Identifying the Active Species in Li-Na Dual-Ion “Saltwater Battery” Based on Spinel Lithium Manganese Oxide, Sodium Titanium Phosphate and Aqueous Electrolyte
Abstract
1. Introduction
2. Experimental
2.1. Electrode Preparation
2.2. Electrolyte Preparation
2.3. Cyclic Voltammetry
2.4. Full Cell Measurement
2.5. Electrolyte Ion Analysis
2.6. X-ray Diffraction (XRD)
3. Results and Discussion
3.1. Cyclic Voltammetry
3.2. Full Cell Measurement
3.3. Ion Analysis of Electrolyte
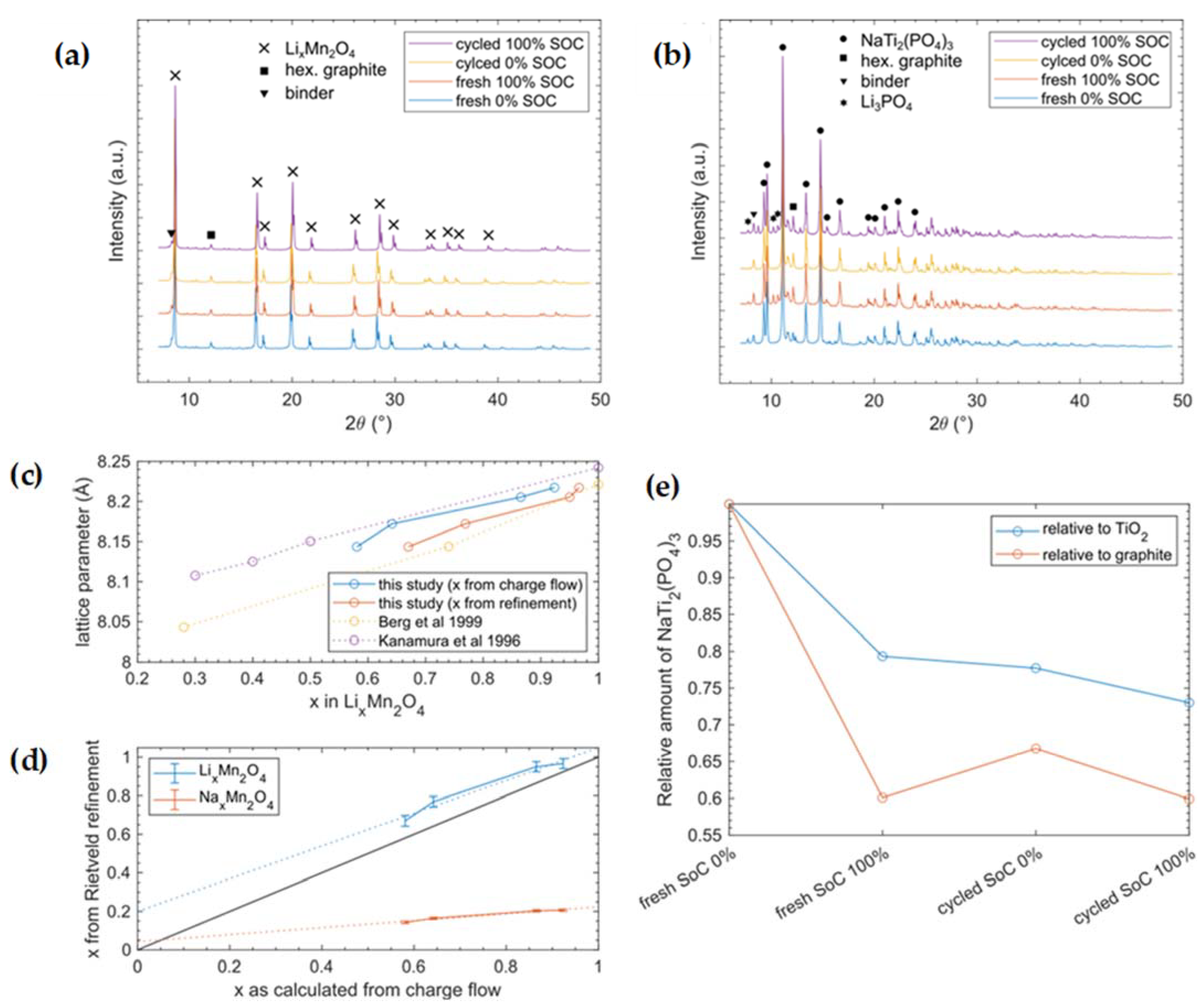
3.4. X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- LiMn2O4(LMO): COD #1514006
- Graphite: ICSD #76767
- LiNaSO4: COD #2106021
- Li2SO4(H2O): COD #1008190
- Na3Li(SO4)2(H20)6: COD #2243890
- NaTi2(PO4)3 (NaTiPO): ICSD #19995
- Li3PO4: ICSD #10257
- TiO2 (Anatase): COD #9008214
- Na2SO4: COD #9004092
- Six coefficients of the Chebyshev-1 background polynomial model;
- Two separate background peaks at 2θ = 8.29° and 8.17° that originated from the binder;
- Lattice parameters and phase fractions of all phases;
- One isotropic microstrain parameter for each phase except for NaTiPO;
- Three anisotropic microstrain parameters for NaTiPO using the generalized model;
- One isotropic atomic displacement factor U for all atoms of the NaTiPO and Li3PO4 phases, respectively;
- All possible atomic positions of the NaTiPO and Li3PO4 phases.
- Six coefficients of the Chebyshev-1 background polynomial model;
- One separate background peak at 2θ = 8.29° that originated from the binder;
- Lattice parameters and phase fractions of all phases;
- One isotropic microstrain parameter for each phase except for LMO and Na3Li(SO4)2(H2O)6;
- Two anisotropic microstrain parameters for LMO using the generalized model;
- No broadening model was used for Na3Li(SO4)2(H2O)6 because its phase fraction was too low;
- One isotropic atomic displacement factor U for all atoms of the LMO phase;
- The x-position of the oxygen atom in the LMO phase;
- The fraction of Li in the LMO phase was set to a value extracted from the summed transferred charge measured by the potentiostat. We assume that the same amount of Li+ was removed from LMO by assuming a transfer ratio of 1 Li+/e−.
References
- Zugschwert, C.; Dundálek, J.; Leyer, S.; Hadji-Minaglou, J.-R.; Kosek, J.; Pettinger, K.-H. The Effect of Input Parameter Variation on the Accuracy of a Vanadium Redox Flow Battery Simulation Model. Batteries 2021, 7, 7. [Google Scholar] [CrossRef]
- Oh, H.G.; Park, S.-K. Co-MOF Derived MoSe2@CoSe2/N-Doped Carbon Nanorods as High-Performance Anode Materials for Potassium Ion Batteries. Int. J. Energy Res. 2022, 46, 10677–10688. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, M.; Liu, X.; Fan, H.; Zhang, Y.; Yang, H.Y. Ultrathin Cobalt Nickel Selenides (Co0.5Ni0.5Se2) Nanosheet Arrays Anchoring on Ti3C2 MXene for High-Performance Na+/K+ Batteries. J. Colloid Interface Sci. 2022, 626, 700–709. [Google Scholar] [CrossRef]
- Li, X.; Liang, H.; Qin, B.; Wang, M.; Zhang, Y.; Fan, H. Rational Design of Heterostructured Bimetallic Sulfides (CoS2/NC@VS4) with VS4 Nanodots Decorated on CoS2 Dodecahedron for High-Performance Sodium and Potassium Ion Batteries. J. Colloid Interface Sci. 2022, 625, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bin, D.; Wang, F.; Tamirat, A.G.; Suo, L.; Wang, Y.; Wang, C.; Xia, Y. Progress in Aqueous Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703008. [Google Scholar] [CrossRef]
- Li, W.-H.; Wu, X.-L. Advanced Cathode Materials in Dual-Ion Batteries: Progress and Prospect. Electrochem. Sci. Adv. 2022, 2, e2100127. [Google Scholar] [CrossRef]
- Wang, X.-T.; Yang, Y.; Guo, J.-Z.; Gu, Z.-Y.; Ang, E.H.; Sun, Z.-H.; Li, W.-H.; Liang, H.-J.; Wu, X.-L. An Advanced Cathode Composite for Co-Utilization of Cations and Anions in Lithium Batteries. J. Mater. Sci. Technol. 2022, 102, 72–79. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Song, Z.; Zhang, H.; Zhang, H.; Wang, Y.; Li, X. A Novel Aqueous Li+ (or Na+)/Br− Hybrid-Ion Battery with Super High Areal Capacity and Energy Density. J. Mater. Chem. A 2019, 7, 13050–13059. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, X.; Zhou, Y.; Wang, S.; Yao, L.; Pan, H.; Su, C.-Y.; Chen, F.; Hou, X. Aqueous Rechargeable Dual-Ion Battery Based on Fluoride Ion and Sodium Ion Electrochemistry. J. Mater. Chem. A 2018, 6, 8244–8250. [Google Scholar] [CrossRef]
- Whitacre, J.F.; Shanbhag, S.; Mohamed, A.; Polonsky, A.; Carlisle, K.; Gulakowski, J.; Wu, W.; Smith, C.; Cooney, L.; Blackwood, D.; et al. A Polyionic, Large-Format Energy Storage Device Using an Aqueous Electrolyte and Thick-Format Composite NaTi 2 (PO 4 ) 3 /Activated Carbon Negative Electrodes. Energy Technol. 2015, 3, 20–31. [Google Scholar] [CrossRef]
- Kalapsazova, M.; Rasheev, H.; Zhecheva, E.; Tadjer, A.; Stoyanova, R. Insights into the Function of Electrode and Electrolyte Materials in a Hybrid Lithium–Sodium Ion Cell. J. Phys. Chem. C 2019, 123, 11508–11521. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.-Y.; Kim, H.; Kim, S.-W.; Kang, K. Aqueous Rechargeable Li and Na Ion Batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.I.; Whitacre, J.F. Capacity Fade of NaTi2(PO4)3 in Aqueous Electrolyte Solutions: Relating PH Increases to Long Term Stability. Electrochim. Acta 2017, 235, 730–739. [Google Scholar] [CrossRef]
- Whitacre, J.F.; Wiley, T.; Shanbhag, S.; Wenzhuo, Y.; Mohamed, A.; Chun, S.E.; Weber, E.; Blackwood, D.; Lynch-Bell, E.; Gulakowski, J.; et al. An Aqueous Electrolyte, Sodium Ion Functional, Large Format Energy Storage Device for Stationary Applications. J. Power Sources 2012, 213, 255–264. [Google Scholar] [CrossRef]
- Wu, W.; Yan, J.; Wise, A.; Rutt, A.; Whitacre, J.F. Using Intimate Carbon to Enhance the Performance of NaTi2(PO4)3 Anode Materials: Carbon Nanotubes vs Graphite. J. Electrochem. Soc. 2014, 161, 561–567. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Cheng, Y.; Feng, K.; Li, X.; Zhang, H. Rational Design and Synthesis of LiTi 2 (PO 4 ) 3−x F x Anode Materials for High-Performance Aqueous Lithium Ion Batteries. J. Mater. Chem. A 2017, 5, 593–599. [Google Scholar] [CrossRef]
- Wu, W.; Mohamed, A.; Whitacre, J.F. Microwave Synthesized NaTi2(PO4)3 as an Aqueous Sodium-Ion Negative Electrode. J. Electrochem. Soc. 2013, 160, 497–504. [Google Scholar] [CrossRef]
- Hou, Z.; Li, X.; Liang, J.; Zhu, Y.; Qian, Y. An Aqueous Rechargeable Sodium Ion Battery Based on a NaMnO2–NaTi2(PO4)3 Hybrid System for Stationary Energy Storage. J. Mater. Chem. A 2015, 3, 1400–1404. [Google Scholar] [CrossRef]
- Sun, D.; Jin, G.; Tang, Y.; Zhang, R.; Xue, X.; Huang, X.; Chu, H.; Wang, H. NaTi2 (PO4)3 Nanoparticles Embedded in Carbon Matrix as Long-Lived Anode for Aqueous Lithium Ion Battery. J. Electrochem. Soc. 2016, 163, A1388–A1393. [Google Scholar] [CrossRef]
- Park, S.I.; Gocheva, I.; Okada, S.; Yamaki, J.-I. Electrochemical Properties of NaTi2(PO4)3 Anode for Rechargeable Aqueous Sodium-Ion Batteries. J. Electrochem. Soc. 2011, 158, 1067–1070. [Google Scholar] [CrossRef]
- Whitacre, J.F.; Tevar, A.; Sharma, S. Na4Mn9O18 as a Positive Electrode Material for an Aqueous Electrolyte Sodium-Ion Energy Storage Device. Electrochem. Commun. 2010, 12, 463–466. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Wang, Y.; Xia, Y. Cycling Stability of Spinel LiMn2O4 with Different Particle Sizes in Aqueous Electrolyte. Electrochim. Acta 2015, 173, 178–183. [Google Scholar] [CrossRef]
- Zhu, Z.; Peelaers, H.; Van de Walle, C.G. Hydrogen-Induced Degradation of NaMnO2. Chem. Mater. 2019, 31, 5224–5228. [Google Scholar] [CrossRef]
- Li, Z.; Young, D.; Xiang, K.; Carter, W.C.; Chiang, Y.-M. Towards High Power High Energy Aqueous Sodium-Ion Batteries: The NaTi2 (PO4)3 /Na0.44MnO2 System. Adv. Energy Mater. 2013, 3, 290–294. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Qiu, B.; Paillard, E.; Ma, C.; Cao, X.; Liu, H.; Stan, M.C.; Liu, H.; Gallash, T.; et al. Durable High-Rate Capability Na0.44MnO2 Cathode Material for Sodium-Ion Batteries. Nano Energy 2016, 27, 602–610. [Google Scholar] [CrossRef]
- Zhan, X.; Shirpour, M. Evolution of Solid/Aqueous Interface in Aqueous Sodium-Ion Batteries. Chem. Commun. 2017, 53, 204–207. [Google Scholar] [CrossRef]
- Sauvage, F.; Laffont, L.; Tarascon, J.-M.; Baudrin, E. Study of the Insertion/Deinsertion Mechanism of Sodium into Na0.44MnO2. Inorg. Chem. 2007, 46, 3289–3294. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Xia, Y.-Y. Aqueous Lithium-Ion Battery LiTi2(PO4)3/LiMn2O4 with High Power and Energy Densities as Well as Superior Cycling Stability**. Adv. Funct. Mater. 2007, 17, 3877–3884. [Google Scholar] [CrossRef]
- Tang, W.; Hou, Y.; Wang, F.; Liu, L.; Wu, Y.; Zhu, K. LiMn 2 O 4 Nanotube as Cathode Material of Second-Level Charge Capability for Aqueous Rechargeable Batteries. Nano Lett. 2013, 13, 2036–2040. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Guo, Z.; Wang, Y.; Wang, C.; Xia, Y. Electrochemical Profile of LiTi2(PO4)3 and NaTi2(PO4)3 in Lithium, Sodium or Mixed Ion Aqueous Solutions. J. Electrochem. Soc. 2016, 163, A904. [Google Scholar] [CrossRef]
- DIN EN ISO/IEC 17025:2018-03; Allgemeine Anforderungen an Die Kompetenz von Prüf- Und Kalibrierlaboratorien. Deutsche Beuth Verlag GmbH: Berlin, Germany, 2018.
- Binnewies, M.; Finze, M.; Jäckel, M.; Schmidt, P.; Willner, H.; Rayner-Canham, G. Allgemeine und Anorganische Chemie; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-45066-6. [Google Scholar]
- Luo, F.; Wei, C.; Zhang, C.; Gao, H.; Niu, J.; Ma, W.; Peng, Z.; Bai, Y.; Zhang, Z. Operando X-Ray Diffraction Analysis of the Degradation Mechanisms of a Spinel LiMn2O4 Cathode in Different Voltage Windows. J. Energy Chem. 2020, 44, 138–146. [Google Scholar] [CrossRef]
- Marchini, F.; Rubi, D.; del Pozo, M.; Williams, F.J.; Calvo, E.J. Surface Chemistry and Lithium-Ion Exchange in LiMn 2 O 4 for the Electrochemical Selective Extraction of LiCl from Natural Salt Lake Brines. J. Phys. Chem. C 2016, 120, 15875–15883. [Google Scholar] [CrossRef]
- Berg, H. Neutron Diffraction Study of Electrochemically Delithiated LiMn2O4 Spinel. Solid State Ion. 1999, 126, 227–234. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Yano, M.; Kuze, S.; Komaba, S. Electrochemical Behavior and Structural Change of Spinel-Type Li[LixMn2−x]O4 (X = 0 and 0.2) in Sodium Cells. Electrochim. Acta 2012, 82, 296–301. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Hu, P.; Qi, J.; Guo, L.; Wang, L. A High-Rate and Ultralong-Life Sodium-Ion Battery Based on NaTi2(PO4)3 Nanocubes with Synergistic Coating of Carbon and Rutile TiO2. Small 2015, 11, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Kabbour, H.; Coillot, D.; Colmont, M.; Masquelier, C.; Mentré, O. α-Na3M2 (PO4)3(M = Ti, Fe): Absolute Cationic Ordering in NASICON-Type Phases. J. Am. Chem. Soc. 2011, 133, 11900–11903. [Google Scholar] [CrossRef]
- Kanamura, K.; Naito, H.; Yao, T.; Takehara, Z. Structural Change of the LiMn2O4 Spinel Structure Induced by Extraction of Lithium. J. Mater. Chem. 1996, 6, 33. [Google Scholar] [CrossRef]
- Jazouli, A.E.; Nadiri, A.; Dance, J.M.; Delmas, C.; Flem, L. Relationships between Structure and Magnetic Properties of Titanium (III) NASICON-Type Phosphates. J. Phys. Chem. Solids 1988, 7, 779–783. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Cui, W.-J.; He, P.; Xia, Y.-Y. Raising the Cycling Stability of Aqueous Lithium-Ion Batteries by Eliminating Oxygen in the Electrolyte. Nat. Chem. 2010, 2, 760–765. [Google Scholar] [CrossRef]
- Chen, L.; Cao, L.; Ji, X.; Hou, S.; Li, Q.; Chen, J.; Yang, C.; Eidson, N.; Wang, C. Enabling Safe Aqueous Lithium Ion Open Batteries by Suppressing Oxygen Reduction Reaction. Nat. Commun. 2020, 11, 2638. [Google Scholar] [CrossRef]
- Song, Y.-J. Recovery of Lithium as Li3PO4 from Waste Water in a LIB Recycling Process. Korean J. Met. Mater. 2018, 56, 755–762. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. It GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]



| Component | Function | Weight Percentage in Anode/% | Weight Percentage in Cathode/% |
|---|---|---|---|
| NaTiPO | Anode intercalation material | 70 | 0 |
| LMO | Cathode intercalation material | 0 | 80 |
| Carbon black | Electrical conduction | 7 | 2 |
| Graphite | Electrical conduction | 3 | 10 |
| Polytetrafluoroethylene | Binder material | 5 | 8 |
| Activated Carbon | Anode additive for cycle stability | 15 | 0 |
| Type | Electrode | Product Name | Supplier |
|---|---|---|---|
| LMO | Cathode | HLM-Y01 | Eachem, Hunan, China |
| NaTiPO | Anode | Customized synthesis according to [10] | |
| Activated carbon | Anode | PAK C-1000C | CarboTech, Essen, Germany |
| Carbon black | Anode and cathode | Super P | Imerys, Willebroek, Belgium |
| Graphite | Anode and cathode | KS6 | Imerys, Bodio, Switzerland |
| Polytetrafluoroethylene | Anode | Algoflon L203 | Solvay, Bollate, Italy |
| Polytetrafluoroethylene | Cathode | Dyneon TF 2021Z | 3M, Burgkirchen, Germany |
| Fresh Electrolyte before Filling | Sample 0% SoC | Sample 100% SoC | |
|---|---|---|---|
| Lithium content in g/L | 11.1 | 13.1 | 14.7 |
| Sodium content in g/L | 58.4 | 69.2 | 70.4 |
| Sample | LixMn2O4 in wt.% | Graphite in wt.% | LiNaSO4 in wt.% | Li2SO4(H2O) in wt.% | Na3Li(SO4)2(H2O)6 in wt.% |
|---|---|---|---|---|---|
| Fresh SoC 0% | 86.9(2) | 7.8(2) | 1.95(9) | 4.5(8) | 2.9(2) |
| Fresh SoC 100% | 87.4(2) | 8.6(2) | 1.3(1) | 2.7(2) | 0.000 |
| Cycled SoC 0% | 86.8(2) | 9.5(2) | 3.2(1) | 0.000 | 0.5(1) |
| Cycled SoC 100% | 87.4(2) | 8.4(2) | 1.4(2) | 2.8(2) | 0.000 |
| Sample | NaTi2(PO4)3 in wt.% | Graphite in wt.% | Na3Li(SO4)2(H2O)6 in wt.% | Li3PO4 in wt.% | TiO2 in wt.% | Na2SO4 in wt.% |
|---|---|---|---|---|---|---|
| Fresh SoC 0% | 86.4(2) | 6.5(2) | 4.4(2) | 0.000 | 2.7(1) | 0.000 |
| Fresh SoC 100% | 76.5(3) | 9.5(2) | 0.000 | 11.0(3) | 3.0(1) | 0.000 |
| Cycled SoC 0% | 86.8(2) | 9.7(2) | 0.000 | 0.000 | 3.5(1) | 0.000 |
| Cycled SoC 100% | 73.5(2) | 9.2(2) | 0.000 | 6.8(2) | 3.1(1) | 7.4(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, J.; Grossmann, L.; Seidlmayer, S.; Pettinger, K.-H.; Gilles, R.; Danzer, M.A. Identifying the Active Species in Li-Na Dual-Ion “Saltwater Battery” Based on Spinel Lithium Manganese Oxide, Sodium Titanium Phosphate and Aqueous Electrolyte. Energies 2023, 16, 4485. https://doi.org/10.3390/en16114485
Schubert J, Grossmann L, Seidlmayer S, Pettinger K-H, Gilles R, Danzer MA. Identifying the Active Species in Li-Na Dual-Ion “Saltwater Battery” Based on Spinel Lithium Manganese Oxide, Sodium Titanium Phosphate and Aqueous Electrolyte. Energies. 2023; 16(11):4485. https://doi.org/10.3390/en16114485
Chicago/Turabian StyleSchubert, Jonathan, Lukas Grossmann, Stefan Seidlmayer, Karl-Heinz Pettinger, Ralph Gilles, and Michael A. Danzer. 2023. "Identifying the Active Species in Li-Na Dual-Ion “Saltwater Battery” Based on Spinel Lithium Manganese Oxide, Sodium Titanium Phosphate and Aqueous Electrolyte" Energies 16, no. 11: 4485. https://doi.org/10.3390/en16114485
APA StyleSchubert, J., Grossmann, L., Seidlmayer, S., Pettinger, K.-H., Gilles, R., & Danzer, M. A. (2023). Identifying the Active Species in Li-Na Dual-Ion “Saltwater Battery” Based on Spinel Lithium Manganese Oxide, Sodium Titanium Phosphate and Aqueous Electrolyte. Energies, 16(11), 4485. https://doi.org/10.3390/en16114485







