Mechanistic Approach towards Designing Covalent Organic Frameworks for Photocatalytic Hydrogen Generation
Abstract
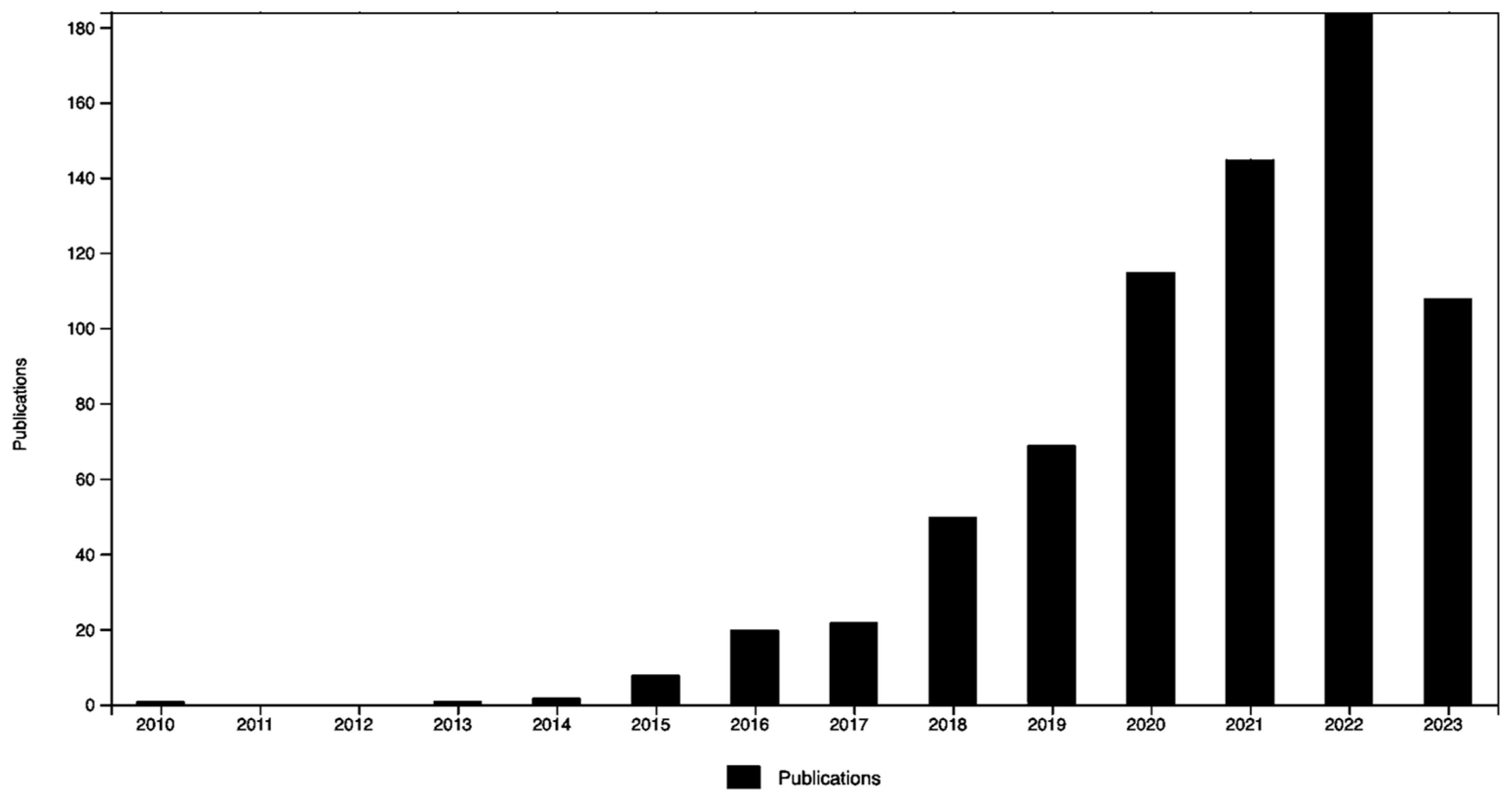
1. Introduction
2. Concept of Photocatalytic Hydrogen Evolution
3. Requirements of Photocatalytic Hydrogen Evolution
4. Challenges in Photocatalytic Hydrogen Evolution
5. Covalent Organic Frameworks (COFs) and Photocatalytic Hydrogen Evolution
6. Design Principles of COFs for Photocatalytic HER
6.1. Extending π-Conjugation
6.2. Incorporating Heteroatoms or Metal Complexes
6.2.1. Incorporating Heteroatoms
6.2.2. Incorporating Metal Complexes/Metals
6.3. Donor–Acceptor (D-A) Configuration
6.4. Hetero-Structural Mixed Linkage Approach
6.5. Miscellaneous Approach
6.5.1. Electron-Transfer Mediators (ETMs)
6.5.2. Graphene like COFs
7. Challenges for the Use of COFs as Photocatalysts for Hydrogen Evolution
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armaroli, N.; Balzani, V. Solar electricity and solar fuels: Status and perspectives in the context of the energy transition. Chem. A Eur. J. 2016, 22, 32–57. [Google Scholar] [CrossRef] [PubMed]
- Bie, C.; Wang, L.; Yu, J. Challenges for photocatalytic overall water splitting. Chem 2022, 8, 1567–1574. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z—What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2019, 120, 919–985. [Google Scholar] [CrossRef]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; Ferrer, B.; García, H. Metal–organic frameworks as photocatalysts for solar-driven overall water splitting. Chem. Rev. 2022, 123, 445–490. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Kibria, M.G.; Mullins, C.B. Metal-free photocatalysts for hydrogen evolution. Chem. Soc. Rev. 2020, 49, 1887–1931. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, S.; Kabumoto, A.; Mizumoto, K.; Pac, C.; Yoshino, K. Poly (p-phenylene)-catalysed photoreduction of water to hydrogen. J. Chem. Soc. Chem. Commun. 1985, 1985, 474–475. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoneda, Y.; Maruyama, T. Ruthenium and nickel complexes of a π-conjugated electrically conducting polymer chelate ligand, poly (2, 2′-bipyridine-5, 5′-diyl), and their chemical and catalytic reactivity. J. Chem. Soc. Chem. Commun. 1992, 1992, 1652–1654. [Google Scholar] [CrossRef]
- Sprick, R.S.; Bonillo, B.; Clowes, R.; Guiglion, P.; Brownbill, N.J.; Slater, B.J.; Blanc, F.; Zwijnenburg, M.A.; Adams, D.J.; Cooper, A.I. Visible-light-driven hydrogen evolution using planarized conjugated polymer photocatalysts. Angew. Chem. Int. Ed. 2016, 55, 1792–1796. [Google Scholar] [CrossRef]
- Yang, C.; Ma, B.C.; Zhang, L.; Lin, S.; Ghasimi, S.; Landfester, K.; Zhang, K.A.; Wang, X. Molecular engineering of conjugated polybenzothiadiazoles for enhanced hydrogen production by photosynthesis. Angew. Chem. Int. Ed. 2016, 55, 9202–9206. [Google Scholar] [CrossRef]
- Li, L.; Hadt, R.G.; Yao, S.; Lo, W.-Y.; Cai, Z.; Wu, Q.; Pandit, B.; Chen, L.X.; Yu, L. Photocatalysts based on cobalt-chelating conjugated polymers for hydrogen evolution from water. Chem. Mater. 2016, 28, 5394–5399. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. 2010, 49, 441–444. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Solangi, N.H.; Karri, R.R.; Mazari, S.A.; Mubarak, N.M.; Jatoi, A.S.; Malafaia, G.; Azad, A.K. MXene as emerging material for photocatalytic degradation of environmental pollutants. Coord. Chem. Rev. 2023, 477, 214965. [Google Scholar] [CrossRef]
- Hu, N.; Cai, Y.; Li, L.; Wang, X.; Gao, J. Amino-functionalized titanium based metal-organic framework for photocatalytic hydrogen production. Molecules 2022, 27, 4241. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Andreozzi, R.; Marotta, R. Hydrogen generation through solar photocatalytic processes: A review of the configuration and the properties of effective metal-based semiconductor nanomaterials. Energies 2017, 10, 1624. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; Cheng, M.; Liu, Z.; Huang, D.; Zhang, G.; Shao, B.; Liang, Q.; Luo, S.; Wu, T. The application of Zeolitic imidazolate frameworks (ZIFs) and their derivatives based materials for photocatalytic hydrogen evolution and pollutants treatment. Chem. Eng. J. 2021, 417, 127914. [Google Scholar] [CrossRef]
- Bai, Y.; Hu, Z.; Jiang, J.X.; Huang, F. Hydrophilic conjugated materials for photocatalytic hydrogen evolution. Chem.–Asian J. 2020, 15, 1780–1790. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Chen, L.; Xu, H.; Xiong, Y. 2D polymers as emerging materials for photocatalytic overall water splitting. Adv. Mater. 2018, 30, 1801955. [Google Scholar] [CrossRef]
- Zhu, G.; Li, X.; Wang, H.; Zhang, L. Microwave assisted synthesis of reduced graphene oxide incorporated MOF-derived ZnO composites for photocatalytic application. Catal. Commun. 2017, 88, 5–8. [Google Scholar]
- Mehta, A.; Rather, R.A.; Belec, B.; Gardonio, S.; Fang, M.; Valant, M. Plastic waste precursor-derived fluorescent carbon and construction of ternary FCs@ CuO@ TiO2 hybrid photocatalyst for hydrogen production and sensing application. Energies 2022, 15, 1734. [Google Scholar]
- Jawhari, A.H. Novel Nanomaterials for Hydrogen Production and Storage: Evaluating the Futurity of Graphene/Graphene Composites in Hydrogen Energy. Energies 2022, 15, 9085. [Google Scholar] [CrossRef]
- Wei, Z.; Mogan, T.R.; Wang, K.; Janczarek, M.; Kowalska, E. Morphology-governed performance of multi-dimensional photocatalysts for hydrogen generation. Energies 2021, 14, 7223. [Google Scholar]
- Khan, N.A.; Humayun, M.; Usman, M.; Ghazi, Z.A.; Naeem, A.; Khan, A.; Khan, A.L.; Tahir, A.A.; Ullah, H. Structural characteristics and environmental applications of covalent organic frameworks. Energies 2021, 14, 2267. [Google Scholar]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar]
- Liu, J.; Wang, N.; Ma, L. Recent advances in covalent organic frameworks for catalysis. Chem. Asian J. 2020, 15, 338–351. [Google Scholar]
- Ma, H.C.; Zou, J.; Li, X.T.; Chen, G.J.; Dong, Y.B. Homochiral covalent organic frameworks for asymmetric catalysis. Chem. A Eur. J. 2020, 26, 13754–13770. [Google Scholar] [CrossRef]
- Wan, C.-P.; Yi, J.-D.; Cao, R.; Huang, Y.-B. Conductive Metal/Covalent Organic Frameworks for CO2 Electro-reduction. Chin. J. Struct. Chem. 2022, 41, 2205001–2205014. [Google Scholar]
- Zhu, L.; Zhang, Y.-B. Crystallization of covalent organic frameworks for gas storage applications. Molecules 2017, 22, 1149. [Google Scholar] [CrossRef]
- Khan, N.A.; Zhang, R.; Wu, H.; Shen, J.; Yuan, J.; Fan, C.; Cao, L.; Olson, M.A.; Jiang, Z. Solid–vapor interface engineered covalent organic framework membranes for molecular separation. J. Am. Chem. Soc. 2020, 142, 13450–13458. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; You, X.; Khan, N.A.; Li, R.; Zhang, R.; Shen, J.; Cao, L.; Long, M.; Liu, Y.; Xu, Z. Photo-tailored heterocrystalline covalent organic framework membranes for organics separation. Nat. Commun. 2022, 13, 3826. [Google Scholar] [CrossRef]
- Khan, N.A.; Zhang, R.; Wang, X.; Cao, L.; Azad, C.S.; Fan, C.; Yuan, J.; Long, M.; Wu, H.; Olson, M.A. Assembling covalent organic framework membranes via phase switching for ultrafast molecular transport. Nat. Commun. 2022, 13, 3169. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Yuan, J.; Wu, H.; Cao, L.; Zhang, R.; Liu, Y.; Li, L.; Rahman, A.U.; Kasher, R.; Jiang, Z. Mixed nanosheet membranes assembled from chemically grafted graphene oxide and covalent organic frameworks for ultra-high water flux. ACS Appl. Mater. Interfaces 2019, 11, 28978–28986. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.S.; Bein, T. Covalent organic frameworks: Structures, synthesis, and applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef]
- Khan, N.A.; Yuan, J.; Wu, H.; Huang, T.; You, X.; Rahman, A.U.; Azad, C.S.; Olson, M.A.; Jiang, Z. Covalent organic framework nanosheets as reactive fillers to fabricate free-standing polyamide membranes for efficient desalination. ACS Appl. Mater. Interfaces 2020, 12, 27777–27785. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Wu, H.; Jinqiu, Y.; Mengyuan, W.; Yang, P.; Long, M.; Rahman, A.U.; Ahmad, N.M.; Zhang, R.; Jiang, Z. Incorporating covalent organic framework nanosheets into polyamide membranes for efficient desalination. Sep. Purif. Technol. 2021, 274, 119046. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Chen, J.; Li, Z.; Yin, Y.; Cao, L.; Zhong, Y.; Wu, H. Covalent organic framework modified polyamide nanofiltration membrane with enhanced performance for desalination. J. Membr. Sci. 2017, 523, 273–281. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Zhao, H.; Li, B.; Ma, H. A two-dimensional cationic covalent organic framework membrane for selective molecular sieving. J. Mater. Chem. A 2018, 6, 13331–13339. [Google Scholar] [CrossRef]
- Zheng, Y.; Shen, J.; Yuan, J.; Khan, N.A.; You, X.; Yang, C.; Zhang, S.; El-Gendi, A.; Wu, H.; Zhang, R. 2D nanosheets seeding layer modulated covalent organic framework membranes for efficient desalination. Desalination 2022, 532, 115753. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, Z.; Tang, L.; Zeng, G.; Xu, P.; Chen, M.; Xiong, T.; Zhou, C.; Li, X. Covalent organic framework photocatalysts: Structures and applications. Chem. Soc. Rev. 2020, 49, 4135–4165. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; Zhu, L.; Wang, H.; Naeem, A.; Khattak, A.M.; Liang, B.; Khan, N.A.; Wei, Z.; Li, L.; Tang, Z. Efficient polysulfide chemisorption in covalent organic frameworks for high-performance lithium-sulfur batteries. Adv. Energy Mater. 2016, 6, 1601250. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, X.; Zhang, J.; Hu, L.; Xu, T.; Li, W.; Chen, L.; Shen, M.; Ren, S.-B.; Han, D.-M. Bandgap engineering of covalent organic frameworks for boosting photocatalytic hydrogen evolution from water. J. Mater. Chem. A 2022, 10, 24620–24627. [Google Scholar] [CrossRef]
- Wan, S.; Gándara, F.; Asano, A.; Furukawa, H.; Saeki, A.; Dey, S.K.; Liao, L.; Ambrogio, M.W.; Botros, Y.Y.; Duan, X. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 2011, 23, 4094–4097. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, K.; Ding, X.; Wang, S.; Zhang, C.; Liu, J.; Zhan, Z.; Cheng, G.; Li, B.; Chen, H. Controlling monomer feeding rate to achieve highly crystalline covalent triazine frameworks. Adv. Mater. 2019, 31, 1807865. [Google Scholar] [CrossRef] [PubMed]
- Stegbauer, L.; Schwinghammer, K.; Lotsch, B.V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014, 5, 2789–2793. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Ryabchuk, V.K.; Kuznetsov, V.N.; Emeline, A.V.; Artem’ev, Y.M.; Kataeva, G.V.; Horikoshi, S.; Serpone, N. Water will be the coal of the future—The untamed dream of Jules Verne for a solar fuel. Molecules 2016, 21, 1638. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R.; López, N.; Palomares, E. Photocatalytic water splitting: Advantages and challenges. Sustain. Energy Fuels 2021, 5, 4560–4569. [Google Scholar] [CrossRef]
- Li, Y.; Song, X.; Zhang, G.; Wang, L.; Liu, Y.; Chen, W.; Chen, L. 2D covalent organic frameworks toward efficient photocatalytic hydrogen evolution. ChemSusChem 2022, 15, e202200901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, W.; Sheng, P.; Zhao, G.; Zhu, D. Organic donor–acceptor complexes as novel organic semiconductors. Acc. Chem. Res. 2017, 50, 1654–1662. [Google Scholar] [CrossRef]
- Vyas, V.S.; Haase, F.; Stegbauer, L.; Savasci, G.; Podjaski, F.; Ochsenfeld, C.; Lotsch, B.V. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation. Nat. Commun. 2015, 6, 8508. [Google Scholar] [CrossRef]
- Haase, F.; Banerjee, T.; Savasci, G.; Ochsenfeld, C.; Lotsch, B.V. Structure–property–activity relationships in a pyridine containing azine-linked covalent organic framework for photocatalytic hydrogen evolution. Faraday Discuss. 2017, 201, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Pachfule, P.; Acharjya, A.; Roeser, J.; Langenhahn, T.; Schwarze, M.; Schomäcker, R.; Thomas, A.; Schmidt, J. Diacetylene functionalized covalent organic framework (COF) for photocatalytic hydrogen generation. J. Am. Chem. Soc. 2018, 140, 1423–1427. [Google Scholar] [CrossRef]
- Stegbauer, L.; Zech, S.; Savasci, G.; Banerjee, T.; Podjaski, F.; Schwinghammer, K.; Ochsenfeld, C.; Lotsch, B.V. Photocatalysis: Tailor-Made Photoconductive Pyrene-Based Covalent Organic Frameworks for Visible-Light Driven Hydrogen Generation (Adv. Energy Mater. 24/2018). Adv. Energy Mater. 2018, 8, 1870107. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Chong, S.Y.; Little, M.A.; Wu, Y.; Zhu, W.-H.; Clowes, R.; Yan, Y.; Zwijnenburg, M.A.; Sprick, R.S. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 2018, 10, 1180–1189. [Google Scholar] [CrossRef]
- Jin, E.; Lan, Z.; Jiang, Q.; Geng, K.; Li, G.; Wang, X.; Jiang, D. 2D sp2 carbon-conjugated covalent organic frameworks for photocatalytic hydrogen production from water. Chem 2019, 5, 1632–1647. [Google Scholar] [CrossRef]
- Yang, S.; Lv, H.; Zhong, H.; Yuan, D.; Wang, X.; Wang, R. Transformation of Covalent Organic Frameworks from N-Acylhydrazone to Oxadiazole Linkages for Smooth Electron Transfer in Photocatalysis. Angew. Chem. Int. Ed. Engl. 2022, 61, e202115655. [Google Scholar] [CrossRef]
- Yang, J.; Acharjya, A.; Ye, M.Y.; Rabeah, J.; Li, S.; Kochovski, Z.; Youk, S.; Roeser, J.; Grüneberg, J.; Penschke, C. Protonated Imine-Linked Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2021, 60, 19797–19803. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Murakami, R.; Nishida, J.-I.; Tada, H.; Inoue, Y.; Tokito, S.; Yamashita, Y. n-Type organic field-effect transistors with very high electron mobility based on thiazole oligomers with trifluoromethylphenyl groups. J. Am. Chem. Soc. 2005, 127, 14996–14997. [Google Scholar] [CrossRef]
- Li, S.; Ma, R.; Xu, S.; Zheng, T.; Wang, H.; Fu, G.; Yang, H.; Hou, Y.; Liao, Z.; Wu, B. Two-Dimensional Benzobisthiazole-Vinylene-Linked Covalent Organic Frameworks Outperform One-Dimensional Counterparts in Photocatalysis. ACS Catal. 2023, 13, 1089–1096. [Google Scholar] [CrossRef]
- Huang, W.; Hu, Y.; Qin, Z.; Ji, Y.; Zhao, X.; Wu, Y.; He, Q.; Li, Y.; Zhang, C.; Lu, J. Highly crystalline and water-wettable benzobisthiazole-based covalent organic frameworks for enhanced photocatalytic hydrogen production. Natl. Sci. Rev. 2023, 10, nwac171. [Google Scholar] [CrossRef]
- Banerjee, T.; Haase, F.; Savasci, G.; Gottschling, K.; Ochsenfeld, C.; Lotsch, B.V. Single-site photocatalytic H2 evolution from covalent organic frameworks with molecular cobaloxime co-catalysts. J. Am. Chem. Soc. 2017, 139, 16228–16234. [Google Scholar] [CrossRef]
- Artero, V.; Chavarot-Kerlidou, M.; Fontecave, M. Splitting water with cobalt. Angew. Chem. Int. Ed. 2011, 50, 7238–7266. [Google Scholar] [CrossRef]
- Biswal, B.P.; Vignolo-González, H.A.; Banerjee, T.; Grunenberg, L.; Savasci, G.; Gottschling, K.; Nuss, J.; Ochsenfeld, C.; Lotsch, B.V. Sustained solar H2 evolution from a thiazolo [5, 4-d] thiazole-bridged covalent organic framework and nickel-thiolate cluster in water. J. Am. Chem. Soc. 2019, 141, 11082–11092. [Google Scholar] [CrossRef]
- Gottschling, K.; Savasci, G.; Vignolo-González, H.; Schmidt, S.; Mauker, P.; Banerjee, T.; Rovó, P.; Ochsenfeld, C.; Lotsch, B.V. Rational design of covalent cobaloxime–covalent organic framework hybrids for enhanced photocatalytic hydrogen evolution. J. Am. Chem. Soc. 2020, 142, 12146–12156. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Z.; Li, L.; Zhuang, Z.; Bi, J.; Chen, J.; Yu, Y.; Yu, J. Thioether-functionalized 2D covalent organic framework featuring specific affinity to Au for photocatalytic hydrogen production from seawater. ACS Sustain. Chem. Eng. 2019, 7, 18574–18581. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Peh, S.B.; Liu, G.; Kundu, T.; Dong, J.; Ying, Y.; Qian, Y.; Zhao, D. Cobalt-containing covalent organic frameworks for visible light-driven hydrogen evolution. Sci. China Chem. 2020, 63, 192–197. [Google Scholar] [CrossRef]
- Zang, Y.; Wang, R.; Shao, P.-P.; Feng, X.; Wang, S.; Zang, S.-Q.; Mak, T.C. Prefabricated covalent organic framework nanosheets with double vacancies: Anchoring Cu for highly efficient photocatalytic H 2 evolution. J. Mater. Chem. A 2020, 8, 25094–25100. [Google Scholar] [CrossRef]
- Gao, M.-Y.; Li, C.-C.; Tang, H.-L.; Sun, X.-J.; Dong, H.; Zhang, F.-M. Boosting visible-light-driven hydrogen evolution of covalent organic frameworks through compositing with MoS2: A promising candidate for noble-metal-free photocatalysts. J. Mater. Chem. A 2019, 7, 20193–20200. [Google Scholar] [CrossRef]
- Thote, J.; Aiyappa, H.B.; Deshpande, A.; Diaz Diaz, D.; Kurungot, S.; Banerjee, R. A Covalent Organic Framework–Cadmium Sulfide Hybrid as a Prototype Photocatalyst for Visible-Light-Driven Hydrogen Production. Chem. A Eur. J. 2014, 20, 15961–15965. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; He, H.; Sun, L.; Wang, H.; Fang, X.; Zhao, Y.; Zheng, D.; Qi, Y.; Li, Z. In situ photodeposition of platinum clusters on a covalent organic framework for photocatalytic hydrogen production. Nat. Commun. 2022, 13, 1355. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, Z.; Kidkhunthod, P.; Guo, J. Stable Immobilization of Nickel Ions on Covalent Organic Frameworks for Panchromatic Photocatalytic Hydrogen Evolution. Angew. Chem. 2023, 135, e202217527. [Google Scholar] [CrossRef]
- Li, W.; Huang, X.; Zeng, T.; Liu, Y.A.; Hu, W.; Yang, H.; Zhang, Y.B.; Wen, K. Thiazolo [5, 4-d] thiazole-based donor–acceptor covalent organic framework for sunlight-driven hydrogen evolution. Angew. Chem. Int. Ed. 2021, 60, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-B.; Li, S.; Yan, C.-X.; Lin, Q.-Q.; Zhu, F.-C.; Geng, Y.; Dong, Y.-B. A benzothiadiazole-based covalent organic framework for highly efficient visible-light driven hydrogen evolution. Chem. Commun. 2020, 56, 12612–12615. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, L.; Mo, D.; He, F.; Wen, Z.; Wu, X.; Xu, H.; Chen, L. Modulating benzothiadiazole-based covalent organic frameworks via halogenation for enhanced photocatalytic water splitting. Angew. Chem. 2020, 132, 17050–17057. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, Y.; Wang, C.; Zhang, S.; Song, J.; Li, Y.; Ma, S.; Cheng, P.; Zhang, Z.; Chen, Y. Fabrication of robust covalent organic frameworks for enhanced visible-light-driven H2 evolution. ACS Catal. 2021, 11, 2098–2107. [Google Scholar] [CrossRef]
- Ghosh, S.; Nakada, A.; Springer, M.A.; Kawaguchi, T.; Suzuki, K.; Kaji, H.; Baburin, I.; Kuc, A.; Heine, T.; Suzuki, H. Identification of prime factors to maximize the photocatalytic hydrogen evolution of covalent organic frameworks. J. Am. Chem. Soc. 2020, 142, 9752–9762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, W.; Liu, H.; Chen, R.; Pan, Q.; Li, Z.; Zhao, Y. Facile construction of fully sp2-carbon conjugated two-dimensional covalent organic frameworks containing benzobisthiazole units. Nat. Commun. 2022, 13, 100. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, X.; Xu, J.; Jia, X.; Chao, M.; Wang, D.; Zhao, Y. Structural Regulation of Thiophene-Based Two-Dimensional Covalent Organic Frameworks toward Highly Efficient Photocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2023, 15, 16794–16800. [Google Scholar] [CrossRef]
- Liu, H.; Wang, D.; Yu, Z.; Chen, Y.; Li, X.; Zhang, R.; Chen, X.; Wu, L.; Ding, N.; Wang, Y. Fully conjugated two-dimensional sp2-carbon covalent organic frameworks for efficient photocatalytic hydrogen generation. Sci. China Mater. 2023, 66, 2283–2289. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, X.; Zhang, P.; Ke, X.; Yuan, X.; Zhai, L.; Wang, W.; Qin, N.; Cui, C.-X.; Qu, L. Vinylene-linked covalent organic frameworks with manipulated electronic structures for efficient solar-driven photocatalytic hydrogen production. Chin. J. Catal. 2023, 47, 171–180. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, M.; Su, L.; Yu, H.; Wang, C.; Sun, D.; Ding, Y. Donor–Acceptor Covalent–Organic Frameworks Based on Phthalimide as an Electron-Deficient Unit for Efficient Visible-Light Catalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2023, 15, 20310–20316. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.L.; Dong, H.; Meng, X.B.; Tang, H.L.; Yao, Y.H.; Liu, D.Q.; Bai, L.L.; Zhang, F.M.; Wei, J.Z.; Sun, X.J. Effect of different functional groups on photocatalytic hydrogen evolution in covalent-organic frameworks. ChemCatChem 2019, 11, 2313–2319. [Google Scholar] [CrossRef]
- Wang, F.D.; Yang, L.J.; Wang, X.X.; Rong, Y.; Yang, L.B.; Zhang, C.X.; Yan, F.Y.; Wang, Q.L. Pyrazine-Functionalized Donor–Acceptor Covalent Organic Frameworks for Enhanced Photocatalytic H2 Evolution with High Proton Transport. Small 2023, 19, 2207421. [Google Scholar] [CrossRef]
- Liu, C.-X.; Pan, D.-L.; Seo, Y.; Park, S.; Kan, J.-L.; Koo, J.Y.; Choi, W.; Lee, E. Phenanthroimidazole-based Covalent Organic Frameworks with Enhanced Activity for the Photocatalytic Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2023, 6, 1126–1133. [Google Scholar] [CrossRef]
- Guo, X.; Wang, S.; Enkelmann, V.; Baumgarten, M.; Müllen, K. Making benzotrithiophene a stronger electron donor. Org. Lett. 2011, 13, 6062–6065. [Google Scholar] [CrossRef]
- Jeon, J.P.; Kim, Y.J.; Joo, S.H.; Noh, H.J.; Kwak, S.K.; Baek, J.B. Benzotrithiophene-based Covalent Organic Framework Photocatalysts with Controlled Conjugation of Building Blocks for Charge Stabilization. Angew. Chem. Int. Ed. 2023, 62, e202217416. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, T.; Ma, S.; Zhang, Z.; Wu, G.; Wang, J.; Li, Q.; Xia, H.; Yang, S.-W.; Liu, X. Three-Component Donor− π–Acceptor Covalent–Organic Frameworks for Boosting Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2023, 145, 8364–8374. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Dong, A.; Meng, X.; Liu, H.; Li, Y.; Li, P.; Wang, B. Enhancement of Visible-Light-Driven Hydrogen Evolution Activity of 2D π-Conjugated Bipyridine-Based Covalent Organic Frameworks via Post-Protonation. Angew. Chem. 2023, 135, e202300224. [Google Scholar] [CrossRef]
- Wang, H.; Qian, C.; Liu, J.; Zeng, Y.; Wang, D.; Zhou, W.; Gu, L.; Wu, H.; Liu, G.; Zhao, Y. Integrating suitable linkage of covalent organic frameworks into covalently bridged inorganic/organic hybrids toward efficient photocatalysis. J. Am. Chem. Soc. 2020, 142, 4862–4871. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Jiang, F.; Wu, Y. Metal-free 2D-2D black phosphorus/covalent organic framework pn heterojunction for efficient visible-light-driven hydrogen evolution without cocatalysts. Int. J. Hydrog. Energy 2023, 48, 8867–8876. [Google Scholar] [CrossRef]
- Wang, M.; Li, D.; Zhao, Y.; Shen, H.; Chen, B.; Wu, X.; Qiao, X.; Shi, W. Bifunctional black phosphorus: Coupling with hematite for Z-scheme photocatalytic overall water splitting. Catal. Sci. Technol. 2021, 11, 681–688. [Google Scholar] [CrossRef]
- Shen, R.; Liang, G.; Hao, L.; Zhang, P.; Li, X. In-situ Synthesis of Chemically bonded 2D/2D Covalent Organic Frameworks/O-vacancy WO3 Z-Scheme Heterostructure for Photocatalytic Overall Water Splitting. Adv. Mater. 2023, 2023, 2303649. [Google Scholar] [CrossRef]
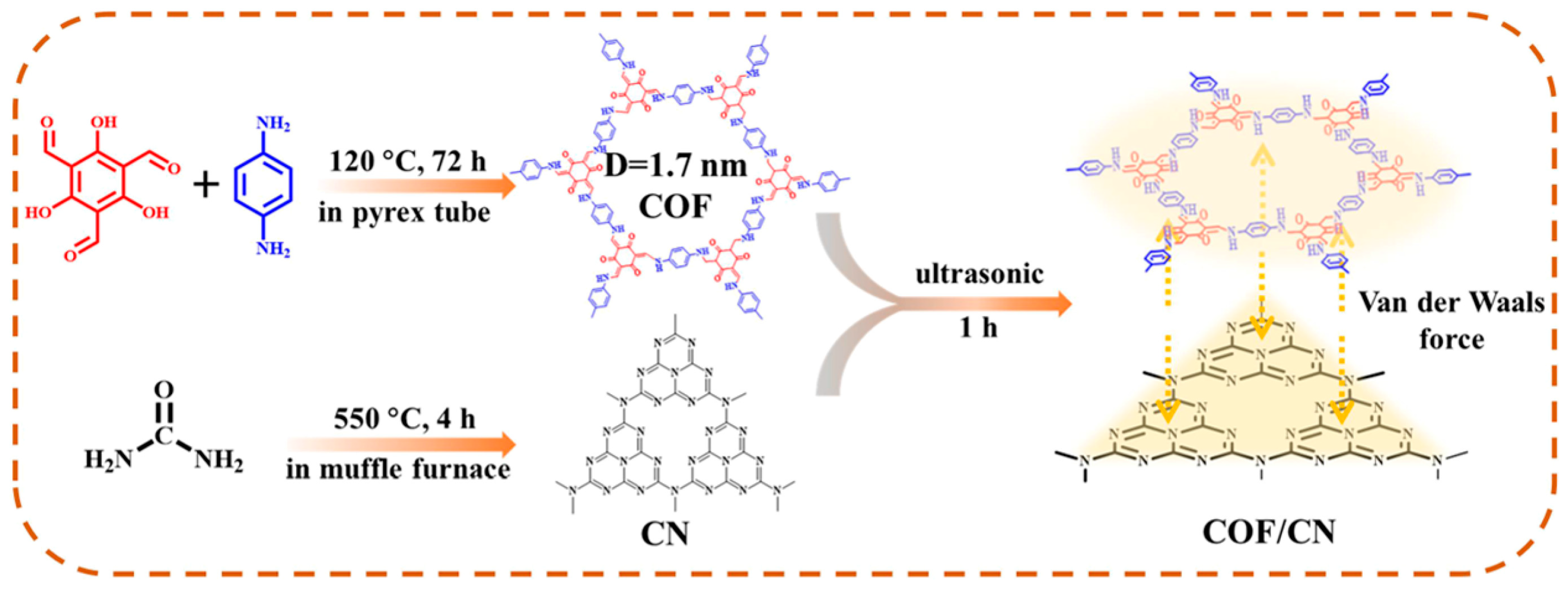
- Liu, Y.; Jiang, L.; Tian, Y.; Xu, Z.; Wang, W.; Qiu, M.; Wang, H.; Li, X.; Zhu, G.; Wang, Y. Covalent organic framework/g-C3N4 van der Waals heterojunction toward H2 production. Inorg. Chem. 2023, 62, 3271–3277. [Google Scholar] [CrossRef]
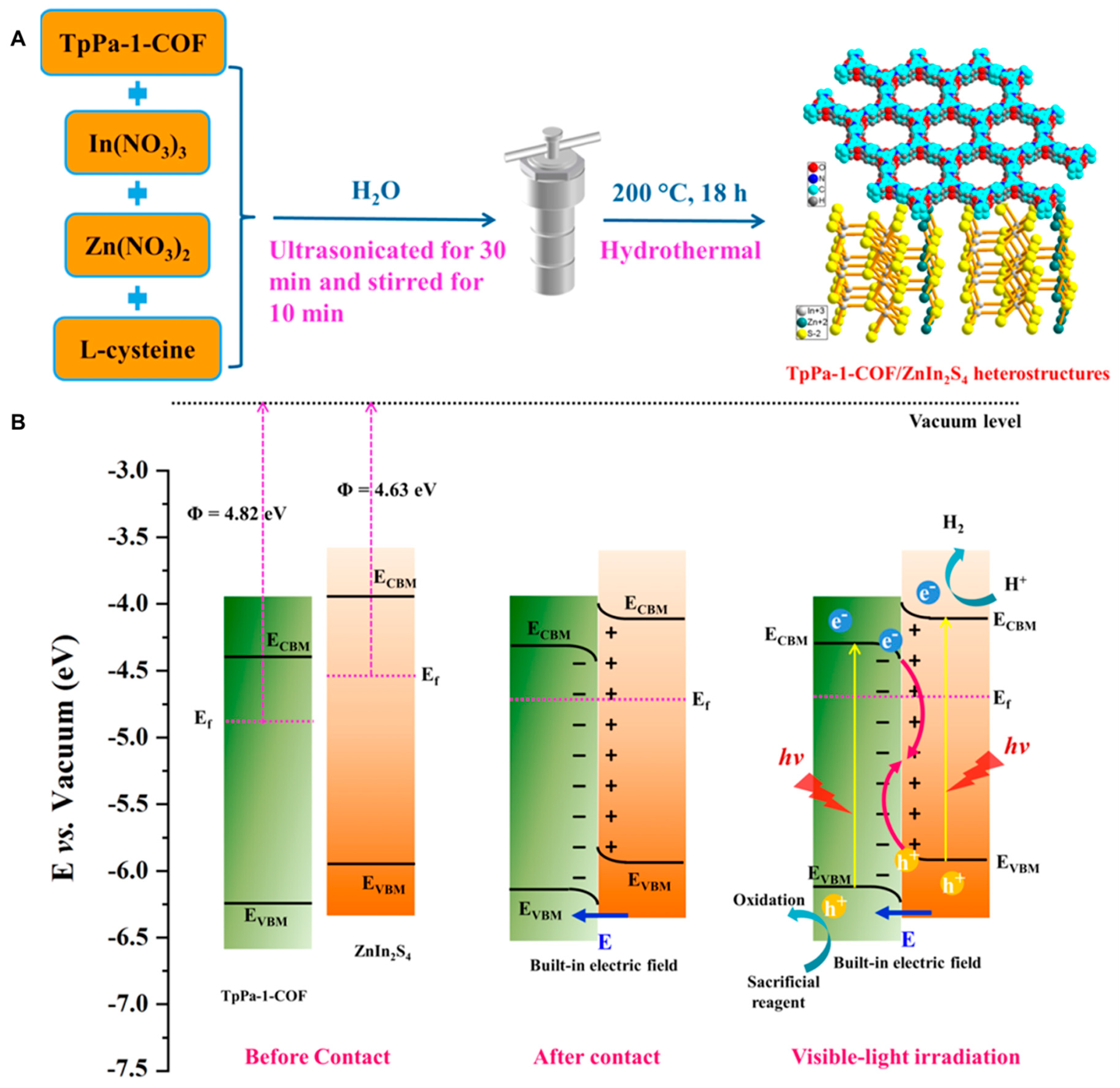
- Dong, P.; Cheng, T.; Zhang, J.-l.; Jiang, J.; Zhang, L.; Xi, X.; Zhang, J. Fabrication of an Organic/Inorganic Hybrid TpPa-1-COF/ZnIn2S4 S-Scheme Heterojunction for Boosted Photocatalytic Hydrogen Production. ACS Appl. Energy Mater. 2023, 6, 1103–1115. [Google Scholar] [CrossRef]
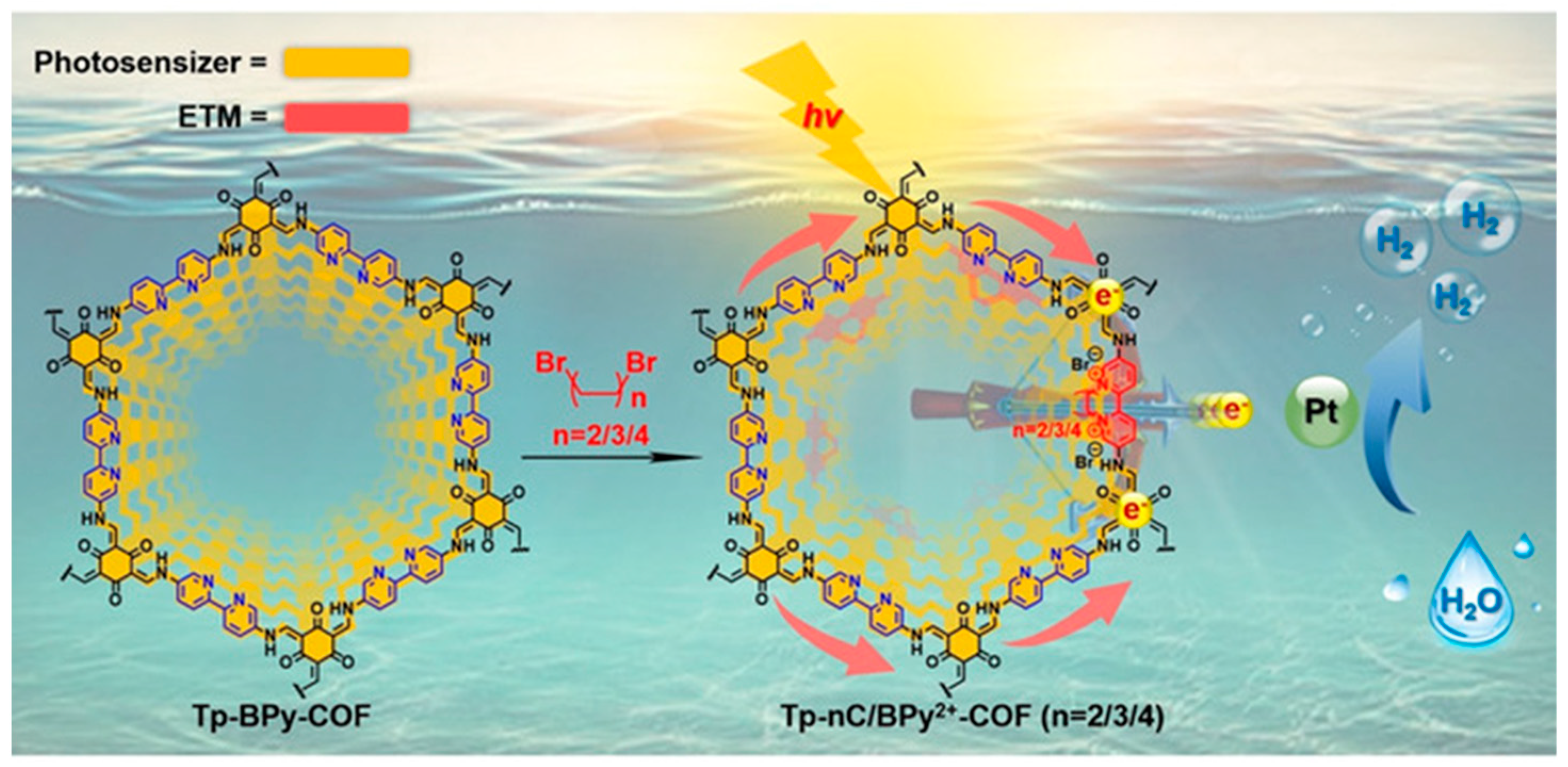
- Mi, Z.; Zhou, T.; Weng, W.; Unruangsri, J.; Hu, K.; Yang, W.; Wang, C.; Zhang, K.A.; Guo, J. Covalent Organic Frameworks Enabling Site Isolation of Viologen-Derived Electron-Transfer Mediators for Stable Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2021, 60, 9642–9649. [Google Scholar] [CrossRef]
- Zhao, Y.; Swierk, J.R.; Megiatto Jr, J.D.; Sherman, B.; Youngblood, W.J.; Qin, D.; Lentz, D.M.; Moore, A.L.; Moore, T.A.; Gust, D. Improving the efficiency of water splitting in dye-sensitized solar cells by using a biomimetic electron transfer mediator. Proc. Natl. Acad. Sci. USA 2012, 109, 15612–15616. [Google Scholar] [CrossRef] [PubMed]
- Altınışık, S.; Yanalak, G.; Hatay Patır, I.; Koyuncu, S. Viologen-Based Covalent Organic Frameworks toward Metal-Free Highly Efficient Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2023, 15, 18836–18844. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hu, R.; Bai, H.; Chen, H.; Lv, F.; Liu, L.; Wang, S.; Tian, H. Efficient conjugated polymer–methyl viologen electron transfer system for controlled photo-driven hydrogen evolution. ACS Appl. Mater. Interfaces 2017, 9, 10355–10359. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, F.; Zhang, W.; Qiang, P.; Yu, K.; Fu, X.; Wu, D.; Bi, S.; Zhang, F. Semiconducting 2D triazine-cored covalent organic frameworks with unsubstituted olefin linkages. J. Am. Chem. Soc. 2019, 141, 14272–14279. [Google Scholar] [CrossRef]
- Bi, S.; Yang, C.; Zhang, W.; Xu, J.; Liu, L.; Wu, D.; Wang, X.; Han, Y.; Liang, Q.; Zhang, F. Two-dimensional semiconducting covalent organic frameworks via condensation at arylmethyl carbon atoms. Nat. Commun. 2019, 10, 2467. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, C.; Bi, S.; Wang, W.; He, Y.; Wu, D.; Liang, Q.; Wang, X.; Zhang, F. Vinylene-linked covalent organic frameworks (COFs) with symmetry-tuned polarity and photocatalytic activity. Angew. Chem. 2020, 132, 24053–24061. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xin, X.; Wang, Y.; Guo, P.; Wang, R.; Wang, B.; Huang, W.; Sobrido, A.J.; Li, X. Internal quantum efficiency higher than 100% achieved by combining doping and quantum effects for photocatalytic overall water splitting. Nat. Energy 2023, 8, 504–514. [Google Scholar] [CrossRef]

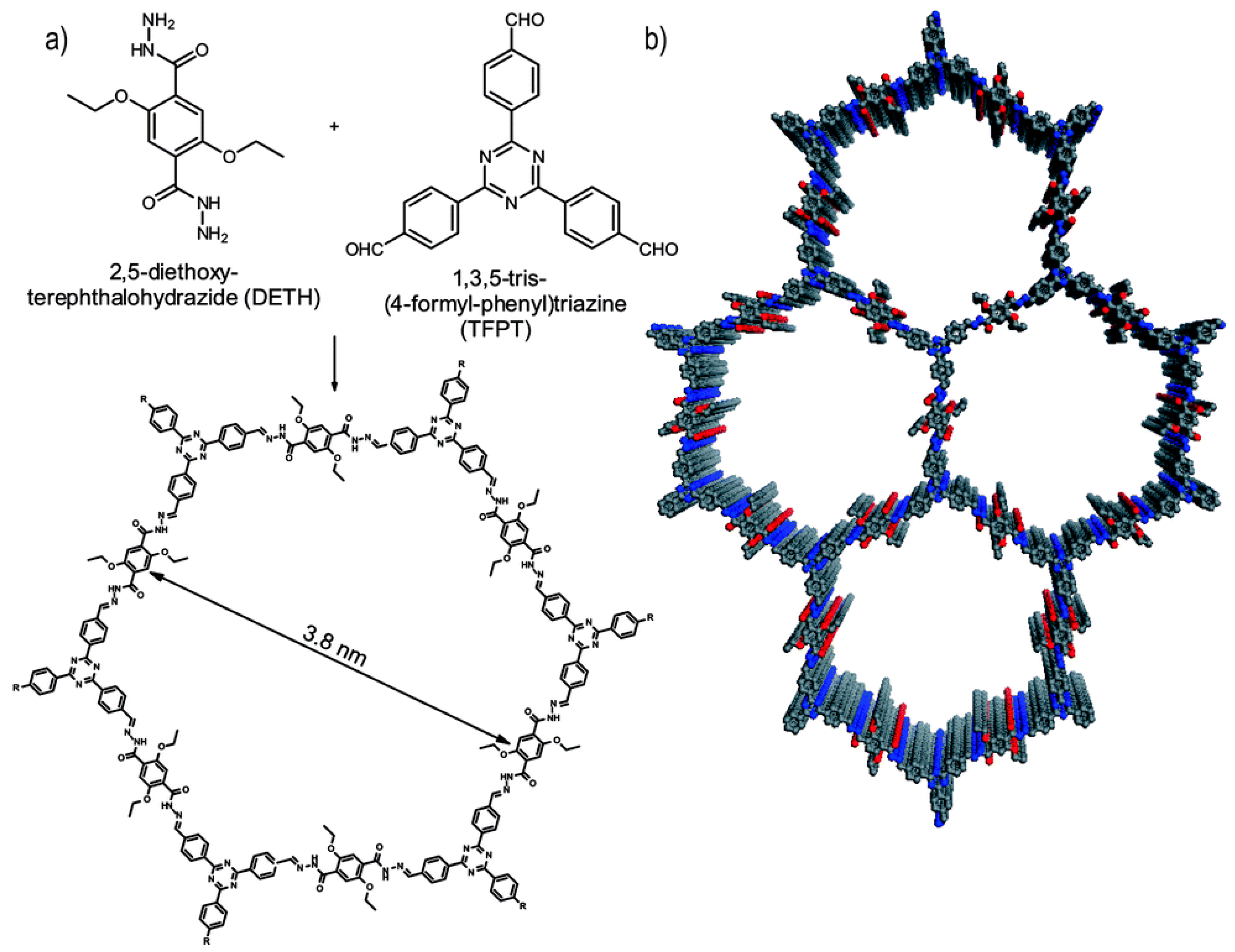


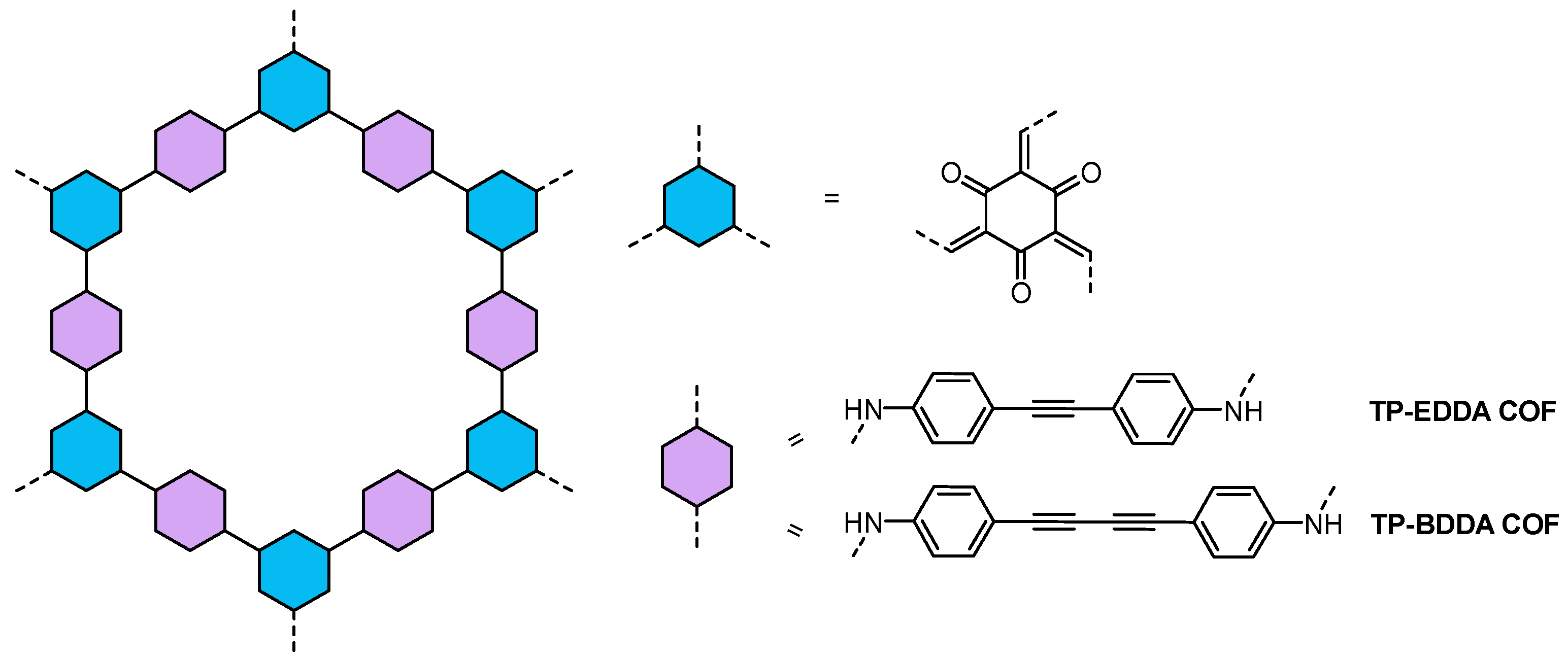

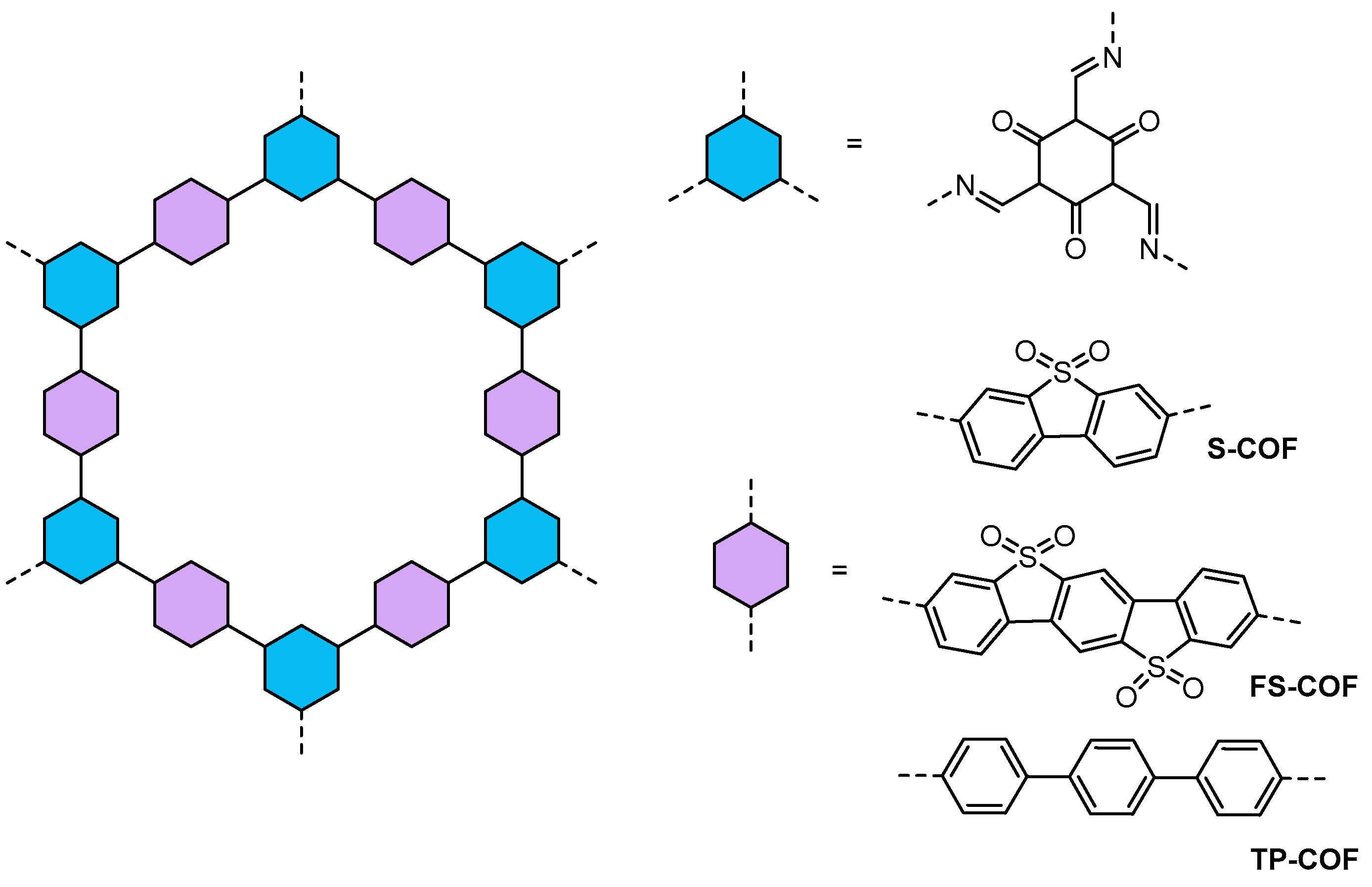




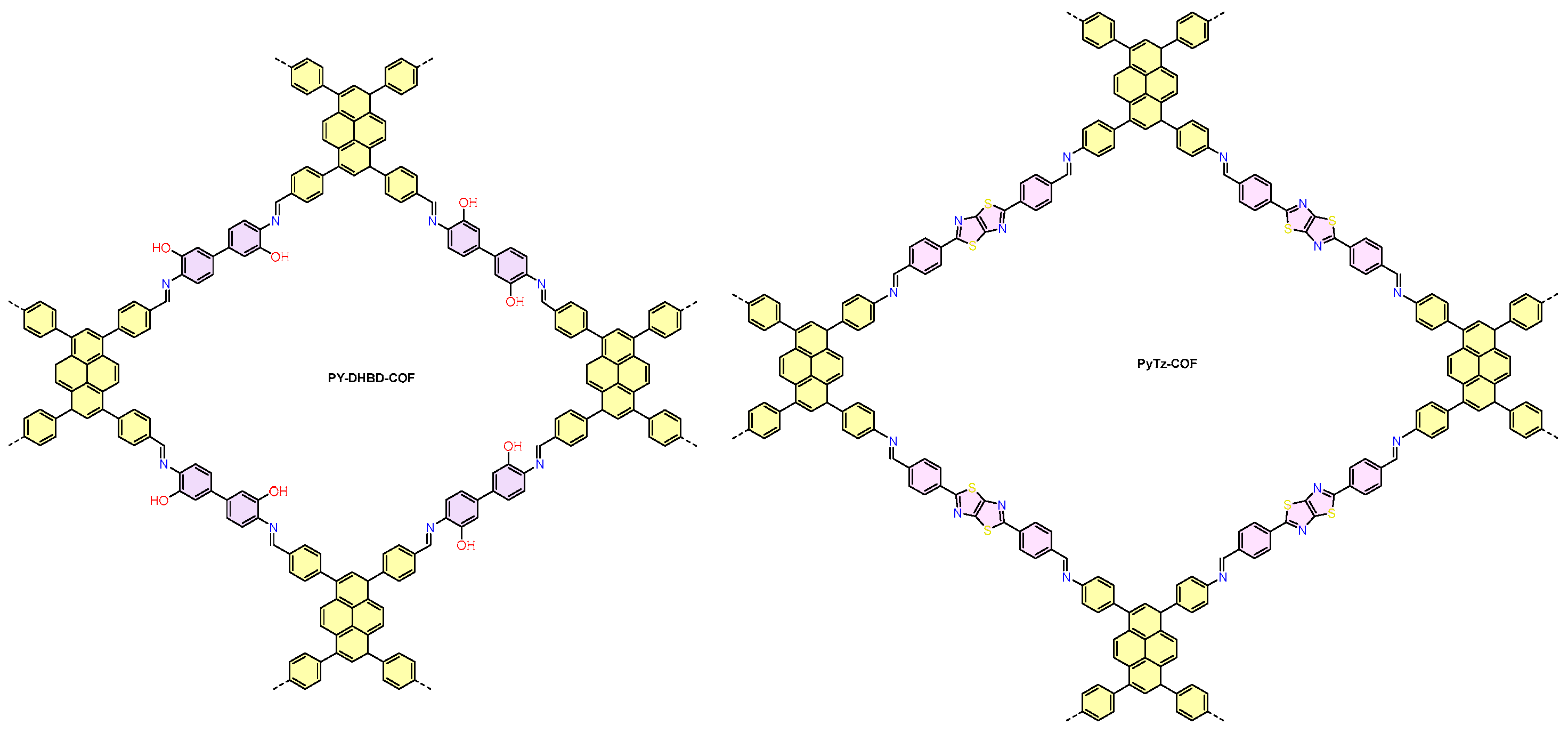





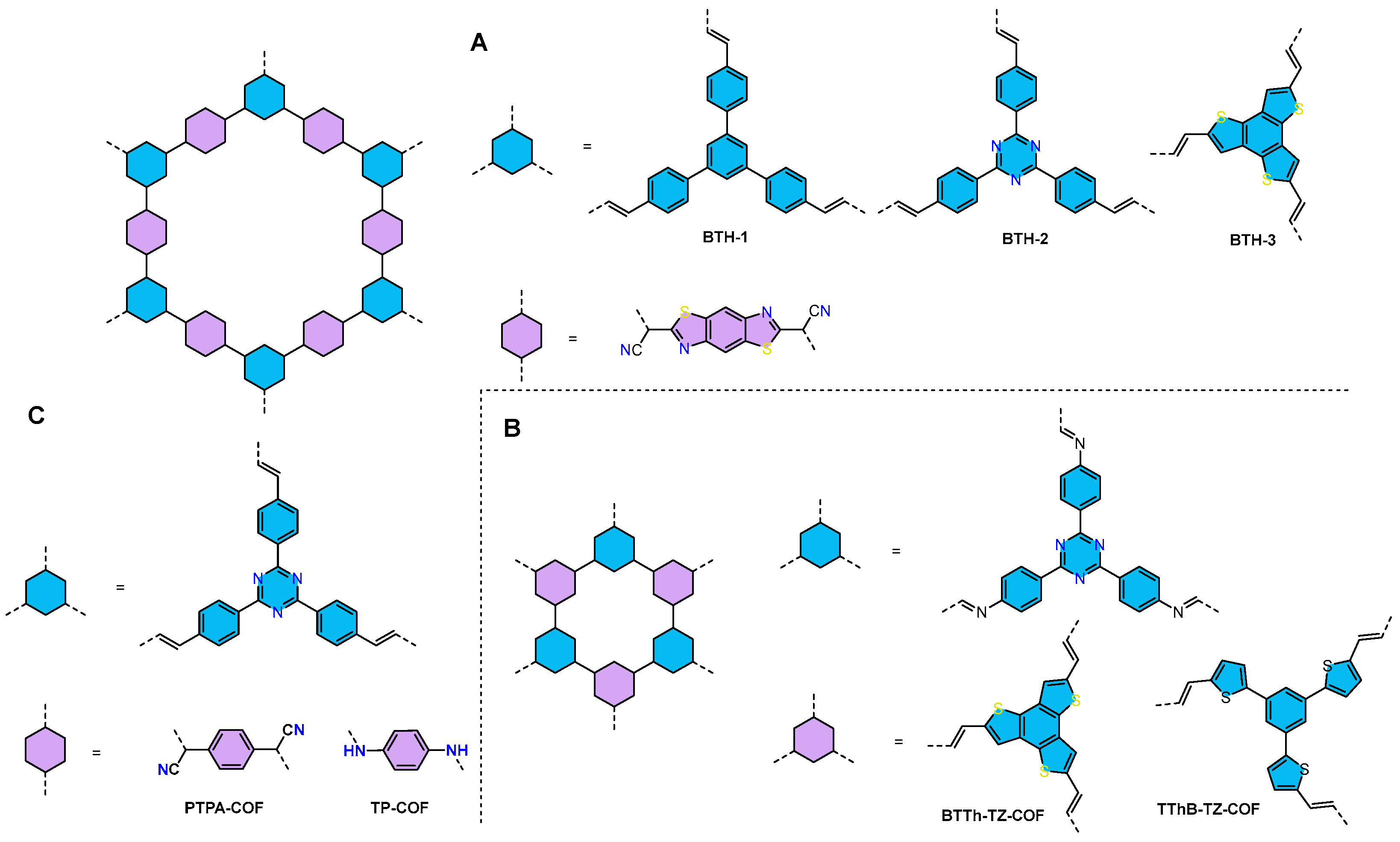
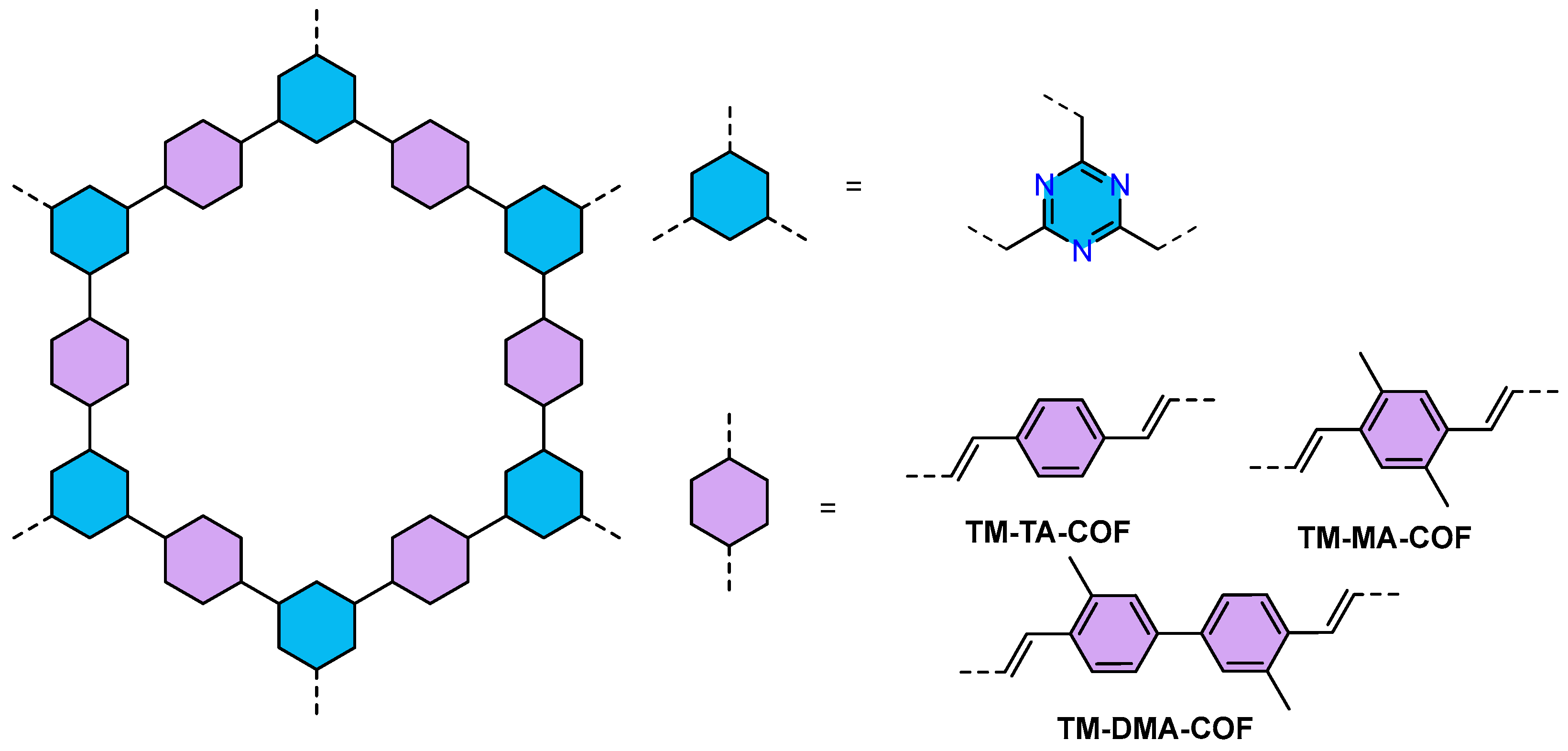
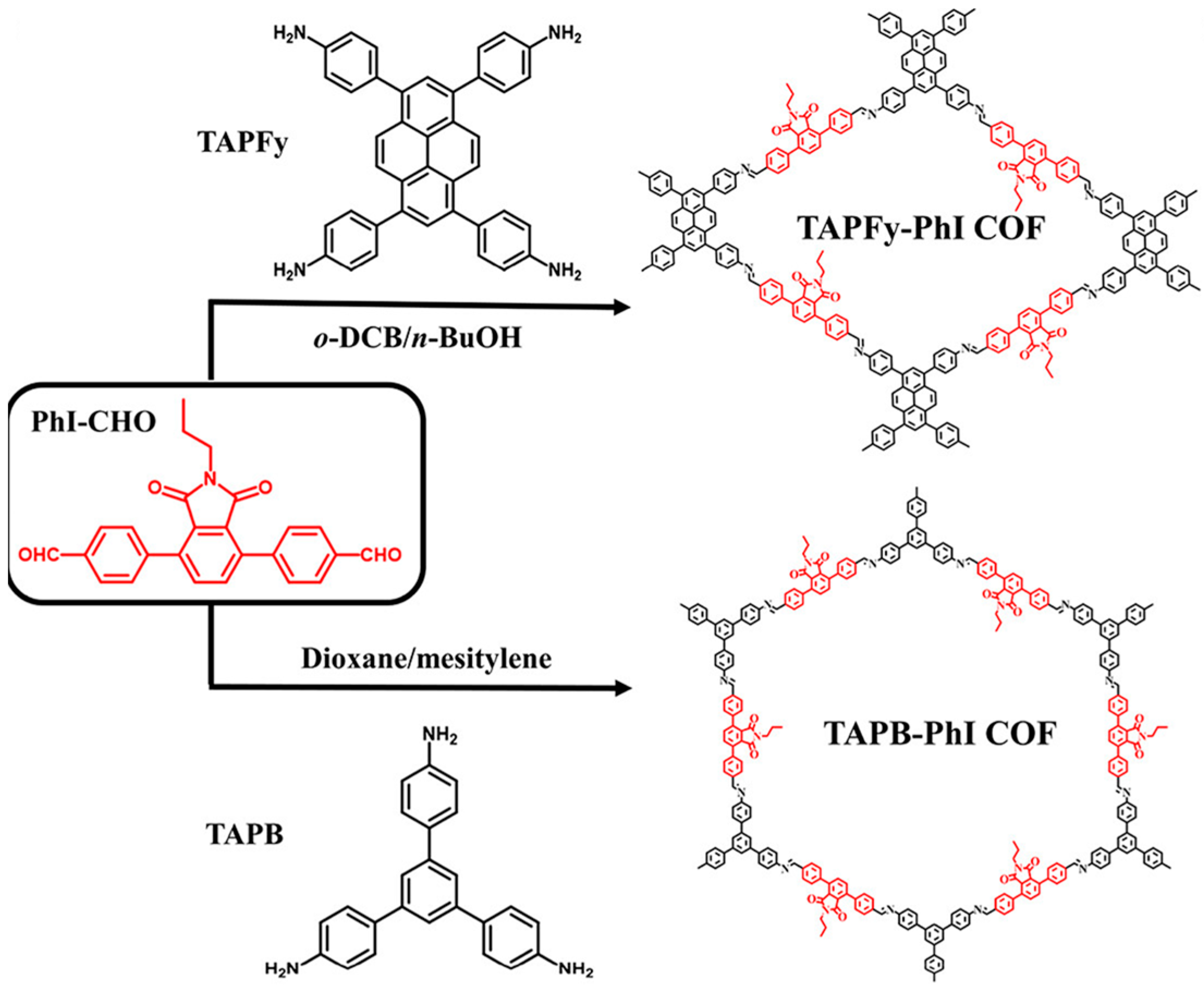
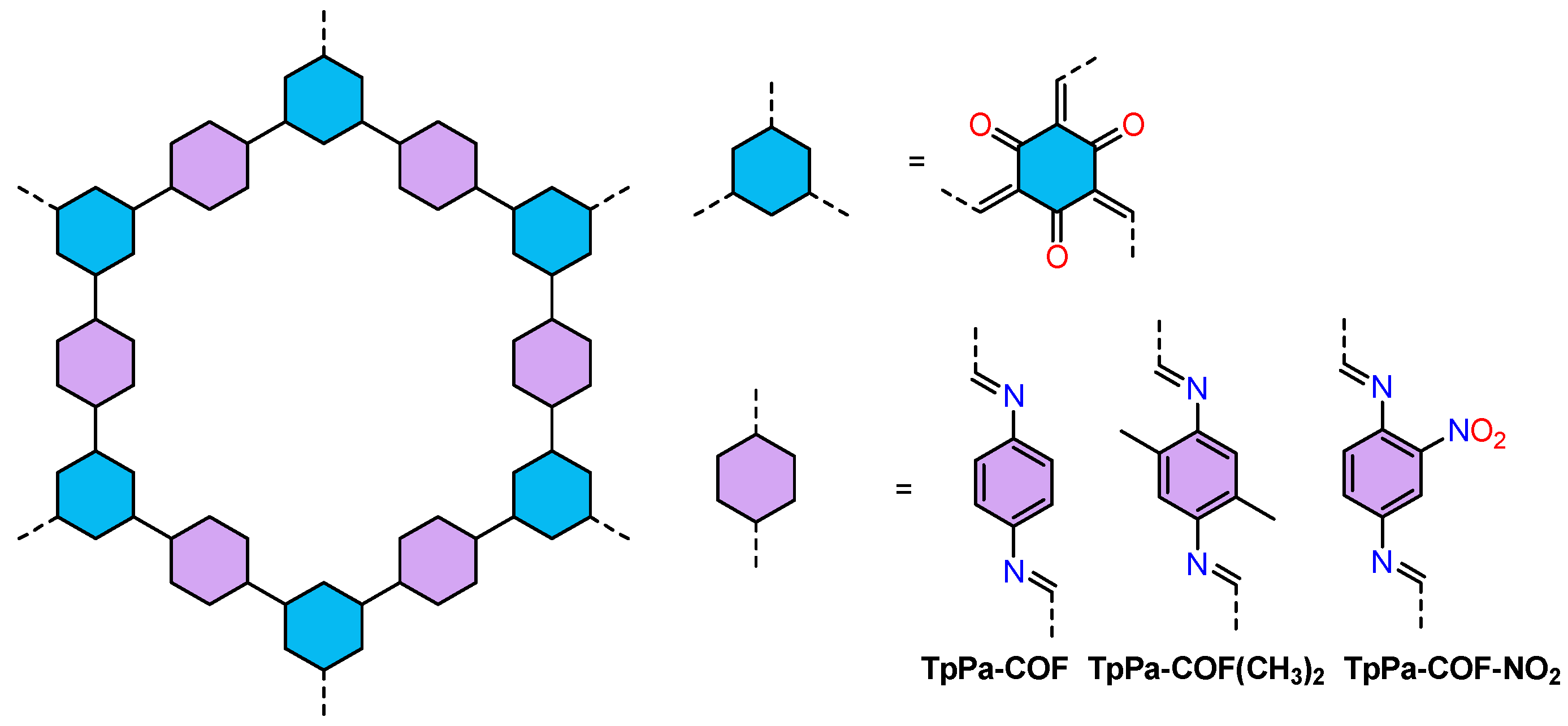


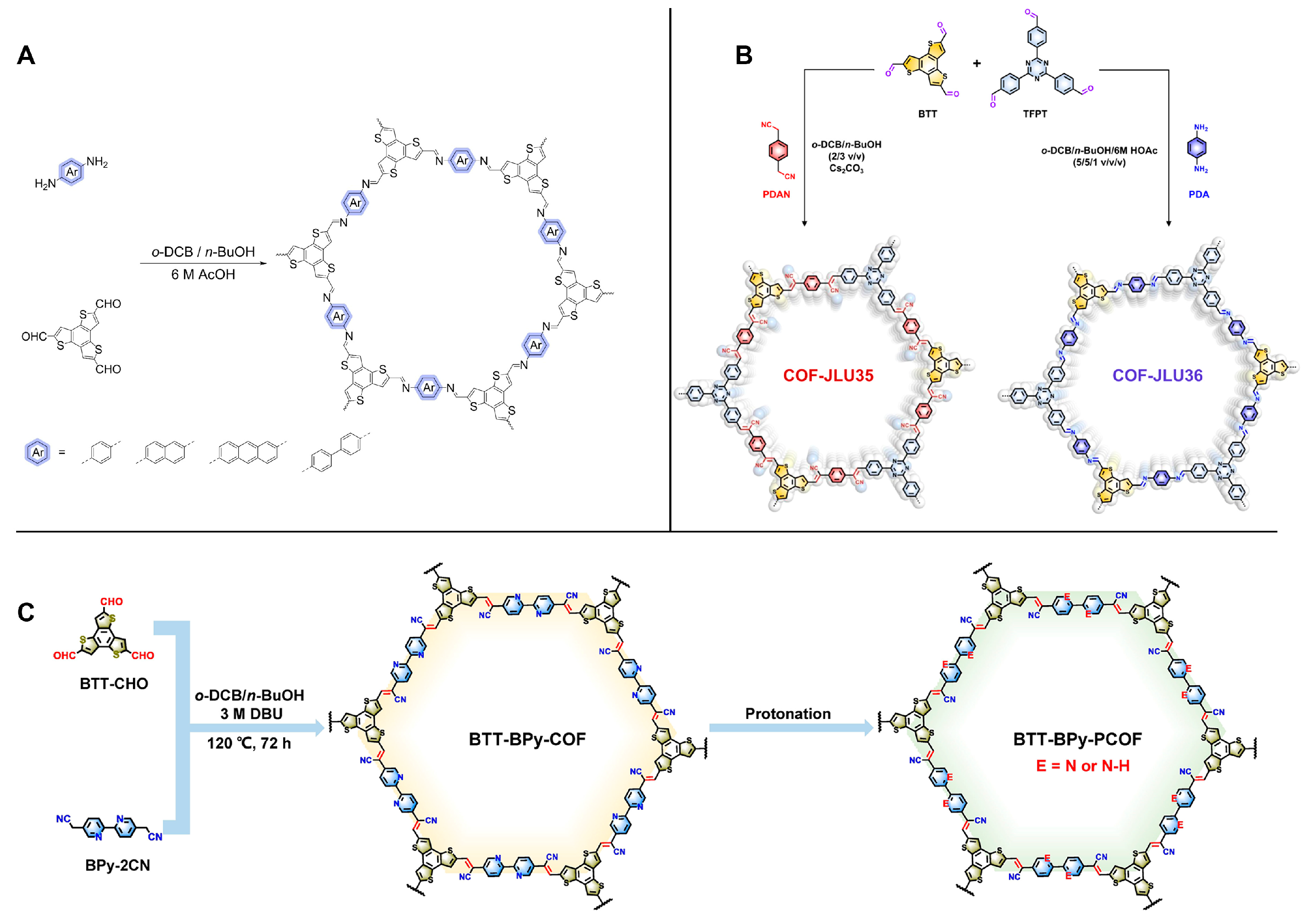
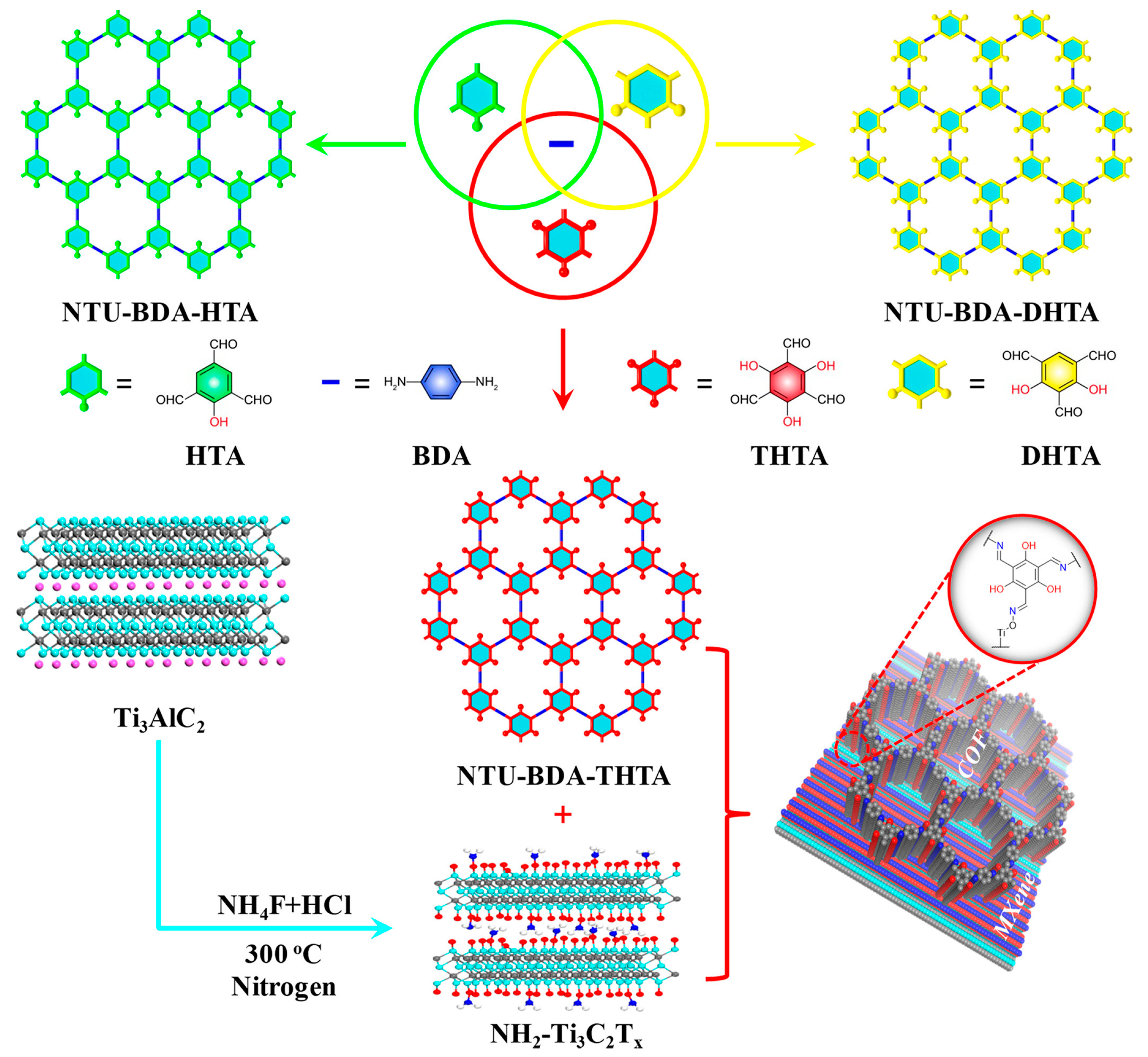

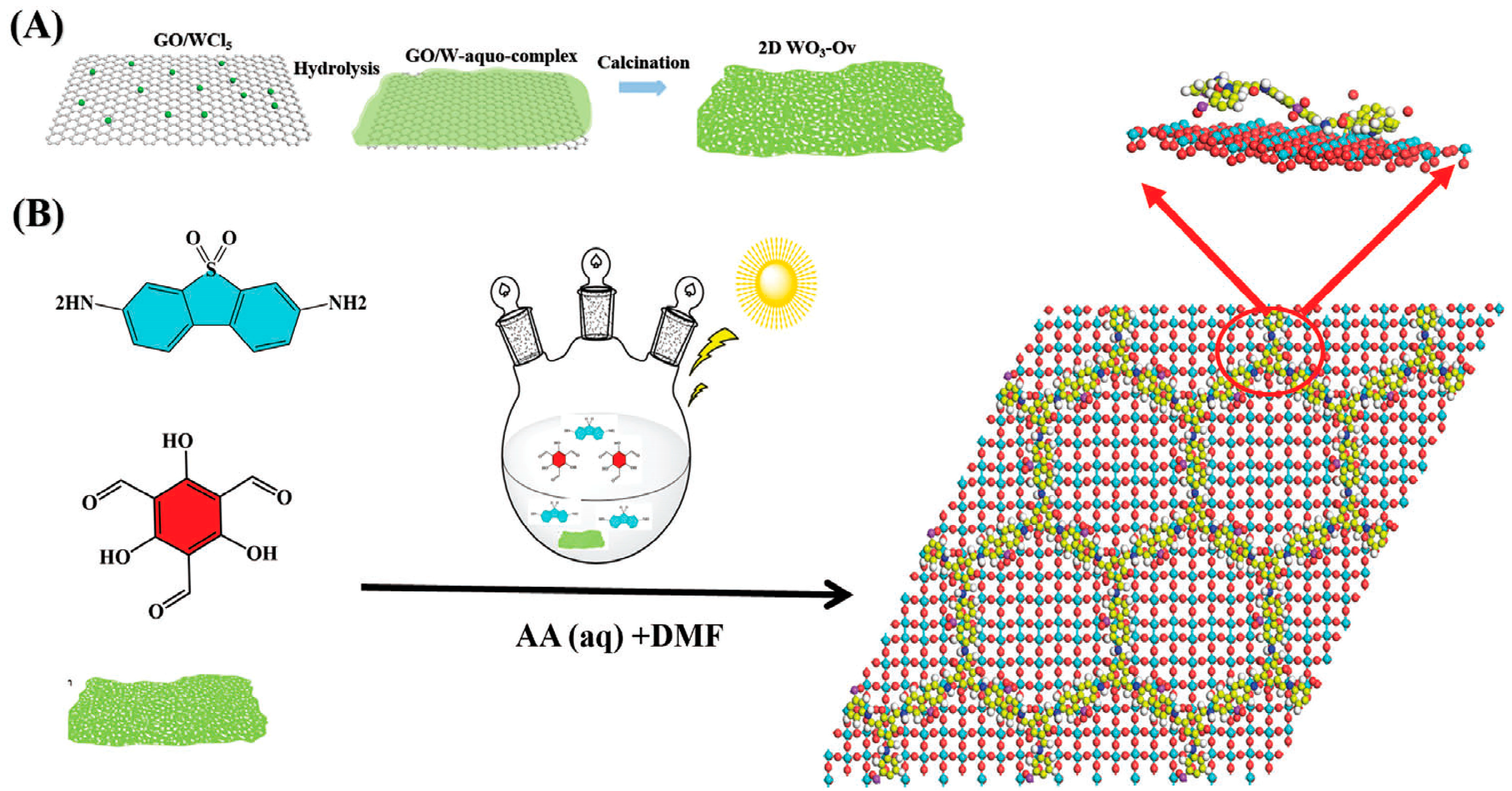

| COFs | Band Gap (eV) | Pore Size (nM) | Metal Co-Catalyst | Sacrificial Element | HER Efficiency |
|---|---|---|---|---|---|
| Extending π-conjugation | |||||
| TFPT-COF49 | 2.8 | 3.8 | Pt | TEOA | 1970 μmol h−1 g−1 |
| Nx-COFs56 | 2.6–2.7 | 3.5 | Pt | TEOA | 1703 μmol h−1 g−1 |
| PTP-COF57 | 2.1 | - | Pt | TEOA | 83.83 μmol h−1 g−1 |
| TP-EDDA58 TP-BDDA | 2.34 2.31 | - - | Pt | TEOA | 324 ± 10 µmol h−1 g−1 30 ± 5 μmol h−1 g−1 |
| Incorporating heteroatoms | |||||
| A(B/N/Y)PY-COF59 | 1.94–1.92 | 2–2.22 | Pt | TEOA | 98 µmol h−1 g−1 |
| S-COF, FS-COF and TP-COF60 | - | 2.90–2.76 | Pt | AA | 10,100 μmol g−1 h−1 16,300 μmol g−1 h−1 (WS5F) 15,800 μmol h−1 m−2 (a thin film of FS-COF) |
| sp2c-COF sp2c-COFERDN61 | 1.90 1.85 | 1.9 | Pt | TEOA | 2120 μmol g−1 h−1 1360 μmol g−1 h−1 |
| ODA-COF62 | 2.00 | - | Pt | TEOA | 2615 μmol g−1 h−1 |
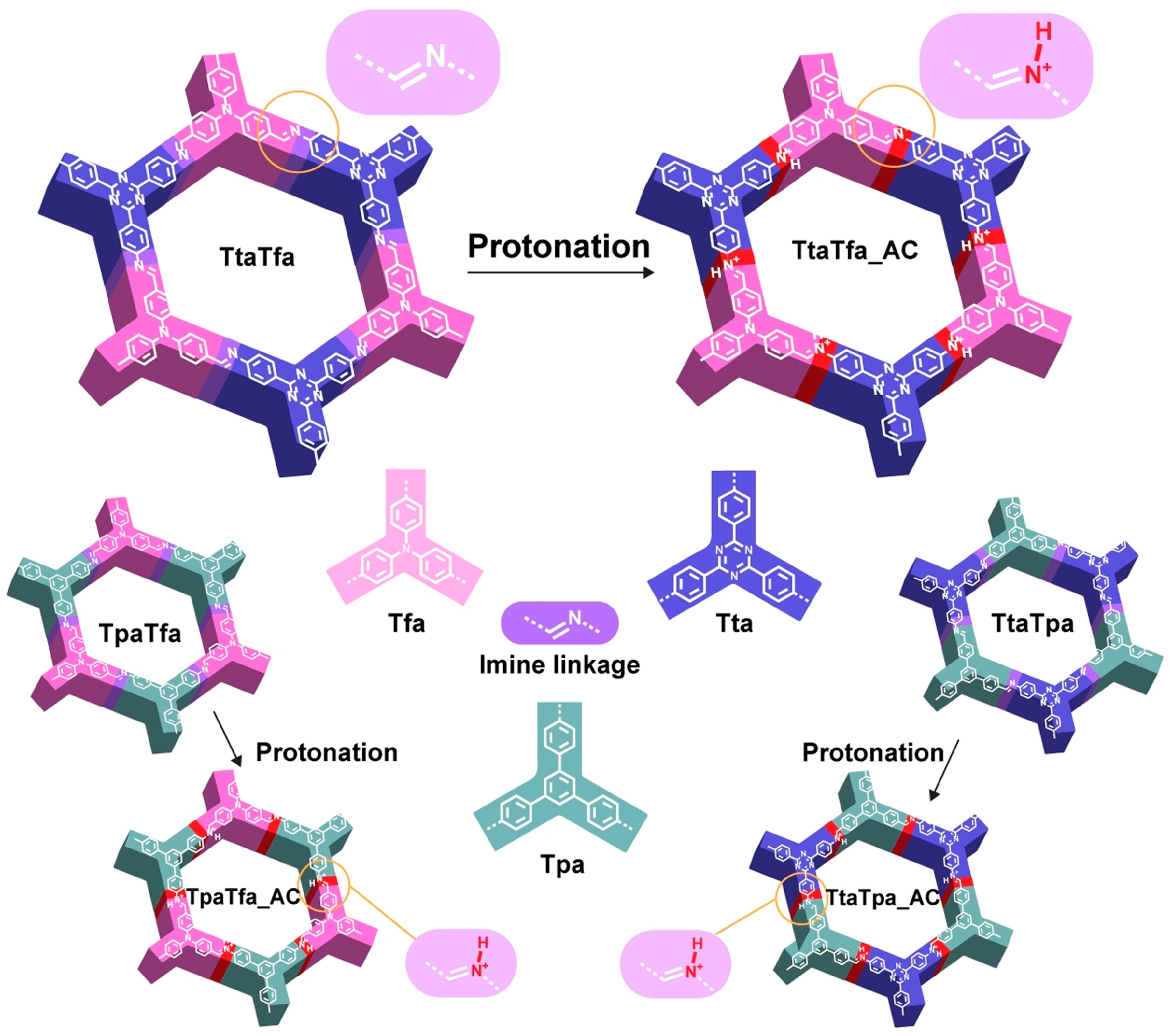
| TtaTfa/TpaTfa/TtaTpa-COF63 | 2.73–2.60 (2.22–1.89 H+) | 1.74 | 20.7 ± 2.7 mmol g−1 h−1 | ||
| v-COF-NS165 | 1.85 | 1.7/1.9 | Pt | AA | 4.4 mmol h−1 g−1 |
| COF-BBT66 | 2.0 | 2.3 | Pt | AA | 48.7 mmol g−1 h−1 |
| Incorporating metal complexes/metals | |||||
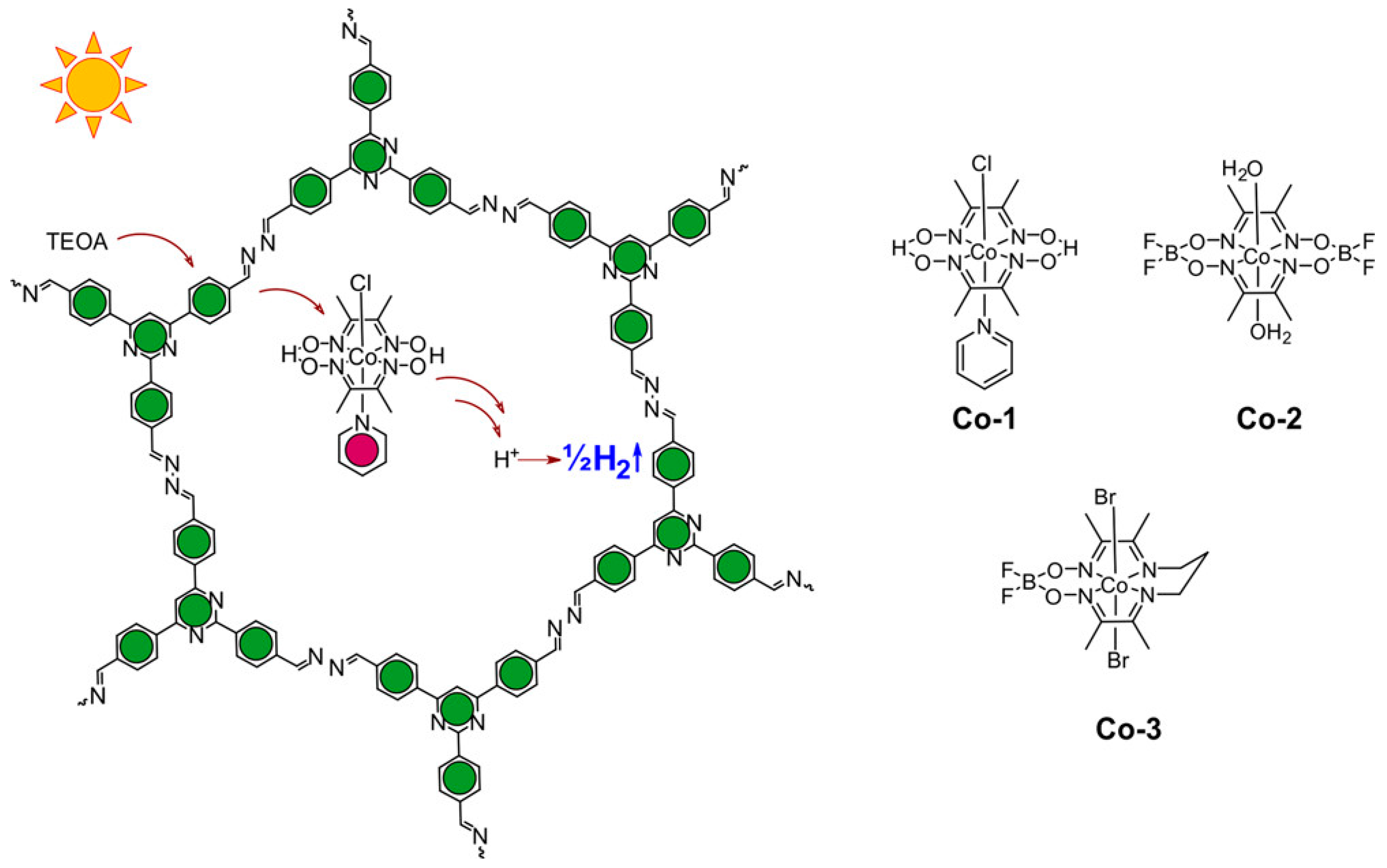
| azine-linked N2-COF68 | - | - | molecular cobaloxime | TEOA | 782 μmol g−1 h−1 |
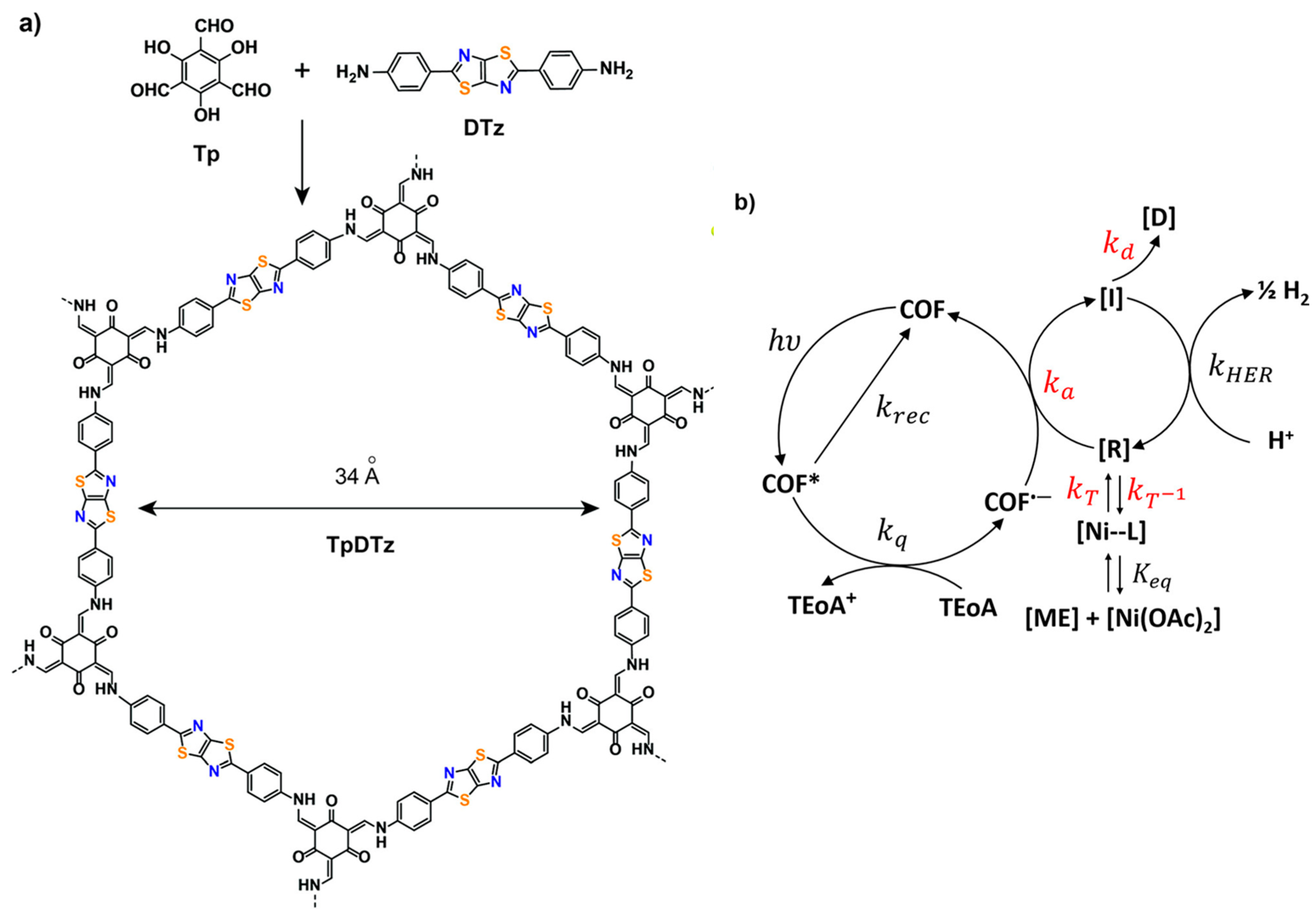
| TpDTz COF69 | 2.07 | 3.4 | NiME cluster | TEOA | 941 μmol h−1 g−1 |
| pCOF1070 | - | 2.3 | cobaloxime catalyst | TEOA | 163 μmol h−1 g−1 |
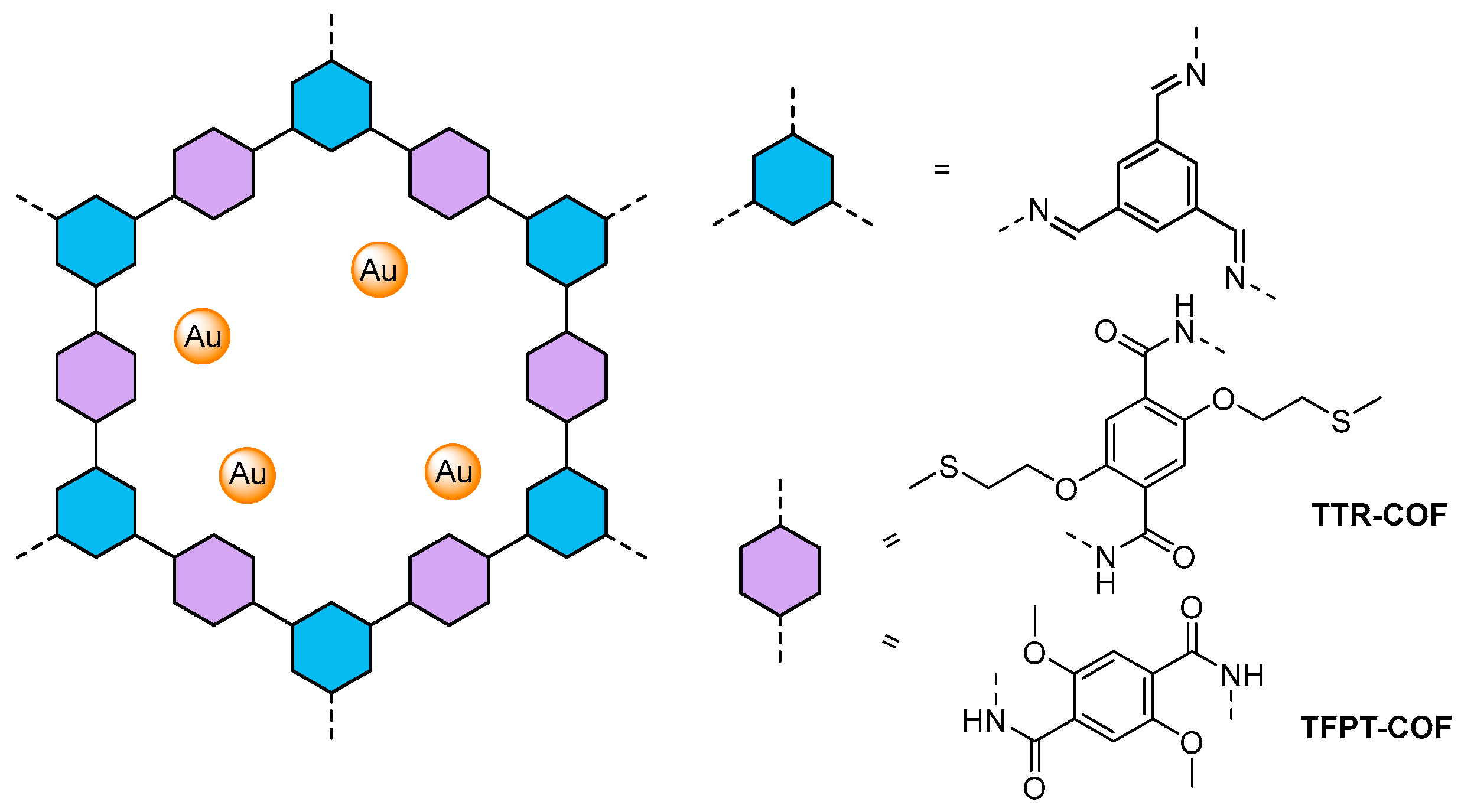
| TTR-COF71 | 2.71 | 2.95 | Au | TEOA | 1720 μmol h−1 g−1 |
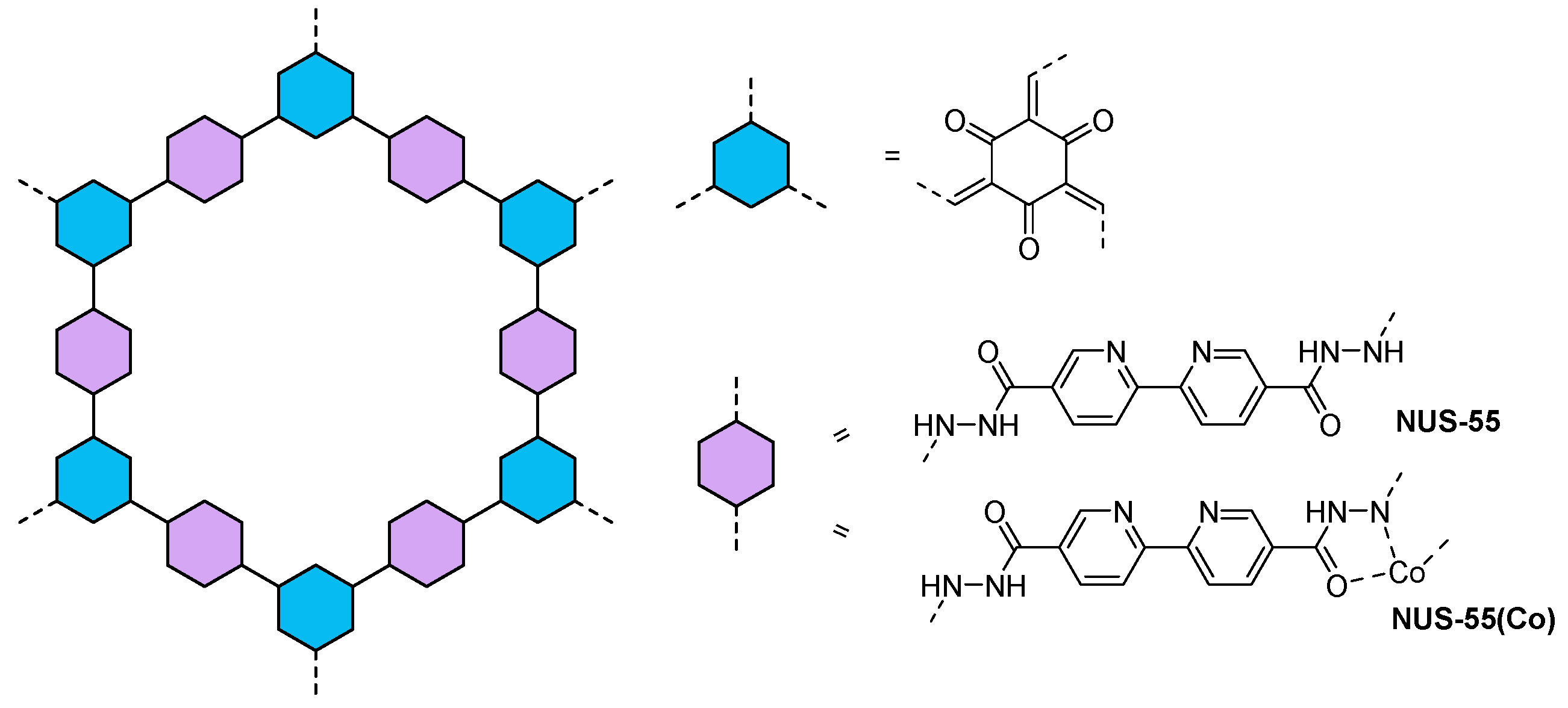
| NUS-55(Co)72 | - | 2.9 | [Co(bpy)3]Cl2 | TEA | 2480 µmol g−1 h−1 |
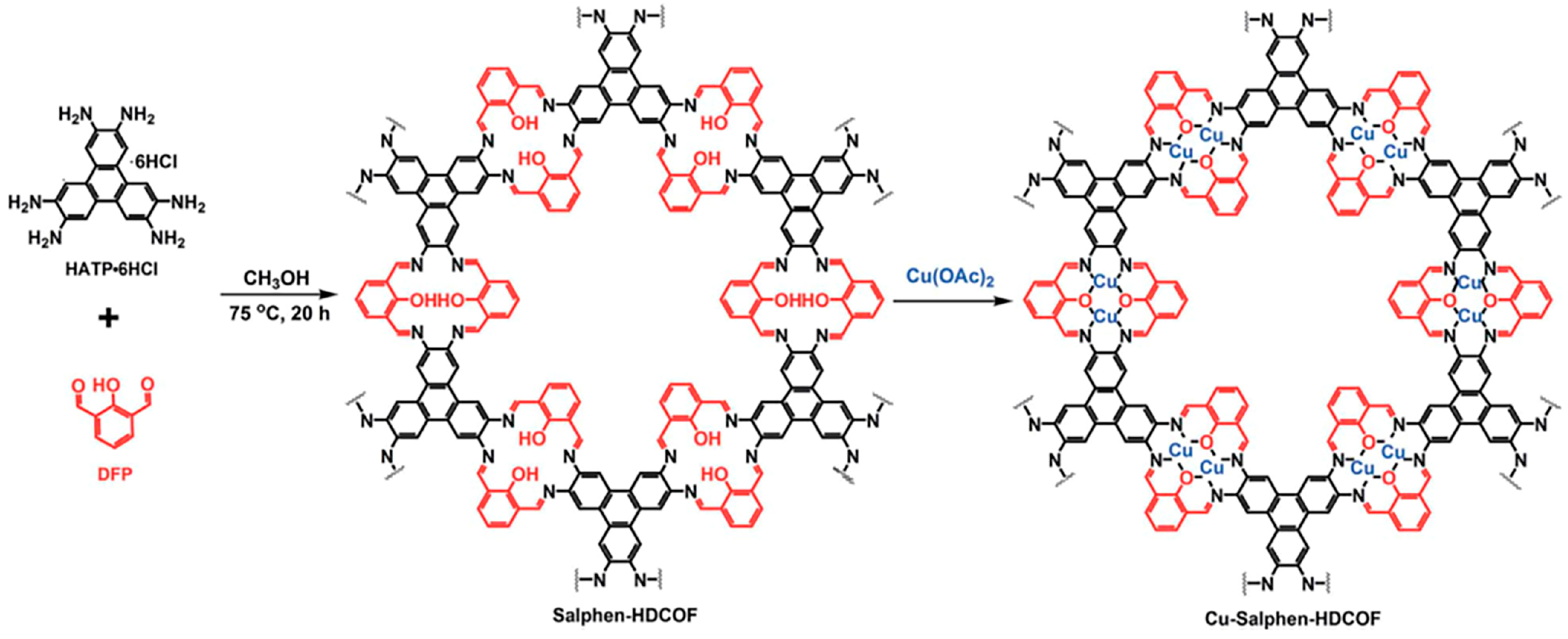
| Cu-salphen-HDCOF-NSs73 | 1.62 | 1.5 | Cu | TEA | 36.99 mmol g−1 h−1 |
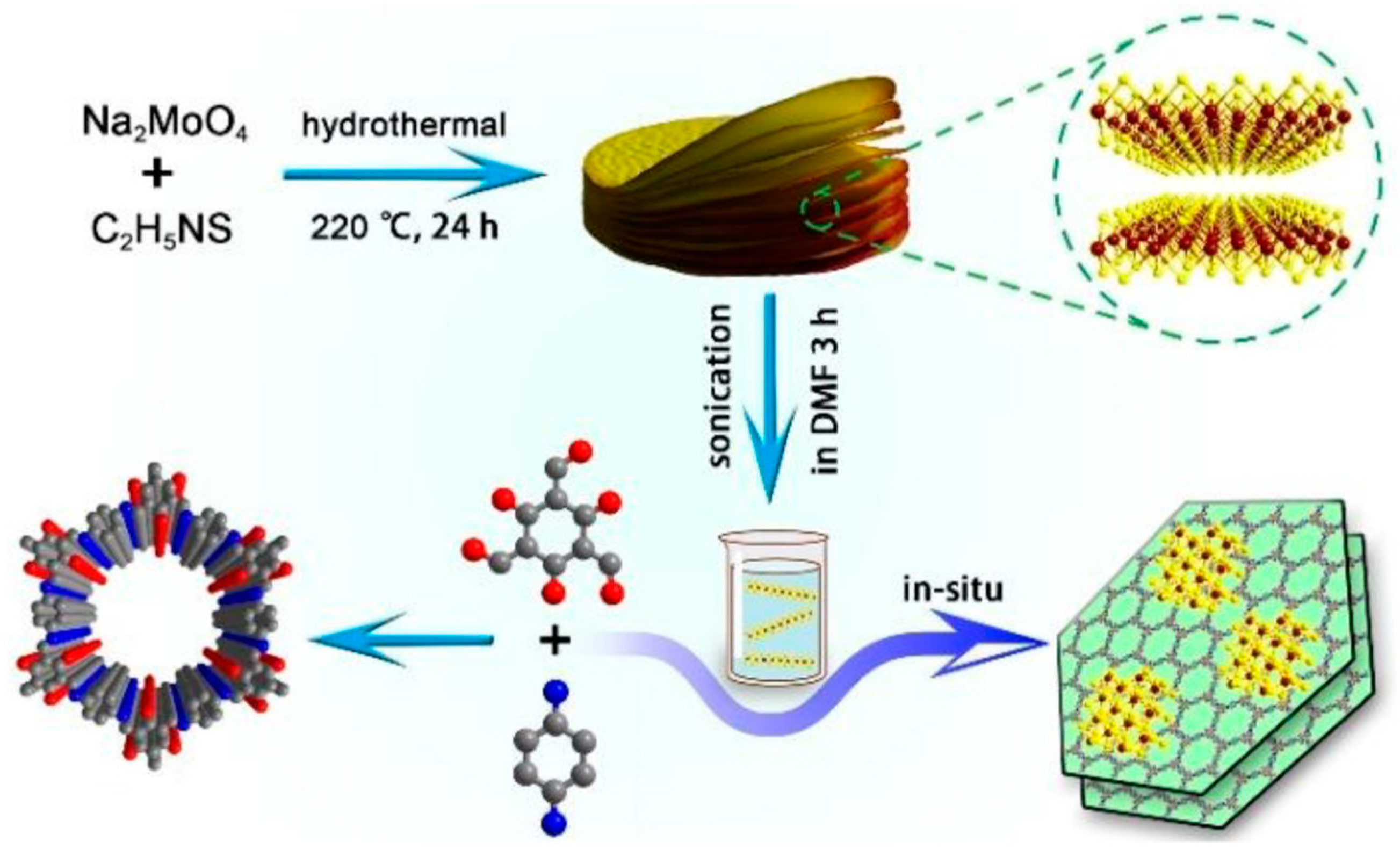
| MoS2/TpPa-1-COF74 | 2.14 | - | MoS2 | AA | 55.85 μmol h−1 |
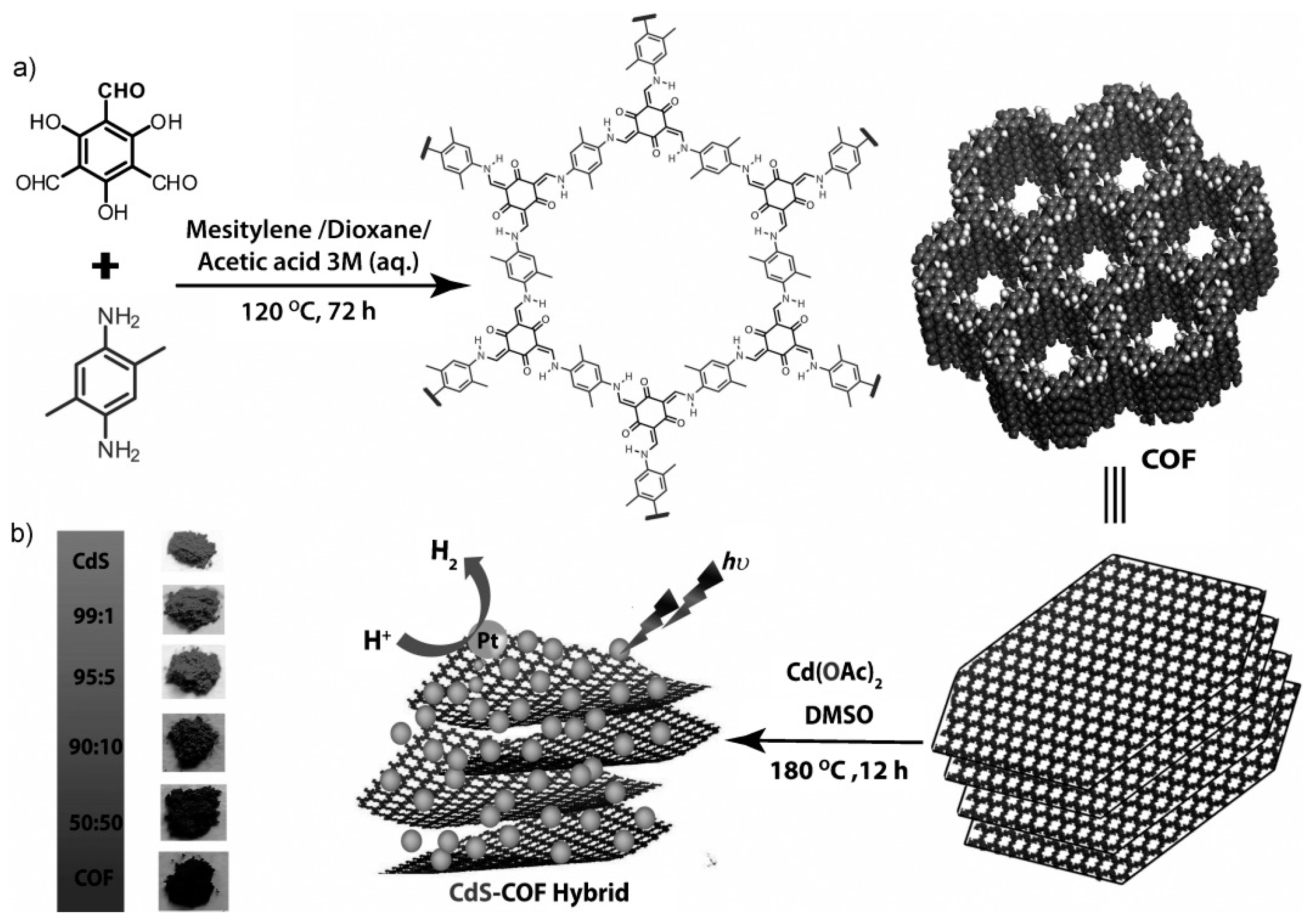
| CdS-COF75 | 2.52 | - | Pt | LA | 3678 μmol h−1 g−1 |
| PY-DHBD-COF76 | 2.28 | Pt | AA | 71,160 μmol g−1 h−1 | |
| TpBpy-Ni 2%77 | 1.84 | - | Pt | AA | 51,300 μmol h−1 g−1 |
| Donor–Acceptor (D–A) configuration | |||||
| PyTz-COF78 | 2.20 | ≈3.2 | Pt | AA | 2072.4 μmol g−1 h−1 |
| BT-TAPT-COF79 | 2.35 | 2.3 | Pt | TEOA | 949 μmol g−1 h−1 |
| Py-ClTP-BT-COF80 | 2.36 | 3.25 | Pt | AA | 177.50 μmol h−1 g−1 |
| NKCOF-10881 | 1.8 | 3.5 | Pt | AA | 120 μmol h−1 |
| BtCOF15082 | 2.5 | - | Pt | TEOA | 750 ± 25 µmol h−1 g−1 |
| BTH-3 COF83 | 2.02 | - | Pt | AA | 15.1 mmol h−1 g−1 |
| BTTh-TZ-COF84 | 2.32 | 1.35 | Pt | AA | 5.22 mmol h−1 g−1 |
| PTPA-COF TP-COF85 | 2.31 2.41 | - - | Pt | TEOA | 36 μmol h−1 g−1 29.12 mmol h−1 g−1 |
| TM-DMA-COF86 | 2.07 | 1.98 | Pt | AA | 4300 mmol h−1 g−1 |
| TAPFy-PhI COF87 | 2.21 | 2.26 | Pt | AA | 1763 μmol g−1 h−1 |
| TpPa−COF−(CH3)288 | 2.06 | 0.48 | Pt | NaAA | 8.33 mmol g−1 h−1 |
| PyPz-COF89 | 2.05 | 2.8 | Pt | AA | 7542 μmol g−1 h−1 |
| BTT-PDA, BTT-NDA, BTT-AnthDA, and BTT-BPhDA92 | 2.15 2.16 2.00 2.21 | 2.48 2.83 3.23 3.23 | Pt | AA | 2.04 mmol h−1 g−1 5.22 mmol h−1 g−1 4.23 mmol h−1 g−1 3.27 mmol h−1 g−1 |
| COF-JUL35 COF-JUL3693 | 1.85 2.12 | 2.7 | Pt | A | 70.8 ± 1.9 mmol h−1 g−1 |
| BTT-Bpy-COF94 | 1.41 | 2.85 | Pt | AA | 15.8 mmol h−1 g−1 |
| Hetero-structural mixed linkage approach/Miscellaneous approach | |||||
| NTU-BDA-THAT NTU-BDA-THAT + NH2-Ti3C2Tx95 | 1.81 | 1.42 2.09 | Pt | AA | 1.47 μmol g−1 h−1 14,228.1 μmol g−1 h−1 |
| 2D-2D BP/TpPa-1-COF96 | - | - | - | - | 456.7 µmol h−1 g−1 |
| TSCOFW98 | 1.95 | 2.6 | Pt | AA | 593 mmol h−1 g−1 |
| 2D/2D COF/g-C3N499 | - | 1.7 | Pt | TEOA | 449.64 μmol h−1 |
| TpPa-1-COF/ZnIn2S4100 | - | - | ZnIn2S4 | Na2S + Na2SO3 | 853 μmol g−1 h−1 |
| Tp-nC/BPy2+-COF101 | - | - | Pt | AA | 34,600 μmol h−1 g−1 |
| TPCBP X-COF X = Butyl103 | 2.36 | 2.95–3.71 | Pt | TEOA | |
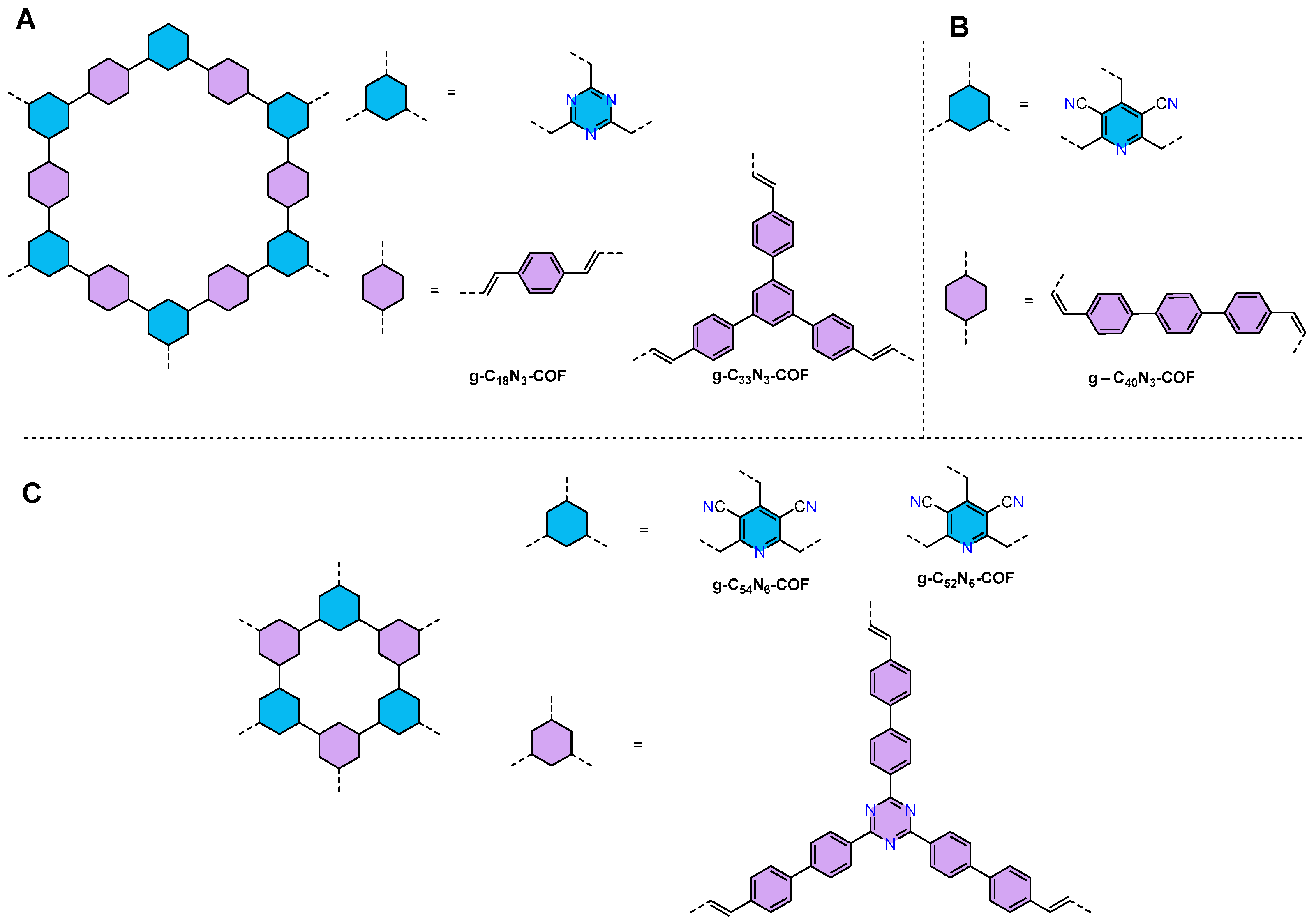
| g-C18N3-COF105 | 2.42 | 1.72 | Pt | AA | 14.6 mol h−1 |
| g-C40N3-COF106 | 2.36 | 3.3 | Pt | TEOA | 206 µmol h−1 |
| g-C54N6-COF107 | 2.03 | 2.28 | Pt | TEOA | 2518.9 μmol h−1 g−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.A.; Azad, C.S.; Luo, M.; Chen, J.; Kesharwani, T.; Badshah, A.; Wang, D. Mechanistic Approach towards Designing Covalent Organic Frameworks for Photocatalytic Hydrogen Generation. Energies 2023, 16, 5888. https://doi.org/10.3390/en16165888
Khan NA, Azad CS, Luo M, Chen J, Kesharwani T, Badshah A, Wang D. Mechanistic Approach towards Designing Covalent Organic Frameworks for Photocatalytic Hydrogen Generation. Energies. 2023; 16(16):5888. https://doi.org/10.3390/en16165888
Chicago/Turabian StyleKhan, Niaz Ali, Chandra S. Azad, Mengying Luo, Jiahui Chen, Tanay Kesharwani, Amir Badshah, and Dong Wang. 2023. "Mechanistic Approach towards Designing Covalent Organic Frameworks for Photocatalytic Hydrogen Generation" Energies 16, no. 16: 5888. https://doi.org/10.3390/en16165888
APA StyleKhan, N. A., Azad, C. S., Luo, M., Chen, J., Kesharwani, T., Badshah, A., & Wang, D. (2023). Mechanistic Approach towards Designing Covalent Organic Frameworks for Photocatalytic Hydrogen Generation. Energies, 16(16), 5888. https://doi.org/10.3390/en16165888








