Large-Scale Experimental Simulations of In Situ Coal Gasification in Terms of Process Efficiency and Physicochemical Properties of Process By-Products
Abstract
1. Introduction
2. Problem Statement
3. Experimental
3.1. Materials and Methods
Preparation of Coal Blocks for Gasification
3.2. Installation for the Ex-Situ Coal Gasification
3.3. Measurements of Gaseous Media
3.4. Experimental Procedure
3.5. UCG Char Characterization
4. Results and Discussion
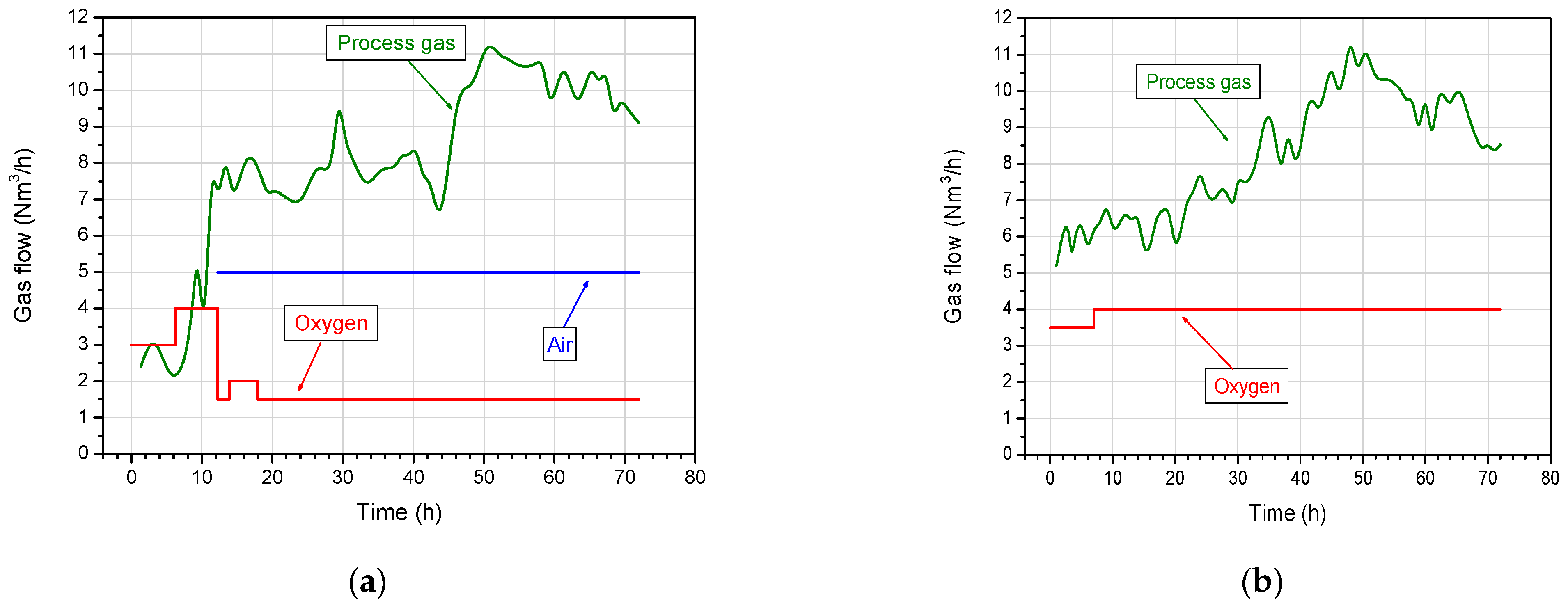
4.1. Oxidants Supply Rates and Gas Production
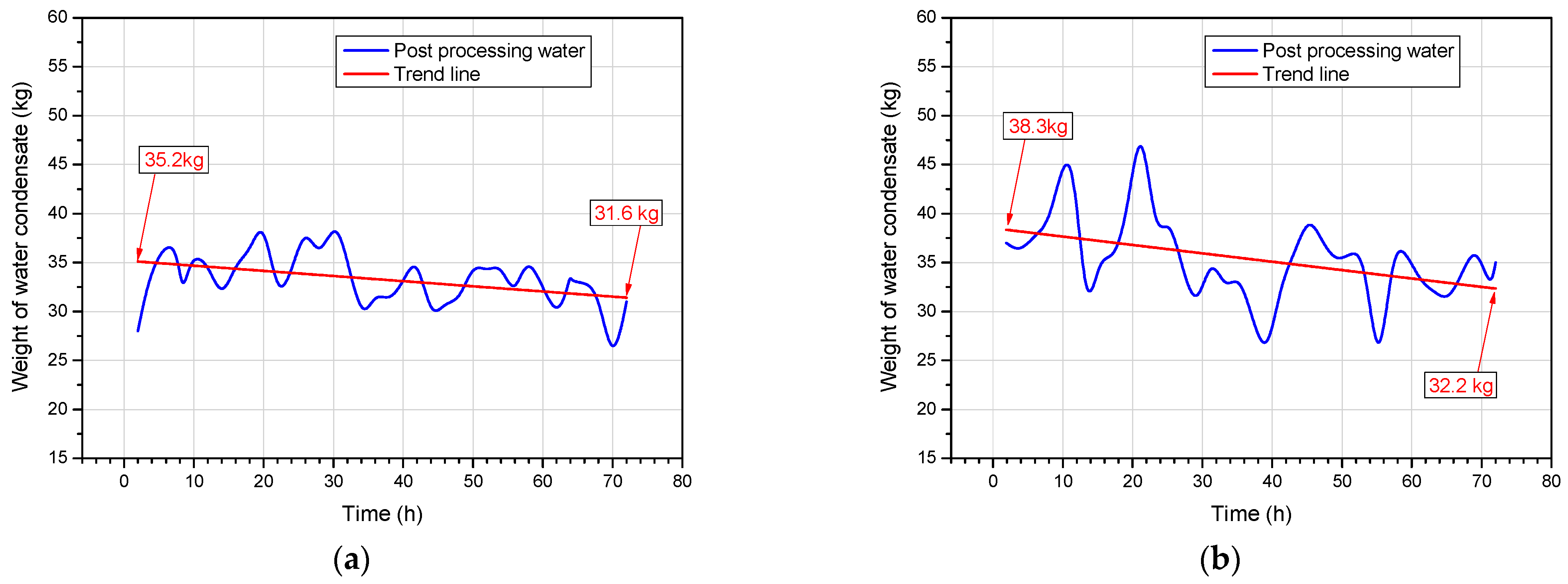
4.2. Weight of Water Condensate
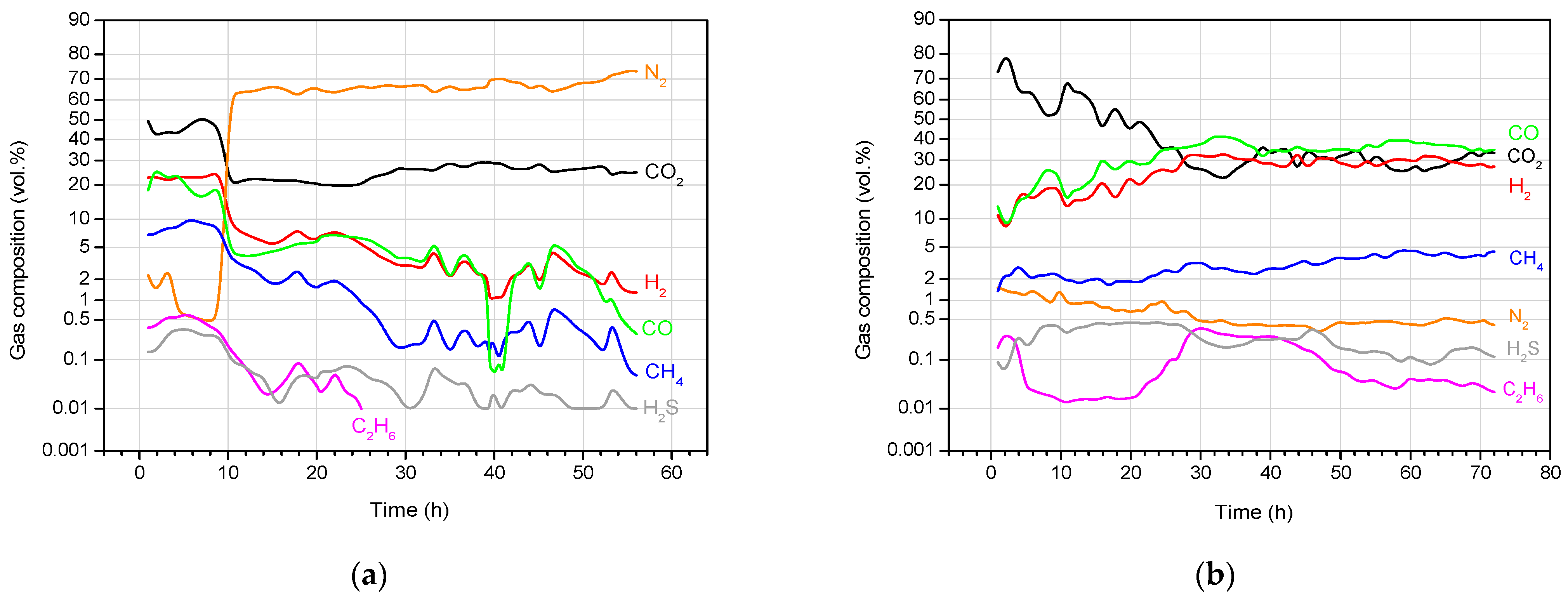
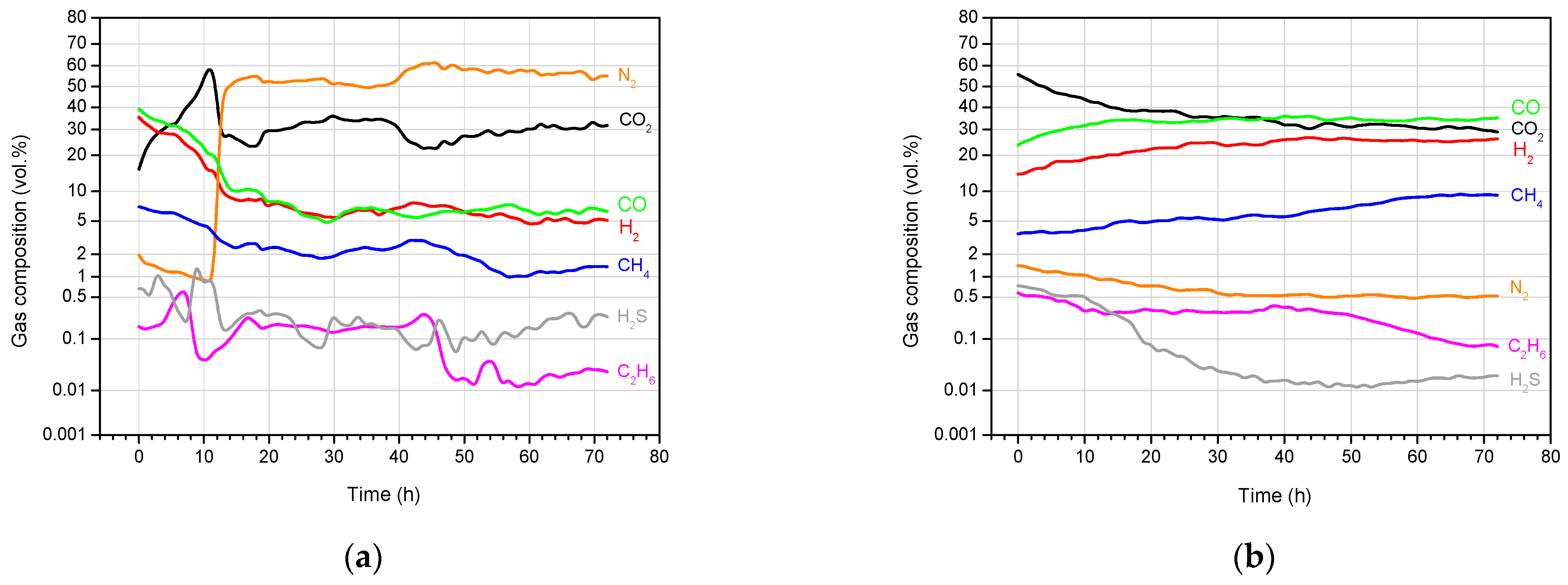
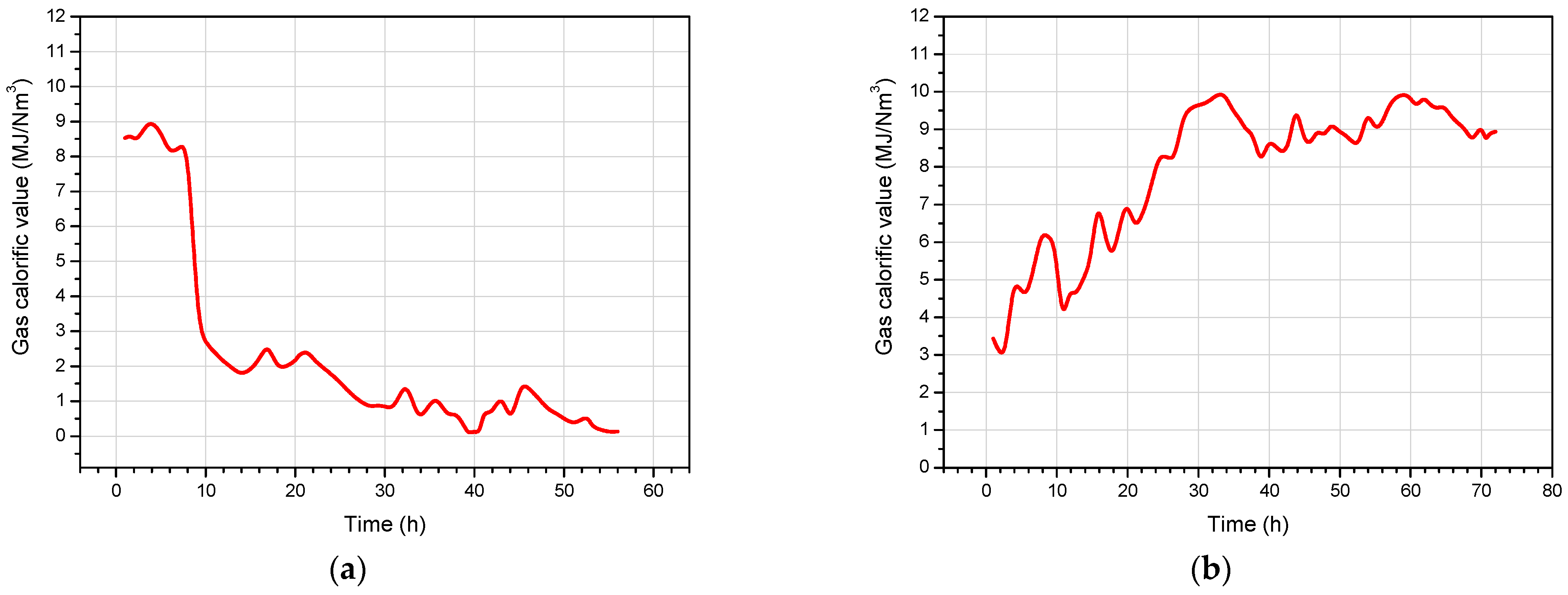
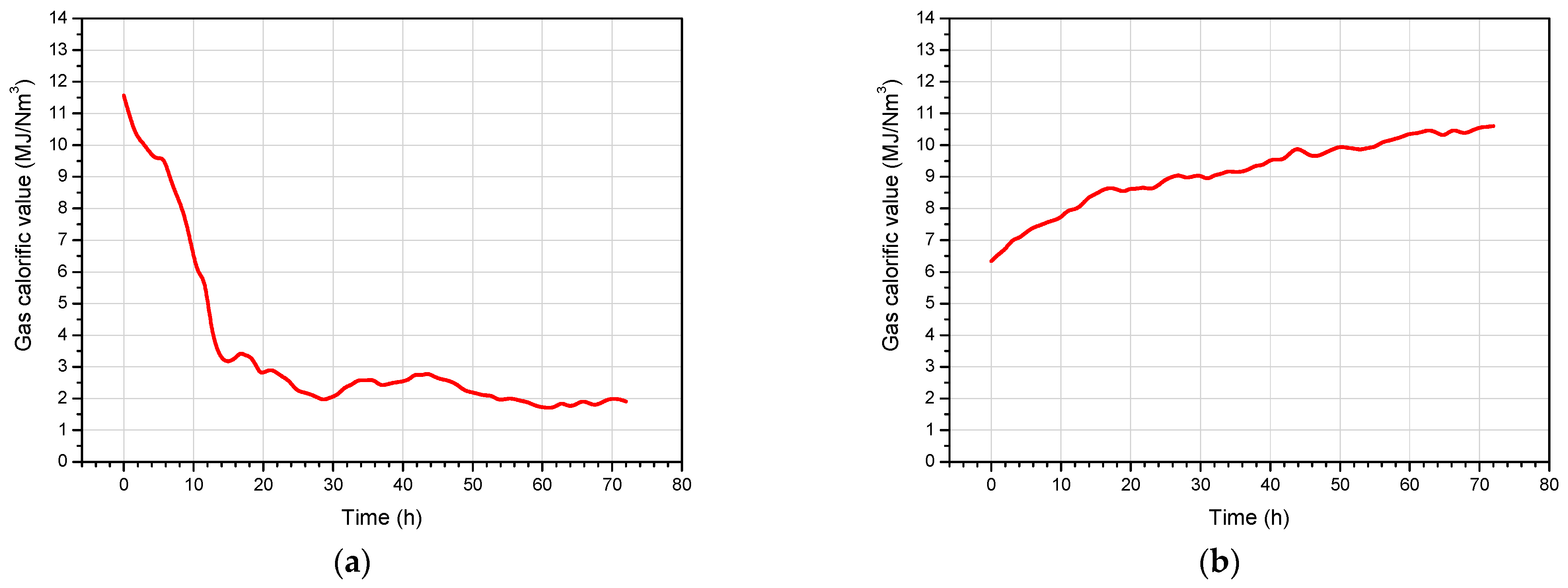
4.3. Product Gas Composition and Gas Calorific Value
4.4. Temperature Distribution
4.5. Process Balance Data
4.6. Physico-Chemical Analysis of Chars
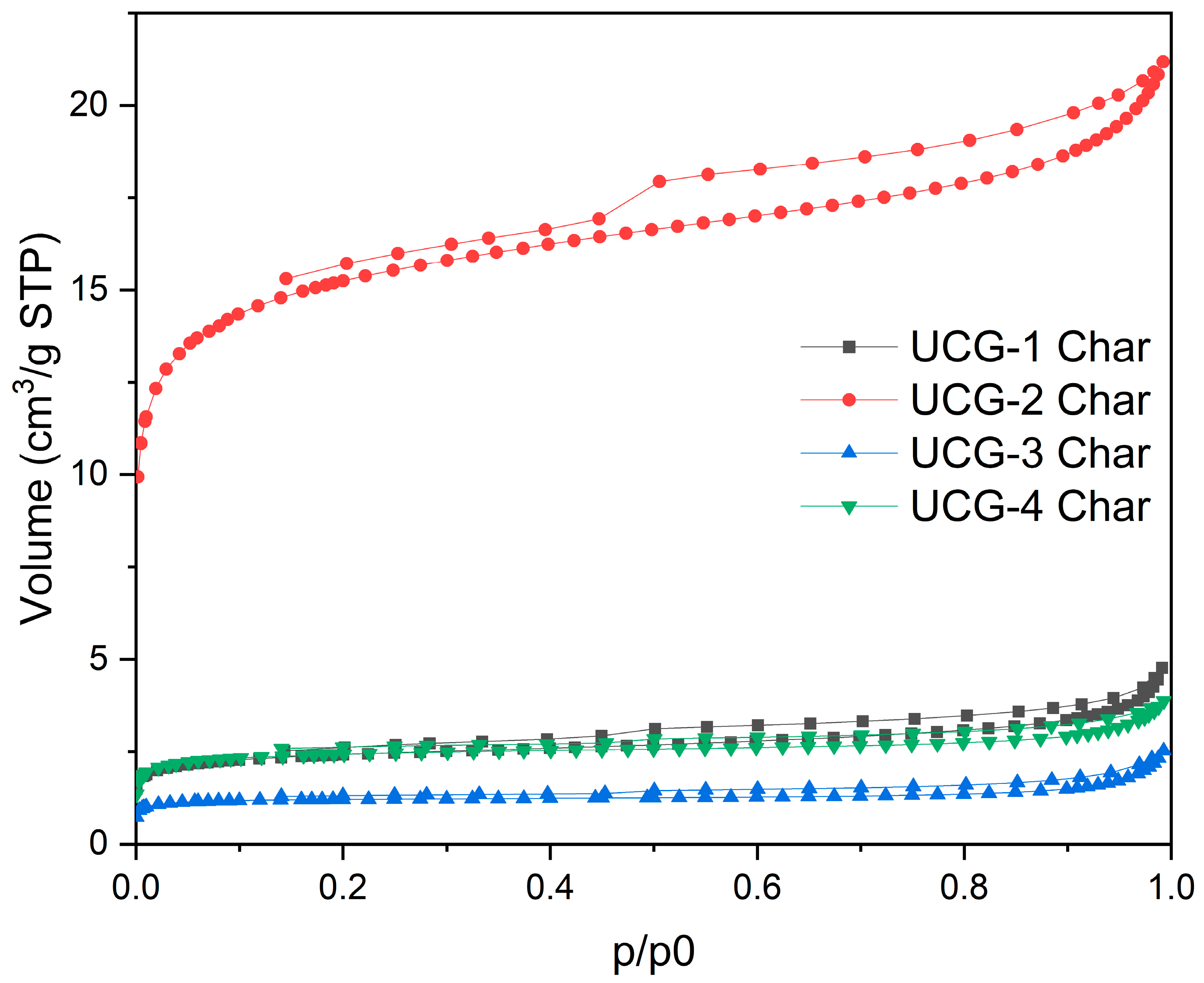
4.7. Textural Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olness, D.U.; Gregg, D.W. The Historical Development of Underground Coal Gasification; Technical Report UCRL-52283; California University, Lawrence Livermore Lab: Livermore, CA, USA, 1977. [Google Scholar]
- Klimenko, A.Y. Early Ideas in Underground Coal Gasification and Their Evolution. Energies 2009, 2, 456–476. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Bazmi, A.A.; Zahedi, G. Underground coal gasification: From fundamentals to applications. Prog. Energy Combust. Sci. 2013, 39, 189–214. [Google Scholar] [CrossRef]
- Blinderman, M.S.; Anderson, B. Underground coal gasification for power generation: Efficiency and CO2-emissions. In Proceedings of the 12th International Conference on Coal Science, Cairns, Australia, 2–6 November 2003; p. 13. [Google Scholar]
- Green, M. Recent developments and current position of underground coal gasification. Proc. Inst. Mech. Eng. Part A J. Power Energy. 2018, 232, 39–46. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, B.; Hou, Y.; Duan, T.-H.; Yang, L.; Wang, Y. Comparative environmental benefits of power generation from underground and surface coal gasification with carbon capture and storage. J. Clean. Prod. 2021, 310, 127383. [Google Scholar] [CrossRef]
- Kostúr, K.; Laciak, M.; Durdan, M. Some Influences of Underground Coal Gasification on the Environment. Sustainability 2018, 10, 1512. [Google Scholar] [CrossRef]
- Mokhahlane, L.S.; Wagner, N.J.; Vermeulen, D. Evidence of coal conversion in the Majuba underground coal gasification pilot plant, South Africa. Coal Combust. Gasif. J. 2022. [Google Scholar] [CrossRef]
- Perkins, G. Underground coal gasification—Part I: Field demonstrations and process performance. Prog. Energy Combust. Sci. 2018, 67, 158–187. [Google Scholar] [CrossRef]
- Perkins, G. Underground coal gasification—Part II: Fundamental phenomena and modeling. Prog. Energy Combust. Sci. 2018, 67, 234–274. [Google Scholar] [CrossRef]
- Pershad, S.; Pistorius, J.; van der Riet, M. Majuba underground coal gasification project. In Underground Coal Gasification and Combustion; Blinderman, M.S., Klimenko, A.Y., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 469–502. [Google Scholar]
- Takyi, S.A.; Zhang, Y.; Si, M.; Zeng, F.; Li, Y.; Tontiwachwuthikul, P. Current status and technology development in implementing low carbon emission energy on underground coal gasification (UCG). Front. Energy Res. 2023, 10, 1051417. [Google Scholar] [CrossRef]
- Sripada, P.; Khan, M.M.; Ramasamy, S.; Trivedi, J.; Gupta, R. Influence of coal properties on the CO2 adsorption capacity of coal gasification residues. Energy Sci. Eng. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Sarhosis, V.; Yang, D.; Sheng, Y.; Kempka, T. Coupled hydro-thermal analysis of underground coal gasification reactor cool down for subsequent CO2 storage. Energy Procedia 2013, 40, 428–436. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Z.; Ali, S.M.F. Feasibility of carbon dioxide storage in post-burn underground coal gasification cavities. Appl. Energy 2019, 252, 113479. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Z.; Ali, S.M.F.; Zhang, J.; Chen, Y.; Chen, S. Storing carbon dioxide in deep unmineable coal seams for centuries following underground coal gasification. J. Clean. Prod. 2022, 378, 134565. [Google Scholar] [CrossRef]
- Kapusta, K.; Wiatowski, M.; Thomas, H.R.; Zagorščak, R.; Sadasivam, S.; Masum, S.; Kempka, T.; Otto, C.; Basa, W.; Szyja, M.; et al. Experimental simulations of methane-oriented underground coal gasification using hydrogen–The effect of coal rank and gasification pressure on the hydrogasification process. Int. J. Hydrog. Energy 2023, 48, 921–932. [Google Scholar] [CrossRef]
- Kumari, G.; Vairakannu, P. Laboratory scale studies on CO2 oxy-fuel combustion in the context of underground coal gasification. J. CO2 Util. 2017, 21, 177–190. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, H.; Chen, Y.; Zhao, T. Coal char characteristics variation in the gasification process and its influencing factors. Energy Explor. Exploit. 2020, 38, 1–15. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, D.; Li, Y.; Tian, H.; Liang, J. Pyrolysis characteristics of large-scale bituminous coal. J. Anal. Appl. Pyrolysis 2021, 158, 105060. [Google Scholar] [CrossRef]
- Xi, J.; Linag, J.; Sheng, X.; Shi, L.; Li, S. Characteristics of lump lignite pyrolysis and the influence of temperature on lignite swelling in underground coal gasification. J. Anal. Appl. Pyrolysis 2016, 117, 228–235. [Google Scholar] [CrossRef]
- Wiatowski, M.; Kapusta, K.; Nowak, J.; Szyja, M.; Basa, W. An exsitu underground coal gasification experiment with a siderite interlayer: Course of the process, production gas, temperatures and energy efficiency. Int. J. Coal Sci. Technol. 2021, 8, 1447–1460. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Z.; Wang, J.; Liang, L.; Tu, C.; Zhang, Q.; Huang, W. Study on pyrolysis behavior of the coal fractions based on macro maceral separation. Fuel 2021, 305, 121572. [Google Scholar] [CrossRef]
- Yu, G.; Fan, X.; Liang, P.; Zhao, G.M.; Hu, X. Online characterization of pyrolysis products and kinetics study for the pyrolysis of a coal. J. Anal. Appl. Pyrolysis 2021, 160, 105376. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Z.; Sun, F.; Xu, H.; Wu, J.; Fu, F. Pyrolysis characteristics and microstructure evolution of different coal types. Energy Explor. Exploit. 2023, 41, 154–169. [Google Scholar] [CrossRef]
- PN-G-04560:1998 Standard; Paliwa Stałe—Oznaczanie Zawartości Wilgoci, Części Lotnych Oraz Popiołu Analizatorem Automatycznym. Solid Fuels—Determination of Moisture, Volatile Matter and Ash by Automatic Analyzer. Polish Committee for Standardization: Warsaw, Poland, 1998. (In Polish)
- PN-G-04516:1998 Standard; Paliwa Stałe—Oznaczanie Zawartości Części Lotnych Metodą Wagową. Solid Fuels—Gravimetric Determination of Volatile Matter. Polish Committee for Standardization: Warsaw, Poland, 1998. (In Polish)
- PN-G-04584:2001 Standard; Paliwa Stałe—Oznaczanie Zawartości Siarki Całkowitej i Popiołowej Automatycznymi Analizatorami. Solid Fuels—Determination of Total and Ash Sulfur Content with Automatic Analyzers. Polish Committee for Standardization: Warsaw, Poland, 2001. (In Polish)
- PN-G-04513:1981 Standard; Paliwa Stałe—Oznaczanie Ciepła Spalania i Obliczanie Wartości Opałowej. Solid Fuels—Determination of the Calorific Value and Calorific Value Calculation. Polish Committee for Standardization: Warsaw, Poland, 1991. (In Polish)
- PN-ISO 7404-5:2002 Standard; Metody Analizy Petrograficznej Węgla Kamiennego (Bitumicznego) i Antracytu—Część 5: Metoda Mikroskopowa Oznaczania Refleksyjności Witrynitu. Methods of Petrographic Analysis of Bituminous Coal and Anthracite—Part 5: Microscopic Method for Determination of Vitrinite Reflectivity. Polish Committee for Standardization: Warsaw, Poland, 2002. (In Polish)
- PN-G-04571:1998 Standard; Paliwa Stałe—Oznaczanie Zawartości Węgla, Wodoru i Azotu Automatycznymi Analizatorami—Metoda Makro. Solid Fuels—Determination of Carbon, Hydrogen and Nitrogen Content with Automatic Analyzers—Macro Method. Polish Committee for Standardization: Warsaw, Poland, 1998. (In Polish)
- International Classification of In-Seam Coals. Available online: https://unece.org/fileadmin/DAM/energy/se/pdfs/coal/1998_International_Classification_of_In-Seam_Coals_-January_1998.pdf (accessed on 22 May 2023).
- Róg, L. Vitrinite reflectance as a measure of the range of influence of the temperature of a georeactor on rock mass during underground coal gasification. Fuel 2018, 224, 94–100. [Google Scholar] [CrossRef]
- Lett, R.G.; Ruppel, T.C. Coal, Chemical and Physical Properties in Encyclopedia of Energy; Elsevier: Amsterdam, The Netherlands, 2004; pp. 411–423. [Google Scholar]
- PN-G-04511:1980 Standard; Paliwa Stałe—Oznaczanie Zawartości Wilgoci. Solid Fuels—Determination of Moisture Content. Polish Committee for Standardization: Warsaw, Poland, 1980. (In Polish)
- PN-ISO 18283:2006+PN-ISO 18283:2006/AC1:2009 Standard; Hard Coal and Coke—Manual Sampling. Polish Committee for Standardization: Warsaw, Poland, 2006.
- UNE 32111:1995 Standard; Hard Coal and Anthracite. Determination of Ash Content with Automatic Programable Furnances. UNE: Madrid Spain, 1995.
- ISO 589:2008 Standard; Hard Coal—Determination of Total Moisture. ISO: Geneva, Switzerland, 2008.
- Kardaś, D. From biomass towards syngas. Trans. Inst. Fluid-Flow Mach. 2015, 127, 63–89. [Google Scholar]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Dai, H.; Zhao, H.; Chen, S.; Jiang, B. A Microwave-Assisted Boudouard Reaction: A Highly Effective Reduction of the Greenhouse Gas CO2 to Useful CO Feedstock with Semi-Coke. Molecules 2021, 26, 1507. [Google Scholar] [CrossRef]
- Sadasivam, S.; Zagorščak, R.; Thomas, H.R.; Kapusta, K.; Stańczyk, K. Experimental study of methane-oriented gasification of semi-anthracite and bituminous coals using oxygen and steam in the context of underground coal gasification (UCG): Effects of pressure, temperature, gasification reactant supply rates and coal rank. Fuel 2020, 268, 117330. [Google Scholar] [CrossRef]
- Kapusta, K.; Wiatowski, M.; Stańczyk, K.; Zagorščak, R.; Thomas, H.R. Large-scale Experimental Investigations to Evaluate the Feasibility of Producing Methane-Rich Gas (SNG) through Underground Coal Gasification Process. Effect of Coal Rank and Gasification. Energies 2020, 13, 1334. [Google Scholar] [CrossRef]
- Otto, C.; Steding, S.; Tranter, M.; Gorka, T.; Hámor-Vidó, M.; Basa, W.; Kapusta, K.; Kalmár, I.; Kempka, T. Numerical Analysis of Potential Contaminant Migration from Abandoned In Situ Coal Conversion Reactors. Adv. Geosci. 2022, 58, 55–66. [Google Scholar] [CrossRef]
- Otto, C.; Kempka, T. Synthesis gas composition prediction for underground coal gasification using a thermochemical equilibrium modeling approach. Energies 2020, 13, 1171. [Google Scholar] [CrossRef]
- Makarova, A.A.; Kaliberda, I.V.; Kovalev, D.A.; Pershin, I.M. Modeling a Production Well Flow Control System Using the Example of the Verkhneberezovskaya Area. In Proceedings of the Conference of Russian Young Researchers in Electrical and Electronic Engineering (ElConRus), Saint Petersburg, Russia, 25–28 January 2022; pp. 760–764. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier Rodriguez-Reinoso, J.P.F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Usefulness of CO2 adsorption at 273 K for the characterization of porous carbons. Carbon N. Y. 2004, 42, 1231–1236. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]



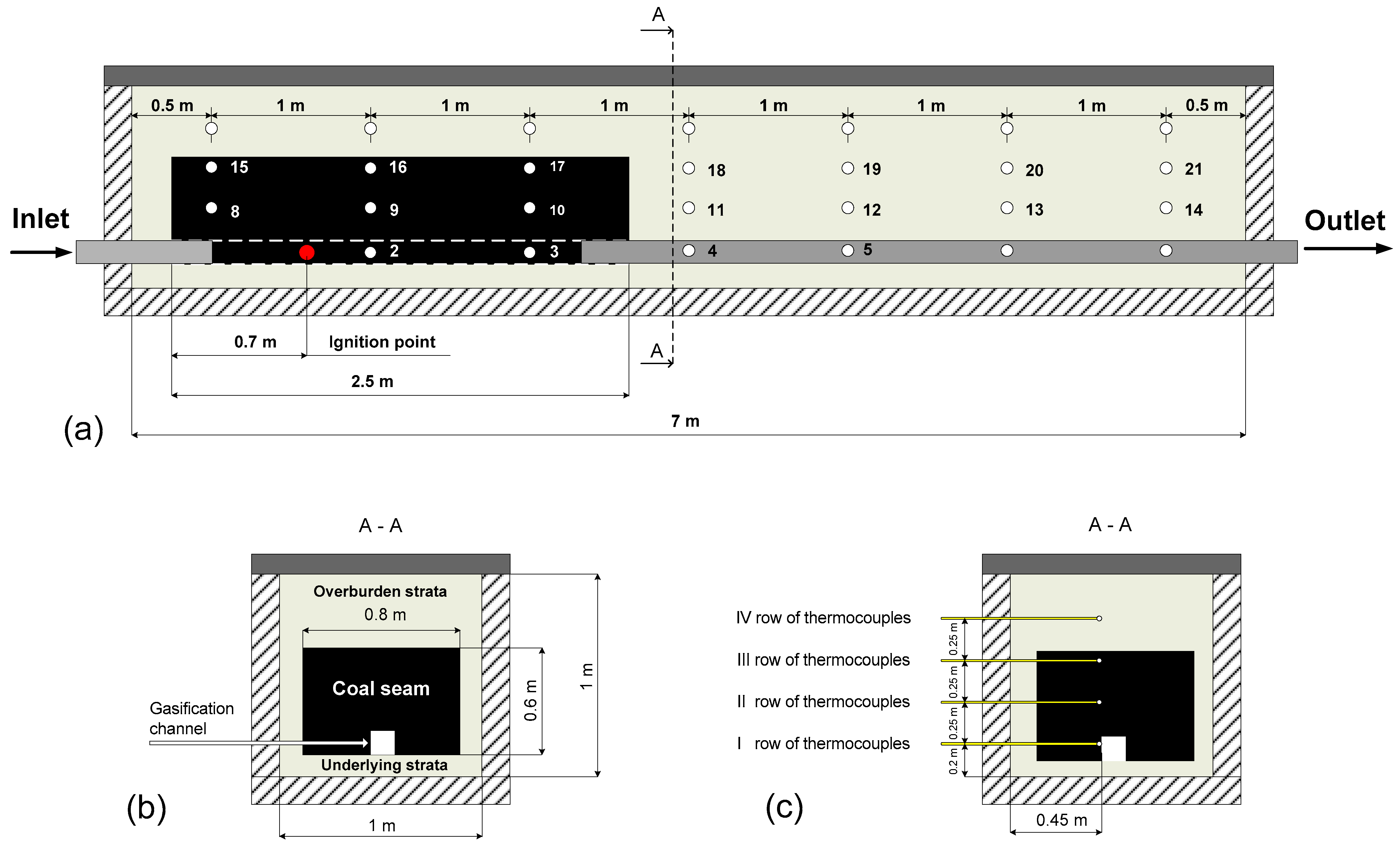







| Parameter | Coal | Method | Reference Document | |
|---|---|---|---|---|
| “Piast-Ziemowit” | “Wesoła” | |||
| As received | ||||
| Total moisture Wtr (%) | 8.50 | 3.73 | Thermogravimetric | PN-G-04560:1998 [26] |
| Ash Atr (%) | 7.56 | 2.14 | Thermogravimetric | PN-G-04560:1998 [26] |
| Volatile matter content Vra (%) | 30.15 | 30.40 | Gravimetric | PN-G-04516:1998 [27] |
| Total sulphur Str (%) | 0.98 | 0.21 | High temperature combustion with IR detection | PN-G-04584:2001 [28] |
| Calorific value Qr (kJ/kg) | 25,786 | 31,458 | Calculations | PN-G-04513:1981 [29] |
| Analytical | ||||
| Moisture Wa (%) | 7.47 | 3.49 | Thermogravimetric | PN-G-04560:1998 [26] |
| Ash Aa (%) | 7.64 | 2.15 | Thermogravimetric | PN-G-04560:1998 [26] |
| Volatile matter content Va (%) | 30.49 | 30.12 | Gravimetric | PN-G-04516:1998 [27] |
| Reflectance of vitrinite R (%) | 0.57 | 0.83 | Microscopic | PN-ISO 7404-5:2002 [30] |
| Lower heating value Qa (kJ/kg) | 26,103 | 31,543 | Calorimetric | PN-G-04513:1981 [29] |
| Total sulphur Sta (%) | 0.99 | 0.21 | High temperature combustion with IR detection | PN-G-04584:2001 28] |
| Carbon Ca (%) | 68.62 | 82.01 | High temperature combustion with IR detection | PN-G-04571:1998 [31] |
| Hydrogen Ha (%) | 4.30 | 5.18 | High temperature combustion with IR detection | PN-G-04571:1998 [31] |
| Nitrogen Na (%) | 1.08 | 2.24 | High temperature combustion with thermal conductivity detection | PN-G-04571:1998 [31] |
| Oxygen Oa (%) | 10.20 | 4.83 | Oxygen calculated as: | |
| Measured Value | Control Method |
|---|---|
| Gas temperature inside the reactor | Thermocouple Pt10Rh-Pt |
| Temperature at the reactor inlet, outlet and scrubber | Resistance sensor Pt100 |
| Pressure | WIKA digital transmitter IS-20-S |
| Flow of process gas | ELSTER bellows gas meter BK-G10M |
| Composition of process gases | Agilent 3000 A gas chromatograph |
| Flow of oxygen | Bronkhorst EL-FLOW mass flow controller, model F-202AV-M20-RAD |
| Experiment Number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Coal origin | “Piast-Ziemowit” mine (Poland) | “Wesoła” mine (Poland) | ||
| Coal type | Bituminous | Bituminous | ||
| Gasifying agent | OEA | Oxygen | OEA | Oxygen |
| Installation pressure | Ambient | Ambient | Ambient | Ambient |
| Coal block dimensions (m) | 0.6 × 0.8 × 2.5 | 0.5 × 0.7 × 2.0 | 0.5 × 0.7 × 2.0 | 0.6 × 0.8 × 2.5 |
| Mass of coal inside the reactor (kg) | 1225 | 687 | 830 | 1365 |
| Experiment duration (h) | 56 | 72 | 72 | 72 |
| Amount of coal gasified (kg) | 140.9 | 323.94 | 165.3 | 292.1 |
| Gasification rate (kg/h) | 2.52 | 4.50 | 2.29 | 4.06 |
| Wastewater produced * (kg) | 234 (1018) | 364 (1372) | 189 (1197) | 261 (1269) |
| Wastewater production rate (kg/h) | 4.18 | 5.01 | 2.63 | 3.63 |
| Wastewater outflow (kg/kg gasified coal) | 1.66 | 1.12 | 1.14 | 0.89 |
| Moisture content in the sand layer after UCG process (%) | 7.0 | 4.2 | 6.5 | 5.7 |
| Exp. No. | Gasification Reagent | Process Gas Yield (Nm3) | Average Gas Production Rate (Nm3/h) | Concentration (vol, %) | Average Calorific Value (MJ/Nm3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | H2 | CH4 | CO | C2H6 | H2S | N2 | |||||
| 1 | OEA | 487.0 | 8.69 | 27.94 | 7.11 | 2.12 | 6.42 | 0.09 | 0.07 | 56.25 | 2.25 |
| 2 | Oxygen | 556.3 | 7.73 | 38.75 | 25.47 | 2.98 | 31.81 | 0.11 | 0.25 | 0.63 | 7.96 |
| 3 | OEA | 567.7 | 7.86 | 30.87 | 9.50 | 2.57 | 10.72 | 0.13 | 0.27 | 45.94 | 3.44 |
| 4 | Oxygen | 584.9 | 8.12 | 36.10 | 23.24 | 6.17 | 33.41 | 0.27 | 0.13 | 0.68 | 9.14 |
| Exp. No. | Gas Yield (Nm3) | Average Gas Production Rate (Nm3/h) | Energy in Gas (MJ) | Amount of Coal Gasified (kg) | Gasification Rate (kg/h) | Energy of Consumed Coal (MJ) | Energy Efficiency (%) |
|---|---|---|---|---|---|---|---|
| 1 | 486.95 | 8.69 | 1095.6 | 140.9 | 2.52 | 3634.3 | 30.1 |
| 2 | 556.26 | 7.73 | 4427.8 | 323.9 | 4.50 | 8353.0 | 53.0 |
| 3 | 567.7 | 7.86 | 1925.9 | 165.3 | 2.29 | 5198.9 | 37.6 |
| 4 | 584.9 | 8.12 | 5348.0 | 292.1 | 4.06 | 9190.3 | 58.2 |
| Parameter (Unit) | Sample | |||
|---|---|---|---|---|
| UCG-1 Char | UCG-2 Char | UCG-3 Char | UCG-4 Char | |
| Elemental analysis | ||||
| Moisture (wt.%) | 3.29 | 3.88 | 2.51 | 0.80 |
| Ash (wt.%) | 12.33 | 21.32 | 4.62 | 6.24 |
| C (wt.%) | 83.58 | 76.41 | 92.59 | 90.81 |
| H (wt.%) | 1.09 | 0.24 | 1.20 | 0.77 |
| N (wt.%) | 1.30 | 0.73 | 1.44 | 1.42 |
| S (wt.%) | 1.25 | 2.06 | 0.21 | 0.21 |
| ICP-MS analysis | ||||
| Na (%) | 2.8 | 3.0 | 1.0 | 2.9 |
| Mg (%) | 1.7 | 0.7 | 9.4 | 7.6 |
| Al (%) | 17.0 | 12.5 | 3.4 | 8.6 |
| K (%) | 0.6 | 0.3 | 0.2 | 0.9 |
| Ca (%) | 5.5 | 0.9 | 17.0 | 13.7 |
| V (ppm) | 327 | 388 | 57 | 69 |
| Cr (ppm) | 239 | 117 | 235 | 450 |
| Mn (ppm) | 1317 | 243 | - | - |
| Mn (%) | - | - | 0.3 | 0.2 |
| Fe (%) | 3.7 | 2.5 | 12.0 | 11.2 |
| Cu (ppm) | 587 | 192 | 475 | 562 |
| Zn (ppm) | 179 | 133 | 450 | 494 |
| As (ppm) | 53 | 12 | 11 | 11.9 |
| P (%) | 1.2 | 0.0 | -- | -- |
| Ti (%) | 0.8 | 0.4 | 0.1 | 0.3 |
| Pb (ppm) | 101 | 84 | 26 | 102 |
| Sample | N2 Adsorption | CO2 Adsorption | ||
|---|---|---|---|---|
| VT (cm3/g) | SBET (m2/g) | VDR,N2 (cm3/g) | VDR,CO2 (cm3/g) | |
| UCG-1 Char | 0.01 | 9 | 0.00 | 0.11 |
| UCG-2 Char | 0.03 | 57 | 0.02 | 0.06 |
| UCG-3 Char | 0.00 | 5 | 0.00 | 0.04 |
| UCG-4 Char | 0.01 | 9 | 0.00 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiatowski, M.; Kapusta, K.; Strugała-Wilczek, A.; Stańczyk, K.; Castro-Muñiz, A.; Suárez-García, F.; Paredes, J.I. Large-Scale Experimental Simulations of In Situ Coal Gasification in Terms of Process Efficiency and Physicochemical Properties of Process By-Products. Energies 2023, 16, 4455. https://doi.org/10.3390/en16114455
Wiatowski M, Kapusta K, Strugała-Wilczek A, Stańczyk K, Castro-Muñiz A, Suárez-García F, Paredes JI. Large-Scale Experimental Simulations of In Situ Coal Gasification in Terms of Process Efficiency and Physicochemical Properties of Process By-Products. Energies. 2023; 16(11):4455. https://doi.org/10.3390/en16114455
Chicago/Turabian StyleWiatowski, Marian, Krzysztof Kapusta, Aleksandra Strugała-Wilczek, Krzysztof Stańczyk, Alberto Castro-Muñiz, Fabián Suárez-García, and Juan Ignacio Paredes. 2023. "Large-Scale Experimental Simulations of In Situ Coal Gasification in Terms of Process Efficiency and Physicochemical Properties of Process By-Products" Energies 16, no. 11: 4455. https://doi.org/10.3390/en16114455
APA StyleWiatowski, M., Kapusta, K., Strugała-Wilczek, A., Stańczyk, K., Castro-Muñiz, A., Suárez-García, F., & Paredes, J. I. (2023). Large-Scale Experimental Simulations of In Situ Coal Gasification in Terms of Process Efficiency and Physicochemical Properties of Process By-Products. Energies, 16(11), 4455. https://doi.org/10.3390/en16114455









