Determination and Application of Archie Model Parameters in Hydrate Formation under Different Temperature Gradients
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
2.2. Experimental Materials
2.3. Experimental Procedure
2.4. Calculation Methods
2.4.1. Hydrate Resistivity
2.4.2. Hydrate Saturation
3. Result and Discussion
3.1. The Fitting of m and n Values in Archie Formula under Different Temperature Gradient Conditions
3.2. The Range and Applicability Analysis of m and n Values under Different Temperature Gradient Conditions
3.2.1. The Range of m and n Values in Archie Model under Different Temperature Gradients
3.2.2. Hydrate Saturation Calculated by Gas Consumption Method under Different Temperature Gradient Conditions
3.2.3. Comparison of Dynamic Hydrate Saturation Calculated by Optimized Archie Model and Gas Consumption Method
3.3. Determination of Hydrate Saturation and Its Distribution in Different Layers Based on the Optimized Archie Model
3.3.1. Hydrate Saturation in Different Layers under Different Temperature Gradient Conditions Calculated by Archie Model
3.3.2. The Saturation Variation and Distribution of Hydrate Formation Process under Different Temperature Gradients Are Simulated by Archie Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.R.; Li, M.L.; Wang, S.Y. The present situation and prospect of exploration and development of combustible ice in permafrost in China. Eng. Res.-Eng. Interdiscip. Perspect. 2007, 10, 440–450. [Google Scholar]
- Wei, J.; Fang, Y.; Lu, H. Distribution and characteristics of natural gas hydrates in the Shenhu Sea Area, South China Sea. Mar. Pet. Geol. 2018, 98, 622–628. [Google Scholar] [CrossRef]
- Nobes, D.C.; Davis, E.E.; Villinger, H. Prediction of Bulk Physical Properties of Oceanic Sediments at Depth from Sea-Floor Geophysical Measurements. Abstract 1986, 70, 941. [Google Scholar]
- Gabitto, J.F.; Tsouris, C. Physical properties of gas hydrates: A review. J. Thermodyn. 2010, 2010, 271291. [Google Scholar] [CrossRef]
- Archie, G.E. The electrical resistivity log as an aid in determining some reservoir characteristics. Trans. AIME 1942, 146, 54–62. [Google Scholar] [CrossRef]
- Sweeney, S.A.; Jennings, H.Y., Jr. Effect of wettability on the electrical resistivity of carbonate rock from a petroleum reservoir. J. Phys. Chem. 1960, 64, 551–553. [Google Scholar] [CrossRef]
- Waxman, M.H.; Smits, L.J.M. Electrical conductivities in oil-bearing shaly sands. Soc. Pet. Eng. J. 1968, 8, 107–122. [Google Scholar] [CrossRef]
- Li, F.G.; Sun, C.Y.; Li, S.L.; Chen, G.J.; Guo, X.Q.; Yang, L.Y.; Zhang, K. Experimental studies on the evolvement of electrical resistivity during methane hydrate formation in sediments. Energy Fuels 2012, 26, 6210–6217. [Google Scholar] [CrossRef]
- Perez-Rosales, C. On the relationship between formation resistivity factor and porosity. Soc. Pet. Eng. J. 1982, 2, 531–536. [Google Scholar] [CrossRef]
- Glover, P. Geophysical Properties of the Near Surface Earth: Electrical Properties-ScienceDirect. Treatise Geophys. 2015, 11, 89–137. [Google Scholar]
- Han, Y.; Zhou, C.; Yu, J.; Li, C.; Hu, F.; Xu, H.; Yuan, C. Experimental investigation on the effect of wettability on rock-electricity response in sandstone reservoirs. Fuel 2019, 239, 1246–1257. [Google Scholar] [CrossRef]
- Wyllie, M.R.J.; Gregory, A.R. Formation factors of unconsolidated porous media: Influence of particle shape and effect of cementation. J. Pet. Technol. 1953, 5, 103–110. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, J.; Cai, H. Evaluation Method for Identifying Low resistance reservoir based on main control factors. J. Xi’an ShiYou Univ. 2017, 32, 35–39. [Google Scholar]
- Zhao, J.; Liu, C.; Li, C. Pore-Scale Investigation of the Electrical Property and Saturation Exponent of Archie’s Law in Hydrate-Bearing Sediments. J. Mar. Sci. Eng. 2022, 10, 111. [Google Scholar] [CrossRef]
- Pearson, C.; Murphy, J.; Hermes, R. Acoustic and resistivity measurements on rock samples containing tetrahydrofuran hydrates: Laboratory analogues to natural gas hydrate deposits. J. Geophys. Res. Solid Earth 1986, 91, 14132–14138. [Google Scholar] [CrossRef]
- Jackson, P.D.; Smith, D.T.; Stanford, P.N. Resistivity-porosity-particle shape relationships for marine sands. Geophysics 1978, 43, 1250–1268. [Google Scholar] [CrossRef]
- Shankar, U.; Riedel, M. Assessment of gas hydrate saturation in marine sediments from resistivity and compressional-wave velocity log measurements in the Mahanadi Basin, India. Mar. Pet. Geol. 2014, 58, 265–277. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Li, C. Fractal analyses on saturation exponent in Archie’s law for electrical properties of hydrate-bearing porous media. J. Pet. Sci. Eng. 2021, 196, 107642. [Google Scholar] [CrossRef]
- Anderson, W. Nettability Literature Survey—Part 3: The Effects d Nettability on the Electrical Properties of Porous Media. J. Pet. 1986, 38, 1371–1378. [Google Scholar]
- Spangenberg, E. Modeling of the influence of gas hydrate content on the electrical properties of porous sediments. J. Geophys. Res. Solid Earth 2001, 106, 6535–6548. [Google Scholar] [CrossRef]
- Pandey, L.; Sain, K.; Joshi, A.K. Estimate of gas hydrate saturations in the Krishna-Godavari basin, eastern continental margin of India, results of expedition. Mar. Pet. Geol. 2019, 108, 581–594. [Google Scholar] [CrossRef]
- Collett, T.S.; Bird, K.J.; Kvenvolden, K.A. Geological interrelations relative to gas hydrates within the North Slop of Alaska. US Geol. Surv. Open File Rep. 1988, 88, 150. [Google Scholar]
- Chen, L.T.; Li, N.; Sun, C.Y.; Chen, G.J.; Koh, C.A.; Sun, B.J. Hydrate formation in sediments from free gas using a one-dimensional visual simulator. Fuel 2017, 197, 298–309. [Google Scholar] [CrossRef]
- Kim, H.S.; Cho, G.C.; Lee, J.Y.; Kim, S.J. Geotechnical and geophysical properties of deep marine fine-grained sediments recovered during the second Ulleung Basin Gas Hydrate expedition, East Sea, Korea. Mar. Pet. Geol. 2013, 47, 56–65. [Google Scholar] [CrossRef]
- Liu, C.; Meng, Q.; He, X. Characterization of natural gas hydrate recovered from Pearl River Mouth basin in South China Sea. Mar. Pet. Geol. 2015, 61, 14–21. [Google Scholar] [CrossRef]
- Yuan, Q. Using soil auger to improve the cutting ring method to accurately determine soil bulk density and porosity. China Hortic. Abstr. 2014, 30, 25–26. [Google Scholar]
- Gao, L.; Wang, Y.J.; Xing, L.C. Numerical experiments of TDR probe optimization and hydrate saturation measurement based on finite element model. Lab. Res. Explor. 2022, 41, 43–49. [Google Scholar]
- Santamarina, J.C.; Ruppel, C. The impact of hydrate saturation on the mechanical, electrical, and thermal properties of hydrate-bearing sand, silts, and clay. In Geophysical Characterization of Gas Hydrates; Society of Exploration Geophysicists: Tulsa, OK, USA, 2010; pp. 373–384. [Google Scholar]
- Chen, G.J.; Guo, T.M. A new approach to gas hydrate modelling. Chem. Eng. J. 1998, 71, 145–151. [Google Scholar] [CrossRef]
- Bai, Z.; Tan, M.; Shi, Y. An improved saturation evaluation method of Chang 8 tight sandstone reservoir in Longdong West area of Ordos Basin, China. Energy Explor. Exploit. 2022, 40, 97–111. [Google Scholar] [CrossRef]
- Feng, C.; Yang, Z.; Feng, Z. A novel method to estimate resistivity index of tight sandstone reservoirs using nuclear magnetic resonance logs. J. Nat. Gas Sci. Eng. 2020, 79, 103358. [Google Scholar] [CrossRef]
- Lu, H.; Zeng, H.; Ripmeester, J.A. Sediment control on the saturation level of gas hydrate in nature environments. In Proceedings of the 6th International Conference on Gas Hydrates (ICGH 2008), Vancouver, BC, Canada, 6–10 July 2008. [Google Scholar]
- Chuvilin, E.M.; Kozlova, E.; Skolotneva, T.S. Experimental simulation of frozen hydratecontaining sediments formation. In Proceedings of the Fifth International Conference on Gas Hydrates, Trondheim, Norway, 13–16 June 2005; pp. 13–16. [Google Scholar]
- Spangenberg, E.; Kulenkampff, J. Pore space hydrate formation in a glass bead sample from methane dissolved in wate. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef]
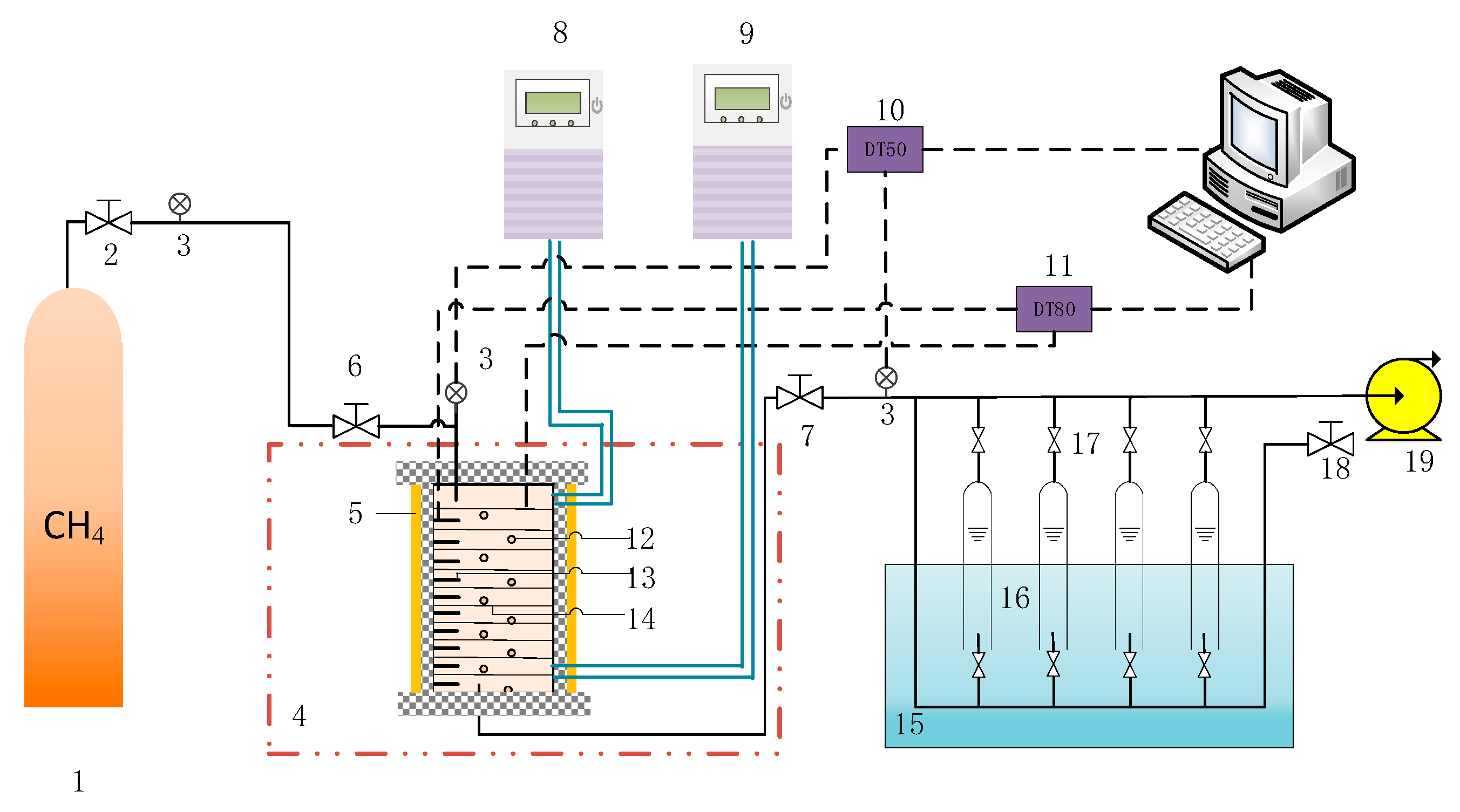
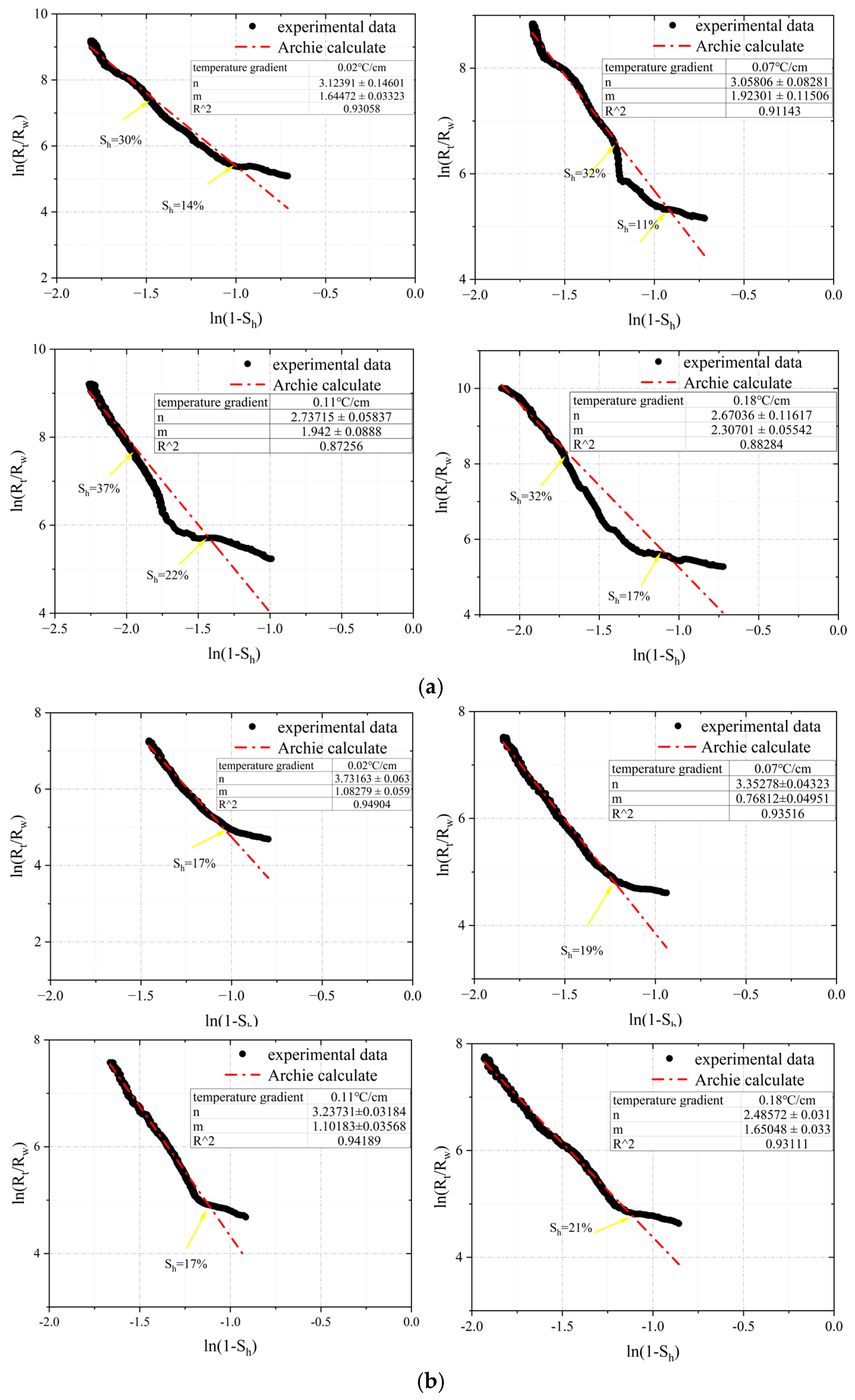
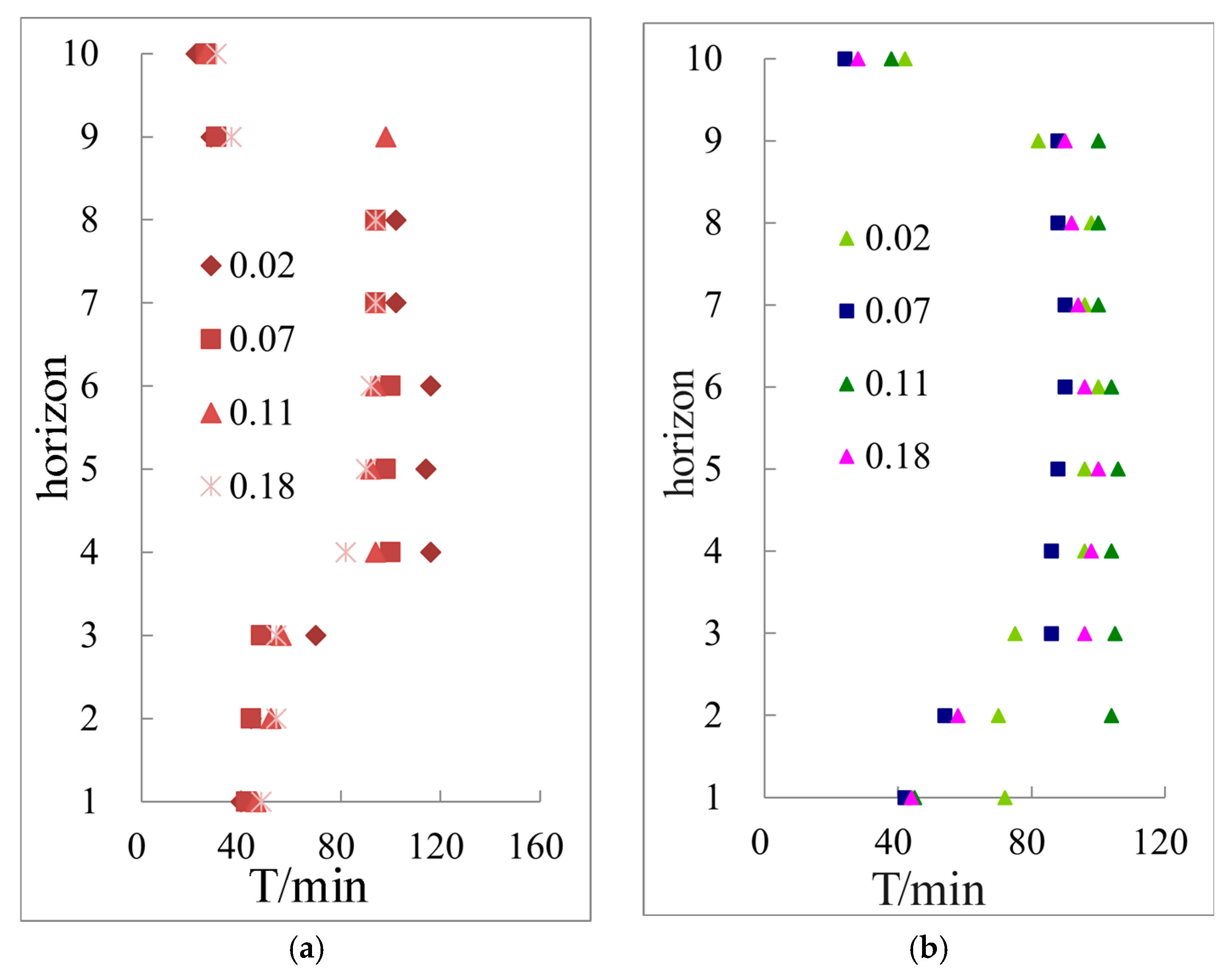
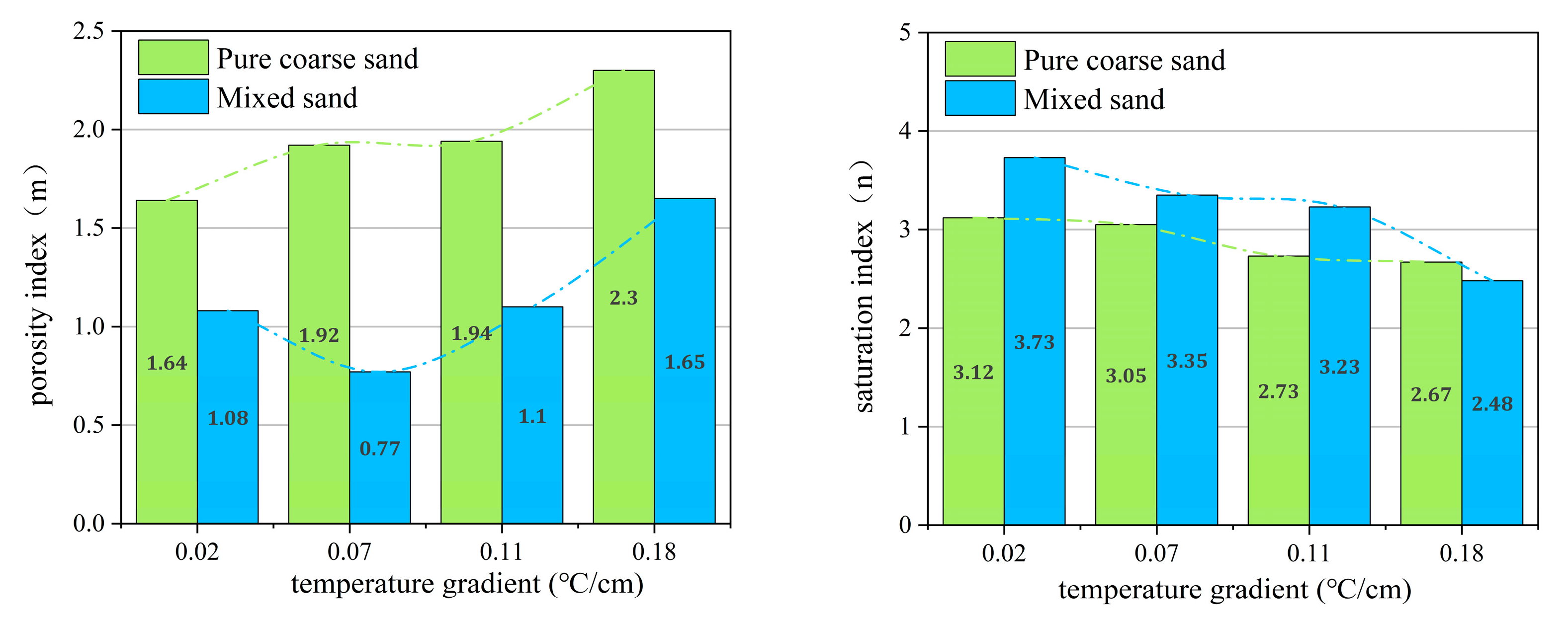
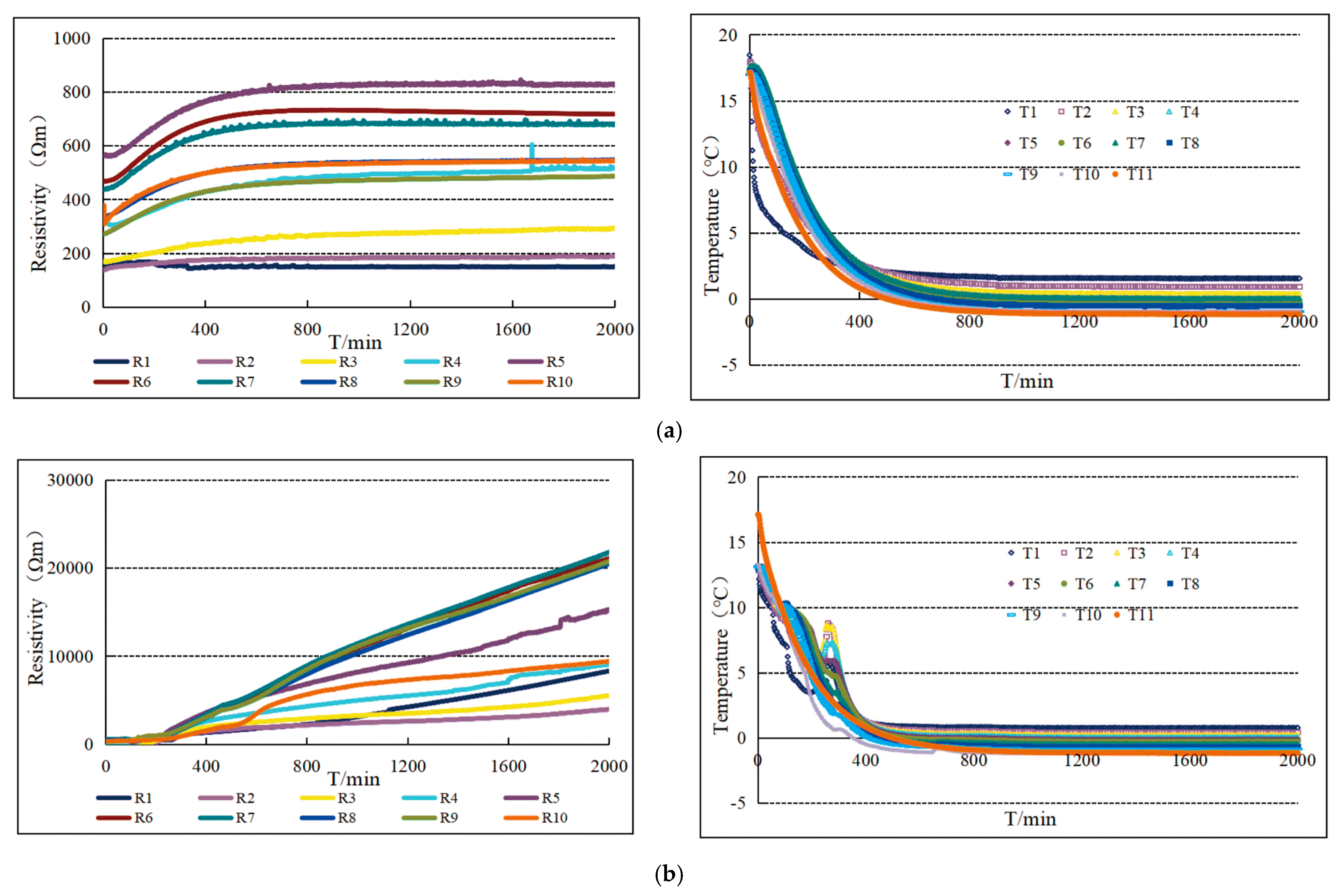
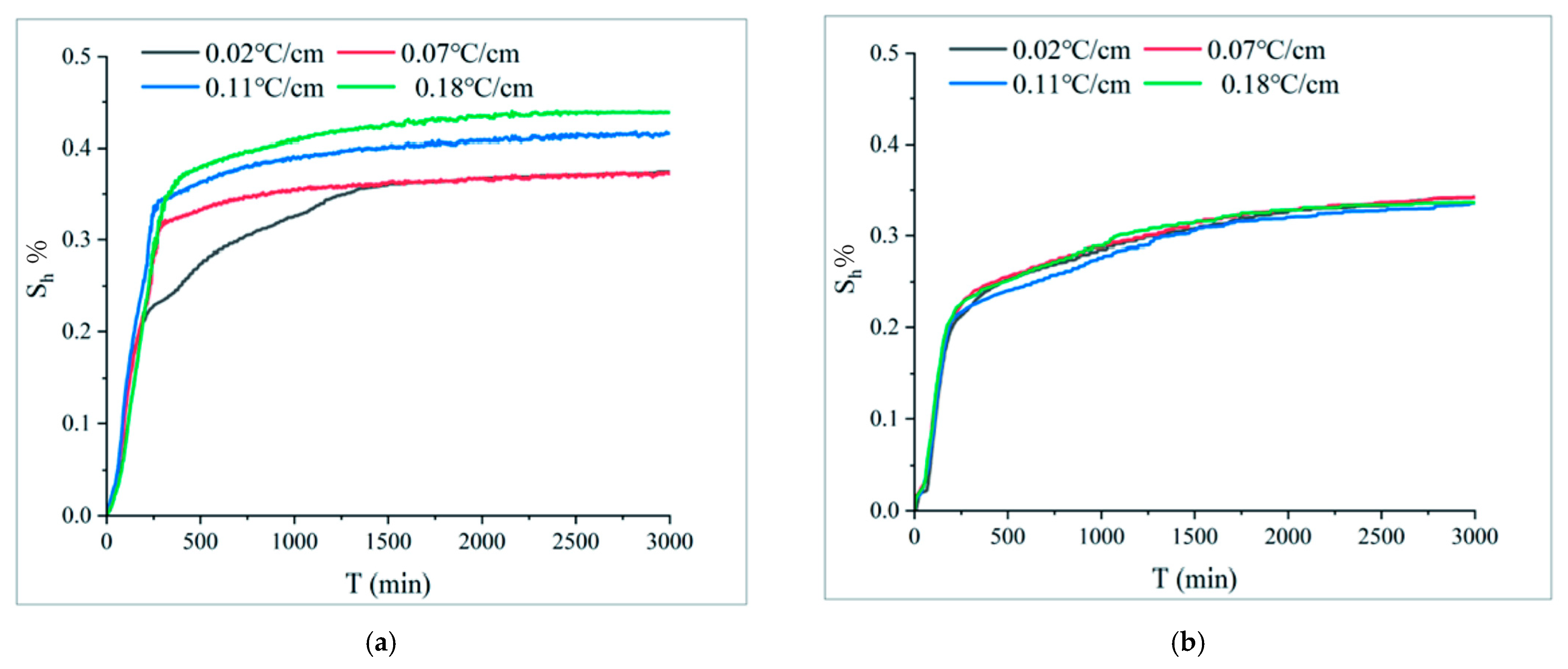
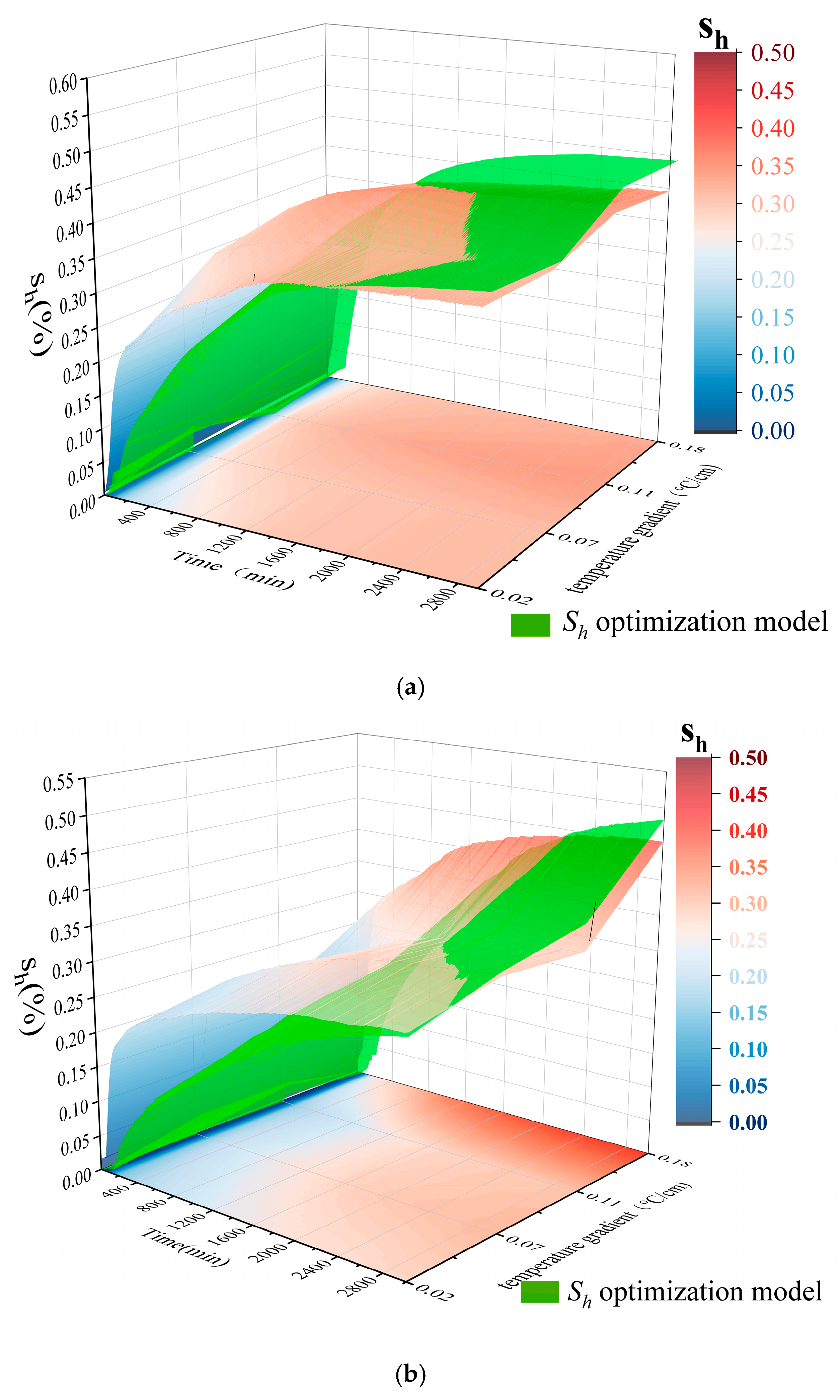


| Sample Composition | Particle Size | Density (g/mL) |
|---|---|---|
| coarse sand | 0.5–1 mm | 1.6 |
| silt | 300 mesh | 2.6 |
| Experimental Materials | Specification | Source |
|---|---|---|
| methane | purity 99.99% | Chengdu, China Zhongke Kate Co., Ltd. |
| quartz sand | SiO2 | Taihang Horticulture and McLean reagent |
| deionized water | conductivity 18.25 MΩ·cm | laboratory made |
| alcohol | purity 95.0% | Chengdu, China Zhongke Kate Co., Ltd. |
| Sand Ratio | Sand Weight (g) | Dry Sand Volume (mL) | Water Volume (mL) | Pore Volume (mL) | φ0 (%) | Sw (%) |
|---|---|---|---|---|---|---|
| 100% coarse sand | 1116.7 | 451.3 | 167.3 | 342.1 | 42.0% | 48.9% |
| 1116.2 | 450.9 | 167.7 | 341.9 | 42.1% | 49.0% | |
| 1116.4 | 450.8 | 167.2 | 342.7 | 42.1% | 49.1% | |
| 1116.2 | 451.3 | 168.1 | 342.5 | 42.2% | 49.4% | |
| Mixed sand | 1370.2 | 538.89 | 134.66 | 281.27 | 34.2% | 47.87% |
| 1371.1 | 539.48 | 133.72 | 280.68 | 34.2% | 47.64% | |
| 1370.4 | 538.99 | 135.12 | 281.39 | 34.3% | 48.05% | |
| 1369.9 | 538.78 | 135.13 | 281.38 | 34.3% | 48.05% |
| Sand Ratio | Temperature Gradient (°C/cm) | Initial Pressure (Mpa) | Final Pressure (Mpa) | Initial Temperature (°C) | Sh |
|---|---|---|---|---|---|
| 100% coarse sand | 0.02 | 8.84 | 6.23 | 13.19 | 0.374 |
| 0.07 | 8.656 | 6.04 | 13.0 | 0.373 | |
| 0.11 | 8.89 | 6.02 | 13.06 | 0.420 | |
| 0.18 | 9.01 | 6.25 | 13.41 | 0.400 | |
| Mixed sand | 0.02 | 8.87 | 7.13 | 13.09 | 0.319 |
| 0.07 | 8.83 | 7.08 | 13.09 | 0.344 | |
| 0.11 | 8.79 | 7.06 | 12.98 | 0.339 | |
| 0.18 | 8.08 | 7.02 | 13.13 | 0.457 |
| Horizon | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100% coarse sand | estimated directly | 0.57 | 0.54 | 0.46 | 0.42 | 0.35 | 0.34 | 0.33 | 0.35 | 0.40 | 0.40 |
| optimization model | 0.56 | 0.46 | 0.39 | 0.43 | 0.39 | 0.39 | 0.39 | 0.39 | 0.45 | 0.43 | |
| Mixed sand | estimated directly | 0.33 | 0.30 | 0.27 | 0.29 | 0.30 | 0.33 | 0.28 | 0.26 | 0.21 | 0.19 |
| optimization model | 0.38 | 0.34 | 0.29 | 0.33 | 0.35 | 0.37 | 0.33 | 0.32 | 0.27 | 0.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, J.; Jiao, W.; Teng, Y.; Zhan, J.; Zhang, P. Determination and Application of Archie Model Parameters in Hydrate Formation under Different Temperature Gradients. Energies 2023, 16, 5517. https://doi.org/10.3390/en16145517
Wang Y, Liu J, Jiao W, Teng Y, Zhan J, Zhang P. Determination and Application of Archie Model Parameters in Hydrate Formation under Different Temperature Gradients. Energies. 2023; 16(14):5517. https://doi.org/10.3390/en16145517
Chicago/Turabian StyleWang, Yingmei, Jie Liu, Wenze Jiao, Yadong Teng, Jing Zhan, and Peng Zhang. 2023. "Determination and Application of Archie Model Parameters in Hydrate Formation under Different Temperature Gradients" Energies 16, no. 14: 5517. https://doi.org/10.3390/en16145517
APA StyleWang, Y., Liu, J., Jiao, W., Teng, Y., Zhan, J., & Zhang, P. (2023). Determination and Application of Archie Model Parameters in Hydrate Formation under Different Temperature Gradients. Energies, 16(14), 5517. https://doi.org/10.3390/en16145517






