A Comparative Analysis of Two-Phase Flow Boiling Heat Transfer Coefficient and Correlations for Hydrocarbons and Ethanol
Abstract
:1. Introduction
2. Experimental Studies on Hydrocarbons and Pure Ethanol
2.1. Description of Experimental Work on Hydrocarbons
| Author/Year | Fluids | Tube Material/Inside Diameter (mm) | Saturation Temperature/Vapor Quality | Heat Flux (kW/m2) | Mass Flux (kg/m2s) |
|---|---|---|---|---|---|
| Yunos et al. (2017) [23] | R290 | Horizontal/single circular stainless-steel tube din = 7.6 | Tsat = 6–20 x = 0.01–0.15 | q = 5–22 | G = 200–650 |
| Chien et al. (2016) [21] | R290, R32, R410a | Horizontal/stainless steel tube(microchannel) din = 0.3 mm, 1.5 | Tsat = 10 x = 0.1–dry out | q = 10–20 | G = 200–500 |
| Kanizwa et al. (2016) [24] | R600a, R134a, R245fa | stainless steel tube din = 0.38–2.6 | Tsat = 22 x = 0.01–0.69 | q = 46–100 | G = 240–400 |
| Wang et al. (2014) [12] | R290 | Horizontal, copper tube din = 6 | Tsat = −35–−1.9 x = 0.14–0.75 | q = 11.7–87.1 | G = 62–104 |
| Del Col et al. (2014) [9] | R290 | Horizontal, copper mini-channel din = 0.96 | Tsat = 31 x = 0.05–0.6 | q = 10–315 | G = 100–600 |
| Wen et al. (2014) [25] | R600a | Horizontal, circular pipe within dispersed-copper porous inserts. din = 0.168–0.506 | Tsat = 10 x = 0.076–0.87 | q = 12–65 | G = 120–1100 |
| Copetti et al. (2013) [26] | R600a, R134a | Horizontal mini-channel/smooth stainless-steel tube din = 2.6 | Tsat = 22 x = 0.076–0.87 | q = 44–95 | G = 240–440 |
| Maqbool et al. (2011) [11] | R290 | Vertical, stainless steel mini-channel din = 1.7 | Tsat = 23, 33, 43 x = 0–1 | q = 5–280 | G = 100–500 |
| Choi et al. (2009) [15] | R290 | Horizontal, smooth stainless steel mini-channels din = 1.5, 3 | Tsat =0, 5, 10 x = 0–1 | q = 5–20 | G = 50–400 |
| Wen et al. (2005) [20] | R290, R600, R290/R600 | Horizontal/copper tube din = 2.46 | Tsat = 6 x = 0–0.86 | q = 5–21 | G = 250–500 |
| Shin et al. (1997) [17] | R22, R32, R134a, R290, R600a refrigerant mixtures | Horizontal/stainless steel tube din = 7.7 | Tsat = 12 x = 0.05–0.7 | q = 10–30 | G = 424–583 |
2.2. Description of Experimental Work on Ethanol
| Author/Year | Fluids | Tube Material/Inside Diameter (mm) | Saturation Temperature/Vapor Quality | Heat Flux (kW/m2) | Mass Flux (kg/m2s) |
|---|---|---|---|---|---|
| Mastrullo et al. (2018) [27] | Ethanol | Horizontal stainless-steel tube din = 6.0 | Tsat = 64.5–85.8 x = 0.11–0.91 | q = 10–40.3 | G = 85–127 |
| Vasileiadou et al. (2017) [29] | Ethanol, Deionized water, 5% v/v Ethanol/water | Borosilicate glass square channel din = 5 | Tsat = 40 | q = 2.8–6.1 | G = 0.3–1 |
| Robertson et al. (1988) [28] | Ethanol | Vertical copper tube din = 10 | Tsat = 88.6 x = 0.03–0.6 | q = 25.5–1.4.6 | G = 145–290 |
3. Assessment of Previous Correlations
Review of Flow Boiling Heat Transfer Coefficient Correlations
| Author (Year) | Correlations |
|---|---|
| ElFaham and Tang (2022) [35] | is calculated using Equation (5) |
| Saitoh et al. (2007) [31] | is calculated using Equation (4) is calculated using Equation (5) |
| Choi et al. (2007) [34] | is calculated using Equation (4) is calculated using Equation (5) |
| Yoon et al. (2004) [36] | where is calculated using Equation (4) is calculated using Equation (5) |
| Wattelet et al. (1994) [37] | is calculated using Equation (4) is calculated using Equation (5) |
| Liu–Winterton(1991) [22] | is calculated using Equation (4) is calculated using Equation (5) |
| Jung et al. (1989) [32] | is calculated using Equation (5) |
| Bennett and Chen (1980) [33] | is calculated using Equation (1) is calculated using Equation (5) |
| Chen (1966) [30] |
4. Results and Discussion
4.1. Assessment of Existing Correlations
4.2. Comparison to Hydrocarbons Dataset
4.3. Comparison to Ethanol Dataset
4.4. Comparison to Propane (R290) Dataset
5. Conclusions
- A database was created based on 11 published papers from 10 independent laboratories for hydrocarbons (R290, R600, and R600a). This evaluation comprises 900 flow boiling heat transfer coefficient data points for hydrocarbons. Moreover, a dataset of 720 experimental data points was collected for ethanol’s flow boiling heat transfer coefficients.
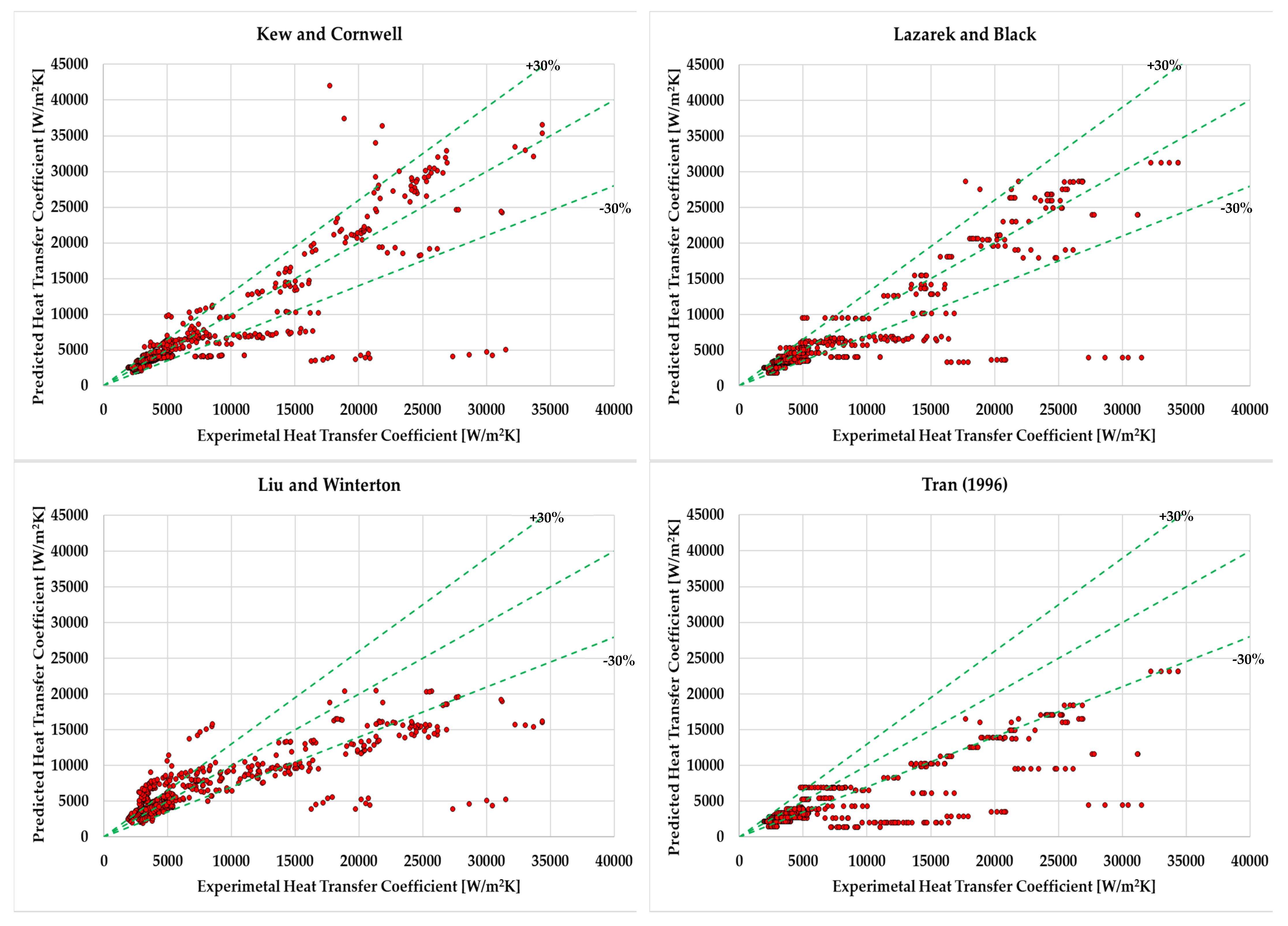
- It was found that for the hydrocarbons Kew and Cornwell [45] (24.6%), Lazarek, and Black [39] (25.7%), correlation has achieved the least mean absolute deviation, which is less than 30%. However, Liu and Winterton [22] (33.1%), ElFaham and Tang [35] (36.7%), and Tran (38.2%) had the tendency to show relatively low Mean Absolute deviation. On the other hand, Agostini et al. [42], Sun and Mishima [41], Chaddock and Brunemann [63], and Bennet and Chen [33] were out of prediction, and their results were unsatisfactory.
- It has been observed that among the assessed correlations for ethanol, ElFaham and Tang [35] achieved the lowest mean absolute deviation (15.3%). Nevertheless, Chen [30] (25%), Liu and Winterton [22] (25.1%), and YU [43] (25.7%) exhibited a range of mean absolute deviation less than 30%, which is considered to be in an outstanding position.
- Each correlation developed using its own data, fluids, geometry, and operating conditions. As a result, no specific universal prediction method exists. This study assessed the same correlations for different fluids to benchmark its findings, demonstrating that each fluid has a varied performance for prediction. Therefore, when comparing Table 6, Table 7 and Table 8, each correlation appears in a different place.
Funding
Conflicts of Interest
Nomenclature
| Roman | Abbreviations | ||
| cp | Specific heat capacity [J/kg·K] | MAE | Mean absolute error |
| d | Diameter [m] | MRE | Mean relative error |
| E | Convective enhancement factor [–] | STD | Standard deviation |
| S | Nucleate boiling suppression factor [–] | Subscripts | |
| G | Mass flux [kg/m2·s] | in | Inner |
| g | Acceleration of gravity [m/s2] | cr | Critical |
| h | Heat transfer coefficient [W/m2·K] | L | Liquid Phase |
| i | Specific enthalpy [J/kg] | b | Bulk or bottom |
| K | Thermal conductivity [W/m·K] | v | Vapor Phase |
| m | Mass flow rate [kg/s] | Sat | Saturation |
| M | Molecular mass [kg/kmol] | sp | Single phase |
| P | Pressure [Pa] | tp | Two-phase |
| q | Heat flux [W/m2] | nb | Nucleate boiling |
| PR | Reduced pressure | Pred | Predicted |
| T | Temperature [K] | Exp | Experimental |
| x | Vapor quality [–] | w | wall |
| Xtt | Martinelli parameter [–] | Dimensionless numbers | |
| R | Wattelet reduction parameter | Re | Reynolds number [–] |
| Ms | Suppression factor multiplier [–] | Pr | Prandtl number [–] |
| C | Chisholm parameter | We | Weber number [–] |
| F | Convection two-phase multiplier | Bo | boiling number [–] |
| Greek | Fr | Froude number [–] | |
| Density [kg/m3] | Nu | Nusselt number [–] | |
| Viscosity [kg/m·s] | CO | Convection number [–] | |
| Two-phase frictional multiplier [–] | Nconf | Confinement number [–] | |
| Surface tension [N/m] | Bd | Bond Number [–] | |
| Latent heat of vaporization | |||
| Difference between wall and saturation temperatures |
References
- Lorentzen, G. The use of natural refrigerants: A complete solution to the CFC/HCFC predicament. Int. J. Refrig. 1995, 18, 190–197. [Google Scholar]
- Zhang, D.; He, Z.; Guan, J.; Tang, S.; Shen, C. Heat transfer and flow visualization of pulsating heat pipe with silica nanofluid: An experimental study. Int. J. Heat Mass Transf. 2022, 183, 122100. [Google Scholar]
- Cavallini, A. Working fluids for mechanical refrigeration—Invited paper presented at the 19th International Congress of Refrigeration, The Hague, August 1995. Int. J. Refrig. 1996, 19, 485–496. [Google Scholar]
- Dzigbor, A.; Chimphango, A. Evaluating the potential of using ethanol/water mixture as a refrigerant in adsorption cooling system by using activated carbon-sodium chloride composite adsorbent. Int. J. Refrig. 2019, 97, 132–142. [Google Scholar]
- Thome, J.R.; Cheng, L.; Ribatski, G.; Vales, L.F. Flow boiling of ammonia and hydrocarbons: A state-of-the-art review. Int. J. Refrig. 2008, 31, 603–620. [Google Scholar]
- Granryd, E. Hydrocarbons as refrigerants—An overview. Int. J. Refrig. 2001, 24, 15–24. [Google Scholar] [CrossRef]
- Thome, J.R. Boiling of new refrigerants: A state-of-the-art review. Int. J. Refrig. 1996, 19, 435–457. [Google Scholar]
- Liu, C.; Sun, Z.; Zhang, Z.; Shi, J.; Chen, J. Literature review of condensation and evaporation of R290. In Proceedings of the International Refrigeration and Air Conditioning Conference, West Lafayette, IN, USA, 11–14 July 2016. [Google Scholar]
- Del Col, D.; Bortolato, M.; Bortolin, S. Comprehensive experimental investigation of two-phase heat transfer and pressure drop with propane in a minichannel. Int. J. Refrig. 2014, 47, 66–84. [Google Scholar]
- Tibiriçá, C.B.; Ribatski, G. Flow patterns and bubble departure fundamental characteristics during flow boiling in microscale channels. Exp. Therm. Fluid Sci. 2014, 59, 152–165. [Google Scholar] [CrossRef]
- Maqbool, M.H.; Palm, B.; Khodabandeh, R.; Rizwan Ali, R. Saturated flow boiling heat transfer characteristics of propane in a smooth vertical minichannel up to dryout incipience. In Proceedings of the 23rd IIR Congress of Refrigeration, Prague, Czech Republic, 21–26 August 2011; pp. 2794–2801. [Google Scholar]
- Wang, S.; Gong, M.; Chen, G.; Sun, Z.; Wu, J. Two-phase heat transfer and pressure drop of propane during saturated flow boiling inside a horizontal tube. Int. J. Refrig. 2014, 41, 200–209. [Google Scholar]
- Kandlikar, S.G.; Steinke, M.E. Flow Boiling Heat Transfer Coefficient In Minichannels−Correlation and Trends. In International Heat Transfer Conference Digital Library; Begel House Inc.: New York, NY, USA, 2002. [Google Scholar]
- Jung, D.; Lee, H.; Bae, D.; Oho, S. Nucleate boiling heat transfer coefficients of flammable refrigerants. Int. J. Refrig. 2004, 27, 409–414. [Google Scholar] [CrossRef]
- Choi, K.-I.; Pamitran, A.; Oh, J.-T.; Saito, K. Pressure drop and heat transfer during two-phase flow vaporization of propane in horizontal smooth minichannels. Int. J. Refrig. 2009, 32, 837–845. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, M.H.; Palm, B.; Khodabandeh, R. Investigation of two phase heat transfer and pressure drop of propane in a vertical circular minichannel. Exp. Therm. Fluid Sci. 2013, 46, 120–130. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, M.S.; Ro, S.T. Experimental study on forced convective boiling heat transfer of pure refrigerants and refrigerant mixtures in a horizontal tube. Int. J. Refrig. 1997, 20, 267–275. [Google Scholar]
- Gungor, K.E.; Winterton, R. A general correlation for flow boiling in tubes and annuli. Int. J. Heat Mass Transf. 1986, 29, 351–358. [Google Scholar]
- Thome, J.; Shakir, S. A new correlation for nucleate pool boiling of aqueous mixtures. In Heat Transfer: Pittsburgh 1987; 1987. [Google Scholar]
- Wen, M.-Y.; Ho, C.-Y. Evaporation heat transfer and pressure drop characteristics of R-290 (propane), R-600 (butane), and a mixture of R-290/R-600 in the three-lines serpentine small-tube bank. Appl. Therm. Eng. 2005, 25, 2921–2936. [Google Scholar] [CrossRef]
- Chien, N.-B.; Vu, P.-Q.; Choi, K.-I.; Oh, J.-T. An experimental investigation of convective boiling heat transfer using alternative and natural refrigerants inside horizontal microchannels. In Proceedings of the International Refrigeration and Air Conditioning Conference, West Lafayette, IN, USA, 11–14 July 2016. [Google Scholar]
- Liu, Z.; Winterton, R. A general correlation for saturated and subcooled flow boiling in tubes and annuli, based on a nucleate pool boiling equation. Int. J. Heat Mass Transf. 1991, 34, 2759–2766. [Google Scholar] [CrossRef]
- Yunos, Y.M.; Ghazali, N.M.; Pamitran, A.S.; Novianto, S. Analysis of the Two-Phase Heat Transfer Coefficient of Propane in a Small Channel. Energy Procedia 2017, 105, 4635–4640. [Google Scholar]
- Kanizawa, F.T.; Tibiriçá, C.B.; Ribatski, G. Heat transfer during convective boiling inside microchannels. Int. J. Heat Mass Transf. 2016, 93, 566–583. [Google Scholar] [CrossRef]
- Wen, M.-Y.; Jang, K.-J.; Ho, C.-Y. The characteristics of boiling heat transfer and pressure drop of R-600a in a circular tube with porous inserts. Appl. Therm. Eng. 2014, 64, 348–357. [Google Scholar]
- Copetti, J.; Macagnan, M.; Zinani, F. Experimental study on R-600a boiling in 2.6 mm tube. Int. J. Refrig. 2013, 36, 325–334. [Google Scholar] [CrossRef]
- Mastrullo, R.; Mauro, A.; Revellin, R.; Viscito, L. Flow boiling heat transfer and pressure drop of pure ethanol (99.8%) in a horizontal stainless steel tube at low reduced pressures. Appl. Therm. Eng. 2018, 145, 251–263. [Google Scholar] [CrossRef]
- Steiner, D.; Taborek, J. Flow boiling heat transfer in vertical tubes correlated by an asymptotic model. Heat Transf. Eng. 1992, 13, 43–69. [Google Scholar] [CrossRef]
- Vasileiadou, P.; Sefiane, K.; Karayiannis, T.G.; Christy, J.R. Flow boiling of ethanol/water binary mixture in a square mini-channel. Appl. Therm. Eng. 2017, 127, 1617–1626. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.C. Correlation for boiling heat transfer to saturated fluids in convective flow. Ind. Eng. Chem. Process Des. Dev. 1966, 5, 322–329. [Google Scholar]
- Saitoh, S.; Daiguji, H.; Hihara, E. Correlation for boiling heat transfer of R-134a in horizontal tubes including effect of tube diameter. Int. J. Heat Mass Transf. 2007, 50, 5215–5225. [Google Scholar] [CrossRef]
- Jung, D.-S.; McLinden, M.; Radermacher, R.; Didion, D. A study of flow boiling heat transfer with refrigerant mixtures. Int. J. Heat Mass Transf. 1989, 32, 1751–1764. [Google Scholar] [CrossRef]
- Bennett, D.L.; Chen, J.C. Forced convective boiling in vertical tubes for saturated pure components and binary mixtures. AIChE J. 1980, 26, 454–461. [Google Scholar] [CrossRef]
- Choi, K.-I.; Pamitran, A.S.; Oh, J.-T. Two-phase flow heat transfer of CO2 vaporization in smooth horizontal minichannels. Int. J. Refrig. 2007, 30, 767–777. [Google Scholar] [CrossRef]
- Elfaham, M. A Development of A General Heat Transfer Correlation for Boiling Two-Phase Flow of Pure Ethanol Based on Experimental Datasets and Correlations. Bachelor’s Thesis, University of North Dakota, Grand Forks, ND, USA, 2022. [Google Scholar]
- Yoon, S.H.; Cho, E.S.; Hwang, Y.W.; Kim, M.S.; Min, K.; Kim, Y. Characteristics of evaporative heat transfer and pressure drop of carbon dioxide and correlation development. Int. J. Refrig. 2004, 27, 111–119. [Google Scholar] [CrossRef]
- Wattelet, J.; Chato, J.; Souza, A.; Christoffersen, B. Evaporative characteristics of R-134a, MP-39, and R-12 at low mass fluxes. ASHRAE Trans. 1993. [Google Scholar]
- Kutateladze, S.S. Boiling heat transfer. Int. J. Heat Mass Transf. 1961, 4, 31–45. [Google Scholar] [CrossRef]
- Lazarek, G.; Black, S. Evaporative heat transfer, pressure drop and critical heat flux in a small vertical tube with R-113. Int. J. Heat Mass Transf. 1982, 25, 945–960. [Google Scholar] [CrossRef]
- Hamdar, M.; Zoughaib, A.; Clodic, D. Flow boiling heat transfer and pressure drop of pure HFC-152a in a horizontal mini-channel. Int. J. Refrig. 2010, 33, 566–577. [Google Scholar] [CrossRef]
- Sun, L.; Mishima, K. An evaluation of prediction methods for saturated flow boiling heat transfer in mini-channels. Int. J. Heat Mass Transf. 2009, 52, 5323–5329. [Google Scholar] [CrossRef]
- Agostini, B.; Bontemps, A. Vertical flow boiling of refrigerant R134a in small channels. Int. J. Heat Fluid Flow 2005, 26, 296–306. [Google Scholar]
- Yu, W.; France, D.; Wambsganss, M.; Hull, J. Two-phase pressure drop, boiling heat transfer, and critical heat flux to water in a small-diameter horizontal tube. Int. J. Multiph. Flow 2002, 28, 927–941. [Google Scholar] [CrossRef]
- Warrier, G.R.; Dhir, V.K.; Momoda, L.A. Heat transfer and pressure drop in narrow rectangular channels. Exp. Therm. Fluid Sci. 2002, 26, 53–64. [Google Scholar] [CrossRef]
- Kew, P.A.; Cornwell, K. Correlations for the prediction of boiling heat transfer in small-diameter channels. Appl. Therm. Eng. 1997, 17, 705–715. [Google Scholar] [CrossRef]
- Tran, T.; Wambsganss, M.; Chyu, M.-C.; France, D. A correlation for nucleate flow boiling in small channels. In Compact Heat Exchangers for the Process Industries; Begel House Inc.: Danbury, CT, USA, 1997. [Google Scholar]
- Tran, T.; Wambsganss, M.; France, D. Small circular-and rectangular-channel boiling with two refrigerants. Int. J. Multiph. Flow 1996, 22, 485–498. [Google Scholar] [CrossRef]
- Kenning, D.; Cooper, M. Saturated flow boiling of water in vertical tubes. Int. J. Heat Mass Transf. 1989, 32, 445–458. [Google Scholar] [CrossRef]
- Forster, H.; Zuber, N. Dynamics of vapor bubbles and boiling heat transfer. AIChE J. 1955, 1, 531–535. [Google Scholar] [CrossRef]
- Stephan, K.; Abdelsalam, M. Heat-transfer correlations for natural convection boiling. Int. J. Heat Mass Transf. 1980, 23, 73–87. [Google Scholar] [CrossRef]
- Chisholm, D. A theoretical basis for the Lockhart-Martinelli correlation for two-phase flow. Int. J. Heat Mass Transf. 1967, 10, 1767–1778. [Google Scholar] [CrossRef]
- ElFaham, M.; Tang, C.C. Review of Datasets and Correlations for Two-Phase Flow Boiling Heat Transfer of Pure Ethanol and Ethanol/Water Binary Mixtures. In Proceedings of the Heat Transfer Summer Conference, Philadelphia, PA, USA, 11–13 July 2022. [Google Scholar]
- Assael, M.; Polimatidou, S. Measurements of the viscosity of alcohols in the temperature range 290–340 K at pressures up to 30 MPa. Int. J. Thermophys. 1994, 15, 95–107. [Google Scholar] [CrossRef]
- Marsh, K.N.; Perkins, R.A.; Ramires, M.L. Measurement and Correlation of the Thermal Conductivity of Propane from 86 K to 600 K at Pressures to 70 MPa. J. Chem. Eng. Data 2002, 47, 932–940. [Google Scholar] [CrossRef]
- Perkins, R.A.; Ramires, M.L.; Nieto de Castro, C.A.; Cusco, L. Measurement and correlation of the thermal conductivity of butane from 135 K to 600 K at pressures to 70 MPa. J. Chem. Eng. Data 2002, 47, 1263–1271. [Google Scholar] [CrossRef]
- Perkins, R.A. Measurement and Correlation of the Thermal Conductivity of Isobutane from 114 K to 600 K at Pressures to 70 MPa. J. Chem. Eng. Data 2002, 47, 1272–1279. [Google Scholar] [CrossRef]
- Oh, H.-K.; Son, C.-H. Evaporation flow pattern and heat transfer of R-22 and R-134a in small diameter tubes. Heat Mass Transf. 2011, 47, 703–717. [Google Scholar] [CrossRef]
- Hu, H.; Ding, G.; Huang, X.-C.; Deng, B.; Gao, Y.-F. Experimental investigation and correlation of two-phase heat transfer of R410a/oil mixture flow boiling in a 5-mm microfin tube. J. Enhanc. Heat Transf. 2011, 18, 209–220. [Google Scholar] [CrossRef]
- Wojtan, L.; Ursenbacher, T.; Thome, J.R. Investigation of flow boiling in horizontal tubes: Part II—Development of a new heat transfer model for stratified-wavy, dryout and mist flow regimes. Int. J. Heat Mass Transf. 2005, 48, 2970–2985. [Google Scholar] [CrossRef]
- Lavin, J.G.; Young, E.H. Heat transfer to evaporating refrigerants in two-phase flow. AIChE J. 1965, 11, 1124–1132. [Google Scholar] [CrossRef] [Green Version]
- Pujol, L.; Stenning, A.H. Coefficients have been measured in a heat transfer loop consisting of two upward and two downward sections formed by bending a twenty. Cocurrent Gas-Liq. Flow 1969, 401. [Google Scholar]
- Li, W.; Wu, Z. A general criterion for evaporative heat transfer in micro/mini-channels. Int. J. Heat Mass Transf. 2010, 53, 1967–1976. [Google Scholar] [CrossRef]
- Chaddock, J.B.; Brunemann, H. Forced Convection Boiling of Refrigerants in Horizontal Tubes: Phase 3; Laboratories, 1967. [Google Scholar]


| Author (Year) | Correlations |
|---|---|
| Hamdar et al. (2010) [40] | |
| Sun and Mishima (2009) [41] | |
| Agostini et al. (2005) [42] | |
| Yu et al. (2002) [43] | |
| Warrier et al. (2002) [44] | |
| Kew and Cornwell (1997) [45] | |
| Tran et al. (1997) [46] | |
| Tran et al. (1996) [47] | |
| Kenning and Cooper (1989) [48] | is calculated using Equation (5) |
| Lazarek and Black (1982) [39] |
| Dimensionless Number | Equation |
|---|---|
| Reynolds number for liquid phase | |
| Boiling number | Bo = |
| Bond number | Bd = |
| Weber number for Liquid phase | |
| Froude number for liquid phase | |
| Lockhart–Martinelli parameter | |
| Convection number | |
| Confinement number |
| Correlations (Year) | MAE (%) | MRE (%) | Correlations (Year) | MAE (%) | MRE (%) |
|---|---|---|---|---|---|
| Kew and Cornwell [45] (1997) | 24.6 | −12.89 | Wattelet [37] (1994) | 67.98 | 54.04 |
| Lazarek and Black [39] (1982) | 25.73 | −18.72 | Kenning Copper [48] (1989) | 68.59 | 38.71 |
| Liu and Winterton [22] (1991) | 33.02 | −3.33 | Oh and Son [57] (2011) | 73.27 | 19.26 |
| ElFaham and Tang [35] (2022) | 36.69 | −6.16 | Hu et al. [58] (2011) | 80.39 | 15 |
| Tran [47] (1996) | 38.16 | −36.22 | Gungor and Winterton [19] (1986) | 83.51 | 75.43 |
| Yoon [36] (2004) | 40.64 | 14.02 | Chen [30] (1966) | 83.75 | 75.31 |
| Wojtan et al. [59] (2005) | 42.54 | −41.39 | Lavin and Young [60] (1965) | 83.8 | 49.16 |
| Hamdar [40] (2010) | 47.01 | −5.01 | Jung [32] (1989) | 94.97 | 61.01 |
| Warrier [44] (2002) | 51.25 | −40.72 | Choi [34] (2007) | 104.56 | 73.16 |
| Pujol and Stenning [61] (1969) | 56.12 | 10.5 | Bennett and Chen [33] (1980) | 104.6 | 74.28 |
| Li and Wu [62] (2010) | 57.64 | 13.22 | Chaddock and Brunemann [63] (1967) | 104.8 | 83.69 |
| Saitoh [31] (2007) | 60.57 | 27.7 | Sun and Mishima [41] (2009) | 116.8 | 96.85 |
| YU [43] (2002) | 65.03 | −9.56 | Kew and Cornwell [45] (1997) | 186.97 | 177.68 |
| Correlations (Year) | MAE (%) | MRE (%) |
|---|---|---|
| ElFaham and Tang [35] (2022) | 15.29 | −5.83 |
| Chen [30] (1966) | 25.02 | 20 |
| Liu and Winterton [22] (1991) | 25.12 | −14.81 |
| YU [43] (2002) | 25.7 | −8.17 |
| Saitoh [31] (2007) | 26.78 | −10.38 |
| Yoon [36] (2004) | 27.37 | −20.03 |
| Wattelet [37] (1994) | 28.39 | −8.35 |
| Sun and Mishima [41] (2009) | 29.69 | 18.58 |
| Wojtan et al. [59](2005) | 40.57 | −40.57 |
| Jung [32] (1989) | 47.25 | −14.42 |
| Gungor and Winterton [19] (1986) | 52.28 | 51.61 |
| Hu et al. [58] (2011) | 56.76 | 56.01 |
| Hamdar [40] (2010) | 58.56 | −58.57 |
| Bennett and Chen [33] (1980) | 59.6 | 59.75 |
| Kenning Copper [48] (1989) | 64.66 | −51.01 |
| Oh and Son [57] (2011) | 66.01 | −52.44 |
| Pujol and Stenning [61] (1969) | 73.54 | −73.34 |
| Lavin and Young [60] (1965) | 76.43 | −74.91 |
| Chaddock and Brunemann [63] (1967) | 77.27 | −29.97 |
| Warrier [44](2002) | 79.67 | −79.65 |
| Tran [47] (1996) | 83.36 | −83.35 |
| Kew and Cornwell [45] (1997) | 83.91 | −83.91 |
| Tran et al. [46] (1997) | 84.02 | −84.02 |
| Lazarek and black [39] (1982) | 85.17 | −85.17 |
| Choi [34] (2007) | 119.07 | 118.89 |
| Li and Wu [62] (2010) | 574.76 | 569.49 |
| Correlations (Year) | MAE (%) | MRE (%) |
|---|---|---|
| Kew and Cornwell [45] (1997) | 17.66 | −1.85 |
| Lazarek and black [39] (1982) | 18.28 | −9.13 |
| Liu and Winterton [22] (1991) | 31.17 | 5.8 |
| Tran [47] (1996) | 32.4 | −30.4 |
| ElFaham and Tang [35] (2022) | 34.96 | 5.48 |
| Wojtan et al. [59] (2005) | 37.78 | −35.99 |
| Yoon [36] (2004) | 40.94 | 28.97 |
| Hamdar [40] (2010) | 43.11 | 13.32 |
| Warrier [44] (2002) | 48.09 | −35.09 |
| Pujol and Stenning [61] (1969) | 58.25 | 26.35 |
| Li and Wu [62] (2010) | 63.01 | 35.31 |
| Saitoh [31] (2007) | 67.88 | 4.8 |
| Wattelet [37] (1994) | 75.55 | 63.57 |
| Kenning Copper [48] (1989) | 79.25 | 39.85 |
| YU [43] (2002) | 80.66 | 66.6 |
| Oh and Son [57] (2011) | 87.04 | 36.72 |
| Chen [30] (1966) | 96.18 | 76.92 |
| Gungor and Winterton [19] (1986) | 102.31 | 99.98 |
| Hu et al. [58] (2011) | 102.34 | 99.97 |
| Lavin and Young [60] (1965) | 113.88 | 94.54 |
| Jung [32] (1989) | 123.46 | 99.67 |
| Tran et al. [46] (1997) | 128.08 | 111.93 |
| Choi [34] (2007) | 130.29 | 123.6 |
| Bennett and Chen [33] (1980) | 138.48 | 127.86 |
| Chaddock and Brunemann [63] (1967) | 220.54 | 218.07 |
| Sun and Mishima [41] (2009) | 252.15 | 250.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElFaham, M.; Tang, C.C. A Comparative Analysis of Two-Phase Flow Boiling Heat Transfer Coefficient and Correlations for Hydrocarbons and Ethanol. Energies 2023, 16, 5931. https://doi.org/10.3390/en16165931
ElFaham M, Tang CC. A Comparative Analysis of Two-Phase Flow Boiling Heat Transfer Coefficient and Correlations for Hydrocarbons and Ethanol. Energies. 2023; 16(16):5931. https://doi.org/10.3390/en16165931
Chicago/Turabian StyleElFaham, Mohamed, and Clement C. Tang. 2023. "A Comparative Analysis of Two-Phase Flow Boiling Heat Transfer Coefficient and Correlations for Hydrocarbons and Ethanol" Energies 16, no. 16: 5931. https://doi.org/10.3390/en16165931
APA StyleElFaham, M., & Tang, C. C. (2023). A Comparative Analysis of Two-Phase Flow Boiling Heat Transfer Coefficient and Correlations for Hydrocarbons and Ethanol. Energies, 16(16), 5931. https://doi.org/10.3390/en16165931






