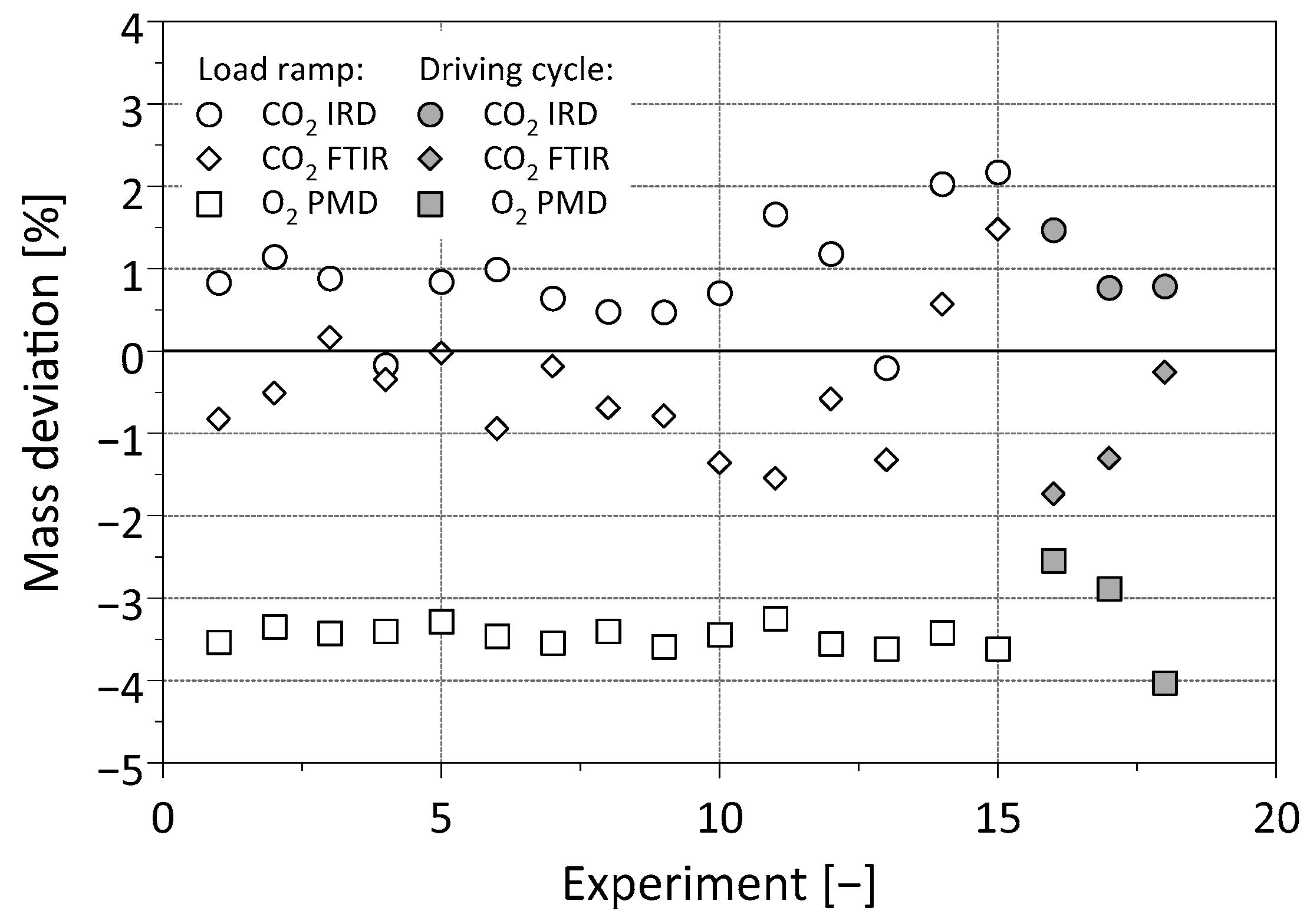
3.1.2. Fuel Cell Operation at Changing Loads
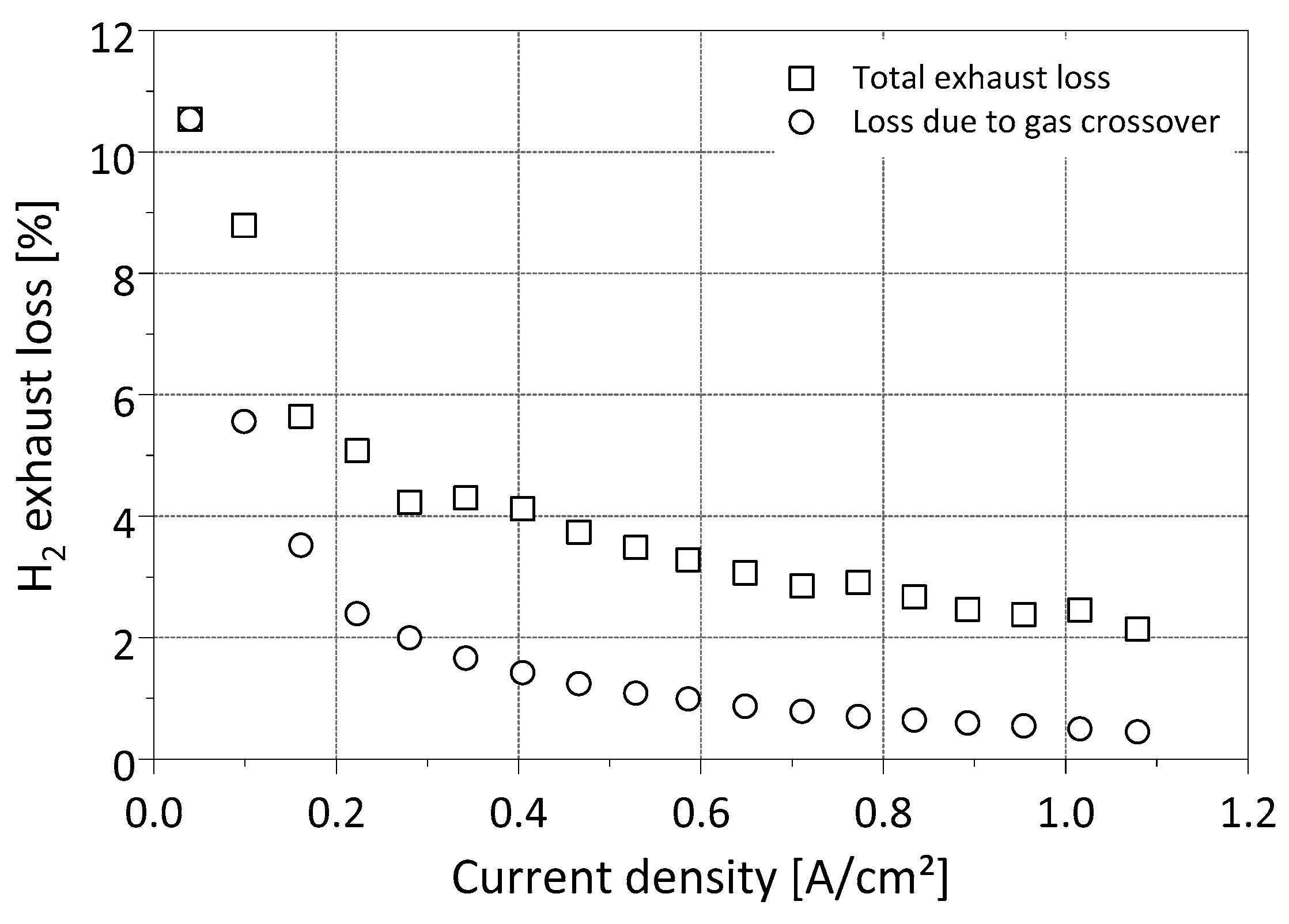
3.2. Exhaust Gas Investigation
3.3. Air Contaminant Experiments
3.3.1. NO Contamination
3.3.2. Mixed Gas Contamination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Ge, M. Review of the Research Status of Cost-Effective Zinc–Iron Redox Flow Batteries. Batteries 2022, 8, 202. [Google Scholar] [CrossRef]
- Aguilar, P.; Groß, B. Battery electric vehicles and fuel cell electric vehicles, an analysis of alternative powertrains as a mean to decarbonise the transport sector. Sustain. Energy Technol. Assess. 2022, 53, 102624. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X. A review of polymer electrolyte membrane fuel cell durability for vehicular applications: Degradation modes and experimental techniques. Energy Convers. Manag. 2019, 199, 112022. [Google Scholar] [CrossRef]
- Abdin, Z.; Webb, C.J.; Gray, E.M. PEM fuel cell model and simulation in Matlab–Simulink based on physical parameters. Energy 2016, 116, 1131–1144. [Google Scholar] [CrossRef]
- Migliardini, F.; Unich, A.; Corbo, P. Experimental comparison between external and internal humidification in proton exchange membrane fuel cells for road vehicles. Int. J. Hydrogen Energy 2015, 40, 5916–5927. [Google Scholar] [CrossRef]
- Kong, I.M.; Jung, A.; Kim, B.J.; Baik, K.D.; Kim, M.S. Experimental study on the start-up with dry gases from normal cell temperatures in self-humidified proton exchange membrane fuel cells. Energy 2015, 93, 57–66. [Google Scholar] [CrossRef]
- Pan, M.; Pan, C.; Li, C.; Zhao, J. A review of membranes in proton exchange membrane fuel cells: Transport phenomena, performance and durability. Renew. Sustain. Energy Rev. 2021, 141, 110771. [Google Scholar] [CrossRef]
- Prokop, M.; Drakselova, M.; Bouzek, K. Review of the experimental study and prediction of Pt-based catalyst degradation during PEM fuel cell operation. Curr. Opin. Electrochem. 2020, 20, 20–27. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.-S.; Wang, H.; Shen, J. A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Werner, C.; Busemeyer, L.; Kallo, J. The impact of operating parameters and system architecture on the water management of a multifunctional PEMFC system. Int. J. Hydrogen Energy 2015, 40, 11595–11603. [Google Scholar] [CrossRef]
- Becker, F.; Pillath, F.; Kallo, J. Cathode Exhaust Gas Recirculation for Polymer Electrolyte Fuel Cell Stack. Fuel Cells 2018, 18, 568–575. [Google Scholar] [CrossRef]
- Rodosik, S.; Poirot-Crouvezier, J.-P.; Bultel, Y. Impact of humidification by cathode exhaust gases recirculation on a PEMFC system for automotive applications. Int. J. Hydrogen Energy 2019, 44, 12802–12817. [Google Scholar] [CrossRef]
- Schultze, M.; Horn, J. Optimization Approach for Cathode Exhaust Gas Conditioning of a Multifunctional PEM Fuel Cell System for the Application in Aircraft; Deutsche Gesellschaft für Luft-und Raumfahrt-Lilienthal-Oberth eV: Bonn, Germany, 2012. [Google Scholar]
- Schultze, M.; Horn, J. Modeling, state estimation and nonlinear model predictive control of cathode exhaust gas mass flow for PEM fuel cells. Control Eng. Pract. 2016, 49, 76–86. [Google Scholar] [CrossRef]
- Kuroda, E.; Yano, M. An Oxygen Balance Method: Fuel Consumption Measurement for Fuel Cell Vehicles based on Exhaust Emissions with No Vehicle Modification. Adv. Automob. Eng. 2016, 5, 1000152. [Google Scholar] [CrossRef]
- Reithuber, P.; Poimer, F.; Brandstätter, S.; Schutting, E.; Buchberger, S.; Trattner, A.; Eichlseder, H. Experimental Investigation of the Influence of NO on a PEM Fuel Cell System and Voltage Recovery Strategies. Energies 2023, 16, 3720. [Google Scholar] [CrossRef]
- Wiegleb, G. Gasmesstechnik in Theorie und Praxis: Messgeräte, Sensoren, Anwendungen, 2nd ed.; Korrigierte und Durchgesehene Auflage; Springer eBook Collection; Springer Vieweg: Wiesbaden, Germany, 2022. [Google Scholar]
- Karimäki, H.; Pérez, L.; Nikiforow, K.; Keränen, T.; Viitakangas, J.; Ihonen, J. The use of on-line hydrogen sensor for studying inert gas effects and nitrogen crossover in PEMFC system. Int. J. Hydrogen Energy 2011, 36, 10179–10187. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- St-Pierre, J.; Zhai, Y.; Angelo, M.S. Effect of Selected Airborne Contaminants on PEMFC Performance. J. Electrochem. Soc. 2014, 161, F280–F290. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Sasmito, A.P.; Shamim, T. Advances in proton exchange membrane fuel cell with dead-end anode operation: A review. Appl. Energy 2019, 252, 113416. [Google Scholar] [CrossRef]


| Model Index | Modification |
|---|---|
| Model-A | Base and reference model |
| Model-B | Model considering the H2 concentration at the cathode, also in the calculation of the exhaust humid air |
| Model-C | Model including the water diffusion through the membrane |
| Model-D | Model considering the N2 diffusion to the anode, resulting in a reduced N2 concentration at the cathode |
| Model-E | A combination of Model-A, -B, -C, and -D |
| O2 [g] | CO2 [mg] | CO [mg] | C3H8 [mg] | NO [mg] | |
|---|---|---|---|---|---|
| At cathode inlet | 139.06 | 3190 | 17 | 28 | 5 |
| Consumed | 65.77 | - | - | - | - |
| Exhausted | 62.26 | 3127 | 0 | n/a | 0 |
| Difference | −11.03 (−7.93%) | −63 (−2%) | −17 (−100%) | n/a | −5 (−100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reithuber, P.; Frühwirth, C.; Buchberger, S.; Eichlseder, H. Investigation of the Proton Exchange Membrane Fuel Cell System Cathode Exhaust Gas Composition Based on Test Bed Measurements. Energies 2023, 16, 6057. https://doi.org/10.3390/en16166057
Reithuber P, Frühwirth C, Buchberger S, Eichlseder H. Investigation of the Proton Exchange Membrane Fuel Cell System Cathode Exhaust Gas Composition Based on Test Bed Measurements. Energies. 2023; 16(16):6057. https://doi.org/10.3390/en16166057
Chicago/Turabian StyleReithuber, Peter, Christian Frühwirth, Simon Buchberger, and Helmut Eichlseder. 2023. "Investigation of the Proton Exchange Membrane Fuel Cell System Cathode Exhaust Gas Composition Based on Test Bed Measurements" Energies 16, no. 16: 6057. https://doi.org/10.3390/en16166057
APA StyleReithuber, P., Frühwirth, C., Buchberger, S., & Eichlseder, H. (2023). Investigation of the Proton Exchange Membrane Fuel Cell System Cathode Exhaust Gas Composition Based on Test Bed Measurements. Energies, 16(16), 6057. https://doi.org/10.3390/en16166057






