A Theoretical Study of NH2 Radical Reactions with Propane and Its Kinetic Implications in NH3-Propane Blends’ Oxidation
Abstract
:1. Introduction
2. Computational Methods
3. Kinetic Modeling
4. Results and Discussion
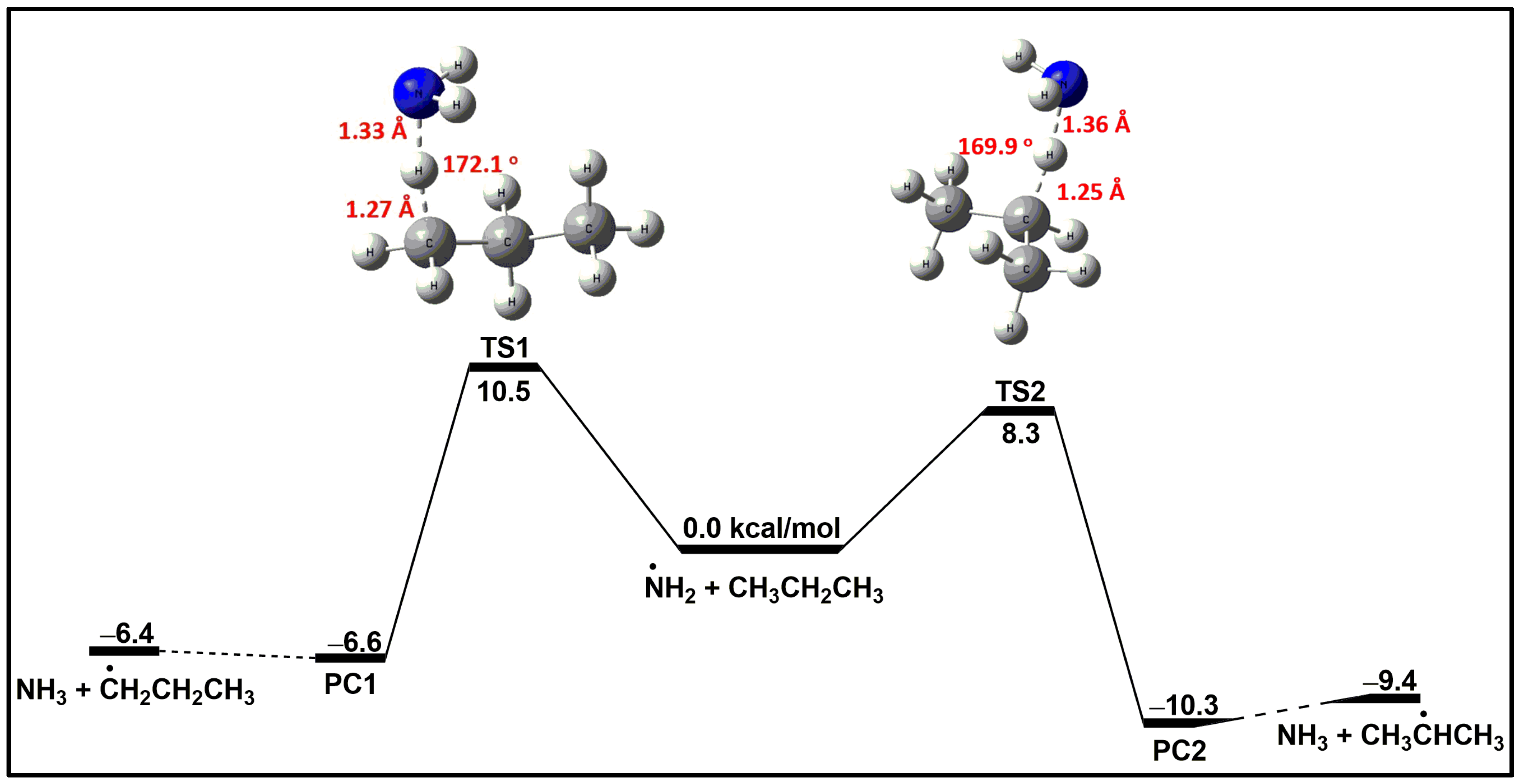
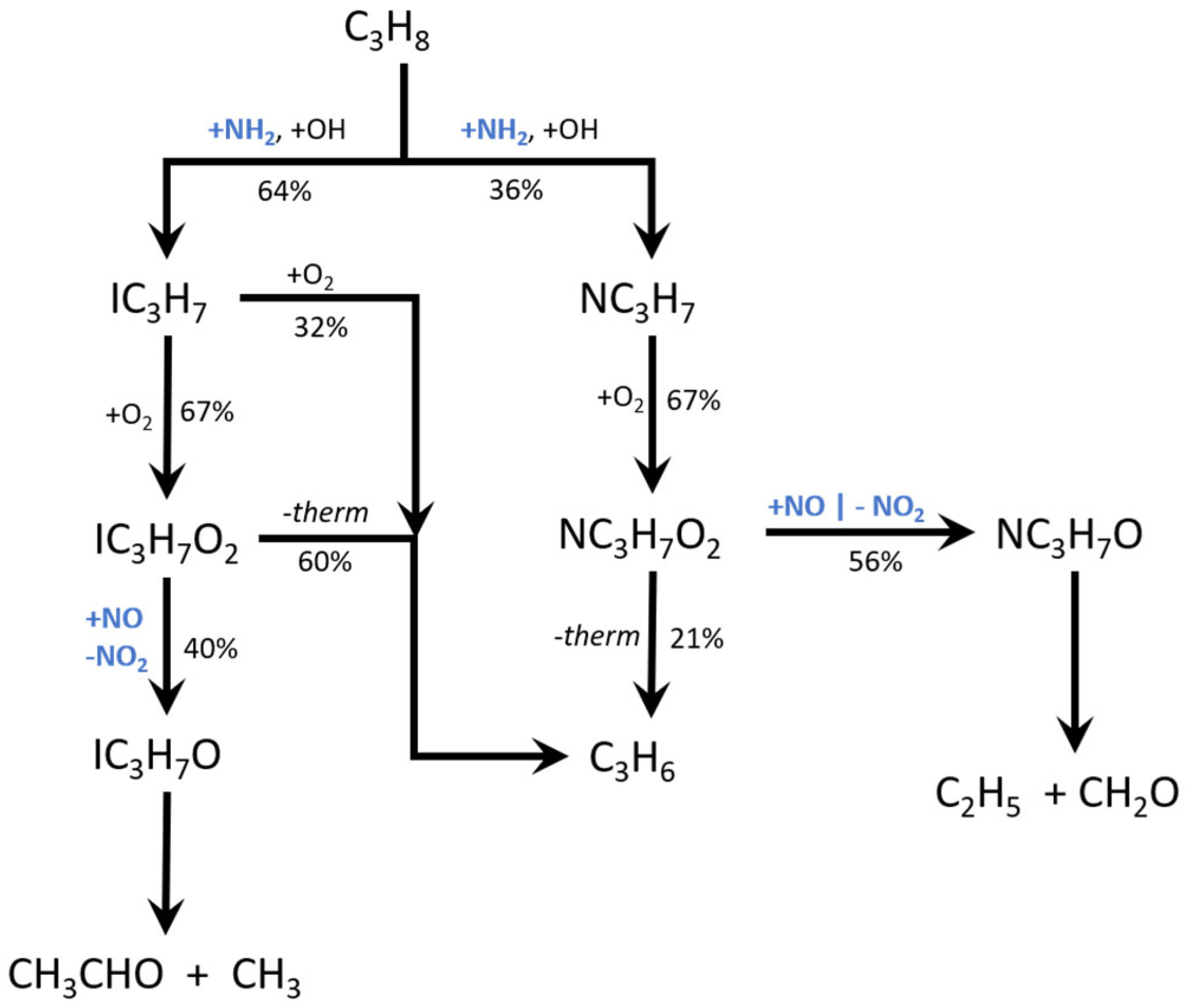
4.1. Potential Energy Surface (PES)
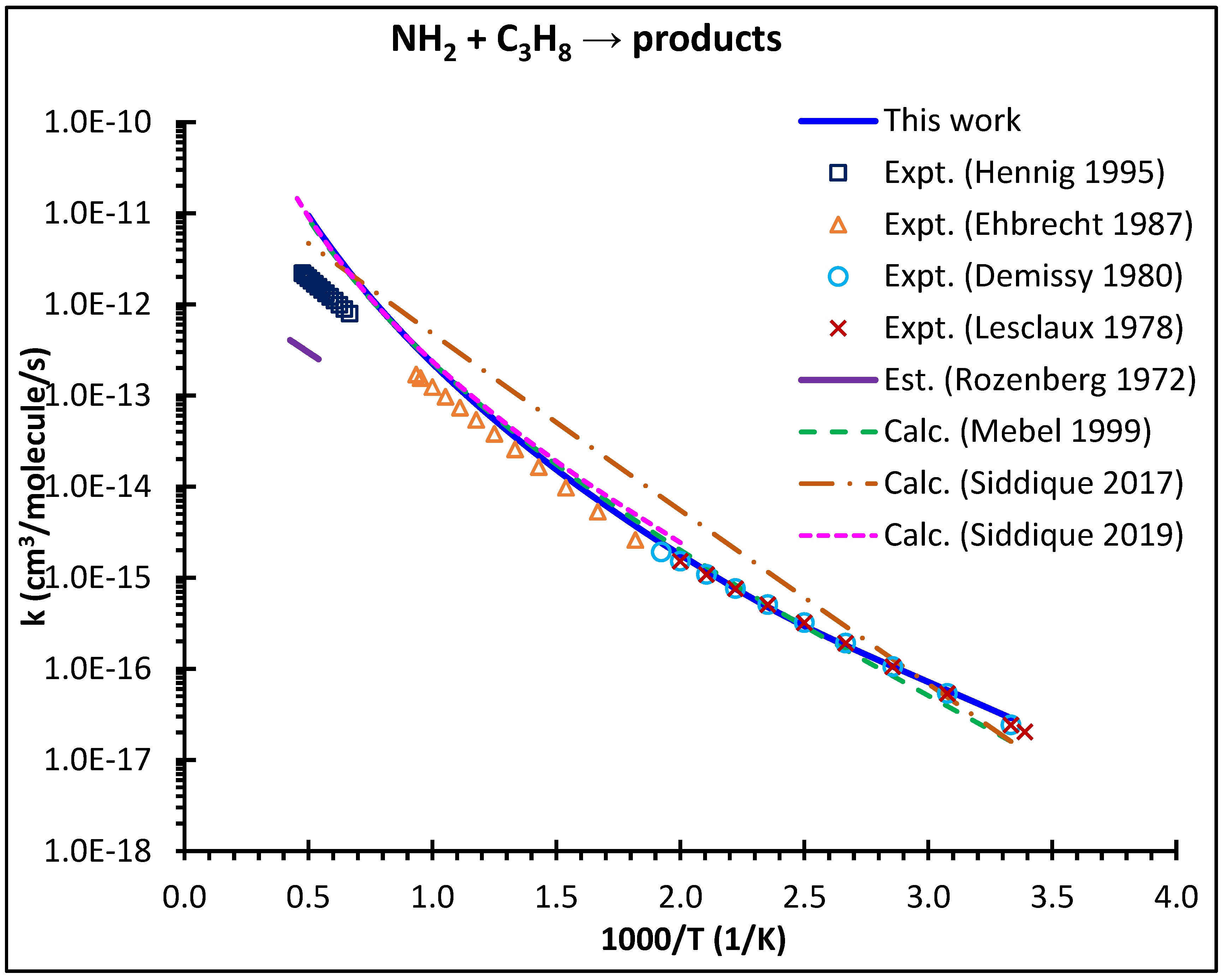
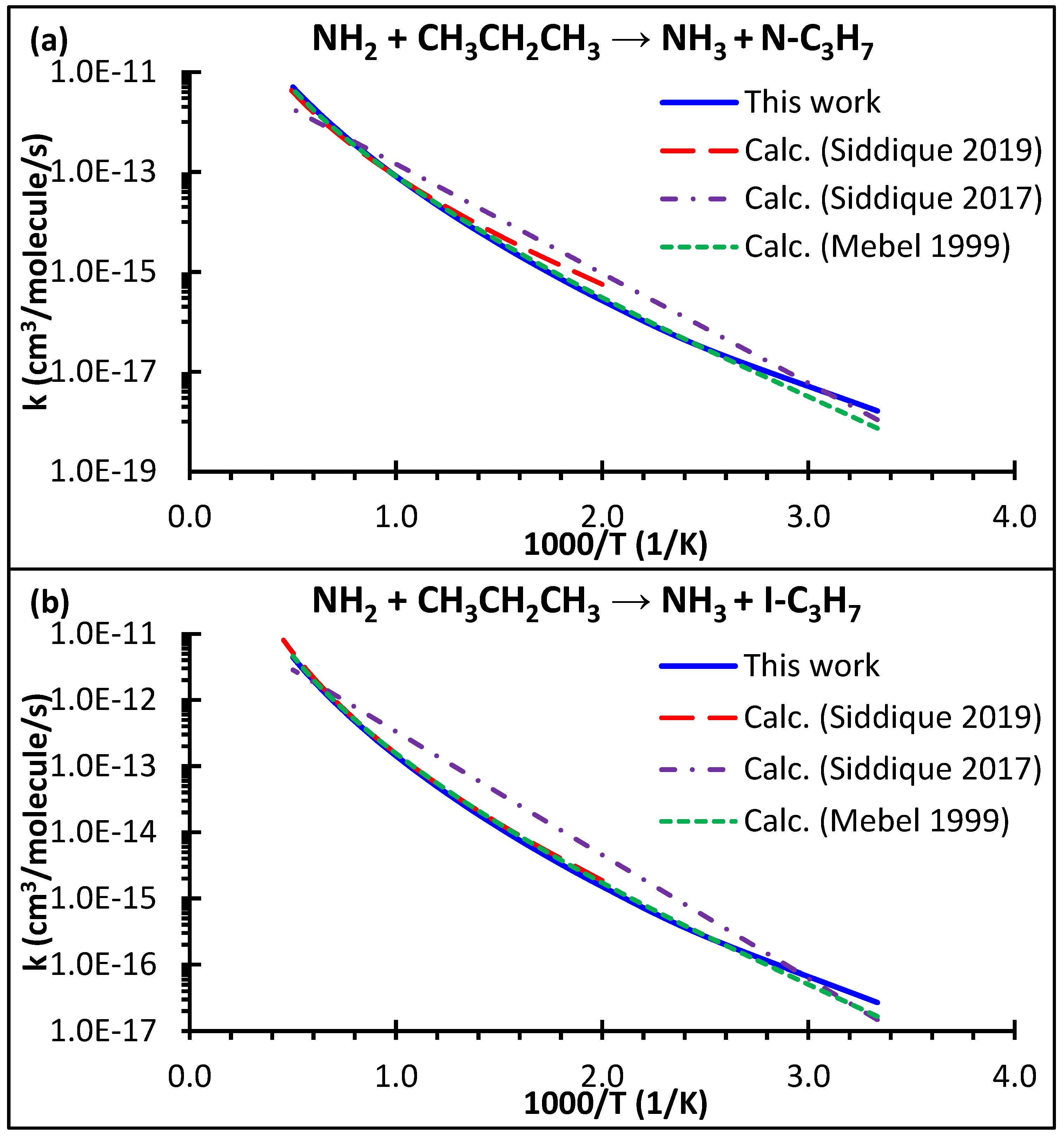
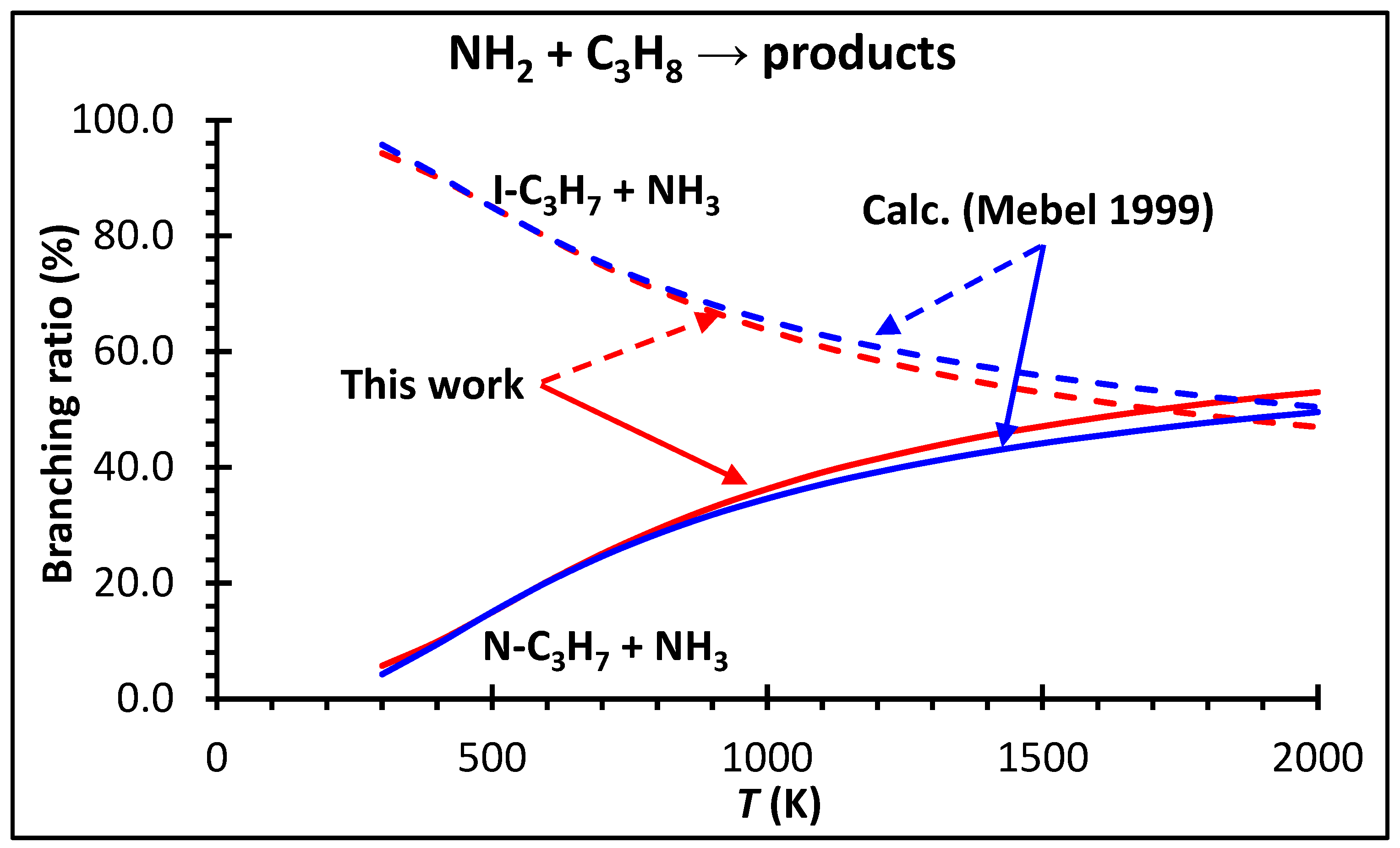
4.2. High-Pressure Limiting Rate Coefficients k∞ (T)
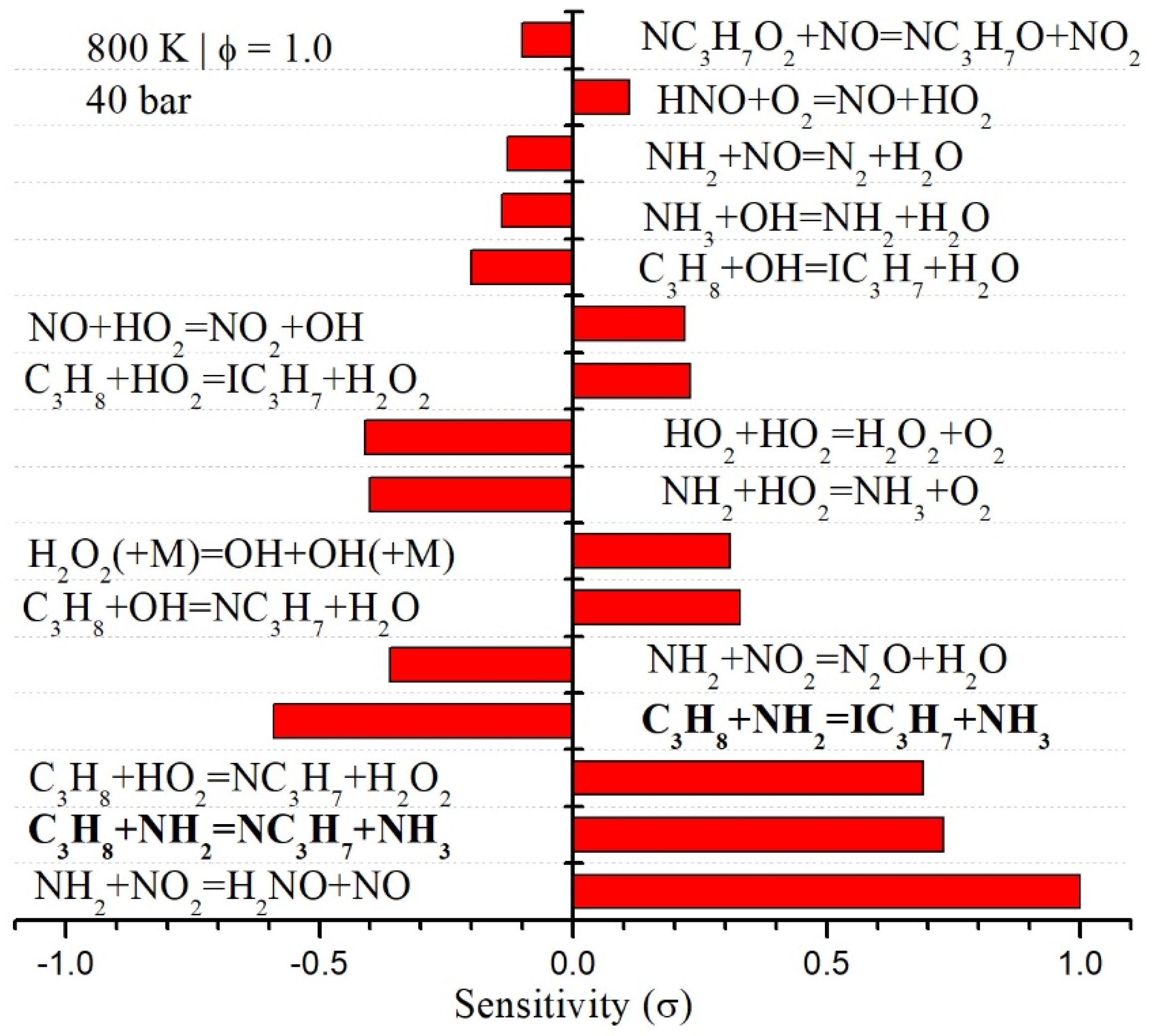
4.3. Kinetic Modeling Implications
4.3.1. Laminar Flame Speed
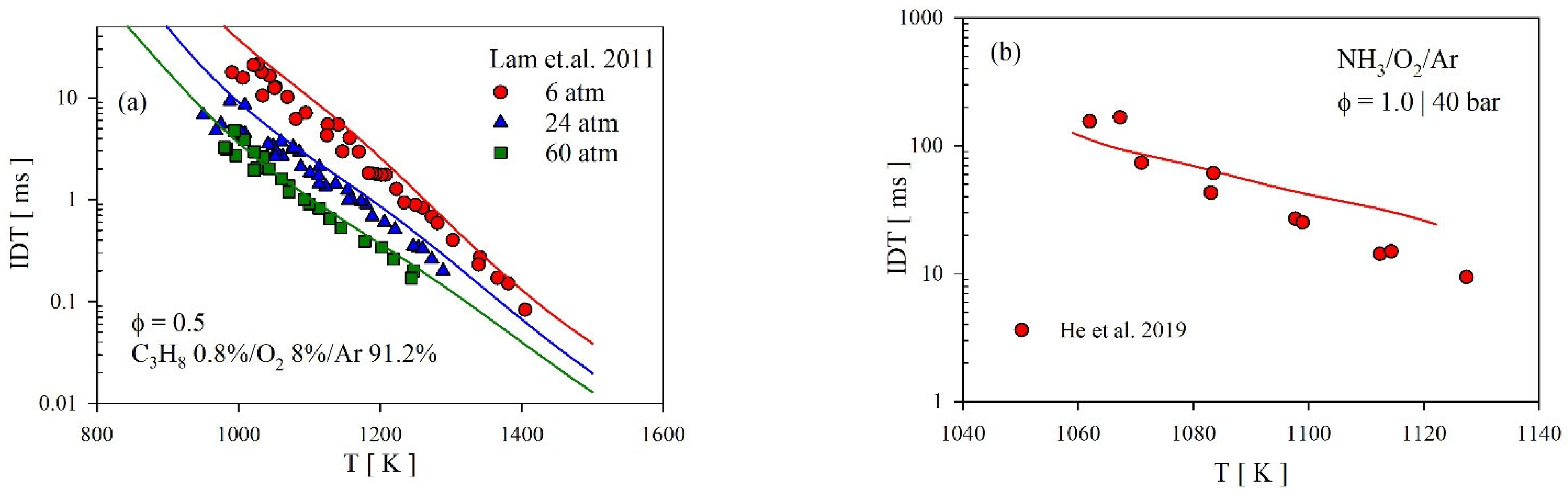
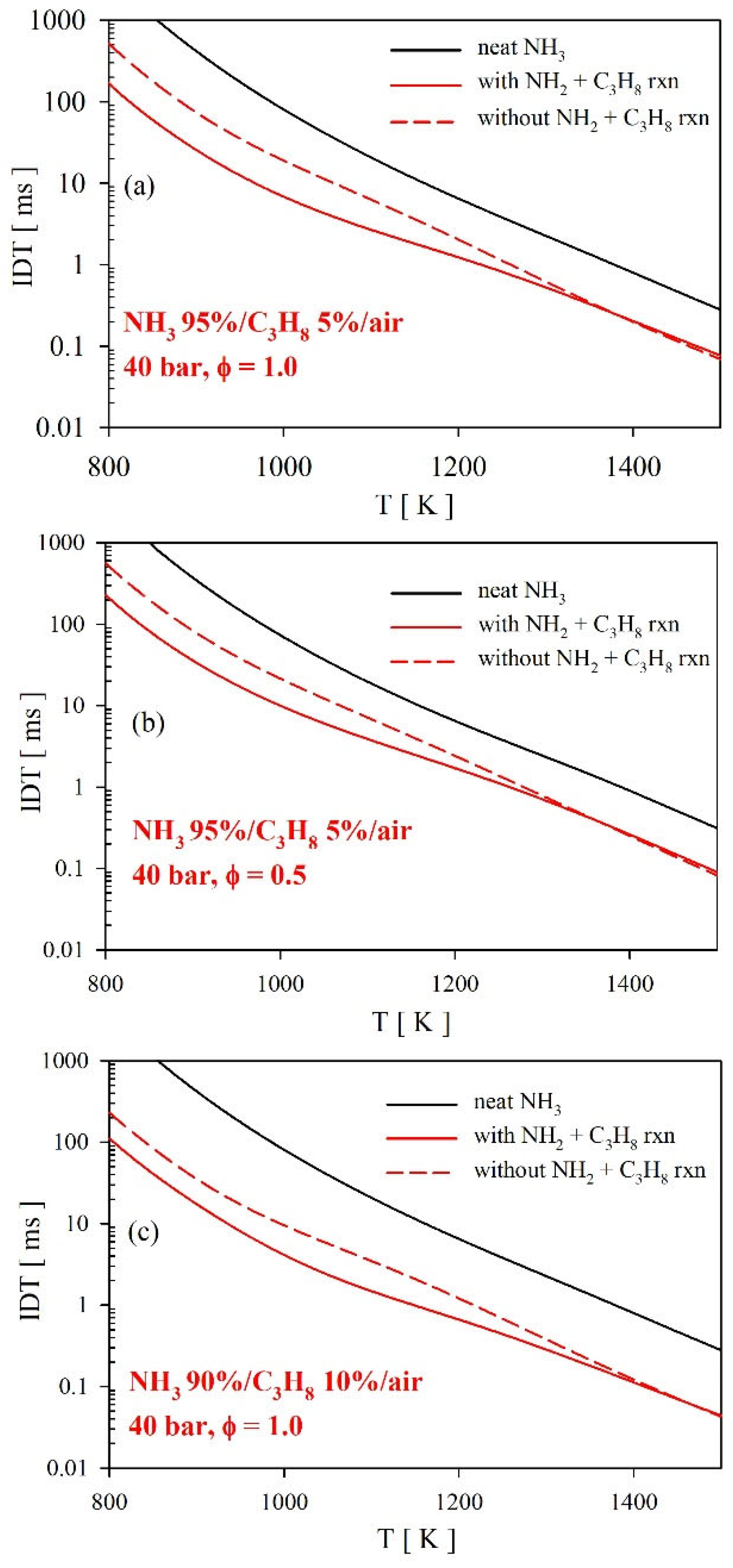
4.3.2. Ignition Delay Time (IDT)
5. Conclusions
- The energetics of the NH2 + C3H8 reaction show strong quantum method dependence. Our quantum approach at the CCSD(T)/cc-pV(T, Q)Z//M06-2X/aug-cc-pVTZ level of theory yielded barrier heights of 10.5 and 8.3 kcal/mol for Reactions (1) and (2), respectively. These barrier height values closely match those of the G2M approaches reported by Mebel and Lin [23].
- Our theory nicely captured the severely curved Arrhenius behavior shown by the experimental literature data. Quantum tunneling and hindered internal rotors corrections are crucial in capturing the experimental rate coefficients at low temperatures.
- Our theoretical predictions of the rate coefficients excellently matched with those of earlier theoretical studies from Mebel and Lin [23] and Siddique et al. [25]. However, all theories severely overpredict the experimental data of Hennig and Wagner [19] at high temperatures. It appears that the reported high-T kinetic data by Hennig and Wagner [19] are affected by the significant loss of the hydrocarbon reactants such that a simple first-order analysis to extract the rate coefficient is not applicable in their case.
- Over the temperature range of 300–2000 K, our theoretical rate coefficients can be given by the following Arrhenius expressions in units of cm3/molecule/s:
- 5.
- We developed a composite kinetic model consisting of NH3 and C3H8 subset chemistries. We incorporated the cross-reactions between the nitrogen and carbon families. Additionally, we updated the rate parameters for the reactions of NH2 radicals with C3H8, as determined in this study. To validate our kinetic model, we compared it against available literature data for the oxidation kinetics of neat C3H8, NH3, and NH3/C3H8 blends across various conditions. Our kinetics model remarkably captured the observed trends of the low- and high-temperature experimental literature data.
- 6.
- The reactions of NH2 radicals with C3H8 significantly affected the low-temperature oxidation behavior of NH3/C3H8 blends. We found that the predicted ignition delay times were shortened considerably by including the NH2 + C3H8 reaction. For instance, at ϕ = 1.0 and 800 K for the 95%NH3/5%C3H8 blend, the model predicted ignition delay time was ~ three times shorter with NH2 + C3H8 reactions compared to when these reactions were excluded. Furthermore, we found that the NH2 + C3H8 reactions did not impact the high-temperature chemistry, as observed for the laminar flame speed of NH3/C3H8 blends.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zamfirescu, C.; Dincer, I. Ammonia as a green fuel and hydrogen source for vehicular applications. Fuel Process. Technol. 2009, 90, 729–737. [Google Scholar] [CrossRef]
- Mercier, A.; Mounaïm-Rousselle, C.; Brequigny, P.; Bouriot, J.; Dumand, C. Improvement of SI engine combustion with ammonia as fuel: Effect of ammonia dissociation prior to combustion. Fuel Commun. 2022, 11, 100058. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Mercier, A.; Brequigny, P.; Dumand, C.; Bouriot, J.; Houillé, S. Performance of ammonia fuel in a spark assisted compression Ignition engine. Int. J. Engine Res. 2022, 23, 781–792. [Google Scholar] [CrossRef]
- Mørch, C.S.; Bjerre, A.; Gøttrup, M.P.; Sorenson, S.C.; Schramm, J. Ammonia/hydrogen mixtures in an SI-engine: Engine performance and analysis of a proposed fuel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Gersen, S.; Glarborg, P.; Mokhov, A.; Levinsky, H. Autoignition studies of NH3/CH4 mixtures at high pressure. Combust. Flame 2020, 218, 19–26. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Giri, B.R.; Issayev, G.; Shrestha, K.P.; Mauss, F.; Farooq, A.; Roberts, W.L. Experimental and Kinetic Modeling Study of Laminar Flame Speed of Dimethoxymethane and Ammonia Blends. Energy Fuels 2020, 34, 14726–14740. [Google Scholar] [CrossRef]
- Issayev, G.; Giri, B.R.; Elbaz, A.M.; Shrestha, K.P.; Mauss, F.; Roberts, W.L.; Farooq, A. Combustion behavior of ammonia blended with diethyl ether. Proc. Combust. Inst. 2021, 38, 499–506. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Lhuillier, C.; Barbosa, A.A.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C.; Seidel, L.; Mauss, F. An experimental and modeling study of ammonia with enriched oxygen content and ammonia/hydrogen laminar flame speed at elevated pressure and temperature. Proc. Combust. Inst. 2021, 38, 2163–2174. [Google Scholar] [CrossRef]
- Issayev, G.; Giri, B.R.; Elbaz, A.M.; Shrestha, K.P.; Mauss, F.; Roberts, W.L.; Farooq, A. Ignition delay time and laminar flame speed measurements of ammonia blended with dimethyl ether: A promising low carbon fuel blend. Renew. Energy 2022, 181, 1353–1370. [Google Scholar] [CrossRef]
- Ronan, P.; Pierre, B.; Christine, M.-R.; Guillaume, D.; Fabien, H. Laminar flame speed of ethanol/ammonia blends–An experimental and kinetic study. Fuel Commun. 2022, 10, 100052. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Giri, B.R.; Elbaz, A.M.; Issayev, G.; Roberts, W.L.; Seidel, L.; Mauss, F.; Farooq, A. A detailed chemical insights into the kinetics of diethyl ether enhancing ammonia combustion and the importance of NOx recycling mechanism. Fuel Commun. 2022, 10, 100051. [Google Scholar] [CrossRef]
- Thomas, D.E.; Shrestha, K.P.; Mauss, F.; Northrop, W.F. Extinction and NO formation of ammonia-hydrogen and air non-premixed counterflow flames. Proc. Combust. Inst. 2023, 39, 1803–1812. [Google Scholar] [CrossRef]
- Giri, B.R.; Shrestha, K.P.; Mai, T.V.T.; Mauss, F.; Huynh, L.K. A theoretical kinetic study of the reactions of NH2 radicals with methanol and ethanol and their implications in kinetic modeling. Int. J. Chem. Kinet. 2023, 55, 3–14. [Google Scholar] [CrossRef]
- Synák, F.; Čulík, K.; Rievaj, V.; Gaňa, J. Liquefied petroleum gas as an alternative fuel. Transp. Res. Procedia 2019, 40, 527–534. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, C.; Wang, D.; Zhang, T.; Zhai, Y.; Wang, S. Experimental and numerical study on laminar burning velocity and premixed combustion characteristics of NH3/C3H8/air mixtures. Fuel 2023, 331, 125936. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, T.; Zhai, Y.; Hou, R.; Tian, Z.Y.; Ji, C. A comparative study on the laminar C1–C4 n-alkane/NH3 premixed flame. Fuel 2022, 324, 124732. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Yu, Z.; Han, X.; He, Y.; Zhu, Y.; Konnov, A.A. Experimental and kinetic modeling study of laminar burning velocity enhancement by ozone additive in NH3+O2+N2 and NH3+CH4/C2H6/C3H8+air flames. Proc. Combust. Inst. 2023, 39, 4237–4246. [Google Scholar] [CrossRef]
- Hennig, G.; Wagner, H.G.G. A Kinetic Study About the Reactions of NH2(2B1) Radicals with Saturated Hydrocarbons in the Gas Phase. Berichte Bunsenges. Phys. Chem. 1995, 99, 863–869. [Google Scholar] [CrossRef]
- Ehbrecht, J.; Hack, W.; Rouveirolles, P.; Wagner, H.G. Hydrogen Abstraction Reactions By NH2(X2B1)-Radicals From Hydrocarbons in the Gas Phase. Berichte Bunsenges. Phys. Chem. 1987, 91, 700–708. [Google Scholar] [CrossRef]
- Lesclaux, R.; Demissy, M. The kinetics of the gas phase reactions of NH2 radicals with alkane and alkyl radicals. J. Photochem. 1978, 9, 110–112. [Google Scholar] [CrossRef]
- Demissy, M.; Lesclaux, R. Kinetics of hydrogen abstraction by amino radicals from alkanes in the gas phase. A flash photolysis-laser resonance absorption study. J. Am. Chem. Soc. 1980, 102, 2897–2902. [Google Scholar] [CrossRef]
- Mebel, A.M. Prediction of absolute rate constants for the reactions of NH2 with alkanes from ab initio G2M/TST calculations. J. Phys. Chem. A 1999, 103, 2088–2096. [Google Scholar] [CrossRef]
- Siddique, K.; Altarawneh, M.; Gore, J.; Westmoreland, P.R.; Dlugogorski, B.Z. Hydrogen Abstraction from Hydrocarbons by NH2. J. Phys. Chem. A 2017, 121, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.; Altarawneh, M.; Saeed, A.; Zeng, Z.; Gore, J.; Dlugogorski, B.Z. Interaction of NH2 radical with alkylbenzenes. Combust. Flame 2019, 200, 85–96. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.K.; Lindstedt, R.P. A comparative ab initio study of hydrogen abstraction from n-propyl benzene. Combust. Flame 2013, 160, 2642–2653. [Google Scholar] [CrossRef]
- Yin, G.; Hu, E.; Zhou, M.; Zhan, H.; Huang, Z. Kinetic Study on the Isomerization and Decomposition of the Alkenyl Radicals of 2,4,4-Trimethyl-1-pentene. Energy Fuels 2020, 34, 14757–14767. [Google Scholar] [CrossRef]
- Giri, B.R.; Mai, T.V.T.; Nguyen, T.T.D.; Szőri, M.; Huynh, L.K.; Farooq, A. Kinetic insights into the reaction of hydroxyl radicals with 1,4-pentadiene: A combined experimental and theoretical study. Combust. Flame 2022, 241, 112153. [Google Scholar] [CrossRef]
- Giri, B.R.; Mai, T.V.T.; Assali, M.; Nguyen, T.T.D.; Nguyen, H.T.; Szori, M.; Huynh, L.K.; Fittschen, C.; Farooq, A. Reaction kinetics of 1,4-cyclohexadienes with OH radicals: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2022, 24, 7836–7847. [Google Scholar] [CrossRef]
- Huynh, L.K.; Mai, T.V.T.; Nguyen, T.T.D.; Nguyen, H.T.; Nguyen, T.T. New mechanistic insights into atmospheric oxidation of aniline initiated by oh radicals. Environ. Sci. Technol. 2021, 55, 7858–7868. [Google Scholar] [CrossRef]
- Safaei, Z.; Shiroudi, A.; Zahedi, E.; Sillanpää, M. Atmospheric oxidation reactions of imidazole initiated by hydroxyl radicals: Kinetics and mechanism of reactions and atmospheric implications. Phys. Chem. Chem. Phys. 2019, 21, 8445–8456. [Google Scholar] [CrossRef] [Green Version]
- Alecu, I.M.; Zheng, J.; Zhao, Y.; Truhlar, D.G. Computational thermochemistry: Scale factor databases and scale factors for vibrational frequencies obtained from electronic model chemistries. J. Chem. Theory Comput. 2010, 6, 2872–2887. [Google Scholar] [CrossRef]
- Hratchian, H.P.; Schlegel, H.B. Accurate reaction paths using a Hessian based predictor-corrector integrator. J. Chem. Phys. 2004, 120, 9918–9924. [Google Scholar] [CrossRef] [PubMed]
- Hratchian, H.P.; Schlegel, H.B. Using Hessian updating to increase the efficiency of a Hessian based predictor-corrector reaction path following method. J. Chem. Theory Comput. 2005, 1, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision D.01. 2009. [Google Scholar]
- Purvis, G.D.; Bartlett, R.J. A full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Scuseria, G.E.; Janssen, C.L.; Schaefer, H.F. An efficient reformulation of the closed-shell coupled cluster single and double excitation (CCSD) equations. J. Chem. Phys. 1988, 89, 7382–7387. [Google Scholar] [CrossRef]
- Scuseria, G.E.; Schaefer, H.F. Is coupled cluster singles and doubles (CCSD) more computationally intensive than quadratic configuration interaction (QCISD)? J. Chem. Phys. 1989, 90, 3700–3703. [Google Scholar] [CrossRef]
- Halkier, A.; Helgaker, T.; Jørgensen, P.; Klopper, W.; Koch, H.; Olsen, J.; Wilson, A.K. Basis-set convergence in correlated calculations on Ne, N2, and H2O. Chem. Phys. Lett. 1998, 286, 243–252. [Google Scholar] [CrossRef]
- Duong, M.V.; Nguyen, H.T.; Truong, N.; Le, T.N.M.; Huynh, L.K. Multi-Species Multi-Channel (MSMC): An Ab Initio-based Parallel Thermodynamic and Kinetic Code for Complex Chemical Systems. Int. J. Chem. Kinet. 2015, 47, 564–575. [Google Scholar] [CrossRef]
- Duong, M.V.; Nguyen, H.T.; Mai, T.V.T.; Huynh, L.K. Global minimum profile error (GMPE)-a least-squares-based approach for extracting macroscopic rate coefficients for complex gas-phase chemical reactions. Phys. Chem. Chem. Phys. 2018, 20, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.M.; Do, S.T.; Huynh, L.K. Algorithm for auto-generation of hindered internal rotation parameters for complex chemical systems. Comput. Theor. Chem. 2017, 1100, 61–69. [Google Scholar] [CrossRef]
- Le, T.H.M.; Tran, T.T.; Huynh, L.K. Identification of hindered internal rotational mode for complex chemical species: A data mining approach with multivariate logistic regression model. Chemom. Intell. Lab. Syst. 2018, 172, 10–16. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Eckart, S.; Elbaz, A.M.; Giri, B.R.; Fritsche, C.; Seidel, L.; Roberts, W.L.; Krause, H.; Mauss, F. A comprehensive kinetic model for dimethyl ether and dimethoxymethane oxidation and NOx interaction utilizing experimental laminar flame speed measurements at elevated pressure and temperature. Combust. Flame 2020, 218, 57–74. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Seidel, L.; Zeuch, T.; Moréac, G.; Dagaut, P.; Mauss, F. On the implications of nitromethane–NOx chemistry interactions for combustion processes. Fuel 2021, 289, 119861. [Google Scholar] [CrossRef]
- Burke, S.M.; Metcalfe, W.; Herbinet, O.; Battin-Leclerc, F.; Haas, F.M.; Santner, J.; Dryer, F.L.; Curran, H.J. An experimental and modeling study of propene oxidation. Part 1: Speciation measurements in jet-stirred and flow reactors. Combust. Flame 2014, 161, 2765–2784. [Google Scholar] [CrossRef] [Green Version]
- Burke, S.M.; Burke, U.; Donagh, R.M.; Mathieu, O.; Osorio, I.; Keesee, C.; Morones, A.; Petersen, E.L.; Wang, W.; DeVerter, T.A.; et al. An experimental and modeling study of propene oxidation. Part 2: Ignition delay time and flame speed measurements. Combust. Flame 2015, 162, 296–314. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.; Goldsmith, C.F. Rate coefficients for fuel + NO2: Predictive kinetics for HONO and HNO2 formation. Proc. Combust. Inst. 2017, 36, 617–626. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Hynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume II-Gas phase reactions of organic species. Atmos. Meas. Tech. 2006, 6, 3625–4055. [Google Scholar] [CrossRef] [Green Version]
- Rozenberg, A.S.; Voronkov, V.G. Rate constants for the interaction of the NH biradical and the aminoradical with alkane molecules. Russ. J. Phys. Chem. 1972, 46, 425. [Google Scholar]
- Vandeputte, A.G.; Sabbe, M.K.; Reyniers, M.F.; Van Speybroeck, V.; Waroquier, M.; Marin, G.B. Theoretical study of the thermodynamics and kinetics of hydrogen abstractions from hydrocarbons. J. Phys. Chem. A 2007, 111, 11771–11786. [Google Scholar] [CrossRef]
- Baulch, D.L.; Cobos, C.J.; Cox, R.A.; Frank, P.; Hayman, G.; Just, T.; Kerr, J.A.; Murrells, T.; Pilling, M.J.; Troe, J.; et al. Evaluated Kinetic Data for Combustion Modeling. Supplement I. J. Phys. Chem. Ref. Data 1994, 23, 847–848. [Google Scholar] [CrossRef] [Green Version]
- Lam, K.Y.; Hong, Z.; Davidson, D.F.; Hanson, R.K. Shock tube ignition delay time measurements in propane/O2/argon mixtures at near-constant-volume conditions. Proc. Combust. Inst. 2011, 33, 251–258. [Google Scholar] [CrossRef]
- He, X.; Shu, B.; Nascimento, D.; Moshammer, K.; Costa, M.; Fernandes, R.X. Auto-ignition kinetics of ammonia and ammonia/hydrogen mixtures at intermediate temperatures and high pressures. Combust. Flame 2019, 206, 189–200. [Google Scholar] [CrossRef]


| Reaction | TS | A | n | E |
|---|---|---|---|---|
| 1. NH2 + C3H8 → NH3 + N-●CH2CH2CH3 | TS1 | 2.19 × 10−26 | 4.52 | 2346.7 |
| 2. NH2 + C3H8 → NH3 + I-CH3CH●CH3 | TS2 | 1.35 × 10−24 | 3.91 | 1684.0 |
| 3. NH2 + C3H8 → products | TS1 + TS2 | 4.00 × 10−26 | 4.47 | 1566.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giri, B.R.; Shrestha, K.P.; Mai, T.V.-T.; Giri, S.; Adil, M.; Naik, R.T.; Mauss, F.; Huynh, L.K. A Theoretical Study of NH2 Radical Reactions with Propane and Its Kinetic Implications in NH3-Propane Blends’ Oxidation. Energies 2023, 16, 5943. https://doi.org/10.3390/en16165943
Giri BR, Shrestha KP, Mai TV-T, Giri S, Adil M, Naik RT, Mauss F, Huynh LK. A Theoretical Study of NH2 Radical Reactions with Propane and Its Kinetic Implications in NH3-Propane Blends’ Oxidation. Energies. 2023; 16(16):5943. https://doi.org/10.3390/en16165943
Chicago/Turabian StyleGiri, Binod Raj, Krishna Prasad Shrestha, Tam V.-T. Mai, Sushant Giri, Mohammad Adil, R. Thirumaleswara Naik, Fabian Mauss, and Lam Kim Huynh. 2023. "A Theoretical Study of NH2 Radical Reactions with Propane and Its Kinetic Implications in NH3-Propane Blends’ Oxidation" Energies 16, no. 16: 5943. https://doi.org/10.3390/en16165943
APA StyleGiri, B. R., Shrestha, K. P., Mai, T. V.-T., Giri, S., Adil, M., Naik, R. T., Mauss, F., & Huynh, L. K. (2023). A Theoretical Study of NH2 Radical Reactions with Propane and Its Kinetic Implications in NH3-Propane Blends’ Oxidation. Energies, 16(16), 5943. https://doi.org/10.3390/en16165943








