Composite Liquid Biofuels for Power Plants and Engines: Review
Abstract
:1. Introduction
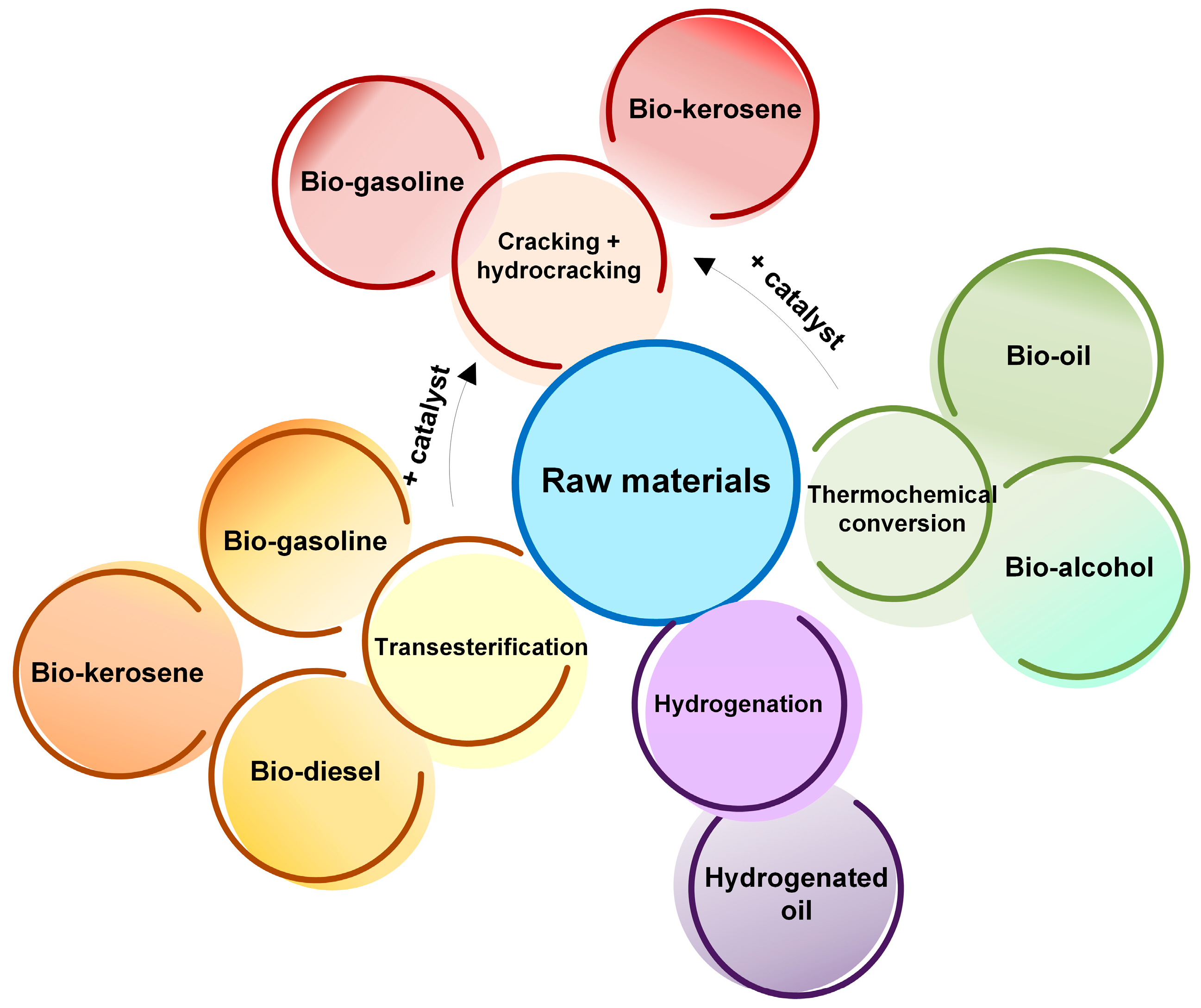
2. Liquid Biofuels: Feedstocks, Production Methods and Properties
3. Atomization
4. Ignition and Combustion Performance of Liquid Biofuels
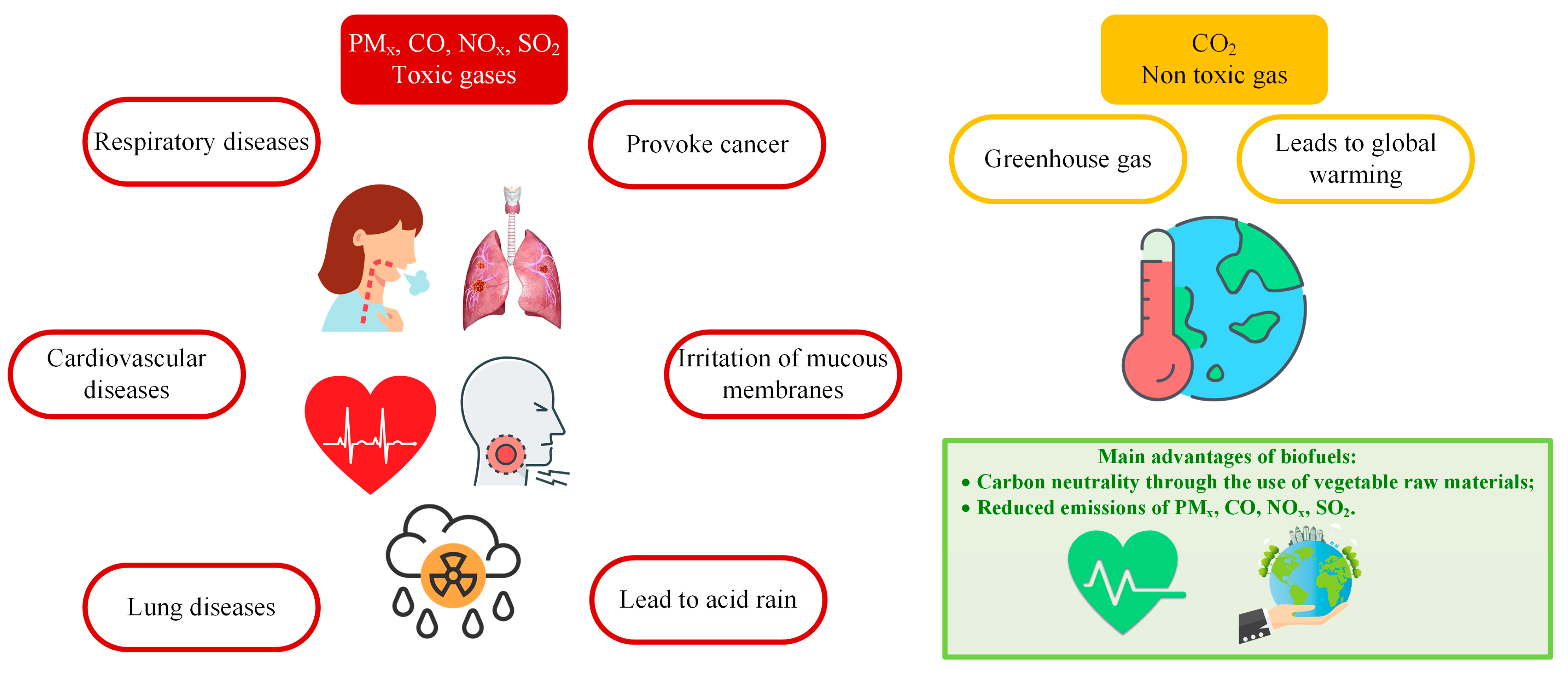
5. Emission Performance
5.1. Diesel-Biofuel Emissions
5.2. Kerosene-Biofuel Emission
5.3. Gasoline-Biofuel Emissions
6. Conclusions
- (i)
- Many of the energy industry aspects are crying out for revision and innovation. In this respect, biofuels are attracting more and more interest. The conducted review has revealed that this area provides tremendous opportunities for replacing fossil fuels of petroleum origin. However, the diversity of raw materials and their processing methods determine the respective variation ranges of end-product properties. Moreover, only some technologies provide high-quality biofuel (transesterification, some types of thermal conversion). More complex technologies (cracking and hydrocracking) serve to create biofuels whose performance is comparable to that of conventional hydrocarbon fuels.
- (ii)
- Spraying, being a practical aspect of using liquid fuels, is an important stage. Data from numerous studies suggest that this stage, in many ways, determines the efficiency of combustion of aerosol, thus, the thermal efficiency of a unit and emissions. Increased viscosity of composite liquid fuels containing biofuel is the main constraint on efficient spraying in a combustion chamber. To solve this problem, researchers have proposed using additives (e.g., ethanol), preliminary heating of the fuel, or transverse injection of gas to provide disruption.
- (iii)
- The carbon neutrality of plant raw materials makes composite biofuels very environmentally attractive. Particular performance characteristics of the engine may vary, and in some cases, the combustion of composite fuels is inferior to that of conventional Diesel fuel, kerosene, and gasoline in emissions. There is a clear trend towards a decrease in the emissions of particulate matter (PM) with an increase in the proportion of biodiesel or bio-kerosene in the liquid fuel composition. At the same time, the yield of CO depends greatly on the operating conditions of the equipment. Generally, the review indicates that CO emissions tend to rise with an increase in the proportion of oils or biofuel to over 15–20%. This is largely attributed to a lower combustion temperature.
- (iv)
- The fuel properties, chemical composition, and presence of impurities affect the spraying, mixing with the air, rates of evaporation, and kinetics of ignition and burnout. The studies conducted with different composite fuels reveal that satisfactory combustion is possible by controlling the fuel density and viscosity, as well as by selecting the composition with a high evaporation rate. The impact of the new kinds of fuels on the condition of components and parts of engines, corrosion, and wear is understudied. The most promising development paths are the reduction of the cost of cracking raw materials, the search for affordable low-viscosity components for fuel blends, upgrade of plants and fuel feeding systems for optimal atomization, evaporation, ignition, and burnout of fuel mixtures.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Altarazi, Y.S.M.; Abu Talib, A.R.; Yu, J.; Gires, E.; Abdul Ghafir, M.F.; Lucas, J.; Yusaf, T. Effects of Biofuel on Engines Performance and Emission Characteristics: A Review. Energy 2022, 238, 121910. [Google Scholar] [CrossRef]
- Chiong, M.C.; Chong, C.T.; Ng, J.H.; Lam, S.S.; Tran, M.V.; Chong, W.W.F.; Jaafar, M.M.N.; Valera-Medina, A. Liquid Biofuels Production and Emissions Performance in Gas Turbines: A Review. Energy Convers. Manag. 2018, 173, 640–658. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, I.; Caliskan, H.; Mori, K. Assessment of Biofuels from Waste Cooking Oils for Diesel Engines in Terms of Waste-to-Energy Perspectives. Sustain. Energy Technol. Assess. 2022, 50, 101839. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Bedaiwy, M.Y.; El-Nagar, A.A.; ElKelawy, M.; Alm-Eldin Bastawissi, H. Ethanol Biofuel Production and Characteristics Optimization from Wheat Straw Hydrolysate: Performance and Emission Study of DI-Diesel Engine Fueled with Diesel/Biodiesel/Ethanol Blends. Renew. Energy 2022, 191, 591–607. [Google Scholar] [CrossRef]
- Pearson, R.J.; Turner, J.W.G. Improving the Use of Liquid Biofuels in Internal Combustion Engines. In Advances in Biorefineries Biomass and Waste Supply Chain Exploitation; Woodhead Publishing: Cambridge, UK, 2014; pp. 389–440. [Google Scholar] [CrossRef]
- Puricelli, S.; Costa, D.; Rigamonti, L.; Cardellini, G.; Casadei, S.; Koroma, M.S.; Messagie, M.; Grosso, M. Life Cycle Assessment of Innovative Fuel Blends for Passenger Cars with a Spark-Ignition Engine: A Comparative Approach. J. Clean. Prod. 2022, 378, 134535. [Google Scholar] [CrossRef]
- Asgarian, F.; Hejazi, S.R.; Khosroshahi, H. Investigating the Impact of Government Policies to Develop Sustainable Transportation and Promote Electric Cars, Considering Fossil Fuel Subsidies Elimination: A Case of Norway. Appl. Energy 2023, 347, 121434. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2021; IEA: Paris, France, 2021. [Google Scholar]
- Martins, L.S.; Guimarães, L.F.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric Car Battery: An Overview on Global Demand, Recycling and Future Approaches towards Sustainability. J. Environ. Manag. 2021, 295, 113091. [Google Scholar] [CrossRef]
- Natkunarajah, N.; Scharf, M.; Scharf, P. Scenarios for the Return of Lithium-Ion Batteries out of Electric Cars for Recycling. Procedia CIRP 2015, 29, 740–745. [Google Scholar] [CrossRef]
- Komnos, D.; Tsiakmakis, S.; Pavlovic, J.; Ntziachristos, L.; Fontaras, G. Analysing the Real-World Fuel and Energy Consumption of Conventional and Electric Cars in Europe. Energy Convers. Manag. 2022, 270, 116161. [Google Scholar] [CrossRef]
- Yu, K.L.; Ong, H.C.; Zaman, H.B. Microalgae Biomass as Biofuel and the Green Applications. Energies 2022, 15, 7280. [Google Scholar] [CrossRef]
- Zhou, Y.; Remón, J.; Pang, X.; Jiang, Z.; Liu, H.; Ding, W. Hydrothermal Conversion of Biomass to Fuels, Chemicals and Materials: A Review Holistically Connecting Product Properties and Marketable Applications. Sci. Total Environ. 2023, 886, 163920. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gholizadeh, M. Progress of the Applications of Bio-Oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Yahya, M.; Dutta, A.; Bouri, E.; Wadström, C.; Uddin, G.S. Dependence Structure between the International Crude Oil Market and the European Markets of Biodiesel and Rapeseed Oil. Renew. Energy 2022, 197, 594–605. [Google Scholar] [CrossRef]
- Krstić, J.B.; Nježić, Z.B.; Kostić, M.D.; Marić, B.D.; Šimurina, O.D.; Stamenković, O.S.; Veljković, V.B. Biodiesel Production from Rapeseed Oil over Calcined Waste Filter Cake from Sugar Beet Processing. Process Saf. Environ. Prot. 2022, 168, 463–473. [Google Scholar] [CrossRef]
- Kiehbadroudinezhad, M.; Merabet, A.; Ghenai, C.; Abo-Khalil, A.G.; Salameh, T. The Role of Biofuels for Sustainable MicrogridsF: A Path towards Carbon Neutrality and the Green Economy. Heliyon 2023, 9, e13407. [Google Scholar] [CrossRef] [PubMed]
- Subhash, G.V.; Sivapirakasam, S.P.; Mohan, S.; Harisivasri Phanindra, K. Production, Characterization and Assessment of Reformulated Bio-Mixture Fuel from a Mixture of Various Raw Feedstock’s and the Effect of n-Butanol as an Additive on Bio-Mixture Blends. Biomass Bioenergy 2021, 154, 106246. [Google Scholar] [CrossRef]
- Elfasakhany, A. Exhaust Emissions and Performance of Ternary Iso-Butanol–Bio-Methanol–Gasoline and n-Butanol–Bio-Ethanol–Gasoline Fuel Blends in Spark-Ignition Engines: Assessment and Comparison. Energy 2018, 158, 830–844. [Google Scholar] [CrossRef]
- Ahmad, S.; Jafry, A.T.; Haq, M.U.; Abbas, N.; Ajab, H.; Hussain, A.; Sajjad, U. Performance and Emission Characteristics of Second-Generation Biodiesel with Oxygenated Additives. Energies 2023, 16, 5153. [Google Scholar] [CrossRef]
- Shanmugam, S.; Hari, A.; Pugazhendhi, A.; Kikas, T. Integrated Catalytic Upgrading of Biomass-Derived Alcohols for Advanced Biofuel Production. Energies 2023, 16, 4998. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Li, D.; Meng, X.; Zhan, G.; Wang, Y.; Zhang, A.; Sun, Y.; Ruan, R.; Ragauskas, A.J. Creating Values from Wastes: Producing Biofuels from Waste Cooking Oil via a Tandem Vapor-Phase Hydrotreating Process. Appl. Energy 2022, 323, 119629. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, K.; Li, J.; Wang, Z. Life-Cycle Assessment of Bio-Jet Fuel Production from Waste Cooking Oil via Hydroconversion. Energies 2022, 15, 6612. [Google Scholar] [CrossRef]
- Velvizhi, G.; Jacqueline, P.J.; Shetti, N.P.; Latha, K.L.; Mohanakrishna, G.; Aminabhavi, T.M. Emerging Trends and Advances in Valorization of Lignocellulosic Biomass to Biofuels. J. Environ. Manag. 2023, 345, 118527. [Google Scholar] [CrossRef] [PubMed]
- Akram, H.A.; Imran, M.; Javaid, A.; Latif, S.; Rizvi, N.B.; Jesionowski, T.; Bilal, M. Pretreatment and Catalytic Conversion of Lignocellulosic and Algal Biomass into Biofuels by Metal Organic Frameworks. Mol. Catal. 2023, 539, 112893. [Google Scholar] [CrossRef]
- Materazzi, M.; Holt, A. Experimental Analysis and Preliminary Assessment of an Integrated Thermochemical Process for Production of Low-Molecular Weight Biofuels from Municipal Solid Waste (MSW). Renew. Energy 2019, 143, 663–678. [Google Scholar] [CrossRef]
- Kułażyński, M.; Jabłoński, S.; Kaczmarczyk, J.; Świątek, Ł.; Pstrowska, K.; Łukaszewicz, M. Technological Aspects of Sunflower Biomass and Brown Coal Co-Firing. J. Energy Inst. 2018, 91, 668–675. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, M.; Leong, Y.K.; Zhang, Y.; Zhang, Z.; Zhang, D. An Experimental Study of the Rheological Properties and Stability Characteristics of Biochar-Algae-Water Slurry Fuels. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 105, pp. 125–130. [Google Scholar]
- Gao, W.; Zhang, M.; Wu, H. Ignition Temperatures of Various Bio-Oil Based Fuel Blends and Slurry Fuels. Fuel 2017, 207, 240–243. [Google Scholar] [CrossRef]
- Soria-Verdugo, A.; Kauppinen, J.; Soini, T.; García-Gutiérrez, L.M.; Pikkarainen, T. Pollutant Emissions Released during Sewage Sludge Combustion in a Bubbling Fluidized Bed Reactor. Waste Manag. 2020, 105, 27–38. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yang, W.; Zhao, Q.; Dai, Y. Evaluation of Combustion Properties and Pollutant Emission Characteristics of Blends of Sewage Sludge and Biomass. Sci. Total Environ. 2020, 720, 137365. [Google Scholar] [CrossRef]
- Li, H.; Chi, H.; Han, H.; Hu, S.; Song, G.; Wang, Y.; He, L.; Wang, Y.; Su, S.; Xiang, J. Comprehensive Study on Co-Combustion Behavior of Pelletized Coal-Biomass Mixtures in a Concentrating Photothermal Reactor. Fuel Process. Technol. 2021, 211, 106596. [Google Scholar] [CrossRef]
- Wang, C.; Bi, H.; Jiang, X.; Jiang, C.; Lin, Q. Experimental Study on Ignition and Combustion of Coal-Rice Husk Blends Pellets in Air and Oxy-Fuel Conditions. J. Energy Inst. 2020, 93, 1544–1558. [Google Scholar] [CrossRef]
- Si, T.; Cheng, J.; Zhou, F.; Zhou, J.; Cen, K. Control of Pollutants in the Combustion of Biomass Pellets Prepared with Coal Tar Residue as a Binder. Fuel 2017, 208, 439–446. [Google Scholar] [CrossRef]
- Yang, R.; Ma, C.; Chen, G.; Cheng, Z.; Yan, B.; Mansour, M. Study on NOx Emission during Corn Straw/Sewage Sludge Co-Combustion: Experiments and Modelling. Fuel 2021, 285, 119208. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Z.; Yu, C.; Xie, G. Conversion Mechanism of Fuel-N during Pyrolysis of Biomass Wastes. Fuel 2019, 246, 42–50. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Scott, J.; Yu, G.; Wang, Z.; Schideman, L.; Zhang, Y.; Strathmann, T.J. Chemical Properties of Biocrude Oil from the Hydrothermal Liquefaction of Spirulina Algae, Swine Manure, and Digested Anaerobic Sludge. Bioresour. Technol. 2011, 102, 8295–8303. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.H.; Grigoras, I.F.; Hoffmann, J.; Toor, S.S.; Daraban, I.M.; Jensen, C.U.; Iversen, S.B.; Madsen, R.B.; Glasius, M.; Arturi, K.R.; et al. Continuous Hydrothermal Co-Liquefaction of Aspen Wood and Glycerol with Water Phase Recirculation. Appl. Energy 2016, 162, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Nazari, L.; Yuan, Z.; Corscadden, K.; Xu, C.C.; He, Q.S. Hydrothermal Liquefaction of Spent Coffee Grounds in Water Medium for Bio-Oil Production. Biomass Bioenergy 2016, 86, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Luo, G.; Zhang, S.; Chen, J. Bio-Oil Production from Eight Selected Green Landscaping Wastes through Hydrothermal Liquefaction. RSC Adv. 2016, 6, 15260–15270. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal Liquefaction of Biomass: A Review of Subcritical Water Technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xie, J.; Liu, H.; Yin, X.; Wu, C. Bio-Oil Production from Hydrothermal Liquefaction of High-Protein High-Ash Microalgae Including Wild Cyanobacteria sp. and Cultivated Bacillariophyta sp. Fuel 2016, 183, 9–19. [Google Scholar] [CrossRef]
- Bach, Q.V.; Sillero, M.V.; Tran, K.Q.; Skjermo, J. Fast Hydrothermal Liquefaction of a Norwegian Macro-Alga: Screening Tests. Algal Res. 2014, 6, 271–276. [Google Scholar] [CrossRef]
- Braza, C.E.M.; Crnkovic, P.M. Physical-Chemical Characterization of Biomass Samples for Application Iin Pyrolysis Process. Currículo Lattes 2014, 37, 523–528. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Production and Characterization of Biodiesel from Algae. Fuel Process. Technol. 2014, 120, 79–88. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Bangjang, T.; Kaewchada, A.; Jaree, A. Biodiesel Production from Rice Bran Oil Fatty Acid Distillate via Supercritical Hydrolysis–Esterification–Transesterification in a Microreactor. Energy Rep. 2023, 9, 5299–5305. [Google Scholar] [CrossRef]
- Kanchanatip, E.; Chansiriwat, W.; Palalerd, S.; Khunphonoi, R.; Kumsaen, T.; Wantala, K. Light Biofuel Production from Waste Cooking Oil via Pyrolytic Catalysis Cracking over Modified Thai Dolomite Catalysts. Carbon Resour. Convers. 2022, 5, 177–184. [Google Scholar] [CrossRef]
- Istadi, I.; Riyanto, T.; Khofiyanida, E.; Buchori, L.; Anggoro, D.D.; Sumantri, I.; Putro, B.H.S.; Firnanda, A.S. Low-Oxygenated Biofuels Production from Palm Oil through Hydrocracking Process Using the Enhanced Spent RFCC Catalysts. Bioresour. Technol. Rep. 2021, 14, 100677. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A Review on Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Roman, F. Biodiesel Production through Esterification Applying Ionic Liquids as Catalysts. Ph.D. Thesis, Instituto Politecnico de Braganca, Bragança, Portugal, 2018. [Google Scholar]
- Luque, R.; Melero, J.A. Advances in Biodiesel Production: Processes and Technologies; Woodhead Publishing: Cambridge, UK, 2012; Volume 288. [Google Scholar]
- Sipos, B.; Kreuger, E.; Svensson, S.E.; Réczey, K.; Björnsson, L.; Zacchi, G. Steam Pretreatment of Dry and Ensiled Industrial Hemp for Ethanol Production. Biomass Bioenergy 2010, 34, 1721–1731. [Google Scholar] [CrossRef] [Green Version]
- Stefanidis, S.D.; Kalogiannis, K.G.; Lappas, A.A. Co-Processing Bio-Oil in the Refinery for Drop-in Biofuels via Fluid Catalytic Cracking. Wiley Interdiscip. Rev. Energy Environ. 2018, 7, e281. [Google Scholar] [CrossRef]
- Amini, Z.; Ong, H.C.; Harrison, M.D.; Kusumo, F.; Mazaheri, H.; Ilham, Z. Biodiesel Production by Lipase-Catalyzed Transesterification of Ocimum Basilicum, L. (Sweet Basil) Seed Oil. Energy Convers. Manag. 2017, 132, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Le-Phuc, N.; Tran, T.V.; Phan, T.T.; Ngo, P.T.; Ha, Q.L.M.; Luong, T.N.; Tran, T.H.; Phan, T.T. High-Efficient Production of Biofuels Using Spent Fluid Catalytic Cracking (FCC) Catalysts and High Acid Value Waste Cooking Oils. Renew. Energy 2021, 168, 57–63. [Google Scholar] [CrossRef]
- Shen, J.; Schmetz, E.; Stiegel, G.J.; Winslow, J.C.; Kornosky, R.M.; Madden, D.R.; Jain, S.C. Early Entrance Coproduction Plant—The Pathway to the Commercial CTL (Coal-to-Liquids) Fuels Production. Stud. Surf. Sci. Catal. 2007, 163, 315–325. [Google Scholar] [CrossRef]
- Rahimi, Z.; Anand, A.; Gautam, S. An Overview on Thermochemical Conversion and Potential Evaluation of Biofuels Derived from Agricultural Wastes. Energy Nexus 2022, 7, 100125. [Google Scholar] [CrossRef]
- He, X. A Novel Hybrid Digestion-Gasification Process Integrated with Membranes for Efficient Conversion of Biomass to Bio-Alcohols. Green Energy Environ. 2021, 6, 15–21. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic Thermochemical Conversion of Biomass for Biofuel Production: A Comprehensive Review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Winayanuwattikun, P.; Kaewpiboon, C.; Piriyakananon, K.; Tantong, S.; Thakernkarnkit, W.; Chulalaksananukul, W.; Yongvanich, T. Potential Plant Oil Feedstock for Lipase-Catalyzed Biodiesel Production in Thailand. Biomass Bioenergy 2008, 32, 1279–1286. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Chen, S.S.; Su, C.H.; Lin, J.H.; Chien, C.C. Enzymatic Production of Biodiesel from Insect Fat Using Methyl Acetate as an Acyl Acceptor: Optimization by Using Response Surface Methodology. Energy Convers. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Prasertsan, P.; Sangkharak, K. Improvement of Biodiesel Production Using Waste Cooking Oil and Applying Single and Mixed Immobilised Lipases on Polyhydroxyalkanoate. Renew. Energy 2020, 162, 1819–1827. [Google Scholar] [CrossRef]
- Kumar, D.; Das, T.; Giri, B.S.; Verma, B. Optimization of Biodiesel Synthesis from Nonedible Oil Using Immobilized Bio-Support Catalysts in Jacketed Packed Bed Bioreactor by Response Surface Methodology. J. Clean. Prod. 2020, 244, 118700. [Google Scholar] [CrossRef]
- Imanparast, S.; Faramarzi, M.A.; Hamedi, J. Production of a Cyanobacterium-Based Biodiesel by the Heterogeneous Biocatalyst of SBA-15@oleate@lipase. Fuel 2020, 279, 118580. [Google Scholar] [CrossRef]
- Li, X.; He, X.Y.; Li, Z.L.; Wang, Y.D.; Wang, C.Y.; Shi, H.; Wang, F. Enzymatic Production of Biodiesel from Pistacia Chinensis Bge Seed Oil Using Immobilized Lipase. Fuel 2012, 92, 89–93. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, B.; Chang, J.; Fu, Y.; Lv, P.; Wang, X. Synthesis of Biodiesel from Waste Cooking Oil Using Immobilized Lipase in Fixed Bed Reactor. Energy Convers. Manag. 2009, 50, 668–673. [Google Scholar] [CrossRef]
- Falowo, O.A.; Oloko-Oba, M.I.; Betiku, E. Biodiesel Production Intensification via Microwave Irradiation-Assisted Transesterification of Oil Blend Using Nanoparticles from Elephant-Ear Tree Pod Husk as a Base Heterogeneous Catalyst. Chem. Eng. Process. Process Intensif. 2019, 140, 157–170. [Google Scholar] [CrossRef]
- Kumar, D.; Das, T.; Giri, B.S.; Rene, E.R.; Verma, B. Biodiesel Production from Hybrid Non-Edible Oil Using Bio-Support Beads Immobilized with Lipase from Pseudomonas Cepacia. Fuel 2019, 255, 115801. [Google Scholar] [CrossRef]
- Sharma, V.; Duraisamy, G. Production and Characterization of Bio-Mix Fuel Produced from a Ternary and Quaternary Mixture of Raw Oil Feedstock. J. Clean. Prod. 2019, 221, 271–285. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic Viscosity of BioDiesel fuel Components and Related Compounds. Influence of Compound Structure and Comparison to PetroDiesel fuel Components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Z.; Kuti, O.A.; Zhang, W.; Nishida, K. Experimental and Analytical Study on Biodiesel and Diesel Spray Characteristics under Ultra-High Injection Pressure. Int. J. Heat Fluid Flow 2010, 31, 659–666. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Wandel, A.P. Macroscopic and Microscopic Characteristics of Biofuel Spray (Biodiesel and Alcohols) in CI Engines: A Review. Fuel 2021, 292, 120303. [Google Scholar] [CrossRef]
- Suraj, C.K.; Sudarshan, G.; Anand, K.; Sundararajan, T. Effects of Autooxidation on the Fuel Spray Characteristics of Karanja Biodiesel. Biomass Bioenergy 2021, 149, 106084. [Google Scholar] [CrossRef]
- Park, S.H.; Cha, J.; Kim, H.J.; Lee, C.S. Effect of Early Injection Strategy on Spray Atomization and Emission Reduction Characteristics in Bioethanol Blended Diesel fueled Engine. Energy 2012, 39, 375–387. [Google Scholar] [CrossRef]
- Gao, Y.; Deng, J.; Li, C.; Dang, F.; Liao, Z.; Wu, Z.; Li, L. Experimental Study of the Spray Characteristics of Biodiesel Based on Inedible Oil. Biotechnol. Adv. 2009, 27, 616–624. [Google Scholar] [CrossRef]
- Chaudhari, V.D.; Kulkarni, A.; Deshmukh, D. Spray Characteristics of Biofuels for Advance Combustion Engines. Clean. Eng. Technol. 2021, 5, 100265. [Google Scholar] [CrossRef]
- Yu, S.; Yin, B.; Deng, W.; Jia, H.; Ye, Z.; Xu, B.; Xu, H. Experimental Study on the Diesel and Biodiesel Spray Characteristics Emerging from Equilateral Triangular Orifice under Real Diesel Engine Operation Conditions. Fuel 2018, 224, 357–365. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, M.; Yan, F.; Chen, L.; Li, G.; Feng, L. Effects of Cavitation Flow and Stagnant Bubbles on the Initial Temporal Evolution of Diesel Spray. Exp. Therm. Fluid Sci. 2017, 87, 69–79. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Z.; Liu, F.; Wang, C.; Sun, Q.; Xu, C. Simulation of Throttling Effect on Cavitation for Nozzle Internal Flow. Fuel 2019, 243, 277–287. [Google Scholar] [CrossRef]
- Jagadale, V.S.; Rao, D.C.K.; Deshmukh, D.; Hanstorp, D.; Mishra, Y.N. Modes of Atomization in Biofuel Droplets Induced by a Focused Laser Pulse. Fuel 2022, 315, 123190. [Google Scholar] [CrossRef]
- Palash, S.M.; Kalam, M.A.; Masjuki, H.H.; Masum, B.M.; Rizwanul Fattah, I.M.; Mofijur, M. Impacts of Biodiesel Combustion on NOx Emissions and Their Reduction Approaches. Renew. Sustain. Energy Rev. 2013, 23, 473–490. [Google Scholar] [CrossRef]
- Evans, M.J.; Proud, D.B.; Medwell, P.R.; Pitsch, H.; Dally, B.B. Highly Radiating Hydrogen Flames: Effect of Toluene Concentration and Phase; Combustion Institute: Pittsburgh, PA, USA, 2021; Volume 38. [Google Scholar]
- Hashimoto, N.; Nishida, H.; Ozawa, Y.; Iwatsubo, T.; Inumaru, J. Influence of Type of Burner on NOx Emission Characteristics from Combustion of Palm Methyl Ester. World Acad. Sci. Eng. Technol. 2009, 3, 565–569. [Google Scholar]
- Jurić, F.; Krajcar, M.; Duić, N.; Vujanović, M. Investigating the Pollutant Formation and Combustion Characteristics of Biofuels in Compression Ignition Engines: A Numerical Study. Therm. Sci. Eng. Prog. 2023, 43, 101939. [Google Scholar] [CrossRef]
- Kim, S.; Im, H.; Yu, J.; Kim, K.; Kim, M.; Lee, T. Biofuel Production from Euglena: Current Status and Techno-Economic Perspectives. Bioresour. Technol. 2023, 371, 128582. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Senthil Kumar, P.; Rangasamy, G.; Varjani, S. CO2 Bio-Mitigation Using Genetically Modified Algae and Biofuel Production towards a Carbon Net-Zero Society. Bioresour. Technol. 2022, 363, 127982. [Google Scholar] [CrossRef]
- Sakthimurugan, V.; Madhu, S. Novel Scenedesmus Obliquus Algae Biofuel Emission and Performance Characterise in Si-DLC Coated Diesel Engine. Mater. Today Proc. 2023, 77, 490–496. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; Rodríguez-Fernández, J. Effect of BioDiesel fuels on Diesel Engine Emissions. Prog. Energy Combust. Sci. 2008, 34, 198–223. [Google Scholar] [CrossRef]
- Oo, C.W.; Shioji, M.; Nakao, S.; Dung, N.N.; Reksowardojo, I.; Roces, S.A.; Dugos, N.P. Ignition and Combustion Characteristics of Various BioDiesel fuels (BDFs). Fuel 2015, 158, 279–287. [Google Scholar] [CrossRef]
- Hidegh, G.T.; Csemány, D.; DarAli, O.; Rizvi, S.A.H.; Ng, J.-H.; Chong, C.T.; Józsa, V. Comparison of Thermophysical Properties and Combustion Characteristics of Various Biodiesels in a Non-MILD Ultra-Low Emission Swirl Burner. Fuel 2023, 334, 126583. [Google Scholar] [CrossRef]
- Setyawan, H.Y.; Zhu, M.; Zhang, Z.; Zhang, D. Ignition and Combustion Characteristics of Single Droplets of a Crude Glycerol in Comparison with Pure Glycerol, Petroleum Diesel, Biodiesel and Ethanol. Energy 2016, 113, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Ciriminna, R.; Pina, C.D.; Rossi, M.; Pagliaro, M. Understanding the Glycerol Market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Tariq, A.I.; Saleh, A.M. An Experimental Investigation into the Combustion Properties, Performance, Emissions, and Cost Reduction of Using Heavy and Light Fuel Oils. Case Stud. Therm. Eng. 2023, 44, 102832. [Google Scholar] [CrossRef]
- Khanjani, A.; Sobati, M.A. Performance and Emission of a Diesel Engine Using Different Water/Waste Fish Oil (WFO) Biodiesel/Diesel Emulsion Fuels: Optimization of Fuel Formulation via Response Surface Methodology (RSM). Fuel 2021, 288, 119662. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Use of Vegetable Oils as I.C. Engine Fuels—A Review. Renew. Energy 2004, 29, 727–742. [Google Scholar] [CrossRef]
- Uddin, S.M.A.; Azad, A.K.; Alam, M.M.; Ahamed, J.U. Performance of a Diesel Engine Run with Mustard-Kerosene Blends. Procedia Eng. 2015, 105, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Chivu, R.M.; Martins, J.; Popescu, F.; Uzuneanu, K.; Ion, I.V.; Goncalves, M.; Codău, T.-C.; Onofrei, E.; Brito, F.P. Turpentine as an Additive for Diesel Engines: Experimental Study on Pollutant Emissions and Engine Performance. Energies 2023, 16, 5150. [Google Scholar] [CrossRef]
- Hossain, A.K.; Refahtalab, P.; Omran, A.; Smith, D.I.; Davies, P.A. An Experimental Study on Performance and Emission Characteristics of an IDI Diesel Engine Operating with Neat Oil-Diesel Blend Emulsion. Renew. Energy 2020, 146, 1041–1050. [Google Scholar] [CrossRef]
- Aljaafari, A.; Fattah, I.M.R.; Jahirul, M.I.; Gu, Y.; Mahlia, T.M.I.; Islam, M.A.; Islam, M.S. Biodiesel Emissions: A State-of-the-Art Review on Health and Environmental Impacts. Energies 2022, 15, 6854. [Google Scholar] [CrossRef]
- Adar, S.D.; Sheppard, L.; Vedal, S.; Polak, J.F.; Sampson, P.D.; Diez Roux, A.V.; Budoff, M.; Jacobs, D.R.; Barr, R.G.; Watson, K.; et al. Fine Particulate Air Pollution and the Progression of Carotid Intima-Medial Thickness: A Prospective Cohort Study from the Multi-Ethnic Study of Atherosclerosis and Air Pollution. PLoS Med. 2013, 10, e1001430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longhin, E.; Holme, J.A.; Gutzkow, K.B.; Arlt, V.M.; Kucab, J.E.; Camatini, M.; Gualtieri, M. Cell Cycle Alterations Induced by Urban PM2.5 in Bronchial Epithelial Cells: Characterization of the Process and Possible Mechanisms Involved. Part. Fibre Toxicol. 2013, 10, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Hsu, A.; Moore, P.K. Actions and Interactions of Nitric Oxide, Carbon Monoxide and Hydrogen Sulphide in the Cardiovascular System and in Inflammation—A Tale of Three Gases! Pharmacol. Ther. 2009, 123, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, R. Carbon Monoxide: Endogenous Production, Physiological Functions, and Pharmacological Applications. Pharmacol. Rev. 2005, 57, 585–630. [Google Scholar] [CrossRef]
- Munawer, M.E. Human Health and Environmental Impacts of Coal Combustion and Post-Combustion Wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Gent, J.F.; Triche, E.W.; Holford, T.R.; Belanger, K.; Bracken, M.B.; Beckett, W.S.; Leaderer, B.P. Association of Low-Level Ozone and Fine Particles With Respiratory Symptoms in Children With Asthma. JAMA 2003, 290, 1859–1867. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Feng, Q.; Mao, Q.; Wang, C.; Li, G.; Che, D. Oxy-Fuel Co-Combustion Performances and Kinetics of Bituminous Coal and Ultra-Low Volatile Carbon-Based Fuels. Int. J. Energy Res. 2021, 45, 1892–1907. [Google Scholar] [CrossRef]
- Hamza, N.H.; Ekaab, N.S.; Chaichan, M.T. Impact of Using Iraqi Biofuel–Kerosene Blends on Coarse and Fine Particulate Matter Emitted from Compression Ignition Engines. Alex. Eng. J. 2020, 59, 1717–1724. [Google Scholar] [CrossRef]
- Mohankumar, S.; Senthilkumar, P. Particulate Matter Formation and Its Control Methodologies for Diesel Engine: A Comprehensive Review. Renew. Sustain. Energy Rev. 2017, 80, 1227–1238. [Google Scholar] [CrossRef]
- Arias, S.; Agudelo, J.R.; Molina, F.J.; Llanos-González, E.; Alcaín, F.J.; Ballesteros, R.; Lapuerta, M. Environmental and Health Risk Implications of Unregulated Emissions from Advanced Biofuels in a Euro 6 Engine. Chemosphere 2023, 313, 137462. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, K.; Nylund, N.O.; Hulkkonen, T.; Tilli, A.; Mikkonen, S.; Saikkonen, P.; Mäkinen, R.; Amberla, A. Emission Performance of Paraffinic HVO Diesel Fuel in Heavy Duty Vehicles; SAE International: Troy, MI, USA, 2011. [Google Scholar] [CrossRef]
- Vojtisek-Lom, M.; Beránek, V.; Mikuška, P.; Křůmal, K.; Coufalík, P.; Sikorová, J.; Topinka, J. Blends of Butanol and Hydrotreated Vegetable Oils as Drop-in Replacement for Diesel Engines: Effects on Combustion and Emissions. Fuel 2017, 197, 407–421. [Google Scholar] [CrossRef]
- Westphal, G.A.; Krahl, J.; Munack, A.; Rosenkranz, N.; Schröder, O.; Schaak, J.; Pabst, C.; Brüning, T.; Bünger, J. Combustion of Hydrotreated Vegetable Oil and Jatropha Methyl Ester in a Heavy Duty Engine: Emissions and Bacterial Mutagenicity. Environ. Sci. Technol. 2013, 47, 6038–6046. [Google Scholar] [CrossRef] [PubMed]
- Beran, M.; Axelsson, L.U. Development and Experimental Investigation of a Tubular Combustor for Pyrolysis Oil Burning. J. Eng. Gas Turbines Power 2015, 137, 031508. [Google Scholar] [CrossRef]
- López Juste, G.; Salvá Monfort, J.J. Preliminary Test on Combustion of Wood Derived Fast Pyrolysis Oils in a Gas Turbine Combustor. Biomass Bioenergy 2000, 19, 119–128. [Google Scholar] [CrossRef]
- Boomadevi, P.; Paulson, V.; Samlal, S.; Varatharajan, M.; Sekar, M.; Alsehli, M.; Elfasakhany, A.; Tola, S. Impact of Microalgae Biofuel on Microgas Turbine Aviation Engine: A Combustion and Emission Study. Fuel 2021, 302, 121155. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Tamilselvan, P.; Jiang, C.; He, Z.; Zhong, W.; Qian, Y.; Wang, Q.; Lu, X. Experimental Study of Combustion and Emission Characteristics of Gasoline Compression Ignition (GCI) Engines Fueled by Gasoline-Hydrogenated Catalytic Biodiesel Blends. Energy 2019, 187, 115931. [Google Scholar] [CrossRef]
- Velavan, A.; Saravanan, C.G.; Vikneswaran, M.; James Gunasekaran, E.; Sasikala, J. Visualization of In-Cylinder Combustion Flame and Evaluation of Engine Characteristics of MPFI Engine Fueled by Lemon Peel Oil Blended Gasoline. Fuel 2020, 263, 116728. [Google Scholar] [CrossRef]
- Manoj Babu, A.; Saravanan, C.G.; Vikneswaran, M.; Edwin Jeo, V.; Sasikala, J. Visualization of In-Cylinder Combustion Using Endoscope in Spark Ignition Engine Fueled with Pine Oil Blended Gasoline. Fuel 2020, 263, 116707. [Google Scholar] [CrossRef]
- Kalligeros, S.; Zannikos, F.; Stournas, S.; Lois, E.; Anastopoulos, G.; Teas, C.; Sakellaropoulos, F. An Investigation of Using Biodiesel/Marine Diesel Blends on the Performance of a Stationary Diesel Engine. Biomass Bioenergy 2003, 24, 141–149. [Google Scholar] [CrossRef]
- Raheman, H.; Phadatare, A.G. Diesel Engine Emissions and Performance from Blends of Karanja Methyl Ester and Diesel. Biomass Bioenergy 2004, 27, 393–397. [Google Scholar] [CrossRef]
- Timko, M.T.; Herndon, S.C.; De La Rosa Blanco, E.; Wood, E.C.; Yu, Z.; Miake-Lye, R.C.; Knighton, W.B.; Shafer, L.; Dewitt, M.J.; Corporan, E. Combustion Products of Petroleum Jet Fuel, a Fischer–Tropsch Synthetic Fuel, and a Biomass Fatty Acid Methyl Ester Fuel for a Gas Turbine Engine. Combust. Sci. Technol. 2011, 183, 1039–1068. [Google Scholar] [CrossRef]
- Nascimento, M.A.R.; Sierra, R.G.A.; Silva Lora, E.E.; Rendon, M.A. Performance and Emission Experimental Evaluation and Comparison of a Regenerative Gas Microturbine Using Biodiesel from Various Sources as Fuel. J. Energy Resour. Technol. Trans. ASME 2011, 133, 022204. [Google Scholar] [CrossRef]

| Component | Moisture Content, % | Ash, % | Vdaf, % | Higher Heating Value, MJ/kg | Cdaf, % | Hdaf, % | Ndaf, % | Std, % | Odaf, % |
|---|---|---|---|---|---|---|---|---|---|
| Cedar nut shells | 13.0 | 1.0 | 69.7 | 21.0 | 51.81 | 6.39 | 0.24 | traces | 41.56 |
| Sunflower seed shells [27] | 10.10 | 1.95–4.2 | 77.72 | 17.37 | 50.26 | 5.98 | 1.28 | 0.25 | 42.23 |
| Chlorella [28] | <1 | 12.3 | 78.9 | - | 48.6 | 7.3 | 9.0 | 0.9 | 34.2 |
| Liquid product of pine pyrolysis [29] | 23.6 | - | - | 18.5 | 42.50 | 7.10 | 0.06 | traces | 50.34 |
| Artichokes [30] | 5.7 | 7.5 | 74.5 | 14.9 | 45.4 | 6.6 | 2.6 | 0.2 | 45.2 |
| Rice husk [31,32,33] | 5.62–6.79 | 14.77–17.82 | 62.61–65.65 | 16.02 | 38.23–49.4 | 4.88–6.20 | 0.40–1.02 | 0.16–0.30 | 33.29–34.15 |
| Peanut shells [31] | 7.88 | 1.60 | 68.10 | 21.42 | 54.9 | 6.10 | 1.37 | 0.10 | 7.47 |
| Bamboo stems [34] | 15.62 | 0.92 | 75.24 | 17.41 | 44.2 | 5.15 | 0.49 | 0.22 | 39.40 |
| Corn straw [35] | 2.16 | 2.68 | 77.64 | - | 40.6 | 5.51 | 0.79 | 0.09 | 52.94 |
| Palm kernel [36] | 2.88 | 5.30 | 75.83 | 49.0 | 5.93 | 34.10 | 2.46 | 0.29 | |
| Spirulina algae [37] | - | - | - | 33.2 | 68.9 | 8.9 | 6.5 | 14.9 | 0.86 |
| Anaerobic sludge [37] | - | - | - | 32.0 | 66.6 | 9.2 | 4.3 | 18.9 | 0.97 |
| Aspen wood [38] | 3.8 | 0.48 | - | 34.3 | 75.2 | 8.2 | 0.05 | 15.8 | 0.3 |
| Spent coffee grounds [39] | - | - | - | 31.0 | 71.2 | 7.1 | 3.0 | 18.7 | - |
| Viburnum odoratissimum [40] | - | - | - | 32.5 | 71.7 | 8.1 | 1.2 | 19.0 | 0.01 |
| Salix alba [40] | - | - | - | 23.1 | 73.7 | 9.2 | 3.1 | 14.1 | 0.01 |
| Garbage [41] | - | 36 | - | 21–36 | 73.6 | 9.1 | 4.6 | - | 12.7 |
| Beech wood [41] | 34.9 | 16.1 | - | 21 | 76.7 | 7.1 | 0.1 | - | 16.1 |
| Phytomass [41] | - | - | - | 5–25 | 76.6 | 7.6 | 2.1 | 0.1 | 13.6 |
| Cyanobacteria [42] | - | - | - | 36.51 | 76.0 | 9.1 | 6.29 | 7.44 | 1.15 |
| Seaweed [43] | - | - | - | 35.97 | 75.5 | 9.16 | 3.65 | 11.66 | 0.62 |
| Coffee husk | 8.44 | 7.4 | - | 16.79 | 43.1 | 5.02 | 1.55 | 32.78 | 0.67 |
| Peanut shell [44] | 7.98 | 12.8 | – | 16.52 | 41.5 | 7.43 | 2.12 | 27.96 | 0.6 |
| Rice husk [44] | 8.19 | 29.53 | – | 15.39 | 31.4 | 6.67 | 1.04 | 23.03 | 0.5 |
| Pine sawdust [44] | 6.9 | 4.71 | – | 17.03 | 45.9 | 7.47 | 0.32 | 34.32 | 0.57 |
| Spirulina [45] | - | - | - | - | 48.1 | 6.97 | 10.14 | 34.13 | 0.66 |
| Chlorella [45] | - | - | - | - | 51.3 | 7.9 | 9.8 | 30.38 | 0.59 |
| Synthesis Method | Parameters | Ref. |
|---|---|---|
| Transesterification | V ≈ 3.8 mL T ≈ 260–320 °C P ≈ 1.2–5.9 MPa | [46] |
| Pyrolysis + cracking | T ≈ 450–500 °C Catalyst: MgCO3 + CaCO3; in relation to 0/100, 10/90, 20/80, 30/70 | [47] |
| Hydrothermal liquefaction | T ≈ 280–370 °C P ≈ 10–25 MPa | [49] |
| Pyrolysis | T ≈ 550–600 °C Catalysts: zeolite, AAEMs | [59] |
| Digestion gasification process | T ≈ 300–350 °C P ≈ 3–8 MPa Catalyst: Ferrite | [58] |
| Fuel/Feedstock | Density, g/cm3 | Viscosity, mm2/s | Heating Value, MJ/kg | Cetane Number | References |
|---|---|---|---|---|---|
| RBOFAD | 0.88 | 5 | 41.7 | – | [46] |
| RBOFAD | 0.89 | 3.8 | - | – | |
| RRBO | 0.87 | 4.14 | - | – | |
| RRBO | 0.89 | 8 | - | – | |
| Comm. Biodiesel | 0.88 | 4.4 | 39.03 | – | |
| Bio-gasoline | – | 0.65 | 43.49 | – | [47] |
| Bio-diesel | – | 3.25 | 40.58 | – | |
| Bio-kerosene | – | 0.84 | 42.61 | – | |
| Bio-crude (pyrolysis) | 0.93 | 71.05 | 22.6 | – | [57] |
| Bio-crude (HLF) | 0.95 | 15.306 | 35.7 | – | |
| Palm oil | – | 3.62 | – | 59.11 | [60] |
| Papaya oil | – | 3.69 | – | 56.27 | |
| Rambutan oil | – | 3.95 | – | 61.17 | |
| Vegetable oil | 0.904 | 5.4 | 38.4 | 49 | [61] |
| Waste cooking oil | 0.86 | 3.8 | 196.2 | – | [62] |
| Hybrid oil | 0.8831 | 7.83 | 38.63 | – | [63] |
| Hybrid oil | 0.88 | 7.85 | 39.51 | – | [63] |
| Cyanobacterium | 0.8 | 2.9 | 35.5 | 56 | [64] |
| Sweet basil | 0.87 | 4.26 | 39.72 | - | [54] |
| Pistacia chinensis seed | 0.88 | 4.15 | 39.8 | 49 | [65] |
| Waste cooking oil | 0.892 | 9.12 | – | 68 | [66] |
| Rubber seed oil | 0.920 | 58.11 | 39.32 | 47.6 | [67] |
| Neem oil | 0.941 | 124.43 | 40.82 | 57.71 | [67] |
| Castor oil | 0.965 | 6.6 | 40.83 | - | [68] |
| Jatropha oil | 0.92 | 37.28 | 38.96 | 21 | [69] |
| Palm oil and sesame oil (biodiesel) | 0.88 | 4.63 | 41.24 | 55.37 | [69] |
| Rubber seed and neem oils (bio-diesel) | 0.897 | 5.94 | 39.66 | 56.53 | [67] |
| Waste cooking oil (bio-diesel) | 0.88 | 4.89 | - | - | [67] |
| Ref. | Fuel | Engine | Main Results |
|---|---|---|---|
| [107] | Kerosene + 10–20% biodiesel; Diesel fuel + 10–20% biodiesel | PM1 emissions fell by up to 81% compared to Diesel fuel; PM2.5 emissions fell by up to 51% compared to Diesel fuel | |
| [109] | Commercial biofuel on the basis of hydrogenated vegetable oils (HVO); Diesel fuel + 20 vol% hydrogenated turpentine (HT20); Diesel fuel + 20 vol% hydrogenated orange oil (HO20); Diesel fuel + 20 vol% polyoxymethylene dimethyl ethers (OME20); 80% HVO + 20% glycerol-based biofuel | Diesel engine | The lowest PAH emissions in all the engine tests were typical of HVO. |
| [119] | Sunflower oil methyl ester/diesel blends | A stationary diesel engine | Emissions of NOx, CO, HC, and particulate matter decreased when using blends based on Diesel fuel and biofuel compared to neat Diesel fuel |
| [120] | Methyl ester of Karanja and 20–80% blends with diesel | Direct injection diesel engine with a single cylinder | Lower emissions of CO and NOx when using composite fuels rather than Diesel fuel |
| [114] | 80% biofuel B-EUO4-B + 20% ethanol; | Gas turbine combustor with a swirl atomizer | Nitrogen oxide emissions decreased almost 4-fold compared to conventional jet fuel JP-4 |
| [115] | Fuel blends on the basis of Jet-A and Spirulina algae biofuel: B20% (20% biofuel with 80% Jet-A); B40% (40% biofuel with 60% Jet-A); B60% (60% biofuel with 40% Jet-A); B80% (80% biofuel with 20% Jet-A); biofuel B100%. | Experimental jet engine | Emissions of CO2 decreased by up to 11% compared to Jet-A; Emissions of CO decreased by up to 35% compared to Jet-A; Emissions of NOx decreased by up to 5% compared to Jet-A; |
| [121] | Jet A + 20%/40% biodiesel (B20/B40) | Turbofan engine (CFM56-7B) | Emissions of NOx decreased for B20 and B40 by 29% and 23%, respectively, against Jet A. |
| [122] | Diesel fuel + 10–50% biodiesel based on palm oil (B10–B50) | MGT (30 kW) | Lower emissions of CO and NOx when using composite fuels rather than Diesel fuel |
| [116] | Gasoline + 20–40% hydrogenated catalytic biodiesel (HCB) | Gasoline engine | Emissions of NOx, CO, and HC decrease when using HCB; PM emissions increase when using HCB |
| [117] | Gasoline + 10–30% lemon peel oil (Lp10–Lp30) | Gasoline engine | CO emissions in the combustion of Lp10 were 5–7% lower than in the combustion of gasoline; NO emissions in the combustion of Lp10 were 5% lower than in the combustion of gasoline; CO2 emissions in the combustion of Lp30 were 4% lower than in the combustion of gasoline; |
| [118] | Gasoline + 10–30% pine oil (Pn10–Pn30) | Gasoline engine | Maximum emissions of CO and minimum emissions of NOx were recorded for the fuel blend with 30 vol% pine oil. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsov, G.; Dorokhov, V.; Vershinina, K.; Kerimbekova, S.; Romanov, D.; Kartashova, K. Composite Liquid Biofuels for Power Plants and Engines: Review. Energies 2023, 16, 5939. https://doi.org/10.3390/en16165939
Kuznetsov G, Dorokhov V, Vershinina K, Kerimbekova S, Romanov D, Kartashova K. Composite Liquid Biofuels for Power Plants and Engines: Review. Energies. 2023; 16(16):5939. https://doi.org/10.3390/en16165939
Chicago/Turabian StyleKuznetsov, Genii, Vadim Dorokhov, Ksenia Vershinina, Susanna Kerimbekova, Daniil Romanov, and Ksenia Kartashova. 2023. "Composite Liquid Biofuels for Power Plants and Engines: Review" Energies 16, no. 16: 5939. https://doi.org/10.3390/en16165939
APA StyleKuznetsov, G., Dorokhov, V., Vershinina, K., Kerimbekova, S., Romanov, D., & Kartashova, K. (2023). Composite Liquid Biofuels for Power Plants and Engines: Review. Energies, 16(16), 5939. https://doi.org/10.3390/en16165939







