Abstract
CO2-assisted gasification of carbon-based waste materials is one of the processes that both utilises waste carbon chemicals and produces CO, which is a highly sought after raw material. In this work, we aimed at finding and clarifying the synergistic effects of simultaneous potassium and Fe, Co, or Ni-driven catalysis. To reveal the behaviour of such systems, a series with different potassium loadings and a fixed second metal loading of 5 wt.% was prepared. The following methods were applied for this purpose: SEM, TEM, EDX, XRD, electron diffraction, and catalytic tests. The most active sample was found to be 3 wt.% K and 5 wt.% Co- or Fe-loaded hydrolysis lignin. The attained CO2 conversion was up to 92%, while the pure lignin sample demonstrated only 62% conversion under the same conditions.
1. Introduction
Since the demand for fossil fuels has grown significantly in recent decades, the importance of the search for alternative fuel sources is clear. Currently, humankind relies mostly on fossil fuels, and because of the increasing human population, the demand for energy (and so for fuels) has grown [1]. Along with the energy source issue, the problem of environmental pollution, and particularly the problem of carbon waste utilisation, has arisen. This leads to a challenge in how to transform waste carbon materials into sources of valuable substances, or at least into not environmentally harmful ones. Otherwise, the problems of climate change (global warming) and other environmental pollution will be enhanced. CO2-assisted gasification of lignin, for example, provides an additional source of valuable carbon monoxide used in the large-scale syntheses of methanol, dimethyl ether, etc., and simultaneously utilises CO2 by converting it into carbon monoxide, methane or more complex products [2,3]. This application can be ecologically valuable. An additional advantage of using lignin is that its involvement in chemical transformations can partly solve the problem of its utilisation [4,5,6].
At the same time, biomass from different sources represent a renewable resource, usually of low cost, with the amounts of production, for example, reaching about 180 billion tonnes per year in the case of lignocellulosic biomass [1,7]. However, such a biomass is not uniform; it consists of cellulose, lignocellulose and lignin. The lignin content can reach up to 30% of the biomass depending on the plant from which it was obtained [8]. Moreover, lignin is not uniform itself; as a natural biopolymer its composition strongly depends on the nature of the raw materials, the conditions of growth (such as temperature, pressure, amount of sun, etc.) and the activity of enzymes regulating the biosynthesis [8]. Despite the attempts to regulate the biosynthesis of lignin from phenylalanine with enzymes by the methods of genetic engineering to unify the composition of this biopolymer [9], this process has not been translated to the industrial scale. So, the inhomogeneity of the raw material should be overcome by traditional methods, particularly by the search for highly active and selective catalysts sufficient for the conversion of inhomogeneous biomass.
The main research efforts are focused on the following groups of catalysts: alkali metals (mostly Na [10,11], especially K [12,13,14] and sometimes Li or Cs [11]), alkali-earth metals (mostly Ca) [15,16,17], transition metals (Ni, Co, Fe [18,19], Zn [20], etc. [21,22]), noble metals and polymetallic catalysts (for example, Na–Fe [23] or Fe–Ni [24] particles or particles deposited on carbon nanofibers [25]) [26,27]. In the case of catalysis in carbon-based material gasification using alkali and alkali-earth metal compounds, K and Na compounds are traditionally used, and the following order of decreasing activity is reported: K2CO3 > KOH > Na2CO3 > NaOH. This shows that potassium species are more preferable than sodium ones and carbonates are more preferable than hydroxides [28,29]. Notably, data on the activity of a particular species are sometimes contradictive and there are no clear relations between composition and catalytic activity. Different compositions and combinations can be found in the literature, e.g., Li/Na and Li/K systems [30] applied with different anions (in the case of potassium, the activity depends on the nature of the anion in the process of petroleum coke steam gasification as follows: CH3COO− > SO42− > CO32− > NO3− > Cl− [31]). Moreover, a comparison of the effects of potassium deposition on samples of lignin and the introduction of K deposited on γ-Al2O3 showed that direct deposition provides a higher lignin conversion during pyrolysis and gasification than in the case of the deposited sample [32]. This seems to result from the limited mobility of active sites in the sample; despite the volatility of alkali metal species being relatively high, the directly deposited metal does not have to be transported to the carbon surface as, for example, in [33]. So, despite attempts, detailed mechanisms of the processes taking place and clear correlations between the composition, structure and properties still need to be further investigated.
Despite the attempts to clarify the mechanism of the catalytic gasification of carbon-based waste materials, a firmly proven mechanism is still to be revealed. Alkali-metal catalysis is widely discussed in the literature; the scheme of the catalytic action of potassium carbonate in the reaction of steam gasification of charcoal was tentatively proposed in [34,35]. However, this kind of mechanism is criticized by other authors because of the thermodynamic instability of the atomic form of potassium at temperatures below 827 °C [36].
Transition metal oxide catalysts are also important [37], and the same problems of the correlation between the composition, structure and catalytic behaviour have arisen and attempts to solve them are being made. For example, it was shown by in situ characterisation that Ni-based samples in biomass gasification formed nanoparticles of zero-valent Ni and the time and temperature at which the process took place decreased [38]. When supported on ceria-zirconia mixed oxides, Ni-based catalysts also have a high catalytic activity in tar removal [39]. Among Fe, Co and Ni, iron catalysts are the least active and their lifetime is the shortest, proposedly resulting from the formation of Fe3O4 species which have much lower activity [21,40]. It could also be the result of coke deposition according to [19]. It was reported that the processes of co-gasification of lignin and cellulose over Ni catalysts can be rather interesting [41,42].
Polymetallic catalysts have good prospects because they provide a controllable modification of the properties of the catalysts and synergetic effects between different sites are possible. For example, it was shown that the iron species in an iron–sodium catalyst can suppress the growth of Na2CO3 crystals and their evaporation at high temperatures, while sodium presence favours the reduction of hematite into α-Fe [43]. Nevertheless, further in-depth investigations of such a synergism of transition metal–alkali metal catalysts are still to be performed.
Despite the complexity of the process of pyrolysis, it can be separated into several stages. The first one is drying of the biomass, resulting in dry mass and water vapour. This process takes place at temperatures below 200 °C. The second one is pyrolysis, producing such products as carbon monoxide, carbon dioxide, light hydrocarbons, tars and carbon residue. This process occurs at temperatures between 200 and 450 °C. The temperature limits are relatively large and may be shifted depending on the atmosphere applied and on the nature of the materials [44,45,46]. In the case of carbon-dioxide-assisted gasification, the gasification itself starts at temperatures above 450 °C and includes the chemical transformations detailed in [47,48,49].
In this work, we decided to focus on the catalytic conversion of hydrolysis lignin as a carbon waste material at temperatures above 450 °C, omitting the investigation of the pyrolysis and tar production processes because these processes have been relatively widely investigated previously by different authors. This article is aimed at the preparation and the physico-chemical investigation of the series of materials based on hydrolysis lignin and Fe, Co, Ni or K species while varying the amounts of alkali or transition metal in the samples in order to try to find the potential synergism of their catalytic properties.
2. Materials and Methods
2.1. Materials
The following reagents were used in this work: Fe(NO3)3·9H2O (99%), Co(NO3)2·6H2O (99%), Ni(NO3)2·6H2O (98%) and KNO3 (99%) from Acros Organics (Antwerp, Belgium), and hydrolysis lignin and bidistilled water. All the reagents were used as purchased without further purification. The hydrolysis lignin originated from the residues from the furfural pilot industrial plant from the wood of oak and elm according to the technical conditions BY 490822905.001-2015. In brief, the hydrolysis of cellulose in the original raw material was conducted using 2% H2SO4 at temperatures of 110–140 °C. The ash content was declared to be 10.1 wt.%.
2.2. Methods
The elemental composition of the samples of starting hydrolysis lignin was investigated with a Perkin Elmer 2400 Series II CHNOS analyser (Waltham, MA, USA). The sample weight was 50 mg. The results of the elemental analysis can be found in our previous work [50].
All the materials were examined by SEM with EDX using a Leo Supra 50VP scanning electron microscope under a low vacuum in a nitrogen atmosphere. EDX data were collected using an energy-dispersive spectrometer INCA Energy (Oxford Instruments, X-Max-80, Abingdon, UK).
Transmission electron microscopy studies of the materials were performed using the JEM-2100 JEOL transmission electron microscope (Tokyo, Japan).
Powder XRD diffraction patterns were collected with a STOE STADI P transmission diffractometer using CuKα1 radiation (λ = 1.54056 Å) monochromatized with a curved germanium (111) monochromator. The samples were examined in the region 2θ = 10–60°, with a step scan of 0.01° and a 10 s counting time per point. Before the examination, the samples were heated at 300 °C for one hour in a flow of CO2 of 30 mL per minute.
The evaluation of the activities of the resulting materials of the gasification process was performed using a quartz flow-type reactor with an internal diameter of 8 mm under a CO2 pressure of 1 atm. The temperature ramp was 10 °C per minute, the temperature range was 100–850 °C and the total flow rate of CO2 was 30 mL per minute. A Bronkhorst EL-FLOW SELECT F-111B gas flow controller was used to vary the gas flow rate. The material loading was 1 g and the particle size was 0.25–0.5 mm. The gas reaction products were analysed using a Chromatek Crystal 5000 gas chromatograph with thermal conductivity detectors, Mss 3163 m × 2 mm columns, a Hayesep Q 80/100 mesh and CaA molecular sieves. The setup used in this work is presented in Figure 1.
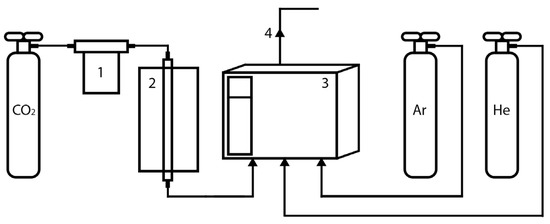
Figure 1.
The setup scheme: (1) Bronkhorst EL-FLOW SELECT F-111B gas flow regulator, (2) furnace with a quartz reactor, (3) gas chromatograph, (4) outlet.
The conversion (X) of carbon dioxide during the tests was calculated by the following formula (Equation (1)):
2.3. Synthetic Procedure
All the samples were prepared as follows: nitrates of Fe, Co or Ni were completely dissolved simultaneously with potassium nitrate in the appropriate amount of water. The amount of hydrolysis lignin (5 g) was impregnated with the appropriate salt solution amount to obtain the desired K and second metal content. The samples were dried at 50 °C for 6 h in an oven. Thus, a series of 16 samples was prepared: the samples with only transition metal loading of 5 wt.% (designated as FeL, CoL and NiL), samples with only potassium loading (0.5KL, 3KL and 5KL, where the digit indicates the wt.% of K added) and mixed samples (0.5K-FeL, 0.5K-CoL, 0.5K-NiL, 1K-FeL, 1K-CoL, 1K-NiL, 3K-FeL, 3K-CoL and 3K-NiL, where the non-alkali metal loading was 5 wt.%). The letter L in the names of the samples means lignin.
Pure lignin was used as a reference sample in the catalytic evaluation of CO2-assisted gasification. Since the focus of this article was on the high-temperature processes in lignin CO2-assisted gasification, we additionally heated the samples of the fresh materials at 300 °C in a CO2 flow of 30 mL per minute for 1 h to get close the investigated materials with the samples that really underwent the catalytic tests in the designed temperature range.
3. Results and Discussion
3.1. SEM with EDX Characterisation
All the samples of lignin with deposited active phases were investigated by SEM with EDX (Figure 2, Figure 3, Figure 4 and Figure 5). Figure 2 shows the distribution of potassium on the surface of lignin. The sample 0.5K-L contains the smallest amount of local potassium agglomerates, the sample 1K-L demonstrates larger amounts of such agglomerates and the sample 3K-L contains many separate areas with a significantly larger potassium concentration. Additionally, it can be noticed that all the samples demonstrate relatively large silica particles with a size of up to 8 nm.
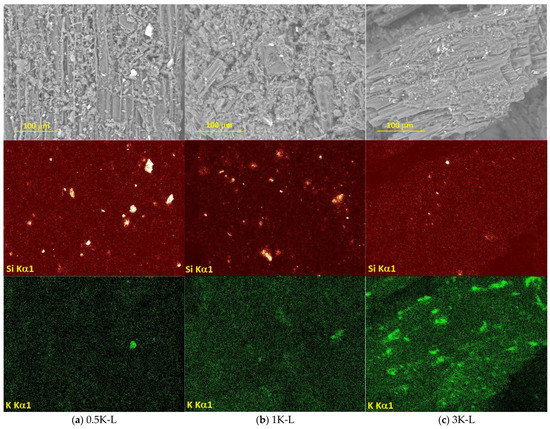
Figure 2.
SEM images of the samples containing (a) 0.5, (b) 1 and (c) 3 wt.% K deposited on the lignin surface and the element distribution maps.

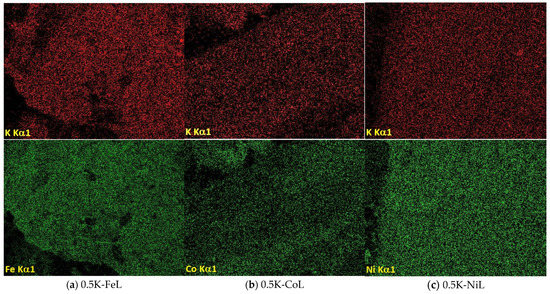
Figure 3.
SEM images of the samples containing 0.5 wt.% K and (a) Fe, (b) Co or (c) Ni, and the element distribution maps.
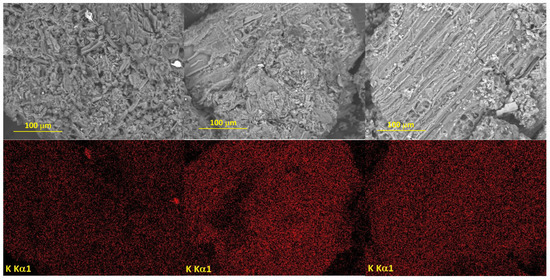
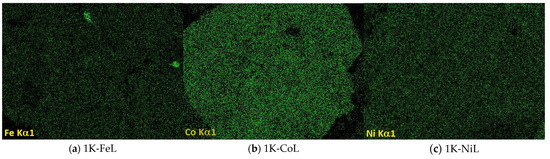
Figure 4.
SEM images of the samples containing 1 wt.% K and (a) Fe, (b) Co or (c) Ni, and the element distribution maps.
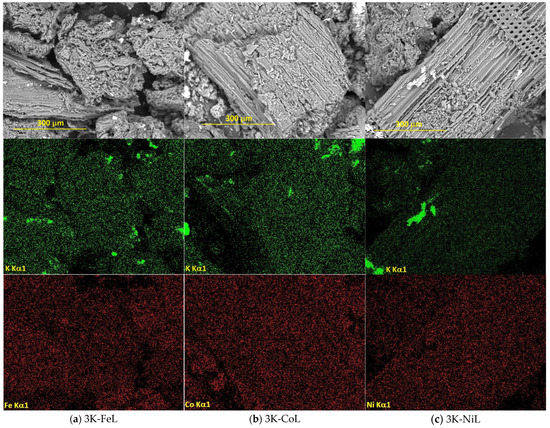
Figure 5.
SEM images of the samples containing 3 wt.% K and (a) Fe, (b) Co or (c) Ni, and the element distribution maps.
3.2. TEM Characterisation
The TEM characterisation (Figure 6) demonstrates that the samples of metal-loaded lignins before the catalytic tests contain particles with different average sizes; observable particles were found in the 3K-NiL sample, while the 3K-FeL and 3K-CoL samples showed no such observable particles, so very small sizes can be tentatively anticipated. After the catalytic tests, the 3K-NiL sample showed nearly no growth in particle size, while in the case of the 3K-CoL sample, large metal particles (up to 100 nm) appeared.
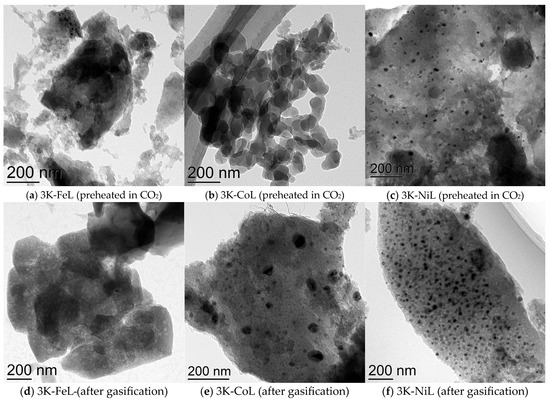
Figure 6.
TEM images of the samples (a–c) heated at 300 °C in a CO2 flow; (d–f) after the catalytic CO2-assisted gasification.
The formation of particles of Ni before heating in CO2 can be tentatively attributed to the worse affinity of this metal to the surface of the carbon material. A similar situation took place in our previous article [51]. The samples after the catalytic tests exhibit observable particles. The tentative hypothesis of the mechanism of the process refers to the formation of nanoparticles of carbides on the carbon materials: the diffusion of carbon through the particles of metal carbide is driven by the gradient of the carbon concentration in the sample. This promotes the reaction between carbon and a CO2 molecule on the surface of carbide, resulting in the formation of two CO molecules [52,53,54,55]. The start of the formation of large Ni particles can be seen in Figure 7.
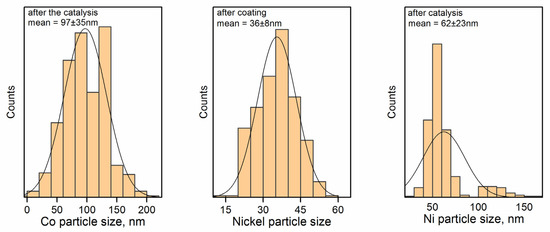
Figure 7.
Particle size distributions for the samples before and after the catalytic tests.
3.3. XRD and Electron Diffraction Characterisation
XRD characterisation was performed for the samples before (Figure 8) the catalytic tests of CO2-assisted gasification. In XRD the patterns, the samples after heating in a CO2 flow at 300 °C demonstrate only the reflexes of the potassium nitrite phase and the silica phase, along with a halo at 20–25° corresponding to an amorphous silica. The presence of the reflexes of the KNO2 phase and the absence of the reflexes of KNO3 indicate that the nitrate anion, being a strong oxidant, was reduced by the lignin components during pre-heating. Additionally, the thermal decomposition reaction of KNO3 may take place under these conditions. It can be noticed that the intensities of the reflexes of KNO2 decrease in the following order: 3K-NiL > 3K-FeL >> 3K-CoL. The 3K-CoL sample has such weak reflexes of the KNO2 phase that they cannot be clearly identified. In the case of the 3K-CoL sample, the electron diffraction pattern (Figure 9) shows the presence of a CoO phase that is not seen by XRD.
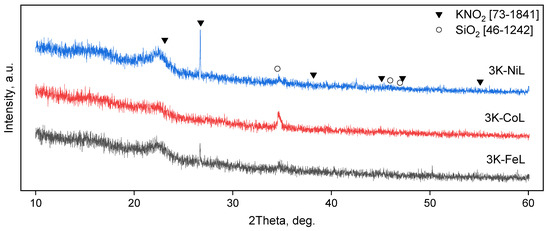
Figure 8.
XRD patterns of the samples after treatment at 300 °C in a CO2 flow.
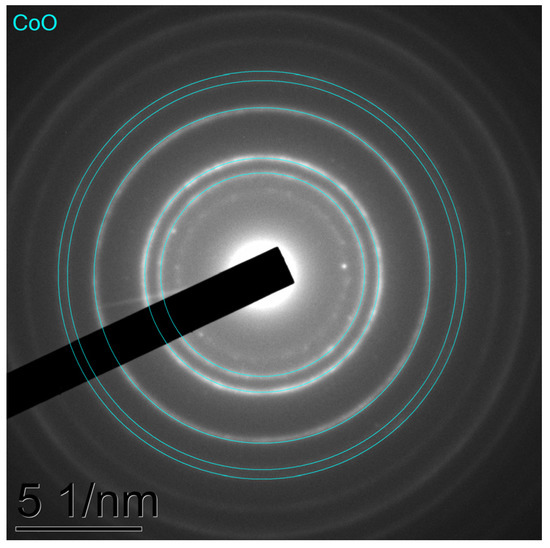
Figure 9.
Electron diffraction patterns obtained via TEM examinations of the 3K-CoL sample after the heating at 300 °C in a CO2 flow.
The samples after catalytic gasification were examined by XRD (Figure 10). It can be easily seen that the 3K-CoL and 3K-NiL samples demonstrate a relatively simple phase composition; only the reflexes of metallic Co or Ni can be seen, along with cristobalite and an amorphous halo from amorphous silica. At the same time, the phase composition of the 3K-FeL sample is much more complex; silica is present in the forms of cristobalite, a-quartz and an amorphous silica phase. Iron species consist of magnetite (Fe3O4) and Fe2O3 according to the diffraction patterns [56], in addition to the Fe2C phase. Its formation can be explained by the presence of large amounts of carbon in the sample. The formation of carbides for iron catalysts is also reported in different works, for example, [57]. Since the cristobalite phase is stable at temperatures above 1470 °C according to the phase diagram for SiO2 [58], it is proposed that the local temperature during gasification may be high enough to let this phase form. Additionally, the presence of the metallic states of cobalt and nickel in the samples after catalytic CO2-assisted gasification (3K-CoL and 3K-NiL) was proven by an electron diffraction analysis (Figure 11).
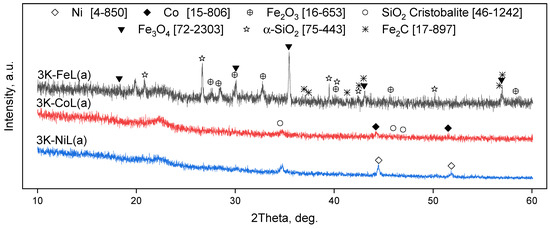
Figure 10.
XRD patterns of the samples after the catalytic tests of CO2-assisted gasification. The ICDD card numbers are indicated in the squared brackets.
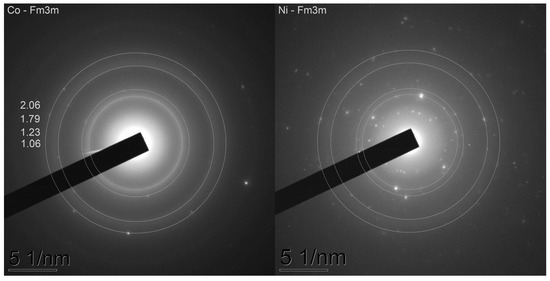
Figure 11.
Electron diffraction patterns obtained via TEM examinations of the samples after catalytic tests of CO2-assisted gasification.
3.4. Catalytic Tests
All the catalytic materials were tested in CO2-assisted gasification (Figure 12 and Figure 13). It can be easily seen that almost all the samples demonstrate activities higher than that for the blank test of just lignin without any additional salts. The exceptions are only the 0.5K-L and 1K-L samples. In the case of fixed potassium loading, a lower catalytic activity was seen for Ni-loaded samples, a higher activity was observed for Fe-loaded ones and the most active samples were Co-loaded catalytic materials. Noticeably, the samples loaded with only transition metals demonstrated increases in CO2 conversion of 10 and 14% for Fe and Co, respectively. At the same time, when loaded with potassium nitrate, these metals demonstrated synergism and demonstrated a 30 and 29% CO2 conversion increase, respectively.
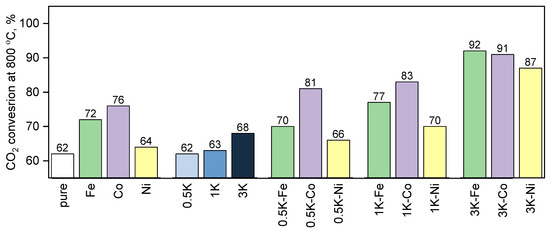
Figure 12.
The results of the catalytic tests of CO2-assisted gasification of hydrolysis lignin at 800 °C.
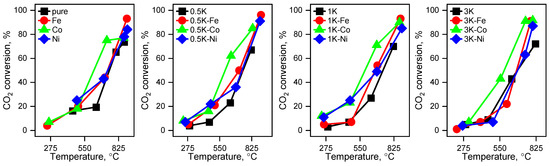
Figure 13.
The temperature dependencies for the catalytic materials in CO2-assisted gasification of hydrolysis lignin.
Even though in the case of potassium loading of 0.5 and 1 wt.%, the activities of the samples with Co were higher than those of the samples with Fe, in the case of 3 wt.% potassium loading, the inverse occurs. Nevertheless, the difference is small and can be interpreted as a value lower than an error of the experiment, which can be estimated as 8 rel.%. Iron-containing samples, demonstrating the lowest conversion level, when combined with 3 wt.% K demonstrate significant improvements in their activity. This may be attributed to a large potassium loading irrespective of the second component, but indeed, the control tests of the series prepared only with potassium as an active component did not prove this hypothesis and here clear synergism can be concluded.
Additionally, the integral values of the catalytic processes were estimated; the integration over the total carbon flows was performed taking into account the mass of carbon in the samples revealed by element analyses (Table 1).

Table 1.
The integral conversions of carbon atoms in hydrolysis lignin during the experiment. An estimation was performed in the temperature region of 500–800 °C.
The yields of CO were revealed both on the basis of CO2 (the efficiency of its transformation) (Figure 13) and on the basis of carbon from lignin (Figure 14) by the integration of material flows over the temperature range of 500–800 °C. As can be seen, the highest conversions took place for the samples loaded with Co (88% vs. 55–57% for the 3K-Co, 3K-Fe and 3K-Ni samples, respectively).
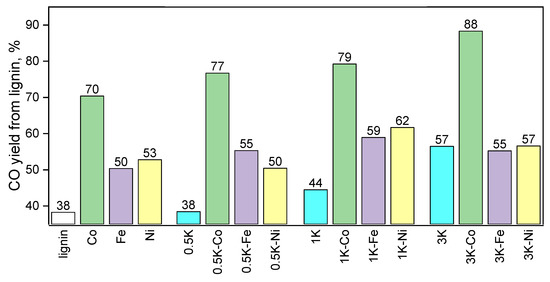
Figure 14.
The yields of CO (lignin basis) in CO2-assisted gasification of hydrolysis lignin.
Taking into account the results of the comparison of the conversions of CO2 at 800 °C, in the case of the samples with 3 wt.% K loading, the order of the samples by decreasing conversion is Fe > Co > Ni, and this dependence is not consistent with the results for the other two series (also at 800 °C). Nevertheless, the integral yields of the conversion (Figure 13 and Figure 14) are consistent with the results of the series with 0.5 and 1 wt.% K loading.
Additionally, the order of the samples with 3 wt.% potassium loading with decreasing conversion (Fe > Co > Ni) (Figure 13) is not consistent with the integral yields (Figure 14 and Figure 15). By the way, the estimates based on the instantaneous conversion at high temperatures (800 °C) are not ultimate because the biological raw material has a rather complex composition including different carbon sites, and these carbon atoms can be separated into easy-to-oxidize and difficult-to-oxidize sites. Since CO2 is a mild oxidizing agent, the difference in the ability to be oxidized may significantly influence the rate of carbon oxidation in CO2-assisted oxidation (gasification). As the carbon integral conversion for the 3K-Co sample is relatively high compared to the 3K-Fe and 3K-Ni samples, the fraction of carbon atoms that are capable of being oxidized at 800 °C in the case of the 3K-Co sample, as we believe, is the lowest compared to the other samples. Thus, this results in a lower instantaneous conversion of CO2 at this temperature. Additionally, this hypothesis can be proven indirectly; the TEM images show large particles in the case of the sample after the catalytic test because of the elimination of the carbon matrix preventing sintering in other samples. Additionally, it is interesting to compare our results with the literature data; the results obtained are in accordance with the results in [59] in that the modification of the surface with different agents leads to different possible surface properties. In [60], the potassium promoters in Co/ZSM-5 catalysts act as electron donors and facilitate the formation of a Co active phase, which is in accordance with our data.
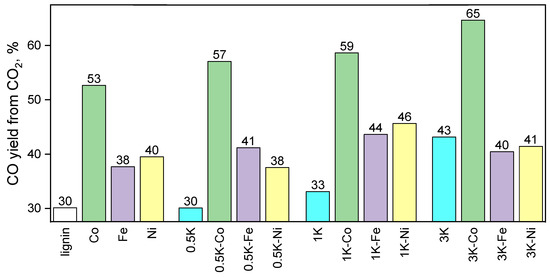
Figure 15.
The yields of CO (CO2 basis) in CO2-assisted gasification of hydrolysis lignin.
4. Conclusions
The investigation of catalytic materials based on hydrolysis lignin in the reaction of CO2-assisted gasification lead to the observation of a significant improvement in the CO2 conversion when a transition metal is deposited on the surface along with potassium, and the higher the potassium loading, the higher the conversion. SEM pictures (both photos and element maps) give no information on any significant difference between the samples; an appearance of “islands” with a high potassium concentration on the surface is predictable from the point of view of the high general concentration. TEM images indicated the following order of the samples regarding their particle size: 3K-CoL >> 3K-NiL > 3K-FeL (for residues from the catalytic tests). However, the Ni-loaded sample has the lowest catalytic activity, while the Co-loaded one exhibits the highest activity. This fact cannot be explained by the particle size, because the general dependence proposes the inverse order, i.e., the smaller the particles, the higher the conversion should be, but we pose a tentative hypothesis that the high activity of the Co-loaded sample is as a result of a high carbon conversion at lower temperatures. Since the design of the experiment was that the temperature increases continuously, possibly the amount of carbon in the reactor at high temperatures in the case of the Co-loaded material is significantly smaller than it is for the less active samples loaded with Ni and Fe. The possible explanation of such a catalytic behaviour can be proposed from XRD and electron diffraction data for the samples after the catalytic tests; the formation of metallic Co and Ni takes place, and the high catalytic activity tentatively could be attributed to these species. No metallic iron can be seen in such a case probably because of its instability under an air atmosphere and additionally because of the abnormal scattering of X-rays when a copper source is used.
We proposed that most of the carbon in the Co-based sample was converted at temperatures lower than 800 °C, while both the less active samples (3K-Fe and 3K-Ni) demonstrate a high instant conversion, indicating that the amount of reactive carbon is still high. The TEM images can provide indirect proof of this hypothesis: large particles in the case of the Co-loaded sample may be the result of significant carbon matrix decay.
Thus, 3 wt.% K and 5 wt.% Co is the optimal catalytic material for CO2-assisted gasification of hydrolysis lignin.
Author Contributions
Conceptualization, L.M.K. and A.L.K.; methodology, A.L.K.; validation, A.A.M., D.A.B. and P.V.S.; formal analysis, A.A.M. and P.V.S.; investigation, A.A.M. and D.A.B.; resources, S.B.P., V.E.P. and E.V.M.; writing—original draft preparation, A.A.M. and D.A.B.; writing—review and editing, A.L.K. and L.M.K.; supervision, L.M.K.; project administration, L.M.K.; funding acquisition, L.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research on the study of catalysts by physicochemical methods was funded by the Ministry of Science and Higher Education of the Russian Federation, project number 075-15-2021-591, and the research related to catalyst preparation and catalytic tests was carried out with financial support from the Russian Science Foundation, grant no. 20-73-10106. L. M. Kustov thanks the «Priority-2030» academic leadership selectivity program, project number K7-2022-062.
Data Availability Statement
Data are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Akbarian, A.; Andooz, A.; Kowsari, E.; Ramakrishna, S.; Asgari, S.; Cheshmeh, Z.A. Challenges and opportunities of lignocellulosic biomass gasification in the path of circular bioeconomy. Bioresour. Technol. 2022, 362, 127774. [Google Scholar] [CrossRef]
- Evdokimenko, N.D.; Kapustin, G.I.; Tkachenko, O.P.; Kalmykov, K.B.; Kustov, A.L. Zn Doping Effect on the Performance of Fe-Based Catalysts for the Hydrogenation of CO2 to Light Hydrocarbons. Molecules 2022, 27, 1065. [Google Scholar] [CrossRef] [PubMed]
- Evdokimenko, N.; Yermekova, Z.; Roslyakov, S.; Tkachenko, O.; Kapustin, G.; Bindiug, D.; Kustov, A.; Mukasyan, A.S. Sponge-like CoNi Catalysts Synthesized by Combustion of Reactive Solutions: Stability and Performance for CO2 Hydrogenation. Materials 2022, 15, 5129. [Google Scholar] [CrossRef] [PubMed]
- Leybo, D.; Firestein, K.L.; Evdokimenko, N.D.; Ryzhova, A.A.; Baidyshev, V.S.; Chepkasov, I.V.; Popov, Z.I.; Kustov, A.L.; Konopatsky, A.S.; Golberg, D.V.; et al. Ball-Milled Processed, Selective Fe/h-BN Nanocatalysts for CO2 Hydrogenation. ACS Appl. Nano Mater. 2022, 5, 16475–16488. [Google Scholar] [CrossRef]
- Kustov, L.M.; Kustov, A.L.; Salmi, T. Processing of lignocellulosic polymer wastes using microwave irradiation. Mendeleev Commun. 2022, 32, 1–8. [Google Scholar] [CrossRef]
- Kustov, L.M.; Tarasov, A.L.; Nissenbaum, V.D.; Kustov, A.L. Dry reforming of lignin: The effect of impregnation with iron. Mendeleev Commun. 2021, 31, 376–378. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem.-Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Alam, M.; DebRoy, T. Reaction between CO2 and coke doped with NaCN. Carbon 1987, 25, 279–288. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B.; Sharrock, P.; Nzihou, A.; Stanmore, B.; Sharrock, P. A review of catalysts for the gasification of biomass char, with some reference to coal. Energy 2013, 58, 305–317. [Google Scholar] [CrossRef]
- Matsunami, J.; Yoshida, S.; Oku, Y.; Yokota, O.; Tamaura, Y.; Kitamura, M. Coal gasification by CO2 gas bubbling in molten salt for solar/fossil energy hybridization. Sol. Energy 2000, 68, 257–261. [Google Scholar] [CrossRef]
- Popa, T.; Fan, M.; Argyle, M.D.; Slimane, R.B.; Bell, D.A.; Towler, B.F. Catalytic gasification of a Powder River Basin coal. Fuel 2013, 103, 161–170. [Google Scholar] [CrossRef]
- Namkung, H.; Yuan, X.; Lee, G.; Kim, D.; Kang, T.J.; Kim, H.T. Reaction characteristics through catalytic steam gasification with ultra clean coal char and coal. J. Energy Inst. 2014, 87, 253–262. [Google Scholar] [CrossRef]
- Hengel, T.D.; Walker, P.L. Catalysis of lignite char gasification by exchangeable calcium and magnesium. Fuel 1984, 63, 1214–1220. [Google Scholar] [CrossRef]
- Devi, T.G.; Kannan, M.P. Calcium catalysis in air gasification of cellulosic chars. Fuel 1998, 77, 1825–1830. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, R.T. Mechanism of alkali and alkaline earth catalyzed gasification of graphite by CO2 and H2O studied by electron microscopy. J. Catal. 1992, 138, 12–23. [Google Scholar] [CrossRef]
- Furimsky, E.; Sears, P.; Suzuki, T. Iron-Catalyzed Gasification of Char in CO2. Energy Fuels 1988, 2, 634–639. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Rivera-Utrilla, J.; Ferro-Garcia, M.A. Gasification of active carbons of different texture impregnated with nickel, cobalt and iron. Carbon 1987, 25, 703–708. [Google Scholar] [CrossRef]
- Gokon, N.; Hasegawa, N.; Kaneko, H.; Aoki, H.; Tamaura, Y.; Kitamura, M. Photocatalytic effect of ZnO on carbon gasification with CO2 for high temperature solar thermochemistry. Sol. Energy Mater. Sol. Cells 2003, 80, 335–341. [Google Scholar] [CrossRef]
- Kurbatova, N.A.; El’Man, A.R.; Bukharkina, T.V. Application of catalysts to coal gasification with carbon dioxide. Kinet. Catal. 2011, 52, 739–748. [Google Scholar] [CrossRef]
- Kodama, T.; Funatoh, A.; Shimizu, K.; Kitayama, Y. Kinetics of metal oxide-catalyzed CO2 gasification of coal in a fluidized-bed reactor for solar thermochemical process. Energy Fuels 2001, 15, 1200–1206. [Google Scholar] [CrossRef]
- Monterroso, R.; Fan, M.; Zhang, F.; Gao, Y.; Popa, T.; Argyle, M.D.; Towler, B.; Sun, Q. Effects of an environmentally-friendly, inexpensive composite iron-sodium catalyst on coal gasification. Fuel 2014, 116, 341–349. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Chen, D.; He, F.; Liu, S.; Zhao, K.; Wei, G.; Zheng, A.; Zhao, Z.; Li, H. Thermodynamic analysis and kinetic investigations on biomass char chemical looping gasification using Fe-Ni bimetallic oxygen carrier. Energy 2017, 141, 1836–1844. [Google Scholar] [CrossRef]
- Xie, Y.; Su, Y.; Wang, P.; Zhang, S.; Xiong, Y. In-situ catalytic conversion of tar from biomass gasification over carbon nanofibers- supported Fe-Ni bimetallic catalysts. Fuel Process. Technol. 2018, 182, 77–87. [Google Scholar] [CrossRef]
- Guo, X.; Li, N.; Zhang, T. Preparation of hydrogen-rich gas from waste polyurethane foam by steam gasification and catalytic reforming in a two-stage fixed bed reactor. J. Mater. Cycles Waste Manag. 2021, 23, 1955–1963. [Google Scholar] [CrossRef]
- Chan, F.L.; Umeki, K.; Tanksale, A. Kinetic study of catalytic steam gasification of biomass by using reactive flash volatilisation. ChemCatChem 2015, 7, 1329–1337. [Google Scholar] [CrossRef]
- Carpio, R.B.; Avendaño, C.I.L.; Basbas, C.A.; Habulan, A.A.; Guerrero, G.A.M.; Maguyon-Detras, M.C.; Bambase, M.E. Assessing the effect of K2CO3 and aqueous phase recycling on hydrothermal liquefaction of corn stover. Bioresour. Technol. Rep. 2022, 18, 14–21. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y.; Oshiki, T.; Kishimoto, T. Low-temperature catalytic hydrothermal treatment of wood biomass: Analysis of liquid products. Chem. Eng. J. 2005, 108, 127–137. [Google Scholar] [CrossRef]
- Meng, L.; Wang, M.; Yang, H.; Ying, H.; Chang, L. Catalytic effect of alkali carbonates on CO2 gasification of Pingshuo coal. Min. Sci. Technol. 2011, 21, 587–590. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Hu, J.; Wang, X.; Chen, H. Effect of catalysts on the reactivity and structure evolution of char in petroleum coke steam gasification. Fuel 2014, 117, 1174–1180. [Google Scholar] [CrossRef]
- Kuchonthara, P.; Vitidsant, T.; Tsutsumi, A. Catalytic effects of potassium on lignin steam gasification with γ-Al2O3 as a bed material. Korean J. Chem. Eng. 2008, 25, 656–662. [Google Scholar] [CrossRef]
- Zhang, D.K.; Poeze, A. Variation of sodium forms and char reactivity during gasification of a south Australian low-rank coal. Proc. Combust. Inst. 2000, 28, 2337–2344. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, W.; Wu, H.; Jensen, P.A.; Song, W.; Du, L.; Li, S. Steam gasification of char derived from penicillin mycelial dreg and lignocellulosic biomass: Influence of P, K and Ca on char reactivity. Energy 2021, 228, 120605. [Google Scholar] [CrossRef]
- Tkachenko, O.P.; Tarasov, A.L.; Mishin, I.V.; Kustov, L.M. Study of Fe- and Ni-Containing Lignins by Diffuse Reflectance IR Spectroscopy and X-ray Diffraction. Russ. J. Phys. Chem. A 2020, 94, 725–730. [Google Scholar] [CrossRef]
- Mross, W.D. Alkali Doping in Heterogeneous Catalysis. Catal. Rev. 1983, 25, 591–637. [Google Scholar] [CrossRef]
- Medvedev, A.A.; Kustov, A.L.; Beldova, D.A.; Kravtsov, A.V.; Kalmykov, K.B.; Sarkar, B.; Kostyukhin, E.M.; Kustov, L.M. Gasification of hydrolysis lignin with CO2 in the presence of Fe and Co compounds. Mendeleev Commun. 2022, 32, 402–404. [Google Scholar] [CrossRef]
- Richardson, Y.; Tanoh, S.T.; Julbe, A.; Blin, J. Improving the kinetics of the CO2 gasification of char through the catalyst/biomass integration concept. Fuel 2015, 154, 217–221. [Google Scholar] [CrossRef]
- Łamacz, A.; Krztoń, A.; Djéga-Mariadassou, G. Steam reforming of model gasification tars compounds on nickel based ceria-zirconia catalysts. Catal. Today 2011, 176, 347–351. [Google Scholar] [CrossRef]
- Bogdan, V.I.; Koklin, A.E.; Kustov, A.L.; Pokusaeva, Y.A.; Bogdan, T.V.; Kustov, L.M. Carbon Dioxide Reduction with Hydrogen on Fe, Co Supported Alumina and Carbon Catalysts under Supercritical Conditions. Molecules 2021, 26, 2883. [Google Scholar] [CrossRef]
- Hanamura, K.; Kameya, Y. Hydrogen-rich gasification of biomass using porous catalyst. Greenh. Gas Control Technol. 2005, 81, 2579–2582. [Google Scholar] [CrossRef]
- Murakami, K.; Sato, M.; Kato, T.; Sugawara, K. Influence of difference in chemical compositions of rice straw on hydrogen formation in nickel-catalyzed steam gasification. Fuel Process. Technol. 2012, 95, 78–83. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, H.; Guo, Q.; Wang, F.; Wang, J. Multiple synergistic effects exerted by coexisting sodium and iron on catalytic steam gasification of coal char. Fuel Process. Technol. 2019, 191, 1–10. [Google Scholar] [CrossRef]
- Patra, D.; Patra, B.R.; Pattnaik, F.; Hans, N.; Kushwaha, A. Recent evolution in green technologies for effective valorization of food and agricultural wastes. In Emerging Trends to Approaching Zero Waste; Hussain, C.M., Singh, S., Goswami, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 103–132. ISBN 978-0-323-85403-0. [Google Scholar]
- Zharova, P.; Arapova, O.V.; Konstantinov, G.I.; Chistyakov, A.V.; Tsodikov, M.V. Kraft Lignin Conversion into Energy Carriers under the Action of Electromagnetic Radiation. J. Chem. 2019, 2019, 6480354. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Nikolaev, S.A.; Chistyakov, A.V.; Bukhtenko, O.V.; Fomkin, A.A. Formation of adsorbents from Fe-containing processing residues of lignin. Microporous Mesoporous Mater. 2020, 298, 110089. [Google Scholar] [CrossRef]
- Tsodikov, M.D.; Ellert, O.G.; Arapova, O.V.; Nikolaev, S.A.; Chistyakov, A.V.; Maksimov, Y.V. Benefit of Fe-containing catalytic systems for dry reforming of lignin to syngas under microwave radiation. Chem. Eng. Trans. 2018, 65, 367–372. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Chistyakov, A.V.; Konstantinov, G.I.; Nikolaev, S.A.; Borisov, R.S.; Levin, I.C.; Maksimov, Y.V.; Gekhman, A.E. Microwave-Stimulated Conversion of a Tar/Lignin Blend into Hydrocarbons in a Plasma-Catalytic Mode. Russ. J. Appl. Chem. 2021, 94, 1513–1524. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Ellert, O.G.; Nikolaev, S.A.; Arapova, O.V.; Konstantinov, G.I.; Bukhtenko, O.V.; Vasil’kov, A.Y. The role of nanosized nickel particles in microwave-assisted dry reforming of lignin. Chem. Eng. J. 2017, 309, 628–637. [Google Scholar] [CrossRef]
- Medvedev, A.A.; Kustov, A.L.; Beldova, D.A.; Kalmykov, K.B.; Mashkin, M.Y.; Shesterkina, A.A.; Dunaev, S.F.; Kustov, L.M. Influence of the Method of Fe Deposition on the Surface of Hydrolytic Lignin on the Activity in the Process of Its Conversion in the Presence of CO2. Int. J. Mol. Sci. 2023, 24, 1279. [Google Scholar] [CrossRef]
- Medvedev, A.A.; Beldova, D.A.; Kalmykov, K.B.; Kravtsov, A.V.; Tedeeva, M.A.; Kustov, L.M.; Dunaev, S.F.; Kustov, A.L. Carbon Dioxide Assisted Conversion of Hydrolysis Lignin Catalyzed by Nickel Compounds. Energies 2022, 15, 6774. [Google Scholar] [CrossRef]
- Arapova, O.V.; Chistyakov, A.V.; Palankoev, T.A.; Bondarenko, G.N.; Tsodikov, M.V. Microwave-Assisted Lignin Conversion to Liquid Products in the Presence of Iron and Nickel. Pet. Chem. 2020, 60, 1019–1025. [Google Scholar] [CrossRef]
- Arapova, O.V.; Ellert, O.G.; Borisov, R.S.; Chistyakov, A.V.; Vasil’kov, A.Y.; Tsodikov, M.V.; Gekhman, A.E. Effect of the Method of Synthesizing a Nickel-Containing Catalyst on Lignin Conversion in Liquid-Phase Hydrodepolymerization. Pet. Chem. 2019, 59, 111–119. [Google Scholar] [CrossRef]
- Fedotov, A.S.; Antonov, D.O.; Uvarov, V.I.; Korchak, V.N.; Tsodikov, M.V.; Moiseev, I.I. Highly selective carbon dioxide gasification of the biomass fermentation products. Dokl. Chem. 2014, 459, 205–208. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Ellert, O.G.; Nikolaev, S.A.; Arapova, O.V.; Bukhtenko, O.V.; Maksimov, Y.V.; Kirdyankin, D.I.; Vasil’kov, A.Y. Fe-containing nanoparticles used as effective catalysts of lignin reforming to syngas and hydrogen assisted by microwave irradiation. J. Nanopart. Res. 2018, 20, 86. [Google Scholar] [CrossRef]
- Minakshi, M. Lithium intercalation into amorphous FePO4 cathode in aqueous solutions. Electrochim. Acta 2010, 55, 9174–9178. [Google Scholar] [CrossRef]
- Liu, R.; Ma, Z.; Sears, J.D.; Juneau, M.; Neidig, M.L.; Porosoff, M.D. Identifying correlations in Fischer-Tropsch synthesis and CO2 hydrogenation over Fe-based ZSM-5 catalysts. J. CO2 Util. 2020, 41, 101290. [Google Scholar] [CrossRef]
- Aasly, K.M.; Terje Myrhaug, E.; Aasly, K.; Malvik, T.; Myrhaug, E. Advanced methods to characterize thermal properties of quartz. INFACON 2007, 11, 381–392. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, D.; Minakshi, M.; Quadsia, S.; Ahuja, R. Activation-Induced Surface Modulation of Biowaste-Derived Hierarchical Porous Carbon for Supercapacitors. Chempluschem 2022, 87, e202200126. [Google Scholar] [CrossRef]
- Liu, R.; Leshchev, D.; Stavitski, E.; Juneau, M.; Agwara, J.N.; Porosoff, M.D. Selective hydrogenation of CO2 and CO over potassium promoted Co/ZSM-5. Appl. Catal. B Environ. 2021, 284, 119787. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).