Abstract
One of the processes that can serve to valorise low-quality biomass and organic waste is hydrothermal carbonization (HTC). It is a thermochemical process that transpires in the presence of water and uses heat to convert wet feedstocks into hydrochar (the solid product of hydrothermal carbonization). In the present experimental study, an improvement consisting of an increased hydrophobic character of HTC-treated biomass is demonstrated through the presentation of enhanced mechanical dewatering at different pressures due to HTC valorisation. As part of this work’s scope, flashing-off of low-quality steam is additionally explored, allowing for the recovery of the physical enthalpy of hot hydrochar slurry. The flashing-off vapours, apart from steam, contain condensable hydrocarbons. Accordingly, a membrane system that purifies such effluent and the subsequent recovery of chemical energy from the retentate are taken into account. Moreover, the biomethane potential is calculated for the condensates, presenting the possibility for the chemical energy recovery of the condensates.
1. Introduction
Hydrothermal carbonisation (HTC) is promising in terms of the valorisation of various types of wet biomass [1,2,3]. HTC is a thermal valorisation process that transpires in subcritical water at elevated temperatures (typically 170 to 260 °C) [4,5,6] with a residence time ranging between 30 min to 2 h [7,8,9,10,11] and at an autogenic pressure, which is higher than the saturation pressure of water. During the HTC process, complex reaction pathways are carried out, with different reactions proceeding in parallel, including hydrolysis, decarboxylation, and dehydration as well as aldol condensation and polymerisation [12,13]. Dehydration is important from the perspective of the global processing efficiency of installations processing wet biomass, as it decreases the number of hydroxyl groups (OH) in the process [14]. From a practical perspective, a loss of hydroxyl groups makes hydrochars more hydrophobic both in terms of decreased equilibrium moisture content [15] and with respect to facilitating mechanical dewatering [14]. Some of the advantages offered by the process include the improved grindability of the hydrochars [16,17], an enhancement in pelletising [16], an increased heating value of the valorised biomass [18,19], and improved sorption capacity for some compounds [20], among others. A positive influence across the whole value chain of wet biomass has been shown using LCA [21,22,23,24]. The HTC process produces liquid by-products, which contain some chemical energy that could be used for the production of biogas [25,26,27].
Ahmed et al. [26] evaluated the influence of HTC process conditions on Capillary Suction Time (CST) and dewaterability with a centrifuge for sewage sludge samples obtained from a wastewater treatment plant in Trento, Italy. HTC at 190 °C with a residence time of 30 min resulted in the CST decreasing from 2.78 s/g/L for raw sewage sludge to 2.67 s/g/L for the HTC-treated material [26]. A further increase in the HTC residence time resulted in a significant decrease in CST, i.e., HTC residence times of one, two, and three hours resulted in CST values as low as 0.38 s/g/L, 0.37 s/g/L, and 0.27 s/g/L, respectively [26]. This is in apparent contradiction to the results reported by Wang et al. [28], who observed an increase in CST for hydrothermally treated samples of sewage sludge from a wastewater treatment plant in Hefei, China, treated at temperatures ranging between 50 °C and 170 °C with a residence time of 30 min. Wang et al. [28] attributed this to the disintegration of flocs caused by the thermal treatment. It seems plausible to suspect that the relatively low temperature and residence time of the hydrothermal treatment applied by Wang et al. [28] was enough to disintegrate the flocs but not enough to decrease the content of oxygenated functional groups (i.e., OH groups) forming on the surface of the hydrochars, which has a maximum at a certain hydrothermal treatment temperature and then decreases with a further temperature increase, as reported by Jain et al. [29]. Gao et al. [30] reported the benefits of the in situ mechanical dewatering of sewage sludge in an HTC reactor, resulting in a reduction of 27.7–59.6% of the moisture content, depending on the HTC severity. Wang et al. [31] also reported a decrease in the moisture content of sewage sludge using in situ dewatering in an HTC reactor and demonstrated that the dewatering performance is significantly better in hot conditions in comparison to dewatering performed after cooling the products to ambient temperature for HTC performed at 180 °C with residence times ranging between 10 and 90 min. Aragon-Briceño et al. [32] demonstrated the positive influence of HTC treatment on mechanical dewatering for the digestate from the anaerobic digestion of the wet fraction of municipal solid waste treated at temperatures ranging from 180 °C to 230 °C with residence times of 30–120 min. Wilk et al. [33] treated effluents after the HTC of sewage sludge with vacuum distillation and demonstrated that the chemical oxygen demand (COD) of the filtrate decreased 32-fold during the process. Czerwińska, Śliz, and Wilk [34] reported that distillation at atmospheric pressure caused a 95% decrease in COD and total organic carbon (TOC). Czerwińska et al. [35] performed a double nanofiltration of effluents after HTC, achieving an 84.5% decrease in COD.
Lisseth et al. [36] proposed recovering heat by using flash vapours as the heat source in an installation with HTC. Such a generation of vapours would also entail the removal of moisture from the hydrochars. This waste stream, i.e., the condensate obtained in the distillation process, can be both a source of water and valuable substances. However, this requires its proper processing. To recover water and high-value substances, membrane pressure-driven processes can be used, among which nanofiltration seems to be the most useful method.
The aims of this work are as follows:
- The optimisation of the dewatering pressure of municipal solid waste digestate after HTC;
- The determination of a possible means of purifying the condensate with nanofiltration membranes after the condensing of vapours flashed for heat recovery after HTC;
- The evaluation of the biomethane potential of flashed vapours.
2. Materials and Methods
Samples of the digestate, produced using the organic fraction of municipal solid waste, were taken from a biogas plant located at the premises of ZGO Gać near Oława in Lower Silesia, Poland. The diagram of the experimental setup for hydrothermal carbonisation (Figure 1) given below shows the autoclave rig. The autoclave was filled with 3.0 litres of wet digestate, which had solid content of 30%. Moisture content was determined with Radwag MA.X2.A (Radom, Poland)at 105 °C.
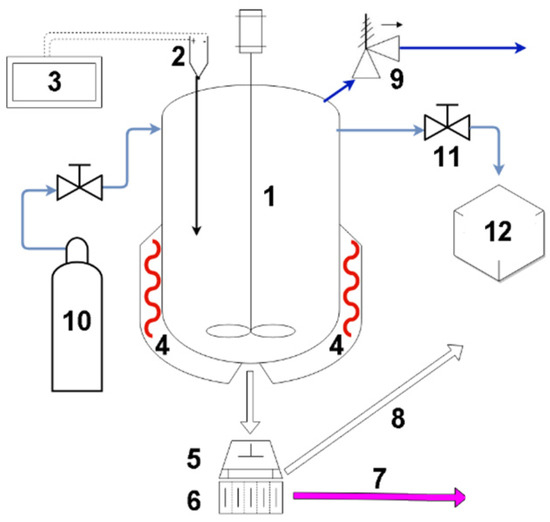
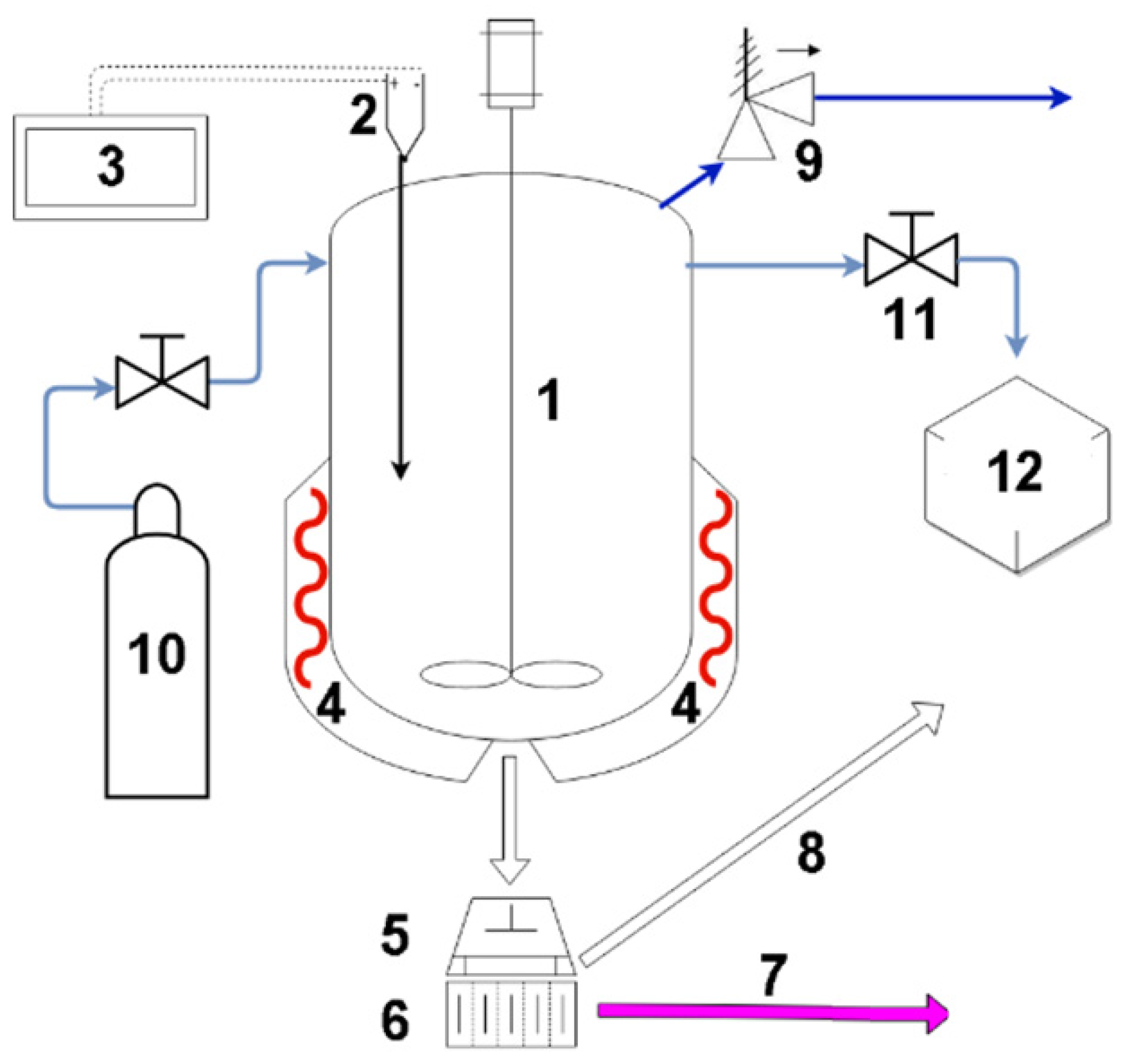
Figure 1.
Diagram of the HTC rig (1—vessel; 2—thermocouple; 3—PLC; 4—heaters; 5—cotton filter; 6—filter’s base; 7—effluent; 8—separated hydrochar; 9—safety valve; 10—purge gas; 11—flashing-off valve; 12—laboratory cooler).
HTC temperature of 200 °C was chosen based on the range between 200 °C and 260 °C [37,38] specified in the literature and by taking into account the design preference in industrial-scale HTC installations for a lower range of pressure, which allows for comparably lower thickness of a reactor’s walls. After heating the biomass with a heating rate of 1.57 °C/min, the biomass was kept in the reactor for 120 min. Subsequently, the heating was halted, and the rig was allowed to cool overnight.
Flashing-off was performed, after the separation of wet hydrochars from liquid effluent, at a temperature of 110 °C. When this temperature had been achieved in the autoclave, the flashing-off valve was slightly opened, and vapours were released into an Allihn-type glass cooler with 1 m of effective length and cooled with tap water. The opening of the valve was kept at a level allowing for a sufficient pressure drop in order to prevent the glass cooler from shattering due to vapour pressure. Condensate was collected for 2 h into 5 containers, with each container being changed after approx. 25 min. Characterisation of each condensate was carried out according to the Standard Methods for the Examination of Water and Wastewater, 23rd Edition. Chemical Oxygen Demand (COD), Biological Oxygen Demand (BOD5), Dissolved Organic Carbon (DOC), pH, conductivity, dry mass, alkaline metal content (Na+, Mg2+, Ca2+, and K+), phosphorus content (total P and PO43−), nitrogen content (total nitrogen, NO3−, and NH4+), sulphate content (SO32−), and halogen content (Cl−, Br−, and F−) were determined. The characteristics of the test solutions (K0–K4) are presented in Table 1.

Table 1.
Properties of the test solutions of condensate.
Treatment of individual condensates was carried out using NPO10P and NPO30P flat nanofiltration membranes from Mann + Hummel Water & Fluid Solutions (Ludwigsburg, Germany). Their characteristics are shown in Table 2.

Table 2.
NF membranes used in the experiments [39].
The membranes were conditioned before the actual membrane filtration process by filtering the redistilled water through the membranes successively under different transmembrane pressures from 0.1 to 0.4 MPa until constant water flux values were obtained.
After each experiment, the membranes were cleaned (chemically regenerated) with 0.1 mol/dm3 of NaOH solution (Avantor Performance Materials Poland S.A., Gliwice, Poland) and rinsed with redistilled water until the initial values of permeate flux were obtained.
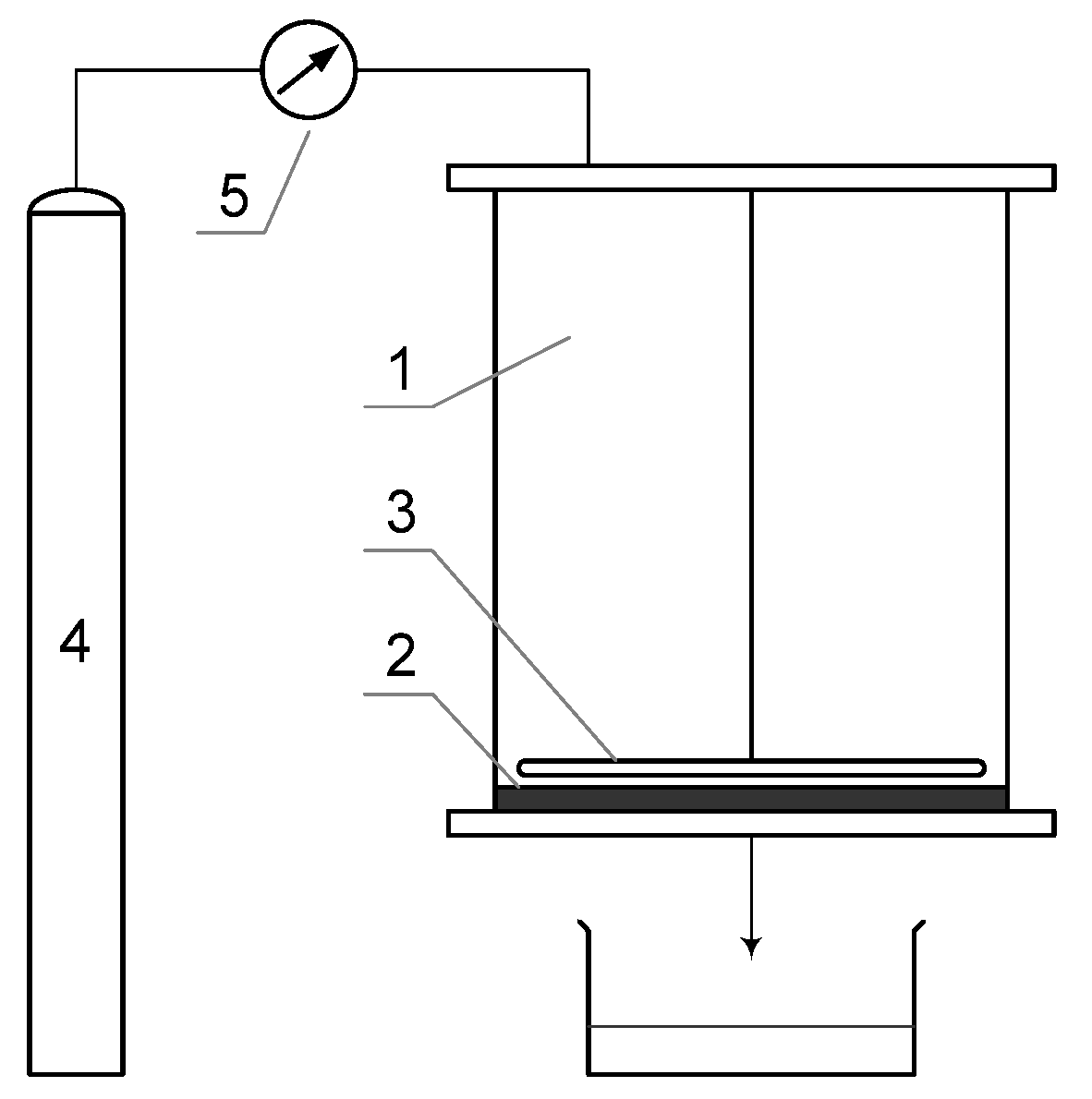
The nanofiltration process was carried out on a test stand equipped with an Amicon 8400 cell produced by Millipore (Figure 2). This cell allows for a dead-end filtration process and is designed to work with flat sheet membranes. The volume of the Amicon 8400 cell is 400 cm3, and the membrane diameter is 76 mm. The cell was placed on an ARE magnetic stirrer produced by OMC Envag (Warsaw, Poland) so that the contaminant concentration was uniform throughout the solution volume. The transmembrane pressure used in this study was 0.3 MPa.
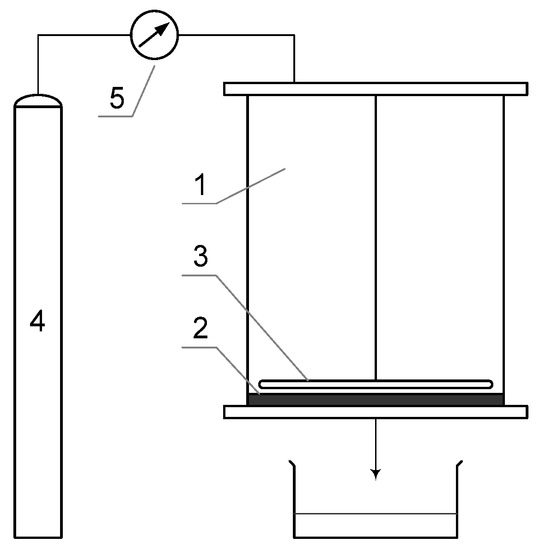
Figure 2.
Amicon 8400 dead-end membrane system (1—filtration cell, 2—membrane, 3—stirrer, 4—pressurized nitrogen cylinder, and 5—pressure valve).
The separation efficiency was evaluated by determining the concentration of impurities in the treated solution and in the purified solution and by determining the value of the reduction factor (removal/retention), R, using the following formula:
where:
cp—concentration of impurities in the treated solution, g/m3;
c0—initial concentration of impurities in the condensate, g/m3.
Equation (2) presents the formula used for the calculation of the theoretical biomethane potential (BMP) in the condensates.
To assess the theoretical BMP of the condensates, the stochiometric Formula (3) of methane oxidation was used. This formula allows for the calculation of the potential amount of methane produced based on the COD balance of a sample [40].
The COD conversion to methane at 35 °C is 0.39 dm3 of CH4 per gram of COD. Furthermore, a second correction factor was applied to render the prediction more accurate. The first correction factor was the DOC-to-COD ratio, which was used since the COD refers to all the organics and inorganics that can be oxidized, while the DOC only refers to the organic carbon compounds that can potentially be converted into methane. The second correction factor was the biodegradability of HTC process water, which was 90%. This factor was based on a comparison of the real BMP reported in previous studies versus the theoretical BMP value from the normal conversion of the stochiometric formula [25,41].
3. Results
The obtained results (Table 3) show that HTC resulted in a reduction in the moisture content of the digestate, as moisture content values of the dewatered hydrochars were much lower in comparison to the moisture content of the raw digestate (75.7%w.b.) and the digestate after dewatering at 3 MPa (65.3%). Furthermore, the increase in dewatering pressure allowed for a further reduction in the moisture content, which allowed achieving 37.1% moisture content after dewatering at a pressure of 10 MPa.

Table 3.
Moisture content of hydrochars after dewatering at different pressures (w.b.—wet basis).
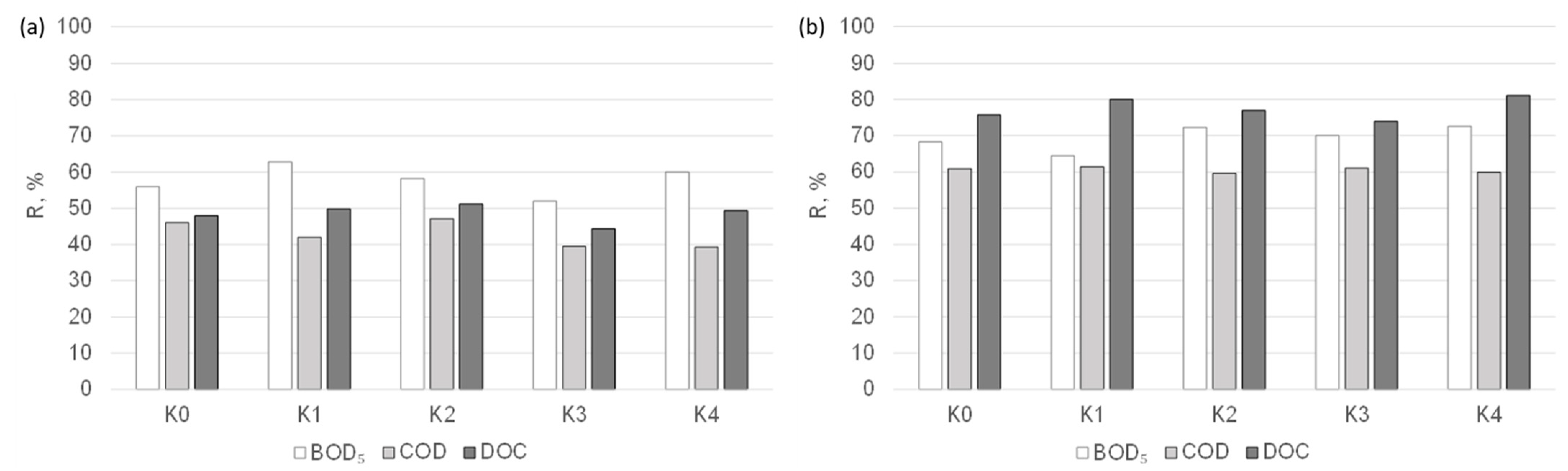
The results of the tests for determining the suitability of nanofiltration membranes for the purification of condensates obtained in the distillation process of the liquid fraction of municipal post-HTC digestate are presented in Figure 3. The effect of the membrane cut-off (MWCO) value on the separation efficiency of the organic compounds from the analysed solutions was analysed. Upon comparing the results obtained, it can be seen that both of the tested nanofiltration membranes can be applied in the treatment of condensates, although a deterioration in the quality of the permeate was observed as the cut-off value increased. In the nanofiltration process, the separation effect of organic macromolecules is determined by the sieve mechanism as well as the solution–diffusion mechanism and electrostatic interactions between the membrane and the solution components. Purification with the NPO10P membrane allowed for the acquisition of the retention coefficients of biological oxygen demand (BOD5), COD, and DOC at levels of up to 62, 47, and 50% and, for the NPO30P membrane, 73, 61, and 82%, respectively. The better separation properties of the NPO30P membrane may be due to its dense structure. According to Kovacs et al. [42], the cut-off value of the NPO10P membrane is higher and is in the range of 1010–1400 Da (with a pore diameter of 0.80–1.29 nm), while that for the NPO30P membrane is 500–700 Da (with a pore diameter of 0.57–0.93 nm).
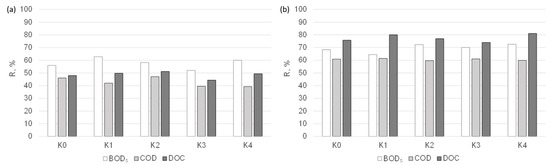
Figure 3.
Effect of nanofiltration membrane type, i.e., (a) NPO10P and (b) NPO30P, on the efficiency of the separation of organic pollutants from condensates obtained from the liquid fraction of municipal post-HTC digestate (Δp = 0.3 MPa).
Upon analysing the results obtained for the individual condensates, it was found that regardless of the type of nanofiltration membrane, the retention coefficients of BOD5, COD, and DOC remained at a relatively constant level, which may mean that the duration of the distillation process had no significant effect on the efficiency of organic compound removal from the analysed liquids.
In this study, the potential for methane production from the retentate through cascade membrane filtration was considered for a more circular approach. This approach has been adopted in some studies that have used the retentates from the membrane filtration of wastewater for anaerobic digestion to produce biogas. Luo et al. [43] suggested this approach in their study, where they applied an ultrafiltration/nanofiltration system in a dairy wastewater model. They did not conduct experimental work but highlighted this approach nonetheless, as the retentate from membrane filtration system contained a high concentration of organics. Chen et al. [44] integrated isoelectric precipitation, nanofiltration, and anaerobic digestion into a cascade, using a model dairy wastewater. The aim of the study was to increase the production of biogas (focused on hydrogen) through concentrating the short chain organics in the retentate and, at the same time, reduce the fouling of the membrane. The result was an increase in hydrogen in the biogas from 32.4% to 58.8%. Campell et al. [45] used HTC-processed water from spent coffee grounds and applied nanofiltration and reverse osmosis to treat the process water and reduce its COD. Both retentates were mixed and subjected to anaerobic digestion, obtaining yields of 0.21 dm3/gCOD. However, one of the main points to consider is that the retentates can contain high concentrations of metals and nutrients (phosphorus and nitrogen) that can inhibit the anaerobic digestion process [46].
Table 4 shows the biomethane potential of the five different retentates. The first sample of condensate (K0) presented the highest and most significant BMP value compared with those of the subsequent samples, which gradually decreased. However, an alternative approach should be considered since nanofiltration has shown significant COD reduction potential. Therefore, it might be beneficial to perform the anaerobic digestion of the retentates remaining after membrane purification.

Table 4.
Biomethane potential (BMP) for subsequently taken condensate samples.
4. Conclusions
This research shows that it is beneficial to increase the dewatering pressure in the dewatering of hydrochars after hydrothermal carbonisation. However, technical difficulties related to increased problems with the strength of the materials used in the construction of dewatering presses should also be considered when selecting dewatering parameters. Flashing-off a share of the liquid by-products seems to be a feasible way to recover the sensible heat of hydrochars after the HTC process. In this study, the condensates had some biomethane potential, which could be utilized to further improve the energy balance of such installations. The nanofiltration membranes, which were made of polyethersulfone, demonstrated good COD removal rates, proving their suitability for the purification of condensates originating from flashed vapours. Overall, the existing possibilities for the purification of condensates make flashing-off a viable dewatering option. We recommend that further research is conducted on the combination of novel in situ dewatering methods presented in the literature with the use of flashing as a heat recovery option in HTC installations.
Author Contributions
Conceptualisation: H.P.-K., A.U. and L.N.; methodology: A.U., C.A.-B. and L.N.; validation: M.K.-K., A.A. and P.S.; formal analysis: A.U., C.A.-B., L.N. and M.W.; investigation: A.U., M.C., M.B. and M.W.; resources: H.P.-K., M.K.-K., E.B. and G.B.; data curation: P.S., M.W., J.M. and A.A.; writing—original draft preparation: A.U., L.N. and C.A.-B.; writing—review and editing: L.N., H.P.-K., M.K.-K., E.B., G.B. and A.P.; visualisation: J.M. and P.S; supervision: H.P.-K., M.K.-K., E.B., G.B. and A.P.; project administration: H.P.-K., A.P. and P.S.; funding acquisition: H.P.-K., A.P., E.B., G.B., P.S. and A.U. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the European Commission, the National Centre for Research and Development (Poland), Nederlandse Organisatie Voor Wetenschappelijk Onderzoek (Netherlands), and the Swedish Research Council Formas for providing funding through the framework of the collaborative international consortium (RECOWATDIG) financed under the 2018 Joint call of the WaterWorks 2017 ERA-NET Cofund. This ERA-NET is an integral part of the activities developed by the Water JPI. National Centre for Research and Development agreement number WATERWORKS2017/I/RECOWATDIG/01/2019.
Data Availability Statement
Data are available on request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Aragón-Briceño, C.I.; Ross, A.B.; Camargo-Valero, M.A. Mass and energy integration study of hydrothermal carbonization with anaerobic digestion of sewage sludge. Renew. Energy 2021, 167, 473–483. [Google Scholar] [CrossRef]
- Shi, W.; Fenton, O.; Ashekuzzaman, S.M.; Daly, K.; Leahy, J.J.; Khalaf, N.; Hu, Y.; Chojnacka, K.; Numviyimana, C.; Healy, M.G. An examination of maximum legal application rates of dairy processing and associated STRUBIAS fertilising products in agriculture. J. Environ. Manag. 2022, 301, 113880. [Google Scholar] [CrossRef]
- Numviyimana, C.; Warchoł, J.; Khalaf, N.; Leahy, J.J.; Chojnacka, K. Phosphorus recovery as struvite from hydrothermal carbonization liquor of chemically produced dairy sludge by extraction and precipitation. J. Environ. Chem. Eng. 2022, 10, 106947. [Google Scholar] [CrossRef]
- Śliz, M.; Wilk, M. A comprehensive investigation of hydrothermal carbonization: Energy potential of hydrochar derived from Virginia mallow. Renew. Energy 2020, 156, 942–950. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba-Rec, I.; Szymańska-Chargot, M. Upgrading of green waste into carbon-rich solid biofuel by hydrothermal carbonization: The effect of process parameters on hydrochar derived from acacia. Energy 2020, 202, 117717. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M.; Wądrzyk, M. Pyrolysis of hydrochar derived from biomass—Experimental investigation. Fuel 2020, 267, 117246. [Google Scholar] [CrossRef]
- Kumar, N.; Weldon, R.; Lynam, J.G. Hydrothermal carbonization of coffee silverskins. Biocatal. Agric. Biotechnol. 2021, 36, 102145. [Google Scholar] [CrossRef]
- Sobek, S.; Tran, Q.-K.; Junga, R.; Werle, S. Hydrothermal carbonization of the waste straw: A study of the biomass transient heating behavior and solid products combustion kinetics. Fuel 2021, 314, 122725. [Google Scholar] [CrossRef]
- Lühmann, T.; Wirth, B. Sewage Sludge Valorization via Hydrothermal Carbonization: Optimizing Dewaterability and Phosphorus Release. Energies 2020, 13, 4417. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal carbonization: Modeling, final properties design and applications: A review. Energies 2018, 11, 216. [Google Scholar] [CrossRef]
- Smith, A.M.; Ekpo, U.; Ross, A.B. The influence of pH on the combustion properties of bio-coal following hydrothermal treatment of swine manure. Energies 2020, 13, 331. [Google Scholar] [CrossRef]
- Djandja, O.S.; Yin, L.-X.; Wang, Z.-C.; Duan, P.-G. From wastewater treatment to resources recovery through hydrothermal treatments of municipal sewage sludge: A critical review. Process Saf. Environ. Prot. 2021, 151, 101–127. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Messineo, A. Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges. Energies 2021, 14, 2962. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonisation of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.B.; Panigrahi, S.; Dubey, B.K. Hydrothermal carbonization of yard waste for solid bio-fuel production: Study on combustion kinetic, energy properties, grindability and flowability of hydrochar. Waste Manag. 2019, 91, 108–119. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers. Manag. 2015, 105, 746–755. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge. A review. Renew. Sustain. Energy Rev. 2022, 154, 111873. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Sieradzka, M.; Magdziarz, A. Thermal upgrading of hydrochar from anaerobic digestion of municipal solid waste organic fraction. Fuel 2022, 324, 124435. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Lorenc-Grabowska, E.; Kordek-Khalil, K.; Rutkowski, P. Hydrothermal and pyrolytic biochars from waste milk thistle (Silybum marianum) extrudates as precursors for production of effective isoproturon adsorbents. J. Water Process Eng. 2020, 37, 101459. [Google Scholar] [CrossRef]
- Mayer, F.; Bhandari, R.; Gäth, S.A. Life cycle assessment on the treatment of organic waste streams by anaerobic digestion, hydrothermal carbonization and incineration. Waste Manag. 2021, 130, 93–106. [Google Scholar] [CrossRef]
- Stobernack, N.; Mayer, F.; Malek, C.; Bhandari, R. Evaluation of the energetic and environmental potential of the hydrothermal carbonization of biowaste: Modeling of the entire process chain. Bioresour. Technol. 2020, 318, 124038. [Google Scholar] [CrossRef]
- Medina-Martos, E.; Istrate, I.R.; Villamil, J.A.; Gálvez-Martos, J.L.; Dufour, J.; Mohedano, Á.F. Techno-economic and life cycle assessment of an integrated hydrothermal carbonization system for sewage sludge. J. Clean. Prod. 2020, 277. [Google Scholar] [CrossRef]
- Mendecka, B.; Lombardi, L.; Micali, F.; de Risi, A. Energy Recovery from Olive Pomace by Hydrothermal Carbonization on Hypothetical Industrial Scale: A LCA Perspective. Waste Biomass Valorization 2020, 11, 5503–5519. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Ross, A.B.B.; Camargo-Valero, M.A.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Ahmed, M.; Andreottola, G.; Elagroudy, S.; Negm, M.S.; Fiori, L. Coupling hydrothermal carbonization and anaerobic digestion for sewage digestate management: Influence of hydrothermal treatment time on dewaterability and bio-methane production. J. Environ. Manag. 2021, 281, 111910. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Hülsemann, B.; Wüst, D.; Oechsner, H.; Lautenbach, A.; Kruse, A. Effect of residence time during hydrothermal carbonization of biogas digestate on the combustion characteristics of hydrochar and the biogas production of process water. Bioresour. Technol. 2021, 333, 125110. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Qian, C.; Jiang, J.-K.; Ye, X.-D.; Yu, H.-Q. Response of extracellular polymeric substances to thermal treatment in sludge dewatering process. Environ. Pollut. 2017, 231, 1388–1392. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Gao, N.; Li, Z.; Quan, C.; Miskolczi, N.; Egedy, A. A new method combining hydrothermal carbonization and mechanical compression in-situ for sewage sludge dewatering: Bench-scale verification. J. Anal. Appl. Pyrolysis 2019, 139, 187–195. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, A. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: Influence of operating conditions and the process energetics. Water Res. 2014, 65, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Briceño, C.; Pożarlik, A.; Bramer, E.; Brem, G.; Wang, S.; Wen, Y.; Yang, W.; Pawlak-Kruczek, H.; Niedźwiecki, Ł.; Urbanowska, A.; et al. Integration of hydrothermal carbonization treatment for water and energy recovery from organic fraction of municipal solid waste digestate. Renew. Energy 2022, 184, 577–591. [Google Scholar] [CrossRef]
- Wilk, M.; Czerwińska, K.; Śliz, M.; Imbierowicz, M. Hydrothermal carbonization of sewage sludge: Hydrochar properties and processing water treatment by distillation and wet oxidation. Energy Rep. 2023, 9, 39–58. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Thermal Disposal of Post-processing Water Derived from the Hydrothermal Carbonization Process of Sewage Sludge. Waste Biomass Valorization 2023, 1–10. [Google Scholar] [CrossRef]
- Czerwińska, K.; Marszałek, A.; Kudlek, E.; Śliz, M.; Dudziak, M.; Wilk, M. The treatment of post-processing liquid from the hydrothermal carbonization of sewage sludge. Sci. Total Environ. 2023, 885, 163858. [Google Scholar] [CrossRef]
- Lisseth, C.; Martinez, M.; Sermyagina, E.; Saari, J.; Silva, M.; Jesus, D.; Cardoso, M.; de Almeida, G.M.; Vakkilainen, E. Biomass and Bioenergy Hydrothermal carbonization of lignocellulosic agro-forest based biomass residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- Wnukowski, M.; Owczarek, P.; Niedźwiecki, Ł. Wet Torrefaction of Miscanthus—Characterization of Hydrochars in View of Handling, Storage and Combustion Properties. J. Ecol. Eng. 2015, 16, 161–167. [Google Scholar] [CrossRef]
- Yan, W.; Hastings, J.T.; Acharjee, T.C.; Coronella, C.J.; Vásquez, V.R. Mass and energy balances of wet torrefaction of lignocellulosic biomass. Energy Fuels 2010, 24, 4738–4742. [Google Scholar] [CrossRef]
- MICRODYN-NADIR. Flat Sheet Membrane Data Sheets—MICRODYN-NADIR n.d. Available online: https://www.microdyn-nadir.com/flat-sheet-membrane-data-sheets/ (accessed on 15 February 2021).
- Tauber, J.; Parravicini, V.; Svardal, K.; Krampe, J. Quantifying methane emissions from anaerobic digesters. Water Sci. Technol. 2019, 80, 1654–1661. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Grasham, O.; Ross, A.B.; Dupont, V.; Camargo-Valero, M.A. Hydrothermal carbonization of sewage digestate at wastewater treatment works: Influence of solid loading on characteristics of hydrochar, process water and plant energetics. Renew. Energy 2020, 157, 959–973. [Google Scholar] [CrossRef]
- Kovacs, Z.; Samhaber, W. Characterization of nanofiltration membranes with uncharged solutes. Membrantechnika 2008, 12, 22–36. [Google Scholar]
- Luo, J.; Ding, L.; Qi, B.; Jaffrin, M.Y.; Wan, Y. A two-stage ultrafiltration and nanofiltration process for recycling dairy wastewater. Bioresour. Technol. 2011, 102, 7437–7442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, J.; Chen, X.; Hang, X.; Shen, F.; Wan, Y. Fully recycling dairy wastewater by an integrated isoelectric precipitation–nanofiltration–anaerobic fermentation process. Chem. Eng. J. 2016, 283, 476–485. [Google Scholar] [CrossRef]
- Campbell, B.S.; Thorpe, R.B.; Peus, D.; Lee, J. Anaerobic digestion of untreated and treated process water from the hydrothermal carbonisation of spent coffee grounds. Chemosphere 2022, 293, 133529. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.-P.; Mandale, S.J.; Oatley-Radcliffe, D.; Lovitt, R.W. Nutrient recovery and fractionation of anaerobic digester effluents employing pilot scale membrane technology. J. Water Process Eng. 2019, 31, 100846. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).