The Limiting Content of Combustibles to Prevent Minestone from the Spreading of Fire
Abstract
1. Introduction
2. Materials and Methods
2.1. Method of Thermal Analysis
2.2. Combustion Calorimetry
3. Results and Discussion
3.1. Combustibles in Minestone
3.2. The Limiting Content of Combustibles to Prevent the Spreading of Fire
3.2.1. Theoretical Approach
3.2.2. Experimental Investigations
3.2.3. Scale Considerations
4. Conclusions
- (i)
- The content of combustible matter in minestone should properly be evaluated as the energetic equivalent of combustibles, EEC, relating oxidation/combustion heat of the combustibles to that of the coal standard.
- (ii)
- As a theoretical limiting content of EECm to prevent the spreading of fire in minestone, a value of 2% can be considered.
- (iii)
- For real conditions of thermally active coal waste dump, the value of EECm of ca 9–10% was suggested as a boundary between fireproof and fire spreading minestone for conditions of Heřmanice tailing coal dump to be investigated.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
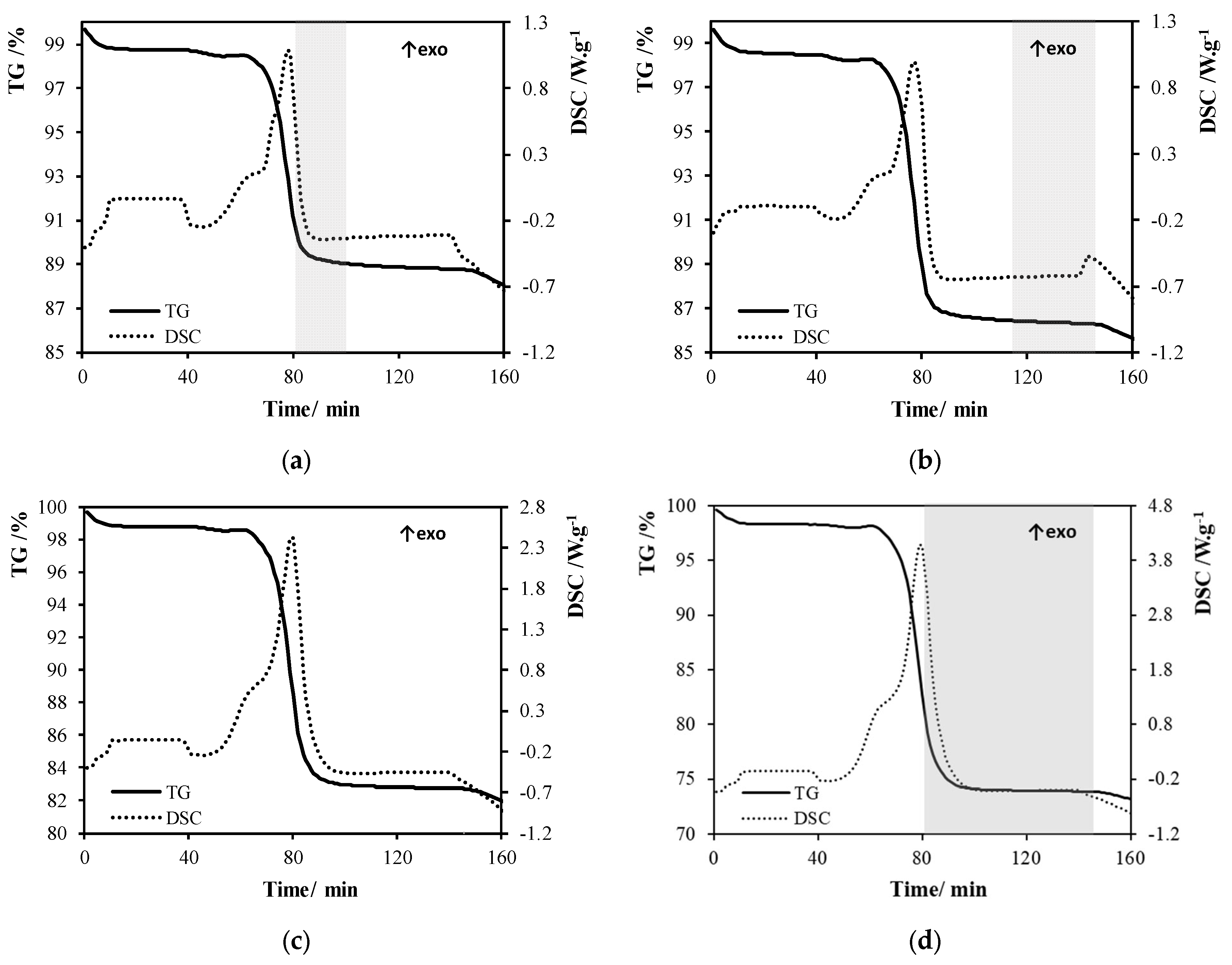
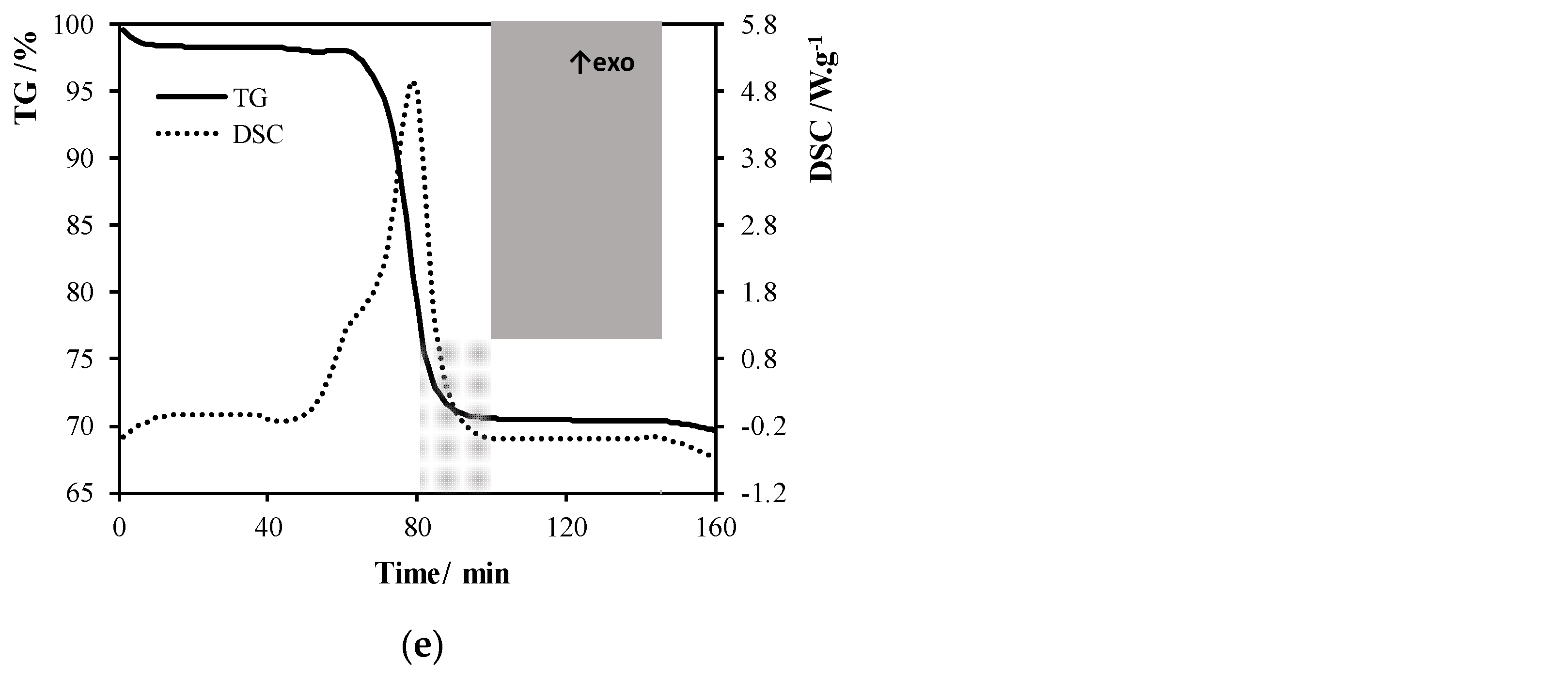
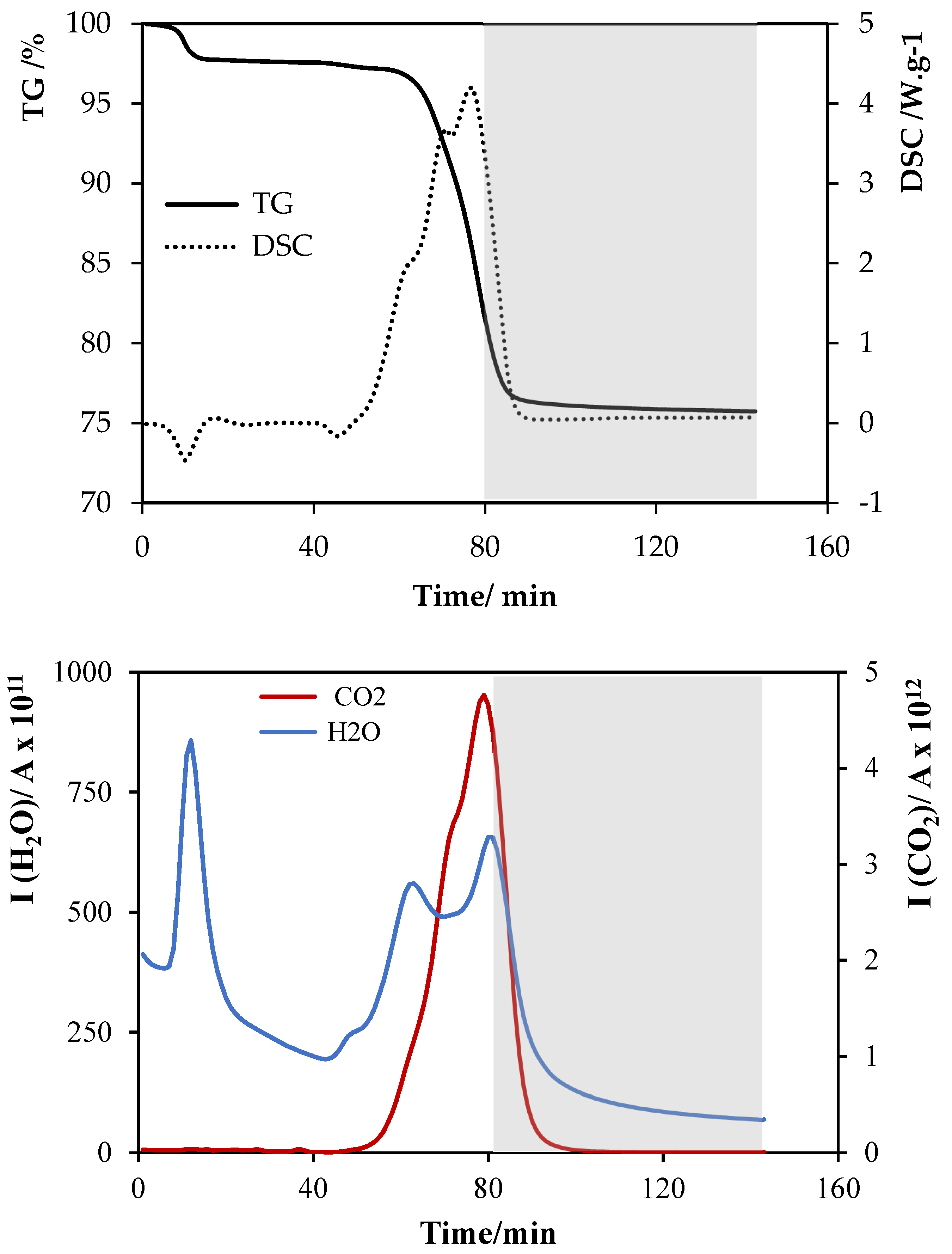
Appendix B

References
- Nádudvari, Á. Thermal Mapping of Self-Heating Zones on Coal Waste Dumps in Upper Silesia (Poland)—A Case Study. Int. J. Coal Geol. 2014, 128–129, 47–54. [Google Scholar] [CrossRef]
- Różański, Z. Management of Mining Waste and the Areas of Its Storage—Environmental Aspects. Miner. Resour. Manag. 2019, 35, 119–142. [Google Scholar]
- Bian, Z.; Dong, J.; Lei, S.; Leng, H.; Mu, S.; Wang, H. The Impact of Disposal and Treatment of Coal Mining Wastes on Environment and Farmland. Environ. Geol. 2009, 58, 625–634. [Google Scholar] [CrossRef]
- Gogola, K.; Rogala, T.; Magdziarczyk, M.; Smoliński, A. The Mechanisms of Endogenous Fires Occurring in Extractive Waste Dumping Facilities. Sustainability 2020, 12, 2856. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, S.; Zhou, B.; Cai, J. Study on Spontaneous Combustion Characteristics of Waste Coal Gangue Hill. Combust. Sci. Technol. 2021, 195, 713–727. [Google Scholar] [CrossRef]
- Wasilewski, S. Monitoring the Thermal and Gaseous Activity of Coal Waste Dumps. Environ. Earth Sci. 2020, 79, 474. [Google Scholar] [CrossRef]
- Smoliński, A.; Dombek, V.; Pertile, E.; Drobek, L.; Gogola, K.; Żechowska, S.W.; Magdziarczyk, M. An Analysis of Self-Ignition of Mine Waste Dumps in Terms of Environmental Protection in Industrial Areas in Poland. Sci. Rep. 2021, 11, 8851. [Google Scholar] [CrossRef] [PubMed]
- Jelínek, P.; Marschalko, M.; Lamich, D.; Yilmaz, I.; Zastěrová, P.; Bednárik, M.; Heviánková, S.; Kyncl, M.; Drusa, M.; Růčková, H. Monitoring and Analysis of Burning in Coal Tailing Dumps: A Case Study from the Czech Republic. Environ. Earth Sci. 2015, 73, 6601–6612. [Google Scholar] [CrossRef]
- Skarżyńska, K.M. Reuse of Coal Mining Wastes in Civil Engineering—Part 1: Properties of Minestone. Waste Manag. 1995, 15, 3–42. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Z.; Wu, D.; Luo, Z.; Zhao, S.; Wang, Y.; Deng, J. Prediction of Coal Self-Ignition Tendency Using Machine Learning. Fuel 2022, 325, 124832. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y. Preparation, Properties and Application of Gel Materials for Coal Gangue Control. Energies 2022, 15, 557. [Google Scholar] [CrossRef]
- Machacek, Z.; Hajovsky, R. Modeling of Temperature Field Distribution in Mine Dumps with Spread Prediction. Elektron. Elektrotechnika 2013, 19, 53–56. [Google Scholar] [CrossRef]
- Hotova, G.; Slovak, V. Quantitative TG-MS Analysis of Evolved Gases during the Thermal Decomposition of Carbon Containing Solids. Thermochim. Acta 2016, 632, 23–28. [Google Scholar] [CrossRef]
- Beaudoin, A. A Comparison of Two Methods for Estimating the Organic Content of Sediments. J. Paleolimnol. 2003, 29, 387–390. [Google Scholar] [CrossRef]
- EN 12879:2000; Characterization of Sludges—Determination of the Loss on Ignition of Dry Mass. iTeh, Inc.: Newark, DE, USA, 2000. (In Czech)
- Miao, S.Q.; Li, H.P.; Chen, G. Temperature Dependence of Thermal Diffusivity, Specific Heat Capacity, and Thermal Conductivity for Several Types of Rocks. J. Therm. Anal. Calorim. 2014, 115, 1057–1063. [Google Scholar] [CrossRef]
- Goto, S.; Yamano, M.; Morita, S.; Kanamatsu, T.; Hachikubo, A.; Kataoka, S.; Tanahashi, M.; Matsumoto, R. Physical and Thermal Properties of Mud-Dominant Sediment from the Joetsu Basin in the Eastern Margin of the Japan Sea. Mar. Geophys. Res. 2017, 38, 393–407. [Google Scholar] [CrossRef]
- Vilímová, Z. Risk Analysis Mine Waste Dumps Affected by Endogenous Burning; DIAMO, Division ODRA, GeoTest Brno: Slatina, Czech Republic, 2010. (In Czech) [Google Scholar]
- Vohlídal, J.; Julák, A.; Štulík, K. Chemické a Analytické Tabulky; Grada Publishing: Praha, Czech Republic, 1999; ISBN 80-7169-855-5. (In Czech) [Google Scholar]





| Sample | LOImuffle (%) | LOITG (%) | EEC (%) |
|---|---|---|---|
| A | 10.0 | 10.0 | 7.4 |
| B | 12.8 | 12.1 | 8.5 |
| D | 17.2 | 16.1 | 16.5 |
| E | 21.3 | 20.6 | 19 |
| F | 25.1 | 24.1 | 20.8 |
| G | 27.8 | 28.3 | 26 |
| Parameter | Laboratory Experiment | In Situ (Heřmanice Dump) | Change in the Limit, EECm |
|---|---|---|---|
| Grain size | ≤0.2 mm | ~0–100 mm | Increase |
| Accessibility/partial pressure of oxygen | Easy/partial pressure of O2 = 3000 kPa | Difficult/partial pressure of O2 = 20 kPa | Increase |
| Dissipation of heat to the surroundings | Marked: (“pseudoizothermal conditions”) | Hindered: (“pseudoadiabatic conditions”) | Decrease |
| Elapsing time of firing | Transient (order of seconds) | Long-term (order of months/years | Decrease |
| Content of moisture | Minimal (air dried): Moisture < 1% | Moisture ~10% * | Increase (by 1% for 10% of moisture) |
| Unburned residue of combustibles | Negligible | About 1% * | Increase (by 1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taraba, B.; Gřunděl, P.; Zelenková, G. The Limiting Content of Combustibles to Prevent Minestone from the Spreading of Fire. Energies 2023, 16, 5054. https://doi.org/10.3390/en16135054
Taraba B, Gřunděl P, Zelenková G. The Limiting Content of Combustibles to Prevent Minestone from the Spreading of Fire. Energies. 2023; 16(13):5054. https://doi.org/10.3390/en16135054
Chicago/Turabian StyleTaraba, Boleslav, Petr Gřunděl, and Gabriela Zelenková. 2023. "The Limiting Content of Combustibles to Prevent Minestone from the Spreading of Fire" Energies 16, no. 13: 5054. https://doi.org/10.3390/en16135054
APA StyleTaraba, B., Gřunděl, P., & Zelenková, G. (2023). The Limiting Content of Combustibles to Prevent Minestone from the Spreading of Fire. Energies, 16(13), 5054. https://doi.org/10.3390/en16135054








