Eco-Friendly Drilling Fluid: Calcium Chloride-Based Natural Deep Eutectic Solvent (NADES) as an All-Rounder Additive
Abstract
1. Introduction
2. Materials and Methods
2.1. In-House Preparation of Calcium–Glycerine (CC:Gly) NADES Molar Ratios
Screening Criteria for Selecting HBD and HBA for NADES
2.2. Drilling Mud Preparation
Drilling Mud Properties
2.3. Shale Stabilization Studies
2.3.1. Bentonite Wafer Preparation
2.3.2. Linear Swell Meter
2.3.3. Shale Recovery Test
2.4. Hydrate Inhibition Study
2.5. Characterization Techniques
2.5.1. Surface Tension
2.5.2. D-Spacing
2.5.3. Zeta Potential Measurement
3. Results and Discussion
3.1. In-House Preparation of NADES
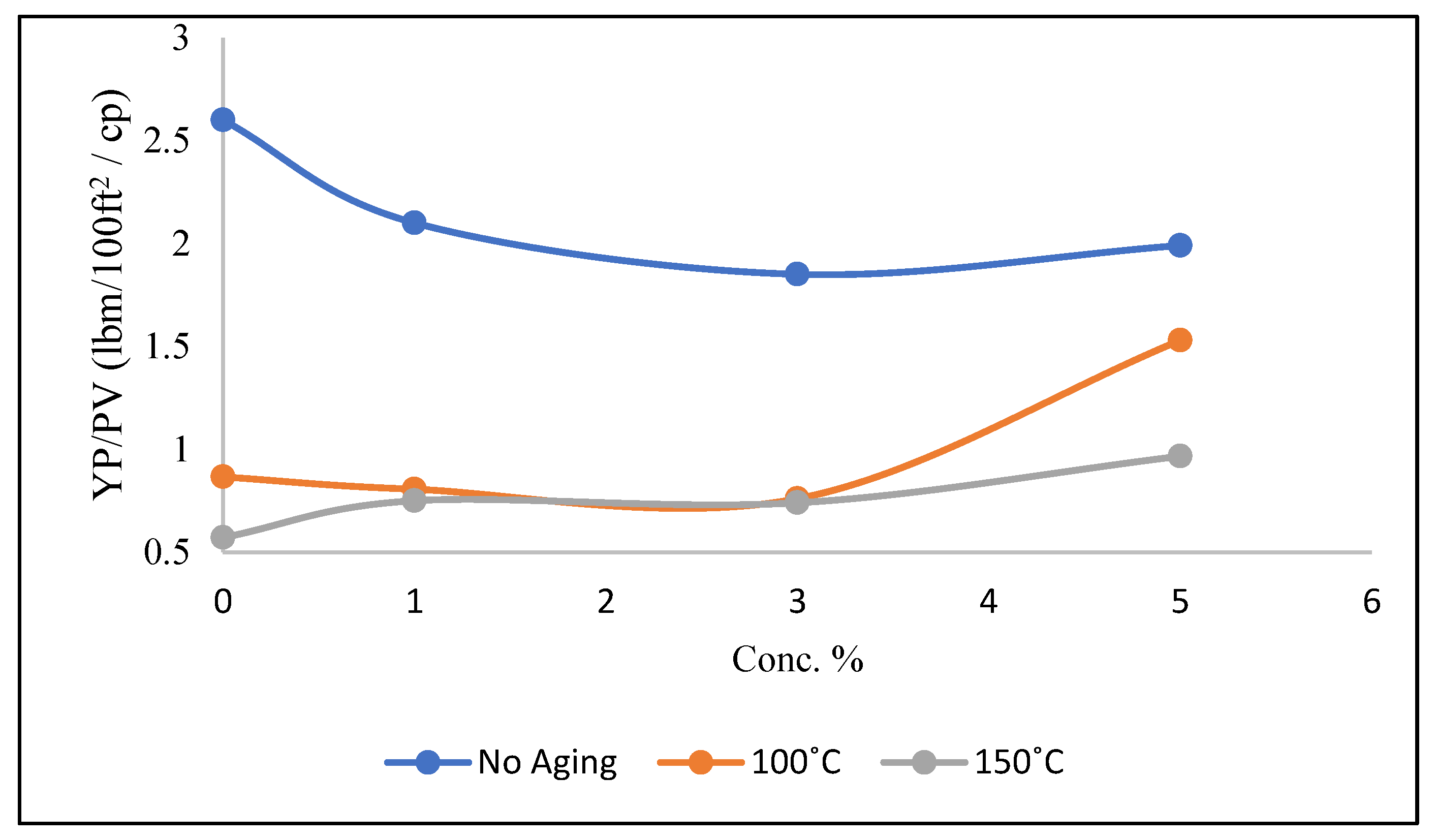
3.2. YP/PV of Mud Samples
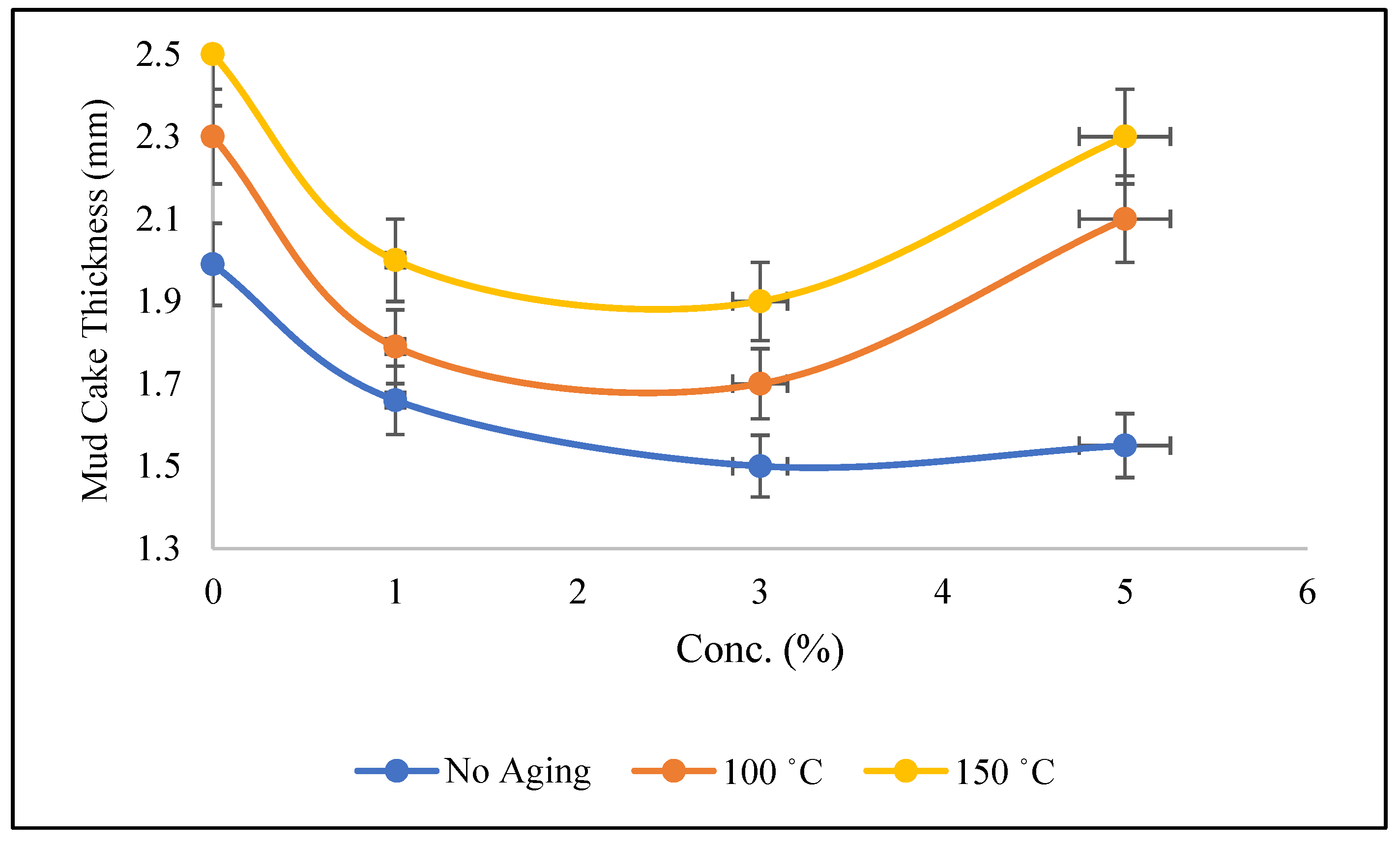
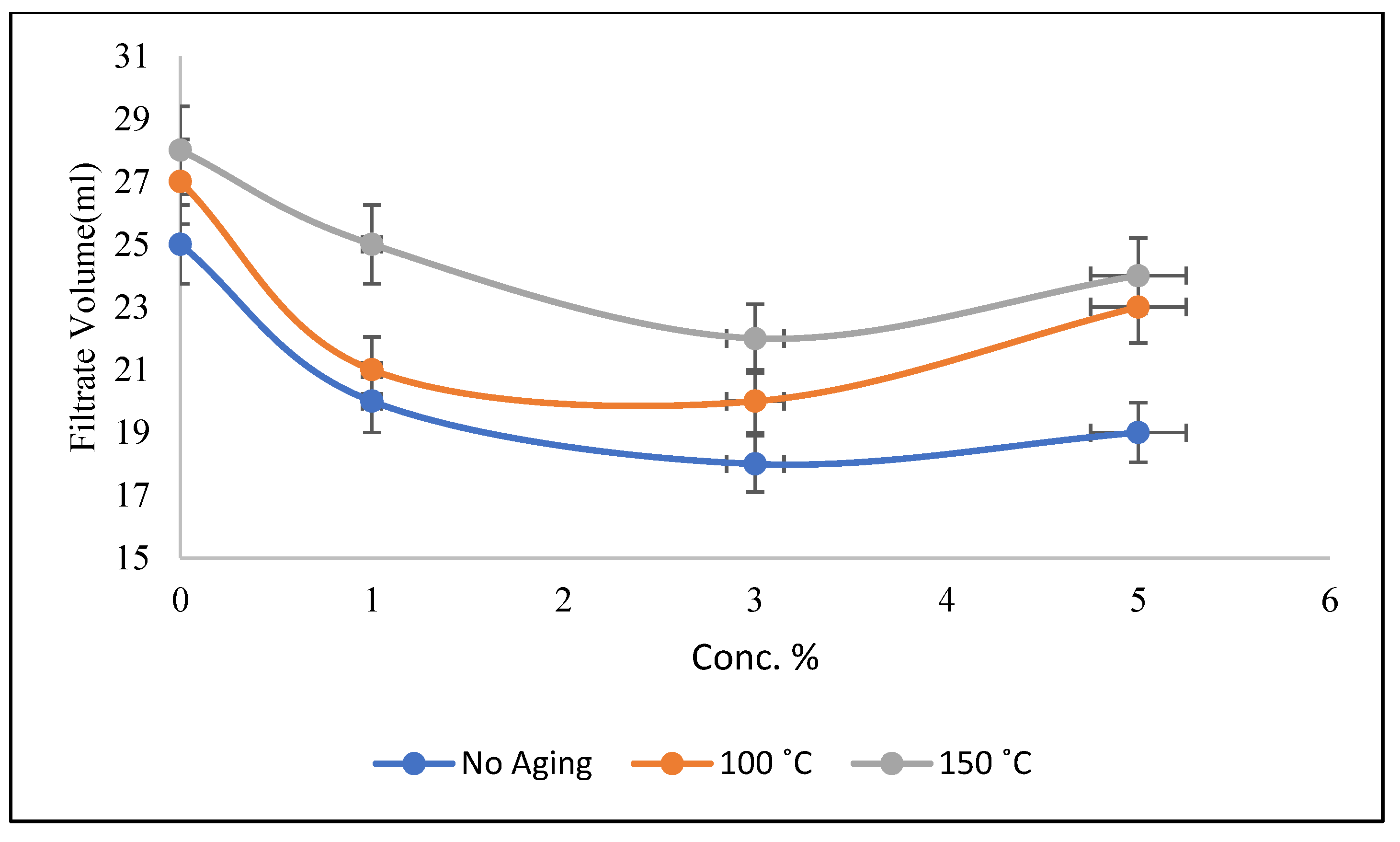
3.3. Filtration Properties of Mud
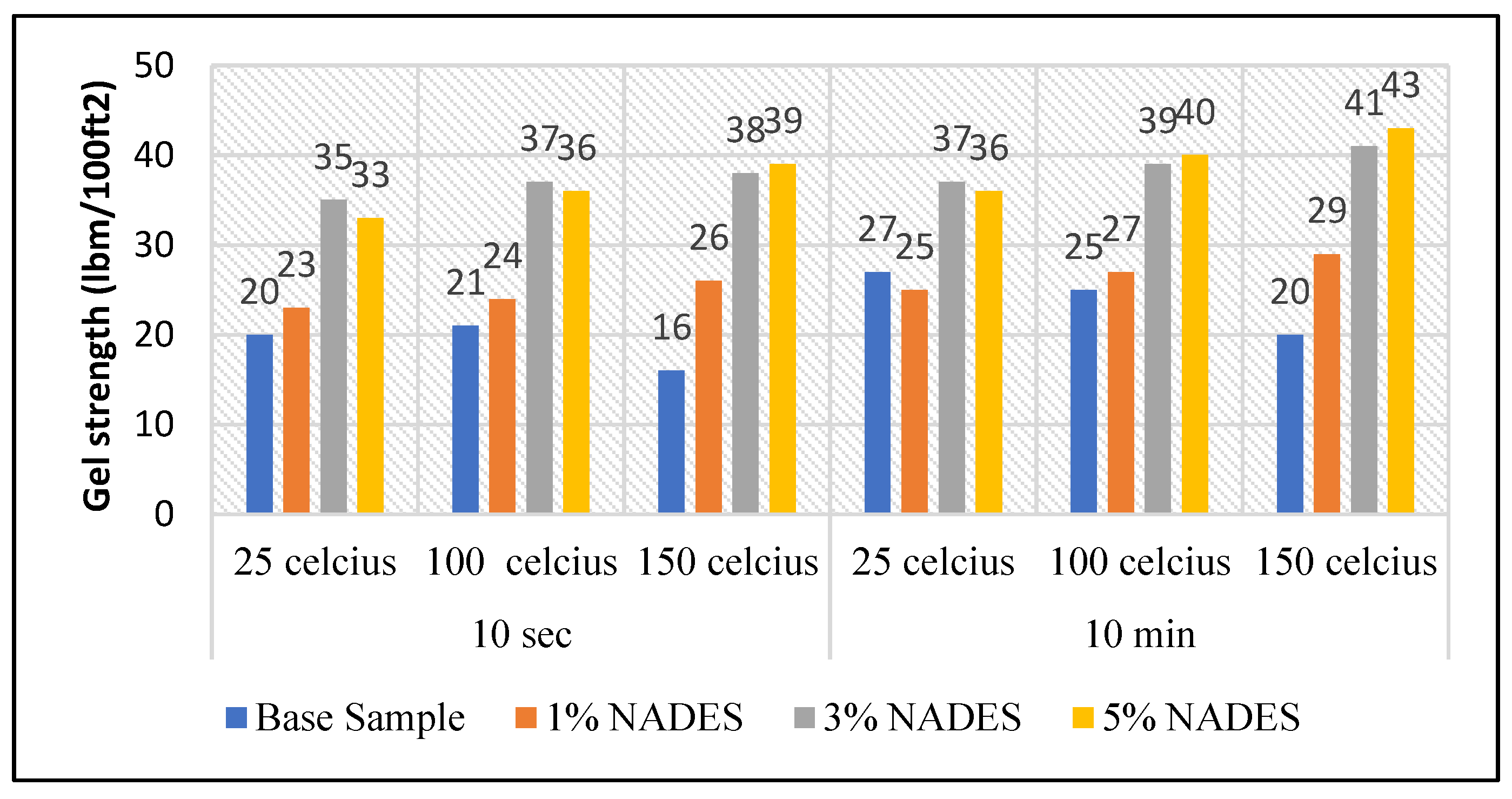
3.4. Gel Strength
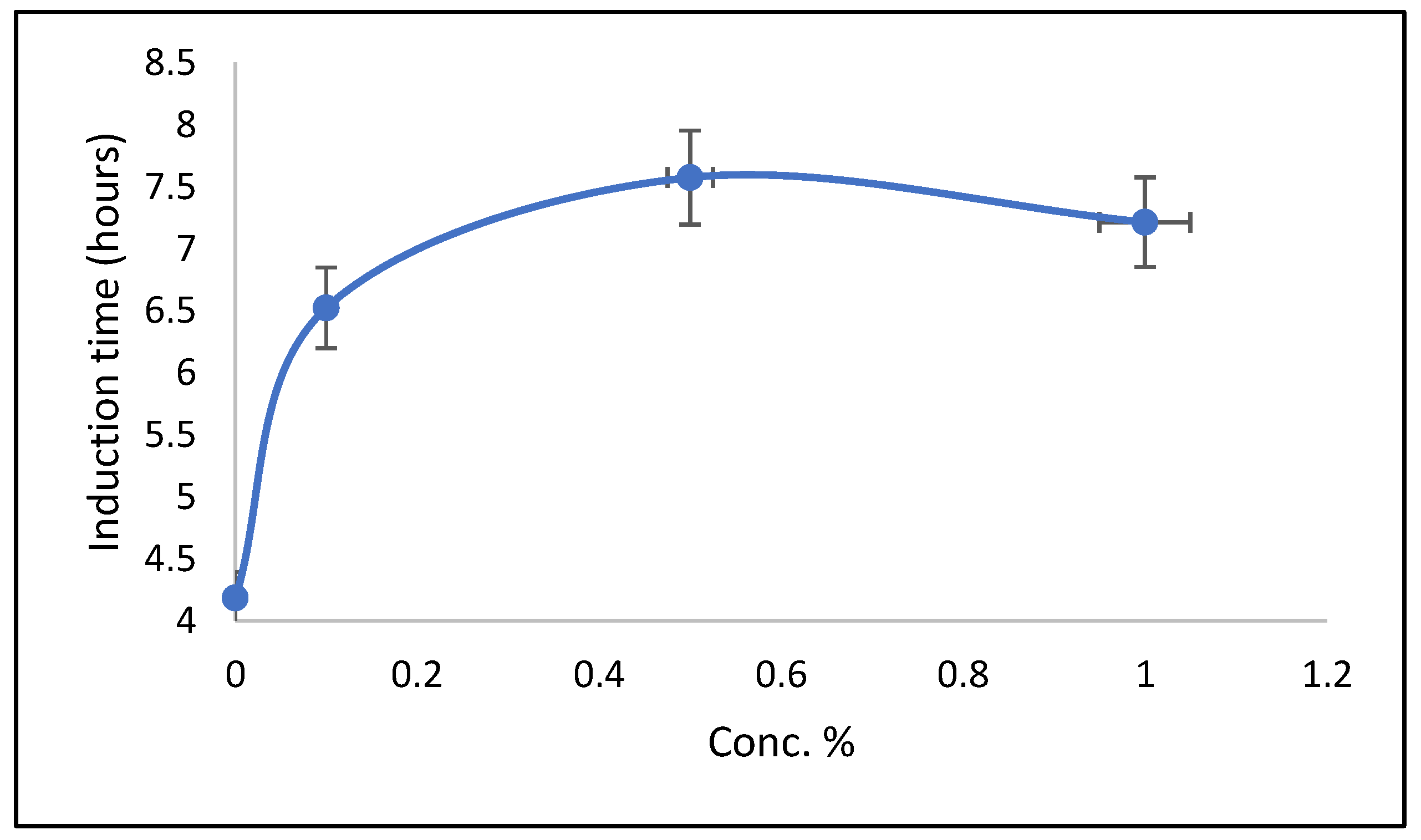
3.5. Induction Time of Hydrate Formation
3.6. Linear Swelling and Shale Recovery Tests (Dispersion Test)
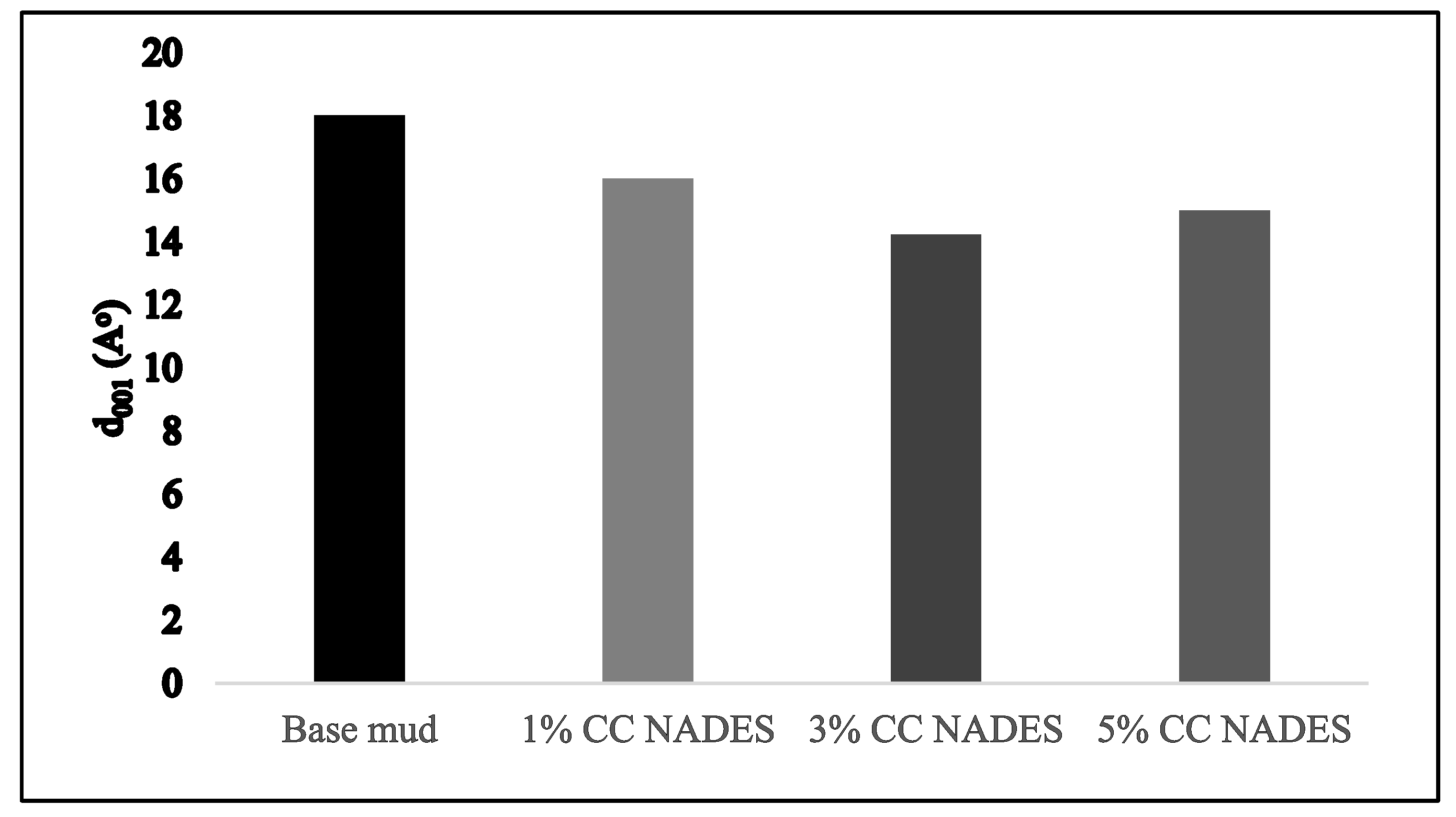
3.7. D-Spacing of Inhibitor-Based Mud
3.8. Surface Tension
3.9. Zeta Potential
3.10. Discussion on Underlying Mechanism
4. Conclusions
- Propelling Forward: It is recommended to conduct a detailed assessment for the comparison and compatibility of the combined effects of NADES and conventional additives on key drilling mud properties such as fluid loss control, shale inhibition, lubricity, rheological behavior, and thermal stability. This evaluation will help quantify the synergistic benefits and optimize the formulation for improved drilling performance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agwu, O.E.; Akpabio, J.U.; Ekpenyong, M.E.; Inyang, U.G.; Asuquo, D.E.; Eyoh, I.J.; Adeoye, O.S. A critical review of drilling mud rheological models. J. Pet. Sci. Eng. 2021, 203, 108659. [Google Scholar] [CrossRef]
- Zhang, J.R.; Xu, M.D.; Christidis, G.E.; Zhou, C.H. Clay minerals in drilling fluids: Functions and challenges. Clay Miner. 2020, 55, 1–11. [Google Scholar] [CrossRef]
- Wastu, A.; Hamid, A.; Samsol, S. The effect of drilling mud on hole cleaning in oil and gas industry. J. Phys. Conf. Ser. 2019, 1402, 022054. [Google Scholar] [CrossRef]
- Rana, A.; Murtaza, M.; Saleh, T.A.; Kamal, M.S.; Mahmoud, M. An efficient, cost-effective, and green natural extract in water-based drilling muds for clay swelling inhibition. J. Pet. Sci. Eng. 2022, 214, 110332. [Google Scholar] [CrossRef]
- Hassan, A.; Murtaza, M.; Alade, O.; Tariq, Z.; Kamal, M.S.; Mahmoud, M. Chapter 3—Interactions of drilling and completion fluids during drilling and completion operations. In Developments in Petroleum Science; Hussein, I.A., Mahmoud, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 78, pp. 41–74. [Google Scholar]
- Zamir, A.; Elraies, K.A.; Rasool, M.H.; Ahmad, M.; Ayoub, M.; Abbas, M.A.; Ali, I. Influence of alkyl chain length in ionic liquid based drilling mud for rheology modification: A review. J. Pet. Explor. Prod. Technol. 2021, 12, 485–492. [Google Scholar] [CrossRef]
- Rahman, M.T.; Negash, B.M.; Danso, D.K.; Idris, A.; Elryes, A.A.; Umar, I.A. Effects of imidazolium-and ammonium-based ionic liquids on clay swelling: Experimental and simulation approach. J. Pet. Explor. Prod. Technol. 2021, 12, 1841–1853. [Google Scholar] [CrossRef]
- Khan, R.A.; Murtaza, M.; Ahmad, H.M.; Abdulraheem, A.; Kamal, M.S.; Mahmoud, M. Development of Novel Shale Swelling Inhibitors Using Hydrophobic Ionic Liquids and Gemini Surfactants for Water-Based Drilling Fluids. In SPE Middle East Oil & Gas Show and Conference; OnePetro: Richardson, TX, USA, 2021. [Google Scholar]
- Khan, R.A.; Murtaza, M.; Abdulraheem, A.; Kamal, M.S.; Mahmoud, M. Imidazolium-Based Ionic Liquids as Clay Swelling Inhibitors: Mechanism, Performance Evaluation, and Effect of Different Anions. ACS Omega 2020, 5, 26682–26696. [Google Scholar] [CrossRef]
- Huang, P.; Jia, H.; Han, Y.; Wang, Q.; Wei, X.; Luo, Q.; Dai, J.; Song, J.; Yan, H.; Liu, D. Designing novel high-performance shale inhibitors by optimizing the spacer length of imidazolium-based bola-form ionic liquids. Energy Fuels 2020, 34, 5838–5845. [Google Scholar] [CrossRef]
- Yang, L.; Yang, X.; Wang, T.; Jiang, G.; Luckham, P.F.; Li, X.; Shi, H.; Luo, J. Effect of alkyl chain length on shale hydration inhibitive performance of vinylimidazolium-based ionic liquids. Ind. Eng. Chem. Res. 2019, 58, 8565–8577. [Google Scholar] [CrossRef]
- Ofei, T.N.; Bavoh, C.B.; Rashidi, A.B. Insight into ionic liquid as potential drilling mud additive for high temperature wells. J. Mol. Liq. 2017, 242, 931–939. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, G.; Shi, Y.; Yang, X. Application of ionic liquid and polymeric ionic liquid as shale hydration inhibitors. Energy Fuels 2017, 31, 4308–4317. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, L.; Yu, P.; Chen, Z. Experimental study on the application of an ionic liquid as a shale inhibitor and inhibitive mechanism. Appl. Clay Sci. 2017, 150, 267–274. [Google Scholar] [CrossRef]
- Jia, H.; Huang, P.; Wang, Q.; Han, Y.; Wang, S.; Zhang, F.; Pan, W.; Lv, K. Investigation of inhibition mechanism of three deep eutectic solvents as potential shale inhibitors in water-based drilling fluids. Fuel 2019, 244, 403–411. [Google Scholar] [CrossRef]
- Rasool, M.H.; Zamir, A.; Elraies, K.A.; Ahmad, M.; Ayoub, M.; Abbas, M.A.; Ali, I. Rheological characterization of potassium carbonate deep eutectic solvent (DES) based drilling mud. J. Pet. Explor. Prod. Technol. 2021, 12, 1785–1795. [Google Scholar] [CrossRef]
- Rasool, M.H.; Zamir, A.; Elraies, K.A.; Ahmad, M.; Ayoub, M.; Abbas, M.A. Potassium carbonate based deep eutectic solvent (DES) as a potential drilling fluid additive in deep water drilling applications. Pet. Sci. Technol. 2021, 39, 612–631. [Google Scholar] [CrossRef]
- Rasool, M.H.; Ahmad, M.; Abbas, M.A. A Double Action PD (Polymer-Deep Eutectic Solvent) Based Shale Inhibitor in Drilling Mud. J. Adv. Res. Fluid Mech. Therm. Sci. 2022, 99, 149–157. [Google Scholar] [CrossRef]
- Ghandi, K. A review of ionic liquids, their limits and applications. Green Sustain. Chem. 2014, 27, 2014. [Google Scholar] [CrossRef]
- Shamsuri, A.A. Ionic liquids: Preparations and limitations. Makara J. Sci. 2011, 14, 101–106. [Google Scholar] [CrossRef]
- Maciel, V.G.; Wales, D.J.; Seferin, M.; Ugaya, C.M.L.; Sans, V. State-of-the-art and limitations in the life cycle assessment of ionic liquids. J. Clean. Prod. 2019, 217, 844–858. [Google Scholar] [CrossRef]
- Torregrosa-Crespo, J.; Marset, X.; Guillena, G.; Ramon, D.J.; Martínez-Espinosa, R.M. New guidelines for testing “Deep eutectic solvents” toxicity and their effects on the environment and living beings. Sci. Total Environ. 2020, 704, 135382. [Google Scholar] [CrossRef]
- Elencovan, V.; Joseph, J.; Yahaya, N.; Samad, N.A.; Raoov, M.; Lim, V.; Zain, N.N.M. Exploring a novel deep eutectic solvents combined with vortex assisted dispersive liquid–liquid microextraction and its toxicity for organophosphorus pesticides analysis from honey and fruit samples. Food Chem. 2022, 368, 130835. [Google Scholar] [CrossRef]
- Rasool, M.H.; Ahmad, M.; Ayoub, M. Selecting Geological Formations for CO2 Storage: A Comparative Rating System. Sustainability 2023, 15, 6599. [Google Scholar] [CrossRef]
- Al-Risheq, D.I.; Shaikh, S.M.; Nasser, M.S.; Almomani, F.; Hussein, I.A.; Hassan, M.K. Influence of combined natural deep eutectic solvent and polyacrylamide on the flocculation and rheological behaviors of bentonite dispersion. Sep. Purif. Technol. 2022, 293, 121109. [Google Scholar] [CrossRef]
- Al-Risheq, D.I.; Nasser, M.; Qiblawey, H.; Hussein, I.A.; Benamor, A. Choline chloride based natural deep eutectic solvent for destabilization and separation of stable colloidal dispersions. Sep. Purif. Technol. 2021, 255, 117737. [Google Scholar] [CrossRef]
- Rasool, M.H.; Zamir, A.; Elraies, K.A.; Ahmad, M.; Ayoub, M.; Abbas, M.A. A Deep Eutectic Solvent based novel drilling mud with modified rheology for hydrates inhibition in deep water drilling. J. Pet. Sci. Eng. 2022, 211, 110151. [Google Scholar] [CrossRef]
- Rasool, M.H.; Zamir, A.; Elraies, K.A.; Ahmad, M.; Ayoub, M.; Abbas, M.A. Investigative review on cutting transportation ability of ionic liquid-based drilling mud. J. Hunan Univ. Nat. Sci. 2021, 48, 2. [Google Scholar]
- Rasool, M.H.; Ahmad, M.; Ayoub, M.; Abbas, M.A. A Novel Ascorbic Acid Based Natural Deep Eutectic Solvent as a Drilling Mud Additive for Shale Stabilization. Processes 2023, 11, 1135. [Google Scholar] [CrossRef]
- Murtaza, M.; Ahmad, H.M.; Zhou, X.; Al-Shehri, D.; Mahmoud, M.; Kamal, M.S. Okra mucilage as environment friendly and non-toxic shale swelling inhibitor in water based drilling fluids. Fuel 2022, 320, 123868. [Google Scholar] [CrossRef]
- Rasool, M.H.; Ahmad, M.; Ayoub, M.; Zamir, A.; Abbas, M.A. A review of the usage of deep eutectic solvents as shale inhibitors in drilling mud. J. Mol. Liq. 2022, 119673, 2022. [Google Scholar]
- API RP 13B-1; Field Testing Water-based Drilling Fluids. American Petroleum Institute (API): Washington, DC, USA, 2003.
- Rasool, M.H.; Ahmad, M. Synthesis and physico-chemical characterization of novel Epsom salt based natural deep Eutectic solvent. Chem. Data Collect. 2023, 44, 101004. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Charact. Nanoparticles Intend. Drug Deliv. 2011, 697, 63–70. [Google Scholar]
- Ahmad, H.M.; Murtaza, M.; Gbadamosi, A.; Kamal, M.S.; Hussain, S.M.S.; Mahmoud, M.; Patil, S. Application of Novel Magnetic Surfactant-Based Drilling Fluids for Clay Swelling Inhibition. Energy Fuels 2023, 37, 8212–8223. [Google Scholar] [CrossRef]
- İşçi, S.; Ünlü, C.H.; Atici, O.; Güngör, N. Rheology and structure of aqueous bentonite-polyvinyl alcohol dispersions. Bull. Mater. Sci. 2006, 29, 449–456. [Google Scholar] [CrossRef]
- Farhadian, A.; Zhao, Y.; Naeiji, P.; Rahimi, A.; Berisha, A.; Zhang, L.; Rizi, Z.T.; Iravani, D.; Zhao, J. Simultaneous inhibition of natural gas hydrate formation and CO2/H2S corrosion for flow assurance inside the oil and gas pipelines. Energy 2023, 269, 126797. [Google Scholar] [CrossRef]
- Lee, D.; Go, W.; Oh, J.; Lee, J.; Jo, I.; Kim, K.-S.; Seo, Y. Thermodynamic inhibition effects of an ionic liquid (choline chloride), a naturally derived substance (urea), and their mixture (deep eutectic solvent) on CH4 hydrates. Chem. Eng. J. 2020, 399, 125830. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Saleh, T.A. Partially aminated acrylic acid grafted activated carbon as inexpensive shale hydration inhibitor. Carbohydr. Res. 2020, 491, 107960. [Google Scholar] [CrossRef]
- Ding, L.; Shi, B.; Liu, Y.; Song, S.; Wang, W.; Wu, H.; Gong, J. Rheology of natural gas hydrate slurry: Effect of hydrate agglomeration and deposition. Fuel 2019, 239, 126–137. [Google Scholar] [CrossRef]
- Rasool, M.H.; Ahmad, M. Understanding Shale Instability through the Lens of Clay Mineralogy and Zeta Potential. Geol. Earth Mar. Sci. 2023, 5, 1–10. [Google Scholar] [CrossRef]




| Mineral | Percentage |
|---|---|
| Quartz | 29% |
| Hematite | 4.7% |
| Carbonates | 9% |
| Smectite | 47% |
| Hatrurite | 4% |
| Sodium Oxide | 1.83% |
| Iron Silicates | 2.17% |
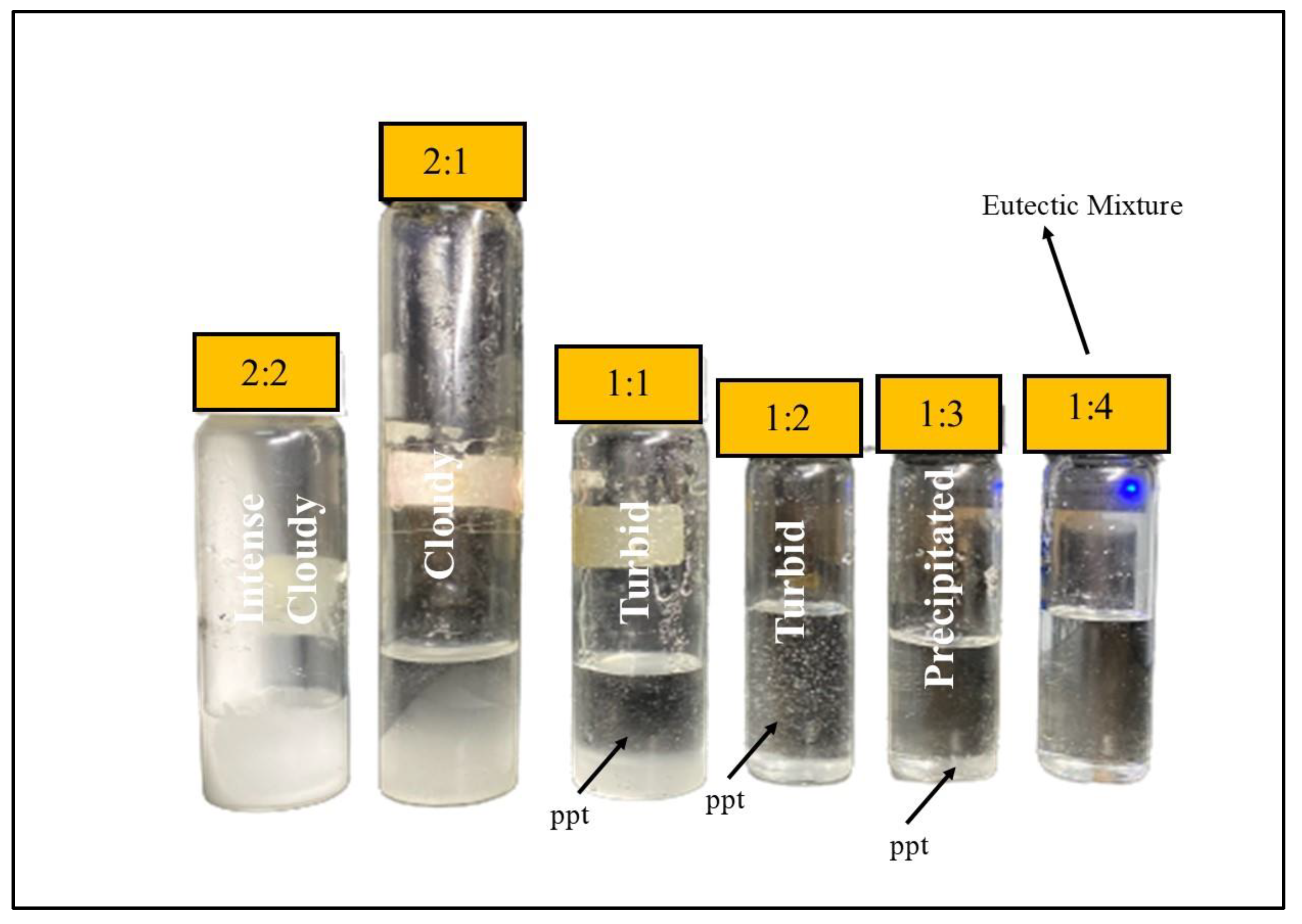
| Molar Ratio | Observation |
|---|---|
| 1:1 | Cloudy and turbid |
| 1:2 | Turbid with ppt |
| 1:3 | Precipitated |
| 1:4 | Transparent, no precipitate (eutectic mixture) |
| 2:1 | Cloudy |
| 2:2 | Intensely cloudy |
| Conc. | Induction Time (h) | Peak Height (Hydrates) | Ice Onset Time | Hydrate Onset Temperature (°C) |
|---|---|---|---|---|
| (mW) | (h) | |||
| Base | 4.185 | 7.948 | 5.439 | −4.136 |
| 1% | 6.523 | 7.284 | 6.214 | −12.525 |
| 3% | 7.573 | 12.715 | 6.361 | −13.393 |
| 5% | 7.213 | 80.97 | 6.42 | −9.631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasool, M.H.; Ahmad, M.; Siddiqui, N.A.; Junejo, A.Z. Eco-Friendly Drilling Fluid: Calcium Chloride-Based Natural Deep Eutectic Solvent (NADES) as an All-Rounder Additive. Energies 2023, 16, 5533. https://doi.org/10.3390/en16145533
Rasool MH, Ahmad M, Siddiqui NA, Junejo AZ. Eco-Friendly Drilling Fluid: Calcium Chloride-Based Natural Deep Eutectic Solvent (NADES) as an All-Rounder Additive. Energies. 2023; 16(14):5533. https://doi.org/10.3390/en16145533
Chicago/Turabian StyleRasool, Muhammad Hammad, Maqsood Ahmad, Numair Ahmed Siddiqui, and Aisha Zahid Junejo. 2023. "Eco-Friendly Drilling Fluid: Calcium Chloride-Based Natural Deep Eutectic Solvent (NADES) as an All-Rounder Additive" Energies 16, no. 14: 5533. https://doi.org/10.3390/en16145533
APA StyleRasool, M. H., Ahmad, M., Siddiqui, N. A., & Junejo, A. Z. (2023). Eco-Friendly Drilling Fluid: Calcium Chloride-Based Natural Deep Eutectic Solvent (NADES) as an All-Rounder Additive. Energies, 16(14), 5533. https://doi.org/10.3390/en16145533







