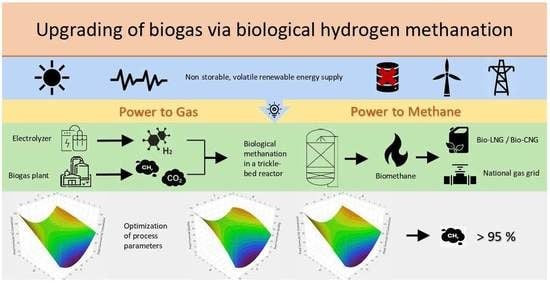
Optimization of Thermodynamic Parameters of the Biological Hydrogen Methanation in a Trickle-Bed Reactor for the Conditioning of Biogas to Biomethane
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Design of Experiment
2.3. Analytical Section
2.4. Calculations
- Methane formation rate (MFR);
- Gas hourly space velocity (GHSV);
- Retention time (RT);
- The conversion rates of H2 and CO2.
3. Results and Discussion
3.1. Operation and Performance Parameters
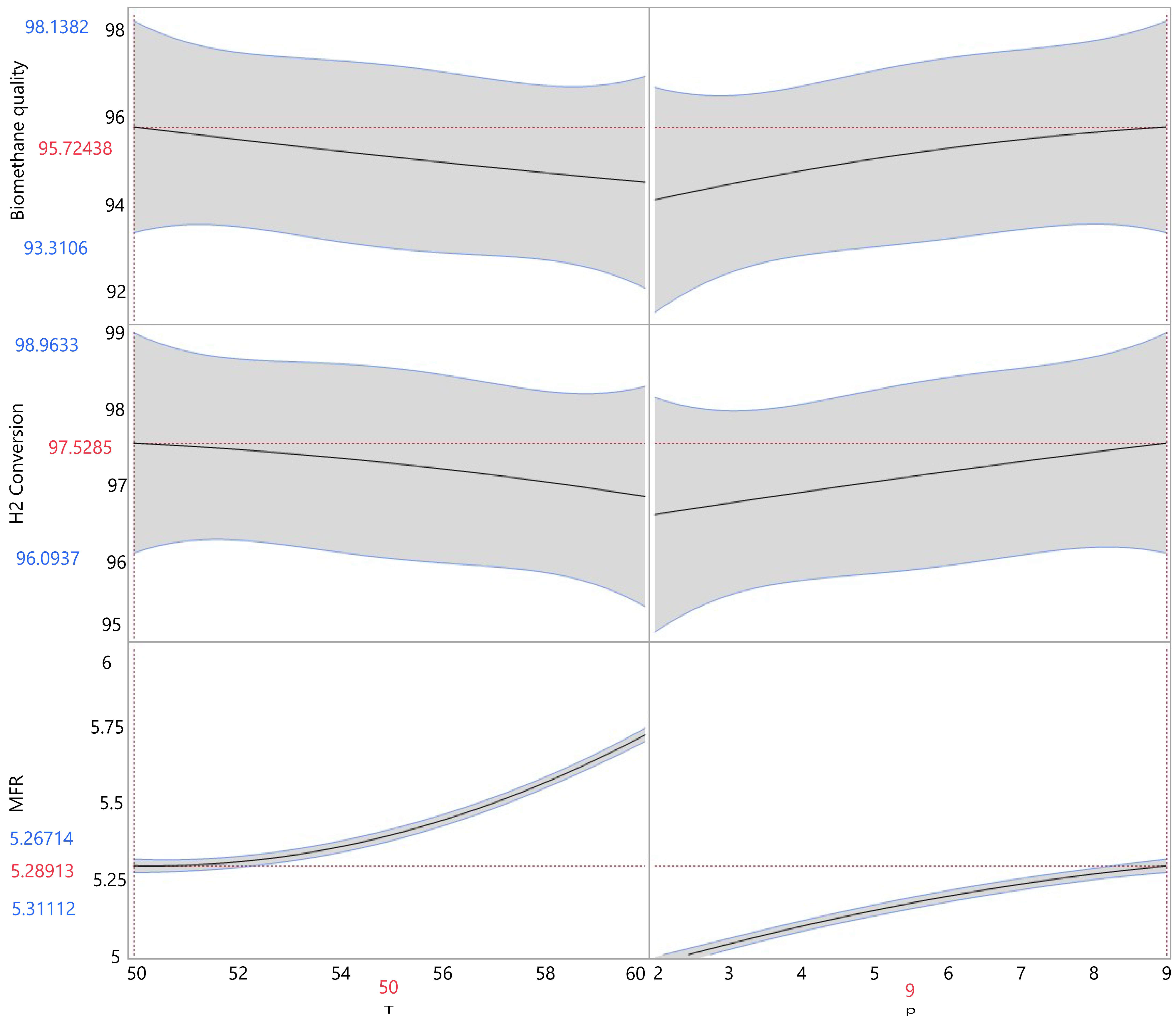
3.2. Optimizing Operating Parameters
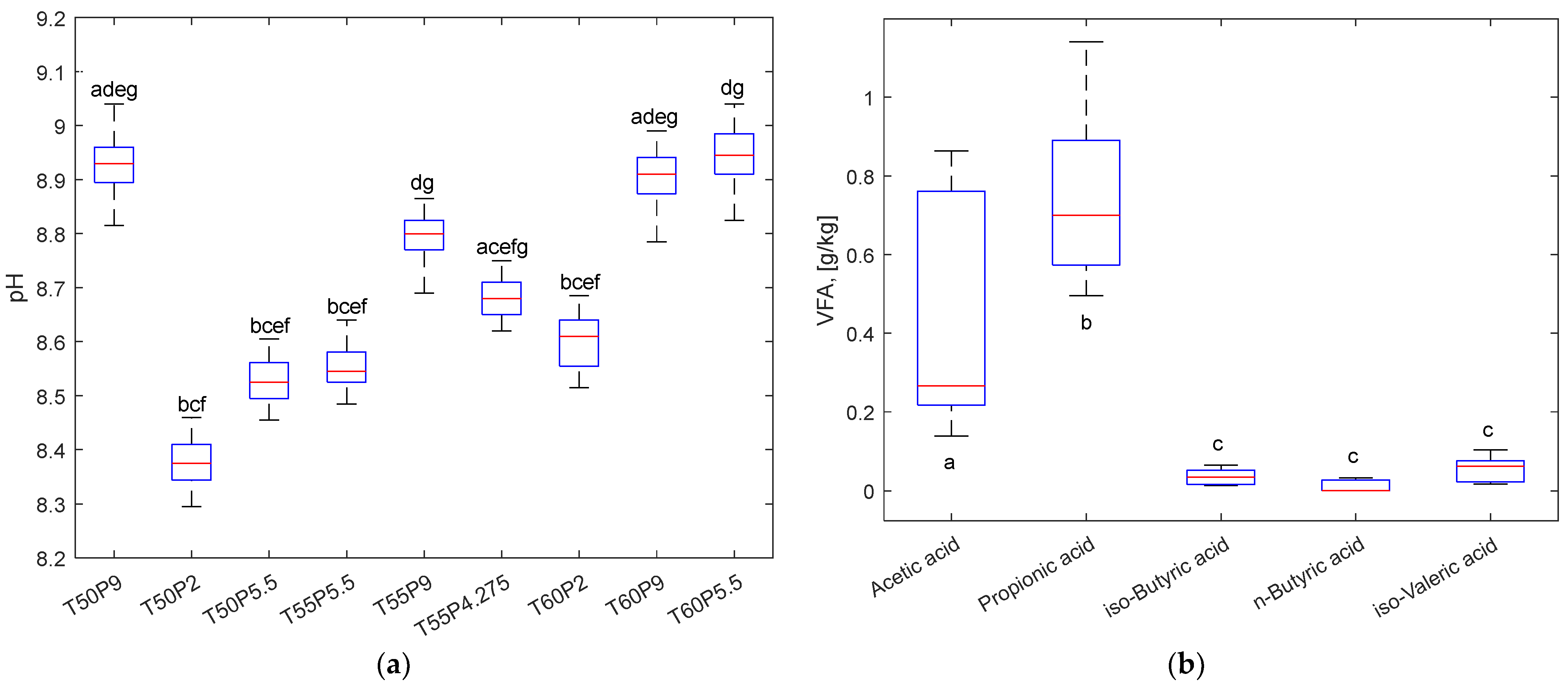
3.3. Analysis of the Process Liquid
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Anaerobic digestion | AD |
| Biological hydrogen methanation | BHM |
| Chemical oxygen demand | COD |
| Combined heat and power units | CHP |
| Design of experiment | DoE |
| Gas hourly space velocity | GHSV |
| High-calorific gas | H-gas |
| Methane formation rate | MFR |
| Low-calorific gas | L-gas |
| Power-to-gas | PtG |
| Retention time | RT |
| Total carbon | TC |
| Total nitrogen | TN |
| Total organic carbon | TOC |
| Trickling-bed reactor | TBR |
| Volatile fatty acid | VFA |
References
- Wellinger, A.; Murphy, J.; Baxter, D. The Biogas Handbook: Science, Production and Applications; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing Limited: Oxford, UK, 2013; ISBN 9780857097415. [Google Scholar]
- Miltner, M.; Makaruk, A.; Harasek, M. Review on available biogas upgrading technologies and innovations towards advanced solutions. J. Clean. Prod. 2017, 161, 1329–1337. [Google Scholar] [CrossRef]
- Pelkmans, L. Implementation of Bioenergy in Germany 2021; Country Reports; IEA Bioenergy: Paris, France, 2021. [Google Scholar]
- Germand Technical and Scientific Associarion for Gas and Water. Technical Rule—Standard: Gas Quality, A; (G 260); Economic and Publishing Company Gas and Water: Bonn, Germany, 2021; Available online: https://shop.wvgw.de/G-260-Technical-Rule-09-2021/511831 (accessed on 3 April 2023).
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for Biogas Upgrading to Biomethane: A Review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, M.; Busch, G. Methanation of hydrogen and carbon dioxide. Appl. Energy 2013, 111, 74–79. [Google Scholar] [CrossRef]
- Ullrich, T.; Lindner, J.; Bär, K.; Mörs, F.; Graf, F.; Lemmer, A. Influence of operating pressure on the biological hydrogen methanation in trickle-bed reactors. Bioresour. Technol. 2018, 247, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, A.; Ullrich, T. Effect of Different Operating Temperatures on the Biological Hydrogen Methanation in Trickle Bed Reactors. Energies 2018, 11, 1344. [Google Scholar] [CrossRef]
- Strübing, D.; Huber, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. High performance biological methanation in a thermophilic anaerobic trickle bed reactor. Bioresour. Technol. 2017, 245, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, M.; Koschack, T.; Busch, G. Biocatalytic methanation of hydrogen and carbon dioxide in an anaerobic three-phase system. Bioresour. Technol. 2015, 178, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Thema, M.; Weidlich, T.; Kaul, A.; Böllmann, A.; Huber, H.; Bellack, A.; Karl, J.; Sterner, M. Optimized biological CO2-methanation with a pure culture of thermophilic methanogenic archaea in a trickle-bed reactor. Bioresour. Technol. 2021, 333, 125135. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.P.; Lindner, J.; Oechsner, H.; Lemmer, A. Effects of target pH-value on organic acids and methane production in two-stage anaerobic digestion of vegetable waste. Bioresour. Technol. 2018, 247, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Rusmanis, D.; O’Shea, R.; Wall, D.M.; Murphy, J.D. Biological hydrogen methanation systems—An overview of design and efficiency. Bioengineered 2019, 10, 604–634. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, K.F.; Kalmokoff, M.L. Nutritional requirements of the methanogenic archaebacteria. Can. J. Microbiol. 1988, 34, 557–576. [Google Scholar] [CrossRef]
- Westerholm, M.; Isaksson, S.; Karlsson Lindsjö, O.; Schnürer, A. Microbial community adaptability to altered temperature conditions determines the potential for process optimisation in biogas production. Appl. Energy 2018, 226, 838–848. [Google Scholar] [CrossRef]
- Chen, H.; Chang, S. Dissecting methanogenesis for temperature-phased anaerobic digestion: Impact of temperature on community structure, correlation, and fate of methanogens. Bioresour. Technol. 2020, 306, 123104. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Goos, P. I-Optimal Versus D-Optimal Split-Plot Response Surface Designs. J. Qual. Technol. 2012, 44, 85–101. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Froment, G.F.; Bischoff, K.B.; De Wilde, J. Chemical Reactor Analysis and Design; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Thema, M.; Weidlich, T.; Hörl, M.; Bellack, A.; Mörs, F.; Hackl, F.; Kohlmayer, M.; Gleich, J.; Stabenau, C.; Trabold, T.; et al. Biological CO2-Methanation: An Approach to Standardization. Energies 2019, 12, 1670. [Google Scholar] [CrossRef]
- Sposob, M.; Wahid, R.; Fischer, K. Ex-Situ biological CO2 methanation using trickle bed reactor: Review and recent advances. Rev. Environ. Sci. Biotechnol. 2021, 20, 1087–1102. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Biological Treatment of Organic Materials for Energy and Nutrients Production—Anaerobic Digestion and Composting; Elsevier: Amsterdam, The Netherlands, 2019; pp. 121–181. ISBN 9780128177105. [Google Scholar]


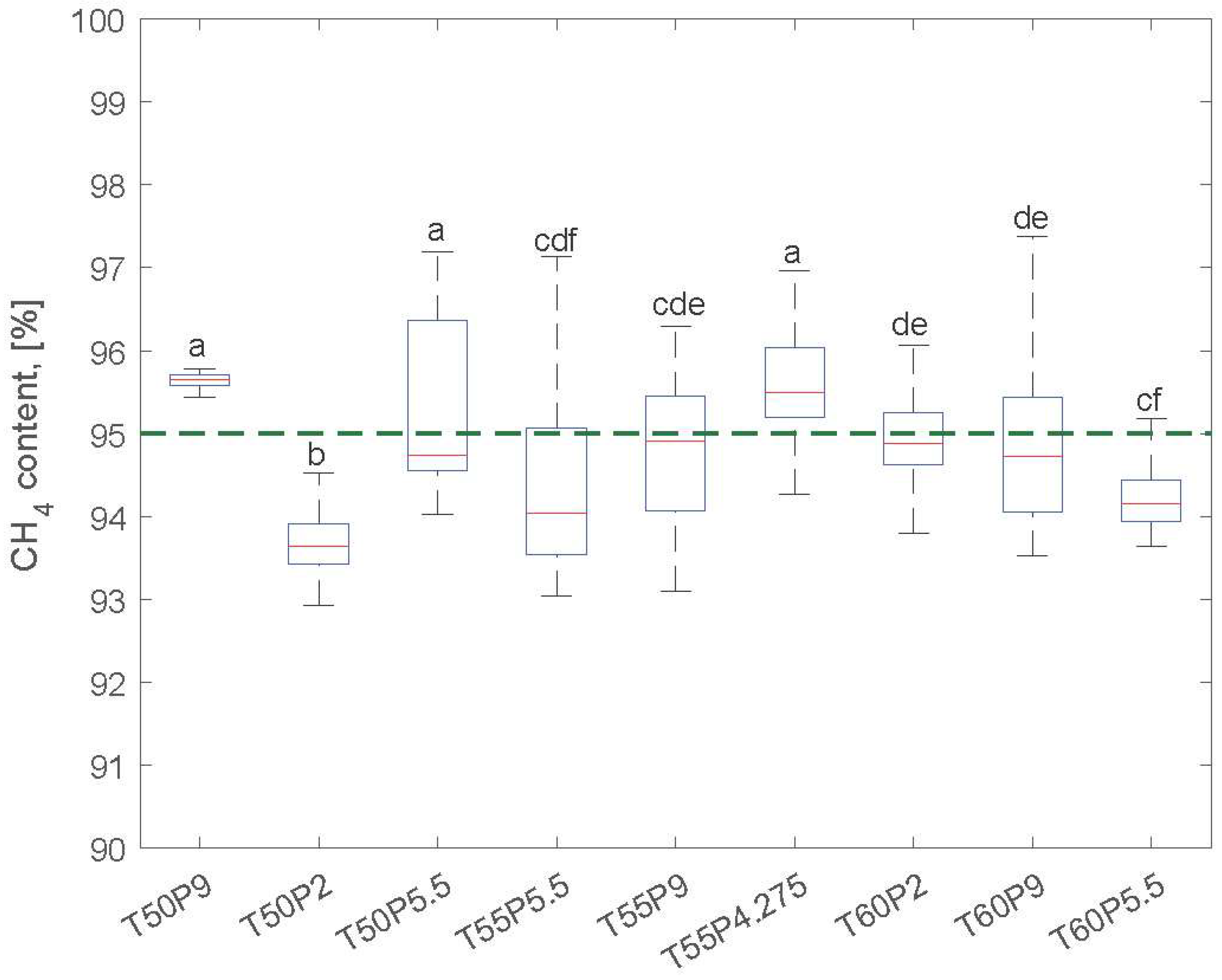


| Run ID | Temperature (°C) | Pressure (bar) |
|---|---|---|
| T50P9 | 50 | 9 |
| T50P2 | 50 | 2 |
| T50P5.5 | 50 | 5.5 |
| T55P5.5 | 55 | 5.5 |
| T55P9 | 55 | 9 |
| T55P4.275 | 55 | 4.275 |
| T60P2 | 60 | 2 |
| T60P9 | 60 | 9 |
| T60P5.5 | 60 | 5.5 |
| Parameters | T50P9 | T50P2 | T50P5.5 | T55P5.5 | T55P9 | T55P4.275 | T60P2 | T60P9 | T60P5.5 |
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 49.801 ± 0.284 | 50.065 ± 0.231 | 50.145 ± 0.201 | 54.983 ± 0.250 | 54.879 ± 0.264 | 54.952 ± 0.253 | 59.742 ± 0.297 | 59.990 ± 0.0 | 59.99 ± 0.0 |
| Pressure (bar) | 9.012 ± 0.018 | 2.061 ± 0.016 | 5.512 ± 0.013 | 5.480 ± 0.022 | 8.998 ± 0.018 | 4.288 ± 0.016 | 2.028 ± 0.011 | 9.046 ± 0.018 | 5.535 ± 0.013 |
| pH | 8.921 ± 0.128 | 8.458 ± 0.101 | 8.689 ± 0.131 | 8.745 ± 0.106 | 8.837 ± 0.082 | 8.762 ± 0.111 | 8.673 ± 0.106 | 9.013 ± 0.088 | 8.949 ± 0.138 |
| Flow rate H2 (L/h) | 11.250 | 11.250 | 11.250 | 11.250 | 11.250 | 11.250 | 11.250 | 11.250 | 11.250 |
| Flow rate CO2 (L/h) | 2.810 | 2.810 | 2.810 | 2.810 | 2.810 | 2.810 | 2.810 | 2.810 | 2.810 |
| Flow rate CH4 (L/h) | 3.440 | 3.440 | 3.440 | 3.440 | 3.440 | 3.440 | 3.440 | 3.440 | 3.440 |
| MFR (m3/(m3·d)) | 6.376 ± 0.369 | 5.545 ± 0.224 | 5.687 ± 0.266 | 5.701 ± 0.186 | 5.755 ± 0.337 | 5.794 ± 0.363 | 6.076 ± 0.229 | 5.906 ± 0.241 | 6.001 ± 0.386 |
| CH4total 1 (%) | 95.614 ± 0.151 | 93.675 ± 0.452 | 95.614 ± 1.632 | 94.386 ± 1.137 | 94.784 ± 0.781 | 95.626 ± 0.563 | 95.015 ± 0.714 | 94.929 ± 0.992 | 94.214 ± 0.360 |
| CH4conv 2 (%) | 48.102 ± 1.017 | 46.574 ± 0.996 | 47.425 ± 1.543 | 47.584 ± 1.670 | 48.483 ± 1.657 | 47.285 ± 0.834 | 47.203 ± 1.201 | 50.000 ± 1.443 | 48.873 ± 0.965 |
| H2:CO2 | 4.072 ± 0.003 | 4.106 ± 0.009 | 4.068 ± 0.031 | 4.095 ± 0.024 | 4.089 ± 0.015 | 4.070 ± 0.013 | 4.083 ± 0.014 | 4.087 ± 0.0719 | 4.100 ± 0.007 |
| H2conv (%) | 97.535 ± 0.166 | 96.394 ± 0.274 | 97.288 ± 0.579 | 96.790 ± 0.827 | 97.042 ± 0.525 | 97.608 ± 0.382 | 97.127 ± 0.354 | 97.115 ± 0.679 | 96.616 ± 0.264 |
| CO2conv (%) | 99.767 ± 0.026 | 99.600 ± 0.137 | 99.569 ± 0.164 | 99.820 ± 0.085 | 99.884 ± 0.033 | 99.784 ± 0.081 | 99.811 ± 0.037 | 99.942 ± 0.008 | 99.895 ± 0.021 |
| RT (h) | 5.579 | 1.240 | 3.409 | 3.357 | 5.494 | 2.610 | 1.203 | 5.411 | 3.307 |
| GHSV (h−1) | 1.082 | 1.082 | 1.082 | 1.082 | 1.082 | 1.082 | 1.082 | 1.082 | 1.082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holl, E.; Oskina, A.; Baier, U.; Lemmer, A. Optimization of Thermodynamic Parameters of the Biological Hydrogen Methanation in a Trickle-Bed Reactor for the Conditioning of Biogas to Biomethane. Energies 2023, 16, 4720. https://doi.org/10.3390/en16124720
Holl E, Oskina A, Baier U, Lemmer A. Optimization of Thermodynamic Parameters of the Biological Hydrogen Methanation in a Trickle-Bed Reactor for the Conditioning of Biogas to Biomethane. Energies. 2023; 16(12):4720. https://doi.org/10.3390/en16124720
Chicago/Turabian StyleHoll, Elena, Anastasia Oskina, Urs Baier, and Andreas Lemmer. 2023. "Optimization of Thermodynamic Parameters of the Biological Hydrogen Methanation in a Trickle-Bed Reactor for the Conditioning of Biogas to Biomethane" Energies 16, no. 12: 4720. https://doi.org/10.3390/en16124720
APA StyleHoll, E., Oskina, A., Baier, U., & Lemmer, A. (2023). Optimization of Thermodynamic Parameters of the Biological Hydrogen Methanation in a Trickle-Bed Reactor for the Conditioning of Biogas to Biomethane. Energies, 16(12), 4720. https://doi.org/10.3390/en16124720