Optimization Study on Salinity Gradient Energy Capture from Brine and Dilute Brine
Abstract
1. Introduction
2. Methods and Experimental Verifications
2.1. Models
2.1.1. Ion Migration Models of a RED Stack
2.1.2. Optimization Models of a RED Stack
2.2. Experiment
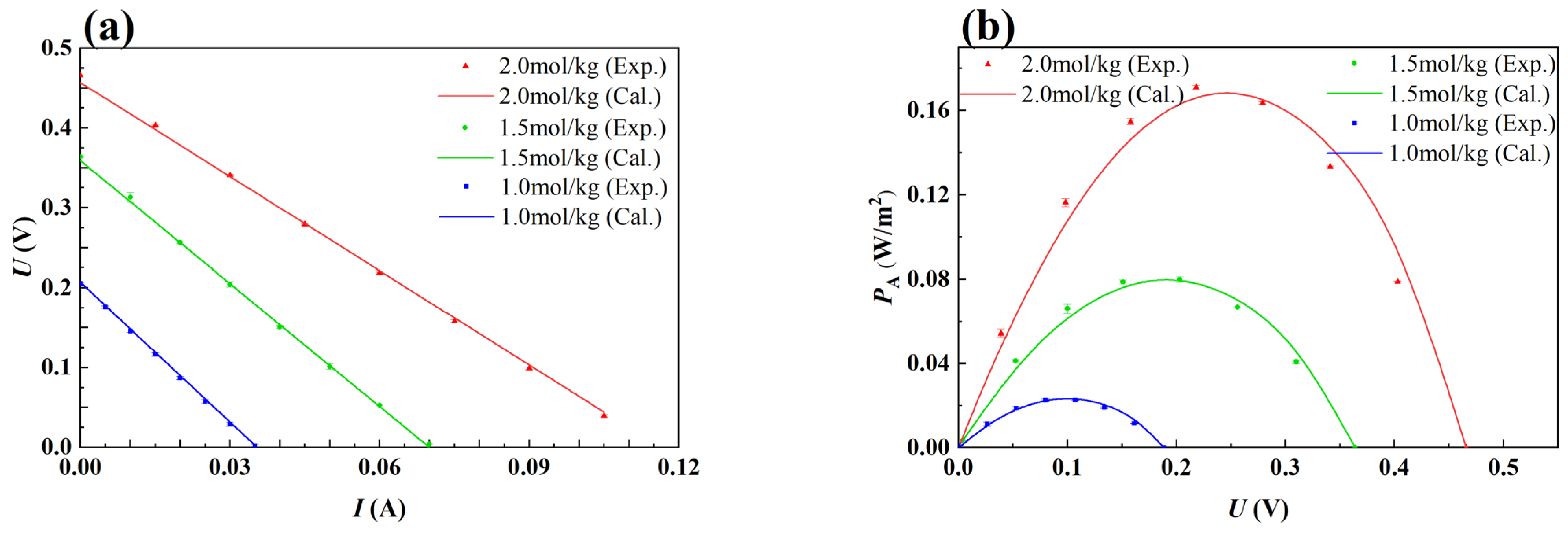
2.3. Model Verification
3. Results and Analysis
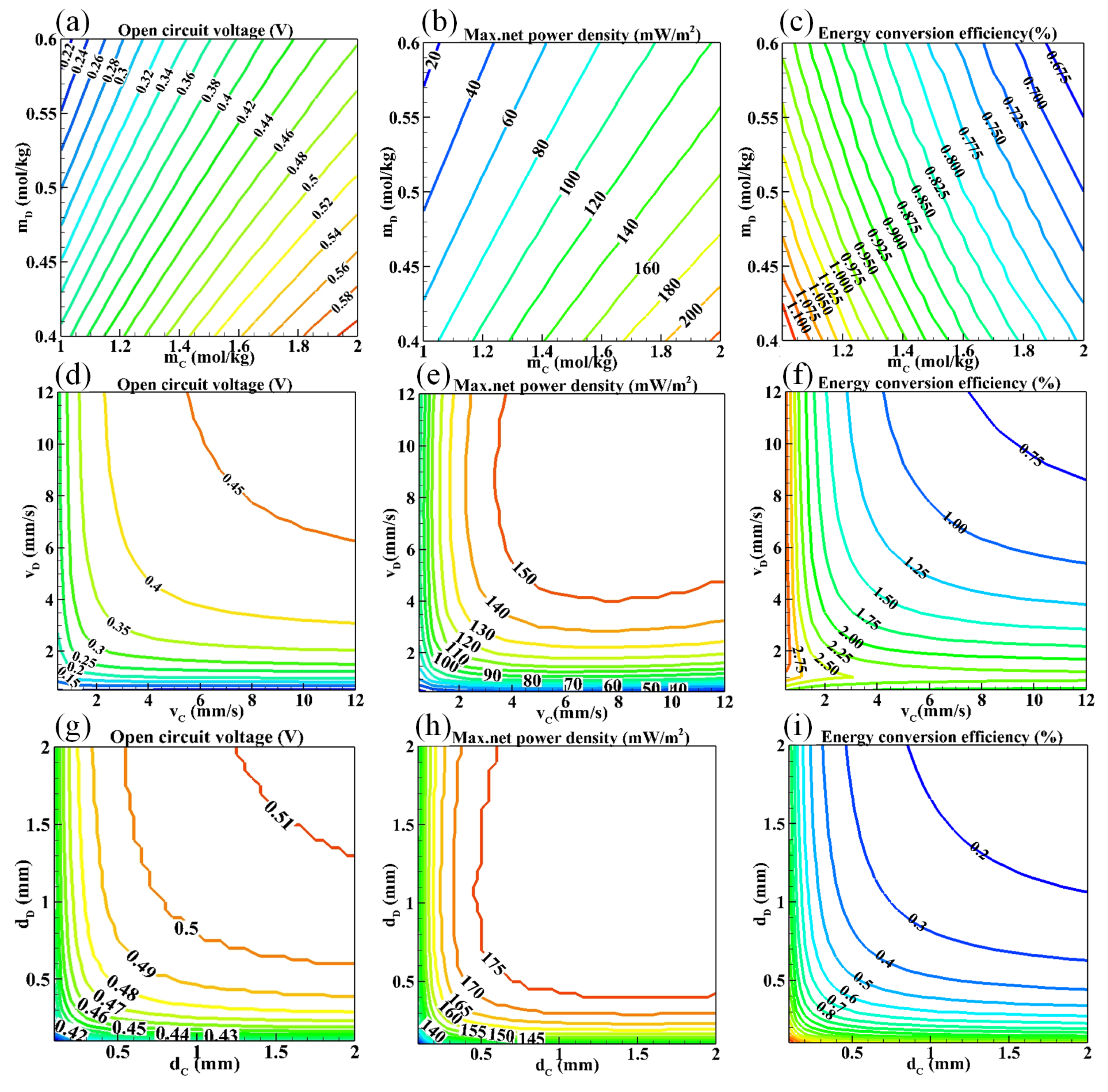
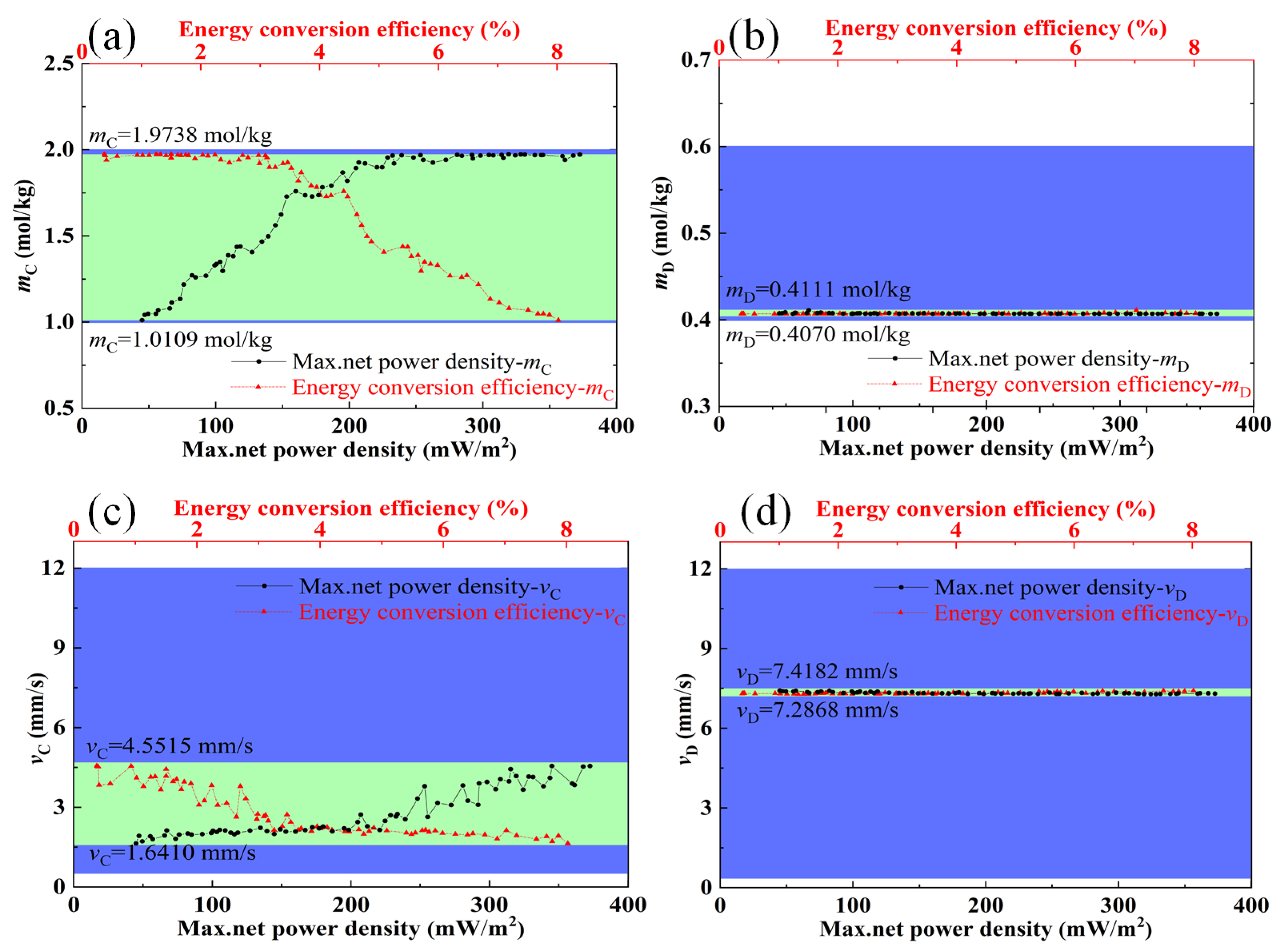
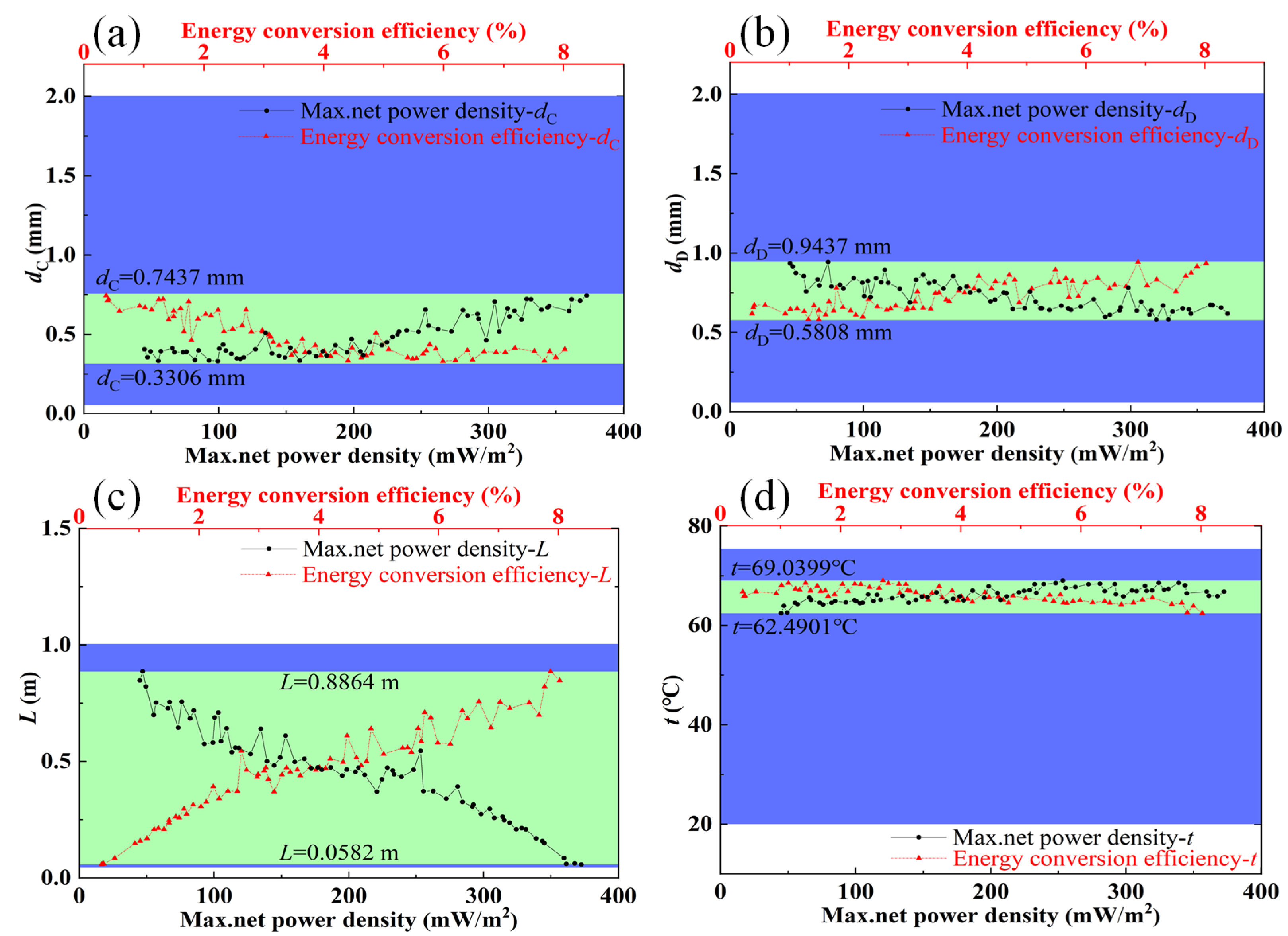
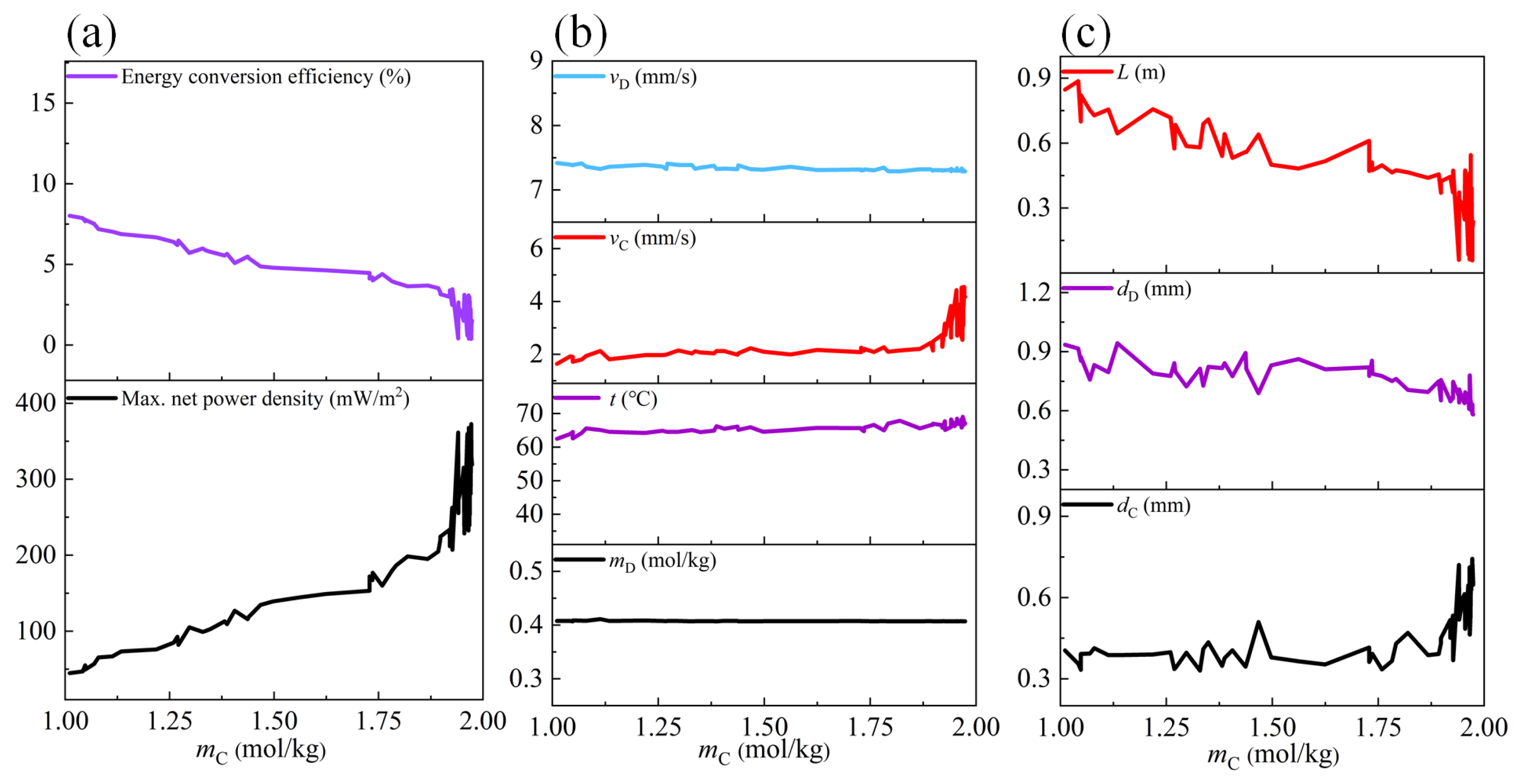
3.1. Influences of Feed Parameters and Stack Sizes on Performances of a RED Stack
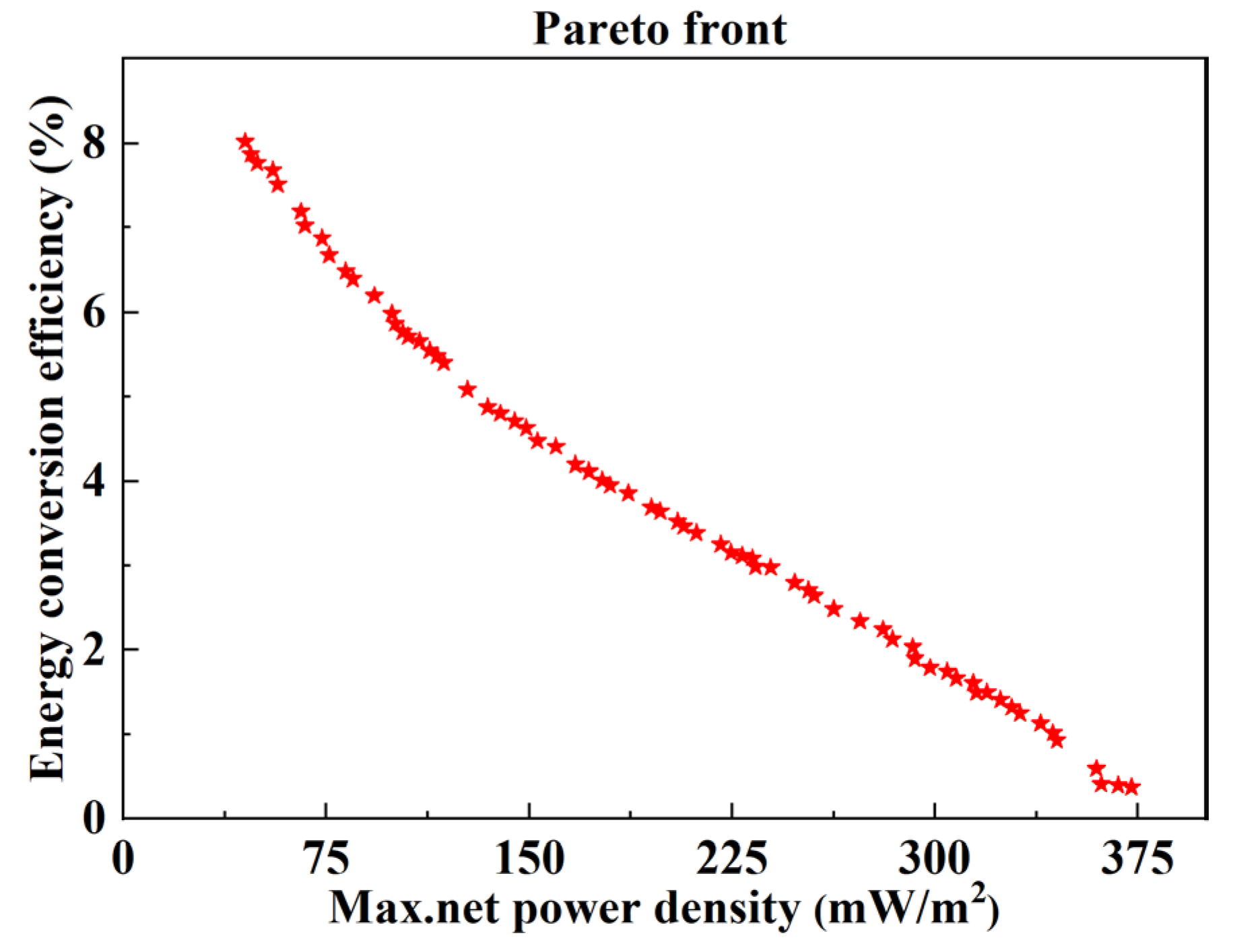
3.2. Optimization Analysis
4. Conclusions
- (1)
- The concentration of feed solutions has the most significant effect on the maximum net power density of the stack, whereas the flow velocity and the length of the electrode plate have the most obvious effect on the energy conversion efficiency of the stack.
- (2)
- The optimal ranges of feed parameters (concentration, flow velocity, and temperature) and stack sizes (the compartment thickness and the length of electrode plate) were obtained, in which the maximum net power density and energy conversion efficiency could be coordinated and relatively larger. The optimal ranges of the flow velocity of the concentrated solution and feed solution temperature are 1.6 mm/s,−4.6 mm/s, and 62–69 °C, respectively.
- (3)
- The optimal compartment thickness and the length of the electrode plate are obtained based on the discharge concentration of concentrated seawater from different desalination plants. The maximum net power density and energy conversion efficiency can be coordinated when the flow velocity of the dilute solution and the concentration of the dilute solution are approximately 7.3 mm/s and 0.4 mol/kg, respectively.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nomenclature | |
| A | Area, m2 |
| b | Width of compartments in a RED unit, m |
| c | Concentration, mol/L |
| Dc | The permeability coefficient of ion exchange membrane, m2/s |
| d | Thickness of ions exchange membrane or spacer, m |
| E | Electromotive force, V |
| F | Faraday constant, 96485 C/mol |
| fy | Spacer shadow factor |
| fm | Correction factor of resistance for ion exchange membrane |
| G | Gibbs free energy, W |
| I | Output current of a stack, A |
| J | Molar flux, mol/(m2·s) |
| j | Current density, A/m2 |
| L | Length of electrodes, m |
| Lp | Permeability coefficient of ion exchange membranes to water, kg2/(J·m2·s) |
| M | Molar mass, kg/mol |
| m | Mass molar concentration, mol/kg |
| N | Number of cell pairs in a RED stack |
| nh | Number of water molecules carried by an ion |
| OCV | Open circuit voltage, V |
| PA | Power density, W/m2 |
| PAn | Net power density, W/m2 |
| Pg | Output power, W |
| Pn | Net output power, W |
| Pp | Hydraulic loss power, W |
| Pr | Pressure, Pa |
| R | Gas constant, 8.31432 J/(mol·K) |
| r | Theresistance of a stack, Ω |
| T | Kelvin temperature, K |
| t | Centigrade temperature, °C |
| U | Output voltage of a stack, V |
| v | Velocity of feed solution, m/s |
| z | Ionic valence |
| Greek symbols | |
| α | Selective permeability coefficient |
| β | Correction factor of selective permeability coefficient |
| γ | Average ionic activity coefficient of solution |
| Δ | Delta |
| η | Energy conversion efficiency |
| Λ | Molar conductivity, S·m2/mol |
| μ | Dynamic viscosity, Pa·s |
| ρ | Density, kg/m3 |
| υ | Number of ions in the solution of 1 mole of solute |
| Φ | Volume flow of solution, m3/s |
| φ | Osmotic coefficient of water |
| Superscripts and subscripts | |
| AEM | Anion exchange membrane |
| C | Concentrated solution |
| CEM | Cation exchange membrane |
| cell | Cell pairs |
| co | Same electrical properties as fixed charges in ion exchange membranes |
| cou | Opposite electrical properties as fixed charges in ion exchange mem branes |
| D | Dilute solution |
| el | Electrodes |
| eosm | Electroosmosis |
| M | Mean |
| Max | Maximum |
| Min | Minimum |
| o | Original |
| osm | Osmosis |
| RED | Reverse electrodialysis |
| tot | Total |
| w | Water |
References
- Baggio, G.; Qadir, M.; Smakhtin, V. Freshwater availability status across countries for human and ecosystem needs. Sci. Total Environ. 2021, 792, 148230. [Google Scholar] [CrossRef]
- Prajapati, M.; Shah, M.; Soni, B.; Parikh, S.; Sircar, A.; Balchandani, S.; Thakore, S.; Tala, M. Geothermal-solar integrated groundwater desalination system: Current status and future perspective. Groundw. Sustain. Dev. 2021, 12, 100506. [Google Scholar] [CrossRef]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.; Missimer, T.; Lattemann, S. Membrane-based seawater desalination: Present and future prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Garrote-Moreno, A.; Fernández-Torquemada, Y.; Sánchez-Lizaso, J.L. Salinity fluctuation of the brine discharge affects growth and survival of the seagrass Cymodocea nodosa. Mar. Pollut. Bull. 2014, 81, 61–68. [Google Scholar] [CrossRef]
- Tedesco, M.; Brauns, E.; Cipollina, A.; Micale, G.; Modica, P.; Russo, G.; Helsen, J. Reverse electrodialysis with saline waters and concentrated brines: A laboratory investigation towards technology scale-up. J. Membr. Sci. 2015, 492, 9–20. [Google Scholar] [CrossRef]
- Yaroshchuk, A. Optimal hydrostatic counter-pressure in Pressure-Retarded Osmosis with composite/asymmetric membranes. J. Membr. Sci. 2015, 477, 157–160. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, W.; Hatzell, M.C.; Logan, B.E. Energy Recovery from Solutions with Different Salinities Based on Swelling and Shrinking of Hydrogels. Environ. Sci. Technol. 2014, 48, 7157–7163. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Wick, G.L.; Isaacs, J.D. Salinity Gradient Power: Utilizing Vapor Pressure Differences. Science 1979, 206, 452–454. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kim, Y.; Logan, B.E. Energy Capture from Thermolytic Solutions in Microbial Reverse-Electrodialysis Cells. Science 2012, 335, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; Wessells, C.D.; Cui, Y.; Mantia, F.L. A Desalination Battery. Nano Lett. 2012, 12, 839–843. [Google Scholar] [CrossRef]
- Pattle, R.E. Production of Electric Power by mixing Fresh and Salt Water in the Hydroelectric Pile. Nature 1954, 174, 660. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Leitz, F.B. Electric Power from Differences in Salinity: The Dialytic Battery. Science 1976, 191, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Lacey, R.E. Energy by reverse electrodialysis. Ocean Eng. 1980, 7, 1–47. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Doubled Power Density from Salinity Gradients at Reduced Intermembrane Distance. Environ. Sci. Technol. 2011, 45, 7089–7095. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, S.; Wu, X.; Wu, D.; Jin, D.; Wang, P.; Xu, L.; Leng, Q. Exergy analysis for the multi-effect distillation—Reverse electrodialysis heat engine. Desalination 2019, 467, 158–169. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, D.; Bai, Y.; Zhang, J.; Gong, M. Energy and exergy analysis of heat to salinity gradient power conversion in reverse electrodialysis heat engine. Energy Convers. Manag. 2022, 252, 115068. [Google Scholar] [CrossRef]
- Wu, X.; Ren, Y.; Zhang, Y.; Xu, S.; Yang, S. Hydrogen production from water electrolysis driven by the membrane voltage of a closed-loop reverse electrodialysis system integrating air-gap diffusion distillation technology. Energy Convers. Manag. 2022, 268, 115974. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Xu, S.; Leng, Q.; Wang, S. A serial system of multi-stage reverse electrodialysis stacks for hydrogen production. Energy Convers. Manag. 2022, 251, 114932. [Google Scholar] [CrossRef]
- Scialdone, O.; D’Angelo, A.; De Lumè, E.; Galia, A. Cathodic reduction of hexavalent chromium coupled with electricity generation achieved by reverse-electrodialysis processes using salinity gradients. Electrochim. Acta 2014, 137, 258–265. [Google Scholar] [CrossRef]
- Leng, Q.; Xu, S.; Wu, X.; Wang, S.; Jin, D.; Wang, P.; Wu, D.; Dong, F. Electrochemical removal of synthetic methyl orange dyeing wastewater by reverse electrodialysis reactor: Experiment and mineralizing model. Environ. Res. 2022, 214, 114064. [Google Scholar] [CrossRef]
- Wick, G.L. Power from salinity gradients. Energy 1978, 3, 95–100. [Google Scholar] [CrossRef]
- Avci, A.H.; Tufa, R.A.; Fontananova, E.; Di Profio, G.; Curcio, E. Reverse Electrodialysis for energy production from natural river water and seawater. Energy 2018, 165, 512–521. [Google Scholar] [CrossRef]
- Long, R.; Li, B.; Liu, Z.; Liu, W. Performance analysis of reverse electrodialysis stacks: Channel geometry and flow rate optimization. Energy 2018, 158, 427–436. [Google Scholar] [CrossRef]
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy consumption and environmental impact assessment of desalination plants and brine disposal strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Jianbo, L.; Chen, Z.; Kai, L.; Li, Y.; Xiangqiang, K. Experimental study on salinity gradient energy recovery from desalination seawater based on RED. Energy Convers. Manag. 2021, 244, 114475. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Zhang, C.; Wang, H.; Kong, X. Power production from seawater and discharge brine of thermal desalination units by reverse electrodialysis. Appl. Energy 2022, 314, 118977. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Wang, H.; Li, M.; Wang, L.; Kong, X. The Effect of Trace Ions on the Performance of Reverse Electrodialysis Using Brine/Seawater as Working Pairs. Front. Energy Res. 2022, 10, 919878. [Google Scholar] [CrossRef]
- Kang, S.; Li, J.; Wang, Z.; Zhang, C.; Kong, X. Salinity gradient energy capture for power production by reverse electrodialysis experiment in thermal desalination plants. J. Power Sources 2022, 519, 230806. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Wang, Z.; Bai, Z.; Kong, X. Salinity gradient energy harvested from thermal desalination for power production by reverse electrodialysis. Energy Convers. Manag. 2022, 252, 115043. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Wang, Z.; Wang, H.; Bai, Z.; Kong, X. Power harvesting from concentrated seawater and seawater by reverse electrodialysis. J. Power Sources 2022, 530, 231314. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Choi, J.; Kim, J.; Jeong, N. Optimization of the number of cell pairs to design efficient reverse electrodialysis stack. Desalination 2021, 497, 114676. [Google Scholar] [CrossRef]
- Simões, C.; Pintossi, D.; Saakes, M.; Brilman, W. Optimizing multistage reverse electrodialysis for enhanced energy recovery from river water and seawater: Experimental and modeling investigation. Adv. Appl. Energy 2021, 2, 100023. [Google Scholar] [CrossRef]
- Ciofalo, M.; La Cerva, M.; Di Liberto, M.; Gurreri, L.; Cipollina, A.; Micale, G. Optimization of net power density in Reverse Electrodialysis. Energy 2019, 181, 576–588. [Google Scholar] [CrossRef]
- Long, R.; Li, B.; Liu, Z.; Liu, W. Reverse electrodialysis: Modelling and performance analysis based on multi-objective optimization. Energy 2018, 151, 1–10. [Google Scholar] [CrossRef]
- Veerman, J.; Post, J.W.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reducing power losses caused by ionic shortcut currents in reverse electrodialysis stacks by a validated model. J. Membr. Sci. 2008, 310, 418–430. [Google Scholar] [CrossRef]
- Hu, J.; Xu, S.; Wu, X.; Wu, D.; Jin, D.; Wang, P.; Leng, Q. Multi-stage reverse electrodialysis: Strategies to harvest salinity gradient energy. Energy Convers. Manag. 2019, 183, 803–815. [Google Scholar] [CrossRef]
- Giacalone, F.; Catrini, P.; Tamburini, A.; Cipollina, A.; Piacentino, A.; Micale, G. Exergy analysis of reverse electrodialysis. Energy Convers. Manag. 2018, 164, 588–602. [Google Scholar] [CrossRef]
- Veerman, J.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reverse electrodialysis: A validated process model for design and optimization. Chem. Eng. J. 2011, 166, 256–268. [Google Scholar] [CrossRef]
- Güler, E.; Elizen, R.; Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Performance-determining membrane properties in reverse electrodialysis. J. Membr. Sci. 2013, 446, 266–276. [Google Scholar] [CrossRef]
- Veerman, J.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reverse electrodialysis: Evaluation of suitable electrode systems. J. Appl. Electrochem. 2010, 40, 1461–1474. [Google Scholar] [CrossRef]


| Descriptions | Formulas | Unit |
|---|---|---|
| Effective selective permeability coefficient of AEM | - | |
| Effective selective permeability coefficient of CEM | - | |
| Correction factor | - |
| Descriptions | Formulas | Unit |
|---|---|---|
| Resistance of the concentrated compartment | [36] | Ω·m2 |
| Resistance of the dilute compartment | [36] | Ω·m2 |
| Correction factor | [36] | - |
| Resistance of AEM | [36] | Ω·m2 |
| Resistance of CEM | [36] | Ω·m2 |
| Descriptions | Formulas | Unit |
|---|---|---|
| Output current | A | |
| Gross power | W | |
| Power loss | W | |
| Pressure loss in concentrated solution compartment | Pa | |
| Pressure loss in dilute solution compartment | Pa | |
| Net power | W | |
| Gross power density | W/m2 | |
| Net power density | W/m2 | |
| Energy conversion efficiency | - | |
| Maximum Gibbs free energy | W |
| Parameters | mC (mol/kg) | mD (mol/kg) | t (°C) | vC (mm/s) | vD (mm/s) | dC (mm) | dD (mm) | L (m) |
|---|---|---|---|---|---|---|---|---|
| Upper limit | 2.0 | 0.6 | 70 | 12.0 | 12.0 | 2.0 | 2.0 | 1.00 |
| Lower limit | 1.0 | 0.4 | 25 | 0.5 | 0.5 | 0.1 | 0.1 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Xiao, Z.; Zhang, J.; Zhang, X.; Liu, X.; Liu, X.; Cui, J.; Li, J. Optimization Study on Salinity Gradient Energy Capture from Brine and Dilute Brine. Energies 2023, 16, 4643. https://doi.org/10.3390/en16124643
Gao H, Xiao Z, Zhang J, Zhang X, Liu X, Liu X, Cui J, Li J. Optimization Study on Salinity Gradient Energy Capture from Brine and Dilute Brine. Energies. 2023; 16(12):4643. https://doi.org/10.3390/en16124643
Chicago/Turabian StyleGao, Hailong, Zhiyong Xiao, Jie Zhang, Xiaohan Zhang, Xiangdong Liu, Xinying Liu, Jin Cui, and Jianbo Li. 2023. "Optimization Study on Salinity Gradient Energy Capture from Brine and Dilute Brine" Energies 16, no. 12: 4643. https://doi.org/10.3390/en16124643
APA StyleGao, H., Xiao, Z., Zhang, J., Zhang, X., Liu, X., Liu, X., Cui, J., & Li, J. (2023). Optimization Study on Salinity Gradient Energy Capture from Brine and Dilute Brine. Energies, 16(12), 4643. https://doi.org/10.3390/en16124643







