Three-Dimensional CFD Simulation of a Proton Exchange Membrane Electrolysis Cell
Abstract
1. Introduction
2. Mathematical Modelling
- Laminar flow regime, because of the low fluid velocity both in channels and porous diffusion media. The calculated Reynolds number in the examined cases is approximately 1000 in the channels, confirming this assumption.
- Ideal gases behaviour, given the relatively low pressure and temperature.
- The gravitational force is not considered.
- The gas diffusion layer (GDL) and CL are treated as isotropic and homogeneous porous media, characterized by effective permeability, uniform porosity, and tortuosity.
- Butler–Volmer kinetics govern the electrochemical reaction at the anode and cathode.
- The membrane is an impermeable solid medium, and the water flux is modelled by sorption reactions at the interfaces adjacent to CLs.
- The simulations are steady state since the objective is to analyse the time-independent cell’s performance at different voltages, aiming at understanding its stationary operation and numerically reproducing the polarization curve.
2.1. Porous Media Modelling
2.2. Membrane Modelling
2.3. Electrochemical Modelling
3. 3D-CFD Model
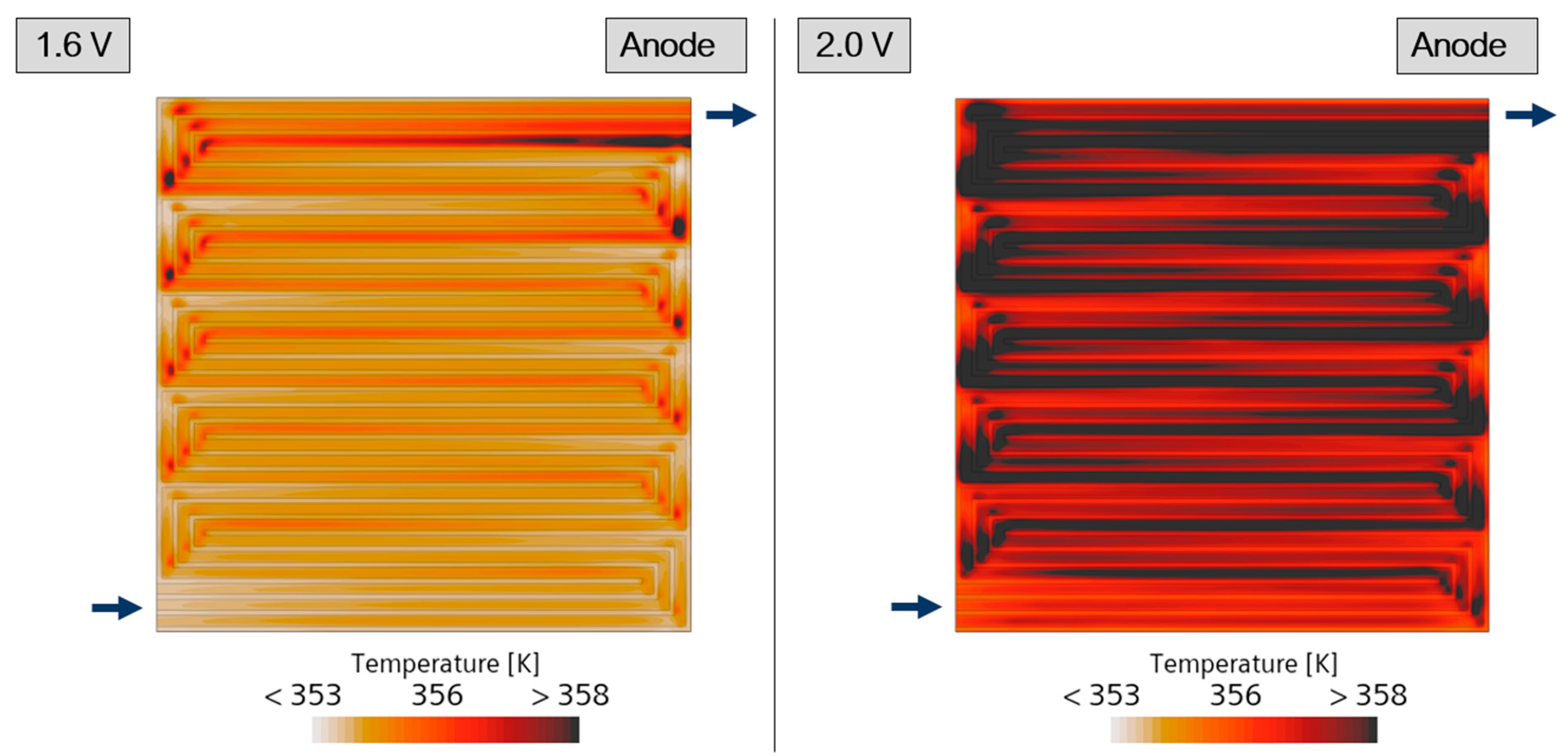
4. Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| a | activity |
| BP | Bipolar Plate |
| c | concentration [mol/m3] |
| cp | specific heat [J/kg/K] |
| CL | catalyst layer |
| CFD | Computational Fluid Dynamics |
| EW | Equivalent molecular weight of dry membrane [kg/mol] |
| F | Faraday constant [C/mol] |
| force [N] | |
| GDL | Gas Diffusion Layer |
| LHV | Lower Heating Value [MJ/kg] |
| i | (superficial) current density [A/m2] |
| j | volumetric current density [A/m3] |
| K | permeability [m2] |
| k | thermal conductivity [W/m/K] |
| Mass flow [kg/s] | |
| M | millions |
| MMP | Mixture multi-phase |
| p | pressure [Pa] |
| P | Power [W] |
| PEM | Proton Exchange Membrane |
| PEMEC | Proton Exchange Membrane Electrolyser Cell |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| R | universal gas constant [J/mol/K] |
| RES | renewable energy sources |
| RH | Relative humidity |
| S | entropy, source terms |
| SMR | Steam Methane Reforming |
| T | temperature [K] |
| velocity [m/s] | |
| Greek Symbols | |
| α | charge transfer coefficient; volume fraction |
| γ | pressure scaling coefficients; membrane water absorption/desorption rate [1/s] |
| δ | thickness [m] |
| ε | porosity |
| specific active surface of the catalyst [1/m] | |
| η | overpotential [V] |
| contact angle [°] | |
| κ | electric conductivity [S/m] |
| λ | water content |
| μ | dynamic viscosity [kg/m/s] |
| ρ | density [kg/m3] |
| σ | ionic conductivity [S/m]; surface tension [N/m] |
| τ | tortuosity |
| φ | potential [V] |
| Subscripts and superscripts | |
| a | anode |
| c | cathode |
| e | electrolyte |
| el | electric |
| eff | effective |
| eq | equilibrium |
| g | gas |
| i | ionomer |
| in | inertial |
| l | liquid |
| m | membrane |
| n | the n-th phase |
| p | porous |
| pt | platinum |
| ref | reference |
| rl | relative |
| s | solid |
| v | viscous |
| w | water |
References
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Solar Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, T. Optimization and economic evaluation of a PEM electrolysis system considering its degradation in variable-power operations. Appl. Energy 2022, 324, 119760. [Google Scholar] [CrossRef]
- Olivier, P.; Bourasseau, C.; Bouamama, P.B. Low-temperature electrolysis system modelling: A review. Renew. Sustain. Energy Rev. 2017, 78, 280–300. [Google Scholar] [CrossRef]
- Choi, P.; Bessarabov, D.G.; Datta, R. A simple model for solid polymer electrolyte (SPE) water electrolysis. Solid. State Ion. 2004, 175, 535–539. [Google Scholar] [CrossRef]
- Diaz, D.F.R.; Valenzuela, E.; Wang, Y. A component-level model of polymer electrolyte membrane electrolysis cells for hydrogen production. Appl. Energy 2022, 321, 119398. [Google Scholar] [CrossRef]
- D’Adamo, A.; Haslinger, M.; Corda, G.; Höflinger, J.; Fontanesi, S.; Lauer, T. Modelling Methods and Validation Techniques for CFD Simulations of PEM Fuel Cells. Processes 2021, 9, 688. [Google Scholar] [CrossRef]
- Nie, J.; Chen, Y. Numerical modeling of three-dimensional two-phase gas–liquid flow in the flow field plate of a PEM electrolysis cell. Int. J. Hydrogen Energy 2010, 35, 3183–3197. [Google Scholar] [CrossRef]
- Wang, C.Y. Fundamental Models for Fuel Cell Engineering. Chem. Rev. 2004, 104, 4727–4765. [Google Scholar] [CrossRef]
- Ruiz, D.D.H.; Sasmito, A.P.; Shamim, T. Numerical Investigation of the High Temperature PEM Electrolyzer: Effect of Flow Channel Configurations. ECS Trans. 2013, 58, 99–112. [Google Scholar] [CrossRef]
- Toghyani, S.; Afshari, E.; Baniasadi, E. Metal foams as flow distributors in comparison with serpentine and parallel flow fields in proton exchange membrane electrolyzer cells. Electrochim. Acta 2018, 290, 506–519. [Google Scholar] [CrossRef]
- Toghyani, S.; Afshari, E.; Baniasadi, E. Three-dimensional computational fluid dynamics modeling of proton exchange membrane electrolyzer with new flow field pattern. J. Therm. Anal. Calorim. 2018, 135, 1911. [Google Scholar] [CrossRef]
- Toghyani, S.; Afshari, E.; Baniasadi, E.; Atyabi, S.A. Thermal and electrochemical analysis of different flow field patterns in a PEM electrolyzer. Electrochim. Acta 2018, 267, 234–245. [Google Scholar] [CrossRef]
- Ma, Z.; Witteman, L.; Wrubel, J.A.; Bender, G. A comprehensive modeling method for proton exchange membrane electrolyzer development. Int. J. Hydrogen Energy 2021, 46, 17627–17643. [Google Scholar] [CrossRef]
- D’Adamo, A.; Riccardi, M.; Locci, C.; Romagnoli, M.; Fontanesi, S. Numerical Simulation of a High Current Density PEM Fuel Cell; SAE Technical Papers; SAE International: Warrendale, PA, USA, 2020. [Google Scholar] [CrossRef]
- Harvey, D.; Pharoah, J.G.; Karan, K. A comparison of different approaches to modelling the PEMFC catalyst layer. J. Power Sources 2008, 179, 209–219. [Google Scholar] [CrossRef]
- Lafmejani, S.S.; Olesen, A.C.; Kær, S.K. VOF modelling of gas–liquid flow in PEM water electrolysis cell micro-channels. Int. J. Hydrogen Energy 2017, 42, 16333–16344. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, G.; Xie, B.; Tongsh, C.; Jiao, K. Integration of the detailed channel two-phase flow into three-dimensional multi-phase simulation of proton exchange membrane electrolyzer cell. Int. J. Green Energy 2021, 18, 541–555. [Google Scholar] [CrossRef]
- Corda, G.; Fontanesi, S.; d’Adamo, A. Methodology for PEMFC CFD Simulation Including the Effect of Porous Parts Compression. Int. J. Hydrogen Energy 2022, 47, 14658–14673. [Google Scholar] [CrossRef]
- Corda, G.; Fontanesi, S.; D’Adamo, A. Numerical Comparison of the Performance of Four Cooling Circuit Designs for Proton Exchange Membrane Fuel Cells (PEMFCs); SAE Technical Papers; SAE International: Warrendale, PA, USA, 2022. [Google Scholar] [CrossRef]
- D’Adamo, A.; Corda, G. Numerical Simulation of Advanced Bipolar Plates Materials for Hydrogen-Fueled PEM Fuel Cell; SAE Technical Papers; SAE International: Warrendale, PA, USA, 2022. [Google Scholar] [CrossRef]
- D’Adamo, A.; Riccardi, M.; Borghi, M.; Fontanesi, S. CFD Modelling of a Hydrogen/Air PEM Fuel Cell with a Serpentine Gas Distributor. Processes 2021, 9, 564. [Google Scholar] [CrossRef]
- Hao, L.; Cheng, P. Lattice Boltzmann simulations of anisotropic permeabilities in carbon paper gas diffusion layers. J. Power Sources 2009, 186, 104–114. [Google Scholar] [CrossRef]
- Tabuchi, Y.; Shiomi, T.; Aoki, O.; Kubo, N.; Shinohara, K. Effects of heat and water transport on the performance of polymer electrolyte membrane fuel cell under high current density operation. Electrochim. Acta 2010, 56, 352–360. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Berg, P. On the modeling of water transport in polymer electrolyte membrane fuel cells. Electrochim. Acta 2009, 54, 6913–6927. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer Electrolyte Fuel Cell Model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Chang, Y.; Qin, Y.; Yin, Y.; Zhang, J.; Li, X. Humidification strategy for polymer electrolyte membrane fuel cells – A review. Appl. Energy 2018, 230, 643–662. [Google Scholar] [CrossRef]
- Marangio, F.; Santarelli, M.; Calì, M. Theoretical model and experimental analysis of a high pressure PEM water electrolyser for hydrogen production. Int. J. Hydrogen Energy 2009, 34, 1143–1158. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.; Lee, K.S. One-dimensional dynamic modeling of a high-pressure water electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2013, 38, 2596–2609. [Google Scholar] [CrossRef]
- Awasthi, A.; Scott, K.; Basu, S. Dynamic modeling and simulation of a proton exchange membrane electrolyzer for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 14779–14786. [Google Scholar] [CrossRef]
- Bessarabov, D.; Wang, H.; Li, H.; Zhao, N. (Eds.) PEM Electrolysis for Hydrogen Production: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
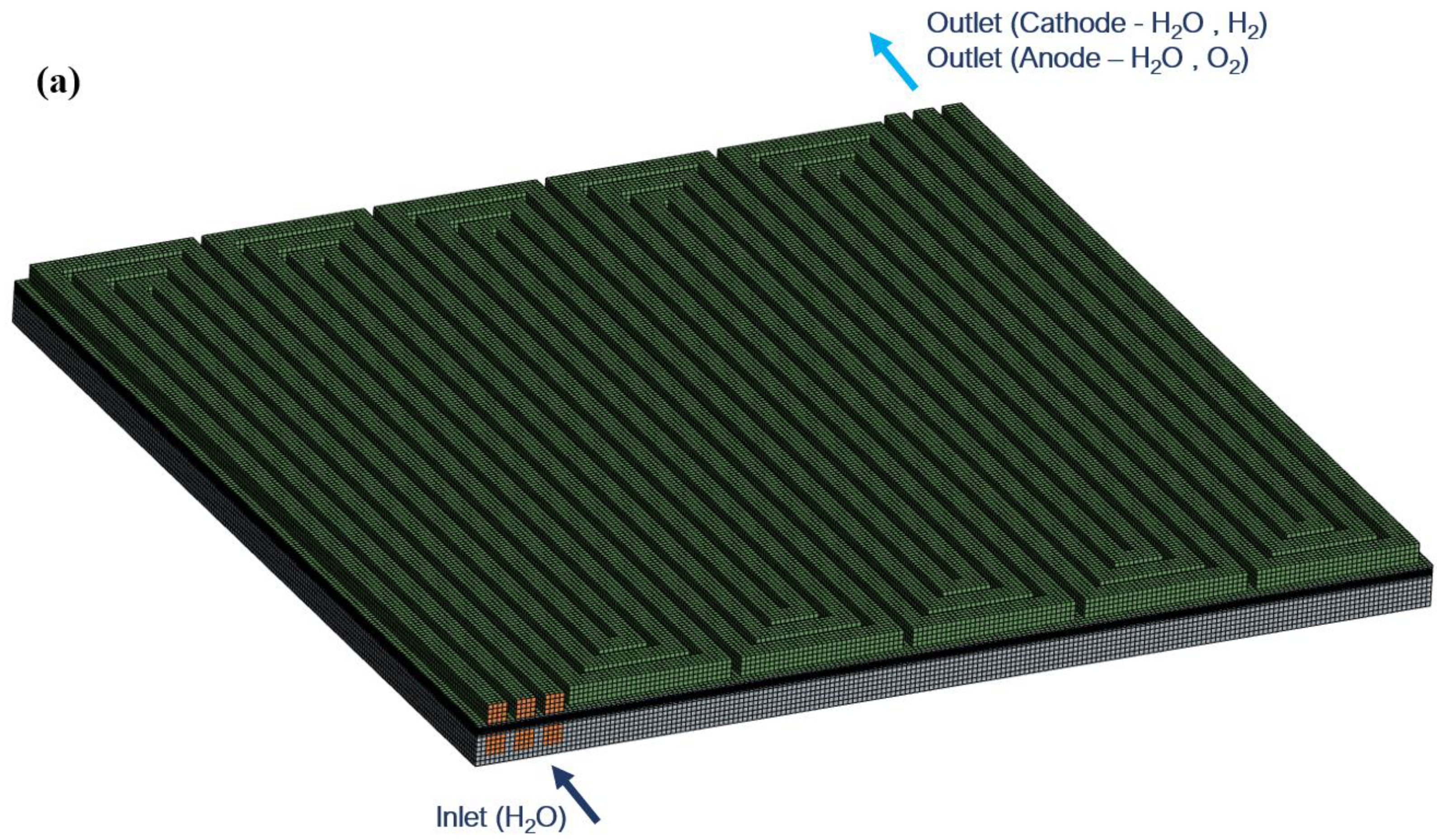

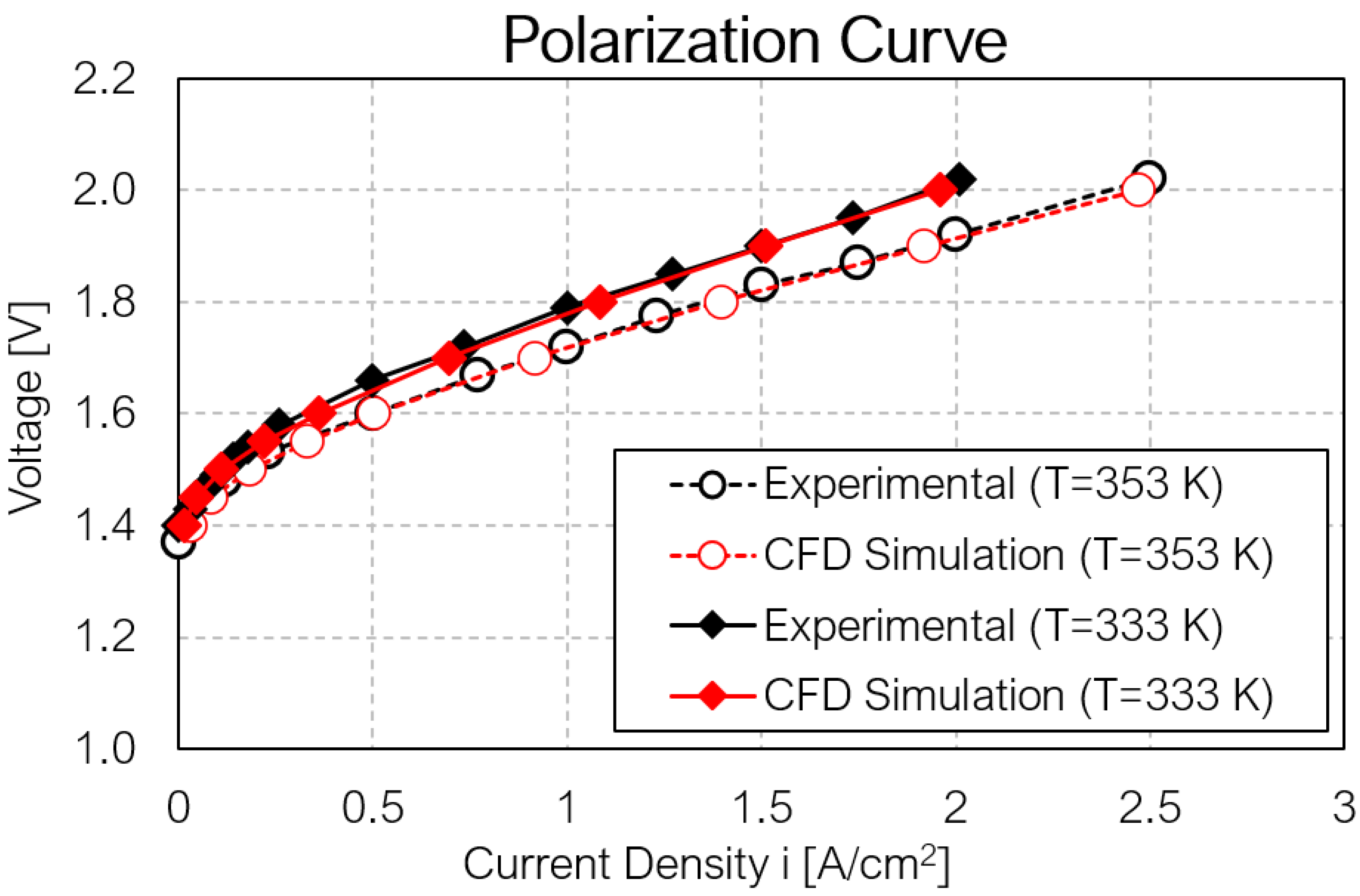
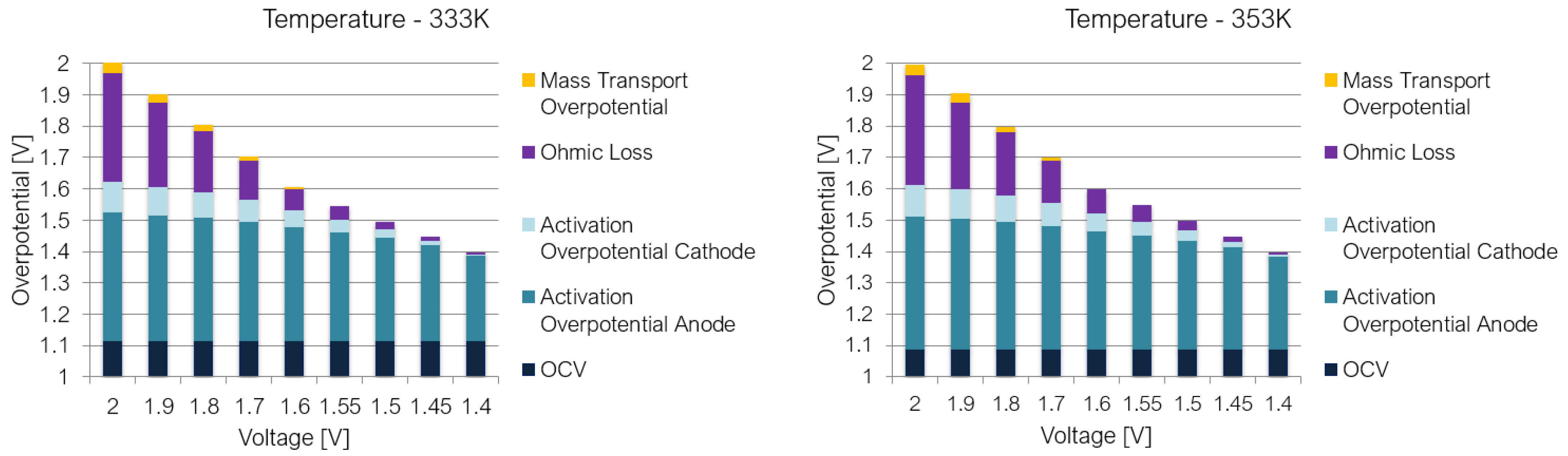
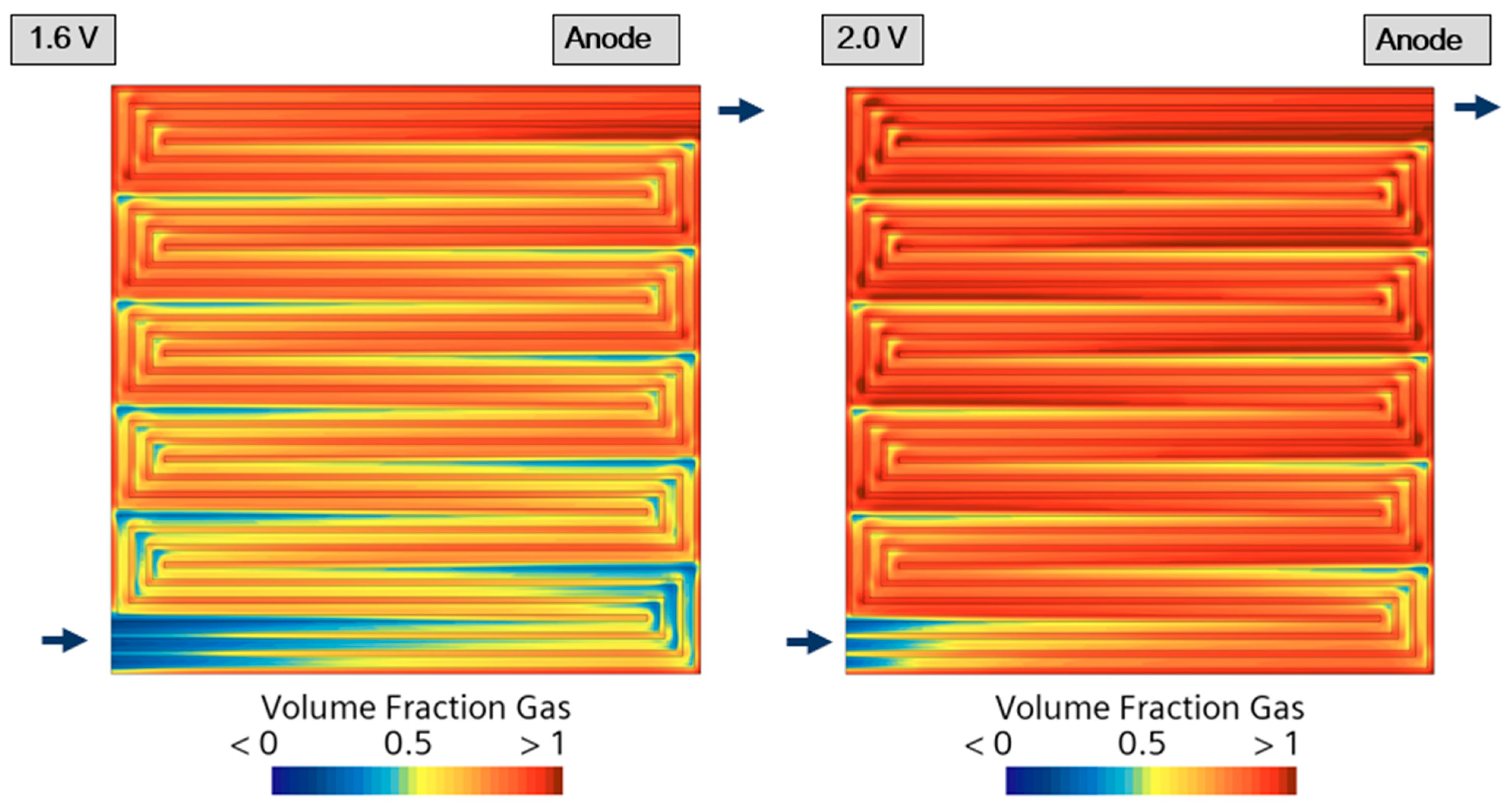
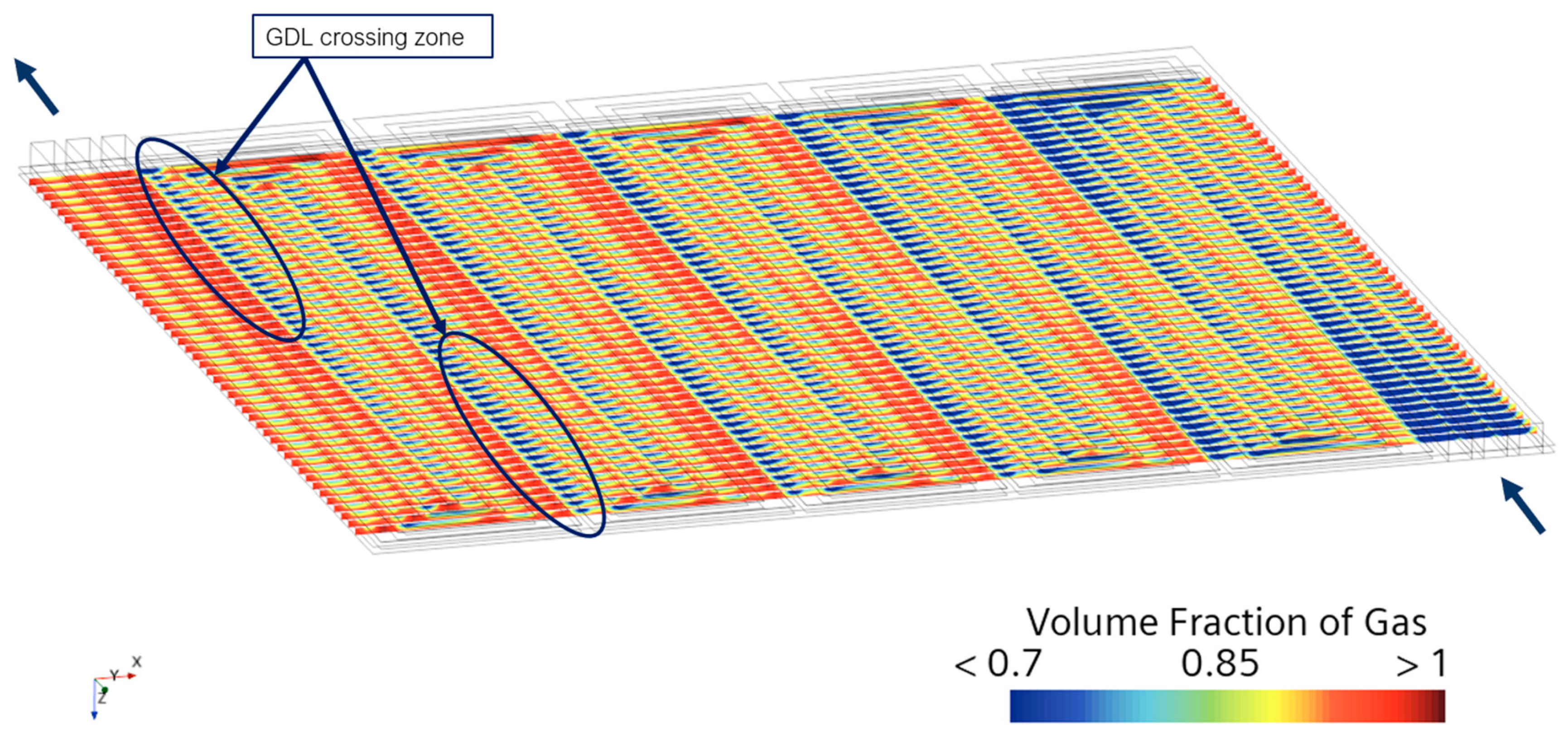
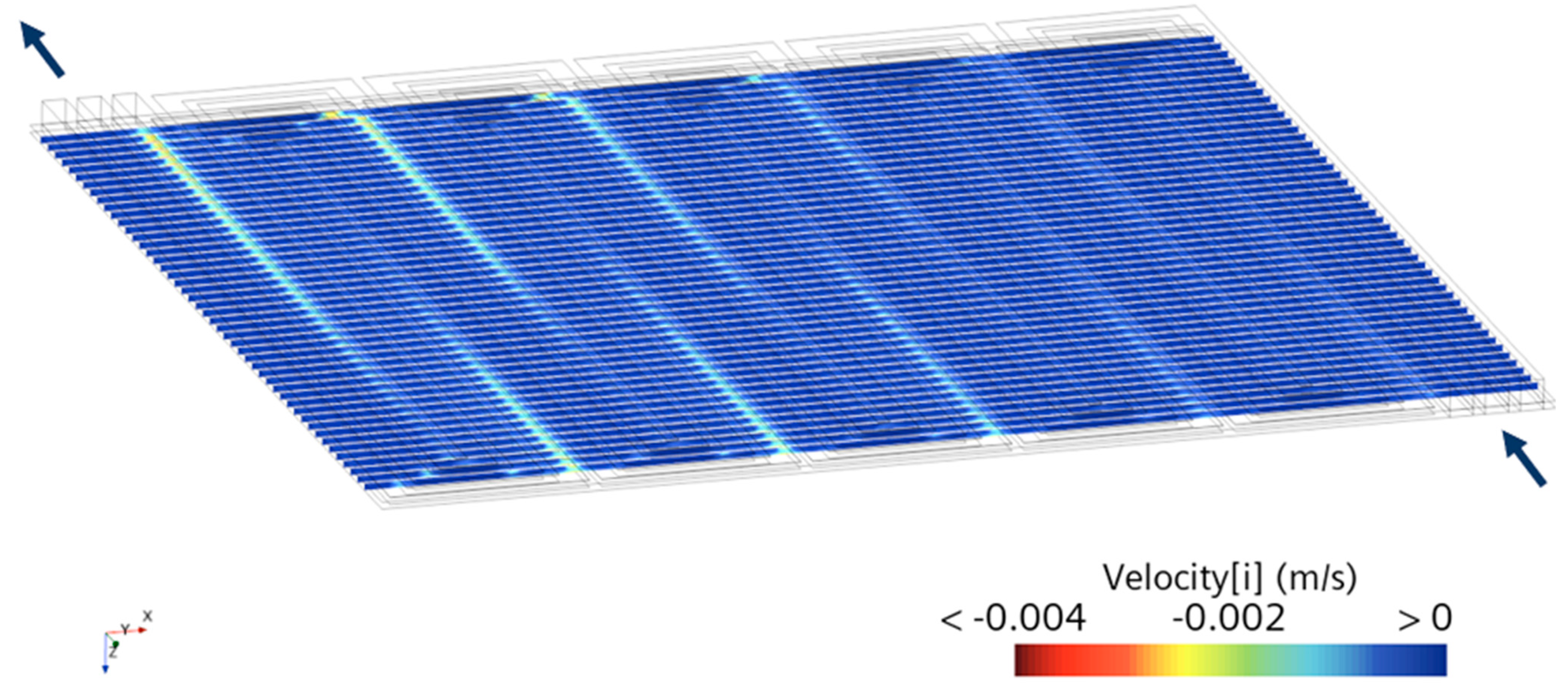

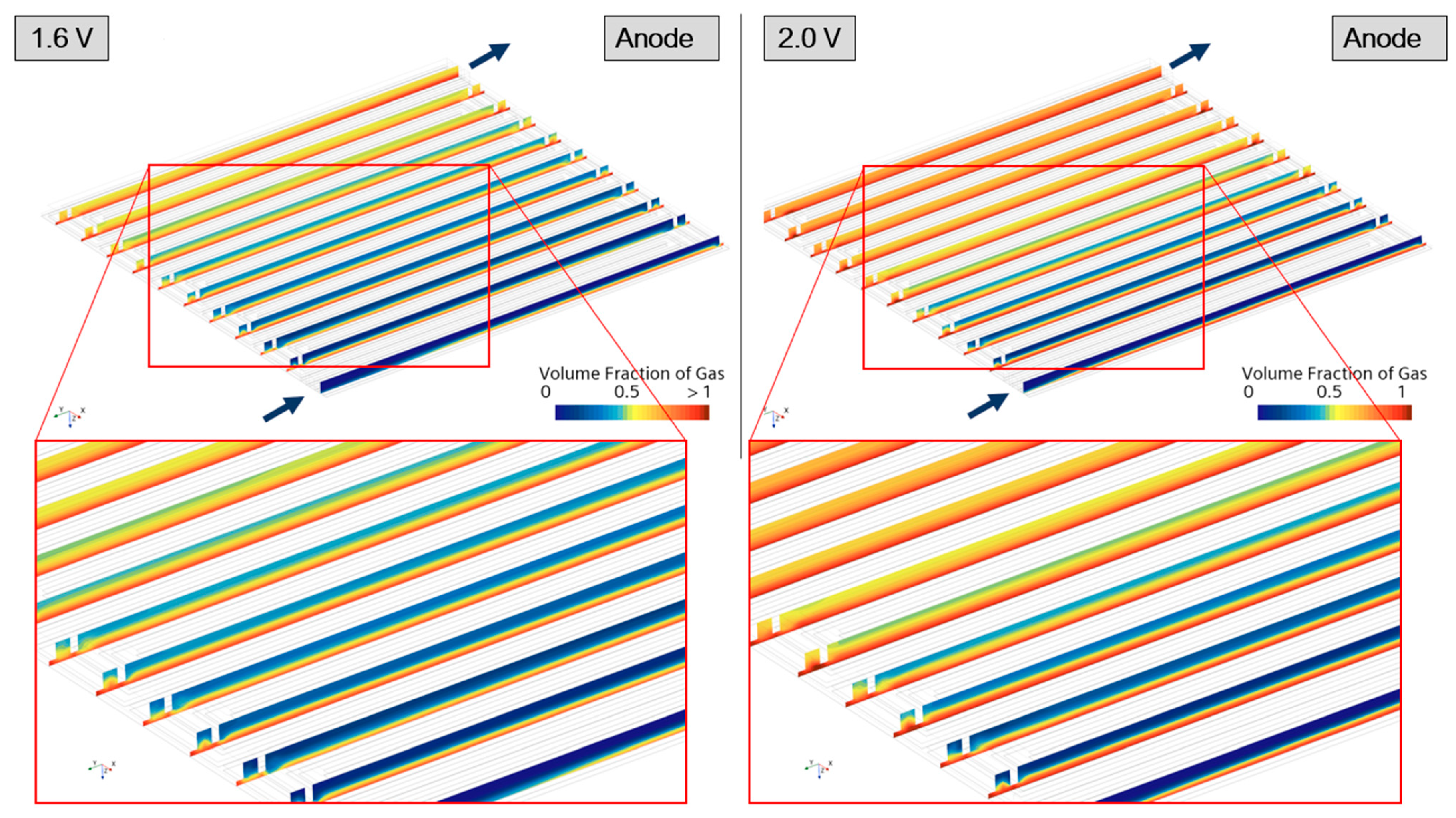


| Governing Equation | Source Term Specification |
|---|---|
| Continuity equation: (1) | |
| Momentum equation: (2) | |
| Species transport: (3) | |
| Energy transport: (4) | Solid parts (membrane): |
| Charge transport: (5) (6) | ; ; ; |
| Component Dimensions | Value |
|---|---|
| Channel height | 1 mm |
| Channel width | 1 mm |
| BP width between channels | 0.5 mm |
| BP height | 1.5 mm |
| GDL thickness | 300 μm |
| CL thickness | 12 μm |
| Membrane thickness | 30 μm |
| Physical Properties of FC Main Components | Value |
|---|---|
| GDL Density (solid phase) Electrical conductivity Thermal conductivity Permeability Contact angle θc Porosity εGDL CL Porosity εCL Permeability Contact angle θc Specific active area Ionomer Density Ion. conductivity Spec. heat Th. conductivity Volume fraction Pt/C Density El. conductivity Spec. heat Th. conductivity Volume fraction BP Density El. conductivity Spec. heat Th. conductivity Membrane Density Ion. conductivity Spec. heat Th. conductivity | 2250 kg/m3 500 S/m 24 W/m/K 4 × 10−12 m2 110° 0.7 0.4 4 × 10−13 m2 110° 2000 kg/m3 Equation (12) 903.0 J/kg/K 0.445 W/m/K 0.4 2250.0 kg/m3 500.0 S/m 707.68 J/kg/K 10 W/m/K 0.6 2250 kg/m3 20,000 S/m 707.68 J/kg/K 20 W/m/K 2000 kg/m3 Equation (12) 903 J/kg/K 0.445 W/m/K |
| Boundary Conditions | Value |
|---|---|
| Cathode Channel Inlet Water flowrate Temperature Volume fraction of water Outlet Pressure Anode Channel Inlet Water flowrate Temperature Volume fraction of water Outlet Pressure Cathode BP Top Electric potential Temperature Bottom Electric potential Temperature | 5 mL/min 353 K/333 K 1 101,325 Pa 50 mL/min 353 K/333 K 1 101,325 Pa 0 V 353 K/333 K (Fixed by the cooling system) From 1.4 V to 2.0 V 353 K/333 K (Fixed by the cooling system) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corda, G.; Cucurachi, A.; Fontanesi, S.; d’Adamo, A. Three-Dimensional CFD Simulation of a Proton Exchange Membrane Electrolysis Cell. Energies 2023, 16, 5968. https://doi.org/10.3390/en16165968
Corda G, Cucurachi A, Fontanesi S, d’Adamo A. Three-Dimensional CFD Simulation of a Proton Exchange Membrane Electrolysis Cell. Energies. 2023; 16(16):5968. https://doi.org/10.3390/en16165968
Chicago/Turabian StyleCorda, Giuseppe, Antonio Cucurachi, Stefano Fontanesi, and Alessandro d’Adamo. 2023. "Three-Dimensional CFD Simulation of a Proton Exchange Membrane Electrolysis Cell" Energies 16, no. 16: 5968. https://doi.org/10.3390/en16165968
APA StyleCorda, G., Cucurachi, A., Fontanesi, S., & d’Adamo, A. (2023). Three-Dimensional CFD Simulation of a Proton Exchange Membrane Electrolysis Cell. Energies, 16(16), 5968. https://doi.org/10.3390/en16165968








