Using the Magnetic Anisotropy Method to Determine Hydrogenated Sections of a Steel Pipeline
Abstract
1. Introduction
2. General Information
3. Effect of Hydrogenation on the Magnetic Properties of Steel
4. Materials and Methods
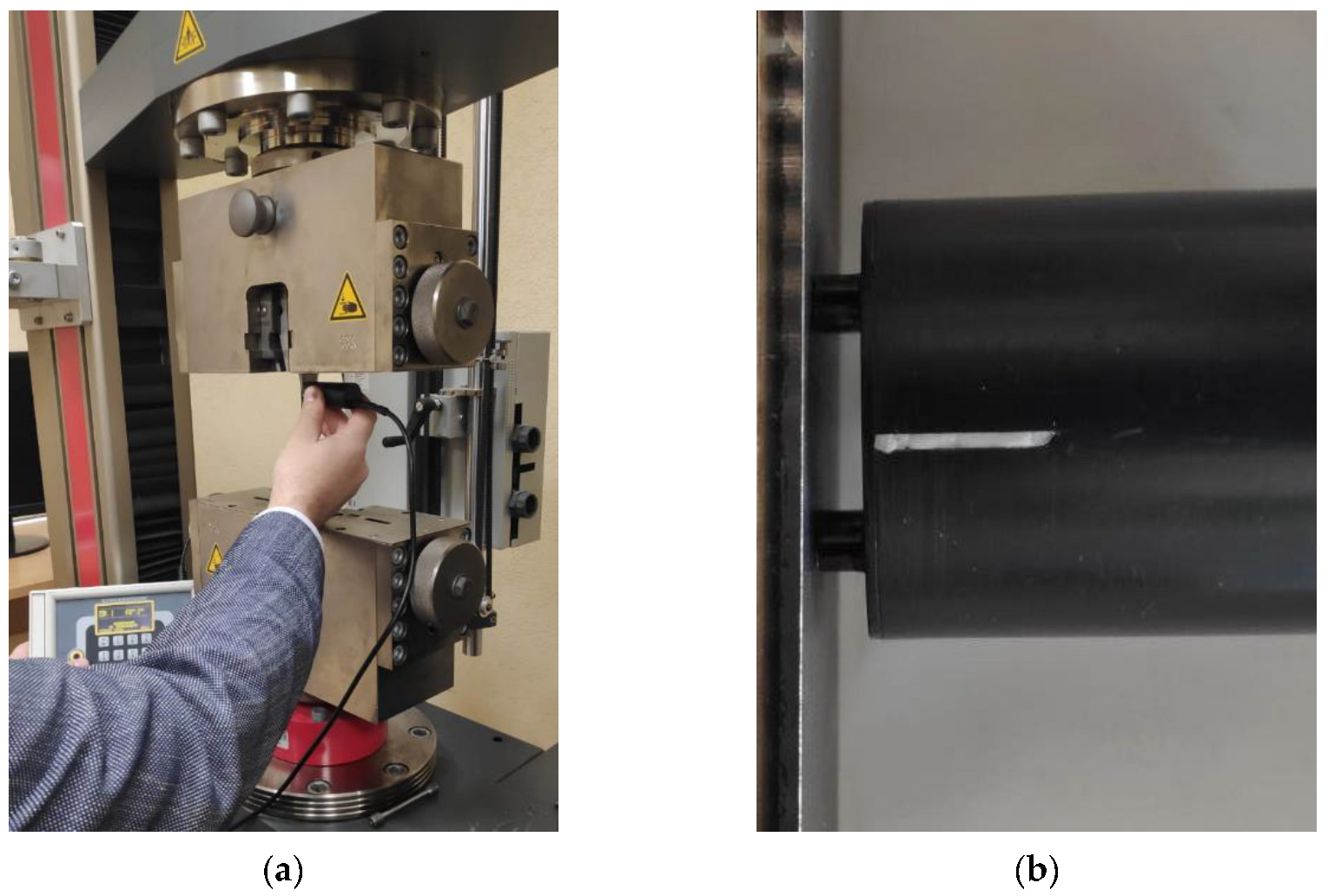
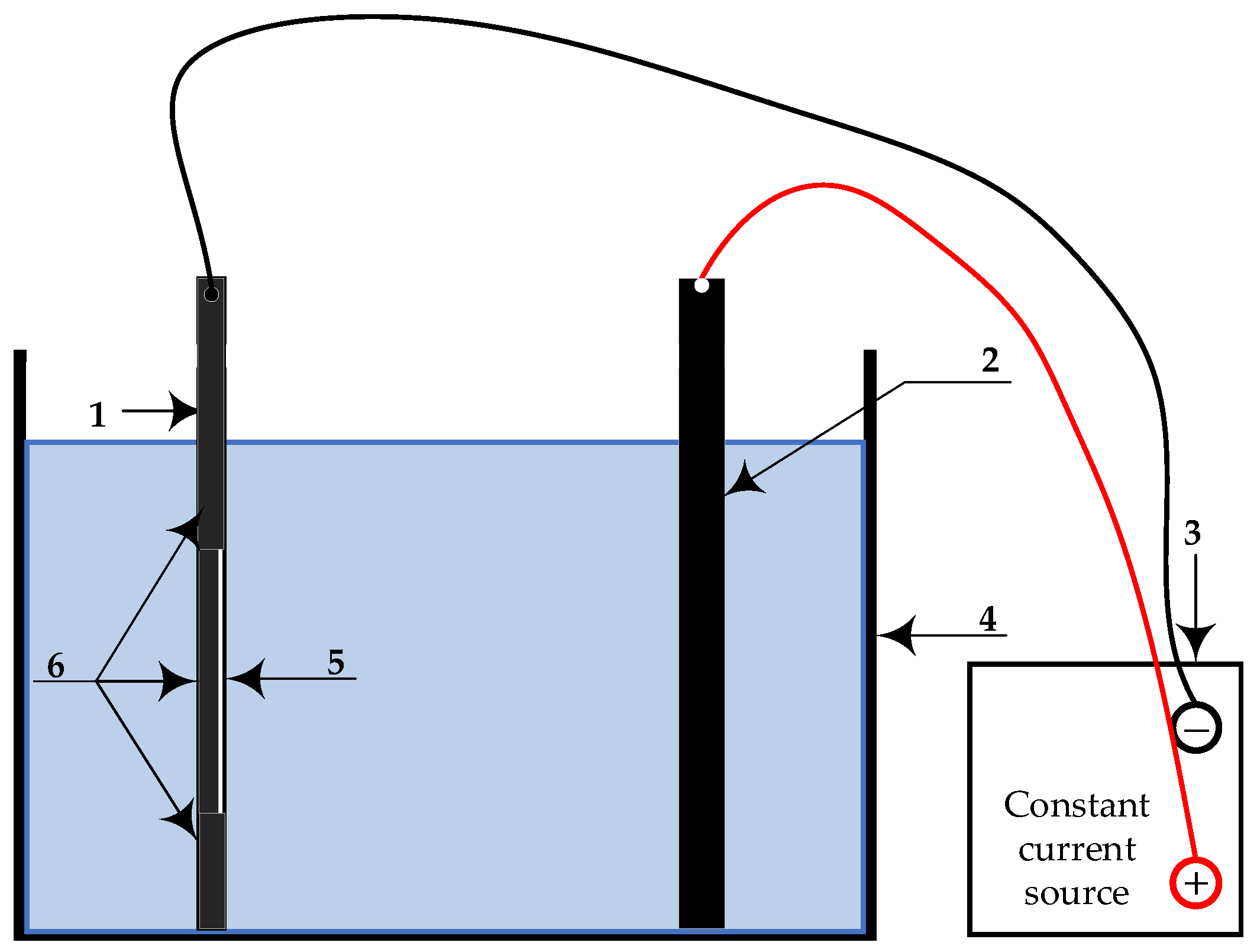
4.1. Test Preparation
4.2. Testing
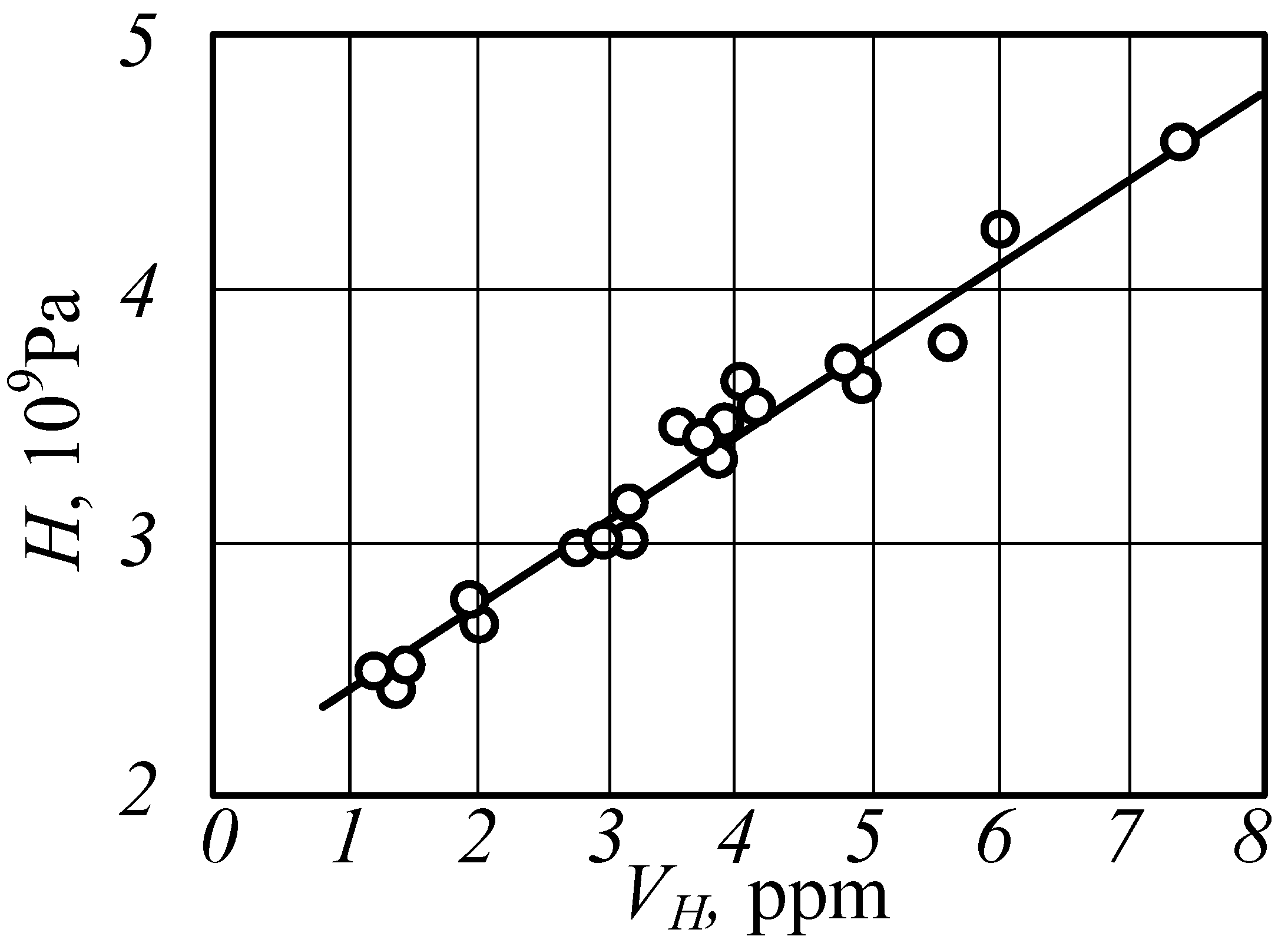
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moroz, L.S.; Chechulin, B.B. Hydrogen Brittleness of Metals; Gordon, L.M., Ed.; Metallurgy Publishing House: Moscow, Russia, 1967. [Google Scholar]
- Kolachev, S.V. Hydrogen Brittleness of Metals; Metallurgy Publishing House: Moscow, Russia, 1985. [Google Scholar]
- Litvinenko, V.S.; Tsvetkov, P.S.; Dvoynikov, M.V.; Buslaev, G.V. Barriers to Implementation of Hydrogen Initiatives in the Context of Global Energy Sustainable Development. J. Min. Inst. 2020, 244, 428–438. [Google Scholar] [CrossRef]
- Balitskii, A.I.; Ivaskevich, L.M.; Balitskii, O.A. Rotor Steels Crack Resistance and Fracture Behavior for Hydrogen Targeted Materials Ever-Widening Database. Eng. Fract. Mech. 2022, 260, 108168. [Google Scholar] [CrossRef]
- Pyshmintsev, I.Y.; Gizatullin, A.B.; Devyaterikova, N.A.; Laev, K.A.; Tsvetkov, A.S.; Al’khimenko, A.A.; Shaposhnikov, N.O.; Kurakin, M.K. Preliminary Assessment of the Possibility to Use Large-Diameter Pipes of X52 Steel for Transportation of Pure Gaseous Hydrogen under Pressure. Izv. Ferr. Metall. 2023, 66, 35–42. [Google Scholar] [CrossRef]
- Anijdan, S.H.M.; Sabzi, M.; Park, N.; Lee, U. Sour Corrosion Performance and Sensitivity to Hydrogen Induced Cracking in the X70 Pipeline Steel: Effect of Microstructural Variation and Pearlite Percentage. Int. J. Press. Vessel. Pip. 2022, 199, 104759. [Google Scholar] [CrossRef]
- Balitskii, A.I.; Dmytryk, V.V.; Ivaskevich, L.M.; Balitskii, O.A.; Glushko, A.V.; Medovar, L.B.; Abramek, K.F.; Stovpchenko, G.P.; Eliasz, J.J.; Krolikowski, M.A. Improvement of the Mechanical Characteristics, Hydrogen Crack Resistance and Durability of Turbine Rotor Steels Welded Joints. Energies 2022, 15, 6006. [Google Scholar] [CrossRef]
- Beloglazov, I.; Morenov, V.; Leusheva, E.; Gudmestad, O.T. Modeling of Heavy-Oil Flow with Regard to Their Rheological Properties. Energies 2021, 14, 359. [Google Scholar] [CrossRef]
- Silvestrov, S.A.; Gumerov, K.M. Change of Mechanical Properties of Pipe Metal in Hydrogen-Containing Media. In Proceedings of the VIII Ural Scientific and Practical Conference “Welding. Renovation. Tribotechnics”; Nizhny Tagil Institute of Technology (branch) UrFU, Nizhny Tagil, Russia, 2–3 February 2017. [Google Scholar]
- Lisin, Y.V.; Neganov, D.A.; Surikov, V.I.; Gumerov, K.M. Research of Changes in the Properties of Pipeline Metal in the Process of Operation: Generalization of the Results and Prospective Developments of the Ufa Scientific School. Sci. Technol. Oil Oil Prod. Pipeline 2017, 7, 22–30. [Google Scholar] [CrossRef]
- Silvestrov, S.A.; Gumerov, A.K. Latent Period of Stress Corrosion Cracking Development at the Main Pipelines. Probl. Gather. Treat. Transp. Oil Oil Prod. 2018, 113, 95–113. [Google Scholar] [CrossRef]
- Bolobov, V.I.; Latipov, I.U.; Zhukov, V.S.; Kasyanenko, S.V.; Nikulin, V.E.; Popov, G.G.; Sumin, E.I. Role of hydrogen in corrosion and sulfide cracking of pipelines. GAS Ind. Russ. 2023, 850, 90–99. [Google Scholar]
- Zemenkova, M.Y.; Chizhevskaya, E.L.; Zemenkov, Y.D. Intelligent Monitoring of the Condition of Hydrocarbon Pipeline Transport Facilities Using Neural Network Technologies. J. Min. Inst. 2022, 258, 933–944. [Google Scholar] [CrossRef]
- Tan, N.; Zhou, L.; Zheng, W.; Song, H.; Sun, Z.; Wang, Z.; Wang, G.; Wang, G.; Zhang, L.; Zhou, X. Using Finite Element Method for Stress-Strain Evaluation of Commonly Used Buried Pipelines in Fault. Energies 2022, 15, 1655. [Google Scholar] [CrossRef]
- Goldobina, L.A.; Orlov, P.S. Analysis of the Causes of Corrosion Damage in Underground Pipelines and New Solutions for Increasing the Corrosion Resistance of Steel. J. Min. Inst. 2016, 219, 459–464. [Google Scholar] [CrossRef]
- Aginey, R.; Firstov, A. Improving the Method for Assessment of Bending Stresses in the Wall of an Underground Pipeline. J. Min. Inst. 2022. Online first. [Google Scholar] [CrossRef]
- Baktizin, R.N.; Zaripov, R.M.; Korobkov, G.E.; Masalimov, R.B. Assessment of Internal Pressure Effect, Causing Additional Bending of the Pipeline. J. Min. Inst. 2020, 242, 160–168. [Google Scholar] [CrossRef]
- Karyakina, E.; Shammazov, I.; Voronov, V.; Shalygin, A. The Simulation of Ultra-High Molecular Weight Polyethylene Cryogenic Pipeline Stress-Strain State. Mater. Sci. Forum 2021, 1031, 132–140. [Google Scholar] [CrossRef]
- Cristescu, N.; Craciun, E.-M.; Soós, E. Mechanics of Elastic Composites; Chapman & Hall/CRC: Boca Raton, FL, USA, 2004; ISBN 1584884428. [Google Scholar]
- Shammazov, I.; Dzhemilev, E.; Sidorkin, D. Improving the Method of Replacing the Defective Sections of Main Oil and Gas Pipelines Using Laser Scanning Data. Appl. Sci. 2022, 13, 48. [Google Scholar] [CrossRef]
- Palaev, A.G.; Nosov, V.V.; Krasnikov, A.A. Simulating Distribution of Temperature Fields and Stresses in Welded Joint Using ANSYS. Sci. Technol. Oil Oil Prod. Pipeline Transp. 2022, 12, 353–361. [Google Scholar]
- Lyubchik, A.N.; Krapivsky, E.I.; Bolshunova, O.M. Forecasting the Technical Condition of Main Pipelines Based on the Analysis of Emergency Situations. J. Min. Inst. 2011, 192, 153–156. [Google Scholar]
- Sultanbekov, R.; Beloglazov, I.; Islamov, S.; Ong, M.C. Exploring of the Incompatibility of Marine Residual Fuel: A Case Study Using Machine Learning Methods. Energies 2021, 14, 8422. [Google Scholar] [CrossRef]
- Vidas, L.; Castro, R.; Pires, A. A Review of the Impact of Hydrogen Integration in Natural Gas Distribution Networks and Electric Smart Grids. Energies 2022, 15, 3160. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Schipachev, A. Manifestation of Incompatibility of Marine Residual Fuels: A Method for Determining Compatibility, Studying Composition of Fuels and Sediment. J. Min. Inst. 2022. Online first. [Google Scholar] [CrossRef]
- Shammazov, I.; Karyakina, E. The LNG Flow Simulation in Stationary Conditions through a Pipeline with Various Types of Insulating Coating. Fluids 2023, 8, 68. [Google Scholar] [CrossRef]
- Skalskyi, V.; Nazarchuk, Z.; Stankevych, O.; Klym, B. Influence of Occluded Hydrogen on Magnetoacoustic Emission of Low-Carbon Steels. Int. J. Hydrogen Energy 2023, 48, 6146–6156. [Google Scholar] [CrossRef]
- Zhukov, S.V.; Zhukov, V.S. RU 2195636 C2 Method for Determining Mechanical Stresses and Device for Its Implementation 2002. Available online: https://www.elibrary.ru/item.asp?id=37897742 (accessed on 7 July 2023).
- Zhukov, S.V.; Zhukov, V.S. RU 2079825 C1 Device for Measuring Mechanical Stresses in Metal Products 1997. Available online: https://www.elibrary.ru/item.asp?id=38062207 (accessed on 7 July 2023).
- Rajnak, M.; Wu, Z.; Dolnik, B.; Paulovicova, K.; Tothova, J.; Cimbala, R.; Kurimsky, J.; Kopcansky, P.; Sunden, B.; Wadso, L.; et al. Magnetic Field Effect on Thermal, Dielectric, and Viscous Properties of a Transformer Oil-Based Magnetic Nanofluid. Energies 2019, 12, 4532. [Google Scholar] [CrossRef]
- Evstratikova, Y.I.; Nikulin, V.E. Control of Residual Weld Stresses Using Magnetic Anisotropic Method After Applying Ultrasonic Impact Treatment. Weld. Diagn. 2019, 4, 38–42. [Google Scholar]
- Stepanov, M.A.; Stepanov, A.P. Evaluation of the Distribution of Bending Stresses and Defects Within Symmetrical Cross Sections of a Steel Beam. Mod. Technol. Syst. Anal. Model. 2019, 63, 22–31. [Google Scholar]
- Sakai, Y.; Unishi, H.; Yahata, T. Non-Destructive Method of Stress Evaluation in Linepipes Using Magnetic Anisotropy Sensor. In JFE Technical Report; JFE Steel Corporation: Tokyo, Japan, 2004; pp. 47–53. [Google Scholar]
- Korneev, A.V.; Treschev, A.A. Accounting for the Influence of a Hydrogen-Containing Environment on the Stress-Strain State of Materials Based on Titanium Alloys. News Tula State Univ. Eng. Sci. 2009, 116–123. Available online: https://cyberleninka.ru/article/n/uchet-vliyaniya-vodorodosoderzhaschey-sredy-na-napryazhenno-deformirovannoe-sostoyanie-materialov-na-osnove-titanovyh-splavov (accessed on 7 July 2023).
- Glikman, L.A.; Snezhkova, T.N. On the Occurrence of Residual Stresses at the Electrolytic Saturation of the Steel Surface with Hydrogen. J. Tech. Phys. 1952, 22, 1104–1108. [Google Scholar]
- Shchegoleva, T.A.; Glukhova, Z.L.; Vetchinov, A.V. Technique of Experimental Research of Form Change of Matellic Plates. J. Theor. Appl. Mech. 2021, 76, 25–31. [Google Scholar]
- Smyalovsky, M.; Shklyarskaya-Smyalovskaya, Z. Principle of a New Method for Investigating the Diffusion of Cathodic Hydrogen. Izv. AN SSSR 1954, 226–229. [Google Scholar]
- Khizhnyakov, V.I. On the Controlling Role of the Current Density of Cathodic Protection in the Formation of Corrosion and Stress-Corrosion Defects on the External Surface of the Main Gas and Oil Pipelines. Tomsk State Univ. J. 2013, 18, 2248–2252. [Google Scholar]
- Galaktionova, N.A. Hydrogen in Metals; Metallurgy Publishing House: Moscow, Russia, 1967. [Google Scholar]
- Schipachev, A.M.; Dmitrieva, A.S. Application of the Resonant Energy Separation Effect at Natural Gas Reduction Points in Order to Improve the Energy Efficiency of the Gas Distribution System. J. Min. Inst. 2021, 248, 253–259. [Google Scholar] [CrossRef]
- Fetisov, V.; Shalygin, A.V.; Modestova, S.A.; Tyan, V.K.; Shao, C. Development of a Numerical Method for Calculating a Gas Supply System during a Period of Change in Thermal Loads. Energies 2022, 16, 60. [Google Scholar] [CrossRef]
- Karpenko, G.V.; Kripyakevich, R.I. Effect of Hydrogen on the Properties of Steel; Metallurgizdat: Moscow, Russia, 1962. [Google Scholar]
- Shevchenko, G.I. Magnetic Anisotropic Sensors; Energy: Moscow, Russia, 1967. [Google Scholar]
- Capelle, J.; Gilgert, J.; Dmytrakh, I.; Pluvinage, G. Sensitivity of Pipelines with Steel API X52 to Hydrogen Embrittlement. Int. J. Hydrogen Energy 2008, 33, 7630–7641. [Google Scholar] [CrossRef]
- Li, H.L.; Gao, K.W.; Qiao, L.J.; Wang, Y.B.; Chu, W.Y. Strength Effect in Stress Corrosion Cracking of High-Strength Steel in Aqueous Solution. Corrosion 2001, 57, 295–299. [Google Scholar] [CrossRef]
- Dey, S.; Mandhyan, A.K.; Sondhi, S.K.; Chattoraj, I. Hydrogen Entry into Pipeline Steel under Freely Corroding Conditions in Two Corroding Media. Corros. Sci. 2006, 48, 2676–2688. [Google Scholar] [CrossRef]
- Latipov, I.U.; Sherstneva, A.O.; Popov, G.G.; Bolobov, V.I. Analysis of Existing Techniques for Hydrogenation and Testing of Steel Specimens for the Effect of Hydrogen. GAS Ind. Russ. 2022, 836, 36–43. [Google Scholar]
- Karpenko, G.V.; Kripyakevich, R.I. Influence of Steel Polarization on Its Mechanical Properties. Rep. Acad. Sci. USSR 1958, 120, 827–829. [Google Scholar]
- Irzhov, G.G.; Golovanenko, S.A.; Sergeeva, T.K. Resistance to Hydrogen Embrittlement of Steels for Main Pipelines. Physicochem. Mech. Mater. 1982, 4, 89–93. [Google Scholar] [CrossRef]
- Efimenko, E.A.; Sidorenko, A.V.; Ivanova, Y.S.; Sarafanova, Y.A. Evaluation of the Hydrogen Embrittlement Degree for Low-Carbon Steels. ISJAEE 2003, 9, 22–25. [Google Scholar]
- Li, X. Hydrogen Effects on X80 Steel Mechanical Properties Measured by Tensile and Impact Testing; University of South Florida: Tampa, FL, USA, 2016. [Google Scholar]
- Hardie, D.; Charles, E.A.; Lopez, A.H. Hydrogen Embrittlement of High Strength Pipeline Steels. Corros. Sci. 2006, 48, 4378–4385. [Google Scholar] [CrossRef]
- Newman, J.F.; Shreir, L.L. Role of Hydrides in Hydrogen Entry into Steel from Solutions Containing Promoters. Corros. Sci. 1969, 9, 631–641. [Google Scholar] [CrossRef]
- Lunarska, E.; Ososkov, Y.; Jagodzinsky, Y. Correlation between Critical Hydrogen Concentration and Hydrogen Damage of Pipeline Steel. Int. J. Hydrogen Energy 1997, 22, 279–284. [Google Scholar] [CrossRef]
- Morozov, A.N. Hydrogen and Nitrogen in Steel, 2nd ed.; Metallurgy: Moscow, Russia, 1968. [Google Scholar]
- Sahakyan, L.S.; Efremov, A.P.; Soboleva, I.A. Protection Oilfield Equipment from Corrosion: A Workers’ Handbook; Nedra: Moscow, Russia, 1985. [Google Scholar]
- Cheng, Y.F. Analysis of Electrochemical Hydrogen Permeation through X-65 Pipeline Steel and Its Implications on Pipeline Stress Corrosion Cracking. Int. J. Hydrogen Energy 2007, 32, 1269–1276. [Google Scholar] [CrossRef]
- Beloglazov, S.V. Hydrogenation of Steel in Electrochemical Processes; Leningrad University Press: Leningrad, Russia, 1975. [Google Scholar]
- Djukic, M.B.; Bakic, G.M.; Sijacki Zeravcic, V.; Sedmak, A.; Rajicic, B. The Synergistic Action and Interplay of Hydrogen Embrittlement Mechanisms in Steels and Iron: Localized Plasticity and Decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Zhang, T.; Chu, W.Y.; Gao, K.W.; Qiao, L.J. Study of Correlation between Hydrogen-Induced Stress and Hydrogen Embrittlement. Mater. Sci. Eng. A 2003, 347, 291–299. [Google Scholar] [CrossRef]


| No. | Deformation, 10−3 m, after | The Amount of Released Hydrogen, 10−5 m3/kg, as a Result of Vacuum Annealing at a Temperature, °K | ||||||
|---|---|---|---|---|---|---|---|---|
| Saturation with Hydrogen | Hydrogen Saturation and Vacuum Annealing at Temperature, °K | |||||||
| 300 s | in a Day | 323 | 473 | 923 | 323 | 473 | 923 | |
| 1 | 0.46 | 0.47 | 0.44 | 0.44 | 0.49 | 41 | 18.4 | - |
| 2 | 0.88 | 0.90 | 0.87 | 0.87 | 0.97 | 117 | 1.8 | 3.5 |
| 3 | 1.2 | 1.2 | 1.17 | 1.2 | 1.34 | 137 | - | 29.1 |
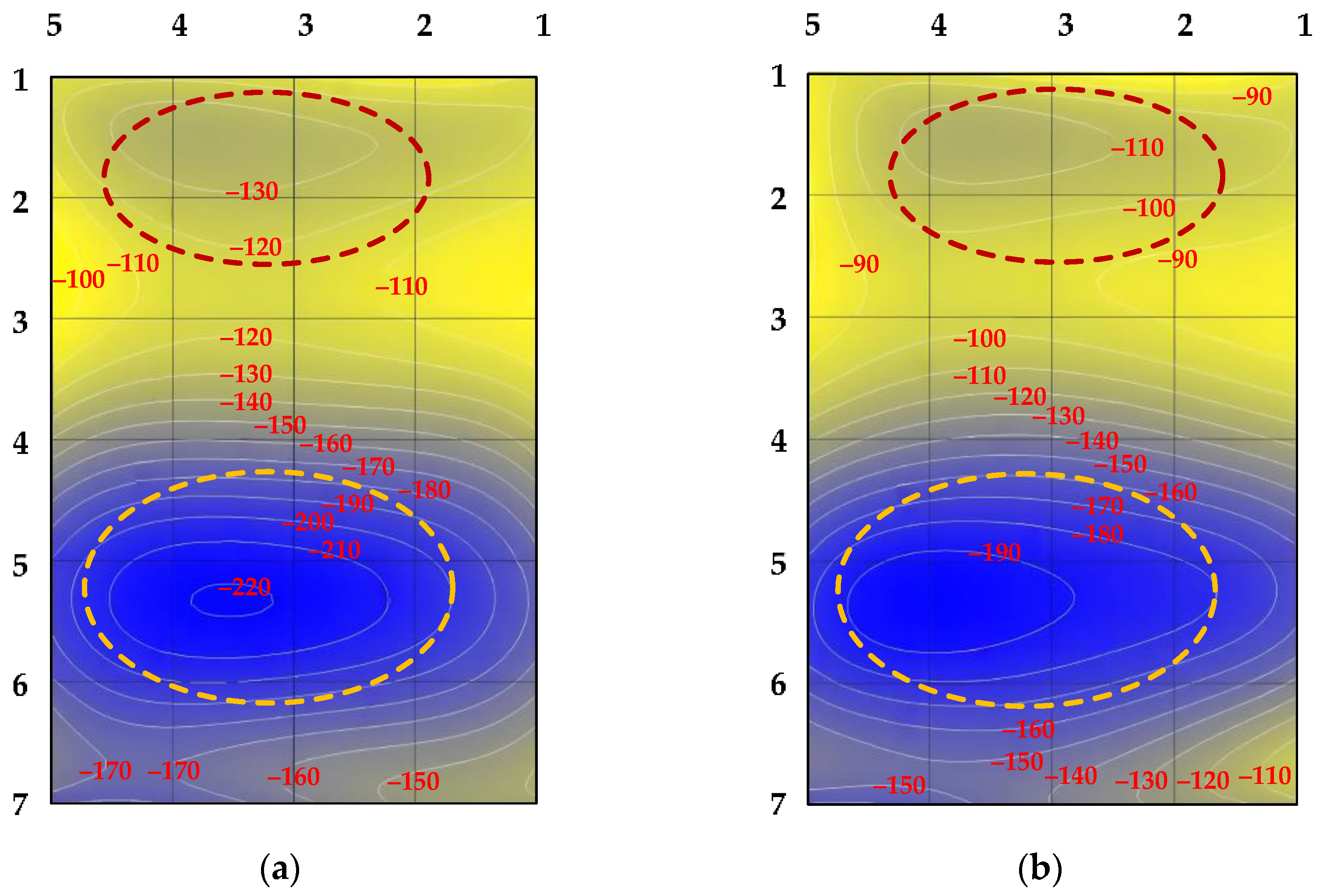
| Specimen No. | j, A/m2 | t, min | The Value of the DPMS at Three Points of the Specimens | |||
|---|---|---|---|---|---|---|
| Original (DPMS0) | Hydrogenated | Δ | Δav | |||
| 1 | 500 | 30 | −273 | −250 | 23 | 24 |
| −275 | −246 | 29 | ||||
| −291 | −270 | 21 | ||||
| 2 | 90 | −98 | −67 | 31 | 29 | |
| −127 | −100 | 27 | ||||
| −132 | −103 | 29 | ||||
| 3 | 90 | −226 | −206 | 20 | 26 | |
| −251 | −220 | 31 | ||||
| −267 | −240 | 27 | ||||
| 4 | 223 | −162 | −140 | 32 | 27 | |
| −198 | −175 | 23 | ||||
| −214 | −188 | 26 | ||||
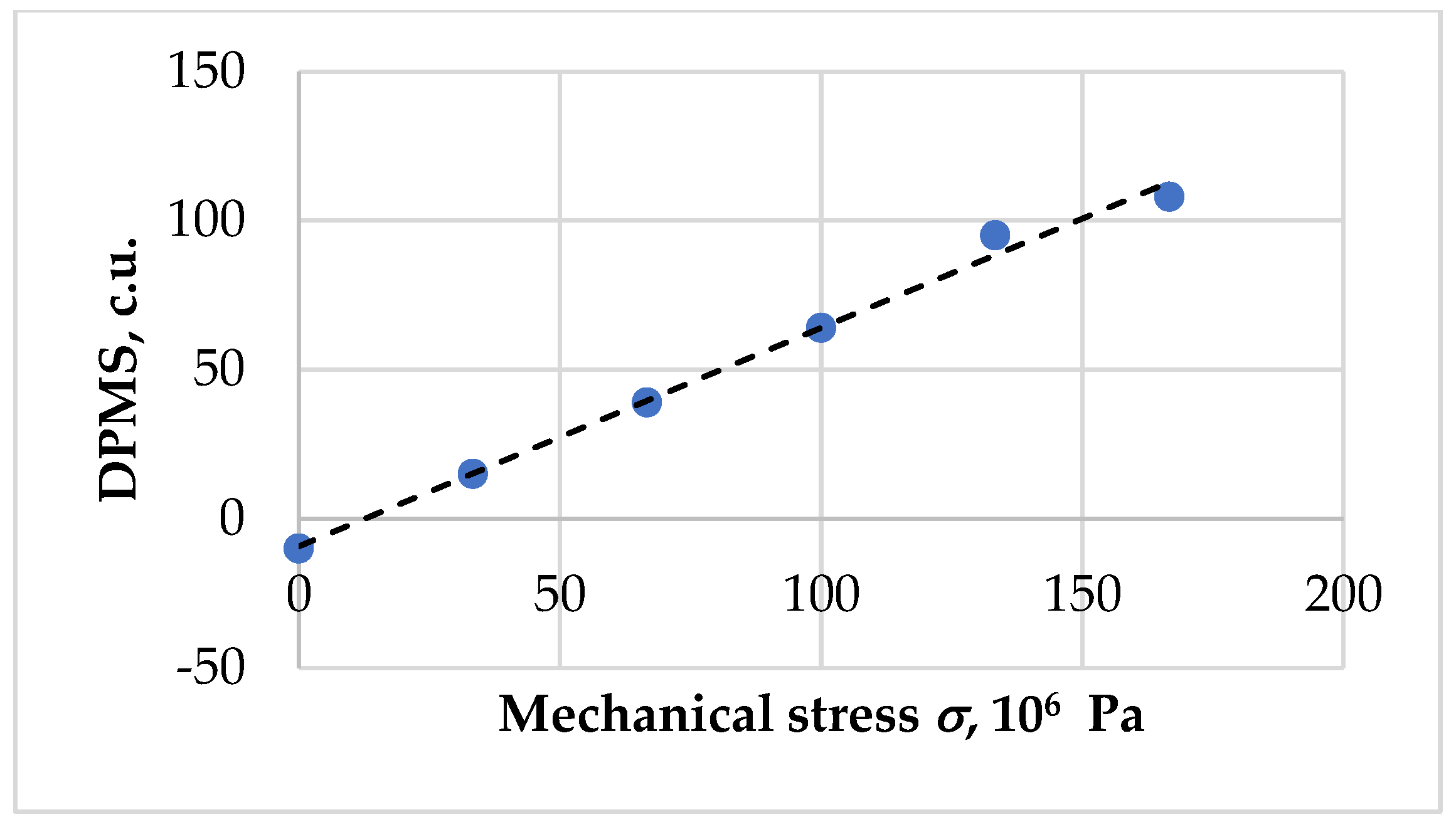
| Δav1 = 26.5 σ1 = 37 × 106 Pa | ||||||
| 5 | 1360 | 15 | −118 | −84 | 34 | 33 |
| −131 | −85 | 46 | ||||
| −131 | −113 | 18 | ||||
| 6 | 45 | −118 | −74 | 44 | 35 | |
| −131 | −106 | 25 | ||||
| −131 | −96 | 35 | ||||
| 7 | 180 | −231 | −201 | 30 | 31 | |
| −299 | −262 | 37 | ||||
| −299 | −272 | 27 | ||||
| Δav2 = 33 σ2 = 46 × 106 Pa | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolobov, V.I.; Latipov, I.U.; Zhukov, V.S.; Popov, G.G. Using the Magnetic Anisotropy Method to Determine Hydrogenated Sections of a Steel Pipeline. Energies 2023, 16, 5585. https://doi.org/10.3390/en16155585
Bolobov VI, Latipov IU, Zhukov VS, Popov GG. Using the Magnetic Anisotropy Method to Determine Hydrogenated Sections of a Steel Pipeline. Energies. 2023; 16(15):5585. https://doi.org/10.3390/en16155585
Chicago/Turabian StyleBolobov, Victor I., Il’nur U. Latipov, Valentin S. Zhukov, and Gregory G. Popov. 2023. "Using the Magnetic Anisotropy Method to Determine Hydrogenated Sections of a Steel Pipeline" Energies 16, no. 15: 5585. https://doi.org/10.3390/en16155585
APA StyleBolobov, V. I., Latipov, I. U., Zhukov, V. S., & Popov, G. G. (2023). Using the Magnetic Anisotropy Method to Determine Hydrogenated Sections of a Steel Pipeline. Energies, 16(15), 5585. https://doi.org/10.3390/en16155585






