Silica Nanoparticle Formation from Supercritical Geothermal Sources
Abstract
1. Introduction
2. The Precipitation Process
- (1)
- Increase in Supersaturation [31]
- The solubility/equilibrium concentration of minerals in a solution are specific for the mineral and may depend on the temperature, density, pH, ionic strength of the solution, degree of hydration and degree of dissociation. Any disruption in these factors may change the chemical equilibrium. Solubility relates to the chemical potential and change in Gibbs free energy upon a reaction. It is usually expressed through ion activity as an equilibrium constant (“K”);
- (2)
- Nucleation is either homogenous or heterogeneous. In homogenous nucleation, the monomeric units collide spontaneously until there are enough molecules organized in the right order to form a stable nucleus, where more material is incorporated, and a particle can grow. In heterogeneous nucleation, nuclei form on a foreign substance, such as a surface or an existing particle in the solution;
- There exists a time period that elapses between the establishment of supersaturation in a system and the detectable appearance of solid particles. This is the time required for the chemical reaction to take place and for the progressive formation of many nuclei and their overgrowth to macroscopical sizes, and it is called the induction time. At comparable supersaturation, homogenous nucleation has a longer induction time than heterogeneous nucleation because the energy barrier for homogenous nucleation is higher than that for heterogeneous nucleation;
- The speeds of the chemical reaction, nucleation process and crystal growth are defined as the reaction kinetics and they may be affected by several factors, including the degree of supersaturation, temperature, pressure, pH, concentration and composition of the mixture;
- (3)
- Growth [29]
- Growth can be either surface growth, Ostwald ripening or agglomeration. In surface growth, molecules are added to the surface. The process can be limited either by the diffusion of molecules to the surface or by chemical absorption onto the surface. In the case of strictly structured growth with a high concentration and relatively low supersaturations, the latter is often limiting. When Ostwald ripening occurs, it is often because the radius of the curvature affects the solubility such that smaller particles are dissolved in favor of the growth of larger particles. This effect is observed in the generation of silica particles in liquid and often results in a relatively monodisperse solution. Agglomeration occurs when two or more particles collide to form larger particles. This mechanism is very relevant when supersaturation is high because the critical radius decreases with increasing supersaturation and a large number of very small particles are formed initially. The shape of the growth curve can help determine what mechanism is dominating. Interplay between growth and agglomeration is necessary for the full cementation of the new particles, but the collision rate is often limiting when determining the agglomeration rate;
- (4)
- Particulate Deposition [28]
- Deposition can occur via molecular solidification directly onto a surface, or by solid particles formed in the bulk fluid and transported to a surface. In the latter case, deposition onto a surface may be governed by one or more of the following mechanisms: inertial impaction, diffusion (Brownian or eddy), gravitation, thermophoresis, interception or diffusiophoresis;
- (5)
- Material Buildup
- The scale thickness as a function of time is dependent on the reaction kinetics, deposition rates, scale microstructure and hydrodynamic factors. In cases in which the deposited layer is porous, it may be torn off completely or partly and carried with the stream. The degree of re-entrainment will depend on changes in the shear stress, the presence of erosive particles and the crystalline structure of the scale. It may be difficult to determine whether the limiting factor for scale growth is the probability of particle cementation (to the wall and each other), or the rate of particle transport to the surface.
3. Solubility
3.1. Silica Solubility in Natural and Pure Liquid Water
3.2. Physical and Chemical Properties of Supercritical Geothermal Fluids
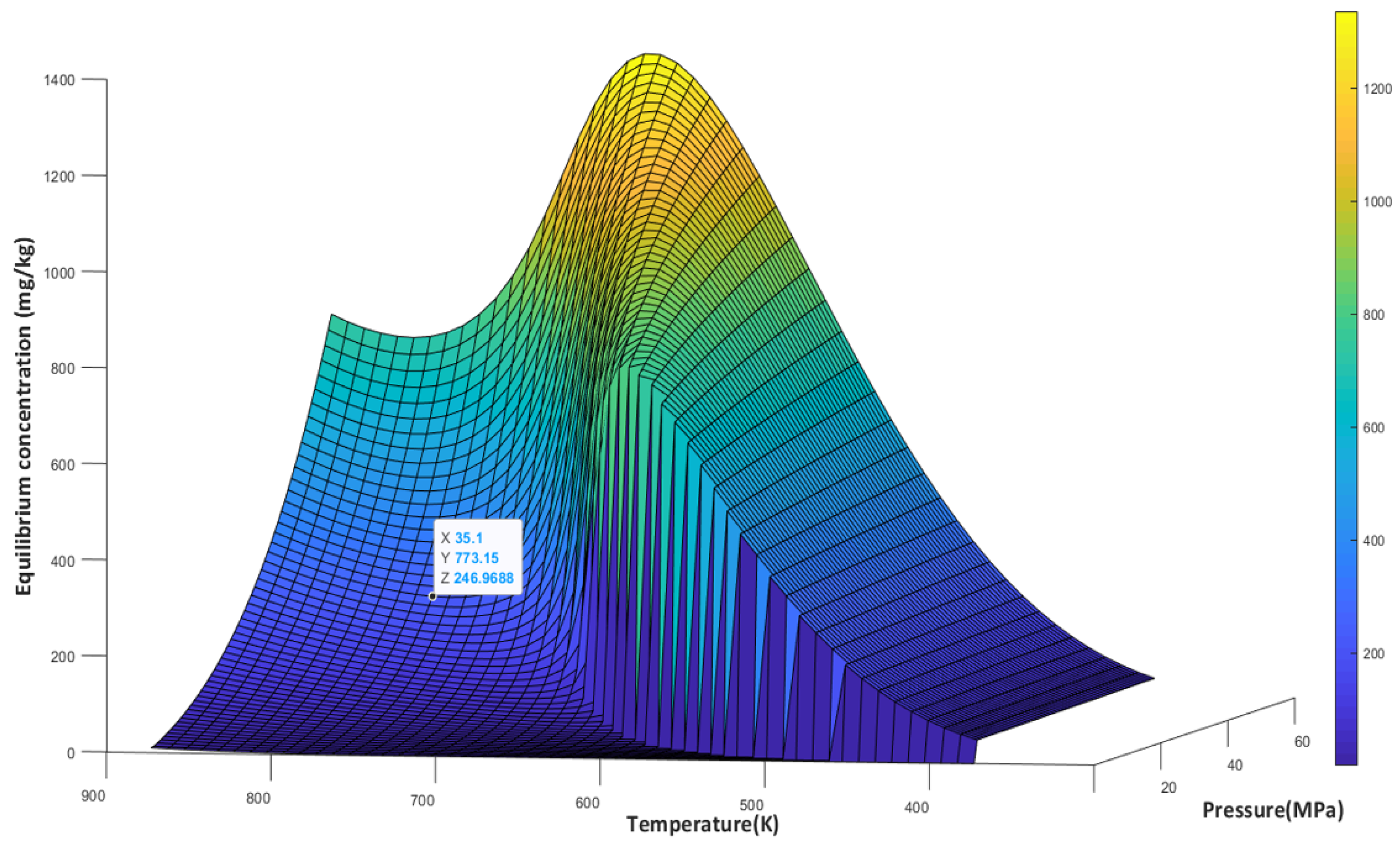
3.3. Silica Solubility in Supercritical and Superheated Steam
4. Kinetics
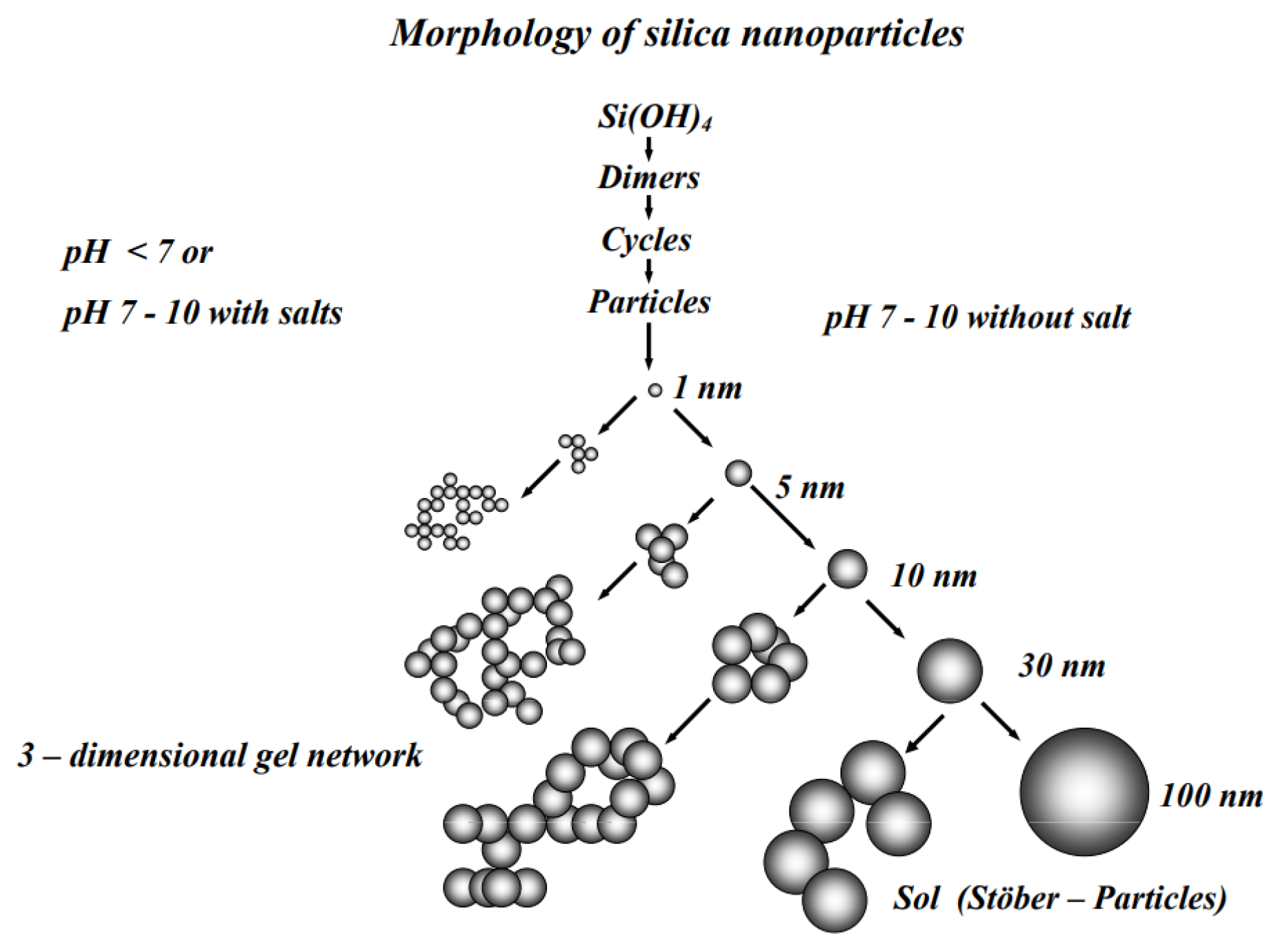
4.1. Forms of Silica Precipitate
4.2. Kinetics Experiments Involving Silica Precipitation in Liquid Water
4.3. The Path from Dissolved Si(OH)4 to Solid SiO2 Based on Liquid-Water Experimental Results
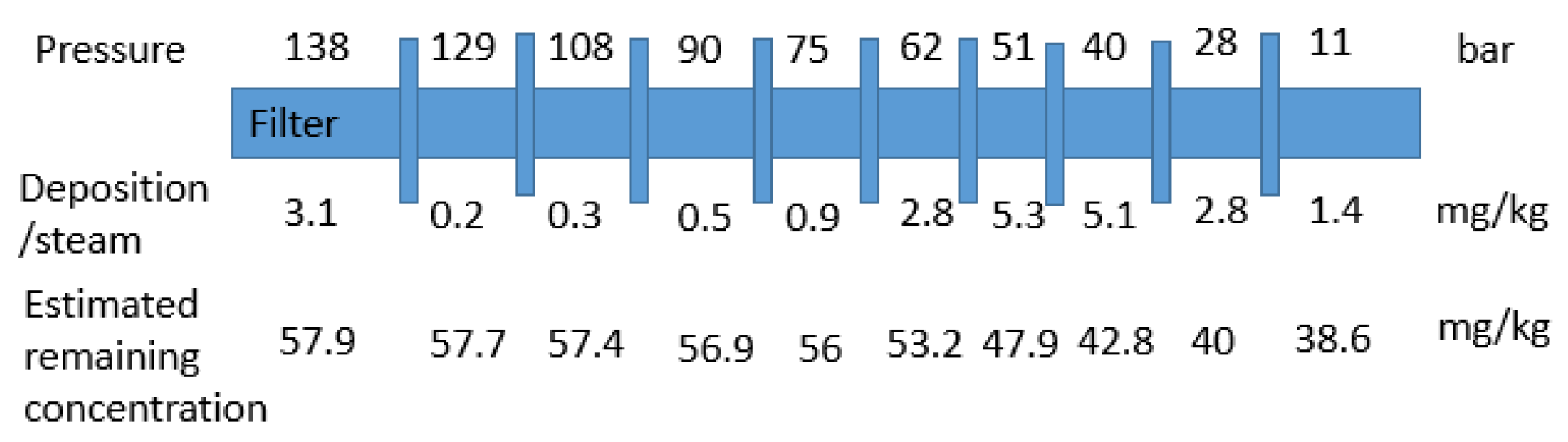
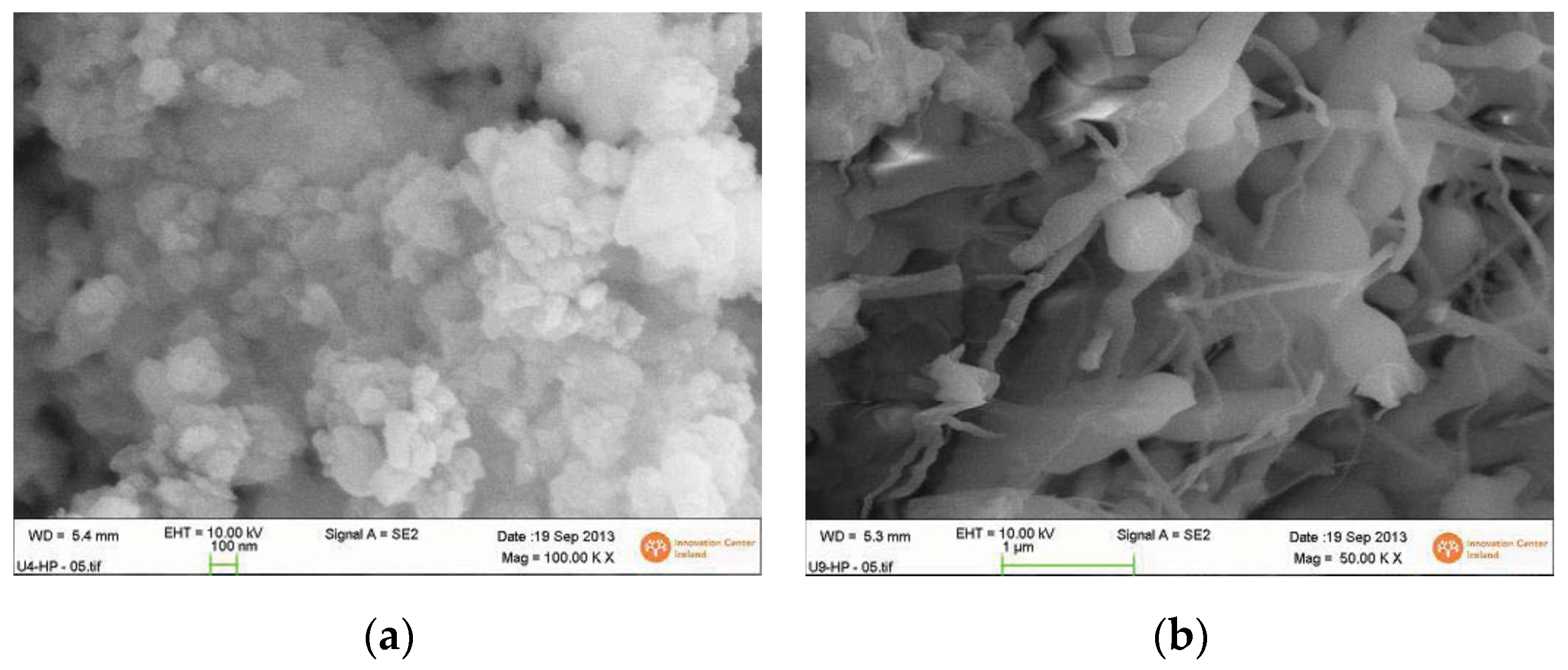
4.4. Observations of Silica Precipitation from Depressurized Superheated Steam
5. Discussion
- Nucleation, resulting in a population balance () that can be calculated as a function of time using classical nucleation theory;
- Growth into nanocolloids () via agglomeration. The population balance develops at a rate depending on the size of the critical nucleus generated, the number of solid particles in the solution and the flow characteristics;
- Deposition then determines the remaining amount of precipitate in the steam (). Deposition onto surfaces follows the laws of mass transport and can be calculated using numerical modeling via CFD (Computational Fluid Dynamics) tools, such as ANSYS FLUENT or OpenFoam. The rate of deposition will depend on the size and characteristics of the particles, in addition to the flow characteristics.
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reinsch, T.; Dobson, P.; Asanuma, H.; Huenges, E.; Poletto, F.; Sanjuan, B. Utilizing supercritical geothermal systems: A review of past ventures and ongoing research activities. Geothermal Energy 2017, 5, 16. [Google Scholar] [CrossRef]
- Battistelli, A.; Finsterle, S.; Marcolini, M.; Pan, L. Modeling of coupled wellbore-reservoir flow in steam-like supercritical geothermal systems. Geothermics 2020, 86, 101793. [Google Scholar] [CrossRef]
- Asanuma, H.; Mogi, T.; Tsuchiya, N.; Watanabe, N.; Naganawa, S.; Ogawa, Y.; Fujimitsu, Y.; Kajiwara, T.; Osato, K.; Shimada, K.; et al. Japanese Supercritical Geothermal Project for Drastic Increase of Geothermal Power Generation in 2050. In Proceedings of the World Geothermal Congress 2020+1, Reykjavik, Iceland, 30 March–27 October 2021. [Google Scholar]
- Tsuchiya, N. Geological Model and Potential of Supercritical Geothermal Reservoir. In Proceedings of the World Geothermal Congress 2020+1, Reykjavik, Iceland, 30 March–27 October 2021. [Google Scholar]
- Feng, G.; Wang, Y.; Xu, T.; Wang, F.; Shi, Y. Multiphase flow modeling and energy extraction performance for supercritical geothermal systems. Renew. Energy 2021, 173, 442–454. [Google Scholar] [CrossRef]
- Yapparova, B.; Lamy-Chappuis, S.W.; Scott, T.; Driesner, A. Peaceman-type well model for the 3D Control Volume Finite Element Method and numerical simulations of supercritical geothermal resource utilization. Geothermics 2022, 105, 102516. [Google Scholar] [CrossRef]
- Watanabe, K.; Watanabe, N.; Watanabe, N.; Sakaguchi, K.; Aichi, M.; Ouchi, H.; Asanuma, H. A numerical study on the creation of artificial supercritical geothermal reservoirs by hydraulic fracturing. Geothermics 2022, 105, 102500. [Google Scholar] [CrossRef]
- Oka, D.; Tamura, M.; Mogi, T.; Nakagawa, M.; Takahashi, H.; Ohzono, M.; Ichiyanagi, M. Conceptual model of supercritical geothermal system in Shiribeshi Region, Hokkaido, Japan. Geothermics 2023, 108, 102617. [Google Scholar] [CrossRef]
- Zierenberg, R.A.; Friðleifsson, G.Ó.; Elders, W.A.; Schiffman, P.; Fowler, A.P.G. Active Basalt Alteration at Supercritical Conditions in a Seawater-Recharged Hydrothermal System: IDDP-2 Drill Hole, Reykjanes, Iceland. AGU Adv. Earth Space Sci. 2021, 22, e2021GC009747. [Google Scholar]
- Óskarsson, F. Composition of Reservoir Fluids in Well IDDP-2. In Proceedings of the World Geothermal Congress 2020+1, Reykjavik, Iceland, 30 March–27 October 2021. [Google Scholar]
- Wang, Y.; Xu, T.; Cheng, Y.; Feng, G. Prospects for Power Generation of the Doublet Supercritical Geothermal System in Reykjanes Geothermal Field, Iceland. Energies 2022, 15, 8466. [Google Scholar] [CrossRef]
- DiPippo, R. Geothermal Power Generation—Developments and Innovation, 1st ed.; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Gunnarsson, I.; Arnórsson, S. Silica scaling: The main obstacle in efficient use of high-temperature geothermal fluids. In Proceedings of the International Geothermal Conference, Reykjavík, Iceland, 14–17 September 2003. [Google Scholar]
- Bahadori, A.; Vuthaluru, H.B. Prediction of silica carry-over and solubility in steam of boilers using a simple correlation. Appl. Therm. Eng. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Krikorian, O.H. Thermodynamics of the Silica-Steam System; Lawrence Radiation Laboratory, University of California: Livermore, CA, USA, 1970. [Google Scholar]
- Friðleifsson, G.Ó.; Pálsson, B.; Albertsson, A.L.; Stefánsson, B.; Gunnlaugsson, E.; Ketilsson, J.; Gíslason, Þ. IDDP-1 Drilled into Magma—World’s First Magma-EGS System Created. In Proceedings of the World Geothermal Congress, Melbourne, Australia, 19–24 April 2015. [Google Scholar]
- Ármannsson, H.; Fridriksson, T.; Gudfinnsson, G.H.; Ólafsson, M.; Óskarsson, F.; Thorbjörnsson, D. IDDP—The chemistry of the IDDP-01 well fluids in relation to the geochemistry of the Krafla geothermal system. Geothermics 2013, 49, 66–75. [Google Scholar] [CrossRef]
- Marrkússon, S.; Einarsson, K.; Pálsson, B. Landsvirkjun, IDDP 1, Flow Test 2010–2012, LV-2013-050; Landsvirkjun, Ed.; Landsvirkjun: Reykjavík, Iceland, 2013; p. 341. [Google Scholar]
- Karlsdottir, S.N.; Ragnarsdottir, K.R.; Moller, A.; Thorbjornsson, I.O.; Einarsson, A. On-site erosion–corrosion testing in superheated geothermal steam. Geothermics 2014, 51, 170–181. [Google Scholar] [CrossRef]
- Fridriksson, T.; Stefánsson, A.; Óskarsson, F.; Eyjólfsdóttir, E.; Sigurdsson, Ó. Fluid Chemistry Scenarios Anticipated for IDDP-2 to be Drilled in Reykjanes, Iceland. In Proceedings of the World Geothermal Congress, Melbourne, Australia, 19–24 April 2015. [Google Scholar]
- Bordvik, S.; Næss, E. Comparative Analysis of Energy Extraction Systems for High Temperature, High Pressure Geothermal Steam Considering Silica Precipitation World Geothermal Congress 2020. In Proceedings of the World Geothermal Congress 2020+1, Reykjavik, Iceland, 30 March–27 October 2021; pp. 1–14. [Google Scholar]
- Fournier, R.O.; Potter, R.W. A revised and expanded silica (quartz) geothermometer. Geotherm. Resour. Counc. Bull. 1982, 11, 3–12. [Google Scholar]
- Plyasunov, A.V. Thermodynamics of Si(OH)4 in the vapor phase of water: Henry’s and vapor–liquid distribution constants, fugacity and cross virial coefficients. Geochim. Cosmochim. Acta 2012, 77, 215–231. [Google Scholar] [CrossRef]
- Palmer, D.A.; Fernández-Prini, R.; Harvey, A.H. Aqueous Systems at Elevated Temperatures and Pressures—Physical Chemistry in Water, Steam and Hydrothermal Solutions; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Hack, A.C.; Thompson, A.B.; Aerts, M. Phase Relations Involving Hydrous Silicate Melts, Aqueous Fluids, and Minerals. Mineral. Geochem. 2007, 65, 129–185. [Google Scholar] [CrossRef]
- Bordvik, S.; Næss, E.; Ucar, S.; Erp, T.V. Predicting silica deposition from superheated pressurized steam using numerical modelling of classical nucleation theory, agglomeration and deposition. Energies, 2023; in review. [Google Scholar]
- Amjad, Z.; Demadis, K. Mineral Scales and Deposits; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Bott, T.R. Fouling of Heat Exchangers; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Lewis, A.; Seckler, M.; Kramer, H.; Rosmalen, G.V. Industrial Crystallization, Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- García, A.V. Measurement and Modeling of Scaling Minerals, Department og Chemical Engineering. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2005. [Google Scholar]
- Sato, N. Chemical Energy and Exergy—An Introduction to Chemical Thermodynamics for Engineers; Elsevier Science: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Langmuir, D. Aqueous Environmental Geochemistry, 1st ed.; Prentice Hall: Hoboken, NJ, USA, 1996. [Google Scholar]
- Driessche, A.E.S.V.; Kellermeier, M.; Benning, L.G.; Gebauer, D. New Perspectives on Mineral Nucleation and Growth—From Solution Precursors to Solid Materials; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Dove, P.M.; Rimstidt, J.D. Silica-Water Interactions. In Silica-Physical Behavior, Geochemistry, and Materials Applications; Heaney, P.J., Prewitt, C.T., Gibbs, G.V., Eds.; De Gruyter: Berlin, Germany, 1994. [Google Scholar]
- Brown, K. Thermodynamics and kinetics of silica scaling. In Proceedings of the International Workshop on Mineral Scaling, Manila, Philippines, 25–27 May 2011. [Google Scholar]
- Amjad, Z.; Zuhl, R.W. Silica Control in Industrial Water Systems with a New Polymeric Dispersant; Association of Water Technologies, Inc., Annual Convention & Exposition; The Lubrizol Corporation: Wickliffe, OH, USA, 2009; Volume 9. [Google Scholar]
- André, L.; Devau, N.; Pedenaud, P.; Azaroual, M. Silica precipitationkinetics: The role of solid surface complexation mechanisms integrating the magnesium effects from 25 to 300 °C. Procedia Earth Planet. Sci. 2017, 17, 217–220. [Google Scholar] [CrossRef]
- Kokhanenko, P. Hydrodynamics and Chemistry of Silica Scale Formation in Hydrogeothermal Systems. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2014. [Google Scholar]
- Fournier, R.O.; Rosenbauer, R.J.; Bischoff, J.L. The solubility of quartz in aqueous sodium chloride solution at 350 °C and 180 to 500 bars. Geochim. Cosmochim. 1982, 46, 1975–1978. [Google Scholar] [CrossRef]
- Churakov, S. Physical-Chemical Properties of Complex Natural Fluids. Ph.D. Thesis, Bauingenieurwesen und Angewandte Geowissenschaften, der Technischen Universität Berlin, Berlin, Germany, 2001; pp. 1–152. [Google Scholar]
- Fournier, R.O.; Thompson, J.M. Composition of steam in the system NaCl-KCl-H2O-quartz at 600 °C. Geochim. Cosmochim. Acta 1993, 57, 4365–4375. [Google Scholar] [CrossRef]
- Mori, T.; Sato, M.; Shimoike, Y.; Notsu, K. High SiF4/HF ratio detected in Satsuma-Iwojima volcano’s plume by remote FT-IR observation. Earth Planets Space 2014, 54, 249–256. [Google Scholar] [CrossRef]
- Hoog, J.C.M.D.; Bergen, M.J.V.; Jacobs, M.H.G. Vapour-phase crystallisation of silica from SiF4-bearing volcanic gases. Ann. Geophys. 2005, 48, 775–785. [Google Scholar]
- Iler, R.K. Chemistry of Silica- Solubility, Polymerization, Colloid and Surface Properties and Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Brady, E.L. Chemical nature of silica carried by steam. J. Phys. Chem. 1953, 57, 706–710. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Opila, E.J.; Myers, D.L.; Copland, E.H. Thermodynamics of gas species in the Si-O-H system. J. Chem. Thermodyn. 2005, 37, 1130–1137. [Google Scholar] [CrossRef]
- Plyasunov, A.V. Thermodynamic properties of H4SiO4 in the ideal gas state as evaluated from experimental data. Geochim. Cosmochim. Acta 2011, 75, 3853–3865. [Google Scholar] [CrossRef]
- Fournier, R.O.; Potter, R.W. An equation correlating the solubility of quartz in water from 25” to 900 °C at pressures up to 10,000 bars. Geochim. Cosmochim. Acta 1982, 46, 1969–1973. [Google Scholar] [CrossRef]
- Fournier, R.O. A method of calculating quartz solubilities in aqueous sodium chloride solutions. Geochim. Cosmochim. Acta 1982, 47, 579–586. [Google Scholar] [CrossRef]
- Fournier, R.O.; Rowe, J.J. The solubility of amorphous silica in water at high temperatures and high pressures. Am. Mineral. 1977, 62, 1052–1056. [Google Scholar]
- Harvey, A.H.; Bellows, J.C. Evaluation and Correlation of Steam Solubility Data for Salts and Minerals of Interest in the Power Industry; NIST—National Institute of Standards and Technology: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Morey, G.W.; Hesselgesser, J.M. The solubility of some minerals in superheated steam at high pressures. Econ. Geol. 1951, 46, 821–835. [Google Scholar] [CrossRef]
- Heitmann, H.G. Die Loslichkeit von Kieselsaure in Wasser und Wasserdampf sowie ihr Einfluss auf Turbinenverkieselungen. Ph.D. Theisis, Technische Hochschule Karlsruhe, Karlsruhe, Germany,, 1964. [Google Scholar]
- Martynova, O.I.; Fursenko, V.F.; Popov, A.S. The study of the dissolved silica distribution between water and steam. Teploenergetika 1972, 12, 51–53. (In Russian) [Google Scholar]
- Martynova, O.I. Some problems of the solubility of involatile inorganic compounds in water vapour at high temperatures and pressures. Russ. J. Inorg. Chem. 1964, 38, 587–592. [Google Scholar]
- Martynova, O.I.; Popov, A.S.; Fursenko, V.F. Boundary lines of phase equilibrium diagrams of the silicon dioxide—Water system. Teploenergetika 1975, 5, 66–68. (In Russian) [Google Scholar]
- Straub, F.G.; Grabowski, H.A. Silica deposition in steam turbines. Trans. Am. Soc. Mech. Eng. 1945, 67, 309–316. [Google Scholar] [CrossRef]
- Manning, C.E. The solubility of quartz in H2O in the lower crust and upper mantle. Geochim. Cosmochim. Acta 1994, 58, 4831–4839. [Google Scholar] [CrossRef]
- Dolejš, D.; Manning, C.E. Thermodynamic model for mineral solubility in aqueous fluids: Theory, calibration and application to model fluid-flow systems. Geofluids 2010, 10, 20–40. [Google Scholar]
- Bergna, H.E.; Roberts, W.O. Colloidal Silica: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Setiawan, F.A.; Rahayuningsih, E.; Petrus, H.T.B.M.; Nurpratama, M.I.; Perdana, I. Kinetics of silica precipitation in geothermal brine with seeds addition: Minimizing silica scaling in a cold re-injection system. Geotherm. Energy 2019, 7, 22. [Google Scholar] [CrossRef]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Zarrouka, S.J.; Woodhursta, B.C.; Morris, C. Silica scaling in geothermal heat exchangers and its impact on pressure drop and performance: Wairakei binary plant, New Zealand. Geothermics 2014, 51, 445–459. [Google Scholar] [CrossRef]
- Henley, R.W. pH and silica scaling control in geothermal field development. Geothermics 1983, 12, 307–321. [Google Scholar] [CrossRef]
- Heuvel, D.B.V.D.; Gunnlaugsson, E.; Gunnarsson, I.; Stawskia, T.M.; Peacock, C.L.; Benning, L.G. Understanding amorphous silica scaling under well-constrained conditions inside geothermal pipelines. Geothermics 2018, 76, 231–241. [Google Scholar] [CrossRef]
- Goto, K. Effect of pH on polymerization of silicic acid. J. Phys. Chem. 1956, 60, 1007–1008. [Google Scholar] [CrossRef]
- Weres, O.; Yee, A.; Tsao, L. Kinetics of Silica Polymerization. J. Colloid Interface Sci. 1981, 84, 379–402. [Google Scholar] [CrossRef]
- Rimstidt, J.D.; Barnes, H.L. The kinetics of silica-water reactions. Geochim. Cosmochim. Acta 1980, 44, 1683–1699. [Google Scholar] [CrossRef]
- Crerar, D.A.; Axtmann, E.V. Growth and ripening of silica polymers in aqueous solutions. Geochim. Cosm. Acta 1982, 45, 1259–1266. [Google Scholar] [CrossRef]
- Renders, P.J.N.; Gammons, C.H.; Barnes, H.L. Precipitation and dissolution rate constants for cristobalite from 150 to 300 °C. Geochim. Cosmochim. Acta 1995, 59, 77–85. [Google Scholar] [CrossRef]
- Dove, P.M.; Han, N.; Wallace, A.F.; Yoreo, J.J.D. Kintetics of amorphous silica dissolution and the paradox of the silica polymorphs. Proc. Natl. Acad. Sci. USA 2008, 105, 9903–9908. [Google Scholar] [CrossRef]
- Icopini, G.A.; Brantley, S.L.; Heaney, P.J. Kinetics of silica oligomerization and nanocolloid formation as a function of pH and ionic strength at 25 °C. Geochim. Cosmochim. Acta 2005, 69, 293–303. [Google Scholar] [CrossRef]
- Tobler, D.J.; Shaw, S.; Benning, L.G. Quantification of initial steps of nucleation and growth of silica nanoparticles: An in-situ SAXS and DLS study. Geochim. Cosmochim. Acta 2009, 73, 5377–5393. [Google Scholar] [CrossRef]
- Bohlmann, E.G.; Mesmer, R.E.; Berlinski, P. Kinetics of silica deposition from simulated geothermal brines. Soc. Pet. Eng. J. 1980, 20, 239–248. [Google Scholar] [CrossRef][Green Version]
- Carroll, S.; Mroczek, E.; Alai, M.; Ebert, M. Amorphous silica precipitation (60 to 120 °C):Comparison of laboratory and field rates. Geochim. Cosmochim. Acta 1998, 62, 1379–1396. [Google Scholar] [CrossRef]
- Conrad, C.F.; Icopini, G.A.; Yasuhara, H.; Bandstra, J.Z.; Brantley, S.L.; Heaney, P.J. Modeling the kinetics of silica nanocolloid formation andprecipitation in geologically relevant aqueous solutions. Geochim. Cosmochim. Acta 2007, 71, 531–542. [Google Scholar] [CrossRef]
- Tobler, D.J.; Benning, L.G. In-situ and time resolved nucleation and growth of silica nanoparticles forming under simulated geothermal conditions. Geochim. Cosmochim. Acta 2013, 114, 156–168. [Google Scholar] [CrossRef]
- Heuvel, D.B.V.D.; Gunnlaugsson, E.; Gunnarsson, I.; Benning, L.G. Microstructural and chemical variation in silica-rich precipitates at the Hellisheii geothermal power plant. Mineral. Mag. 2014, 78, 1381–1389. [Google Scholar]
- Guzman, R.D.; Leon, A.C.C.D.; Advincula, R.; Baltazar, A.D. Characterization of Nanocolloidal Silica Formation of Untreated and Treated Simulated Geothermal Brine through Various Particle Size and Zeta Potential Measurement Techniques. In Proceedings of the World Geothermal Congress, Melbourne, Australia, 19–24 April 2015. [Google Scholar]
- Dixit, C.; Bernard, M.-L.; Sanjuan, B.; André, L.; Gaspard, S. Experimental study on the kinetics of silica polymerization during cooling of the Bouillante geothermal fluid (Guadeloupe, FrenchWest Indies). Chem. Geol. 2016, 442, 97–112. [Google Scholar] [CrossRef]
- Bird, G.; Boon, J.; Stone, T. Silica transport during steam injection into oil sands: 1. Dissolution and precipitation kinetics of quartz: New results and review of existing data. Chem. Geol. 1986, 54, 69–80. [Google Scholar] [CrossRef]
- Tobler, D.J.; Stawski, T.M.; Benning, L.G. Silica and Alumina Nanophases: Natural Processes and Industrial Applications; Link, S., Ed.; New Perspectives on Mineral Nucleation and Growth; Springer: Berlin/Heidelberg, Germany, 2016; pp. 293–316. [Google Scholar]
- Noguera, C.; Fritz, B.; Clément, A. Precipitation mechanism of amorphous silica nanoparticles: A simulation approach. J. Colloid Interface Sci. 2015, 448, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Scherer, G.W. The Physics and Chemistry of Sol-Gel Processing, 1st ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Nestor, J. Silica and Titania Nanodispersions. Chapter 5; In Nanocolloids—A Meeting Point for Scientists and Technologists; Elsevier: Amsterdam, The Netherlands, 2016; p. 536. [Google Scholar]
- Papirer, E. Adsorption on Silica Surfaces, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Chauhan, V.; Gudjonsdottir, M.; Saevarsdottir, G. Silica deposition in superheated geothermal systems. In 43rd Workshop on Geothermal Reservoir Engineering; Stanford University: Stanford, CA, USA, 2018; pp. 1–6. [Google Scholar]
- Chauhan, V. Superheated Steam Scrubbing and Utilization for Power Generation. Ph.D. Thesis, School of Science and Engineering, Reykjavik University, Reykjavik, Iceland, 2019. [Google Scholar]
- Benning, L.G.; Waychunas, G.A. Nucleation, Growth, and Aggregation of Mineral Phases: Mechanisms and Kinetic Controls; Brantley, S.L., Kubicki, J.D., White, A.F., Eds.; Kinetics of Water-Rock Interaction; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordvik, S.; Næss, E. Silica Nanoparticle Formation from Supercritical Geothermal Sources. Energies 2023, 16, 5981. https://doi.org/10.3390/en16165981
Bordvik S, Næss E. Silica Nanoparticle Formation from Supercritical Geothermal Sources. Energies. 2023; 16(16):5981. https://doi.org/10.3390/en16165981
Chicago/Turabian StyleBordvik, Silje, and Erling Næss. 2023. "Silica Nanoparticle Formation from Supercritical Geothermal Sources" Energies 16, no. 16: 5981. https://doi.org/10.3390/en16165981
APA StyleBordvik, S., & Næss, E. (2023). Silica Nanoparticle Formation from Supercritical Geothermal Sources. Energies, 16(16), 5981. https://doi.org/10.3390/en16165981





