Progress on Phenanthroimidazole Derivatives for Light-Emitting Electrochemical Cells: An Overview
Abstract
:1. Introduction
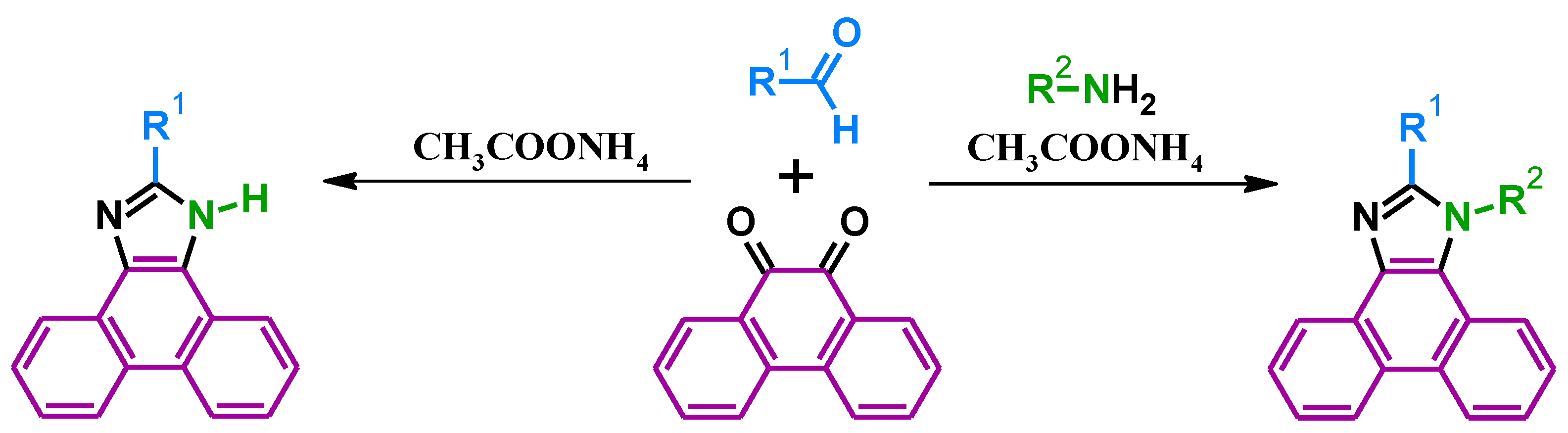
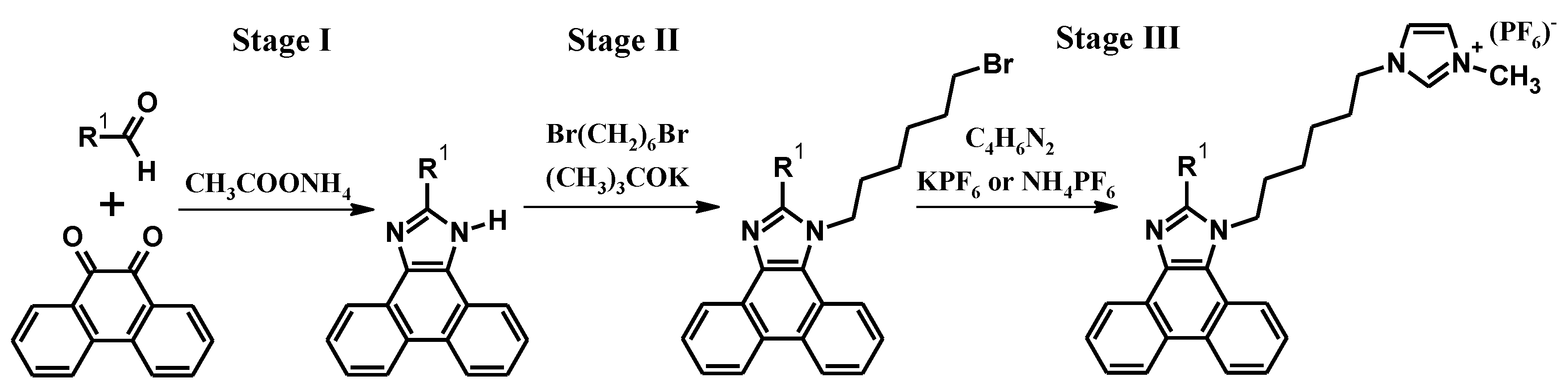
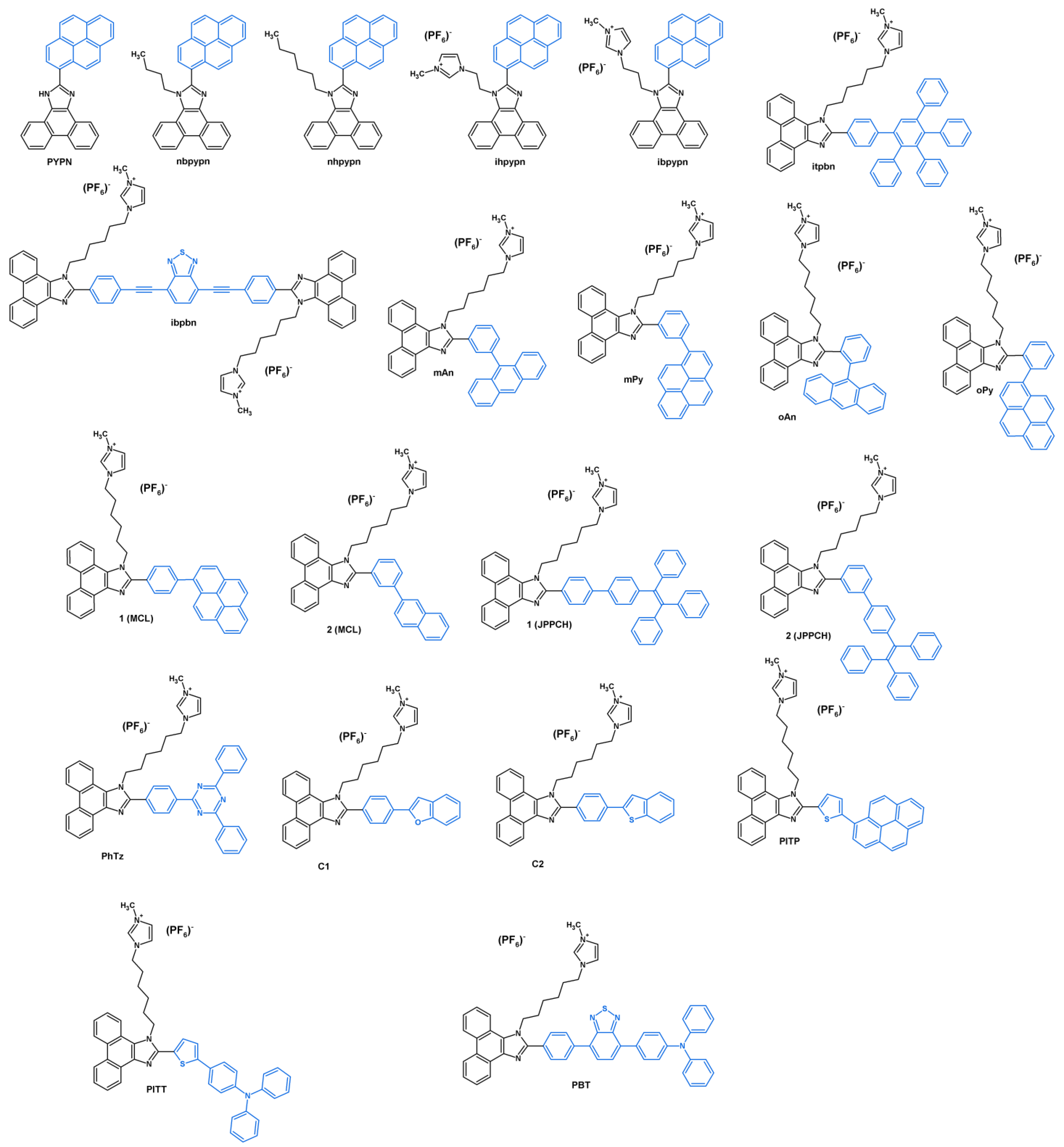
2. Synthesis
3. Thermal Properties
4. Redox Behavior
5. DFT Calculations
6. Optical Properties
7. Electroluminescence Properties and Device Characterization
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sayed, E.T.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Mdallal, A.; Rezk, A.; Abdelkareem, M.A. Renewable Energy and Energy Storage Systems. Energies 2023, 16, 1415. [Google Scholar] [CrossRef]
- Sha, M.-Z.; Pu, Y.-J.; Yin, H.; Hao, X.-T. Recent Progress of Indoor Organic Photovoltaics—From Device Performance to Multifunctional Applications. Org. Electron. 2023, 114, 106736. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Q.; Ma, K.; Shen, W.; Belfiore, L.A.; Bao, X.; Tang, J. Recent Developments of Polymer Solar Cells with Photovoltaic Performance over 17%. Adv. Funct. Mater. 2023, 33, 2213324. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, H.; Huang, J.; Xie, H. Natural Materials for Sustainable Organic Solar Cells: Status and Challenge. Adv. Funct. Mater. 2023, 33, 2213910. [Google Scholar] [CrossRef]
- Solak, E.K.; Irmak, E. Advances in Organic Photovoltaic Cells: A Comprehensive Review of Materials, Technologies, and Performance. RSC Adv. 2023, 13, 12244–12269. [Google Scholar] [CrossRef] [PubMed]
- Fara, L.; Chilibon, I.; Craciunescu, D.; Diaconu, A.; Fara, S. Review: Heterojunction Tandem Solar Cells on Si-Based Metal Oxides. Energies 2023, 16, 3033. [Google Scholar] [CrossRef]
- Mohamed El Amine, B.; Zhou, Y.; Li, H.; Wang, Q.; Xi, J.; Zhao, C. Latest Updates of Single-Junction Organic Solar Cells up to 20% Efficiency. Energies 2023, 16, 3895. [Google Scholar] [CrossRef]
- Tarique, W.B.; Uddin, A. A Review of Progress and Challenges in the Research Developments on Organic Solar Cells. Mater. Sci. Semicond. Process. 2023, 163, 107541. [Google Scholar] [CrossRef]
- Ahmad, N.; Liang, G.; Fan, P.; Zhou, H. Anode Interfacial Modification for Non-Fullerene Polymer Solar Cells: Recent Advances and Prospects. Infomat 2022, 4, e12370. [Google Scholar] [CrossRef]
- Woo, J.Y.; Park, M.; Jeong, S.; Kim, Y.; Kim, B.; Lee, T.; Han, T. Advances in Solution-Processed OLEDs and Their Prospects for Use in Displays. Adv. Mater. 2023, 34, 2207454. [Google Scholar] [CrossRef]
- Miao, W.; Hsiao, F.; Sheng, Y.; Lee, T.; Hong, Y.; Tsai, C.; Chen, H.; Liu, Z.; Lin, C.; Chung, R.; et al. Microdisplays: Mini-LED, Micro-OLED, and Micro-LED. Adv. Opt. Mater. 2023, 11, 2300112. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Tang, Y.-Q.; Cai, X.-Y.; Tang, J.-X.; Li, Y.-Q. Solution-Processed OLEDs for Printing Displays. Mater. Chem. Front. 2023, 7, 1166–1196. [Google Scholar] [CrossRef]
- Zuo, P.; Qu, Y.-K.; Zheng, Q.; Liao, L.-S.; Jiang, Z.-Q. Sensitized Organic Light-Emitting Diodes: Towards High Efficiency and Long Lifetimes. Mater. Chem. Front. 2023, 7, 1760–1780. [Google Scholar] [CrossRef]
- Zhou, Z.; Xie, X.; Sun, Z.; Wang, X.; An, Z.; Huang, W. Recent Advances in Metal-Free Phosphorescent Materials for Organic Light-Emitting Diodes. J. Mater. Chem. C 2023, 11, 3143–3161. [Google Scholar] [CrossRef]
- Yadav, S.; Mittal, P.; Negi, S. Recent Advancements over a Decade for Organic Light-Emitting Diodes: From Structural Diversity, Role of Layers, Colour Emission, Material Classification, Performance Improvement, Fabrication to Applications. Bull. Mater. Sci. 2022, 45, 109. [Google Scholar] [CrossRef]
- Kanagaraj, S.; Puthanveedu, A.; Choe, Y. Small Molecules in Light-Emitting Electrochemical Cells: Promising Light-Emitting Materials. Adv. Funct. Mater. 2020, 30, 1907126. [Google Scholar] [CrossRef]
- Subeesh, M.S.; Shanmugasundaram, K.; Sunesh, C.D.; Won, Y.S.; Choe, Y. Utilization of a Phenanthroimidazole Based Fluorophore in Light-Emitting Electrochemical Cells. J. Mater. Chem. C 2015, 3, 4683–4687. [Google Scholar] [CrossRef]
- Subeesh, M.S.; Shanmugasundaram, K.; Sunesh, C.D.; Nguyen, T.P.; Choe, Y. Phenanthroimidazole Derivative as an Easily Accessible Emitter for Non-Doped Light-Emitting Electrochemical Cells. J. Phys. Chem. C 2015, 119, 23676–23684. [Google Scholar] [CrossRef]
- Subeesh, M.S.; Shanmugasundaram, K.; Sunesh, C.D.; Chitumalla, R.K.; Jang, J.; Choe, Y. Host–Dopant System to Generate Bright Electroluminescence from Small Organic Molecule Functionalized Light-Emitting Electrochemical Cells. J. Phys. Chem. C 2016, 120, 12207–12217. [Google Scholar] [CrossRef]
- Subeesh, M.S.; Nguyen, T.P.; Choe, Y. Blue Light-Emitting Electrochemical Cells Based on Angularly Structured Phenanthroimidazole Derivatives. J. Phys. Chem. C 2017, 121, 14811–14818. [Google Scholar] [CrossRef]
- Son, M.; Choe, Y. Phenanthroimidazole Derivatives for Single Component Blue Light-Emitting Electrochemical Cells. Mol. Cryst. Liq. Cryst. 2017, 654, 234–243. [Google Scholar] [CrossRef]
- Park, J.; Shanmugasundaram, K.; John, J.C.; Choe, Y. Aggregation Induced Emission Small Molecules for Blue Light-Emitting Electrochemical Cells. J. Photochem. Photobiol. A Chem. 2019, 374, 10–15. [Google Scholar] [CrossRef]
- Puthanveedu, A.; Shanmugasundaram, K.; John, J.C.; Choe, Y. Novel Triazine-Based Donor–Acceptor Ionic Green Emitters for Nondoped Light-Emitting Electrochemical Cells. J. Phys. Chem. C 2020, 124, 19273–19281. [Google Scholar] [CrossRef]
- John, J.C.; Shanmugasundaram, K.; Puthanveedu, A.; Rao, C.V.S.B.; Gopakumar, G.; Choe, Y. Introduction of Heterocyclic Ring to Phenanthroimidazole Moiety for Efficient Blue Emitting Ionic Small Molecule LECs. Org. Electron. 2020, 87, 105939. [Google Scholar] [CrossRef]
- Youssef, K.; Li, Y.; O’Keeffe, S.; Li, L.; Pei, Q. Fundamentals of Materials Selection for Light-Emitting Electrochemical Cells. Adv. Funct. Mater. 2020, 30, 1909102. [Google Scholar] [CrossRef]
- Yang, Z.-P.; Su, H.-C. Recent Advances in Optical Engineering of Light-Emitting Electrochemical Cells. Adv. Funct. Mater. 2020, 30, 1906788. [Google Scholar] [CrossRef]
- Ràfols-Ribé, J.; Robinson, N.D.; Larsen, C.; Tang, S.; Top, M.; Sandström, A.; Edman, L. Self-Heating in Light-Emitting Electrochemical Cells. Adv. Funct. Mater. 2020, 30, 1908649. [Google Scholar] [CrossRef] [Green Version]
- Pashaei, B.; Karimi, S.; Shahroosvand, H.; Pilkington, M. Molecularly Engineered Near-Infrared Light-Emitting Electrochemical Cells. Adv. Funct. Mater. 2020, 30, 1908103. [Google Scholar] [CrossRef]
- Nannen, E.; Frohleiks, J.; Gellner, S. Light-Emitting Electrochemical Cells Based on Color-Tunable Inorganic Colloidal Quantum Dots. Adv. Funct. Mater. 2020, 30, 1907349. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Been, H.; John, J.C.; Puthanveedu, A.; Pharm, N.N.T.; Lee, S.G.; Choe, Y. Simple Luminescent Phenanthroimidazole Emitters for Solution-Processed Non-Doped Organic Light-Emitting Electrochemical Cells. New J. Chem. 2021, 45, 19338–19346. [Google Scholar] [CrossRef]
- John, J.C.; Shanmugasundaram, K.; Gopakumar, G.; Choe, Y. Bright and Efficient Red Light-Emitting Electrochemical Cells with Nondoped Organic Small Molecules: A New Approach. ACS Photonics 2022, 9, 203–210. [Google Scholar] [CrossRef]
- Yoo, J.; Li, S.; Kim, D.-H.; Yang, J.; Choi, M.K. Materials and Design Strategies for Stretchable Electroluminescent Devices. Nanoscale Horiz. 2022, 7, 801–821. [Google Scholar] [CrossRef] [PubMed]
- Yasuji, K.; Sakanoue, T.; Yonekawa, F.; Kanemoto, K. Visualizing Electroluminescence Process in Light-Emitting Electrochemical Cells. Nat. Commun. 2023, 14, 992. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Shangguan, R.; Huang, H.; Tu, G.; Wang, L.; Zhu, X. Synthesis, Characterization, Physical Properties, and Blue Electroluminescent Device Applications of Phenanthroimidazole Derivatives Containing Anthracene or Pyrene Moiety. Dye. Pigment. 2014, 101, 93–102. [Google Scholar] [CrossRef]
- Wang, J.; Lou, X.; Liu, Y.; Zhao, G.; Islam, A.; Wang, S.; Ge, Z. Controllable Molecular Configuration for Significant Improvement of Blue OLEDs Based on Novel Twisted Anthracene Derivatives. Dye. Pigment. 2015, 118, 137–144. [Google Scholar] [CrossRef]
- Tang, X.; Bai, Q.; Peng, Q.; Gao, Y.; Li, J.; Liu, Y.; Yao, L.; Lu, P.; Yang, B.; Ma, Y. Efficient Deep Blue Electroluminescence with an External Quantum Efficiency of 6.8% and CIEy < 0.08 Based on a Phenanthroimidazole–Sulfone Hybrid Donor–Acceptor Molecule. Chem. Mater. 2015, 27, 7050–7057. [Google Scholar] [CrossRef]
- Shan, T.; Gao, Z.; Tang, X.; He, X.; Gao, Y.; Li, J.; Sun, X.; Liu, Y.; Liu, H.; Yang, B.; et al. Highly Efficient and Stable Pure Blue Nondoped Organic Light-Emitting Diodes at High Luminance Based on Phenanthroimidazole-Pyrene Derivative Enabled by Triplei-Triplet Annihilation. Dye. Pigment. 2017, 142, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Shi, J.; Xu, X.; Lu, Y.; Sun, Q.; Xue, S.; Yang, W. Tuning the Optoelectronic Properties of Phenothiazine-Based D–A-Type Emitters through Changing Acceptor Pattern. Dye. Pigment. 2017, 147, 6–15. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Yan, X.; Zhang, Y.; Zhang, Z.; Wang, Y. Structurally Simple Non-Doped Sky-Blue OLEDs with High Luminance and Efficiencies at Low Driving Voltages. J. Mater. Chem. C 2017, 5, 1973–1980. [Google Scholar] [CrossRef]
- Thanikachalam, V.; Sarojpurani, E.; Jayabharathi, J.; Jeeva, P. Efficient Phenanthroimidazole-Styryl-Triphenylamine Derivatives for Blue OLEDs: A Combined Experimental and Theoretical Study. New J. Chem. 2017, 41, 2443–2457. [Google Scholar] [CrossRef]
- Chen, W.-C.; Yuan, Y.; Xiong, Y.; Rogach, A.L.; Tong, Q.-X.; Lee, C.-S. Aromatically C6- and C9-Substituted Phenanthro[9,10-d]Imidazole Blue Fluorophores: Structure–Property Relationship and Electroluminescent Application. ACS Appl. Mater. Interfaces 2017, 9, 26268–26278. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewska, B.; Gordel, M.; Szeremeta, J.; Grela, I.; Samoć, M. Photostability of Push-Pull Phenanthroimidazole Derivative upon One- and Two-Photon Excitation. Dye. Pigment. 2017, 136, 150–160. [Google Scholar] [CrossRef]
- Kula, S.; Szlapa-Kula, A.; Kotowicz, S.; Filapek, M.; Bujak, K.; Siwy, M.; Janeczek, H.; Maćkowski, S.; Schab-Balcerzak, E. Phenanthro[9,10-d]Imidazole with Thiophene Rings toward OLEDs Application. Dye. Pigment. 2018, 159, 646–654. [Google Scholar] [CrossRef]
- Tagare, J.; Vaidyanathan, S. Recent Development of Phenanthroimidazole-Based Fluorophores for Blue Organic Light-Emitting Diodes (OLEDs): An Overview. J. Mater. Chem. C 2018, 6, 10138–10173. [Google Scholar] [CrossRef]
- Kothavale, S.; Bhalekar, S.; Sekar, N. Highly Fluorescent Blue-Green Emitting Phenanthroimidazole Derivatives: Detail Experimental and DFT Study of Structural and Donating Group Effects on Fluorescence Properties. Dye. Pigment. 2018, 159, 209–221. [Google Scholar] [CrossRef]
- Zhu, Z.-L.; Ni, S.-F.; Chen, W.-C.; Chen, M.; Zhu, J.-J.; Yuan, Y.; Tong, Q.-X.; Wong, F.-L.; Lee, C.-S. Tuning Electrical Properties of Phenanthroimidazole Derivatives to Construct Multifunctional Deep-Blue Electroluminescent Materials. J. Mater. Chem. C 2018, 6, 3584–3592. [Google Scholar] [CrossRef]
- Kula, S.; Szlapa-Kula, A.; Filapek, M.; Bujak, K.; Kotowicz, S.; Siwy, M.; Grzelak, J.; Szalkowski, M.; Maćkowski, S.; Schab-Balcerzak, E. Novel Phenanthro[9,10-d]Imidazole Derivatives—Effect of Thienyl and 3,4-(Ethylenedioxy)Thienyl Substituents. Synth. Met. 2019, 251, 40–48. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Panimozhi, S.; Thanikachalam, V. Asymmetrically Twisted Phenanthrimidazole Derivatives as Host Materials for Blue Fluorescent, Green and Red Phosphorescent OLEDs. Sci. Rep. 2019, 9, 17555. [Google Scholar] [CrossRef] [Green Version]
- Kula, S.; Ledwon, P.; Maroń, A.M.; Siwy, M.; Grzelak, J.; Szalkowski, M.; Maćkowski, S.; Schab-Balcerzak, E. Synthesis, Photophysical Properties and Electroluminescence Characterization of 1-Phenyl-1H-Phenanthro[9,10-d]Imidazole Derivatives with N-Donor Substituents. Dye. Pigment. 2021, 192, 109437. [Google Scholar] [CrossRef]
- Kula, S.; Krawczyk, P.; Filapek, M.; Maroń, A.M. Influence of N-Donor Substituents on Physicochemical Properties of Phenanthro[9,10-d]Imidazole Derivatives. J. Lumin. 2021, 233, 117910. [Google Scholar] [CrossRef]
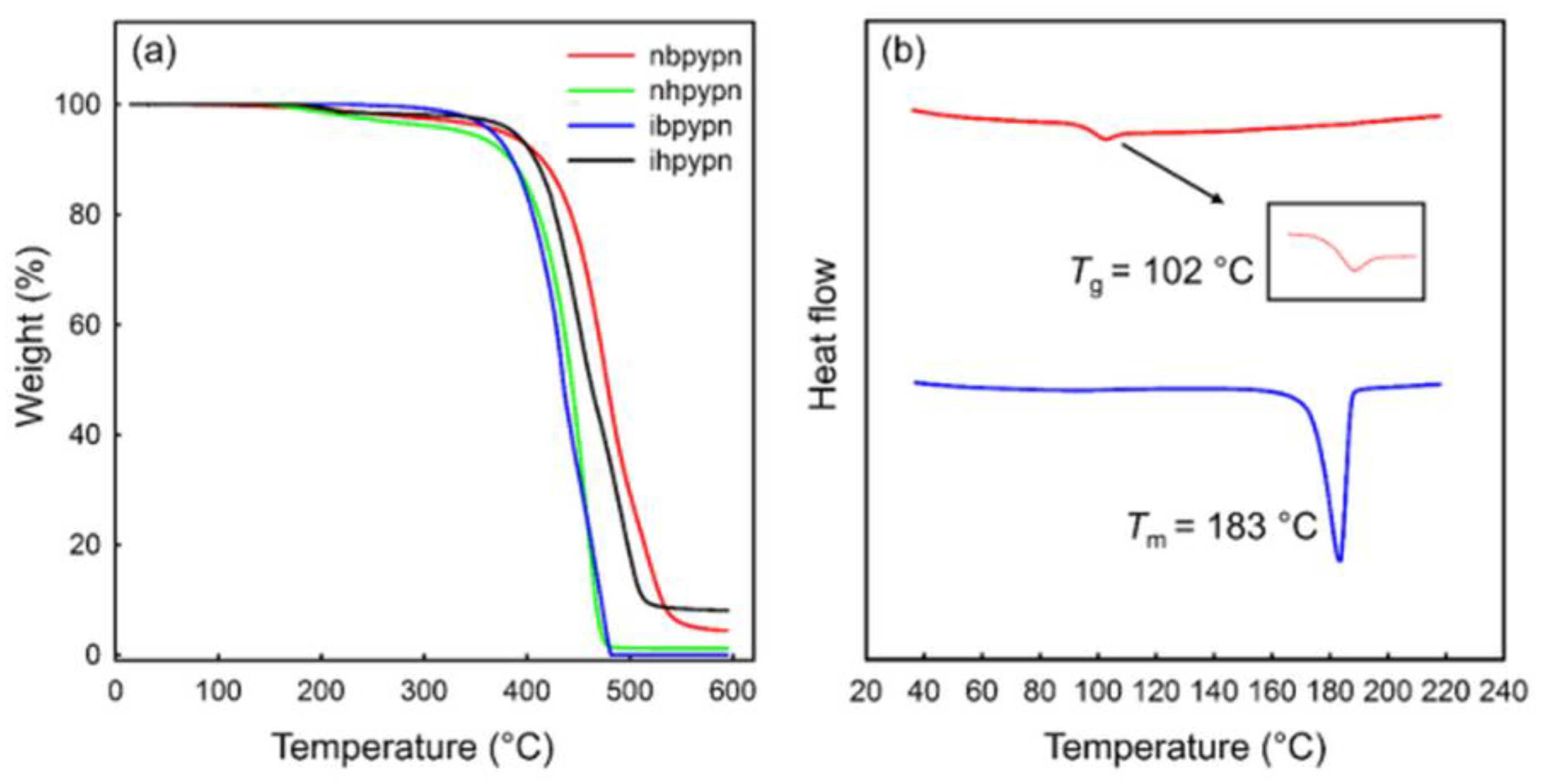
| Code | Tm (°C) (Melting Point) | Tg (°C) (Glass Transition Temperature) | Td (°C) (5% Weight Loss) | Ref. |
|---|---|---|---|---|
| PYPN | 319 | 160 | 426 | [17] |
| nhpypn | not detected | not detected | 339 | [18] |
| nbpypn | 183 | 102 | 376 | [18] |
| ihpypn | not detected | not detected | 386 | [18] |
| ibpypn | not detected | not detected | 366 | [18] |
| ibpbn | - | - | - | [19] |
| itpbn | - | - | - | [19] |
| mPy | not detected | 112 | 398 | [20] |
| mAn | not detected | not detected | 376 | [20] |
| oPy | not detected | not detected | 376 | [20] |
| oAn | not detected | 114 | 364 | [20] |
| 1 (MCL) | - | 131 | 416 | [21] |
| 2 (MCL) | - | 99 | 375 | [21] |
| 1 (JPPCH) | - | 127 | 337 | [22] |
| 2 (JPPCH) | - | 116 | 336 | [22] |
| PhTz | - | 120 | 370 | [23] |
| C1 | - | 125 | 360 | [24] |
| C2 | - | 115 | 335 | [24] |
| PITP | - | 133 | 377 | [30] |
| PITT | - | 143 | 386 | [30] |
| PBT | - | 190 | 357 | [31] |
| Code | Eonset (Ox) (V) | Eonset (Red) (V) | HOMO (eV) | LUMO (eV) | Ref. |
|---|---|---|---|---|---|
| PYPN | 0.99 | - | −5.30 | −2.40 | [17] |
| nhpypn | - | - | - | - | [18] |
| nbpypn | - | - | - | - | [18] |
| ihpypn | 0.99 | - | −5.33 | −2.24 | [19] |
| ibpypn | - | - | - | - | [18] |
| ibpbn | 1.00 | - | −5.34 | −2.75 | [19] |
| itpbn | 0.98 | - | −5.32 | −2.00 | [19] |
| mPy | 1.23 * | −0.75 * | −5.57 | −3.59 | [20] |
| mAn | 1.23 * | −0.80 * | −5.57 | −3.54 | [20] |
| oPy | 1.16 * | −0.79 * | −5.50 | −3.55 | [20] |
| oAn | 1.00 * | −0.80 * | −5.34 | −3.54 | [20] |
| 1 (MCL) | - | - | −5.58 | −2.37 | [21] |
| 2 (MCL) | - | - | −5.63 | −2.37 | [21] |
| 1 (JPPCH) | 1.21 | - | −5.61 | −2.46 | [22] |
| 2 (JPPCH) | 1.26 | - | −5.66 | −2.36 | [22] |
| PhTz | 1.20 | −1.33 | −5.60 | −3.07 | [23] |
| C1 | 1.16 | - | −5.56 | −2.33 | [24] |
| C2 | 1.20 | - | −5.60 | −2.36 | [24] |
| PITP | 0.99 | - | −5.39 | −2.62 | [30] |
| PITT | 0.81 | - | −5.21 | −2.40 | [30] |
| PBT | 0.68 | - | −5.08 | −2.72 | [31] |
| Code | HOMO (eV) | LUMO (eV) | Dihedral Angle * (°) | H-LG (eV) | λabs (nm) | λem (nm) | Ref. |
|---|---|---|---|---|---|---|---|
| PYPN | - | - | - | - | - | - | [17] |
| nhpypn | −5.16 | −1.68 | 61 | 3.48 | - | - | [18] |
| nbpypn | −5.16 | −1.68 | 61 | 3.48 | - | - | [18] |
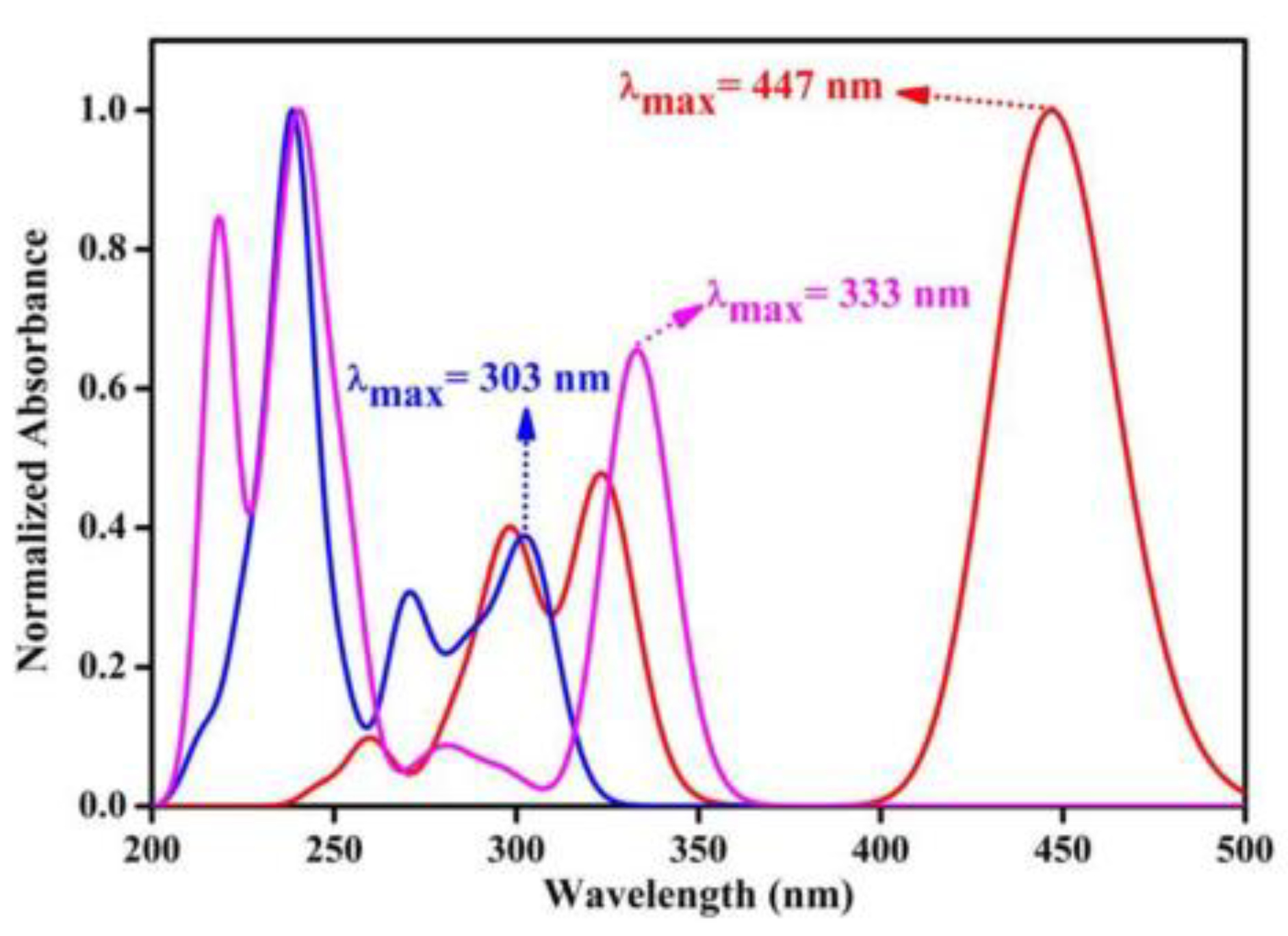
| ihpypn | −5.37 | −1.82 | 59 | 3.55 | 333 | 403 | [19] |
| ibpypn | −5.16 | −1.68 | 61 | 3.48 | - | - | [18] |
| ibpbn | −5.35 | −2.91 | 31 | 2.77 | 447 | 510 | [19] |
| itpbn | −5.43 | −1.37 | 37 | 4.06 | 303 | 369 | [19] |
| mPy | - | - | 35 | - | - | - | [20] |
| mAn | - | - | 35 | - | - | - | [20] |
| oPy | - | - | 59 | - | - | - | [20] |
| oAn | - | - | 66 | - | - | - | [20] |
| 1 (MCL) | - | - | - | - | - | - | [21] |
| 2 (MCL) | - | - | - | - | - | - | [21] |
| 1 (JPPCH) | - | - | - | - | - | - | [22] |
| 2 (JPPCH) | - | - | - | - | - | - | [22] |
| PhTz | −5.50 | −2.14 | 3.36 | [23] | |||
| C1 | −5.42 | −2.25 | - | 3.17 | 391 | - | [24] |
| C2 | −5.44 | −2.27 | - | 3.17 | 391 | - | [24] |
| PITP | −5.31 | −2.55 | - | 2.76 | - | - | [30] |
| PITT | −5.14 | −2.47 | - | 2.67 | - | - | [30] |
| PBT | −7.00 | −5.56 | - | 1.44 | 420−430 nm | - | [31] |
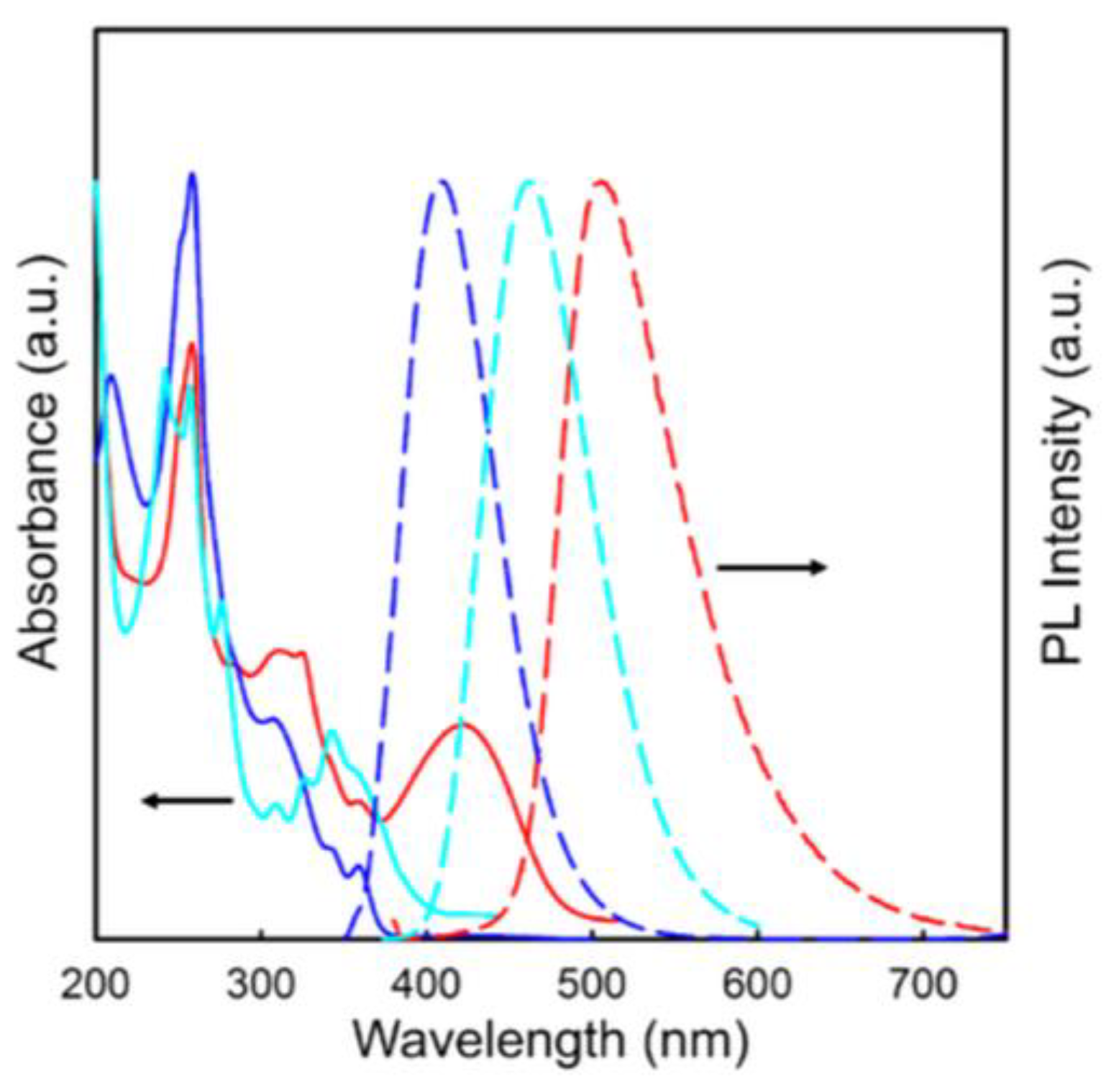
| Code | λabs (nm) | λPL-solution (nm) | Φsolution | Egopt (eV) | λPL-thin film (nm) | Φthin film | Ref. |
|---|---|---|---|---|---|---|---|
| PYPN | 260; 380 | 458 | 0.60 | 2.84 | 477 | 0.30 | [17] |
| nhpypn | 242; 258; 277; 312; 328; 345; 360 | 445 | 0.65 | 3.06 | 459 | 0.31 | [18] |
| nbpypn | 242; 258; 277; 312; 328; 345; 360 | 445 | 0.64 | 3.10 | 462 | 0.30 | [18] |
| ihpypn | 242; 258; 277; 312; 328; 345; 360 | 453 | 0.64 | 3.07 | 463 | 0.36 | [18] |
| ibpypn | 242; 258; 277; 312; 328; 345; 360 | 450 | 0.65 | 3.09 | 458 | 0.35 | [18] |
| ibpbn | 421 | 506 | 0.002 | 2.59 | 583 | - | [19] |
| itpbn | - | 409 | 0.95 | 3.33 | - | - | [19] |
| mPy | - | 381; 397; 494 | - | 3.23 | 449 | 0.15 | [20] |
| mAn | - | - | - | 3.02 | - | 0.10 | [20] |
| oPy | - | - | - | 3.24 | - | 0.25 | [20] |
| oAn | - | - | - | - | - | - | [20] |
| 1 (MCL) | 345 | 455 | - | 3.21 | - | - | [21] |
| 2 (MCL) | 314 | 425 | - | 3.26 | - | - | [21] |
| 1 (JPPCH) | 256; 334; 361 | - | - | 3.15 | 475 | - | [22] |
| 2 (JPPCH) | 256; 314; 356 | - | - | 3.30 | 475 | - | [22] |
| PhTz | 369 | 490 | 0.35 | 2.95 | 500 | 0.27 | [23] |
| C1 | 255; 340; 366 | 428 | 0.46 | 3.23 | 433 | - | [24] |
| C2 | 259; 340; 366 | 433 | 0.66 | 3.24 | 435 | - | [24] |
| PITP | 379 | 492 | 0.65 | 2.81 | 508 | - | [30] |
| PITT | 388 | 480 | 0.60 | 2.77 | 520 | - | [30] |
| PBT | 442 | 619 | 0.46 | 2.36 | 625 | 0.47 | [31] |
| Code | ELmax a (nm) | Von b (V) | Lmax c (cd/m2) | CE d (cd/A) | effmax e (cd/A) | PE f (lm/W) | EQE (%) | CIE g (x;y) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PYPN | 521 | 4.3 | - | - | - | - | - | 0.38; 0.49 | [17] |
| nhpypn | 491 | 6.5 | 278 | 0.31 | 0.38 (62) | - | - | 0.193; 0.362 | [18] |
| nbpypn | 503 | 8.5 | 49 | 0.18 | 0.29 (5.1) | - | - | 0.211; 0.386 | [18] |
| ihpypn | 487 | 5.8 | 711 | 0.18 | 0.2 (98) | - | - | 0.191; 0.309 | [18] |
| ibpypn | 484 | 8.7 | 586 | 0.10 | 0.19 (174) | - | - | 0.181; 0.270 | [18] |
| ihpypn: 0.3% ibpbn | 496 | 6 | 3795 | 0.30 | 0.62 (2986) | - | - | 0.20; 0.33 | [19] |
| ihpypn: 0.6% ibpbn | 496 | 7 | 5016 | 0.73 | 1.35 (1609) | - | - | 0.23; 0.37 | [19] |
| ihpypn: 0.9% ibpbn | 509 | 7.2 | 2239 | 0.48 | 0.81 (434) | - | - | 0.23; 0.39 | [19] |
| ihpypn: 1.2% ibpbn | 532 | 9.2 | 2166 | 0.37 | 1.38 (221) | - | - | 0.29; 0.43 | [19] |
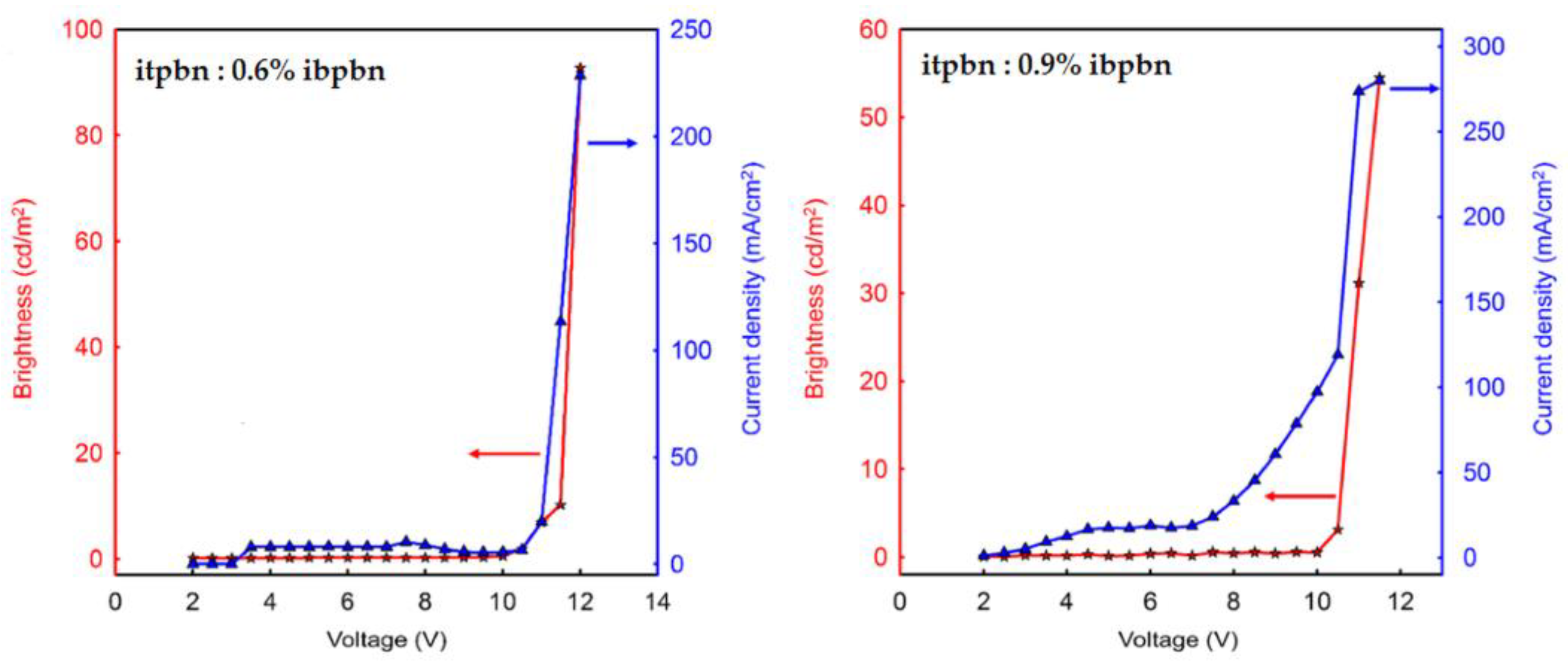
| itpbn: 0.6% ibpbn | - | 10.5 | 92 | 0.04 | 0.04 (92) | - | - | 0.22; 0.23 | [19] |
| itpbn: 0.9% ibpbn | - | 10.5 | 54 | 0.02 | 0.02 (54) | - | - | 0.23; 0.28 | [19] |
| ibpbn | 622 | 4 | 30 | 0.005 | 0.005 (30) | - | - | 0.54; 0.45 | [19] |
| mPy | 485 | 7.0 | 179 | 0.02 | 0.03 (22) | - | - | 0.183; 0.258 | [20] |
| mPy:PEO: LiOTf 1:0.1:0.05 | 485 | 8.25 | 465 | 0.29 | 0.43 (137) | - | - | 0.168; 0.232 | [20] |
| mPy:PEO: LiOTf 1:0.1:0.1 | 485 | 7.0 | 440 | 0.08 | 0.29 (12) | - | - | 0.160; 0.239 | [20] |
| mPy:PEO: LiOTf 1:0.1:0.15 | 485 | 7.0 | 585 | 0.06 | 0.19 (65) | - | - | 0.170; 0.226 | [20] |
| mAn | 467 | 5.0 | 26 | 0.0054 | 0.0054 (26) | - | - | 0.197; 0.236 | [20] |
| mAn:PEO: LiOTf 1:0.1:0.1 | 459 | 6.25 | 83 | 0.02 | 0.05 (9) | - | - | 0.166; 0.173 | [20] |
| oPy | - | - | - | - | - | - | - | - | [20] |
| oAn | - | - | - | - | - | - | - | - | [20] |
| 1 (MCL) | 485 | 5 | 277 | - | - | - | - | 0.170; 0.260 | [21] |
| 2 (MCL) | 450 | 5 | 160 | - | - | - | - | 0.170; 0.160 | [21] |
| 1 (JPPCH) | 474 | - | 644 | 1.97 | - | - | 2.63 | 0.19; 0.26 | [22] |
| 2 (JPPCH) | 474 | - | 1466 | 3.11 | - | - | 2.95 | 0.19; 0.24 | [22] |
| PhTz | 505 | 6 | 1453 | 2.83 | - | 0.63 | 1.65 | 0.28; 0.54 | [23] |
| C1 | 459 | - | 1177 | 1.64 | - | - | 2.39 | 0.18; 0.22 | [24] |
| C2 | 461 | - | 1289 | 1.97 | - | - | 2.71 | 0.19; 0.19 | [24] |
| PITP | 521 | 4.5 | 652 | 1.18 | - | 0.46 | 1.16 | 0.28; 0.39 | [30] |
| PITT | 530 | 4.0 | 886 | 0.91 | - | 0.35 | 0.93 | 0.32; 0.45 | [30] |
| PBT | 622 | 4.0 | 1955 | 4.59 | - | 3.09 | 3.96 | 0.69; 0.30 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szlapa-Kula, A.; Kula, S. Progress on Phenanthroimidazole Derivatives for Light-Emitting Electrochemical Cells: An Overview. Energies 2023, 16, 5194. https://doi.org/10.3390/en16135194
Szlapa-Kula A, Kula S. Progress on Phenanthroimidazole Derivatives for Light-Emitting Electrochemical Cells: An Overview. Energies. 2023; 16(13):5194. https://doi.org/10.3390/en16135194
Chicago/Turabian StyleSzlapa-Kula, Agata, and Slawomir Kula. 2023. "Progress on Phenanthroimidazole Derivatives for Light-Emitting Electrochemical Cells: An Overview" Energies 16, no. 13: 5194. https://doi.org/10.3390/en16135194
APA StyleSzlapa-Kula, A., & Kula, S. (2023). Progress on Phenanthroimidazole Derivatives for Light-Emitting Electrochemical Cells: An Overview. Energies, 16(13), 5194. https://doi.org/10.3390/en16135194






