Enhanced Photocatalytic CO2 Reduction to CH4 Using Novel Ternary Photocatalyst RGO/Au-TNTAs
Abstract
1. Introduction
2. Experimental Overview
2.1. Reagents and Materials
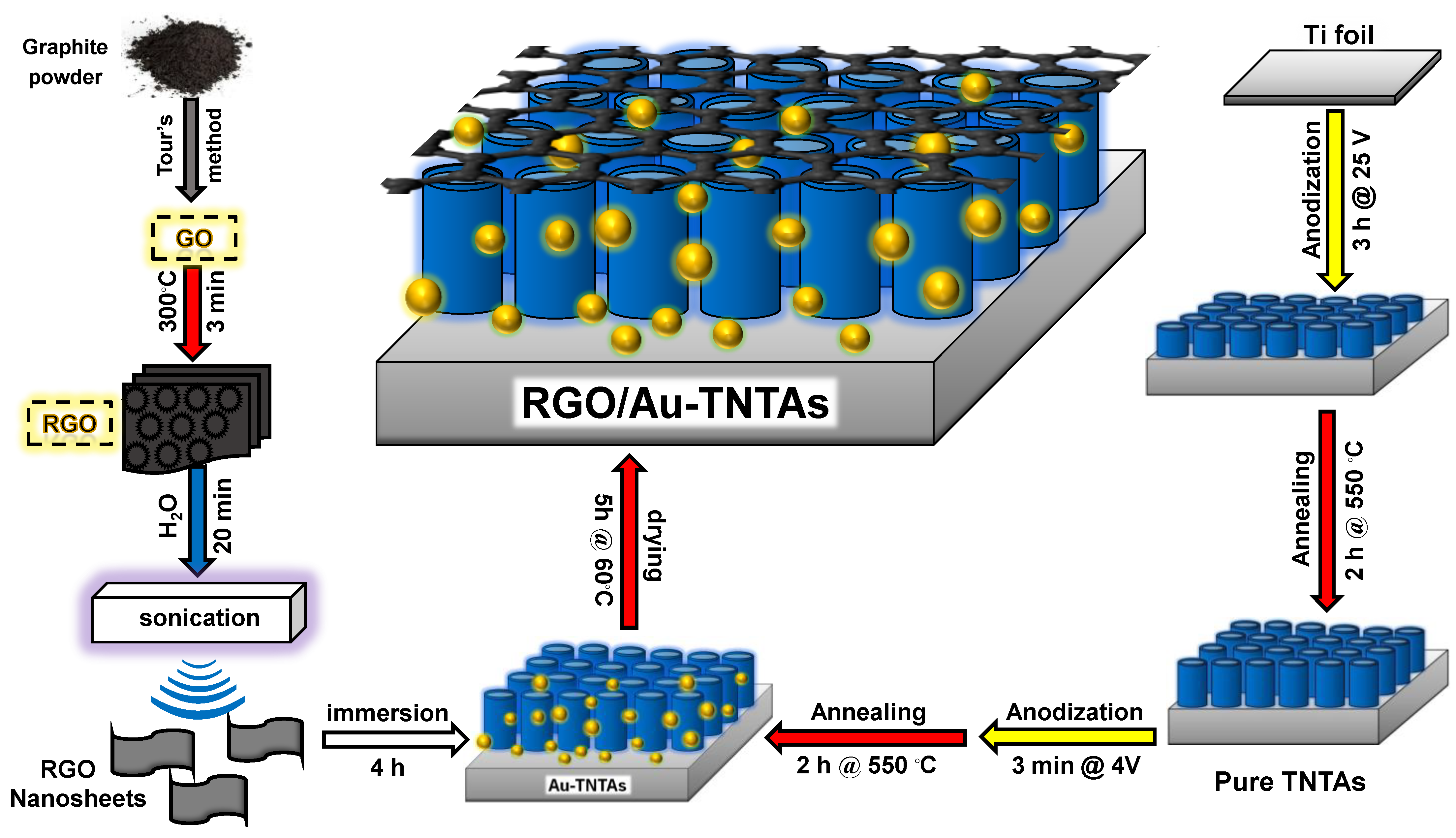
2.2. Preparation of TNT Arrays
2.3. Preparation of Au-Deposited TNT Arrays
2.4. Preparation of GO and RGO
2.5. Preparation of Au and RGO-Modified TNTAs (RGO/Au-TNTAs)
2.6. Characterization
2.7. Quantification of Photocatalytic CO2 Reduction
3. Results and Discussion
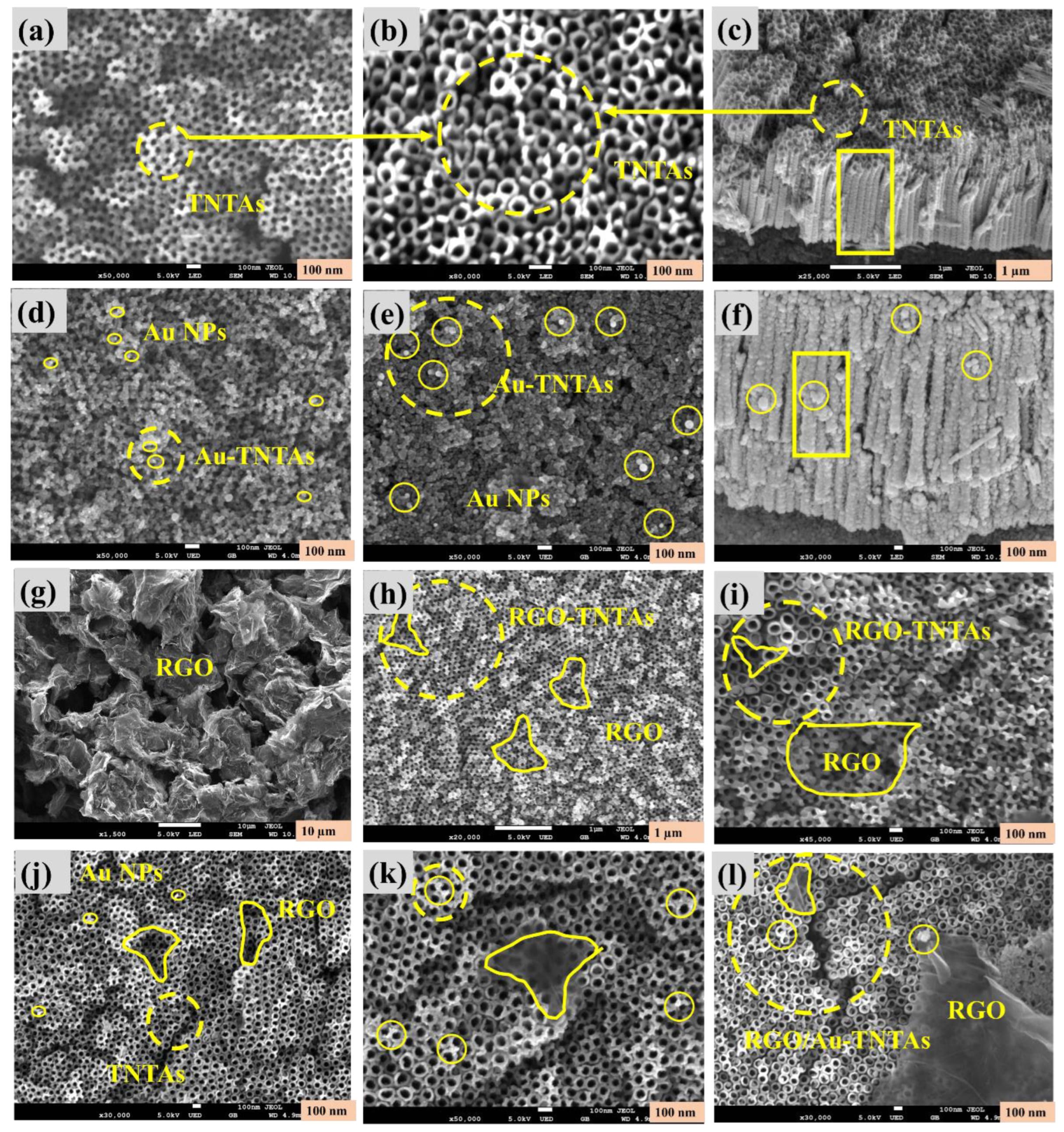
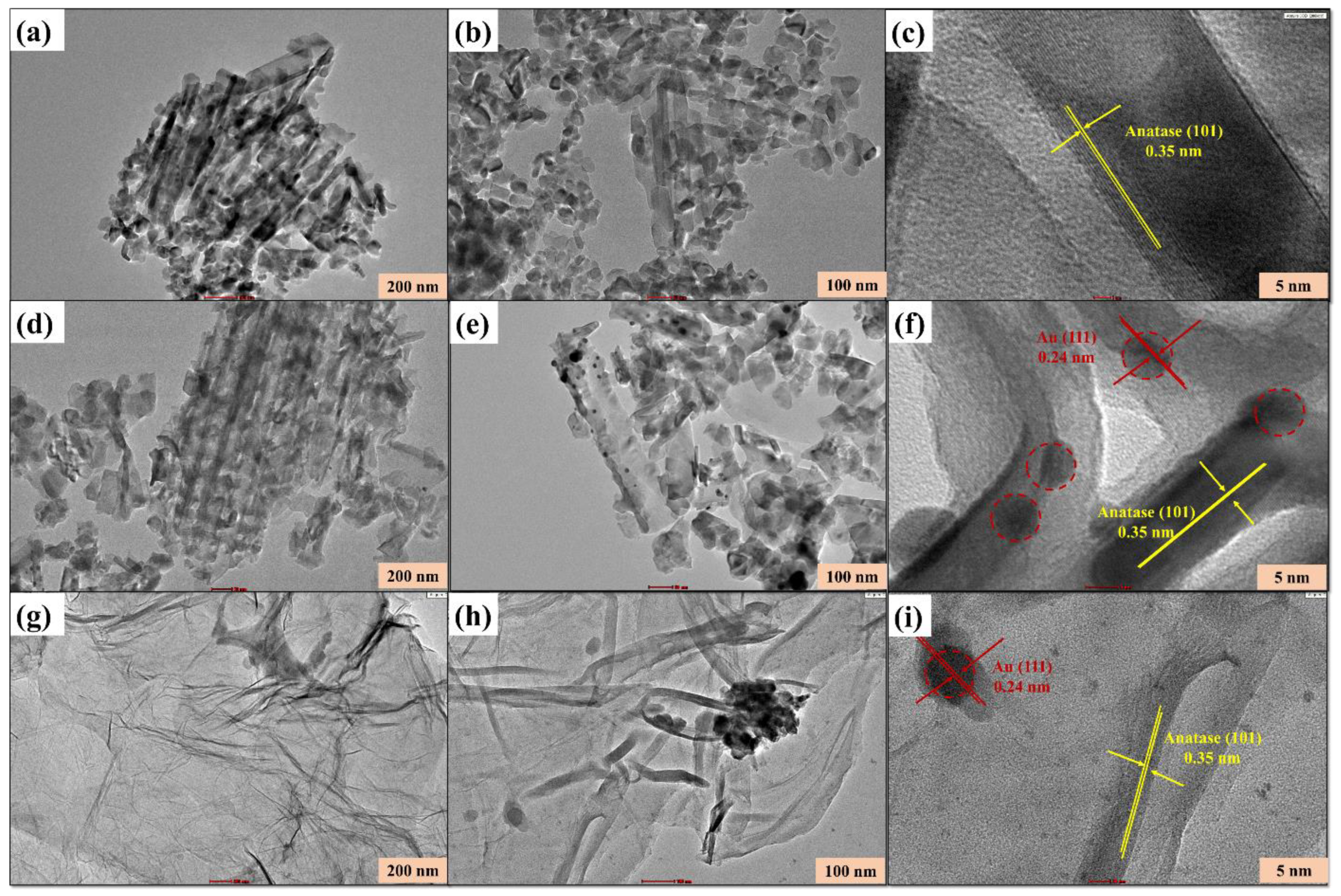
3.1. Morphological Characterization
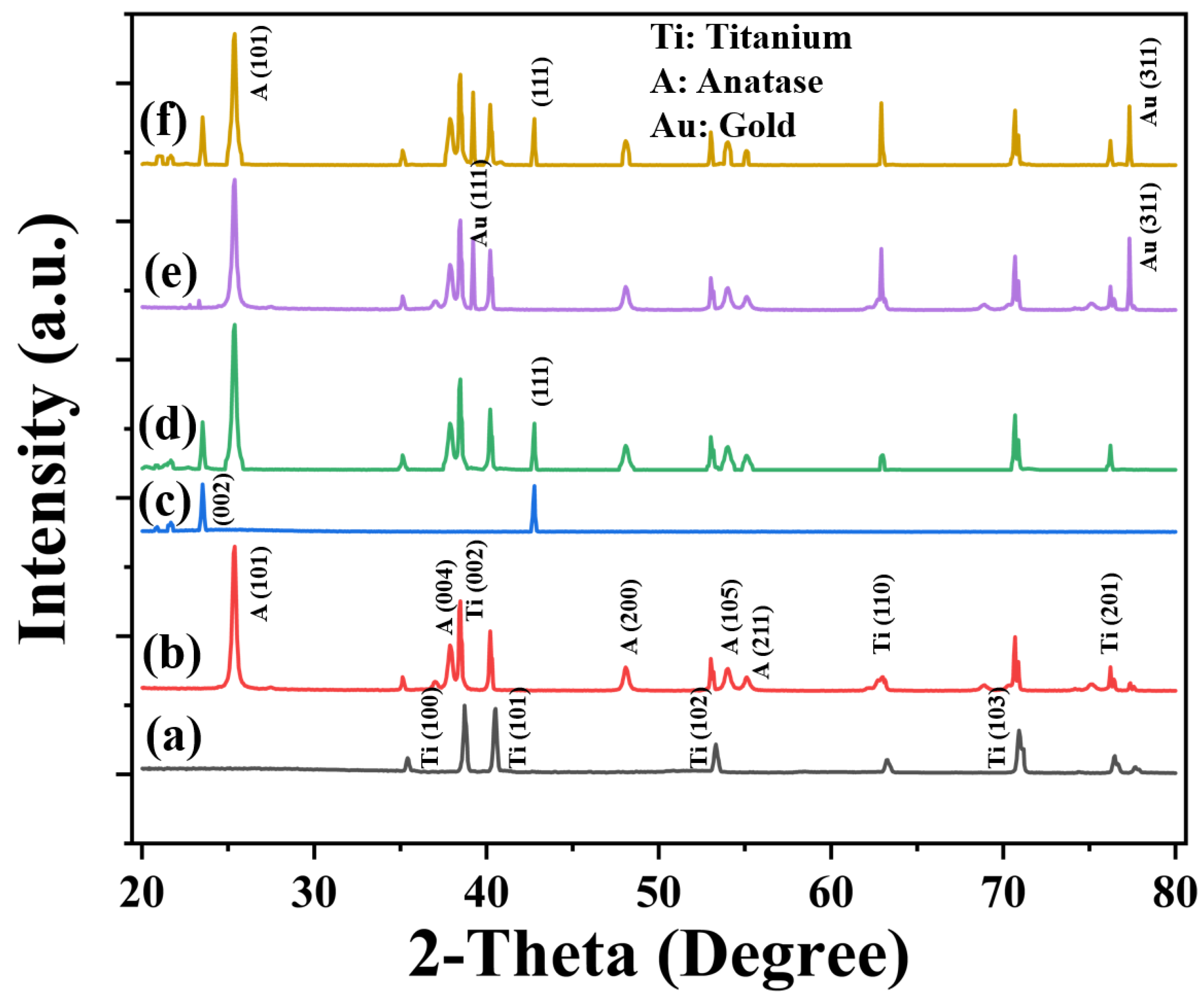
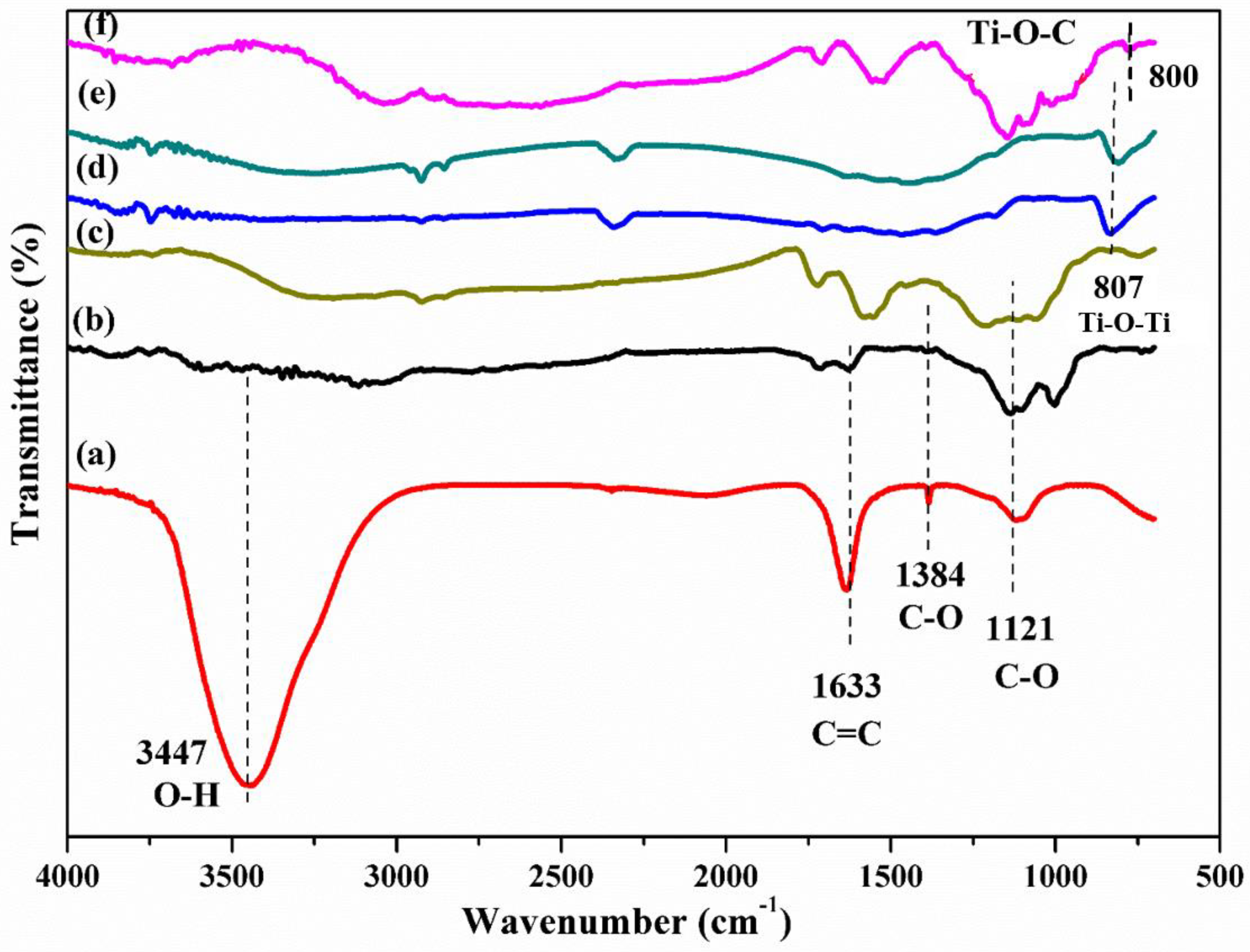
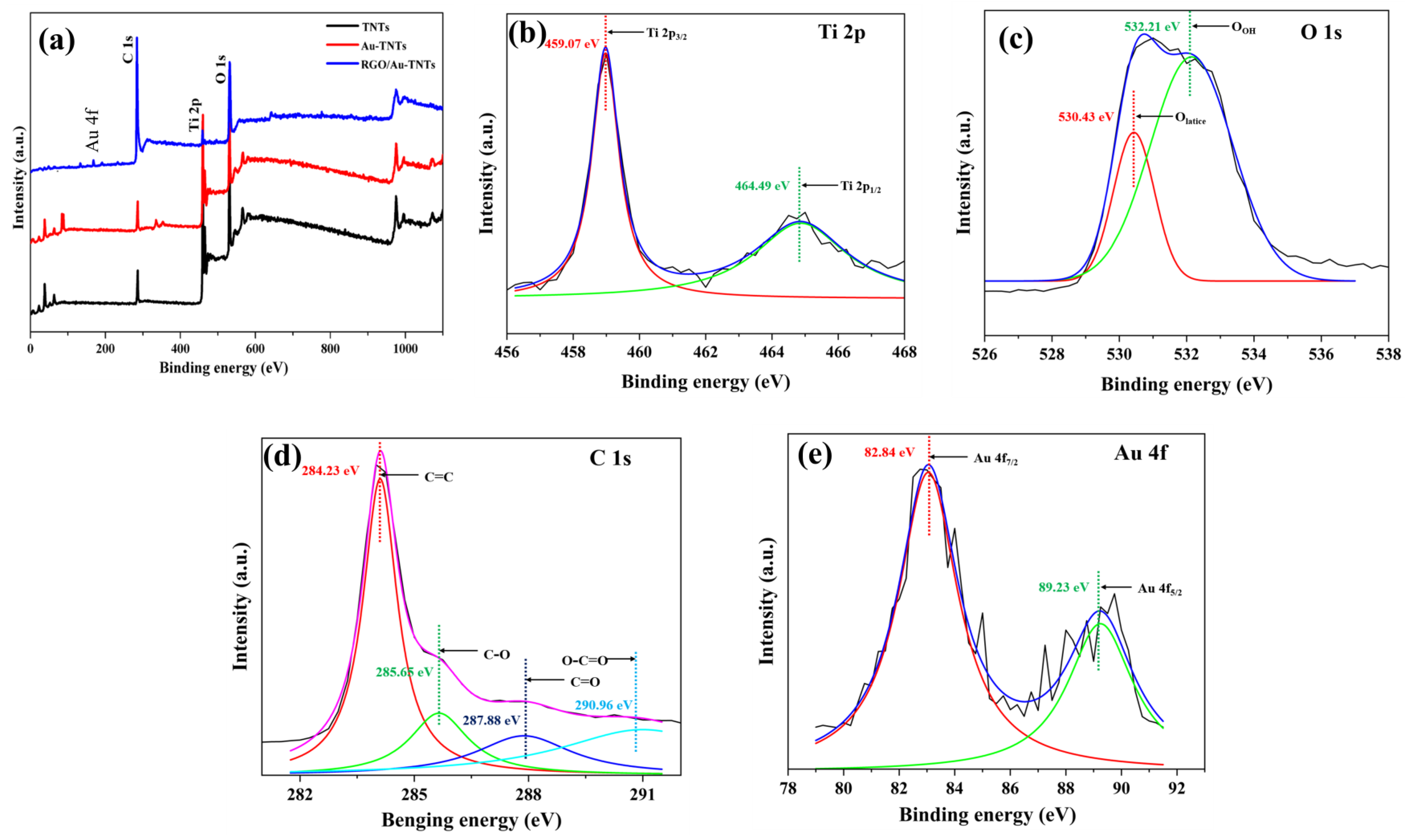
3.2. Surface Analysis
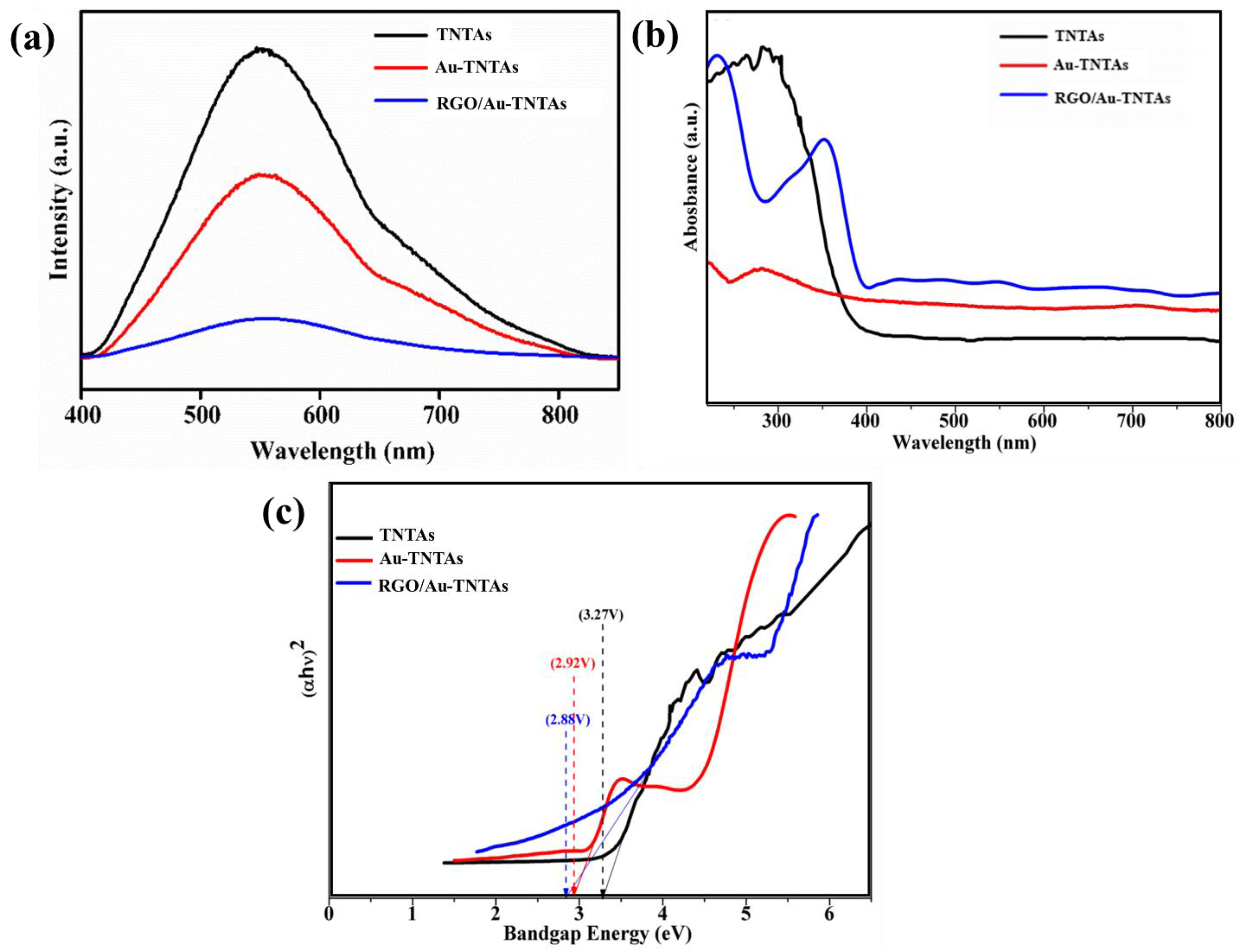
3.3. Optical Analysis
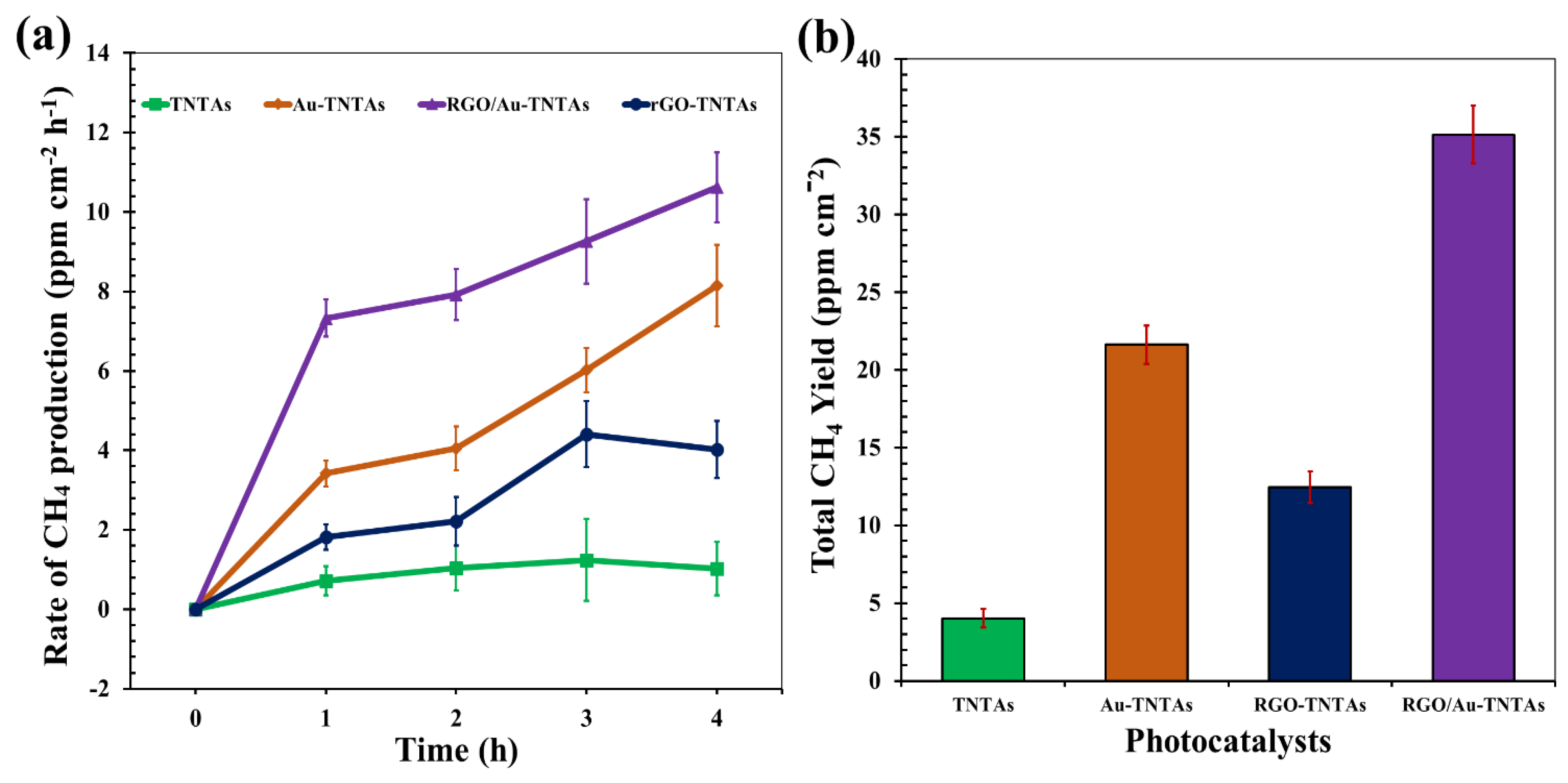
3.4. Phocatalytic CO2 Reduction Performance
3.5. Comparison of CO2 Reduction Rate of TNTAs-Based Photocatalysts
3.6. Proposed Mechanism for Photocatalytic Conversion of CO2 to CH4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, K.Y.; Saini, H.; Pandiarajan, D.; Prashanth, M.K.; Parashuram, L.; Raghu, M.S. Controllable synthesis of TiO2 chemically bonded graphene for photocatalytic hydrogen evolution and dye degradation. Catal. Today 2020, 340, 170–177. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Q.; Tayyab, M.; Zhou, L.; Lei, J.; Zhang, J. Single-atom Pt loaded zinc vacancies ZnO–ZnS induced type-V electron transport for efficiency photocatalytic H2 evolution. Sol. Rrl 2021, 5, 2100536. [Google Scholar] [CrossRef]
- Zhou, Y.; Abazari, R.; Chen, J.; Tahir, M.; Kumar, A.; Ikreedeegh, R.R.; Rani, E.; Singh, H.; Kirillov, A.M. Bimetallic metal–organic frameworks and MOF-derived composites: Recent progress on electro-and photoelectrocatalytic applications. Coord. Chem. Rev. 2022, 451, 214264. [Google Scholar] [CrossRef]
- Wyżga, P.; Tabari, T.; Trochowski, M.; Macyk, W. Optimizing the morphology of titania nanorods for enhanced solar seawater splitting. Results Eng. 2023, 17, 100921. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Min, S.; Irfan, R.M.; Zhu, Q.; Zhou, L.; Lei, J.; Zhang, J. Simultaneous hydrogen production with the selective oxidation of benzyl alcohol to benzaldehyde by a noble-metal-free photocatalyst VC/CdS nanowires. Chin. J. Catal. 2022, 43, 1165–1175. [Google Scholar] [CrossRef]
- Huskinson, B.; Marshak, M.P.; Suh, C.; Er, S.; Gerhardt, M.R.; Galvin, C.J.; Chen, X.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. A metal-free organic–inorganic aqueous flow battery. Nature 2014, 505, 195–198. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tahir, M. A critical review in recent developments of metal-organic-frameworks (MOFs) with band engineering alteration for photocatalytic CO2 reduction to solar fuels. J. CO2 Util. 2021, 43, 101381. [Google Scholar] [CrossRef]
- Alkhatib, I.I.; Garlisi, C.; Pagliaro, M.; Al-Ali, K.; Palmisano, G. Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: A review of strategies and applications. Catal. Today 2020, 340, 209–224. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar]
- Hossen, M.A.; Solayman, H.M.; Leong, K.H.; Sim, L.C.; Nurashikin, Y.; Abd Aziz, A.; Lihua, W.; Monir, M.U. Recent progress in TiO2-Based photocatalysts for conversion of CO2 to hydrocarbon fuels: A systematic review. Results Eng. 2022, 16, 100795. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R. Recent developments of Fe-based metal organic frameworks and their composites in photocatalytic applications: Fundamentals; Synthesis and Challenges. Russ. Chem. Rev. 2022, 91, 1–21. [Google Scholar]
- Liu, G.; Feng, M.; Tayyab, M.; Gong, J.; Zhang, M.; Yang, M.; Lin, K. Direct and efficient reduction of perfluorooctanoic acid using bimetallic catalyst supported on carbon. J. Hazard. Mater. 2021, 412, 125224. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Photo-induced CO2 reduction by CH4/H2O to fuels over Cu-modified g-C3N4 nanorods under simulated solar energy. Appl. Surf. Sci. 2017, 419, 875–885. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Investigating the product distribution behaviour of CO2 methanation through thermodynamic optimized experimental approach using micro/nano structured titania catalyst. Energy Convers. Manag. 2022, 254, 115240. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Amin, N.S. Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers. Manag. 2013, 76, 194–214. [Google Scholar] [CrossRef]
- Adekoya, D.; Tahir, M.; Amin, N.A.S. Recent trends in photocatalytic materials for reduction of carbon dioxide to methanol. Renew. Sustain. Energ. Rev. 2019, 116, 109389. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium Dioxide (TiO₂)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Pan, L.; Xu, Z.; Yue, W.; Zhou, L.; Lei, J.; Zhang, J. One-pot in-situ hydrothermal synthesis of ternary In2S3/Nb2O5/Nb2C Schottky/S-scheme integrated heterojunction for efficient photocatalytic hydrogen production. J. Colloid Interface Sci. 2022, 628, 500–512. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, J.; Dai, K.; Liang, C. Construction of flourinated-TiO2 nanosheets with exposed {001} facets/CdSe-DETA nanojunction for enhancing visible-light-driven photocatalytic H2 evolution. Ceram. Int. 2020, 46, 866–876. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, S.; Chen, R.; Zhu, X.; Liao, Q.; Huang, Y. Copper-decorated TiO2 nanorod thin films in optofluidic planar reactors for efficient photocatalytic reduction of CO2. Int. J. Hydrogen Energy 2017, 42, 9722–9732. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, S.; Song, X.; Luo, S. Photocatalyst based on TiO2 nanotube arrays co-decorated with CdS quantum dots and reduced graphene oxide irradiated by γ rays for effective degradation of ethylene. Appl. Surf. Sci. 2018, 442, 245–255. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tahir, M. Photocatalytic CO2 reduction to CO and CH4 using g-C3N4/RGO on titania nanotube arrays (TNTAs). J. Mater. Sci. 2021, 56, 18989–19014. [Google Scholar] [CrossRef]
- Hossen, M.A.; Solayman, H.M.; Leong, K.H.; Sim, L.C.; Yaacof, N.; Abd Aziz, A.; Lihua, W.; Monir, M.U. A Comprehensive Review on Advances in TiO2 Nanotube (TNT)-Based Photocatalytic CO2 Reduction to Value-Added Products. Energies 2022, 15, 8751. [Google Scholar] [CrossRef]
- Rambabu, Y.; Kumar, U.; Singhal, N.; Kaushal, M.; Jaiswal, M.; Jain, S.L.; Roy, S.C. Photocatalytic reduction of carbon dioxide using graphene oxide wrapped TiO2 nanotubes. Appl. Surf. Sci. 2019, 485, 48–55. [Google Scholar] [CrossRef]
- Santos, J.S.; Fereidooni, M.; Marquez, V.; Arumugam, M.; Tahir, M.; Praserthdam, S.; Praserthdam, P. Single-step fabrication of highly stable amorphous TiO2 nanotubes arrays (am-TNTA) for stimulating gas-phase photoreduction of CO2 to methane. Chemosphere 2022, 289, 133170. [Google Scholar] [CrossRef]
- Tahir, M. Ni/MMT-promoted TiO2 nanocatalyst for dynamic photocatalytic H2 and hydrocarbons production from ethanol-water mixture under UV-light. Int. J. Hydrogen Energy 2017, 42, 28309–28326. [Google Scholar] [CrossRef]
- Azam, M.U.; Tahir, M.; Umer, M.; Jaffar, M.M.; Nawawi, M.G.M. Engineering approach to enhance photocatalytic water splitting for dynamic H2 production using La2O3/TiO2 nanocatalyst in a monolith photoreactor. Appl. Surf. Sci. 2019, 484, 1089–1101. [Google Scholar] [CrossRef]
- Elysabeth, T.; Agriyfani, D.A.; Ibadurrohman, M.; Nurdin, M. Synthesis of Ni-and N-doped titania nanotube arrays for photocatalytic hydrogen production from glycerol–water solutions. Catalysts 2020, 10, 1234. [Google Scholar] [CrossRef]
- Bu, T.; Liu, X.; Chen, R.; Liu, Z.; Li, K.; Li, W.; Peng, Y.; Ku, Z.; Huang, F.; Cheng, Y.B.; et al. Organic/inorganic self-doping controlled crystallization and electronic properties of mixed perovskite solar cells. J. Mater. Chem. A 2018, 6, 6319–6326. [Google Scholar] [CrossRef]
- Cho, H.; Joo, H.; Kim, H.; Kim, J.E.; Kang, K.S.; Yoon, J. Enhanced photocatalytic activity of TiO2 nanotubes decorated with erbium and reduced graphene oxide. Appl. Surf. Sci. 2021, 565, 150459. [Google Scholar] [CrossRef]
- Janekbary, K.K.; Gilani, N.; Pirbazari, A.E. One-step fabrication of Ag/RGO doped TiO2 nanotubes during anodization process with high photocatalytic performance. J. Porous Mater. 2020, 27, 1809–1822. [Google Scholar] [CrossRef]
- Yao, Y.C.; Dai, X.R.; Hu, X.Y.; Huang, S.Z.; Jin, Z. Synthesis of Ag-decorated porous TiO2 nanowires through a sunlight induced reduction method and its enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 387, 469–476. [Google Scholar] [CrossRef]
- Wang, M.; Pang, X.; Zheng, D.; He, Y.; Sun, L.; Lin, C.; Lin, Z. Nonepitaxial growth of uniform and precisely size-tunable core/shell nanoparticles and their enhanced plasmon-driven photocatalysis. J. Mater. Chem. A 2016, 4, 7190–7199. [Google Scholar] [CrossRef]
- Khatun, F.; Abd Aziz, A.; Sim, L.C.; Monir, M.U. Plasmonic enhanced Au decorated TiO2 nanotube arrays as a visible light active catalyst towards photocatalytic CO2 conversion to CH4. J. Environ. Chem. Eng. 2019, 7, 103233. [Google Scholar] [CrossRef]
- Pan, H.; Wang, X.; Xiong, Z.; Sun, M.; Murugananthan, M.; Zhang, Y. Enhanced Photocatalytic CO2 Reduction with Defective TiO2 Nanotubes Modified by Single-Atom Binary Metal Components. Environ. Res. 2021, 198, 111176. [Google Scholar] [CrossRef]
- Montakhab, E.; Rashchi, F.; Sheibani, S. Enhanced photocatalytic activity of TiO2 nanotubes decorated with Ag nanoparticles by simultaneous electrochemical deposition and reduction processes. Appl. Surf. Sci. 2023, 615, 156332. [Google Scholar] [CrossRef]
- Luo, J.; Li, D.; Yang, Y.; Liu, H.; Chen, J.; Wang, H. Preparation of Au/reduced graphene oxide/hydrogenated TiO2 nanotube arrays ternary composites for visible-light-driven photoelectrochemical water splitting. J. Alloys Compd. 2016, 661, 380–388. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tahir, M. Indirect Z-scheme heterojunction of NH2-MIL-125 (Ti) MOF/g-C3N4 nanocomposite with RGO solid electron mediator for efficient photocatalytic CO2 reduction to CO and CH4. J. Environ. Chem. Eng. 2021, 9, 105600. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tahir, M. Facile fabrication of well-designed 2D/2D porous g-C3N4–GO nanocomposite for photocatalytic methane reforming (DRM) with CO2 towards enhanced syngas production under visible light. Fuel 2021, 305, 121558. [Google Scholar] [CrossRef]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent progress on titanium dioxide nanomaterials for photocatalytic applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef]
- Purabgola, A.; Mayilswamy, N.; Kandasubramanian, B. Graphene-based TiO2 composites for photocatalysis & environmental remediation: Synthesis and progress. Environ. Sci. Pollut. Res. 2022, 29, 32305–32325. [Google Scholar]
- Devi, A.D.; Pushpavanam, S.; Singh, N.; Verma, J.; Kaur, M.P.; Roy, S.C. Enhanced methane yield by photoreduction of CO2 at moderate temperature and pressure using Pt coated, graphene oxide wrapped TiO2 nanotubes. Results Eng. 2022, 14, 100441. [Google Scholar] [CrossRef]
- Khatun, F.; Aziz, A.A.; Kafi, A.K.M.; Sim, L.C. Synthesis and characterization of TiO2 nanotube using electrochemical anodization method. Int. J. Eng. Technol. Sci. 2018, 5, 132–139. [Google Scholar]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Grimes, C.A.; In, S.I. Facile fabrication of a noble metal-free photocatalyst: TiO2 nanotube arrays covered with reduced graphene oxide. Carbon 2016, 98, 537–544. [Google Scholar] [CrossRef]
- Zubair, M.; Kim, H.; Razzaq, A.; Grimes, C.A.; In, S.I. Solar spectrum photocatalytic conversion of CO2 to CH4 utilizing TiO2 nanotube arrays embedded with graphene quantum dots. J. CO2 Util. 2018, 26, 70–79. [Google Scholar] [CrossRef]
- Dvorak, F.; Zazpe, R.; Krbal, M.; Sopha, H.; Prikryl, J.; Ng, S.; Hromadko, L.; Bures, F.; Macak, J.M. One-dimensional anodic TiO2 nanotubes coated by atomic layer deposition: Towards advanced applications. Appl. Mater. Today 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Sim, L.C.; Leong, K.H.; Ibrahim, S.; Saravanan, P. Graphene oxide and Ag engulfed TiO2 nanotube arrays for enhanced electron mobility and visible-light-driven photocatalytic performance. J. Mater. Chem. A 2014, 2, 5315–5322. [Google Scholar] [CrossRef]
- Low, J.; Qiu, S.; Xu, D.; Jiang, C.; Cheng, B. Direct evidence and enhancement of surface plasmon resonance effect on Ag-loaded TiO2 nanotube arrays for photocatalytic CO2 reduction. Appl. Surf. Sci. 2018, 434, 423–432. [Google Scholar] [CrossRef]
- Lee, A.R.; Kim, J.Y. Highly Ordered TiO2 Nanotube Electrodes for Efficient Quasi-Solid-State Dye-Sensitized Solar Cells. Energies 2020, 13, 6100. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Z.; Wei, X.; Li, H.; Zhao, G.; Miao, J.; Wu, D.; Liu, B.; Cao, S.; An, D.; et al. Application potential of bone marrow mesenchymal stem cell (BMSCs) based tissue-engineering for spinal cord defect repair in rat fetuses with spina bifida aperta. J. Mater. Sci. Mater. Med. 2016, 27, 77. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Su, K.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Li, Z.; Liang, Y.; Zhu, S.; Yeung, K.W.K.; et al. Near-infrared light photocatalysis and photothermy of carbon quantum dots and au nanoparticles loaded titania nanotube array. Mater. Des. 2019, 177, 107845. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Tayebi, M.; Kolaei, M.; Tayyebi, A.; Masoumi, Z.; Belbasi, Z.; Lee, B.K. Reduced graphene oxide (RGO) on TiO2 for an improved photoelectrochemical (PEC) and photocatalytic activity. Sol. Energy 2019, 190, 185–194. [Google Scholar] [CrossRef]
- Sim, L.C.; Leong, K.H.; Saravanan, P.; Ibrahim, S. Rapid thermal reduced graphene oxide/Pt–TiO2 nanotube arrays for enhanced visible-light-driven photocatalytic reduction of CO2. Appl. Surf. Sci. 2015, 358, 122–129. [Google Scholar] [CrossRef]
- Goddeti, K.C.; Lee, C.; Lee, Y.K.; Park, J.Y. Three-dimensional hot electron photovoltaic device with vertically aligned TiO2 nanotubes. Sci. Rep. 2018, 8, 7330. [Google Scholar] [CrossRef]
- Zhuang, C.; Song, Z.; Yu, Z.; Zhang, C.; Wang, J.; Liu, Y.; Zhao, Q. Photoelectrochemical performance of TiO2 nanotube arrays modified with Ni2P Co-catalyst. Int. J. Hydrog. Energy 2021, 46, 4981–4991. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhou, D.; Wen, J.; Zheng, L.; Liang, W.; Yang, F. Diffusion kinetics of gold in TiO2 nanotube arrays for formation of Au@TiO2 nanotube arrays. RSC Adv. 2016, 6, 48580–48588. [Google Scholar] [CrossRef]
- Montakhab, E.; Rashchi, F.; Sheibani, S. Modification and photocatalytic activity of open channel TiO2 nanotubes array synthesized by anodization process. Appl. Surf. Sci. 2020, 534, 147581. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, H.; Zhao, Y.; Zhong, L.; Zhang, Y.; Wang, Y.; Xue, J.; Li, Y. Au nanoparticle modified three-dimensional network PVA/RGO/TiO2 composite for enhancing visible light photocatalytic performance. Appl. Surf. Sci. 2019, 498, 143855. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhou, D.; Zhao, W.; Qian, X.; Yang, Q.; Sun, T.; Shen, C. Ultra-low Au–Pt Co-decorated TiO2 nanotube arrays: Construction and its improved visible-light-induced photocatalytic properties. Sol. Energy Mater. Sol. Cells 2019, 201, 110065. [Google Scholar] [CrossRef]
- Jumeri, F.A.; Lim, H.N.; Zainal, Z.; Huang, N.M.; Pandikumar, A. Titanium dioxide-reduced graphene oxide thin film for photoelectrochemical water splitting. Ceram. Int. 2014, 40, 15159–15165. [Google Scholar]
- Sim, L.C.; Koh, K.S.; Leong, K.H.; Chin, Y.H.; Abd Aziz, A.; Saravanan, P. In situ growth of g-C3N4 on TiO2 nanotube arrays: Construction of heterostructures for improved photocatalysis properties. J. Environ. Chem. Eng. 2020, 8, 103611. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yan, W.; Zhao, H.; Yang, J. Synthesis of Nb doped TiO2 nanotube/reduced graphene oxide heterostructure photocatalyst with high visible light photocatalytic activity. Appl. Surf. Sci. 2018, 440, 804–813. [Google Scholar] [CrossRef]
- Wang, W.; Yu, J.; Xiang, Q.; Cheng, B. Enhanced photocatalytic activity of hierarchical macro/mesoporous TiO2–graphene composites for photodegradation of acetone in air. Appl. Catal. B 2012, 119, 109–116. [Google Scholar] [CrossRef]
- Zhang, G.; Miao, H.; Hu, X.; Mu, J.; Liu, X.; Han, T.; Fan, J.; Liu, E.; Yin, Y.; Wan, J. A facile strategy to fabricate Au/TiO2 nanotubes photoelectrode with excellent photoelectrocatalytic properties. Appl. Surf. Sci. 2017, 391, 345–352. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Y.; Zheng, W.; Huang, Y.; Xu, F.; Bao, Z. Synergistic photothermal antibacterial therapy enabled by multifunctional nanomaterials: Progress and perspectives. Mater. Chem. Front. 2023, 7, 355–380. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Du, Y.B.; Wang, N.; Li, X.N.; Li, J.; Wu, L.P.; Peng, Q.M.; Li, X.J. A facile synthesis of C3N4-modified TiO2 nanotube embedded Pt nanoparticles for photocatalytic water splitting. Res. Chem. Intermed. 2021, 47, 5175–5188. [Google Scholar] [CrossRef]
- Kumar, P.; Boukherroub, R.; Shankar, K. Sunlight-driven water-splitting using two-dimensional carbon based semiconductors. J. Mater. Chem. A 2018, 6, 12876–12931. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Enhanced photocatalytic H2 production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tang, Z.R.; Xu, Y.J. Multifunctional graphene-based composite photocatalysts oriented by multifaced roles of graphene in photocatalysis. Chin. J. Catal. 2022, 43, 708–730. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, S.; Liu, X.; O’Mullane, A.P. The application and improvement of TiO2 (titanate) based nanomaterials for the photoelectrochemical conversion of CO2 and N2 into useful products. Catal. Sci. Technol. 2021, 11, 768–778. [Google Scholar] [CrossRef]
- Ciocarlan, R.G.; Blommaerts, N.; Lenaerts, S.; Cool, P.; Verbruggen, S.W. Recent Trends in Plasmon-Assisted Photocatalytic CO2 Reduction. ChemSusChem 2023, 16, e202201647. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Feng, S.; Wang, M.; Zhou, Y.; Li, P.; Tu, W.; Zou, Z. Double-shelled plasmonic Ag-TiO2 hollow spheres toward visible light-active photocatalytic conversion of CO2 into solar fuel. Appl. Mater. 2015, 3, 104416. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S. Gold-nanoparticle-modified TiO2 nanowires for plasmon-enhanced photocatalytic CO2 reduction with H2 under visible light irradiation. Appl. Surf. Sci. 2015, 356, 1289–1299. [Google Scholar] [CrossRef]
- Xu, Q.; Xia, Z.; Zhang, J.; Wei, Z.; Guo, Q.; Jin, H.; Tang, H.; Li, S.; Pan, X.; Su, Z.; et al. Recent advances in solar-driven COs reduction over g-C3N4-based photocatalysts. Carbon Energy 2023, 5, e205. [Google Scholar] [CrossRef]


| Photocatalysts | Synthesis Methods of TNTAs and Composite | Photocatalytic Reaction Conditions | Reactants | Total CH4 Yield | Average Yield Rate | Enhancement of CH4 Yield Compared to Pure TNTAs | Ref. |
|---|---|---|---|---|---|---|---|
| TNTAs | Anodization, photo-deposition + immersion | 500 W tungsten–halogen lamp visible light 100 W/m2 5 h | CO2 + H2O vapour | 5.67 µmol m−2 | 1.13 µmol m−2 h−1 | - | [56] |
| RGO-TNTs | 6.89 µmol m−2 | 1.38 µmol m−2 h−1 | 1.22-times | ||||
| Pt-TNTAs | 9.03 µmol m−2 | 1.81 µmol m−2 h−1 | 1.6-times | ||||
| RGO/Pt-TNTAs | 10.96 µmol m−2 | 2.19 µmol m−2 h−1 | 1.94-times | ||||
| TNTAs | Anodization + electrochemical deposition | 100 W Xe lamp visible light 1 h | CO2 + H2O vapour | 1.28 ppm cm−2 | 1.28 ppm cm−2 h−1 | - | [46] |
| RGO-TNTAs | 5.67 ppm cm−2 | 5.67 ppm cm−2 h−1 | 4.4-times | ||||
| TNTAs | Anodization + electrochemical deposition | 100 W Xe lamp visible light 3 h | CO2 + H2O vapour | 1.05 ppm cm−2 | 0.35 ppm cm−2 h−1 | - | [47] |
| GQDs-TNTAs | 5.94 ppm cm−2 | 1.98 ppm cm−2 h−1 | 5.6-times | ||||
| TNTAs | Anodization + immersion | 35 W Xe lamp visible light 20 mW cm−2 4 h | CO2+ H2O vapor | 1713.6 µmol m−2 | 428.4 µmol m−2 h−1 | - | [23] |
| g-C3N4-TNTAs | 1841.5 µmol m−2 | 460.4 µmol m−2 h−1 | 1.13-times | ||||
| g-C3N4-GO-TNTAs | 2470.9 µmol m−2 | 617.7 µmol m−2 h−1 | 1.52-times | ||||
| g-C3N4-RGO-TNTAs | 3322.1 µmol m−2 | 830.5 µmol m−2 h−1 | 1.94-times | ||||
| TNTAs | Anodization + electrophoretic deposition | 400 W metal-halide lamp visible light 10 h | CO2 + H2O vapour | 4700 µmol g−1 | 470 µmol g−1 h−1 | - | [43] |
| GO-TNTAs | 11,500 µmol g−1 | 1150 µmolg−1 h−1 | 2.45-times | ||||
| Pt-TNTAs | 34,320 µmol g−1 | 3432 µmol g−1 h−1 | 7.3-times | ||||
| GO/Pt-TNTAs | 31,430 µmol g−1 | 3143 µmol g−1 h−1 | 6.69-times | ||||
| TNTAs | Anodization, electrochemical-deposition + immersion | 300 W Xe lamp visible light 100 W/m2 4 h | CO2 + H2O vapour | 4.02 ppm cm−2 | 1.01 ppm cm−2 h−1 | - | This study |
| RGO-TNTAs | 12.46 ppm cm−2 | 3.12 ppm cm−2 h−1 | 3.1-times | ||||
| Au-TNTAs | 21.64 ppm cm−2 | 5.41 ppm cm−2 h−1 | 5.38-times | ||||
| RGO/Au-TNTAs | 35.13 ppm cm−2 | 8.78 ppm cm−2 h−1 | 8.75-times |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossen, M.A.; Khatun, F.; Ikreedeegh, R.R.; Muhammad, A.D.; Abd Aziz, A.; Leong, K.H.; Sim, L.C.; Lihua, W.; Monir, M.U. Enhanced Photocatalytic CO2 Reduction to CH4 Using Novel Ternary Photocatalyst RGO/Au-TNTAs. Energies 2023, 16, 5404. https://doi.org/10.3390/en16145404
Hossen MA, Khatun F, Ikreedeegh RR, Muhammad AD, Abd Aziz A, Leong KH, Sim LC, Lihua W, Monir MU. Enhanced Photocatalytic CO2 Reduction to CH4 Using Novel Ternary Photocatalyst RGO/Au-TNTAs. Energies. 2023; 16(14):5404. https://doi.org/10.3390/en16145404
Chicago/Turabian StyleHossen, Md. Arif, Fatema Khatun, Riyadh Ramadhan Ikreedeegh, Aamina Din Muhammad, Azrina Abd Aziz, Kah Hon Leong, Lan Ching Sim, Wu Lihua, and Minhaj Uddin Monir. 2023. "Enhanced Photocatalytic CO2 Reduction to CH4 Using Novel Ternary Photocatalyst RGO/Au-TNTAs" Energies 16, no. 14: 5404. https://doi.org/10.3390/en16145404
APA StyleHossen, M. A., Khatun, F., Ikreedeegh, R. R., Muhammad, A. D., Abd Aziz, A., Leong, K. H., Sim, L. C., Lihua, W., & Monir, M. U. (2023). Enhanced Photocatalytic CO2 Reduction to CH4 Using Novel Ternary Photocatalyst RGO/Au-TNTAs. Energies, 16(14), 5404. https://doi.org/10.3390/en16145404











