Mass Transfer Analysis of CO2-Water-Rock Geochemical Reactions in Reservoirs
Abstract
1. Introduction
2. Mass Transfer Model for Reservoirs
2.1. Model Building
2.1.1. Solute Transport
2.1.2. Precipitation and Dissolution of Minerals
2.1.3. Chemical Reaction Rate
2.1.4. Porosity and Permeability
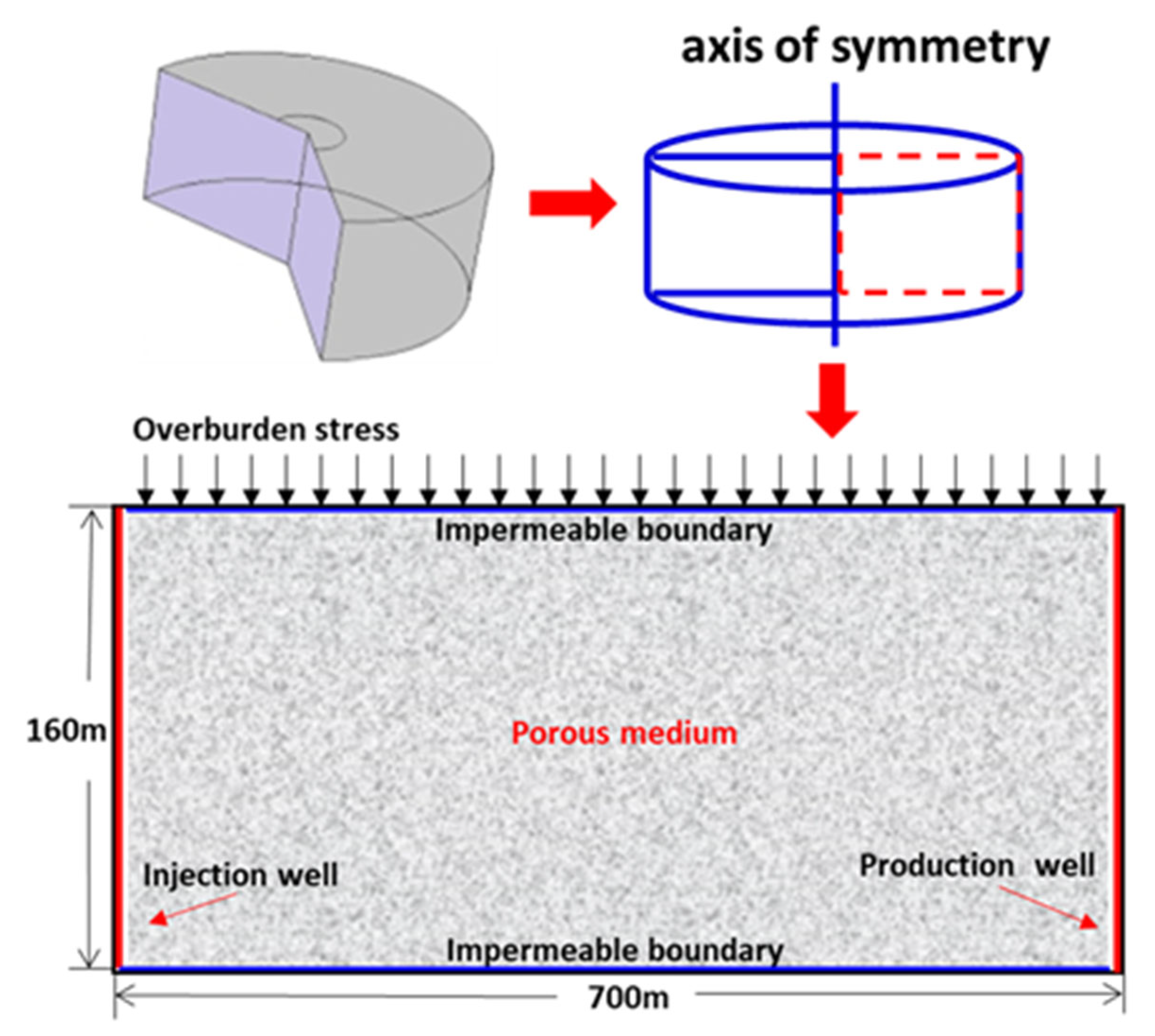
2.2. Initial and Boundary Conditions
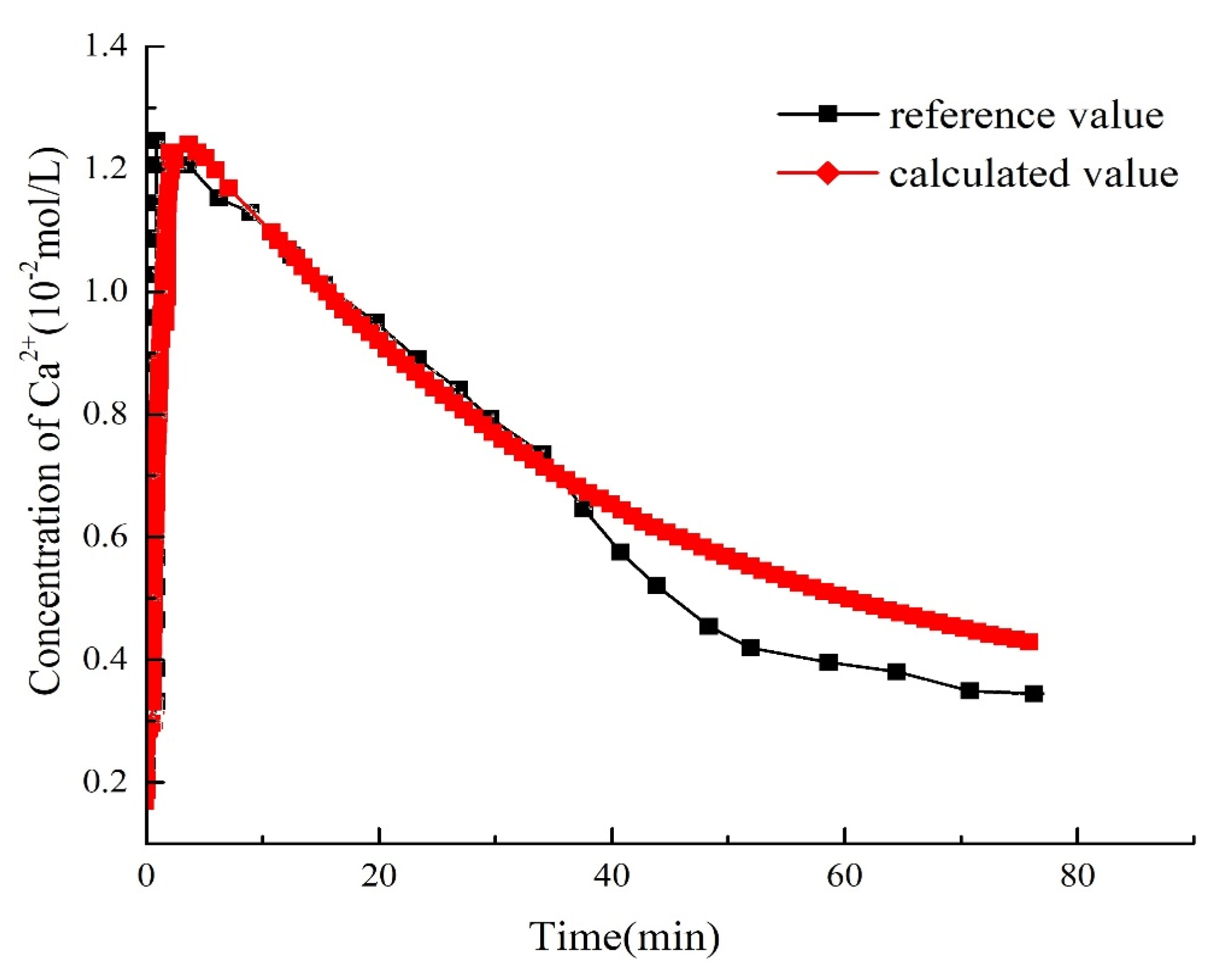
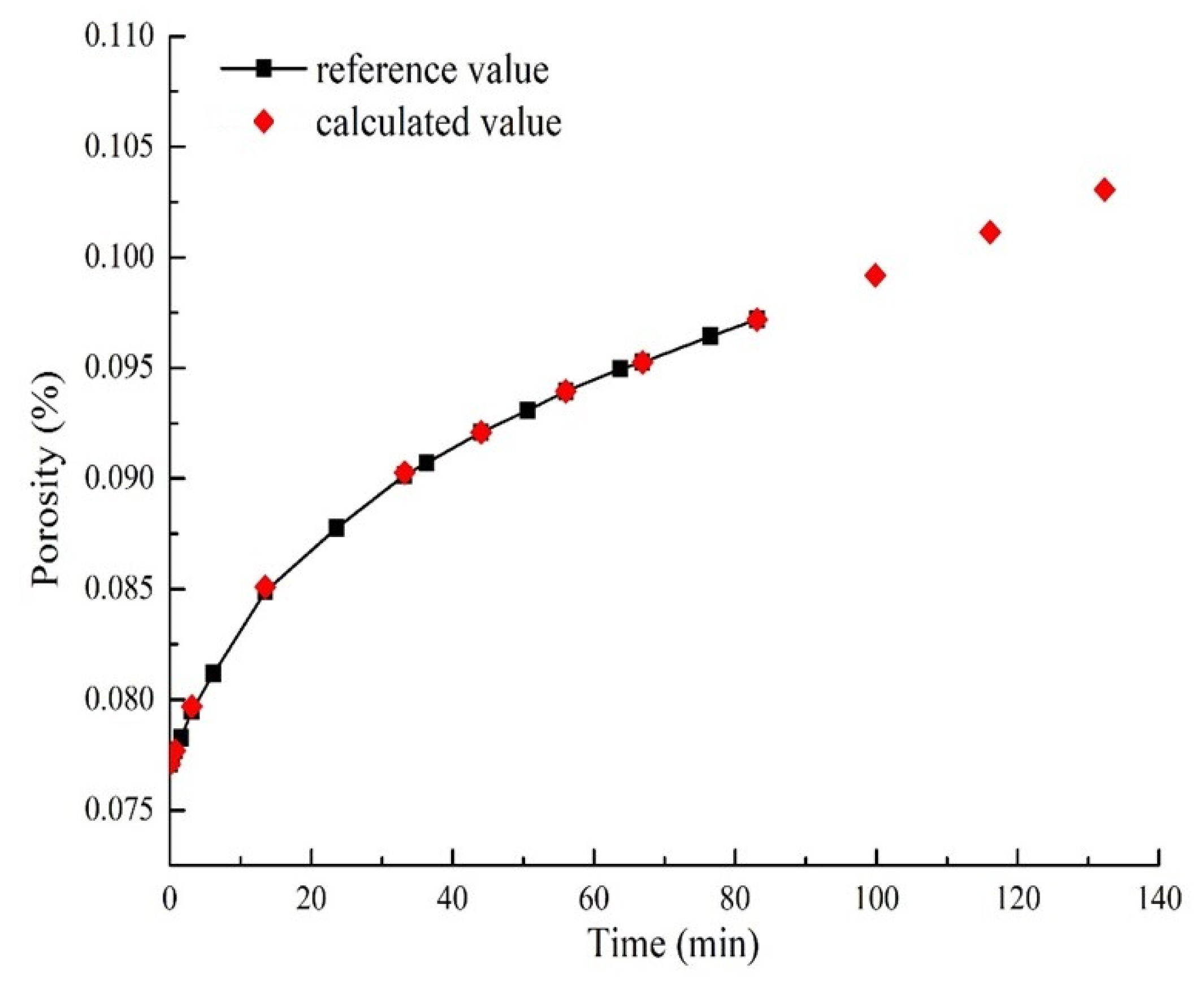
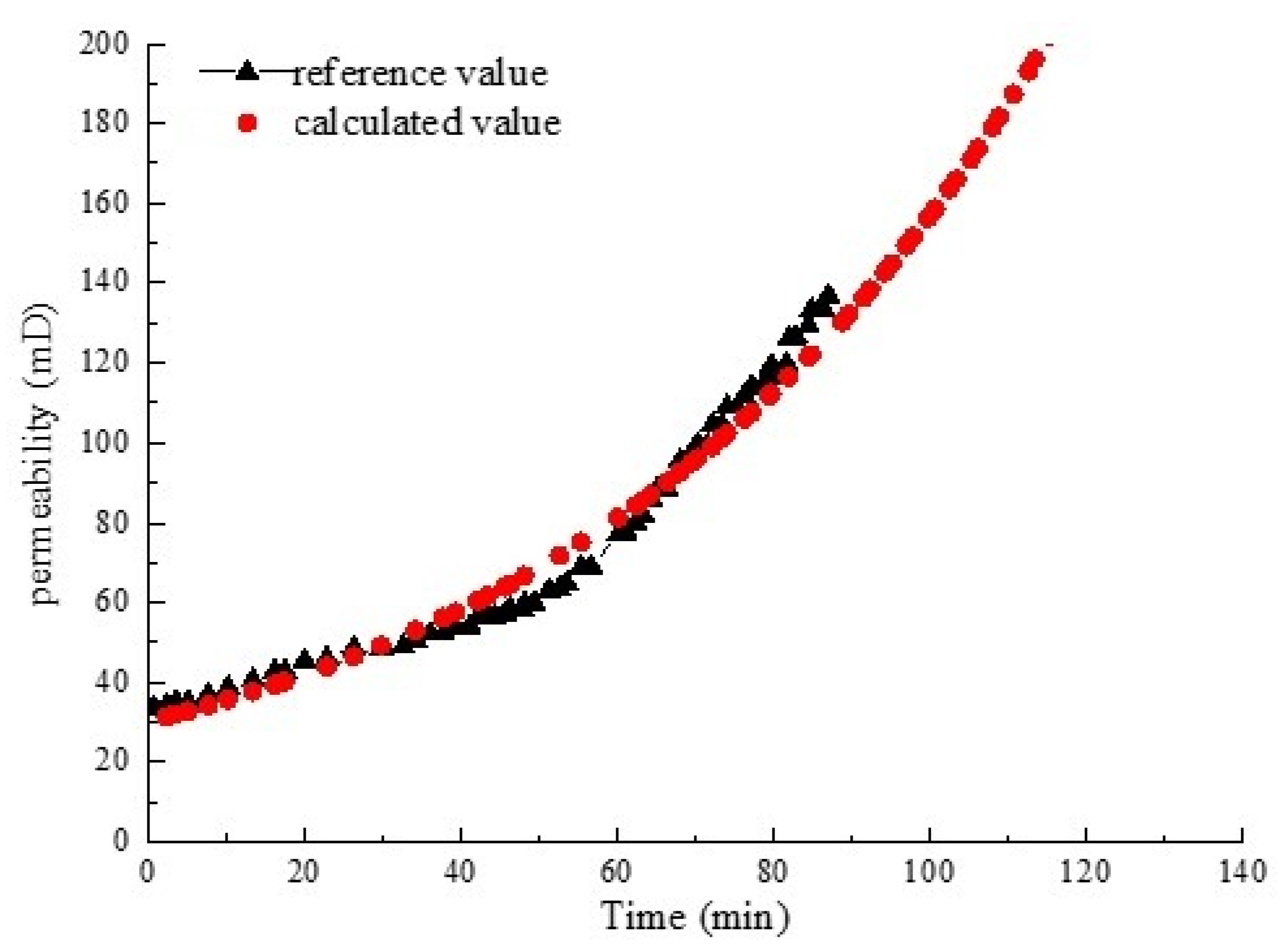
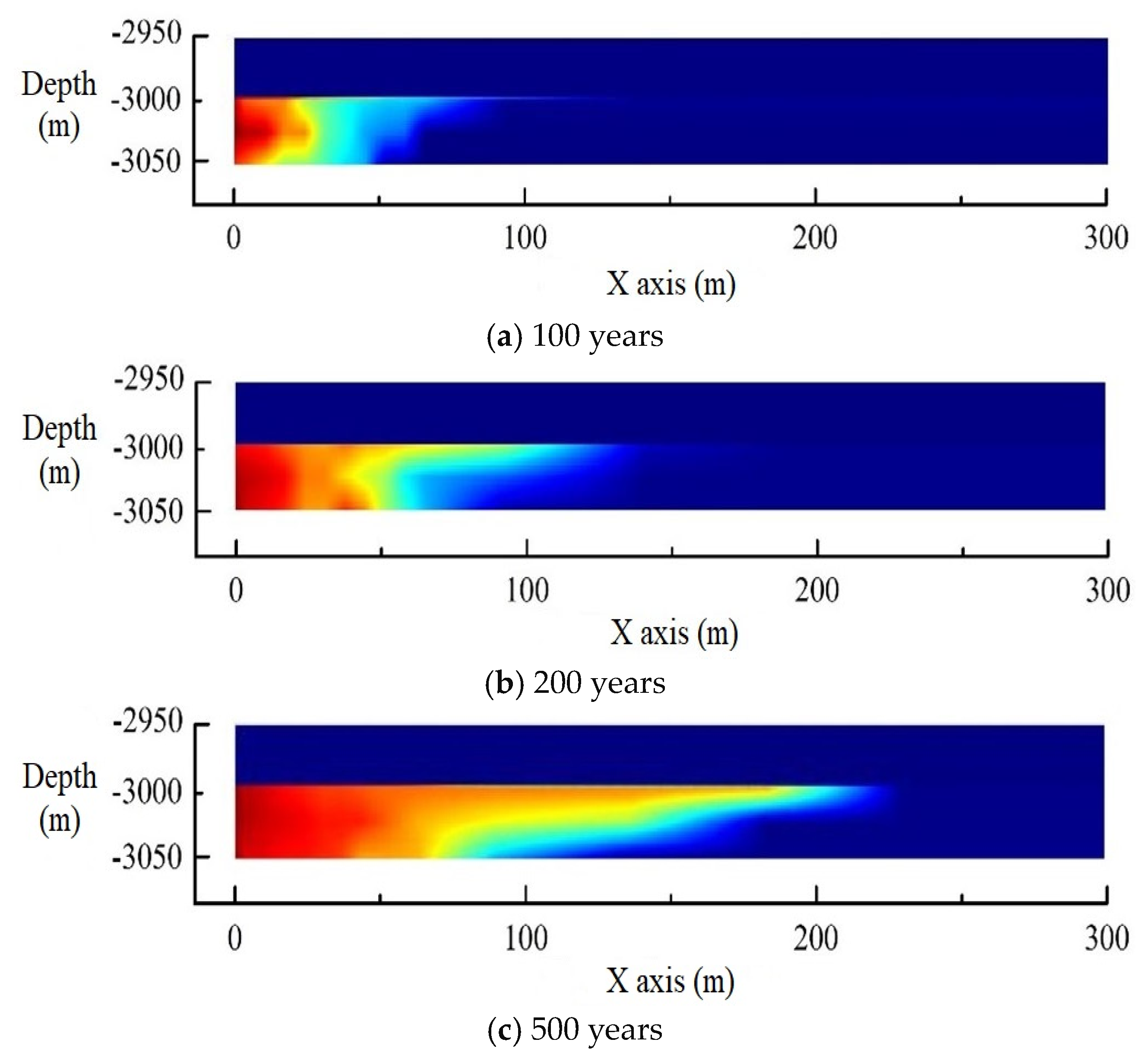
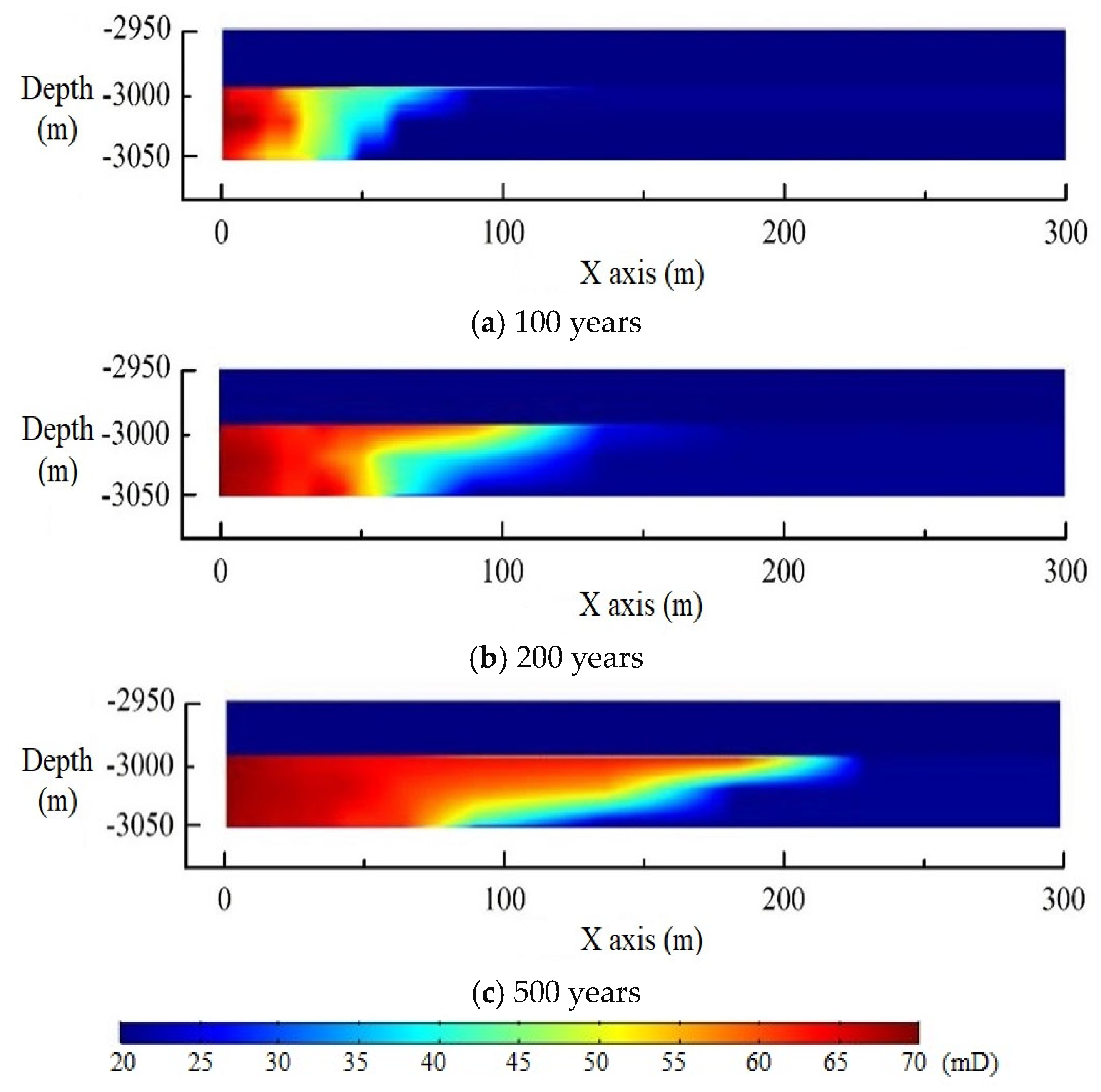
3. Model Validation
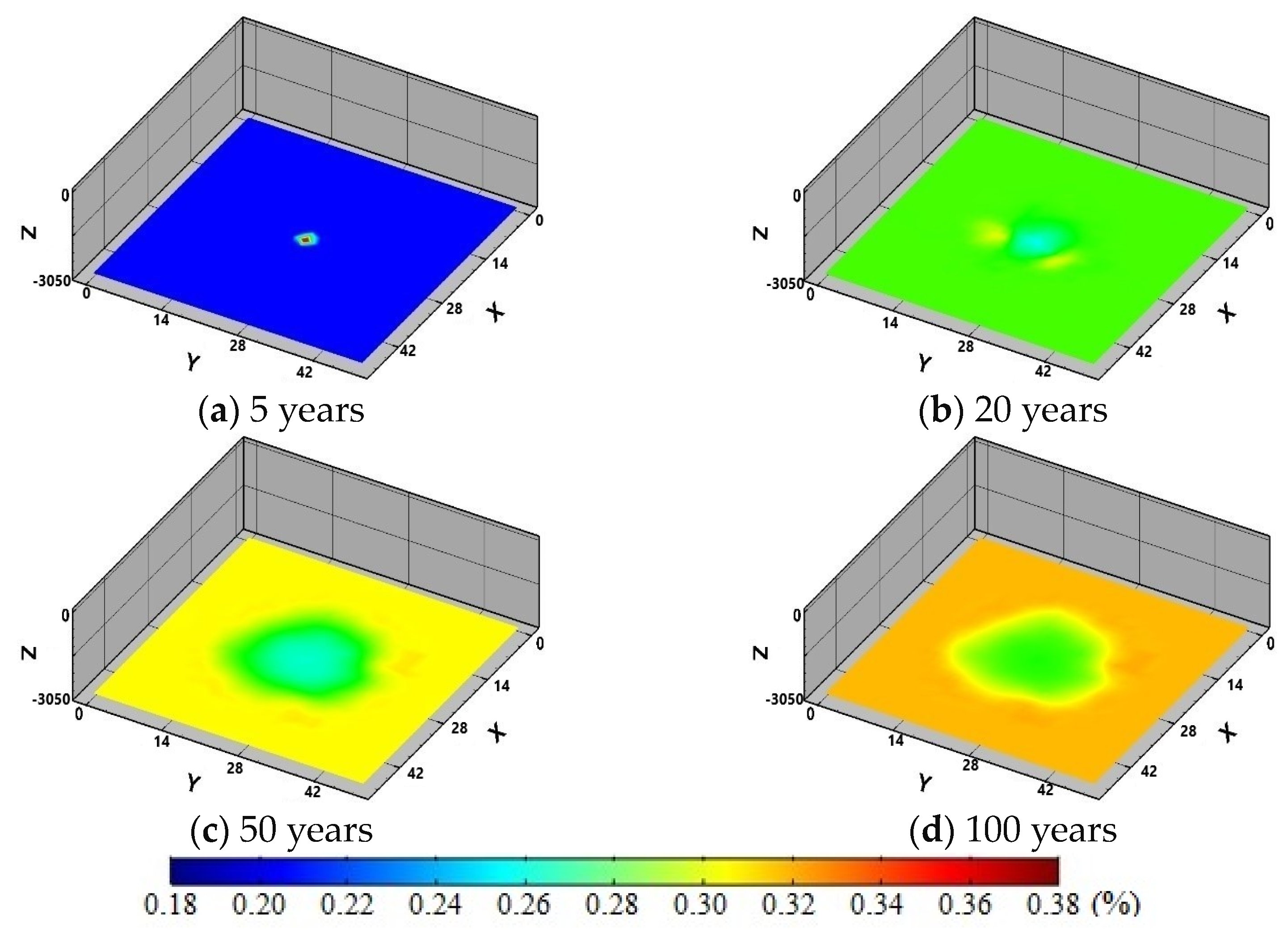
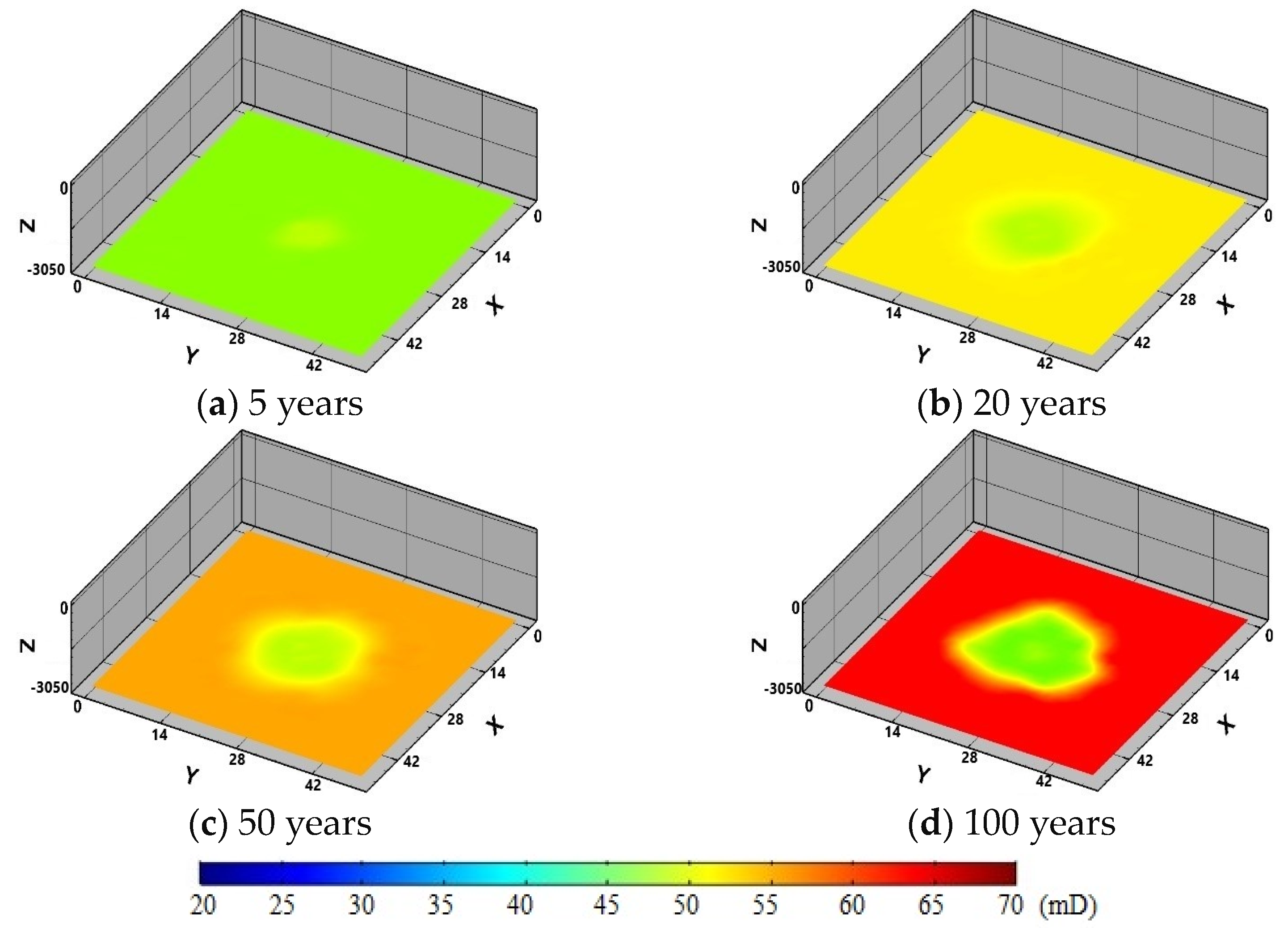
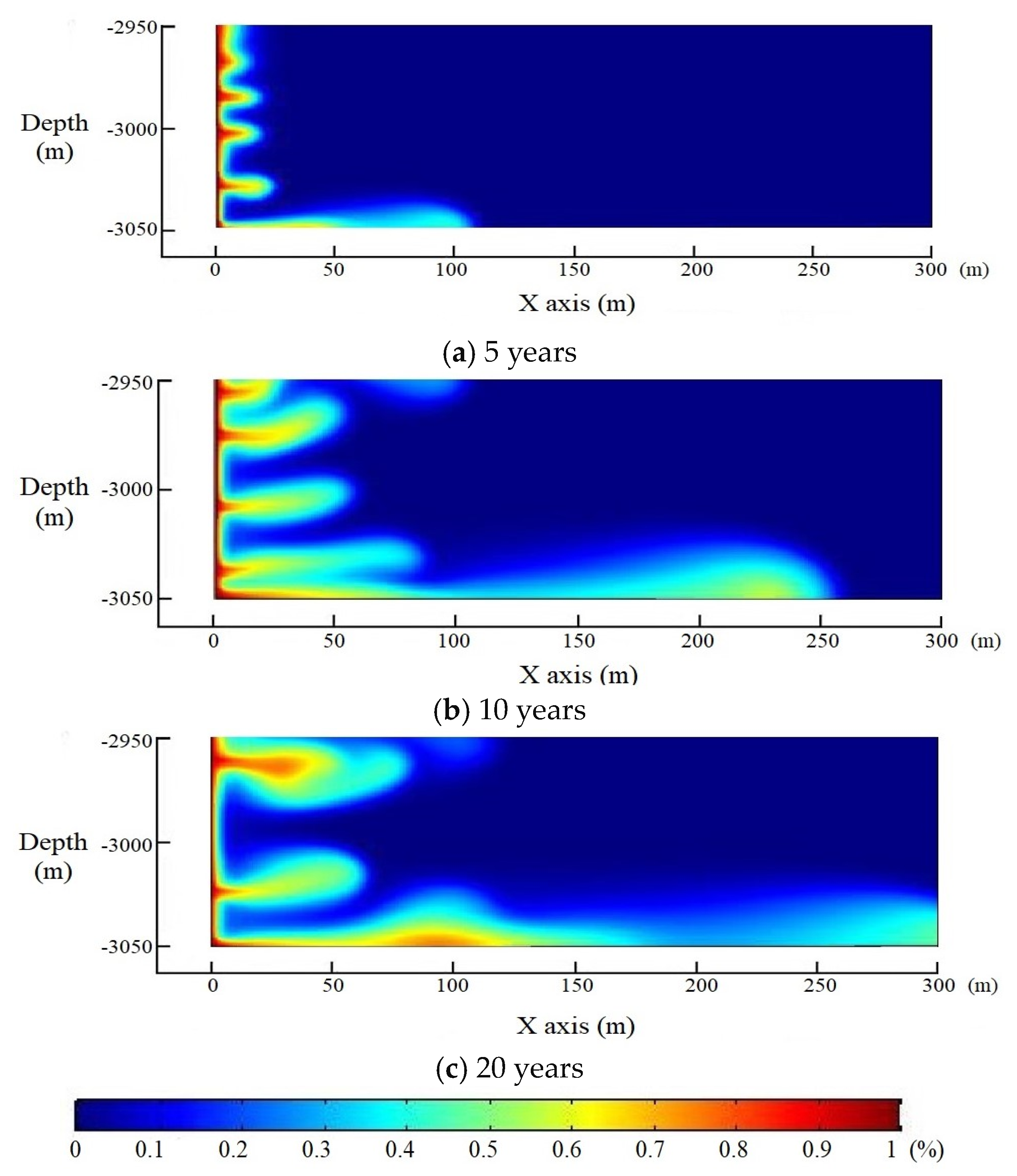
4. Model Application
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, X.; Feng, F.P.; Yan, M.S.; Cong, Z.Y.; Liu, S.Y.; Zhang, Y.H. CO2–Water–Rock Reaction Transport Via Simulation Study of Nanoparticles-CO2 Flooding and Storage. Sustain. Energy Technol. Assess. 2022, 50, 101736. [Google Scholar] [CrossRef]
- Emad, A.; Doaa, S.M.; Yujie, Y.; Stefan, I. Influence of Clay Content on CO2-Rock Interaction and Mineral-Trapping Capacity of Sandstone Reservoirs. Energies 2023, 16, 3489. [Google Scholar]
- Khetib, Y.; Zunez, S. Integrated Pipeline and Wells Transient Behavior of CO2 Injection Operations: Flow Assurance Best Practices. In Proceedings of the PSIG Annual Meeting, San Antonio, TX, USA, 16–19 May 2023; p. PSIG-2325. [Google Scholar]
- Wang, C.; Cui, M.L.; Wang, K.; Wei, Y.; Wang, K.Y.; Gao, H. Full-Scale Experimental Study on the Effect of CO2 Flooding on Storage-Seepage Capacity of Tight Sandstone Reservoirs. ACS Omega 2023, 8, 11897–11907. [Google Scholar] [CrossRef]
- Li, S.Q.; Chen, Z.; Li, W.; Yan, T.; Bi, F.Q.; Tong, Y.S. An FE Simulation of the Fracture Characteristics of Blunt Rock Indenter under Static and Harmonic Dynamic Loadings Using Cohesive Elements. Rock Mech. Rock Eng. 2023, 56, 2935–2947. [Google Scholar] [CrossRef]
- Ao, X.; Wang, B.B.; Rao, Y.X.; Zhang, L.; Wang, Y.; Tang, H.K. Effect of CO2 Corrosion and Adsorption-Induced Strain on Permeability of Oil Shale: Numerical Simulation. Energies 2023, 16, 780. [Google Scholar] [CrossRef]
- Song, R.; Tang, Y.; Wang, Y.; Xie, R.Y.; Liu, J.J. Pore-Scale Numerical Simulation of CO2–Oil Two-Phase Flow: A Multiple-Parameter Analysis Based on Phase-Field Method. Energies 2023, 16, 82. [Google Scholar] [CrossRef]
- Lin, R.; Yu, Z.H.; Zhao, J.Z.; Dai, C.L.; Sun, Y.P.; Ren, L.; Xie, M.K. Experimental Evaluation of Tight Sandstones Reservoir Flow Characteristics under CO2–Brine–Rock Multiphase Interactions: A Case Study in the Chang 6 Layer, Ordos Basin, China. Fuel 2022, 309, 122167. [Google Scholar] [CrossRef]
- Han, X.; Feng, F.P.; Cong, Z.Y. Dynamic Evaluation Method of Caprock Microscopic Sealing in CO2 Sequestration Project. Geofluids 2020, 2020, 2648692. [Google Scholar] [CrossRef]
- Cui, G.D.; Zhu, L.H.; Zhou, Q.C.; Ren, S.R.; Wang, J.Y. Geochemical reactions and their effect on CO2 storage efficiency during the whole process of CO2 EOR and subsequent storage. Int. J. Greenh. Gas Control 2021, 108, 103335. [Google Scholar] [CrossRef]
- Law, D.; Meer, L.; Gunter, W.D. Modeling of Carbon Dioxide Sequestration in Coalbeds: A Numerical Challenge. In Proceedings of the 6th International Conference on Greenhouse Gas Control Technologies, Cairns, Australia, 1 July 2001. [Google Scholar]
- Ketzer, J.M.; Iglesias, R.; Einloft, S.; Dullius, J.; Ligabue, R.; De, L.V. Water–Rock–CO2 Interactions in Saline Aquifers Aimed for Carbon Dioxide Storage: Experimental and Numerical Modeling Studies of the Rio Bonito Formation (Permian), Southern Brazil. Appl. Geochem. 2009, 24, 760–767. [Google Scholar] [CrossRef]
- Andre, L.; Audigane, P.; Azaroual, M.; Menjoz, A. Numerical Modeling of Fluid-Rock Chemical Interactions at the Supercritical CO2-Liquid Interface During CO2 Injection into a Carbonate Reservoir, the Dogger Aquifer (Paris Basin, France). Energy Convers. Manag. 2007, 48, 1782–1797. [Google Scholar] [CrossRef]
- Wandrey, M.; Pellizari, L.; Zettlitzer, M.; Würdemann, H. Microbial Community and Inorganic Fluid Analysis during CO2 Storage within the Frame of CO2SINK–Long-Term Experiments under in Situ Conditions. Energy Procedia 2011, 4, 3651–3657. [Google Scholar] [CrossRef][Green Version]
- Xu, T.; Zheng, L.; Tian, H. Reactive Transport Modeling for CO2 Geological Sequestration. J. Pet. Sci. Eng. 2011, 78, 765–777. [Google Scholar] [CrossRef]
- Credoz, A.; Bildstein, O.; Jullien, M.; Raynal, J.; Pétronin, J.C.; Lillo, M.; Geniaut, G. Experimental and Modeling Study of Geochemical Reactivity between Clayey Caprocks and CO2 in Geological Storage Conditions. Energy Procedia 2009, 1, 3445–3452. [Google Scholar] [CrossRef]
- Gaus, I.; Azaroual, M.; Czernichowski, L.I. Reactive Transport Modelling of the Impact of CO2 Injection on the Clayey Cap Rock at Sleipner (North Sea). Chem. Geol. 2005, 217, 319–337. [Google Scholar] [CrossRef]
- Gherardi, F.; Xu, T.; Pruess, K. Numerical Modeling of Self-limiting and Self-Enhancing Caprock Alteration Induced by CO2 Storage in A Depleted Gas Reservoir. Chem. Geol. 2007, 244, 103–129. [Google Scholar] [CrossRef]
- Angeli, M.; Soldal, M.; Skurtveit, E.; Aker, E. Experimental Percolation of Supercritical CO2 through A Caprock. Energy Procedia 2009, 1, 3351–3358. [Google Scholar] [CrossRef]
- Erickson, K.P.; Lempp, C.; PuLlmann, H. Geochemical and Geo-Mechanical Effects of scCO2 and Associated Impurities on Physical and Petrophysical Properties of Permotriassic Sandstones (Germany): An Experimental Approach. Environ. Earth Sci. 2015, 74, 4719–4743. [Google Scholar] [CrossRef]
- Shiraki, R.; Dunn, T.L. Experimental Study on Water–Rock Interactions during CO2 Flooding in the Tensleep Formation, Wyoming, USA. Appl. Geochem. 2000, 15, 270–279. [Google Scholar] [CrossRef]
- Wigand, M.; Carey, J.W.; Schütt, H.; Spangenberg, E.; Erzinger, J. Geochemical Effects of CO2 Sequestration in Sandstones under Simulated in Situ Conditions of Deep Saline Aquifers. Appl. Geochem. 2008, 23, 2735–2745. [Google Scholar] [CrossRef]
- Bildstein, O.; Kervevan, C.; Lagneau, V.; Delaplace, P.; Credoz, A.; Audigane, P.; Jullien, M. Integrative Modeling of Caprock Integrity in the Context of CO2 Storage: Evolution of Transport and Geochemical Properties and Impact on Performance and Safety Assessment. Oil Gas Sci. Technol. Rev. IFP 2010, 65, 485–502. [Google Scholar] [CrossRef]
- Kang, X.; Hu, W.; Cao, J.; Jin, J.; Wu, H.; Zhao, Y.; Wang, J. Selective Dissolution of Alkali Feldspars and Its Effect on Lower Triassic Sandy Conglomerate Reservoirs in the Junggar Basin, Northwestern China. Geol. J. 2018, 53, 475–499. [Google Scholar] [CrossRef]
- Wang, H.; Alvarado, V.; Bagdonas, D.A.; McLaughlin, J.F.; Kaszuba, J.P.; Grana, D.; Campbell, E.; Ng, K. Effect of CO2-Brine-Rock Reactions on Pore Architecture and Permeability in Dolostone: Implications for CO2 Storage and EOR. Int. J. Greenh. Gas Control 2021, 107, 103283. [Google Scholar] [CrossRef]
- Tao, J.; Meng, S.; Li, D.; Rui, Z.; Liu, H.; Xu, J. Analysis of CO2 Effects on Porosity and Permeability of Shale Reservoirs under Different Water Content Conditions. Geoenergy Sci. Eng. 2023, 226, 211774. [Google Scholar] [CrossRef]
- Lamy-Chappuis, B.; Angus, D.; Fisher, Q.; Grattoni, C.; Yardley, B.W. Rapid Porosity and Permeability Changes of Calcareous Sandstone due to CO2-Enriched Brine Injection. Geophys. Res. Lett. 2014, 41, 399–406. [Google Scholar] [CrossRef]
- Matteo, E.N.; Scherer, G.W. Experimental Study of the Diffusion-Controlled Acid Degradation of Class H Portland Cement. Int. J. Greenh. Gas Control 2012, 7, 181–191. [Google Scholar] [CrossRef]
- Yeh, G.T.; Tripathi, V.S. A Model for Simulating Transport of Reactive Multispecies Components: Model Development and Demonstration. Water Resour. Res. 1991, 27, 3075–3094. [Google Scholar] [CrossRef]
- Koichi, A. Simulation for Memory Effect of Fick’s First Law. Proc. Indian Acad. Sci. Chem. Sci. 2009, 121, 5601–5605. [Google Scholar]
- Li, T.; Gao, H.; Ni, J.; Wang, C.; Cheng, Z.; Xue, J.; Luo, K. Research on the Differential Oil Producing in the Various Scale Pores under Different CO2 Flooding Modes with A Fluid Distribution Pore Classification Method. Energy Fuels 2023, 37, 3775–3784. [Google Scholar] [CrossRef]
- Adebayo, A.R.; Isah, A.; Mahmoud, M.; Al-Shehri, D. Effects of Foam Microbubbles on Electrical Resistivity and Capillary Pressure of Partially Saturated Porous Media. Molecules 2020, 25, 3385. [Google Scholar] [CrossRef]
- Steefel, C.I.; Lasaga, A.C. A Coupled Model for Transport of Multiple Chemical Species and Kinetic Precipitation/Dissolution Reactions with Application to Reactive Flow in Single Phase Hydrothermal Systems. Am. J. Sci. 1994, 294, 529–592. [Google Scholar] [CrossRef]
- Benson, S.M.; Perrin, J.C.; Krause, M.; Kuo, C.W.; Miljkovic, L. Experimental Investigations of Multiphase Flow and Trapping in Saline Aquifers—Annual Report 2007; Stanford University: Stanford, CA, USA, 2008. [Google Scholar]
- Lasaga, A.C.; Soler, J.M.; Ganor, J.; Burch, T.E.; Nagy, K.L. Chemical Weathering Rate Laws and Global Geochemical Cycles. Geochim. Cosmochim. Acta 1994, 58, 2361–2386. [Google Scholar] [CrossRef]
- Genuchten, V. A Close-Form Equation for Predicting the Hydraulic Conductivity of Saturated Soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Kozeny, J. Ueber Kapillare Leitung Des Wassers Im Boden. Sitzungsber Akad. Wiss. Wien 1927, 136, 271–306. [Google Scholar]
- Carman, P.C. Permeability of Saturated Sands, Soils and Clays. J. Agric. Sci. 1939, 29, 263–273. [Google Scholar] [CrossRef]
- Luquot, L.; Gouze, P. Experimental Determination of Porosity and Permeability Changes Induced by Injection of CO2, into Carbonate Rocks. Chem. Geol. 2009, 265, 148–159. [Google Scholar] [CrossRef]
- Ranganathan, P.; Van-Hemert, P.; Rudolph, E.S.J.; Zitha, P.Z. Numerical Modeling of CO2 Mineralisation during Storage in Deep Saline Aquifers. Energy Procedia 2011, 4, 4538–4545. [Google Scholar] [CrossRef]

| Formation Water Composition (mol/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na+ | 1.0 | Ca2+ | 8.25 × 10−3 | Mg2+ | 0.16 × 10−3 | Cl− | 1.0 | CO2 | 0.8 |
| Formation water pH | 3.21 | Mineral composition | Ca0.99Mg0.01CO3 | Chemical reaction equation | |||||
|
Initial pore radius
(μm) |
Initial pore throat ratio
(%) |
Pore throat length ratio
(%) |
Single pore surface density
(kg/m2) |
Initial formation temperature
(°C) |
Initial formation pressure
(MPa) |
Initial porosity
(%) |
Initial permeability
(m2) | Equilibrium constant K | |
| 100 | 5 | 13.3 | 20 | 100 | 10 | 7.5 | 3.5 × 10−14 | 0.168 | |
| Formation Water Composition (mol/kg H2O) | |||||||
|---|---|---|---|---|---|---|---|
| H+ | 1.64 × 10−8 | Al3+ | 5.57 × 10−7 | Ca2+ | 0.1610 | SiO2 | 8.64 × 10−4 |
| Fe2+ | 6.12 × 10−3 | K+ | 0.5100 | Mg2+ | 0.0344 | OH− | 5.66 × 10−5 |
| Main mineral content (%) | |||||||
| Quartz | 0.51 | K-Feldspar | 0.07 | Dolomite | 0.14 | Kaolinite | 0.03 |
| Initial pore radius (μm) | Initial pore throat ratio (%) | Pore throat length ratio (%) | Single pore surface density (kg/m2) | Initial formation temperature (°C) | Initial formation pressure (MPa) | Initial porosity (%) | Initial permeability (m2) |
| 100 | 5 | 15 | 50 | 50 | 28 | 18 | 2.0 × 10−14 |
| Chemical Reaction Equation | Equilibrium Constant K | Reaction Rate Constant λ |
|---|---|---|
| −3.629 | −13.90 | |
| 5.4706 | −12.00 | |
| 1.6727 | −9.222 | |
| −0.344 | −13.00 |
| Quartz | |
| Dolomite | |
| K-Feldspar | |
| Kaolinite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Yan, T.; Han, X.; Qu, J.; Feng, J. Mass Transfer Analysis of CO2-Water-Rock Geochemical Reactions in Reservoirs. Energies 2023, 16, 5862. https://doi.org/10.3390/en16165862
Xu R, Yan T, Han X, Qu J, Feng J. Mass Transfer Analysis of CO2-Water-Rock Geochemical Reactions in Reservoirs. Energies. 2023; 16(16):5862. https://doi.org/10.3390/en16165862
Chicago/Turabian StyleXu, Rui, Tie Yan, Xu Han, Jingyu Qu, and Jinyu Feng. 2023. "Mass Transfer Analysis of CO2-Water-Rock Geochemical Reactions in Reservoirs" Energies 16, no. 16: 5862. https://doi.org/10.3390/en16165862
APA StyleXu, R., Yan, T., Han, X., Qu, J., & Feng, J. (2023). Mass Transfer Analysis of CO2-Water-Rock Geochemical Reactions in Reservoirs. Energies, 16(16), 5862. https://doi.org/10.3390/en16165862






