Effect of GaN-Based Distributed Bragg Reflector on Optical Properties of CH3NH3PbBr3 Crystals
Abstract
1. Introduction
2. Materials and Methods
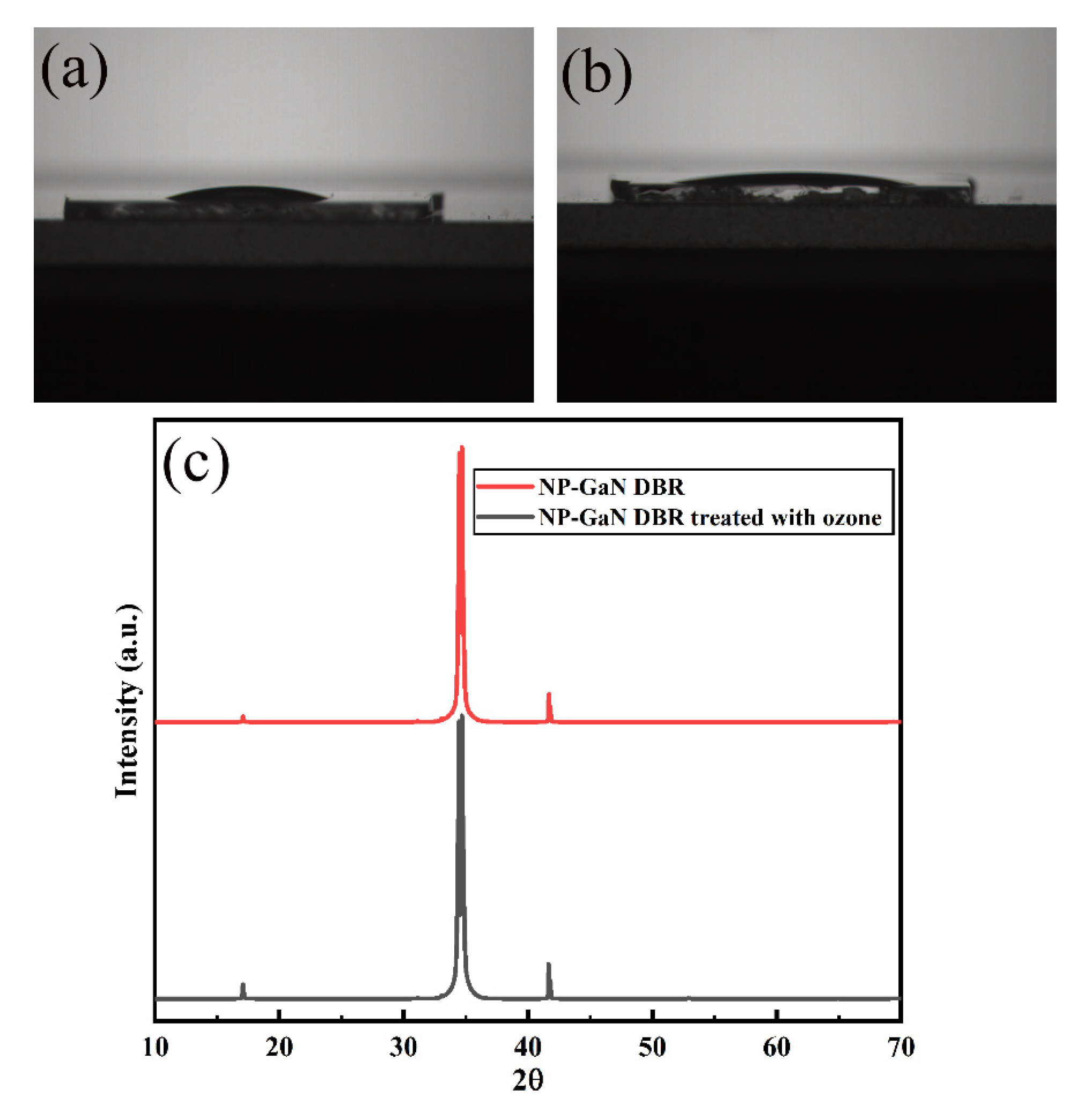
2.1. Fabrication of NP-GaN DBR
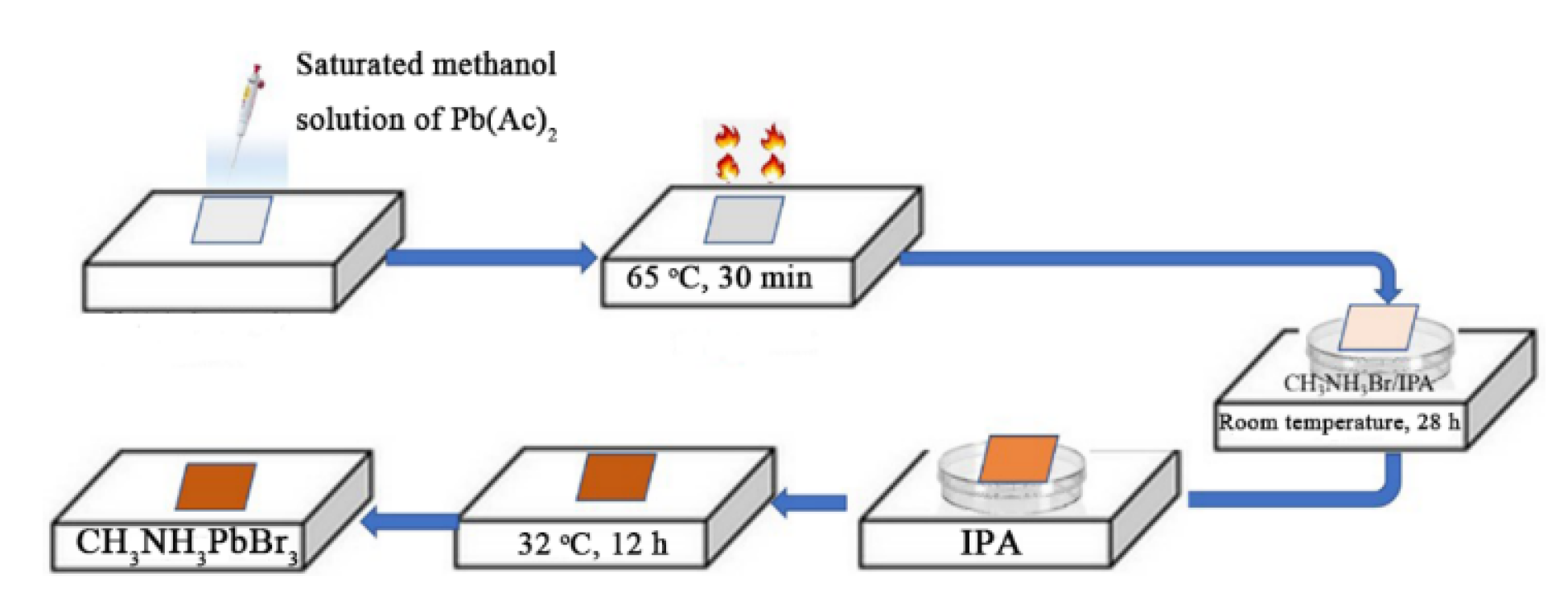
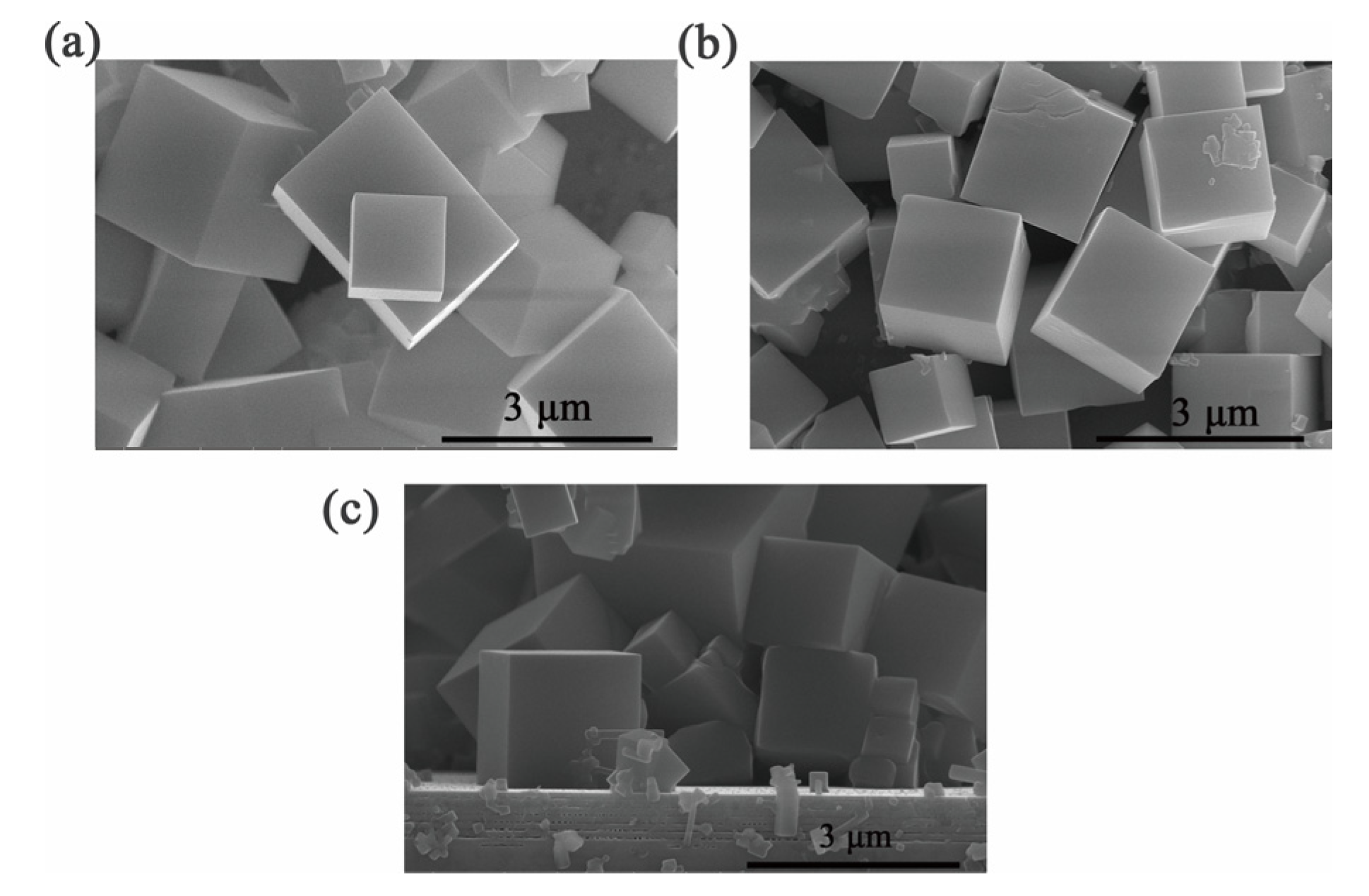
2.2. Preparation of CH3NH3PbBr3 Crystal on the DBR and Reference Substrate
2.3. Materials Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.X.; Tan, Q.S.; Fu, L.B.; Wang, X.G. Coherence dynamics of a two-mode Bose-Einstein condensate coupled with the environment. Phys. Rev. A 2013, 88, 063642. [Google Scholar] [CrossRef]
- Ki, W.; Li, J. A semiconductor bulk material that emits direct white light. J. Am. Chem. Soc. 2008, 130, 8114–8115. [Google Scholar] [CrossRef] [PubMed]
- Ki, W.; Li, J.; Eda, G.; Chhowalla, M. Direct white light emission from inorganic-organic hybrid semiconductor bulk materials. J. Mater. Chem. 2010, 20, 10676–10679. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Liu, M.Z.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Y.; Liu, A.M.; Gao, L.G.; Zhang, C.; Wei, G.Y.; Ma, T.L. Recent progress in metal sulfide-based electron transport layers in perovskite solar cells. Nanoscale 2021, 13, 17272–17289. [Google Scholar] [CrossRef]
- Shoukat, F.; Kang, J.; Khan, Y.; Park, Y.; Lee, J.; Walker, B.; Seo, J. Solution-processed metal ion polyelectrolytes as hole transport materials for efficient inverted perovskite solar cells. Adv. Mater. Interfaces 2023, 2300043. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Lin, J. Layered organic-inorganic hybrid perovskites: Structure, optical properties, film preparation, patterning and templating engineering. Crystengcomm 2010, 12, 2646–2662. [Google Scholar] [CrossRef]
- Chen, J.N.; Zhou, S.S.; Jin, S.Y.; Li, H.Q.; Zhai, T.Y. Crystal organometal halide perovskites with promising optoelectronic applications. J. Mater. Chem. C 2016, 4, 11–27. [Google Scholar] [CrossRef]
- Rong, Y.G.; Mei, A.Y.; Liu, L.F.; Li, X.; Han, H.W. All-solid-state mesoscopic solar cells: From dye-sensitized to perovskite. ACTA Chim. Sin. 2015, 73, 237–251. [Google Scholar] [CrossRef]
- Rezaee, E.; Zhang, W.; Silva, S.R.P. Solvent engineering as a vehicle for high quality thin films of perovskites and their device fabrication. Small 2021, 17, 2008145. [Google Scholar] [CrossRef]
- Li, Y.W.; Meng, L.; Yang, Y.; Xu, G.Y.; Hong, Z.R.; Chen, Q.; You, J.B.; Li, G.; Yang, Y.; Li, Y.F. High-efficiency robust perovskite solar cells on ultrathin flexible substrates. Nat. Commun. 2016, 7, 10214. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Tan, S.; Xue, J.L.; Meng, L.; Lee, J.W.; Yang, Y. Interface and defect engineering for metal halide perovskite optoelectronic devices. Adv. Mater. 2019, 31, 1803515. [Google Scholar] [CrossRef]
- Zhu, H.M.; Fu, Y.P.; Meng, F.; Wu, X.X.; Gong, Z.Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef]
- Wong, A.B.; Lai, M.L.; Eaton, S.W.; Yu, Y.; Lin, E.; Dou, L.; Fu, A.; Yang, P.D. Growth and anion exchange conversion of CH3NH3PbX3 nanorod arrays for light-emitting diodes. Nano Lett. 2015, 15, 5519–5524. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.Z.; Chen, C.; Wu, X.G.; Hu, X.M.; Huang, H.L.; Han, J.B.; Zou, B.S.; Dong, Y.P. Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X = Br, I, Cl) quantum dots: Potential alternatives for display technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Levchuk, I.; Herre, P.; Brandl, M.; Osvet, A.; Hock, R.; Peukert, W.; Schweizer, P.; Spiecker, E.; Batentschuk, M.; Brabec, C.J. Ligand-assisted thickness tailoring of highly luminescent colloidal CH3NH3PbX3 (X = Br and I) perovskite nanoplatelets. Chem. Commun. 2017, 53, 244–247. [Google Scholar] [CrossRef]
- Yan, J.; Ke, X.H.; Chen, Y.L.; Zhang, A.; Zhang, B. Effect of modulating the molar ratio of organic to inorganic content on morphology, optical absorption and photoluminescence of perovskite CH3NH3PbBr3 films. Appl. Surf. Sci. 2015, 351, 1191–1196. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, B.; Chen, Y.L.; Zhang, A.; Ke, X.H. Improving the photoluminescence properties of perovskite CH3NH3PbBr3,Cl-x films by modulating organic cation and chlorine concentrations. ACS Appl. Mater. Interfaces 2016, 8, 12756–12763. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Zhang, R.X.; Guo, Y.; Huan, Y.H.; Xi, J.H.; Li, Y.; Bai, Z.M.; Yan, X.Q. Introducing lead acetate into stoichiometric perovskite lewis acid-base precursor for improved solar cell photovoltaic performance. J. Alloys Compd. 2018, 767, 829–837. [Google Scholar] [CrossRef]
- Mosconi, E.; Azpiroz, J.; Angelis, F. Ab initio molecular dynamics simulations of methylammonium lead iodide perovskite degradation by water. Chem. Mater. 2015, 27, 4885–4892. [Google Scholar] [CrossRef]
- Sharma, R.; Choi, Y.S.; Wang, C.F.; David, A.; Weisbuch, C.; Nakamura, S.; Hu, E.L. Gallium-nitride-based microcavity light-emitting diodes with air-gap distributed Bragg reflectors. Appl. Phys. Lett. 2007, 91, 211108. [Google Scholar] [CrossRef]
- Sharma, R.; Haberer, E.D.; Meier, C.; Hu, E.L.; Nakamura, S. Vertically oriented GaN-based air-gap distributed Bragg reflector structure fabricated using band-gap-selective photoelectrochemical etching. Appl. Phys. Lett. 2005, 87, 051107. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D.; Shen, Y.; Yang, X.; Zhao, C.; Chen, R.; Yang, Z.; Liu, J.; Ma, J.; Xiao, H. Fabrication and applications of wafer-scale nanoporous GaN near-infrared distributed Bragg reflectors. Opt. Mater. 2020, 107, 110093. [Google Scholar] [CrossRef]
- Wang, G.J.; Hong, B.S.; Chen, Y.Y.; Yang, Z.J.; Tsai, T.L.; Lin, Y.S.; Lin, C.F. GaN/AlGaN ultraviolet light-emitting diode with an embedded porous-AlGaN distributed Bragg reflector. Appl. Phys. Express 2017, 10, 122102. [Google Scholar] [CrossRef]
- Ng, H.M.; Doppalapudi, D.; Iliopoulos, E.; Moustakas, T.D. Distributed Bragg reflectors based on AlN/GaN multilayers. Appl. Phys. Lett. 1999, 74, 1036–1038. [Google Scholar] [CrossRef]
- Gacevic, Z.; Fernandez-Garrido, S.; Hosseini, D.; Estrade, S.; Peiro, F.; Calleja, E. InAlN/GaN Bragg reflectors grown by plasma-assisted molecular beam epitaxy. J. Appl. Phys. 2010, 108, 113117. [Google Scholar] [CrossRef]
- Ng, H.M.; Moustakas, T.D.; Chu, S.N.G. High reflectivity and broad bandwidth AlN/GaN distributed Bragg reflectors grown by molecular-beam epitaxy. Appl. Phys. Lett. 2000, 76, 2818–2820. [Google Scholar] [CrossRef]
- Chen, D.; Han, J. High reflectance memebrane-based distributed Bragg reflectors for GaN photonics. Appl. Phys. Lett. 2012, 101, 221104. [Google Scholar] [CrossRef]
- Mishkat-Ul-Masabih, S.; Luk, T.S.; Rishinaramangalam, A.; Monavarian, M.; Nami, M.; Feezell, D. Nanoporous distributed Bragg reflectors on free-standing nonpolar m-plane GaN. Appl. Phys. Lett. 2018, 112, 041109. [Google Scholar] [CrossRef]
- Carreno, J.M.; Passarelli, N.; Otero-Martinez, C.; Polavarapu, L.; Perez, L.A.; Reparaz, J.S.; Alonso, M.I.; Mihi, A. Enhanced Photoluminescence of Cesium Lead Halide Perovskites by Quasi-3D Photonic Crystals. Adv. Opt. Mater. 2022, 10, 2101324. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, C.; Lee, J.; Han, J.; Nurmikko, A. High-Q, low-threshold monolithic perovskite thin-film vertical-cavity lasers. Adv. Mater. 2017, 29, 1604781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Y.; Wang, X.; Song, W.; Luo, X.; Guo, J.; Shi, J.; Cheng, C.; Li, D.; He, L.; et al. High-performance UV–visible photodetectors based on CH3NH3PbI3-xClx/GaN microwire array heterostructures. J. Alloys Compd. 2021, 864, 158710. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Y.; Xiang, P.; Liu, M.; Chen, W.; Han, X.; Hu, G.; Hu, G.; Zang, W.; Lin, X.; et al. Vertical-conducting InGaN/GaN multiple quantum wells LEDs with AlN/GaN distributed Bragg reflectors on Si(111) substrate. Appl. Phys. Express 2014, 7, 042102. [Google Scholar] [CrossRef]
- Zambrano-Serrano, M.A.; Hernandez, C.A.; de Melo, O.; Behar, M.; Gallardo-Hernandez, S.; Casallas-Moreno, Y.L.; Ponce, A.; Hernandez-Robles, A.; Bahena-Uribe, D.; Yee-Rendon, C.M.; et al. Effects of heavy Si doping on the structural and optical properties of n-GaN/AlN/Si(111) heterostructures. Mater. Res. Express 2022, 9, 065903. [Google Scholar] [CrossRef]
- Ni, Y.Q.; He, Z.Y.; Yang, F.; Zhou, D.Q.; Yao, Y.; Zhou, G.L.; Shen, Z.; Zhong, J.; Zhen, Y.; Wu, Z.S. Effect of AlN/GaN superlattice buffer on the strain state in GaN-on-Si(111) system. Jpn. J. Appl. Phys. 2015, 54, 015505. [Google Scholar] [CrossRef]
- Cao, D.Z.; Yang, X.K.; Shen, L.Y.; Zhao, C.C.; Luan, C.N.; Ma, J.; Xiao, H.D. Fabrication and properties of high quality InGaN-based LEDs with highly reflective nanoporous GaN mirrors. Photonics Res. 2018, 6, 1144–1150. [Google Scholar] [CrossRef]
- Lim, K.T.P.; Deakin, C.; Ding, B.N.; Bai, X.Y.; Griffin, P.; Zhu, T.T.; Oliver, R.A.; Credgington, D. Encapsulation of methylammonium lead bromide perovskite in nanoporous GaN. APL Mater. 2019, 7, 021107. [Google Scholar] [CrossRef]
- Huang, H.; Susha, A.S.; Kershaw, S.V.; Hung, T.F.; Rogach, A.L. Control of emission color of high quantum yield CH3NH3PbBr3 perovskite quantum dots by precipitation temperature. Adv. Sci. 2015, 2, 1500194. [Google Scholar] [CrossRef] [PubMed]
- Han, L.P.; Liu, C.; Wu, L.L.; Zhang, J.Q. Observation of the growth of MAPbBr(3) single-crystalline thin film based on space-limited method. J. Cryst. Growth 2018, 501, 27–33. [Google Scholar] [CrossRef]
- Ghosh, S.; Rana, D.; Pradhan, B.; Donfack, P.; Hofkens, J.; Materny, A. Vibrational study of lead bromide perovskite materials with variable cations based on Raman spectroscopy and density functional theory. J. Raman Spectrosc. 2021, 52, 2338. [Google Scholar] [CrossRef]
- Tang, Y.; Yan, N.; Wang, Z.W.; Yuan, H.D.; Xin, Y.L.; Yin, H.F. Precursor solution volume-dependent ligand-assisted synthesis of CH3NH3PbBr3 perovskite nanocrystals. J. Alloys Compd. 2019, 773, 227–233. [Google Scholar] [CrossRef]
- Li, X.J.; Wang, K.Y.; Chen, M.M.; Wang, S.S.; Fan, Y.B.; Liang, T.; Song, Q.H.; Xing, G.C.; Tang, Z.K. Stable whispering gallery mode lasing from solution-processed formamidinium lead bromide perovskite microdisks. Adv. Opt. Mater. 2020, 8, 2000030. [Google Scholar] [CrossRef]
- Xin, Y.; Gao, Z.; Shang, X.; Wu, J.; Yu, D.; Xiu, J.; Li, Z. Effect of thermal annealing on the structural and optical properties of ZnGa2O4 films deposited on sapphire by magnetron sputtering. J. Alloys Compd. 2023, 933, 167760. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, Y.; Zhu, X.; Luo, C.; Jiang, K.; Chen, L.; Zhang, J. Diversity of band gap and photoluminescence properties of lead halide perovskite: A halogen-dependent spectroscopic study. Chem. Phys. Lett. 2018, 699, 93. [Google Scholar] [CrossRef]
- Braun, M.M.; Pilon, L. Effective optical properties of non-absorbing nanoporous thin films. Thin Solid Films 2006, 496, 505–514. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Zhao, C.; Chen, R.; Luan, C.; Xiao, H. Eu-doped Lu2O3 epitaxial films with an embedded nanoporous GaN distributed Bragg reflectors. Ceram. Int. 2021, 47, 22693. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yeh, H.; Lee, C.J.; Chang, W.H. Distributed Bragg reflectors as broadband and large-area platforms for light-coupling enhancement in 2D transition-metal dichalcogenides. ACS Appl. Mater. Interfaces 2018, 10, 16874–16880. [Google Scholar] [CrossRef]
- Lien, D.H.; Kang, J.S.; Amani, M.; Chen, K.; Tosun, M.; Wang, H.P.; Roy, T.; Eggleston, M.S.; Wu, M.C.; Dubey, M.; et al. Engineering light outcoupling in 2D materials. Nano Lett. 2015, 15, 1356–1361. [Google Scholar] [CrossRef]
- Wang, W.J.; Yang, G.F.; Chen, P.; Yu, Z.G.; Liu, B.; Xie, Z.L.; Xiu, X.Q.; Wu, Z.L.; Xu, F.; Xu, Z.; et al. Characteristics of nanoporous InGaN/GaN multiple quantum wells. Superlattices Microst. 2014, 71, 38–45. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, C.R.; Zhou, Y.F.; Wang, X.S.; Liu, B.L.; Wang, X.L.; Lv, Y.J.; Feng, Z.H.; Xu, X.G.; Ji, Z.W. Fabrication and photoluminescence of strong phase-separated InGaN based nanopillar LEDs. Superlattices Microst. 2015, 88, 323–329. [Google Scholar] [CrossRef]
- Chen, R.; Wang, D.; Liu, J.; Feng, B.; Zhu, H.; Han, X.; Luan, C.; Ma, J.; Xiao, H. Ta-Doped Ga2O3 Epitaxial Films on Porous p-GaN Substrates: Structure and Self-Powered Solar-Blind Photodetectors. Cryst. Growth Des. 2022, 22, 5285–5292. [Google Scholar] [CrossRef]
- Cao, D.Z.; Xiao, H.D.; Gao, Q.X.; Yang, X.K.; Luan, C.N.; Mao, H.Z.; Liu, J.Q.; Liu, X.D. Fabrication and improved photoelectrochemical properties of a transferred GaN-based thin film with InGaN/GaN layers. Nanoscale 2019, 9, 11504–11510. [Google Scholar] [CrossRef]
- Chouhan, L.; Ito, S.; Thomas, E.M.; Takano, Y.; Ghimire, S.; Miyasaka, H.; Biju, V. Real-time blinking suppression of perovskite quantum dots by halide vacancy filling. ACS Nano 2021, 15, 2831. [Google Scholar] [CrossRef]
- Park, S.; Kwon, J.; Park, S.; Kwon, O.; Kim, J.; Yoon, S.; Chung, J.; Whang, D.; Park, S.; Lee, D.; et al. Grystallization-induced emission enhancement and amplified spontaneous emission from a CF3-containing excited-state intramolecular-proton-transfer molecule. Adv. Opt. Mater. 2017, 5, 1700353. [Google Scholar] [CrossRef]
- Zhang, Z.; Ghimire, S.; Okamoto, T.; Sachith, B.M.; Sobhanan, J.; Subrahmanyam, C.; Biju, V. Mechano-optical modulation of excitons and carrier recombination in self-assembled halide perovskite quantum dots. ACS Nano 2022, 16, 160. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Duan, Y.; Song, J.; Luo, Z. Effect of GaN-Based Distributed Bragg Reflector on Optical Properties of CH3NH3PbBr3 Crystals. Energies 2023, 16, 4547. https://doi.org/10.3390/en16124547
Jiang F, Duan Y, Song J, Luo Z. Effect of GaN-Based Distributed Bragg Reflector on Optical Properties of CH3NH3PbBr3 Crystals. Energies. 2023; 16(12):4547. https://doi.org/10.3390/en16124547
Chicago/Turabian StyleJiang, Feng, Yiwei Duan, Jiawen Song, and Zhe Luo. 2023. "Effect of GaN-Based Distributed Bragg Reflector on Optical Properties of CH3NH3PbBr3 Crystals" Energies 16, no. 12: 4547. https://doi.org/10.3390/en16124547
APA StyleJiang, F., Duan, Y., Song, J., & Luo, Z. (2023). Effect of GaN-Based Distributed Bragg Reflector on Optical Properties of CH3NH3PbBr3 Crystals. Energies, 16(12), 4547. https://doi.org/10.3390/en16124547






