Selected Technologies of Electrochemical Energy Storage—A Review
Abstract
1. Introduction
2. Classification of Energy Storage Technologies
- The hydrogen energy storage system is basically related to the production and storage of hydrogen. It operates on the principle of water electrolysis. When the electrolyzer is supplied, water is split into hydrogen as the electrical energy carrier and oxygen, which are separated and stored in suitable tanks. It is worth noticing that hydrogen is characterized by the highest specific energy of 33 Wh/kg and a calorific value of 120 MJ/kg.
- SMES—Superconducting Magnetic Energy Storage. These systems are characterized by a short response time (i.e., the time in which the device can react and supply or take energy from the storage) and relatively high power. The basis of this group of energy storage systems’ functioning is accumulation of energy in the magnetic field of induction coils made of superconductors. Due to the fact that these systems operate at very low temperatures (below the temperature of liquid nitrogen), they can be successfully used in the range of very high currents (of the order of kA). Magnetic energy storage tanks are characterized by very high efficiencies of up to 95%, long expected service life (up to 30 years) and are capable of transferring high power (in the order of MW). They are also environmentally friendly due to the lack of toxic consumables and are reliable because there are no moving parts. On the other hand, their disadvantage is low energy density.
- Pumped-storage waterpower plants. These are one of the most popular and the oldest solutions. The main task of a pumped storage power plant is to balance the power in the energy system. Due to the losses in the turbo-set and the loss of evaporated water in the considered power plants, only 70–75% of the energy used to pump water to the upper reservoir is recovered [15]. Taking into account the size of the considered solutions, which depend on topographic and geological factors, these systems require a thorough technical and economic analysis, because the construction of a pumped-storage power plant is a very expensive investment.
- Liquefied Air Energy Storage (LAES) and Compressed Air Energy Storage (CAES). The Liquefied Air Energy Storage (LAES) method consists in using excess energy to compress air, which is then cooled to a very low temperature and liquefied as liquid air. When there is a demand for energy, the liquid air is heated, which causes it to expand and drive a turbine, which in turn generates energy. The efficiency of the energy storage process in liquefied air depends on the possibility of using the waste heat in the process of expanding the working medium and the heat generated in the process of charging the tank with liquefied air. According to paper [16], it is estimated that the real efficiency of the considered solution should reach about 60%. In the second case, CAES, the energy storage process using the mentioned method consists in compressing the air using an engine or turbine, and then storing the compressed air in underground tanks or cisterns. The air is compressed to a pressure of 70 atmospheres. When electricity is required, the compressed air is released to spin a turbine, which in turn powers an electric generator to produce the desired electricity [17]. One of the biggest advantages of CAES is that much larger amounts of energy can be stored with this method of energy storage than in batteries or other forms of energy storage. In addition, CAES has relatively low investment costs and is quite easy to use, which means that it can be used in both large and smaller power systems. However, this technology has some disadvantages. The process of compressing air requires significant amounts of energy, which means that the process can lead to a significant loss of energy in the conversion and storage process. Moreover, this process is usually used to store energy for a short period of time (e.g., hours) [18].
- Flywheel. Electric energy in such a solution can be stored in the form of flywheel kinetic energy. The capacity of such a storage solution depends on the rotating mass, its shape and the speed of rotation. The rotating mass is connected to the motor-generator, which accelerates the wheel during loading and slows down during unloading of the warehouse [19]. Flywheel units can achieve efficiency of over 80%, and their self-discharge rate is less than 3%/h. The use of a superconducting magnetic bearing allows the reduction of the self-discharge rate to less than 0.5%/h [20].
- Thermal method of energy storage. It includes various technologies that use heat as an energy carrier and then store it for later use to produce energy. The principle of operation of such a storage solution is to store heat in a material that has the ability to store a large amount of energy in the form of heat, such as stones or concrete. Thermal energy storage usually consists of two components: a storage element and a heat transfer system. During the energy storage process, thermal energy is supplied to the storage from a heat source such as solar panels or biomass boilers. This energy is then stored in a high heat capacity material that absorbs heat and keeps it constant. When energy is needed, the material is heated, and the heat is sent back to the heating system and used to produce energy or heating [21]. According to the literature, the method is becoming more and more popular in small, distributed installations [22] and in municipal systems [21]. The considered types of energy storage are divided into active and passive storage technologies [21]. Active storage uses sensible heat, latent heat (stores using phase-change materials) and thermochemical reactions. On the other hand, passive warehouses refer to structural elements of the building. Sensible heat accumulators are the most popular and still widely used. These warehouses use the heat capacity and temperature change of the accumulating substances during charging and discharging processes. The amount of the stored heat depends on the mass and specific heat of the substance used for storage and on the temperature difference between the initial and final states. The most popular substance used in sensible heat storage is water due to its high heat capacity and low cost. Water is used in warehouses operating in the temperature range from 20 °C to 70 °C [21].
- Electrical capacity, which characterizes the ability of energy storage devices to store energy. It is defined as the amount of electric charge expressed in [Ah] that can be taken from a fully charged device by discharging it at a temperature of 25 °C with the specified current. In the case of accumulators, it is assumed that the accumulator is discharged with a 20-h rated current (in the literature, capacities are also given for 10, 5-h or 1-h currents), until the appropriate final voltage is obtained (typically 1.75 V/link) [29]. For example, the electrical capacity of a battery depends on many factors, such as:
- the method of battery discharge (applied currents),
- the degree of sulphation of the boards resulting from aging processes and the method of exploitation,
- the environment—primarily the influence of temperature [29,30]. The rated current that affects the electrical capacity of the group of energy storage units under consideration is defined as the quotient of the rated electrical capacity and the discharging time resulting from this capacity. Typically, for batteries this current corresponds to 5% of the nominal capacity given in Ah [29].
- Rated voltage. The value of this parameter depends, for example, on the number and type of cells connected in series inside the battery.
- Internal resistance. This depends primarily on the type of the electrolyte, the design and size of the electrodes and the distance between them, the state of charge of the energy storage, the temperature and its age (in the case of batteries).
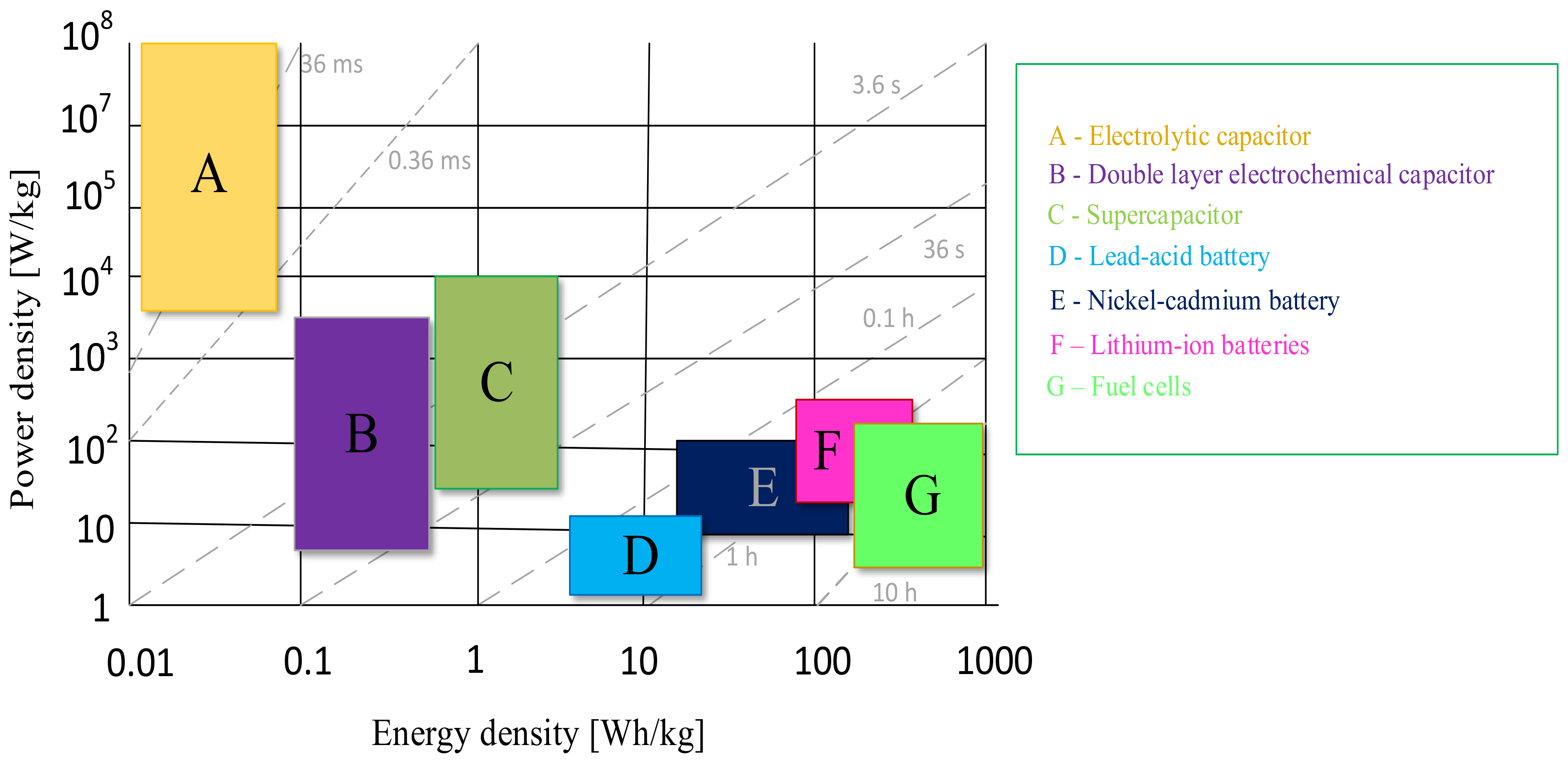
- Energy density. This parameter refers to the amount of energy that an energy storage system can store per unit of mass or volume. This is an important parameter when evaluating the efficiency of energy storage, as it indicates how much energy can be stored in a given system, taking into account its weight or volume.
- Energy storage power density—refers to the ability of an energy storage system to supply or consume energy at a given time. It expresses how quickly energy can be stored or released relative to the mass or volume of the energy store.
- Time and number of charging/discharging cycles, which determine the time needed to deliver energy to the storage (charging) device or to take it from the storage (discharging) device and how many times the energy storage can be charged and discharged.
3. Accumulator and Battery Energy Storage Technologies
3.1. Clasic Battery Energy Storage Technologies
- -
- lead-acid batteries, discussed in Section 3.1.1,
- -
- lithium-ion batteries, described in Section 3.1.2,
- -
- nickel–cadmium batteries, described in Section 3.1.3.
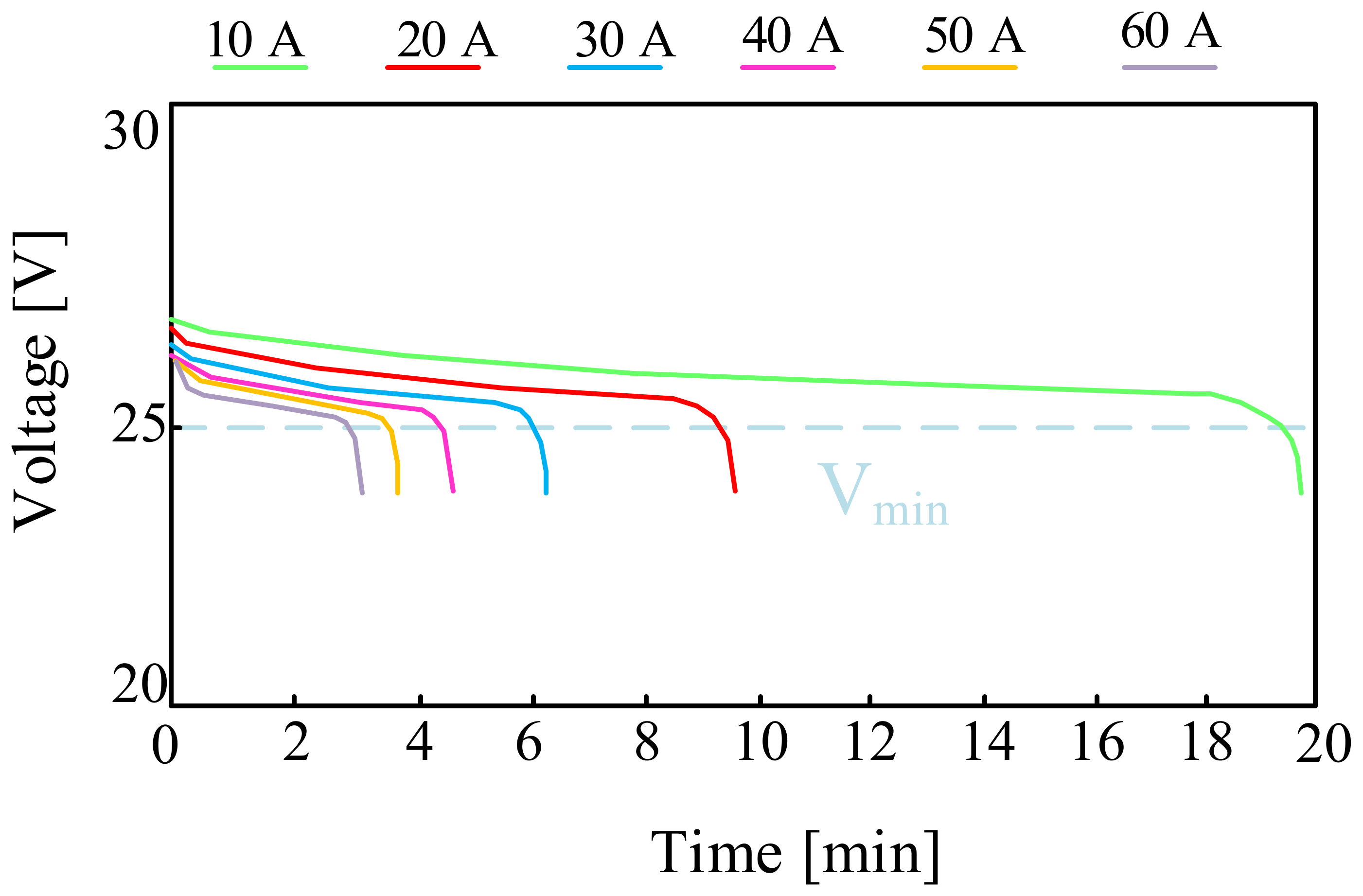
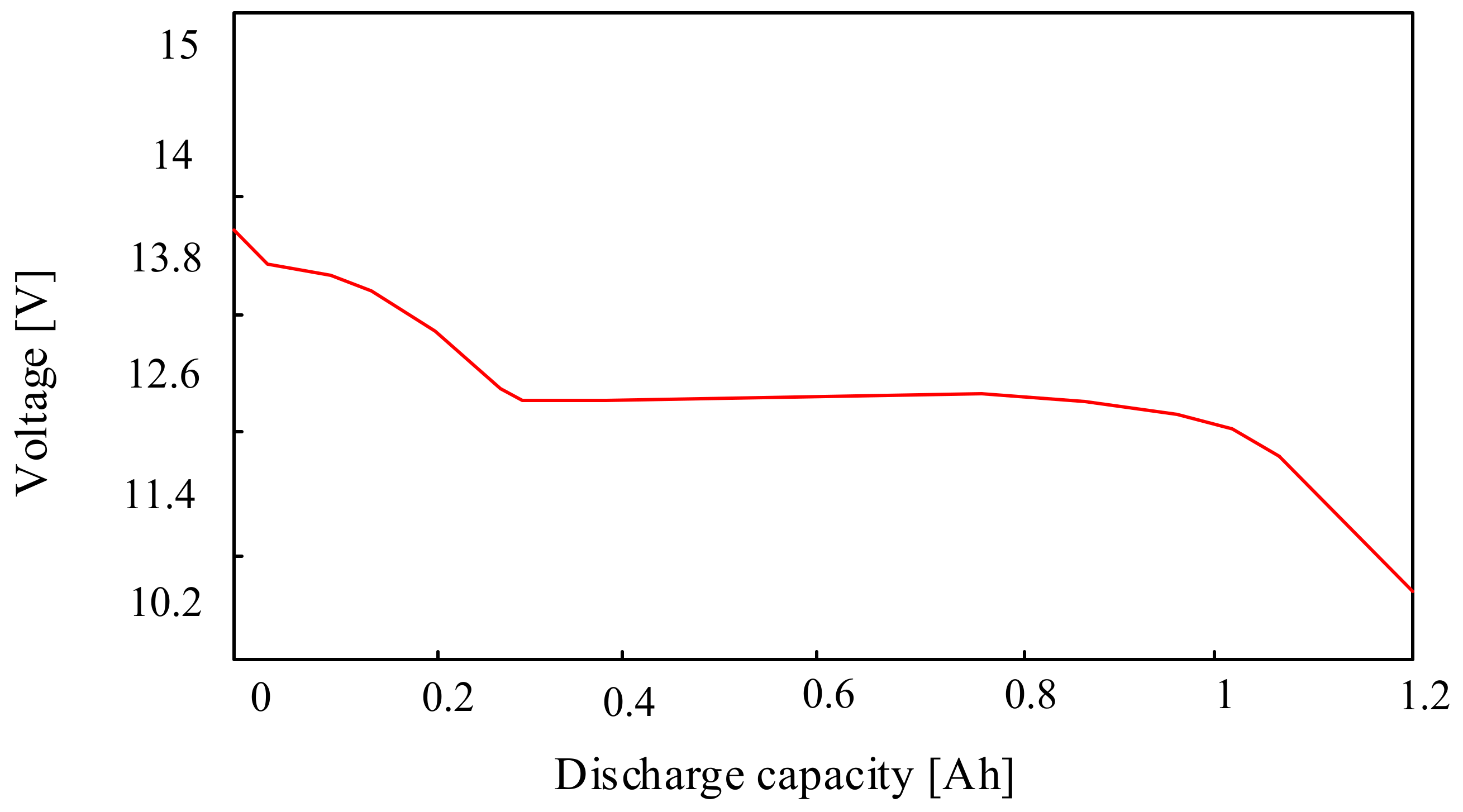
3.1.1. Lead-Acid Batteries
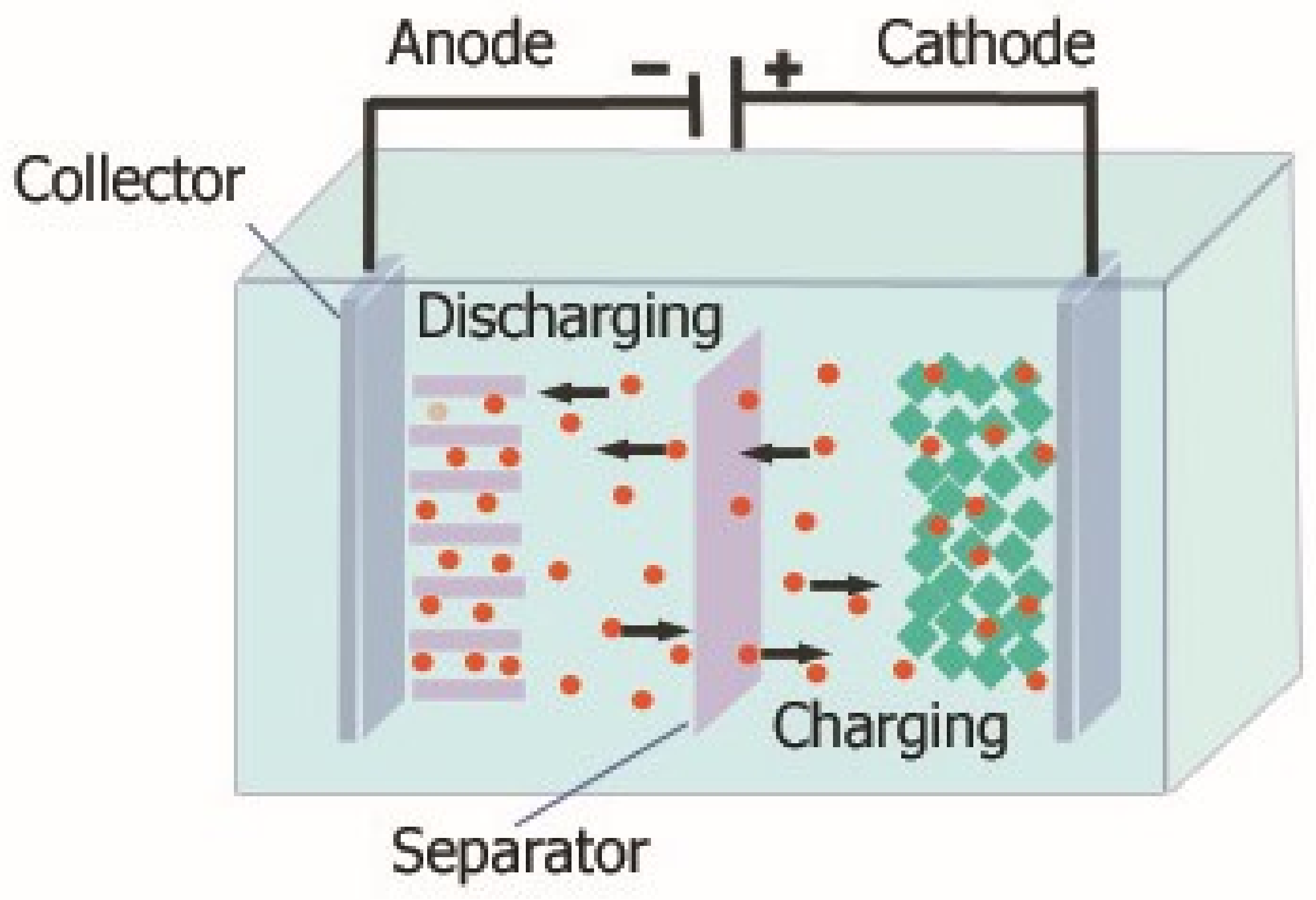
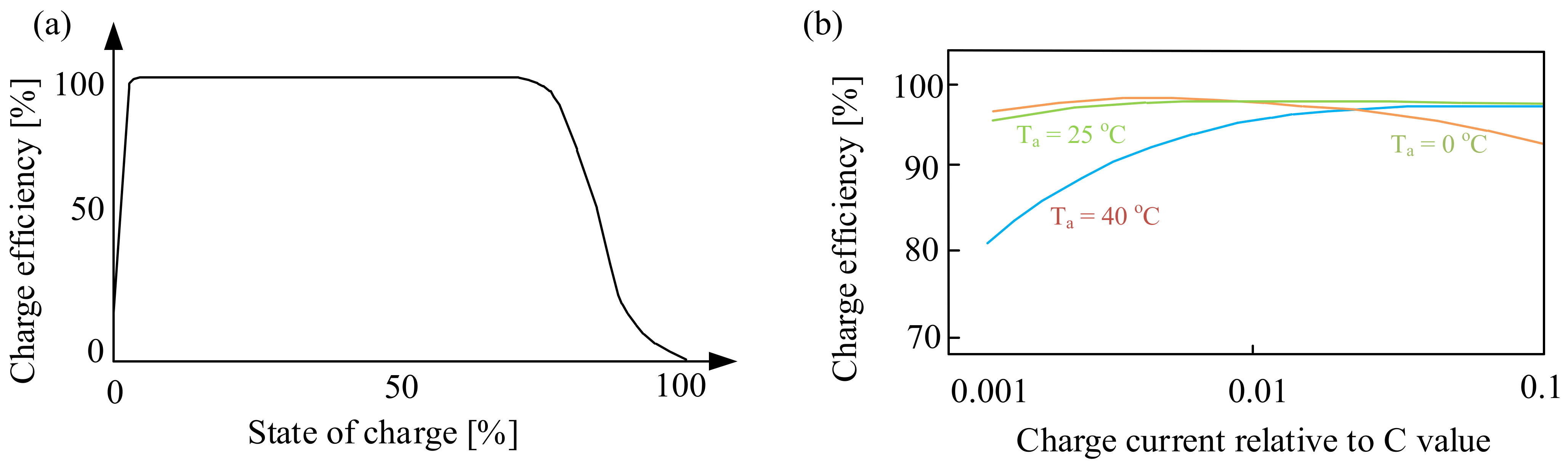
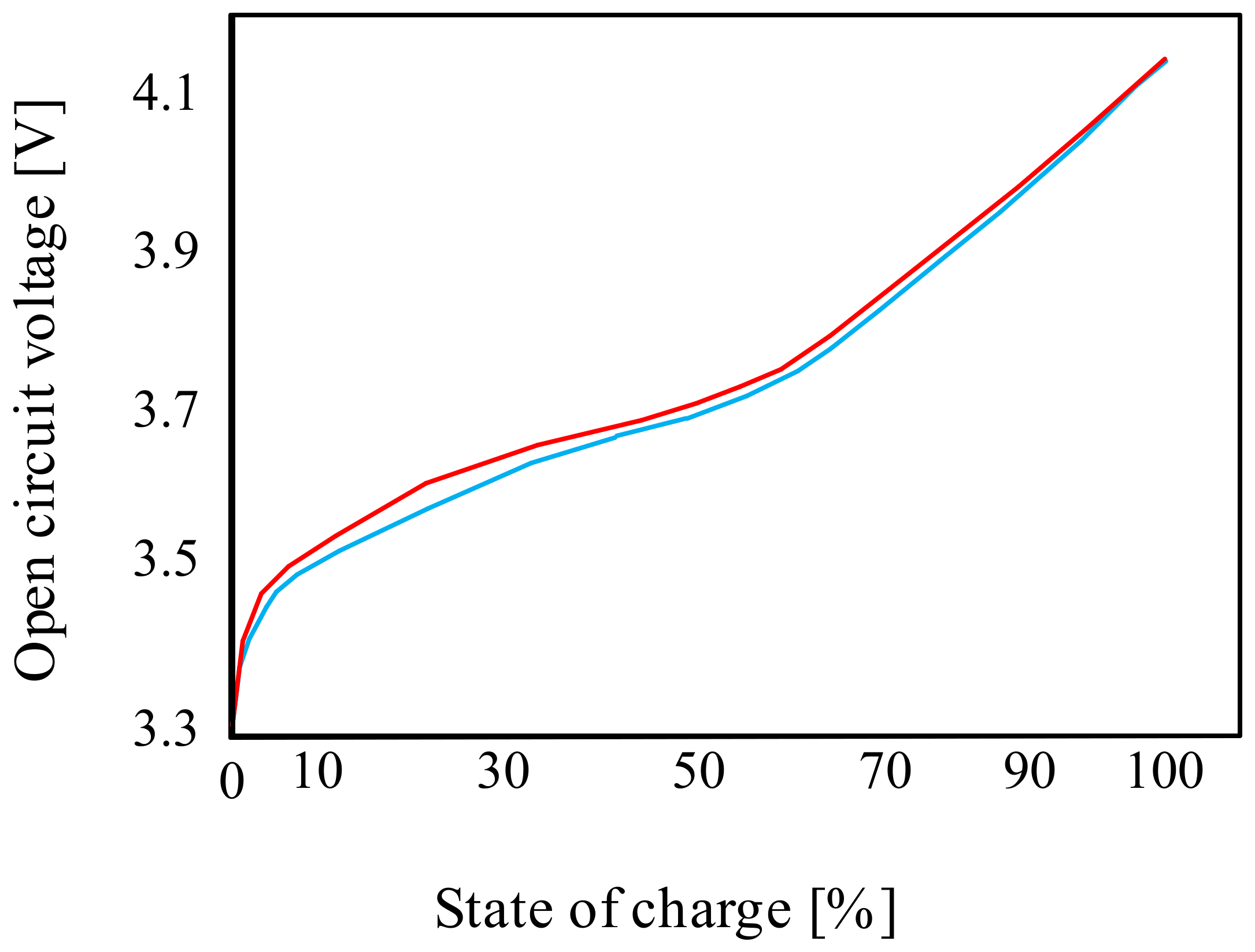
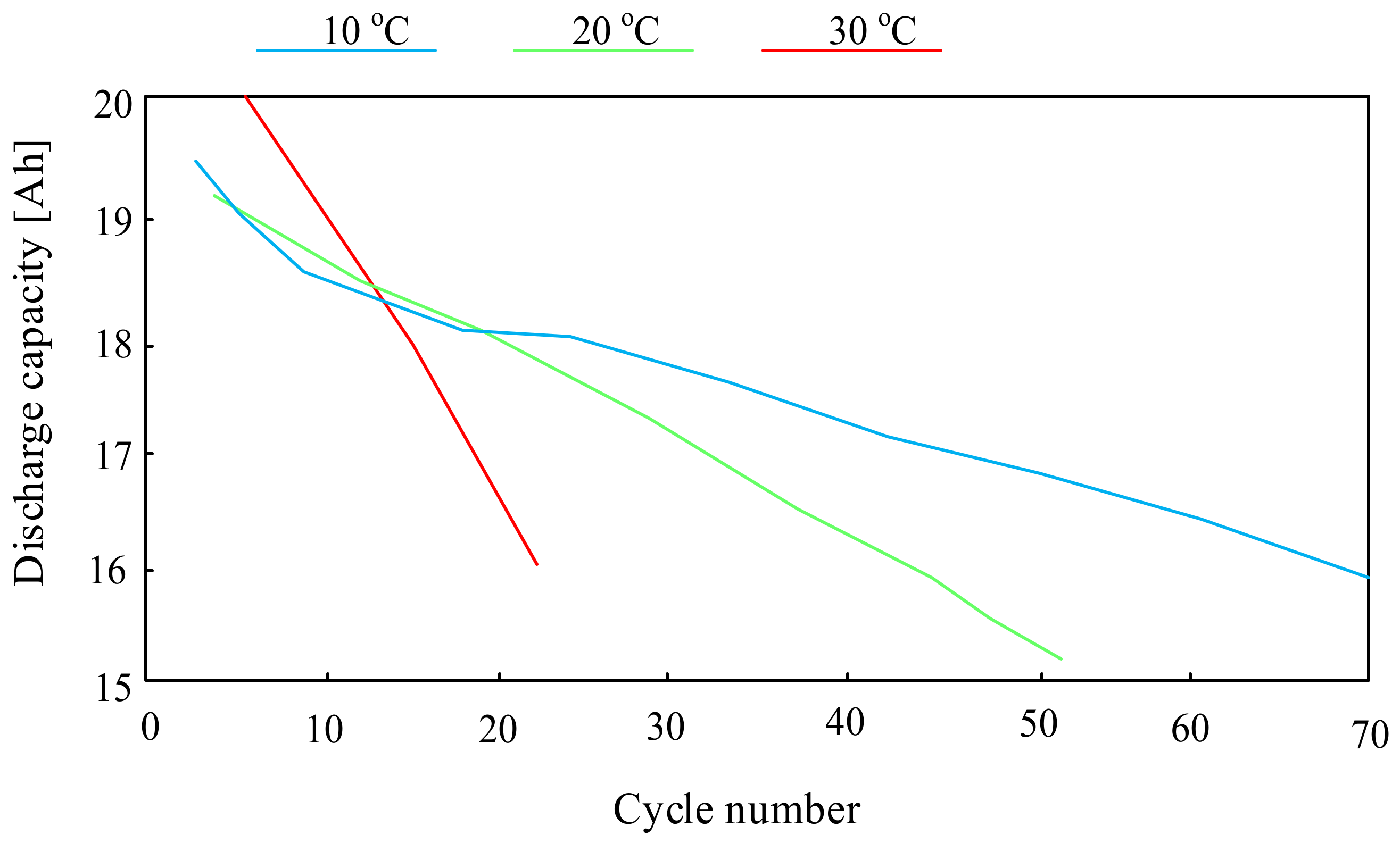
3.1.2. Lithium-Ion Batteries (Li-Ion)
3.1.3. Nickel–Cadmium Batteries (Ni–Cd)
3.2. New Battery Energy Storage Technologies
- -
- lithium–polymer batteries, discussed in Section 3.2.1,
- -
- lithium–sulfur batteries, described in Section 3.2.2,
- -
- metal–air batteries, described in Section 3.2.3,
- -
- sodium–nickel-chloride batteries, described in Section 3.2.4,
- -
- nickel–metal-hydride batteries, described in Section 3.2.5,
- -
- sodium-ion batteries, described in Section 3.2.6,
- -
- sodium–sulfur batteries, described in Section 3.2.7,
- -
- vanadium batteries, described in Section 3.2.8,
- -
- zinc–bromine batteries, described in Section 3.2.9,
- -
- zinc-ion batteries, described in Section 3.2.10.
3.2.1. Lithium Polymer Batteries (Li-Polymer)
3.2.2. Lithium-Sulfur Batteries (Li-S)
3.2.3. Lithium-Polymer-Iron Batteries (LiFePO4)
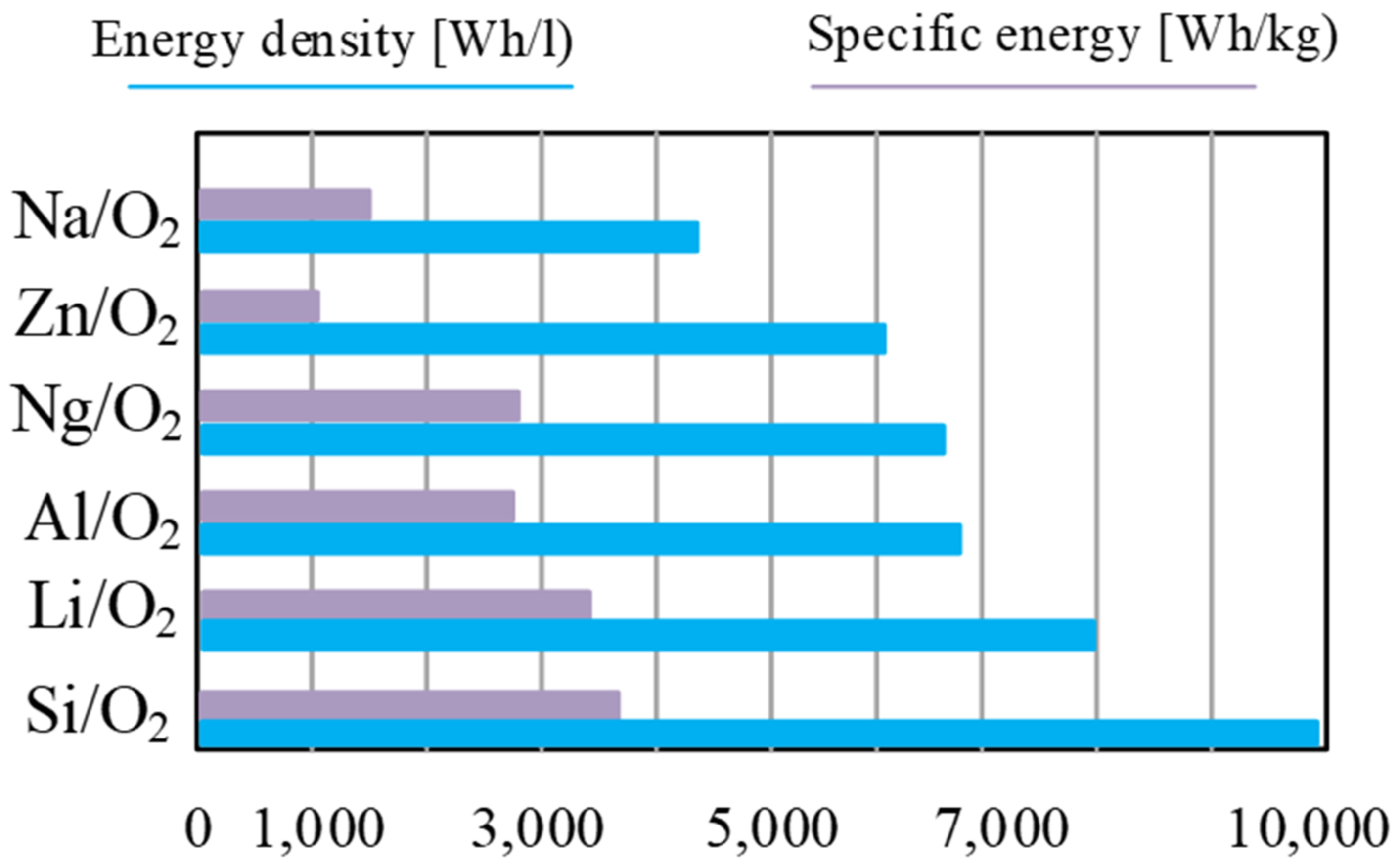
3.2.4. Metal–Air Batteries (Metal–Air)
3.2.5. Sodium–Nickel-Chloride Batteries (Na-NiCl2)
3.2.6. Nickel–Metal-Hydride Batteries (Ni-MH)
3.2.7. Sodium-Ion Batteries (Na-Ion)
3.2.8. Sodium Sulfur Batteries (Na-S)
3.2.9. Vanadium Redox Batteries (VRB)
3.2.10. Zinc–Bromine Batteries (Zn–Br)
3.2.11. Aqueous Zn-Ion Batteries (AZIBs)
4. Supercapacitors
- EDLC double-layer supercapacitors manufactured, among others, by Elna Dynavap
- pseudocapacitors manufactured by, among others, Maxwell PCAP
- hybrids proposed, e.g., by Licap Technologies LC.
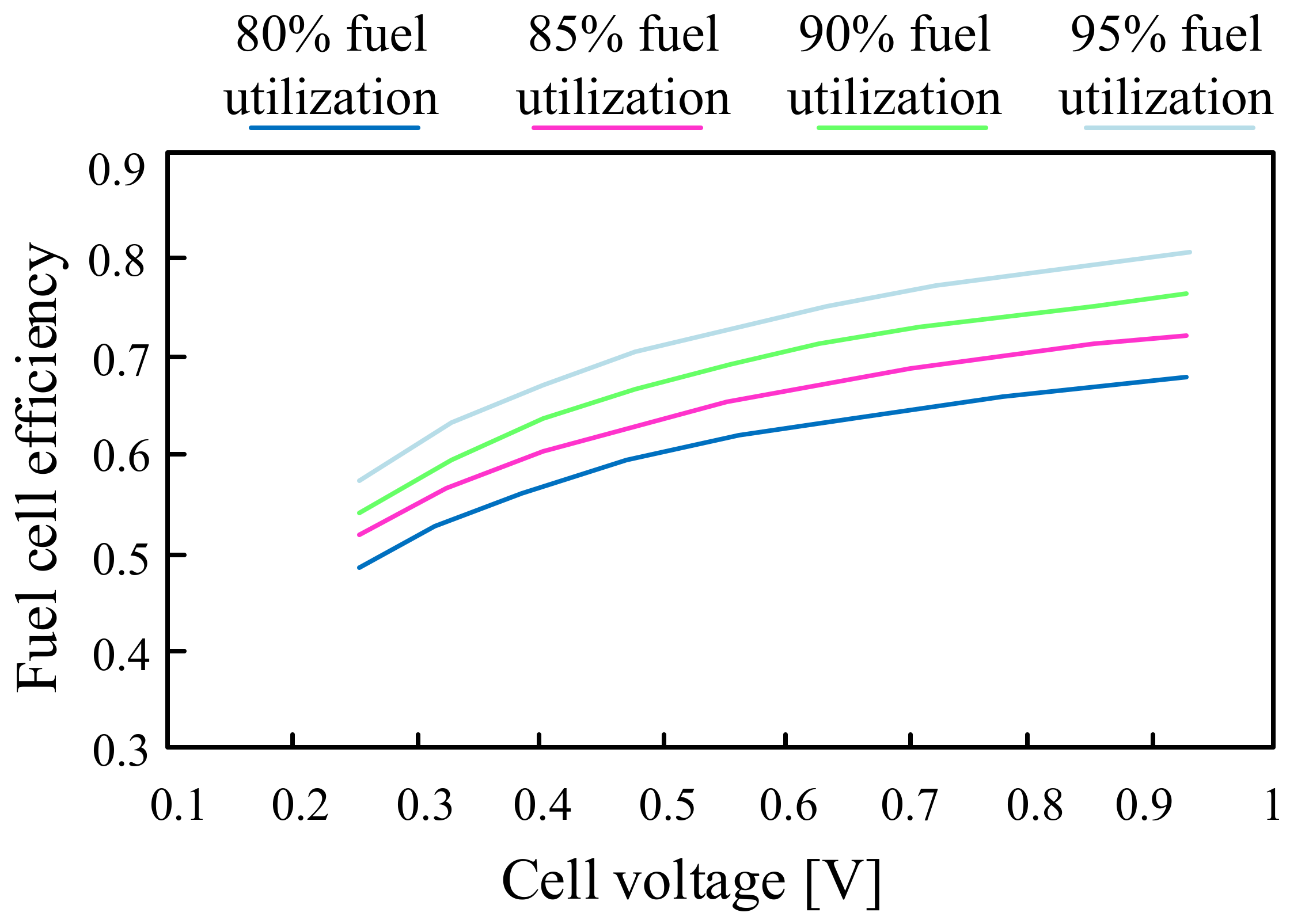
5. Fuel Cells
- alkaline (AFC);
- with phosphoric acid (PAFC);
- solid oxide (SOFC);
- with molten carbonate (MCFC);
- with a proton exchange membrane (PEMFC).
- directly powered by methanol (DMFC)
6. Comparison of Batteries
7. Final Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Climat Trarget Strategy. Available online: https://climate.ec.europa.eu/eu-action/european-green-deal/2030-climate-target-plan_en (accessed on 6 May 2023).
- Jankowska, E. Dekarbonizacja europejskich gospodarek w ujęciu przestrzennym. Stud. Prace WNEiZ US 2016, 45, 265–277. [Google Scholar] [CrossRef]
- Challenges of Energy Storage: TES Global Prospects. Available online: https://www.araner.com/blog/challenges-of-energy-storage (accessed on 6 May 2023).
- Górecki, K.; Dąbrowski, J.; Krac, E. SPICE-aided modeling of daily and seasonal changes in properties of the actual photovoltaic installation. Energies 2021, 14, 6247. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Energy storage systems towards 2050. Energy 2021, 219, 119634. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Nazir, M.S.; Tao, H.; Cao, S.; Ji, R.; Jiang, M.; Yao, L. Integration of energy storage system and renewable energy sources based on artificial intelligence. J. Energy Storage 2021, 40, 102811. [Google Scholar] [CrossRef]
- Nair, N.K.C.; Garimella, N. Battery energy storage systems: Assessment for small-scale renewable energy integration. Energy Build. 2010, 42, 2124–2130. [Google Scholar] [CrossRef]
- Whittingham, M.S. History, evolution, and future status of energy storage. Proc. IEEE 2010, 100, 1518–1534. [Google Scholar] [CrossRef]
- Torres, J.; Moreno-Torres, P.; Navarro, G.; Blanco, M.; Lafoz, M. Fast energy storage systems comparison in terms of energy efficiency for a specific application. IEEE Access 2018, 6, 40656–40672. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Abo-Khalil, A.G.; Maghrabie, H.M.; Elsaid, K.; Abdelkareem, M.A. Battery energy storage systems and SWOT (strengths, weakness, opportunities, and threats) analysis of batteries in power transmission. Energy 2022, 254, 123987. [Google Scholar] [CrossRef]
- Dănilă, E.; Lucache, D.D. History of the first energy storage systems. In Proceedings of the 3rd International Symposium on the History of Electrical Engineering and of Tertiary-Level Engineering Education, Iaşi, Romania, 27–29 October 2010; pp. 27–29. [Google Scholar]
- Mitali, J.; Dhinakaran, S.; Mohamad, A.A. Energy storage systems: A review. Energy Storage Sav. 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Subhashree, C. Review of energy storage system technologies integration to microgrid: Types, control strategies, issues, and future prospects. J. Energy Storage 2022, 48, 103966. [Google Scholar]
- Mostafa, M.M.M.; Abdelmohsen, A.; Reda, A.; Salama, S. High performance of supercapacitor based on alumina nanoparticles derived from Coca-Cola cans. J. Energy Storage 2023, 64, 107168. [Google Scholar] [CrossRef]
- Gouda, M.S.; Shehab, M.; Helmy, S.; Soliman, M.; Salama, R.S. Nickel and cobalt oxides supported on activated carbon derived from willow catkin for efficient supercapacitor electrode. J. Energy Storage 2023, 61, 106806. [Google Scholar] [CrossRef]
- Bartosik, M.; Kamrat, W.; Kaźmierkowski, M.; Lewandowski, W.; Pawlik, M.; Peryt, T.; Skoczkowski, T.; Strupczewski, A.; Szeląg, A. Magazynowanie energii elektrycznej i gospodarka wodorowa. Przegląd Elektrotech. 2016, 12, 334–342. [Google Scholar] [CrossRef]
- Wojciechowski, H. Energy storage technologies: Part II. Instal 2017, 3, 16–26. [Google Scholar]
- Menezes, M.V.P.; Vilasboas, I.F.; da Silva, J.A.M. Liquid air energy storage system (LAES) assisted by cryogenic air rankine cycle (ARC). Energies 2022, 15, 2730. [Google Scholar] [CrossRef]
- Wang, J.; Lu, K.; Ma, L.; Wang, J.; Dooner, M.; Miao, S.; Li, J.; Wang, D. Overview of compressed air energy storage and technology development. Energies 2017, 10, 991. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Ramadan, M.; Abdelkareem, M.A.; Alami, A.H. Compressed air energy storage systems: Components and operating parameters—A review. J. Energy Storage 2021, 34, 102000. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A.; Ramadan, M. Critical review of flywheel energy storage system. Energies 2021, 14, 2159. [Google Scholar] [CrossRef]
- Moseley, P.T.; Nelson, R.F.; Hollenkamp, A.F. The role of carbon in valve-regulated lead–acid battery technology. J. Power Sources 2006, 157, 3–10. [Google Scholar] [CrossRef]
- Magazynowanie Energii Elektrycznej—Mechaniczne Zasobniki Energii. Available online: https://leonardo-energy.pl/artykuly/magazynowanie-energii-elektrycznej-mechaniczne-zasobniki-energii/ (accessed on 6 May 2023).
- Basecq, V.; Michaux, G.; Inard, C.; Blondeau, P. Short-term storage systems of thermal energy for buildings: A review. Adv. Build. Energy Res. 2013, 7, 66–119. [Google Scholar] [CrossRef]
- Shi, M.; Hu, J.; Han, H.; Yuan, X. Design of battery energy storage system based on Ragone curve. In Proceedings of the 2020 4th International Conference on HVDC (HVDC), Xi’an, China, 6–9 November 2020; IEEE: New York, NY, USA, 2020. [Google Scholar]
- Lisowska-Oleksiak, A.; Nowak, A.P.; Wilamowska, M. Superkondensatory jako materiały do magazynowania energii. Acta Energetica 2010, 1, 71–79. [Google Scholar]
- Haas, O.; Cairns, E.J. Electrochemical energy storage. Annu. Rep. Sect. C Phys. Chem. 1999, 95, 163–198. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy storage data reporting in perspective—Guidelines for interpreting the performance of electrochemical energy storage systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Wojciechowski, H. Technologie magazynowania energii: Cz. I. Instal 2017, 2, 20–26. [Google Scholar]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Redox flow batteries for energy storage: Their promise, achievements and challenges. Curr. Opin. Electrochem. 2019, 16, 117–126. [Google Scholar] [CrossRef]
- Bednarek, K.; Bugała, A. Własności użytkowe akumulatorów kwasowo-ołowiowych. Pozn. Univ. Technol. Acad. J. Electr. Eng. 2017, 92, 47–60. [Google Scholar] [CrossRef]
- Plewa, A. Optymalizacja Właściwości Elektrochemicznych Materiału Katodowego LiMn(2)O(4-y)S(y) dla Akumulatorów Litowych. Master’s Thesis, Jagiellonian University, Kraków, Poland, 2014. [Google Scholar]
- Volkov, S.S.; Gumelev, V.Y.; Dmitrievsky, Y.E.; Kitaeva, T.I.; Nikolin, S.V.; Timashev, M.Y.; Tolstoguzov, A.B.; Trukhin, V.V. Investigation of composition and energy processes on the surface of electrodes of a lead-acid accumulator. Bull. Russ. Acad. Sci. Phys. 2010, 74, 272–276. [Google Scholar] [CrossRef]
- Czerwiński, A.; Obrębowski, S.; Kotowski, J.; Rogulski, Z.; Skowroński, J.M.; Krawczyk, P.; Rozmanowski, T.; Bajsert, M.; Przystałowski, M.; Buczkowska-Biniecka, M.; et al. Electrochemical behavior of negative electrode of lead-acid cells based on reticulated vitreous carbon carrier. J. Power Sources 2010, 195, 7524–7529. [Google Scholar] [CrossRef]
- Kutluay, K.; Cadirci, Y.; Ozkazanc, Y.S.; Cadirci, I. A new online state-of-charge estimation and monitoring system for sealed lead-acid batteries in telecommunication power supplies. IEEE Trans. Ind. Electron. 2005, 52, 1315–1327. [Google Scholar] [CrossRef]
- Lithium-Ion Batteries Work Earns Nobel Prize in Chemistry for 3 Scientists. Available online: https://www.nytimes.com/2019/10/09/science/nobel-prize-chemistry.html (accessed on 6 May 2023).
- Shin, S.H.; Yoon, S.J.; So, S.; Kim, T.H.; Hong, Y.T.; Lee, J.Y. Simple and effective modification of absorbed glass mat separator through atmospheric plasma treatment for practical use in AGM lead-acid battery applications. J. Energy Storage 2020, 28, 101187. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, S.; He, Y.B.; Kang, F.; Chen, L.; Li, H.; Yang, Q.H. Solid-state lithium batteries: Safety and prospects. eScience 2022, 2, 138–163. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-art and energy management system of lithium-ion batteries in electric vehicle applications: Issues and recommendations. IEEE Access 2018, 6, 19362–19378. [Google Scholar] [CrossRef]
- Naoki, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar]
- Winter, M.; Barnett, B.; Xu, K. Before Li ion batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Bogusz, P.; Korkosz, M.; Wygonik, P.; Dudek, M.; Lis, B. Analiza wpływu źródła zasilającego na właściwości bezszczotkowego silnika prądu stałego z magnesami trwałymi przeznaczonego do napędu bezzałogowego aparatu latającego. Energia 2015, 2, 2. [Google Scholar] [CrossRef]
- Mazan, B.; Detka, T. Eksperymentalne badanie wpływu temperatury ogniwa litowo-jonowego na pojemność i dokładność obliczeń stopnia naładowania. Napędy Sterow. 2020, 22, 40–45. [Google Scholar]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Chen, W.; Liang, J.; Yang, Z.; Li, G. A review of lithium-ion battery for electric vehicle applications and beyond. Energy Procedia 2019, 158, 4363–4368. [Google Scholar] [CrossRef]
- Lang, J.; Zhang, X.; Liu, B.; Wang, R.; Chen, J.; Yan, X. The roles of graphene in advanced Li-ion hybrid supercapacitors. J. Energy Chem. 2018, 27, 43–56. [Google Scholar] [CrossRef]
- Feng, L.; Tan, C.M.; Pecht, M. Effect of temperature on the aging rate of Li ion battery operating above room temperature. Sci. Rep. 2015, 5, 12967. [Google Scholar]
- Dingsong, B.; An, Z.; Wang, N.; Liu, S.; Yu, X. Research on Influence of Temperature on Capacity Fade of Lithium Ion Battery. IOP Conf. Ser. Earth Environ. Sci. 2021, 791, 012102. [Google Scholar]
- Burzyński, D.; Głuchy, D.; Godek, M. Wpływ temperatury na parametry procesu ładowania z wykorzystaniem technologii Quick Charge oraz trwałość ogniw litowo-jonowych. Pozn. Univ. Technol. Acad. J. Electr. Eng. 2018, 95, 309–320. [Google Scholar] [CrossRef]
- Jeyaseelan, C.; Jain, A.; Khurana, P.; Kumar, D.; Thatai, S. Ni-Cd Batteries. Rechargeable Batteries: History, Progress, and Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2020; Volume 1, pp. 177–194. [Google Scholar]
- Beaudin, M.; Zareipour, H.; Schellenberg, A.; Rosehart, W. Energy storage for mitigating the variability of renewable electricity sources. Energy Storage Smart Grids Plan. Oper. Renew. Var. Energy Resour. 2014, 14, 1–33. [Google Scholar]
- Akumulatory Niklowo-Kadmowe. Available online: https://wamtechnik.pl/produkty/technologie-niklowe/akumulatory-niklowo-kadmowe-ni-cd/ (accessed on 6 May 2023).
- Blumbergs, E.; Serga, V.; Platacis, E.; Maiorov, M.; Shishkin, A. Cadmium recovery from spent Ni-Cd batteries: A brief review. Metals 2021, 11, 1714. [Google Scholar] [CrossRef]
- BU-407: Charging Nickel-Cadmium. Available online: https://batteryuniversity.com/article/bu-407-charging-nickel-cadmium (accessed on 6 May 2023).
- Fattal, J.; Bou Dib, P.; Karami, N. Review on different charging techniques of a lithium polymer battery. In Proceedings of the 2015 Third International Conference on Technological Advances in Electrical, Electronics and Computer Engineering (TAEECE), Beirut, Lebanon, 29 April–1 May 2015. [Google Scholar]
- Lithium-Ion vs Lithium Polymer Battery: Which Is Better? Available online: https://etekware.com/lithium-ion-vs-lithium-polymer-batteries/ (accessed on 6 May 2023).
- The Difference between Lithium Ion and Lithium Polymer Batteries. Available online: https://www.batterypowertips.com/difference-between-lithium-ion-lithium-polymer-batteries-faq/ (accessed on 6 May 2023).
- Lithium-Ion Battery DATA SHEET. Available online: https://www.google.pl/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj44rCIr-b-AhUKl4sKHcDuAPIQFnoECDMQAQ&url=https%3A%2F%2Fwww.ineltro.ch%2Fmedia%2Fdownloads%2FSAAItem%2F45%2F45958%2F36e3e7f3-2049-4adb-a2a7-79c654d92915.pdf&usg=AOvVaw0X3kWXNnwerKpg6qqYAS1C (accessed on 6 May 2023).
- Heydecke, J. Introduction to lithium polymer battery technology. Jauch Quartz GmbH Jauch Battery Solut. GmbH DLache 2018, 24, 78056. [Google Scholar]
- UPF644496 Panasonic. Available online: https://www.datasheets.com/en/search?q=UPF644496%20Panasonic (accessed on 6 May 2023).
- Bogusz, P.; Korkosz, M.; Prokop, J.; Wygonik, P. Badania laboratoryjne ogniw elektrycznych przeznaczonych do zastosowania w napędzie hybrydowym bezzałogowego aparatu latającego. Masz. Elektr. Zesz. Probl. 2012, 2, 111–115. [Google Scholar]
- Baronti, F.; Fantechi, G.; Leonardi, E.; Roncella, R.; Saletti, R. Enhanced model for lithium-polymer cells including temperature effects. In Proceedings of the IECON 2010-36th Annual Conference on IEEE Industrial Electronics Society, Glendale, AR, USA, 7–10 November 2010. [Google Scholar]
- Siczek, K. Ocena możliwości wykorzystania akumulatora litowo–siarkowego do rozruchu silnika spalinowego. Autobusy Tech. Eksploat. Syst. Transp. 2016, 12, 1346–1350. [Google Scholar]
- Choi, Y.-J.; Chung, Y.D.; Baek, C.Y.; Kim, K.W.; Ahn, H.J.; Ahn, J.H. Effects of carbon coating on the electrochemical properties of sulfur cathode for lithium/sulfur cell. J. Power Sources 2008, 184, 548–552. [Google Scholar] [CrossRef]
- Islam, M.M.; Ostadhossein, A.; Borodin, O.; Yeates, A.T.; Tipton, W.W.; Hennig, R.G.; Kumar, N.; van Duin, A.C.T. ReaxFF molecular dynamics simulations on lithiated sulfur cathode materials. Phys. Chem. Chem. Phys. 2015, 17, 3383–3393. [Google Scholar] [CrossRef]
- Sion Introduces a Lithium Sulfur Rechargeable Battery. Available online: https://web.archive.org/web/20090618082627/http://www.evworld.com/article.cfm?storyid=788 (accessed on 5 June 2023).
- Tudron, F.; Akridge, J.; Puglisi, V. Lithium-Sulfur Rechargeable Batteries: Characteristics, State of Development, and Applicability to Powering Portable Electronics. Power Sources 2001. Available online: https://www.researchgate.net/publication/267249582_Lithium-Sulfur_Rechargeable_Batteries_Characteristics_State_of_Development_and_Applicability_to_Powering_Portable_Electronics#fullTextFileContent (accessed on 6 May 2023).
- Shateri, N.; Auger, D.J.; Fotouhi, A.; Brighton, J.; Du, W.; Owen, R.E.; Brett, D.J.L.; Shearing, P.R. Investigation of the effect of temperature on lithium-sulfur cell cycle life performance using system identification and X-ray tomography. Batter. Supercaps 2022, 5, e202200035. [Google Scholar] [CrossRef]
- Wolff, D.; Canals Casals, L.; Benveniste, G.; Corchero, C.; Trilla, L. The effects of lithium sulfur battery ageing on second-life possibilities and environmental life cycle assessment studies. Energies 2019, 12, 2440. [Google Scholar] [CrossRef]
- Kalkan, O.; Celen, A.; Bakirci, K. Experimental and numerical investigation of the LiFePO4 battery cooling by natural convection. J. Energy Storage 2021, 40, 102796. [Google Scholar] [CrossRef]
- Malik, M.; Mathew, M.; Dincer, I.; Rosen, M.A.; McGrory, J.; Fowler, M. Experimental investigation and thermal modelling of a series connected LiFePO4 battery pack. Int. J. Therm. Sci. 2018, 132, 466–477. [Google Scholar] [CrossRef]
- Ghassemi, A.; Hollenkamp, A.F.; Banerjee, P.C.; Bahrani, B. Impact of high-amplitude alternating current on LiFePO4 battery life performance: Investigation of AC-preheating and microcycling effects. Appl. Energy 2022, 314, 118940. [Google Scholar] [CrossRef]
- Yanguang, L.; Lu, J. Metal–air batteries: Will they be the future electrochemical energy storage device of choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar]
- Deepti, A.; Kalpna, V.; Varshney, P. Metal air battery: A sustainable and low cost material for energy storage. J. Phys. Conf. Ser. 2021, 1, 1913. [Google Scholar]
- Neburchilov, V.; Zhang, J. Metal–Air and Metal–Sulfur Batteries: Fundamentals and Applications; CRC Press: Boca, Raton, USA, 2016. [Google Scholar]
- Neburchilov, V.; Zhang, J. Aluminum–Air Batteries Fundamentals and Applications. In Metal–Air and Metal–Sulfur Batteries: Fundamentals and Applications; CRC Press: Boca, Raton, USA, 2016. [Google Scholar]
- Salt Batteries: Opportunities and Applications of Storage Systems Based on Sodium Nickel Chloride Batteries. Available online: https://www.google.pl/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjQ_Nj27-f-AhUC6CoKHV5xCBEQFnoECAgQAQ&url=https%3A%2F%2Fwww.europarl.europa.eu%2FRegData%2Fetudes%2FIDAN%2F2023%2F740064%2FIPOL_IDA(2023)740064_EN.pdf&usg=AOvVaw04AYPgZEVB7Zm6-w2kqa8Q (accessed on 6 May 2023).
- Huang, H.; Xu, R.; Feng, Y.; Zeng, S.; Jiang, Y.; Wang, H.; Luo, W.; Yu, Y. Sodium/potassium-ion batteries: Boosting the rate capability and cycle life by combining morphology, defect and structure engineering. Adv. Mater. 2020, 32, 1904320. [Google Scholar] [CrossRef]
- Akumulatorowe Systemy Magazynowania Energii: Wydajność i Eksploatacja. Część I. Available online: https://leonardo-energy.pl/artykuly/akumulatorowe-systemy-magazynowania-energii-wydajnosc-i-eksploatacja-czesc-i/ (accessed on 6 May 2023).
- Deepika, S. Characteristics and effects of γ-NiOOH on cell performance and a method to quantify it in nickel electrodes. J. Electrochem. Soc. 1998, 145, 116. [Google Scholar]
- Sakaebe, H. Zebra batteries. In Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA.
- Muslimin, S.; Nawawi, Z.; Suprapto, B.Y.; Dewi, T. Comparison of Batteries Used in Electrical Vehicles. In Proceedings of the 5th FIRST T1 T2 2021 International Conference (FIRST-T1-T2 2021), Palembang, Indonesia, 21 October 2021; Atlantis Press: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Baterie Niklowo-Wodorkowe NiMH HT. Available online: https://hybryd.com.pl/pl/informacje/baterie/baterie_niklowo-wodorkowe_nimh_ht/ (accessed on 6 May 2023).
- Nurshahirah Athirah, R.; Nor Farahaida, A.R.; Ammirrul Atiqi Mohd Zainuri, M. Characteristics of lead-acid and nickel metal hydride batteries in uninterruptible power supply operation. Int. J. Power Electron. Drive Syst. 2018, 10, 1520. [Google Scholar]
- Characteristics of Rechargeable Batteries. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjG89KT6dn-AhXQuosKHSYQBfEQFnoECD4QAQ&url=https%3A%2F%2Fwww.ti.com%2Flit%2Fan%2Fsnva533%2Fsnva533.pdf&usg=AOvVaw1EatkQ9GS80jJpkGObEdOR (accessed on 6 May 2023).
- Hu, W.K.; Geng, M.M.; Gao, X.P.; Burchardt, T.; Gong, Z.X.; Noreus, D.; Nakstad, N.K. Effect of long-term overcharge and operated temperature on performance of rechargeable NiMH cells. J. Power Sources 2006, 159, 1478–1483. [Google Scholar] [CrossRef]
- Goikolea, E.; Palomares, V.; Wang, S.; Ruiz de Larramendi, I.; Guo, X.; Wang, G.; Rojo, T. Na-ion batteries—Approaching old and new challenges. Adv. Energy Mater. 2020, 10, 2002055. [Google Scholar] [CrossRef]
- Shan, X.Y.; Wang, Y.; Wang, D.W.; Li, F.; Cheng, H.M. Armoring graphene cathodes for high-rate and long-life lithium ion supercapacitors. Adv. Energy Mater. 2016, 6, 1502064. [Google Scholar] [CrossRef]
- Abraham, K.M. How comparable are sodium-ion batteries to lithium-ion counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Sodium Ion Battery: The Definitive Guide. Available online: https://www.ecolithiumbattery.com/sodium-ion-battery/ (accessed on 6 May 2023).
- Rudola, A.; Wright, C.J.; Barker, J. Reviewing the safe shipping of lithium-ion and sodium-ion cells: A materials chemistry perspective. Energy Mater. Adv. 2021, 2021, 9798460. [Google Scholar] [CrossRef]
- Kirubakaran, A.; Shailendra, J.; Nema, R.K. Renewable and sustainable energy reviews. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Calt Company. Available online: https://www.catl.com/en/news/6013.html (accessed on 6 May 2023).
- Sodium Sulphur Battery. Available online: https://www.google.pl/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjTxcyZver-AhWXyYsKHbevBWYQFnoECBEQAQ&url=https%3A%2F%2Fease-storage.eu%2Fwp-content%2Fuploads%2F2016%2F03%2FEASE_TD_NaS.pdf&usg=AOvVaw1-qwbcDjmPe7HByfxMZkeE (accessed on 6 May 2023).
- Arabkoohsar, A. Classification of energy storage systems. In Future Grid-Scale Energy Storage Solutions; Academic Press: Cambridge, MA, USA, 2023; pp. 1–30. [Google Scholar]
- Krawczyk, G. Zasobniki energii elektrycznej w transporcie szynowym. Autobusy Tech. Eksploat. Syst. Transp. 2013, 14, 1065–1076. [Google Scholar]
- Ye, R.; Henkensmeier, D.; Yoon, S.J.; Huang, Z.; Kim, D.K.; Chang, Z.; Chen, R. Redox flow batteries for energy storage: A technology review. J. Electrochem. Energy Convers. Storage 2018, 15, 010801. [Google Scholar] [CrossRef]
- Iwakiri, I.; Antunes, T.; Almeida, H.; Sousa, J.P.; Figueira, R.B.; Mendes, A. Redox flow batteries: Materials, design and prospects. Energies 2021, 14, 5643. [Google Scholar] [CrossRef]
- Cunha, A.; Martins, J.; Rodrigues, N.; Brito, F.P. Vanadium redox flow batteries: A technology review. Int. J. Energy Res. 2015, 39, 889–918. [Google Scholar] [CrossRef]
- Konev, D.V.; Antipov, A.E.; Loktionov, P.A.; Pichugov, R.D.; Kartashova, N.V.; Glazkov, A.T.; Abunaeva, L.Z.; Andreev, V.N.; Vorotyntsev, M.A. Redox flow batteries: Role in modern electric power industry and comparative characteristics of the main types. Russ. Chem. Rev. 2021, 90, 677. [Google Scholar]
- Li, M.; Li, Z.; Wang, X.; Meng, J.; Liu, X.; Wu, B.; Mai, L. Comprehensive understanding of the roles of water molecules in aqueous Zn-ion batteries: From electrolytes to electrode materials. Energy Environ. Sci. 2021, 14, 3796–3839. [Google Scholar] [CrossRef]
- Singh, V.; Kim, S.; Kang, J.; Byon, H.R. Aqueous organic redox flow batteries. Nano Res. 2019, 12, 1988–2001. [Google Scholar] [CrossRef]
- Głuchy, D.; Kasprzyk, L.; Tomczewski, A. Modelowanie superkondensatorów na potrzeby współpracy z OZE. Pozn. Univ. Technol. Acad. J. Electr. Eng. 2017, 89, 335–345. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Wang, Z.; Sun, F.; Dorrell, D.G. A review of supercapacitor modeling, estimation, and applications: A control/management perspective. Renew. Sustain. Energy Rev. 2018, 81, 1868–1878. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- October 1842: William Grove’s Letter to Faraday Describing a Fuel Cell. Available online: https://www.aps.org/publications/apsnews/201909/history.cfm (accessed on 6 June 2023).
- Żyjewska, U. Rodzaje ogniw paliwowych i ich potencjalne kierunki wykorzystania. Naft. Gaz 2021, 5, 332–339. [Google Scholar] [CrossRef]
- Saad, M.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar]
- Welaya, Y.M.A.; Gohary, M.M.; Nader, R.A. A comparison between fuel cells and other alternatives for marine electric power generation. Int. J. Nav. Archit. Ocean Eng. 2011, 3, 141–149. [Google Scholar] [CrossRef]
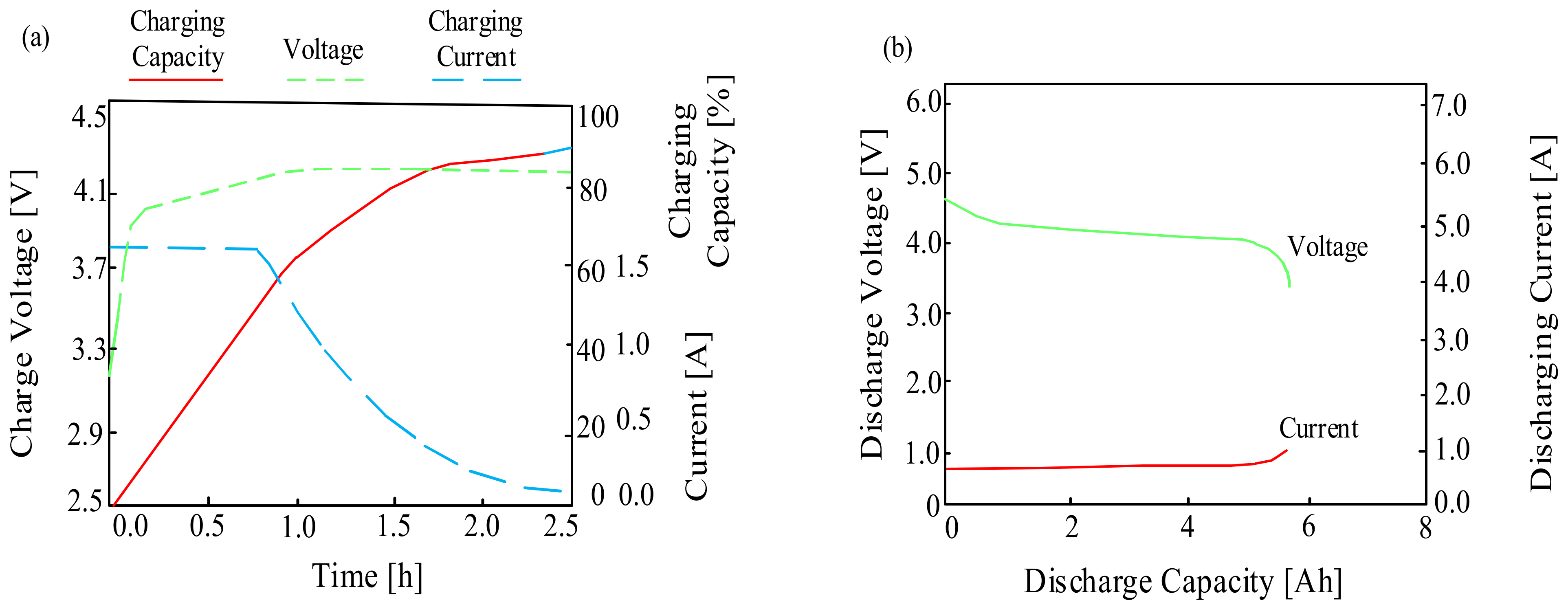
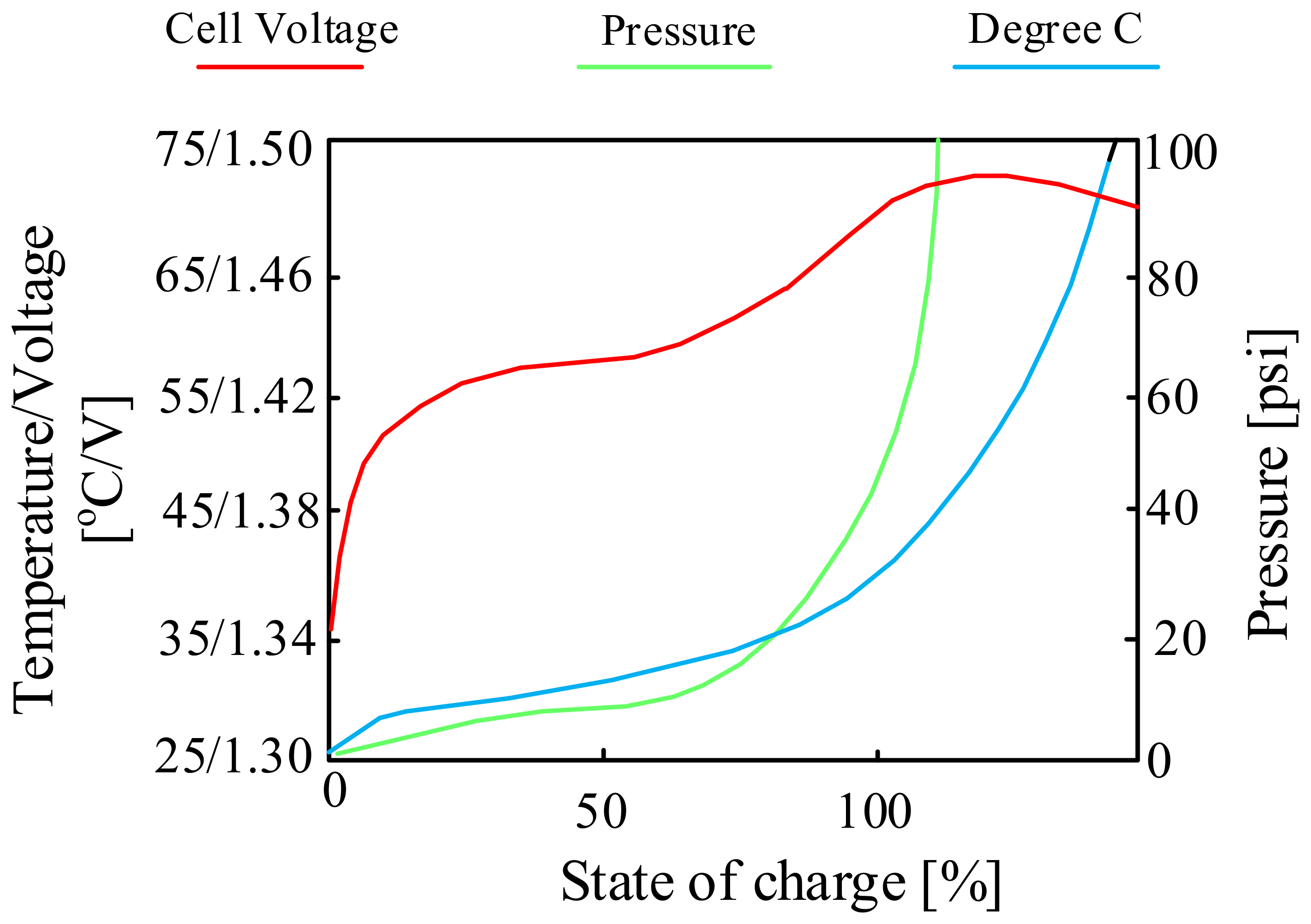
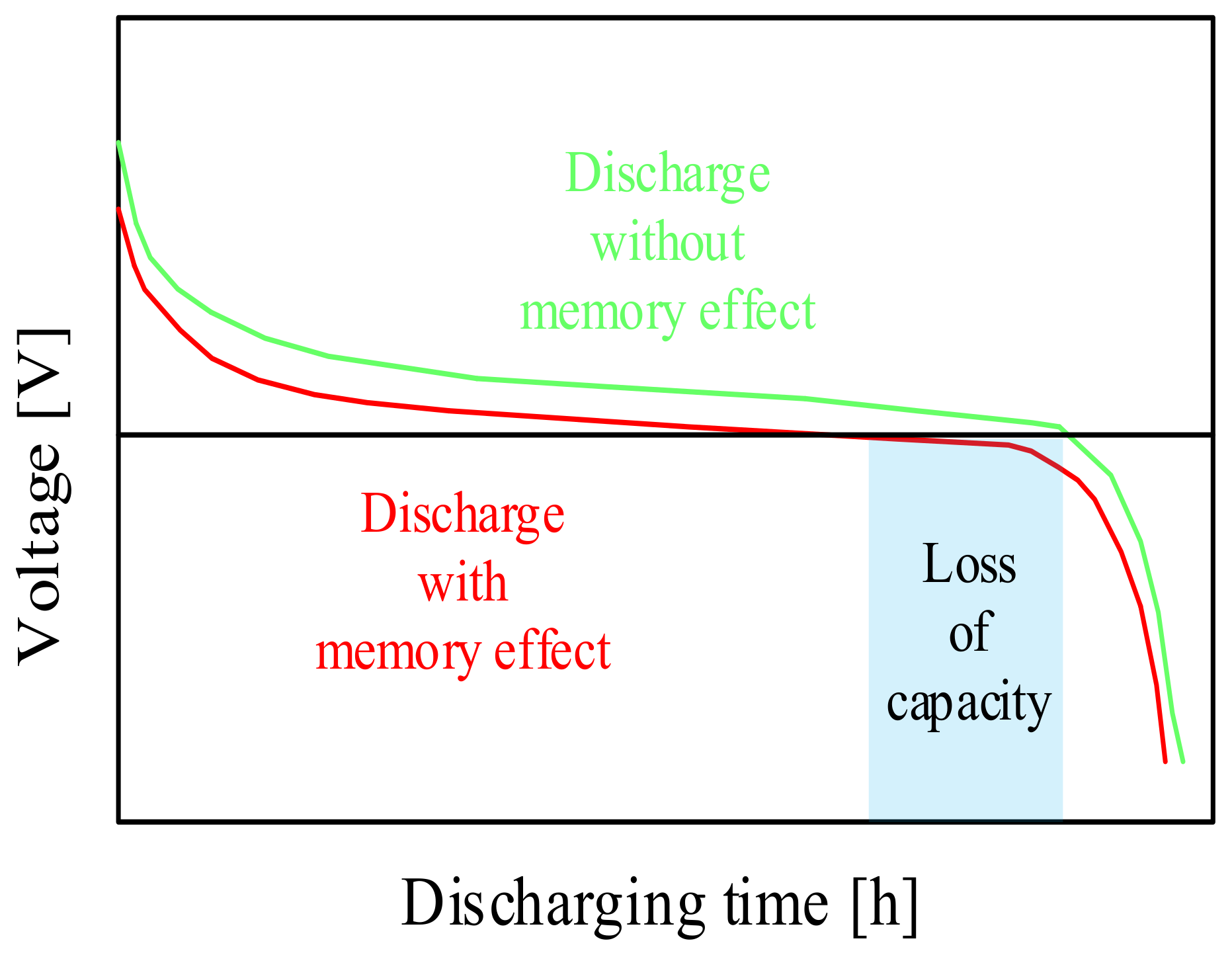
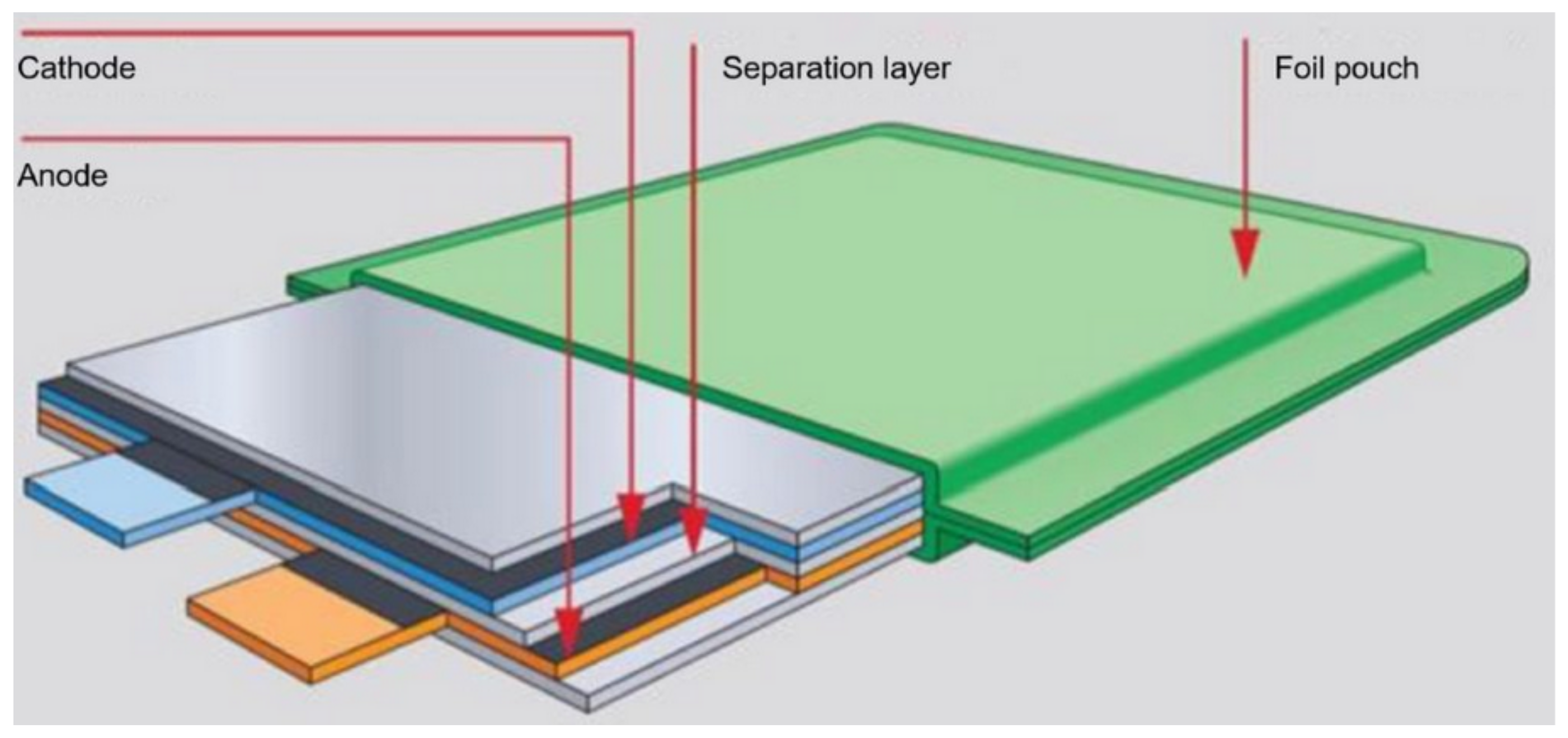



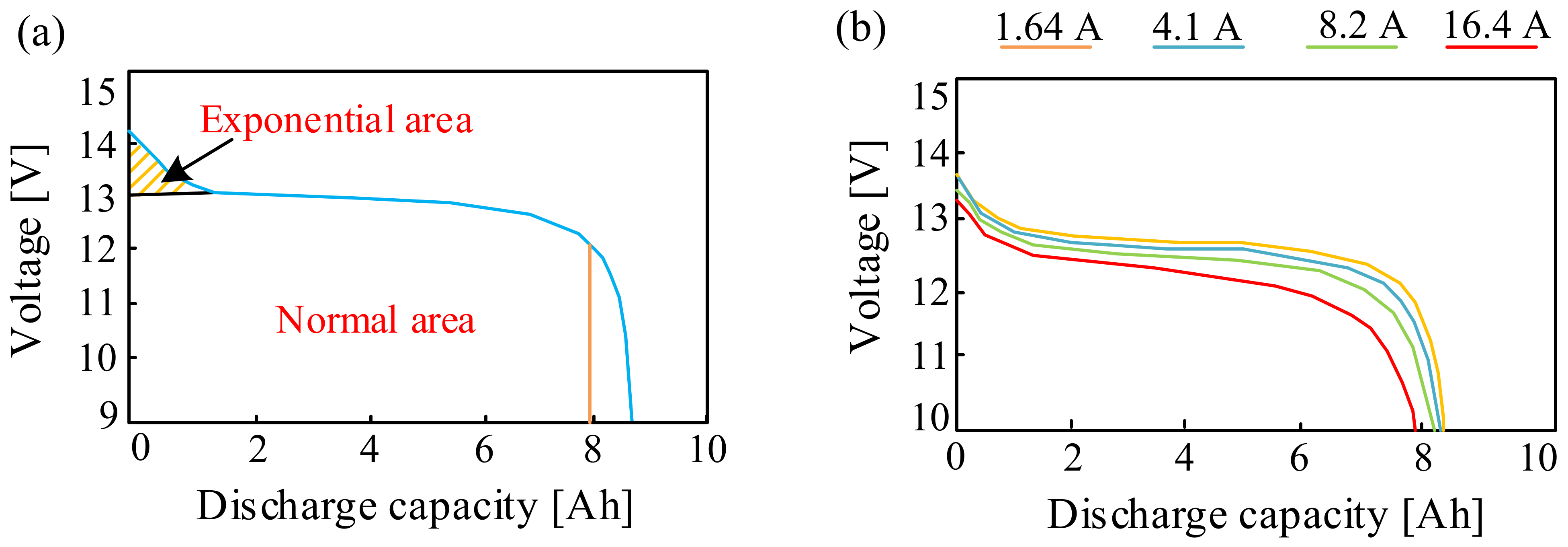

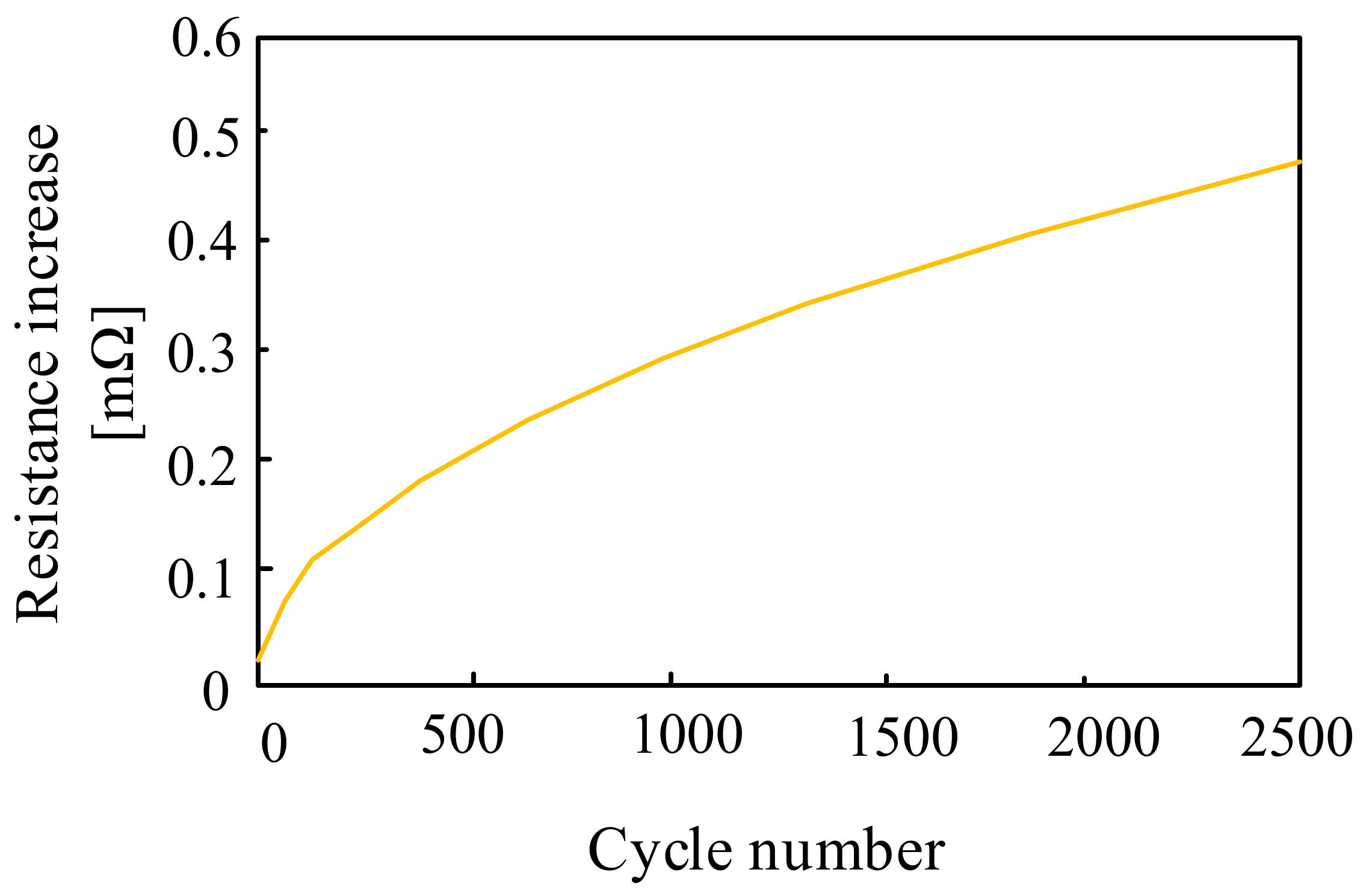
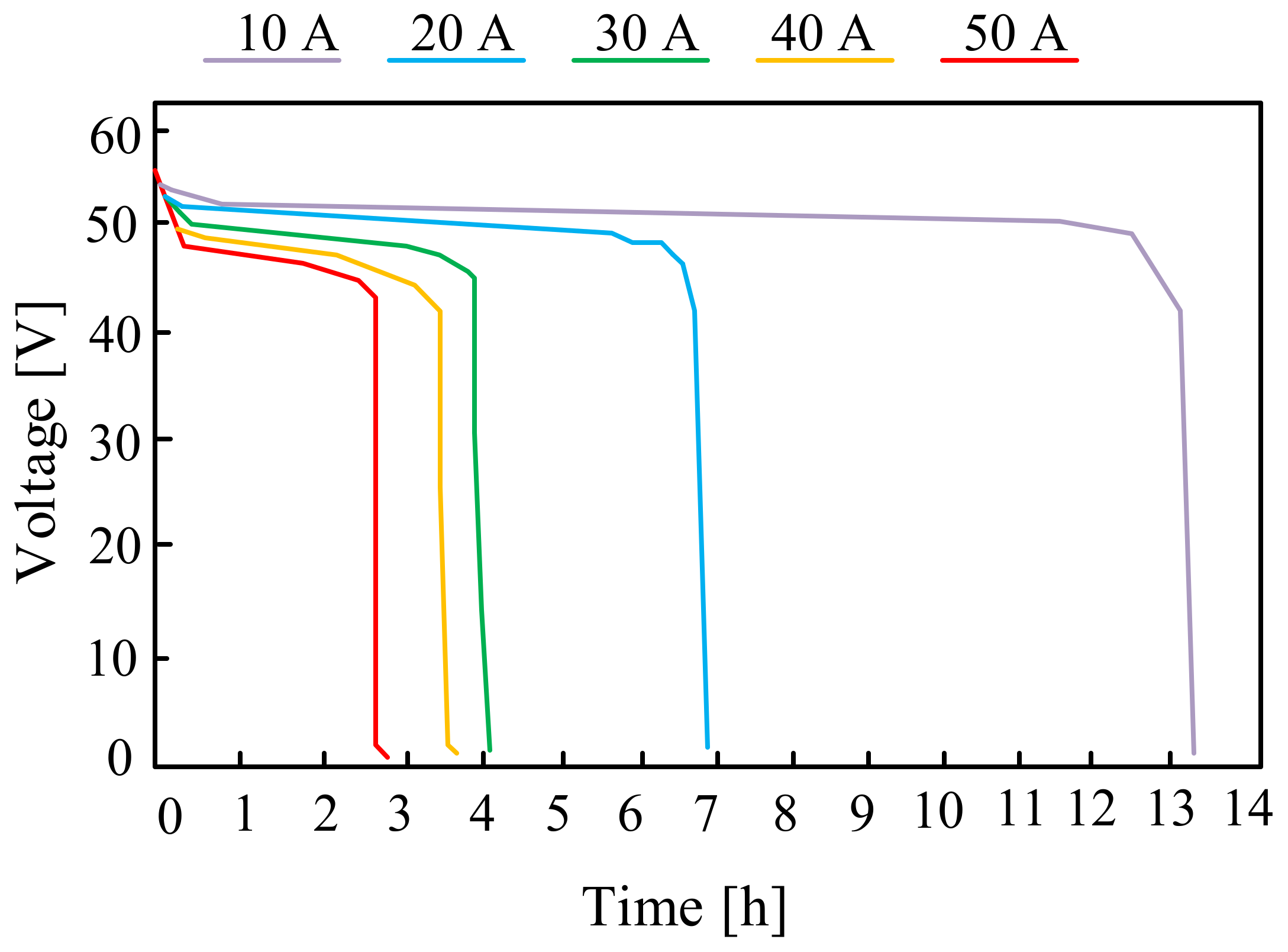

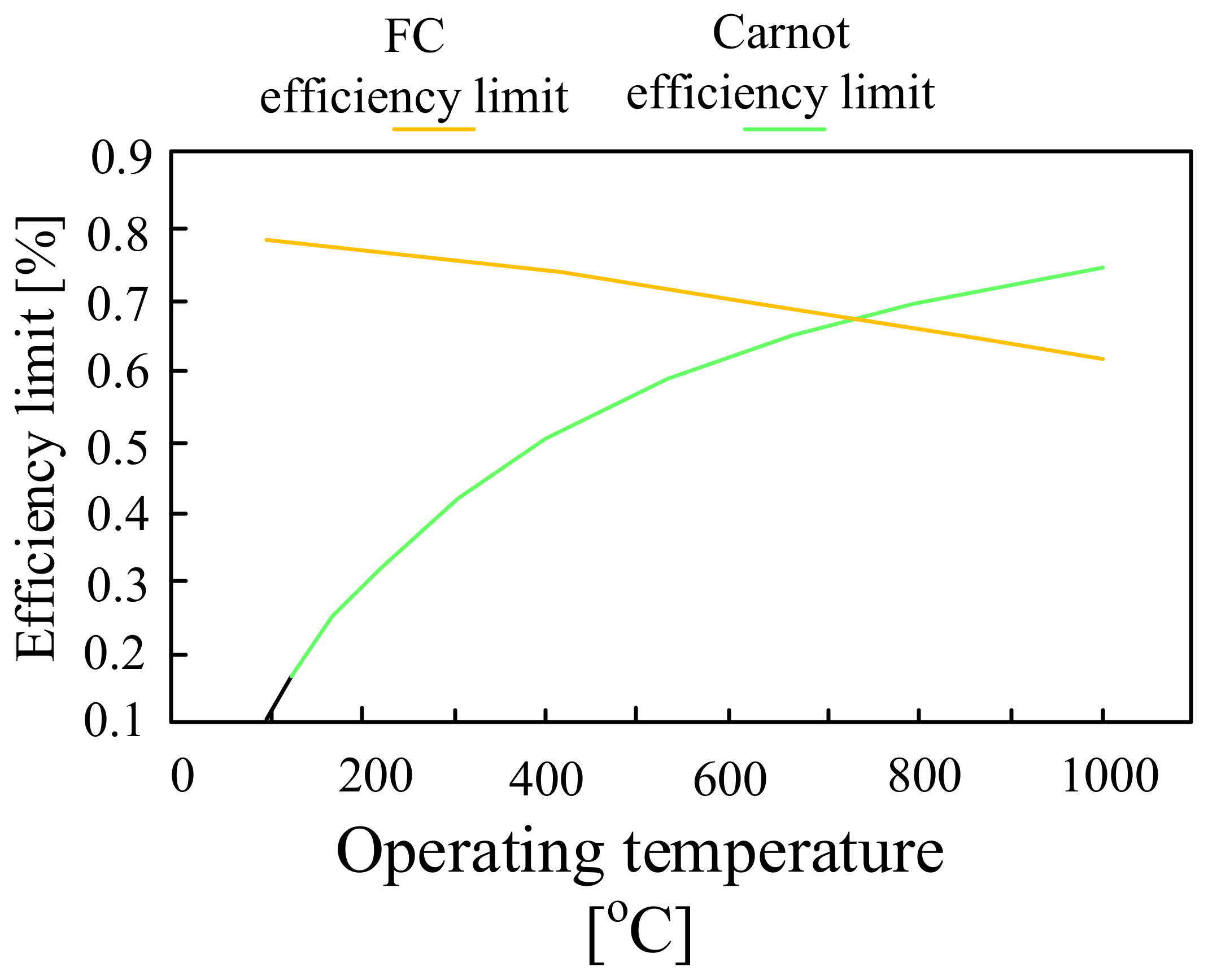

| Battery | Specific Capacity Based on Anode [Ah/g] | Theoretical Voltage [V] | Practical Voltage [V] | Specific Energy Based on Anode [Wh/kg] |
|---|---|---|---|---|
| AAB | 2.98 | 2.71 | 1.3 | 3.874 |
| Li/air | 3.862 | 3.45 | 3.0 | 11.586 |
| Zn/air | 0.82 | 1.65 | 1.1 | 902 |
| Mg/air | 2.205 | 2.93 | 1.3 | 2.867 |
| Fe/air | 0.96 | 1.30 | 1.0 | 960 |
| Ca/air | 1.337 | 3.12 | 2.0 | 2.675 |
| Cell Type | Fuel | Electroly-te/Membrane | Temperature [°C] | Generated Voltage [V] | Electrical Efficiency [%] |
|---|---|---|---|---|---|
| AFC | hydrogen | aqueous solution of potassium hydroxide | 90–100 | 1.0 | 60 |
| PAFC | hydrogen | liquid phosphoric acid | 150–200 | 1.1 | >40 |
| SOFC | hydrogen, carbon monoxide, methane | solid zirconia with an admixture of yttrium oxide | 600–1000 | 0.8–1.0 | 35–43 |
| MCFC | hydrogen, methane, carbon monoxide | a mixture of molten sodium and/or potassium carbonates | 600–700 | 0.7–1.0 | 45 |
| PEMFC | hydrogen | solid polymer. perfluorosulfonic acid | 50–100 | 1.1 | 53–58 |
| DMFC | methanol | solid polymer membrane | 60–200 | 0.2–0.4 | 40 |
| Technology Type | Power Density [kW/m3] | Energy Density [kWh/m3] | Energy Density [Wh/kg] | Cycle Efficiency [%] | Lifetime [Cycles] |
|---|---|---|---|---|---|
| Supercapacitors | 40,000–120,000 | 10 | 1–5 | 90–100 | <1,000,000 |
| Fuel cell | >500 | 500–3000 | 800–10,000 | 35–60% | >1000 |
| Battery | |||||
| Li-ion | 60–10,000 | 90–750 | 60–300 | 85–98 | 250–10,000 |
| Lead-acid | 10–700 | 25–90 | 30–50 | 65–90 | 250–1500 |
| NiCd vented | 75–700 | 15–80 | 15–40 | 60–80 | 1500–3000 |
| NiCd sealed | 80–600 | 60–150 | 50–75 | 80 | 2500 |
| NaNiCl | 15–270 | 100–200 | 100–200 | 80–90 | 1000–2500 |
| NaS | 120–160 | 150–300 | 100–250 | 70–85 | 2500–4500 |
| NiMH sealed | 10–3000 | 25–200 | 30–80 | 65–90 | 500–1200 |
| VRB | 0.5–2 | 20–70 | 15–50 | 60–75 | 1000–3600 |
| Technology | Advantages | Disadvantages |
|---|---|---|
| Supercapacitors | High power density | The life cycle depends on the voltage distribution between the cells and the maximum voltage thresholds; a more invasive solution in terms of safety. Not environmentally neutral |
| Fuel cell | Simple construction, low risk of failure, no negative influence on the environment, long working time, the ability to work with high load current | High cost of materials used to build catalysts, lower efficiency than for batteries |
| Battery | ||
| Li-ion | High energy and power density compared with other batteries; short response time | The lifetime depends on the number of charging and discharging cycles; high price |
| Lead-acid | Low cost; technical maturity | Low energy density; low power density; short response time; short life cycle; high maintenance requirements; toxicity |
| NiCd | Technical maturity | High cost; low energy density; low power density; toxicity; most used nickel electrode battery in the energy storage industry; popular in energy storage applications for power plants (e.g., substation batteries and bulk storage) |
| NaNiCl | Stability of work; safety of use | High production cost; large size; limited availability |
| NaS | High performance; resistance to temperature fluctuations | High price, large sizes; high temperature requirements |
| NiMH | Large capacity, no memory effect, wide range of application | Self-discharge, short lifetime |
| VRB | Large capacitance | Low energy density; low power density, complex design |
| Energy Storage | Electrochemical Technology Used | Power [MW] | Energy [MWh] | Battery Setup |
|---|---|---|---|---|
| Southern California Edision, Rosemead, CA, USA | Lead–acid batteries | 10 | 40 | 8256 × 2600 Ah, 8 parallel chains of 1032 cells |
| Golden Valley Electric Associacion, Fairbanks, AK, USA | Nickel–cadmium batteries | 40 | 6.5 | 13,760 cells, 4 chains of 3440 cells |
| Pacificicorp, Castle Valley, UT, USA | Vanadium batteries | 0.250 | 2 | 5 modules of 50 kW |
| AEP Sodium Sulfur Distibuted energy Storage System at Chemical Station, Charleston, SC, USA | Sodium–sulfur batteries | 1 | 7.2 | 20 modules of 50 kW |
| BYD, Hong Kong, China | Lithium–iron–phosphate cells | 20 | 40 | 60,000 single cells |
| Hornsdale Power Reserve in Hornsdale, Australia | Li-ion cells | 150 | 129 | 2,500,000 Li-ion cells |
| Vestas, Eday Island, Scotland, UK | Nickel–metal–hydride battery | 3 | 2.75 | 12,000 NiMH batteries |
| Kansas City Power & Light Co., Kansas City, MO, USA | Lithium polymer cell | 1 | 1 | n.a. |
| Car Type | Electrochemical Technology Used | Power [kW] | Energy [kWh] | Battery Setup |
|---|---|---|---|---|
| Model S (electric) | Li-ion cells | 285–568 | 75–100 | 4000–7000 connected cells |
| BMW i3 (electric) | Li-ion cells | 125 | 42.2 | 96 Li-ion cells |
| Toyota Prius | Nickel–metal–hybrid cells | 50–60 | 1.31 | 168 nickel–metal–hybrid cells |
| Kia Niro | Li-ion cells | 150 | 64 | 288 Li-ion cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detka, K.; Górecki, K. Selected Technologies of Electrochemical Energy Storage—A Review. Energies 2023, 16, 5034. https://doi.org/10.3390/en16135034
Detka K, Górecki K. Selected Technologies of Electrochemical Energy Storage—A Review. Energies. 2023; 16(13):5034. https://doi.org/10.3390/en16135034
Chicago/Turabian StyleDetka, Kalina, and Krzysztof Górecki. 2023. "Selected Technologies of Electrochemical Energy Storage—A Review" Energies 16, no. 13: 5034. https://doi.org/10.3390/en16135034
APA StyleDetka, K., & Górecki, K. (2023). Selected Technologies of Electrochemical Energy Storage—A Review. Energies, 16(13), 5034. https://doi.org/10.3390/en16135034







