Recent Trends in Highly Porous Structured Carbon Electrodes for Supercapacitor Applications: A Review
Abstract
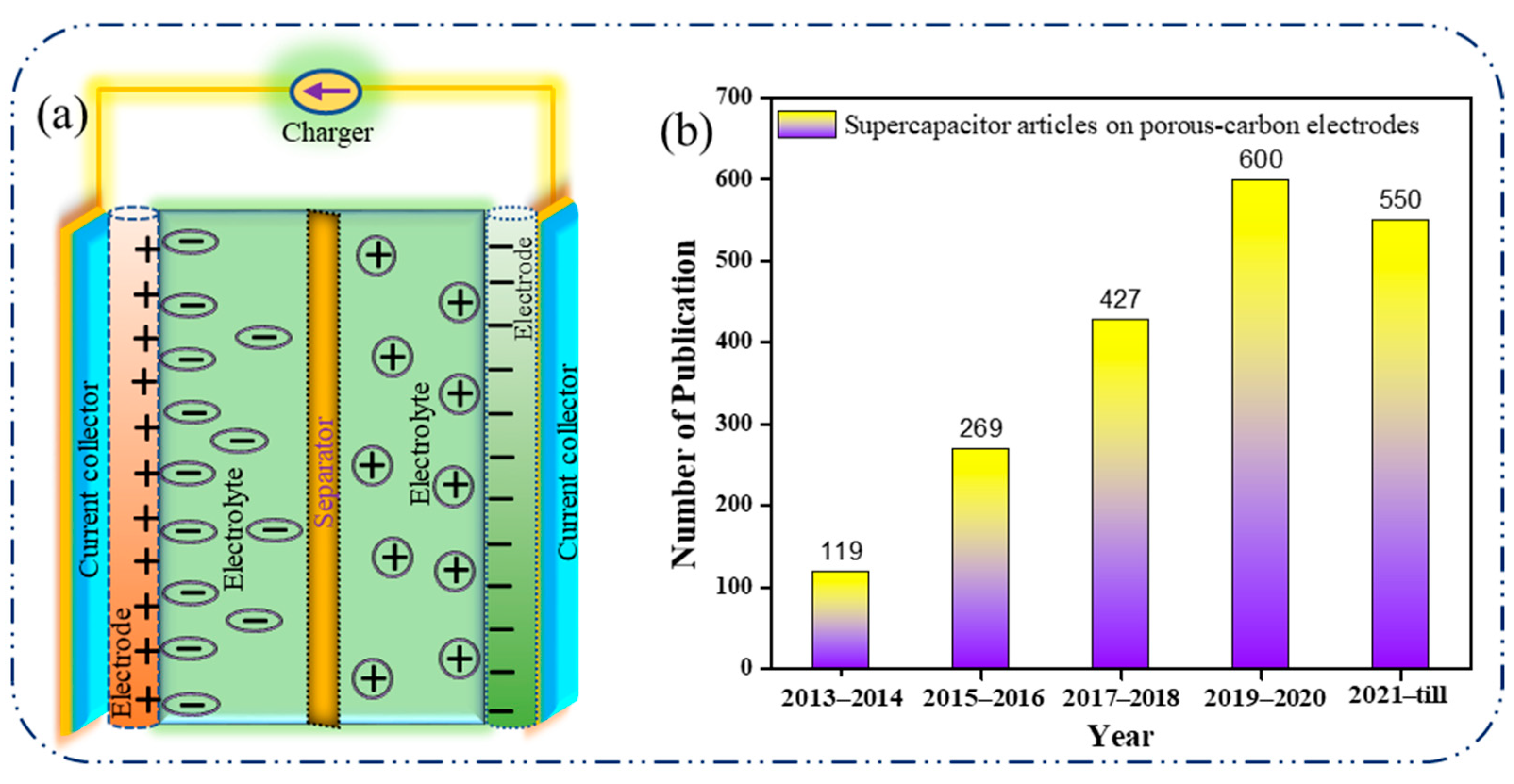
1. Introduction
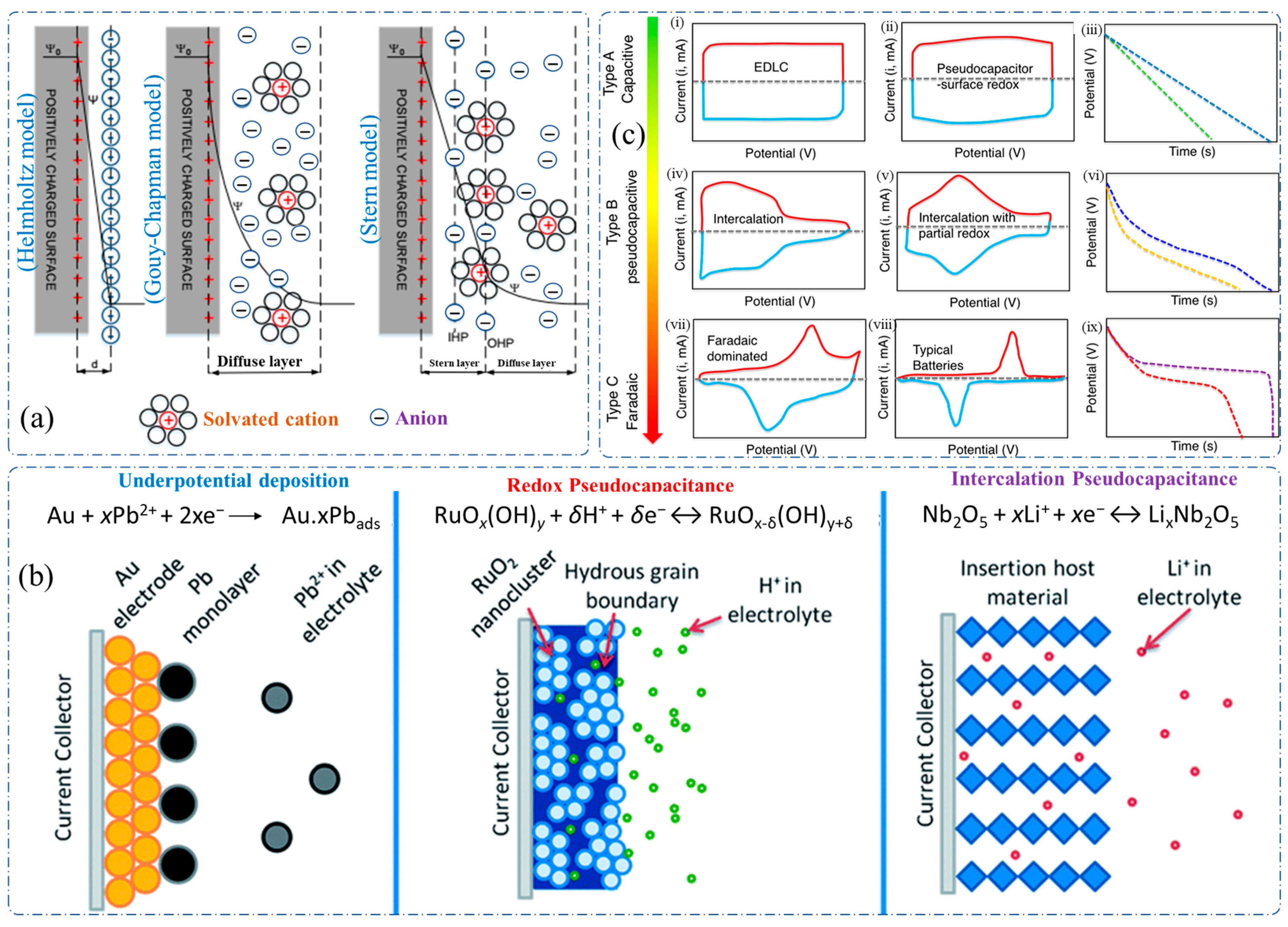
1.1. Mechanism of Supercapacitor
1.1.1. Electric Double-Layer Capacitor (EDLC)
1.1.2. Pseudocapacitance
2. Synthesis of Porous Carbons
2.1. Porous Carbon-Derived from Polymer-Based Materials
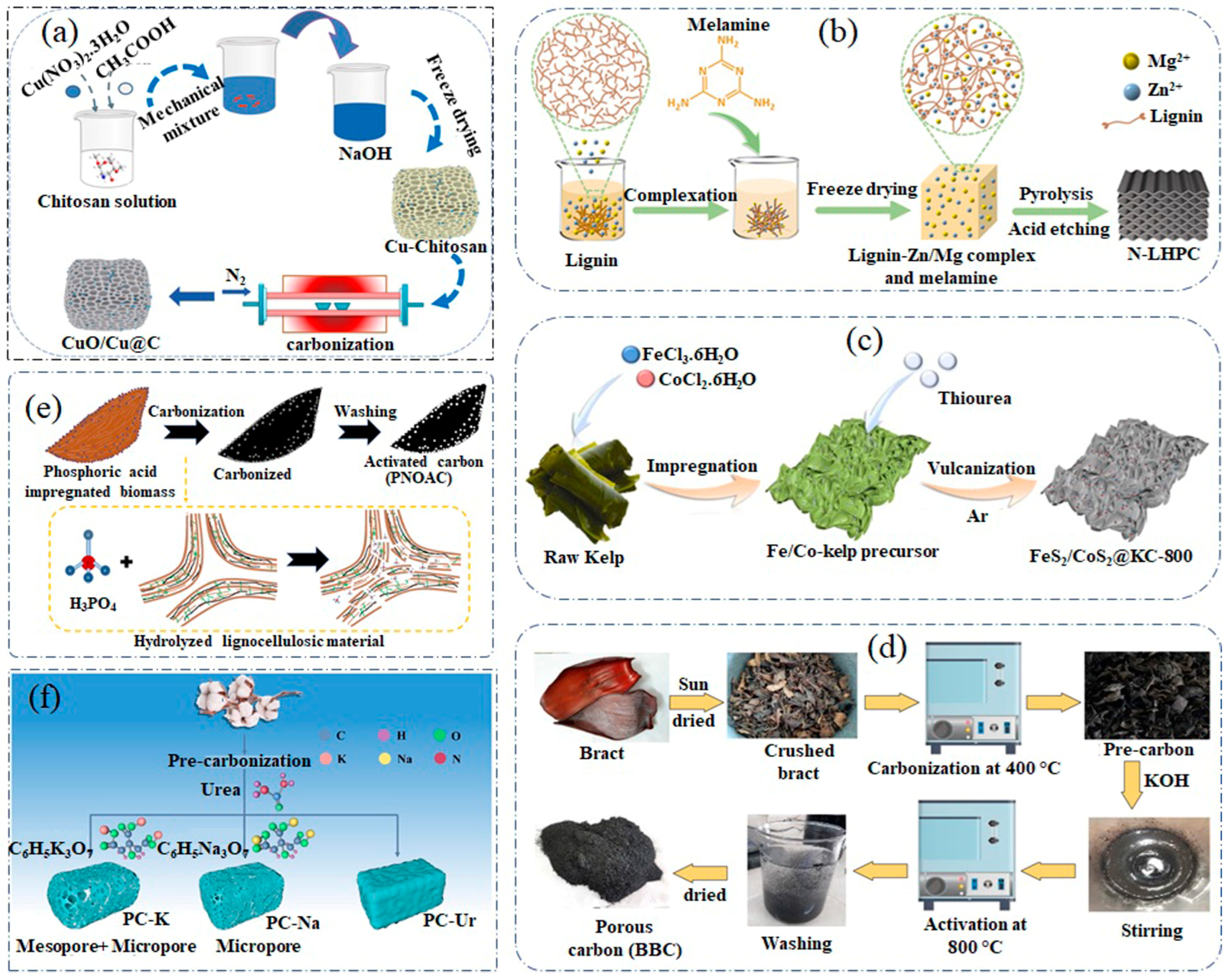
2.2. Porous Carbon-Derived from Biomass
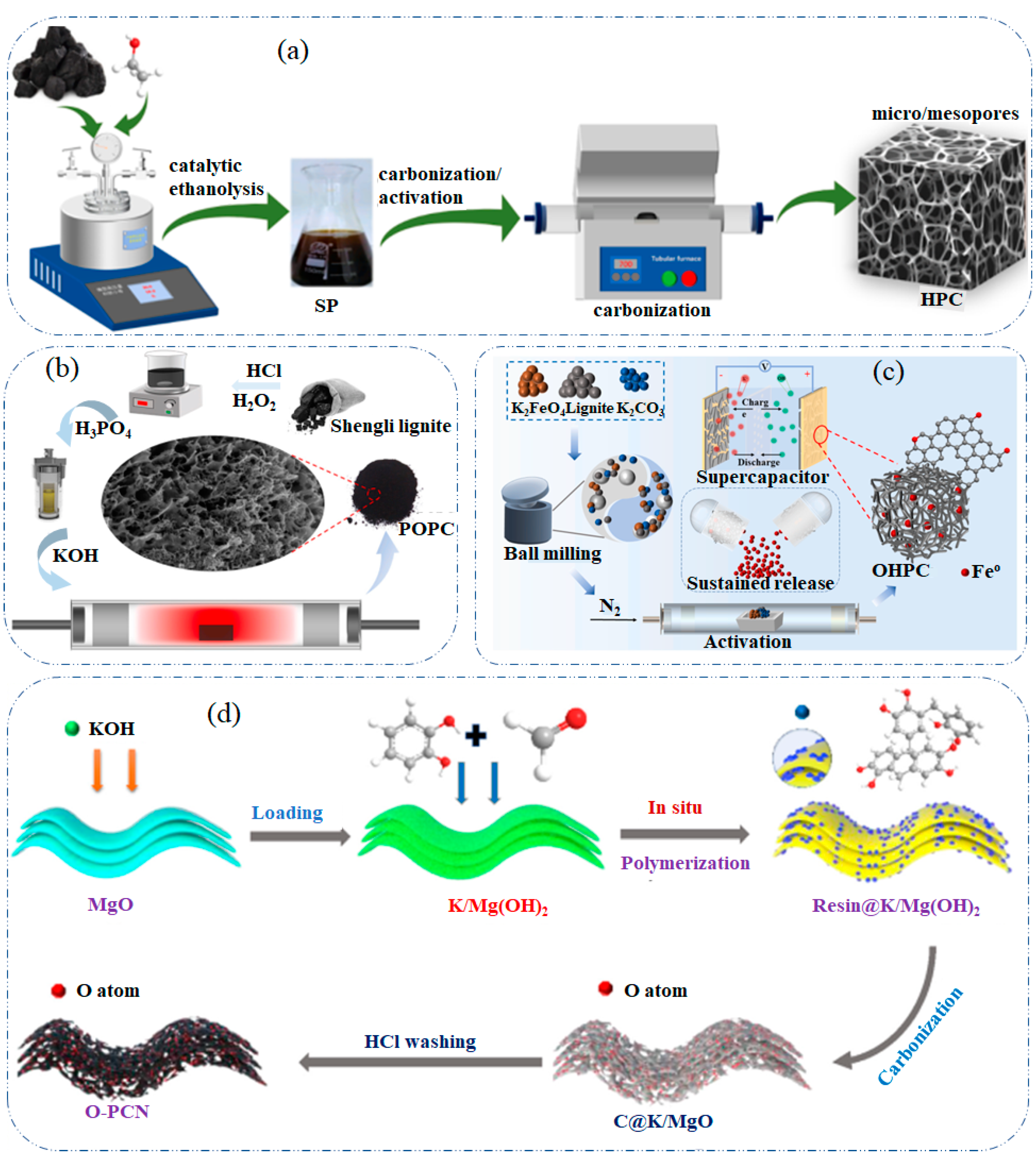
2.3. Porous Carbon-Derived from Lignite
2.4. Porous Carbon-Derived from Metal Salts or Precursors
2.5. Porous Carbon-Derived from Bio-Oil
2.6. Porous Carbon-Derived from Melamine
2.7. Porous Carbon Derived from Others
2.8. Discussion
3. Application of Porous Carbon in a Supercapacitor
3.1. Ultra-High Specific Capacitance-Based Porous Carbon
3.2. High-Specific Capacitance-Based Porous Carbon
3.3. Medium-Specific Capacitance-Based Porous Carbon
3.4. Low-Specific Capacitance-Based Porous Carbon
3.5. Discussion
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Nishchith, B.S.; Kalegowda, Y.; Ashoka, S.; Shreenivasa, L.; Sriram, G.; Kurkuri, M.D.; Ajeya, K.V.; Jung, H.-Y. Electrochemical kinetic study and performance evaluation of surface-modified mesoporous sodium carbonophosphates nanostructures for pseudocapacitor applications. J. Alloys Compd. 2023, 939, 168711. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Kasinathan, D.; Prabhakar, P.; Muruganandam, P.; Wiston, B.R.; Mahalingam, A.; Sriram, G. Solution Processed NiO/MoS2 Heterostructure Nanocomposite for Supercapacitor Electrode Application. Energies 2023, 16, 335. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Shreenivasa, L.; Viswanatha, R.; Ganesan, S.; Kalegowda, Y.; Kurkuri, M.D.; Ashoka, S. Scalable chemical approach to prepare crystalline Mn2V2O7 nanoparticles: Introducing a new long-term cycling cathode material for lithium-ion battery. J. Mater. Sci. Mater. Electron. 2020, 31, 19638–19646. [Google Scholar] [CrossRef]
- Eisa, T.; Abdelkareem, M.A.; Jadhav, D.A.; Mohamed, H.O.; Sayed, E.T.; Olabi, A.G.; Castaño, P.; Chae, K.-J. Critical review on the synthesis, characterization, and application of highly efficient metal chalcogenide catalysts for fuel cells. Prog. Energy Combust. Sci. 2023, 94, 101044. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors Performance Evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Shaheen Shah, S.; Abu Nayem, S.M.; Sultana, N.; Saleh Ahammad, A.J.; Abdul Aziz, M. Preparation of Sulfur-doped Carbon for Supercapacitor Applications: A Review. ChemSusChem 2022, 15, e202101282. [Google Scholar] [CrossRef]
- Sardana, S.; Gupta, A.; Singh, K.; Maan, A.S.; Ohlan, A. Conducting polymer hydrogel based electrode materials for supercapacitor applications. Renew. Sustain. Energy Rev. 2022, 45, 103510. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, Z.; Zhai, S.; Wang, G.; An, Q.; Yang, D. A high-temperature phosphorization for synthesis of core-shell Ni-NixPy@C nanocomposite-immobilized sponge-like P-doped porous carbon with excellent supercapacitance performance. Electrochim. Acta 2019, 309, 197–208. [Google Scholar] [CrossRef]
- Nishchith, B.S.; Kalegowda, Y.; Ashoka, S.; Sriram, G.; Kurkuri, M.D.; Channegowda, M. Two-step synthesis of a-NiCu(OH)2CO3/Na3NiCuCO3PO4: A battery-type electrode for pseudocapacitor applications. New J. Chem. 2023, 47, 4386–4401. [Google Scholar]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-based nanostructured materials and their composites as supercapacitor electrodes. J. Mater. Chem. 2012, 22, 767–784. [Google Scholar] [CrossRef]
- Supriya, S.; Sriram, G.; Ngaini, Z.; Kavitha, C.; Kurkuri, M.; De Padova, I.P.; Hegde, G. The Role of Temperature on Physical–Chemical Properties of Green Synthesized Porous Carbon Nanoparticles. Waste Biomass Valorization 2020, 11, 3821–3831. [Google Scholar] [CrossRef]
- Luo, L.; Lan, Y.; Zhang, Q.; Deng, J.; Luo, L.; Zeng, Q.; Gao, H.; Zhao, W. A review on biomass-derived activated carbon as electrode materials for energy storage supercapacitors. J. Energy Storage 2022, 55, 105839. [Google Scholar] [CrossRef]
- Luo, L.; Lan, Y.; Zhang, Q.; Deng, J.; Zeng, Q.; Gao, H.; Du, G.; Zhao, W. Enhanced capacitance of phosphorus, nitrogen, and oxygen tri-doped balsa wood-based porous carbon for supercapacitors. J. Energy Storage 2023, 58, 106339. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Liu, Q.; Chen, S.; Wang, L.; Gao, F.; Shao, G.; Tian, Y.; Lin, Z.; Yang, W. Robust High-Temperature Supercapacitors Based on SiC Nanowires. Adv. Funct. Mater. 2021, 31, 2008901. [Google Scholar] [CrossRef]
- Priya, D.S.; Kennedy, L.J.; Anand, G.T. Emerging trends in biomass-derived porous carbon materials for energy storage application: A critical review. Mater. Today Sustain. 2023, 21, 100320. [Google Scholar] [CrossRef]
- Kang, M.S.; Heo, I.; Cho, K.G.; Kyung, H.; Kim, H.S.; Lee, K.H.; Yoo, W.C. Coarsening-induced hierarchically interconnected porous carbon polyhedrons for stretchable ionogel-based supercapacitors. Energy Storage Mater. 2022, 45, 380–388. [Google Scholar] [CrossRef]
- Rawat, S.; Mishra, R.K.; Bhaskar, T. Biomass derived functional carbon materials for supercapacitor applications. Chemosphere 2022, 286, 131961. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, M.; Yannopoulos, S.N.; Ioannides, T. Biomass-derived graphene-like materials as active electrodes for supercapacitor applications: A critical review. Chem. Eng. J 2022, 446, 137191. [Google Scholar] [CrossRef]
- Rajeshkumar, L.; Ramesh, M.; Bhuvaneswari, V.; Balaji, D. Carbon nano-materials (CNMs) derived from biomass for energy storage applications: A review. Carbon Lett. 2023, 33, 661–690. [Google Scholar] [CrossRef]
- Zhou, Q.; Yao, H. Recent development of carbon electrode materials for electrochemical supercapacitors. Energy Rep. 2022, 8, 656–661. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Li, L. Applications and challenges of porous carbon with different dimensions in supercapacitors—A mini review. Front. Energy Res. 2022, 10, 951701. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, X.; Luan, P.; Zhang, Z.; Ding, Y.; Cao, J.; Liao, Y. Refilling nitrogen into carbon sponge for enhanced performance of compressible supercapacitor. Diam. Relat. Mater. 2023, 131, 109586. [Google Scholar] [CrossRef]
- Fu, M.; Chen, W.; Lei, Y.; Yu, H.; Lin, Y.; Terrones, M. Biomimetic Construction of Ferrite Quantum Dot/Graphene Heterostructure for Enhancing Ion/Charge Transfer in Supercapacitors. Adv. Mater. 2023, 35, 2300940. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, L.; Sun, X.; Zhang, J.; Hou, L.; Li, L.; Yang, S.; Yuan, C. One-Dimensional Nanostructured Pseudocapacitive Materials: Design, Synthesis and Applications in Supercapacitors. Batter. Supercaps 2019, 2, 820–841. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Mohanty, A.; Balasingam, S.K.; Kim, S.J.; Ramadoss, A. Comprehensive Insight into the Mechanism, Material Selection and Performance Evaluation of Supercapatteries. Nano-Micro Lett. 2020, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Namsheer, K.; Jose, J.R.; Sahoo, S.; Chakraborty, B.; Rout, C.S. Understanding the charge storage mechanism of supercapacitors: In situ/operando spectroscopic approaches and theoretical investigations. J. Mater. Chem. A 2021, 9, 25852–25891. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Kumar, N.; Kim, S.-B.; Lee, S.-Y.; Park, S.-J. Recent Advanced Supercapacitor: A Review of Storage Mechanisms, Electrode Materials, Modification, and Perspectives. Nanomaterials 2022, 12, 3708. [Google Scholar] [CrossRef]
- Conway, B.E. Two-dimensional and quasi-two-dimensional isotherms for Li intercalation and upd processes at surfaces. Electrochim. Acta 1993, 38, 1249–1258. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.-E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.M.; Santino, L.M.; Lu, Y.; Acharya, S.; D’Arcy, J.M. Conducting Polymers for Pseudocapacitive Energy Storage. Chem. Mater. 2016, 28, 5989–5998. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials—Capacitive, Pseudocapacitive, or Battery-like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, H.-X.; Cao, Y.; Yan, X.-H.; Yan, J.; Gao, H.-L.; Luo, H.-W.; Wang, S.-W.; Jia, X.-D.; Kachalova, L.; et al. Recent advances and challenges of electrode materials for flexible supercapacitors. Coord. Chem. Rev. 2021, 438, 213910. [Google Scholar] [CrossRef]
- Xi, Y.; Xiao, Z.; Lv, H.; Sun, H.; Zhai, S.; An, Q. Construction of CuO/Cu-nanoflowers loaded on chitosan-derived porous carbon for high energy density supercapacitors. J. Colloid Interface Sci. 2023, 630, 525–534. [Google Scholar] [CrossRef]
- Saini, S.; Chand, P.; Joshi, A. Biomass derived carbon for supercapacitor applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Lei, D.; Zeng, Y.; Zhong, J.; Chen, J.; Ye, Y.; Wang, W. Ultra-high specific surface area porous carbons derived from Chinese medicinal herbal residues with potential applications in supercapacitors and CO2 capture. Colloids Surf. A 2023, 666, 131327. [Google Scholar] [CrossRef]
- Wang, H.; Yao, L.; Zuo, H.; Ruan, F.; Wang, H. Fabrication of Porous Carbon Nanofibers from Polymer Blends Using Template Method for Electrode-Active Materials in Supercapacitor. Molecules 2023, 28, 2228. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Santander, P.; Butter, B.; Oyarce, E.; Yáñez, M.; Xiao, L.-P.; Sánchez, J. Lignin-based adsorbent materials for metal ion removal from wastewater: A review. Ind. Crops Prod. 2021, 167, 113510. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Slabon, A.; Sipponen, M.H. Lignin–Inorganic Interfaces: Chemistry and Applications from Adsorbents to Catalysts and Energy Storage Materials. ChemSusChem 2020, 13, 4344–4355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hong, S.; Zhang, H.; Chen, Y.; Xu, H.; Wang, X.; Jiang, Z.; Chen, S.; Liu, Y. Toward biomass-based single-atom catalysts and plastics: Highly active single-atom Co on N-doped carbon for oxidative esterification of primary alcohols. Appl. Catal. B 2019, 256, 117767. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Sui, W.; Xu, Y.; Parvez, A.M.; Si, C. Novel metal-lignin assembly strategy for one-pot fabrication of lignin-derived heteroatom-doped hierarchically porous carbon and its application in high-performance supercapacitor. Int. J. Biol. Macromol. 2023, 234, 123603. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, D.; Wang, T.; Guo, Y. Polysaccharide of agar based ultra-high specific surface area porous carbon for superior supercapacitor. Int. J. Biol. Macromol. 2023, 228, 40–47. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Z.; Zhao, Z.; Lv, H.; Zhai, S.; An, Q.; Hao, J. Construction of iron/cobalt disulfides nanoparticles anchored on biomass-derived hierarchically porous carbon for hybrid supercapacitors with ultrahigh energy density. J. Alloys Compd. 2023, 935, 168074. [Google Scholar] [CrossRef]
- Priya, D.S.; Kennedy, L.J.; Anand, G.T. Effective conversion of waste banana bract into porous carbon electrode for supercapacitor energy storage applications. Results Surf. Interfaces 2023, 10, 100096. [Google Scholar] [CrossRef]
- Liu, D.; Xu, G.; Yuan, X.; Ding, Y.; Fan, B. Pore size distribution modulation of waste cotton-derived carbon materials via citrate activator to boost supercapacitive performance. Fuel 2023, 332, 126044. [Google Scholar] [CrossRef]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Wang, M.; You, W.; Manickam, S.; Tao, Y.; Yoon, J.Y.; Sun, X. Hydrodynamic cavitation-assisted preparation of porous carbon from garlic peels for supercapacitors. Ultrason. Sonochem. 2023, 94, 106333. [Google Scholar] [CrossRef]
- Gupta, S.P.; Walke, P.S. Scalable multifunctional ultralight mesoporous micro yarn carbon for excellent durable supercapacitor and tremendous oils sorbent. Chem. Eng. J 2023, 456, 141011. [Google Scholar] [CrossRef]
- Li, L.; Wei, X.-Y.; Shao, C.-W.; Yin, F.; Sun, B.-K.; Liu, F.-J.; Li, J.-H.; Liu, Z.-Q.; Zong, Z.-M. Honeycomb-like N/O self-doped hierarchical porous carbons derived from low-rank coal and its derivatives for high-performance supercapacitor. Fuel 2023, 331, 125658. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Cao, J.-P.; Zhuang, Q.-Q.; Wu, Y.; Zhou, Z.; Wei, Y.-L.; Zhao, X.-Y. Oxygen-enriched porous carbon derived from acid washed and oxidized lignite via H3PO4 hydrothermal for high-performance supercapacitors. Fuel Process. Technol. 2023, 243, 107665. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Lv, L.; Liu, X.; Wang, Z.; Liu, J. Oxygen-enriched hierarchical porous carbons derived from lignite for high-performance supercapacitors. Energy 2023, 269, 126707. [Google Scholar] [CrossRef]
- Yao, Y.; Yu, Y.; Du, C.; Wan, L.; Zhang, Y.; Chen, J.; Xiao, T.; Xie, M. Superbases-templated carbons doped with electrochemically active oxygen as advanced supercapacitor electrodes. J. Colloid Interface Sci. 2023, 630, 487–496. [Google Scholar] [CrossRef]
- Khoramjah, F.; Omidvar, M.; Miresmaieli, M.S.; Dalvand, S.; Asghari, A.; Kambarani, M.; Mohammadi, N. Defective mesoporous carbon coupled with a redox additive electrolyte for high performance supercapacitor. Diam. Relat. Mater. 2023, 132, 109590. [Google Scholar] [CrossRef]
- Wan, L.; Wang, J.; Xie, L.; Sun, Y.; Li, K. Nitrogen-Enriched Hierarchically Porous Carbons Prepared from Polybenzoxazine for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 15583–15596. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhou, X.; Yang, X.; Wu, X.; Zhou, P.; Zhou, J.; Zhuo, S. Self-templating synthesis of porous carbon with phytate salts for supercapacitor application. J. Energy Storage 2023, 57, 106221. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, F.; Cai, Z.; Zhai, B.; Wang, X.; Song, Y. Zn-ethylenediaminetetraacetic acid complex derived N-doped porous carbon for high-performance supercapacitor. J. Energy Storage 2023, 60, 106659. [Google Scholar] [CrossRef]
- Qiu, C.; Zuo, M.; Qiu, D.; Cao, J.; Jia, X.; Li, Y.; Liu, C.; Chen, N.; Chen, X.; Li, M. Unique hierarchical porous carbon nanosheet network for supercapacitors: Ultra-long cycling stability and enhanced electroactivity of oxygen at high temperature. Electrochim. Acta 2023, 437, 141522. [Google Scholar] [CrossRef]
- Li, K.; Nan, D.-h.; Li, Z.-y.; Xie, J.-h.; Ma, S.-w.; Huang, Y.-q.; Lu, Q. Preparation and optimization of nitrogen-doped porous carbon derived from bio-oil distillation residue for high-performance supercapacitors. J. Energy Storage 2023, 57, 106219. [Google Scholar] [CrossRef]
- Xue, B.; Xu, J.; Chen, Z.; Xiao, R. Valorizing high-fraction bio-oil to prepare 3D interconnected porous carbon with efficient pore utilization for supercapacitor applications. Fuel Process. Technol. 2023, 239, 107538. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, S.; Zhao, H.; Zou, Y.; Yu, C.; Zhong, W. Nitrogen-doped interpenetrating porous carbon/graphene networks for supercapacitor applications. Chem. Eng. J 2021, 409, 127891. [Google Scholar] [CrossRef]
- Leng, Q.; Tian, F.; Yuan, Y.; Li, W. N/S Codoped Three-Dimensional Porous Carbons for High-Performance Supercapacitors with Remarkable Rate Performance. Energy Fuels 2023, 37, 5499–5507. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, J.; Jin, C.; Wang, Z.; Wang, T.; Cheng, X.; Ma, C. Effects of temperature, oxygen and steam on pore structure characteristics of coconut husk activated carbon powders prepared by one-step rapid pyrolysis activation process. Bioresour. Technol. 2020, 310, 123413. [Google Scholar] [CrossRef]
- Tong, J.; Wang, J.; Xu, P.; Zhang, S. Nitrogen and oxygen codoped porous carbon based on a synthetic polymer for high-performance solid-state supercapacitors. J. Energy Storage 2023, 58, 106349. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.; Han, W.; Cheng, Y.; Sun, B.; Hou, C.; Zhao, G.; Liu, D.; Chen, G.; Han, J.; et al. Nature-inspired self-activation method for the controllable synthesis of highly porous carbons for high-performance supercapacitors. Carbon 2023, 205, 1–9. [Google Scholar] [CrossRef]
- Bommier, C.; Xu, R.; Wang, W.; Wang, X.; Wen, D.; Lu, J.; Ji, X. Self-activation of cellulose: A new preparation methodology for activated carbon electrodes in electrochemical capacitors. Nano Energy 2015, 13, 709–717. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Yan, L.; Wang, Q.; Li, J.; Zhong, X.; Liu, Q.; Li, Q.; Cui, S.; Xie, G. From pollutant to high-performance supercapacitor: Semi-coking wastewater derived N–O–S self-doped porous carbon. Colloids Surf. A 2023, 657, 130596. [Google Scholar] [CrossRef]
- Geioushy, R.A.; Attia, S.Y.; Mohamed, S.G.; Li, H.; Fouad, O.A. High-performance electrode materials for supercapacitor applications using Ni-catalyzed carbon nanostructures derived from biomass waste materials. J. Energy Storage 2022, 48, 104034. [Google Scholar] [CrossRef]
- Huang, W.; Khalafallah, D.; Ouyang, C.; Zhi, M.; Hong, Z. Strategic N/P self-doped biomass-derived hierarchical porous carbon for regulating the supercapacitive performances. Renew. Energy 2023, 202, 1259–1272. [Google Scholar] [CrossRef]
- Deng, F.; Li, Y.; Zhang, Y.; Zhang, Q.; Li, Y.; Shang, J.; Wang, J.; Gao, R.; Li, R. A popcorn-derived porous carbon optimized by thermal treatment and its outstanding electrochemical performance. J. Energy Storage 2023, 60, 106668. [Google Scholar] [CrossRef]
- Tan, H.; Fan, Y.; Pan, X.; Chen, S.; Tao, H.; Yang, D.; Zhang, W.; Li, Z. Fabrication of tobacco-stem-derived hierarchical porous carbon via synergistic gas exfoliation effect for high-performance supercapacitors. Ind. Crops Prod. 2023, 191, 115981. [Google Scholar] [CrossRef]
- Kumaresan, N.; Alsalhi, M.S.; Karuppasamy, P.; Praveen Kumar, M.; Pandian, M.S.; Arulraj, A.; Peera, S.G.; Mangalaraja, R.V.; Devanesan, S.; Ramasamy, P.; et al. Nitrogen implanted carbon nanosheets derived from Acorus calamus as an efficient electrode for the supercapacitor application. Mol. Catal. 2023, 538, 112978. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, Y.; He, C.; Qiu, Y.; Hou, W.; Lu, B.; Chen, Y.; Huang, B.; Lv, J.; Lin, G. Preparation of biomass-based N, P, and S co-doped porous carbon with high mesoporosity based on the synergistic effect of NaOH/thiourea and melamine phosphate and its application in high performance supercapacitors. J. Anal. Appl. Pyrolysis 2023, 169, 105822. [Google Scholar] [CrossRef]
- Inayat, A.; Albalawi, K.; Rehman, A.-u.; Adnan; Saad, A.Y.; Saleh, E.A.M.; Alamri, M.A.; El-Zahhar, A.A.; Haider, A.; Abbas, S.M. Tunable synthesis of carbon quantum dots from the biomass of spent tea leaves as supercapacitor electrode. Mater. Today Commun. 2023, 34, 105479. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, J.; Huan, Y.; Ren, M.; Wei, T.; Yan, S. 3D stack tubular mesoporous carbon derived from discarded sesame capsule shells for high-performance supercapacitors. Diam. Relat. Mater. 2023, 131, 109562. [Google Scholar] [CrossRef]
- Jiao, S.; Yao, Y.; Zhang, J.; Zhang, L.; Li, C.; Zhang, H.; Zhao, X.; Chen, H.; Jiang, J. Nano-flower-like porous carbon derived from soybean straw for efficient N-S co-doped supercapacitors by coupling in-situ heteroatom doping with green activation method. Appl. Surf. Sci. 2023, 615, 156365. [Google Scholar] [CrossRef]
- Prasankumar, T.; Salpekar, D.; Bhattacharyya, S.; Manoharan, K.; Yadav, R.M.; Campos Mata, M.A.; Miller, K.A.; Vajtai, R.; Jose, S.; Roy, S.; et al. Biomass derived hierarchical porous carbon for supercapacitor application and dilute stream CO2 capture. Carbon 2022, 199, 249–257. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, R.; Yuan, Y.; Hou, R.; Liu, Y.; Ao, W.; Qu, J.; Yu, M.; Song, H.; Dai, J. An environment-friendly strategy to prepare oxygen-nitrogen-sulfur doped mesopore-dominant porous carbons for symmetric supercapacitors. Fuel 2023, 344, 128039. [Google Scholar] [CrossRef]
- Lesbayev, B.; Auyelkhankyzy, M.; Ustayeva, G.; Yeleuov, M.; Rakhymzhan, N.; Maral, Y.; Tolynbekov, A. Modification of Biomass-Derived Nanoporous Carbon with Nickel Oxide Nanoparticles for Supercapacitor Application. J. Compos. Sci. 2023, 7, 20. [Google Scholar] [CrossRef]
- Zhu, W.; Shen, D.; Xie, H. Combination of chemical activation and nitrogen doping toward hierarchical porous carbon from houttuynia cordata for supercapacitors. J. Energy Storage 2023, 60, 106595. [Google Scholar] [CrossRef]


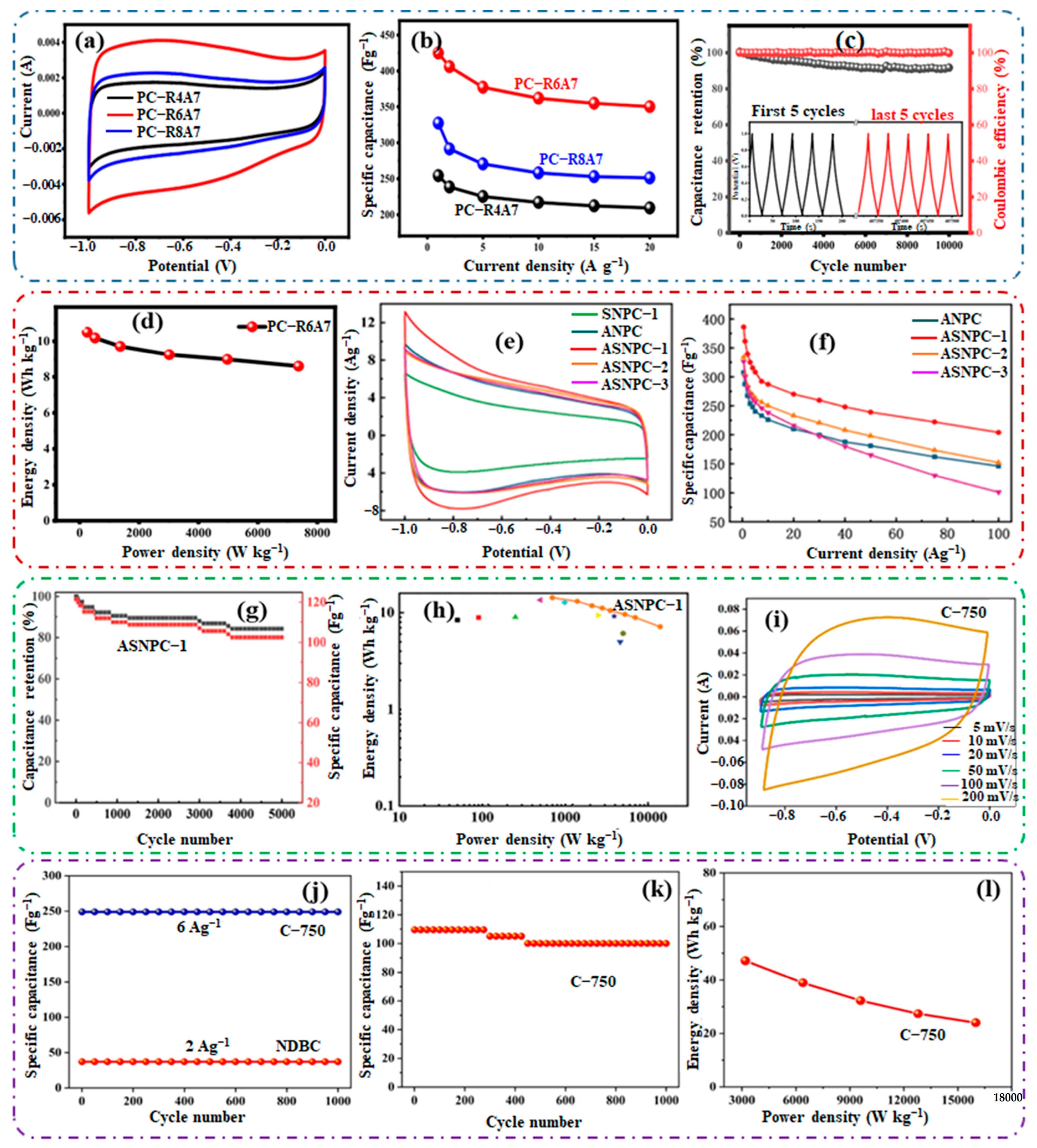
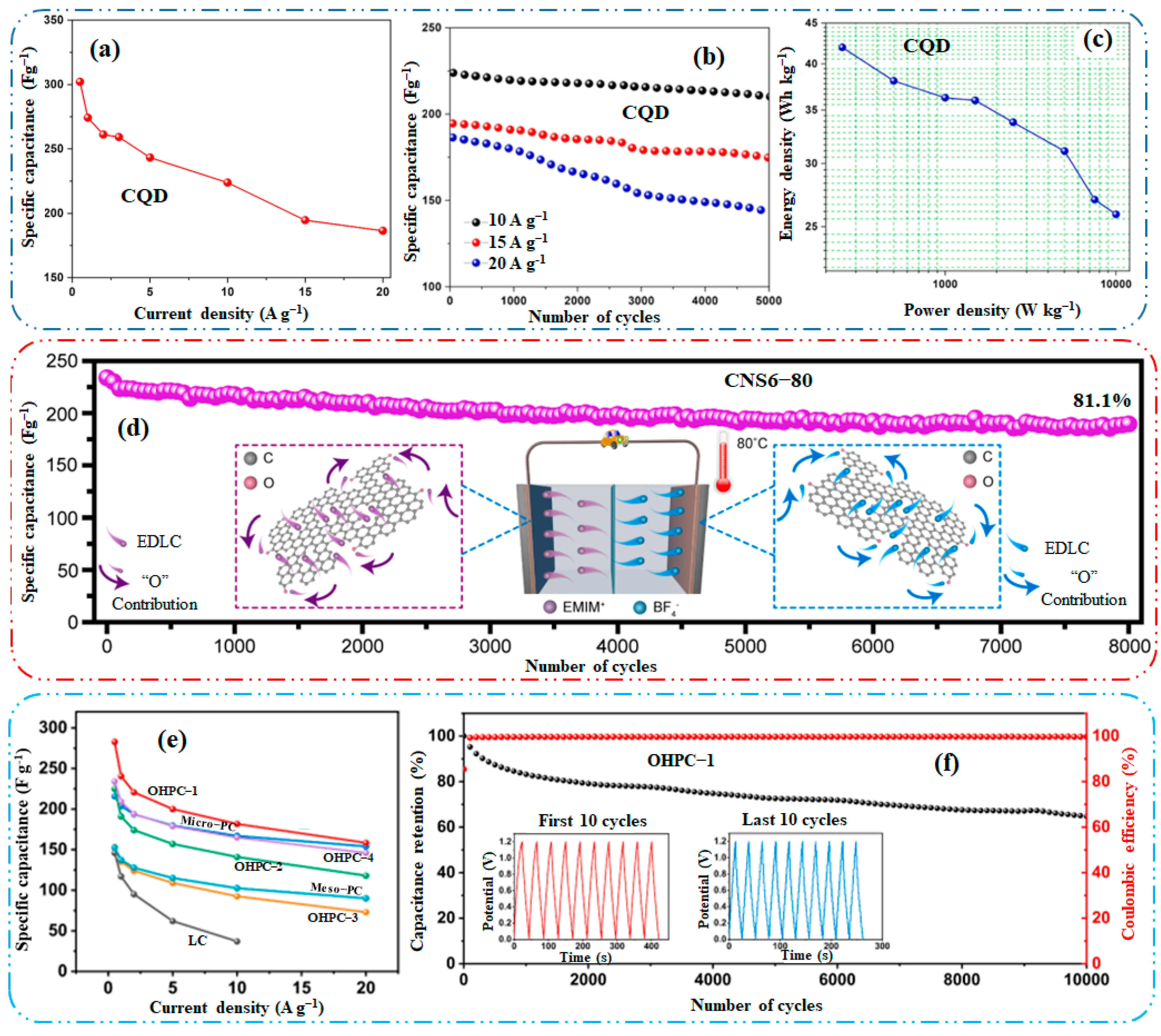
| Porous Carbon | Source | Doping/Activation | Pyrolysis Temp. (°C) | ATM & Time | SBET (m2 g−1) | Cost | Ref. |
|---|---|---|---|---|---|---|---|
| PNOAC | Balsa wood | H3PO4 | 600 | - & 1 h | 1302.9 | Low | [21] |
| NCS | Melamine sponge | Urea/KOH | 950 | N2 & 1 h | 158.1 | Moderate | [30] |
| CuO/Cu@C-700 | Chitosan & Cu(NO3)2·3H2O | - | 700 | N2 & 2 h | 313.2 | High | [47] |
| N-LHPC | Lignin, MgCl2⋅6H2O & ZnCl2 | Melamine | 900 | N2 & 2 h | - | High | [55] |
| NPC | Agar-Arg xerogel | L-arginine/KHCO3 | 800 | N2 & 2 h | 3184.0 | Low | [56] |
| FeS2/CoS2-C | CoCl2·6H2O, FeCl3·6H2O & Kelp | Thiourea | 800 | Ar & 2 h | 468.5 | High | [57] |
| BBC | Banana bract | KOH | 500–800 | N2 & 2 h | 712.1 | Moderate | [58] |
| PC-K | Waste cotton | Potassium citrate | 700 | N2 & 2 h | 1727.9 | Low | [59] |
| HCGP | Garlic peel | Alkali activation | 600 | - & 2 h | 3272.0 | Low | [61] |
| CA | Kapok silk | - | 600–800 | N2 & 2 h | 489.0 | Low | [62] |
| SPLRL | Runbei lignite | KOH | 700 | N2 & 1 h | 3586.0 | Low | [63] |
| POPC | Lignite | KOH | 700 | Ar & 2 h | 2852.0 | Moderate | [64] |
| OHPC | lignite powder | K2FeO4, & K2CO3 | 900 | N2 & 3 h | 1638.0 | Moderate | [65] |
| OPCN | KOH/Mg(OH)2, catechol & formaldehyde | KOH | 700 | Ar & 2 h | 930.0 | Moderate | [66] |
| DMC | Nano silica | KNO3 | 1000 | N2 & 3 h | 1350.0 | Low | [67] |
| PC-K | Zinc acetate | KOH | 800 | N2 & 1 h | 1210.0 | Low | [69] |
| NPC-2 | EDTA & Zinc acetate | - | 850.0 | N2 & 4 h | 1173.8 | Low | [70] |
| CNS | EDTA4Na | KOH | 800 | Ar & 1 h | 3400.0 | Low | [71] |
| BAC | Bio-oil distillation residue | Melamine/KOH | 500–800 | N2 & 1.5h | 1664.1 | Low | [72] |
| HBPC | Bio-oil | KOH | 700–900 | Ar & 1 h | 1758.0 | Moderate | [73] |
| ASNPC | Melamine & SGO | KOH | 800 | N2 & 1 h | 3281.0 | Moderate | [76] |
| SPMA-BQ | Benzoquinone and melamine | KOH | 700–900 | N2 & 2 h | 2154.1 | Moderate | [78] |
| HPC | Glucose & Polyacrylamide | KOH | 900 | - & 1 h | 3381.0 | Moderate | [79] |
| SWPR | Semi-coking wastewater | Na2CO3 | 600–800 | N2 & 1 h | 750.0 | Low | [81] |
| Porous Carbon | SC * (F g−1) | CD (A g−1) | EL | ED (Wh kg−1) | PD (W kg−1) | CS and R | System Built | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PNOAC600-2 | 263.0 | 0.5 | 6 M KOH | 9.2 | 414.0 | 10,000 & 91.4% | Symmetric | Low | [21] |
| NCS | 253.0 | 0.5 | 6 M KOH | 4.2 | 250.0 | 10,000 & 99% | - | Low | [30] |
| CuO/Cu@C-700 | 2479.0 | 0.5 | 6 M KOH | 76.8 | 374.5 | 10,000 & 82.4% | Asymmetric | High | [47] |
| N-LHPC | 235.7 | 0.5 | 6 M KOH | 5.7 | 246.6 | 5000 & 81.3% | Symmetric | Low | [55] |
| NPC-800-4 | 443.0 | 0.5 | 6 M KOH | 35.5 | 450.0 | 20,000 & 99.7% | Symmetric | High | [56] |
| FeS2/CoS2@PC-800 | 3480.4 | 0.5 | 6 M KOH | 200.2 | 463.1 | 10,000 & 94.7% | Asymmetric | Very High | [57] |
| BBC-800 | 472.0 | 1.0 | 1 M Na2SO4 | 86.0 | 1284.0 | 5000 & 93.5% | - | High | [58] |
| PCK | 273.0 | 1.0 | 6 M KOH | 9.9 | 350.0 | 5000 & 98% | Symmetric | Moderate | [59] |
| 1:6-HCGP+N | 227.0 | 10.0 | 6 M KOH | 10.1 | 250.0 | 5000 & 96% | - | Moderate | [61] |
| CA8 | 355.0 | 1.0 | 1 M H2SO4 and IL | 35.0 | 65.0 | 20,000 & 96% | Symmetric | High | [62] |
| SPLRL | 373.0 | 0.5 | 6 M KOH | 11.5 | 125.0 | 10,000 & 97.4% | Symmetric | Moderate | [63] |
| POPC2 | 320.0 | 1.0 | 6 M KOH | 10.7 | 11.1 | 25,000 & 88% | Symmetric | High | [64] |
| OHPC1 | 283.0 | 0.5 | 6 M NaOH | 16.5 | 300.0 | 10,000 & 64.8% | Symmetric | Moderate | [65] |
| OPCN-20 | 375.0 | 1.0 | 6 M KOH | 25.7 | 900.0 | 20,000 & 86.2% | Symmetric | High | [66] |
| DMC | 327.0 | 0.5 | 1 M H2SO4 + 0.01 M AO 45 | 11.4 | 250.0 | 2000 & 74% | Symmetric | Moderate | [67] |
| PC-K4 | 296.0 | 0.5 | 6 M KOH | 10.0 | 230.0 | 8000 & 96.5% | Symmetric | Moderate | [69] |
| NPC2 | 251.9 | 1.0 | 6 M KOH | 8.7 | 248.7 | 10,000 & 100% | - | Moderate | [70] |
| CNS6-80 | 290.0 | 2.0 | IL | 122.0 | 1740.0 | 8000 & 81.1% | Symmetric | Moderate | [71] |
| BAC-10 | 442.0 | 1.0 | 6 M KOH | 14.8 | 48.5 | - | Symmetric | High | [72] |
| SC@KOH | 336.0 | 0.5 | 6 M KOH | 9.54 | 130.0 | 10,000 & 92–98% | Symmetric | Moderate | [73] |
| ASNPC-1 | 386.0 | 0.5 | 6 M KOH | 14.4 | 700.0 | 5000 & 84% | Symmetric | Moderate | [76] |
| SPMA-BQ-5-800 | 414.6 | 0.5 | 6 M KOH | 8.8 | 250.0 | 7000 & 90.2% | Symmetric | High | [78] |
| HPC60 | 419.0 | 0.5 | 6 M KOH | 10.9 | 125.0 | 20,000 & 89.9% | Symmetric | High | [79] |
| SWPR-700-2.5 | 129.5 | 1.0 | 6 M KOH | 5.9 | 124.9 | 10,000 & 99.4% | Symmetric | Very low | [81] |
| So | 508.0 | 1.0 | 6 M KOH | 7.0 | 586.0 | 10,000 & 69% | Asymmetric | Moderate | [82] |
| N/P-HPC-Y:PA(2:1)-800 | 432.0 | 1.0 | 1 M H2SO4 | 13.6 | 500.0 | 10,000 & 93.3% | Symmetric | High | [83] |
| PC-R6A7 | 425.0 | 1.0 | 6 M KOH | 10.5 | 256.0 | 10,000 & 92% | Symmetric | High | [84] |
| TS-HPC | 402.0 | 0.5 | 6 M KOH | 7.0 | 15,789.5 | 10,000 & 97.8% | Symmetric | High | [85] |
| C750 | 354.0 | 1.0 | 6 M KOH | 47.2 | 16,000.0 | 1000 & 91% | Symmetric | High | [86] |
| 850-24-20-5% | 316.0 | 1.0 | 6 M KOH | 16.3 | 489.0 | 5000 & 91.2% | Symmetric | High | [87] |
| CQD | 302.0 | 0.5 | 1 M H2SO4 | 41.9 | 250.0 | 5000 & 93.8% | - | Moderate | [88] |
| TMCK | 222.7 | 1.0 | 2 M KOH | 6.9 | 600.0 | 20,000 & 100% | Symmetric | Moderate | [89] |
| SSL-N/S-K-700 | 220.0 | 0.5 | 1 M KOH | 11.2 | 400.0 | 10,000 & 99% | Symmetric | Low | [90] |
| ACTBG | 212.0 | 1.0 | 1 M KOH | 18.8 | 4900.0 | 15,000 & - | Symmetric | Low | [91] |
| ZMB | 208.0 | 1.0 | 6 M KOH | 2.93 | 61.7 | - | Low | [92] | |
| WS-dAC/NiO-0.005 | 205.0 | 0.5 | 6 M KOH | - | - | 1000 & 98% | - | Low | [93] |
| N-HPC | 144.9 | 0.5 | 6 M KOH | 5.0 | 124.8 | 15,000 & 96.5% | - | Very low | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriram, G.; Kurkuri, M.; Oh, T.H. Recent Trends in Highly Porous Structured Carbon Electrodes for Supercapacitor Applications: A Review. Energies 2023, 16, 4641. https://doi.org/10.3390/en16124641
Sriram G, Kurkuri M, Oh TH. Recent Trends in Highly Porous Structured Carbon Electrodes for Supercapacitor Applications: A Review. Energies. 2023; 16(12):4641. https://doi.org/10.3390/en16124641
Chicago/Turabian StyleSriram, Ganesan, Mahaveer Kurkuri, and Tae Hwan Oh. 2023. "Recent Trends in Highly Porous Structured Carbon Electrodes for Supercapacitor Applications: A Review" Energies 16, no. 12: 4641. https://doi.org/10.3390/en16124641
APA StyleSriram, G., Kurkuri, M., & Oh, T. H. (2023). Recent Trends in Highly Porous Structured Carbon Electrodes for Supercapacitor Applications: A Review. Energies, 16(12), 4641. https://doi.org/10.3390/en16124641







