Numerical Simulation of CO2 Migration and Geochemical Reactions in Shihezi Formation Caprock, China
Abstract
1. Introduction
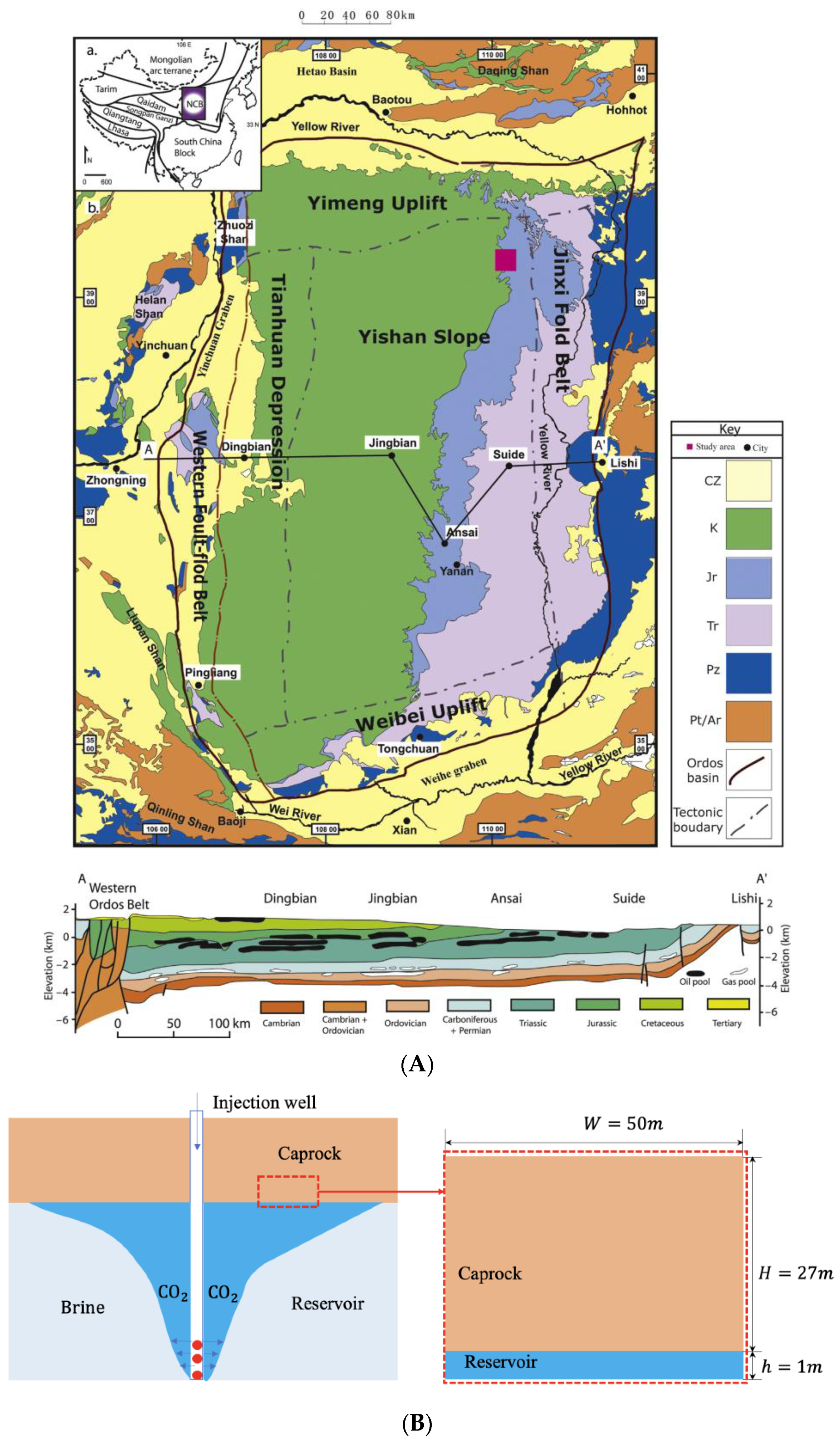
2. Heterogeneity Description of Caprock
3. Conceptual Model
3.1. Description of Simulator
3.2. Geological Storage Project and Numerical Model
3.3. Model Parameters
4. Results and Discussion
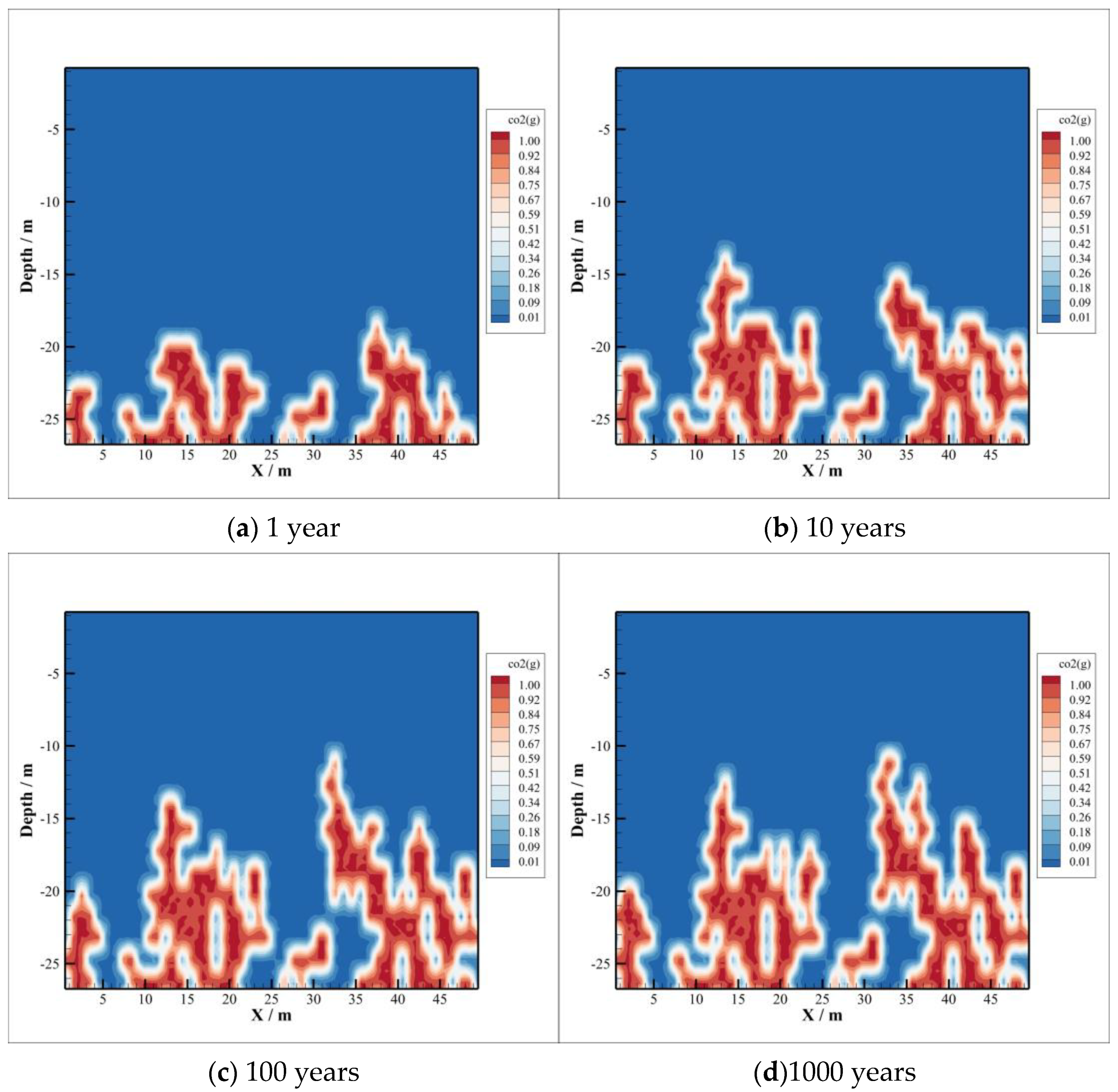
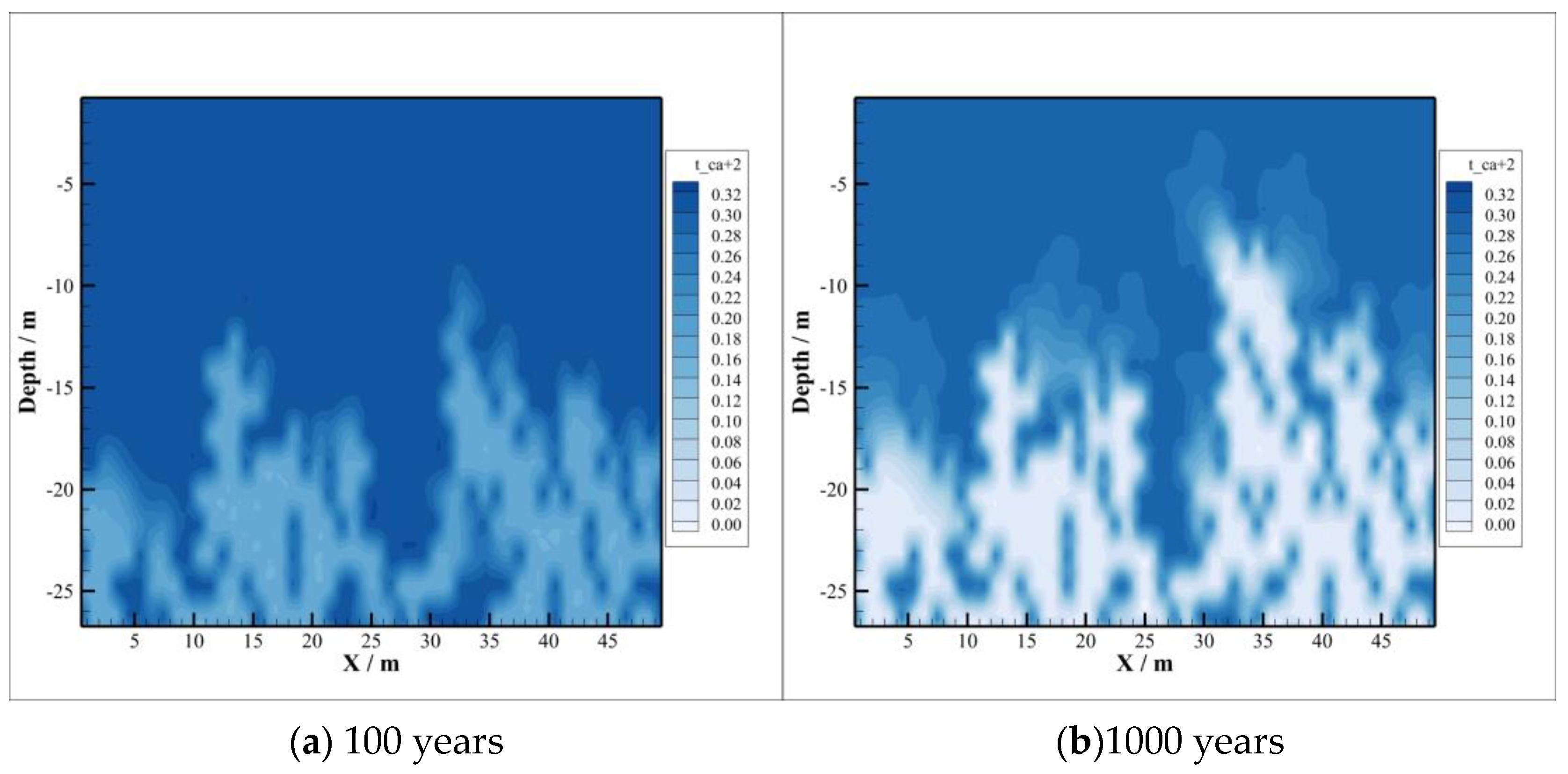
4.1. CO2 Distribution
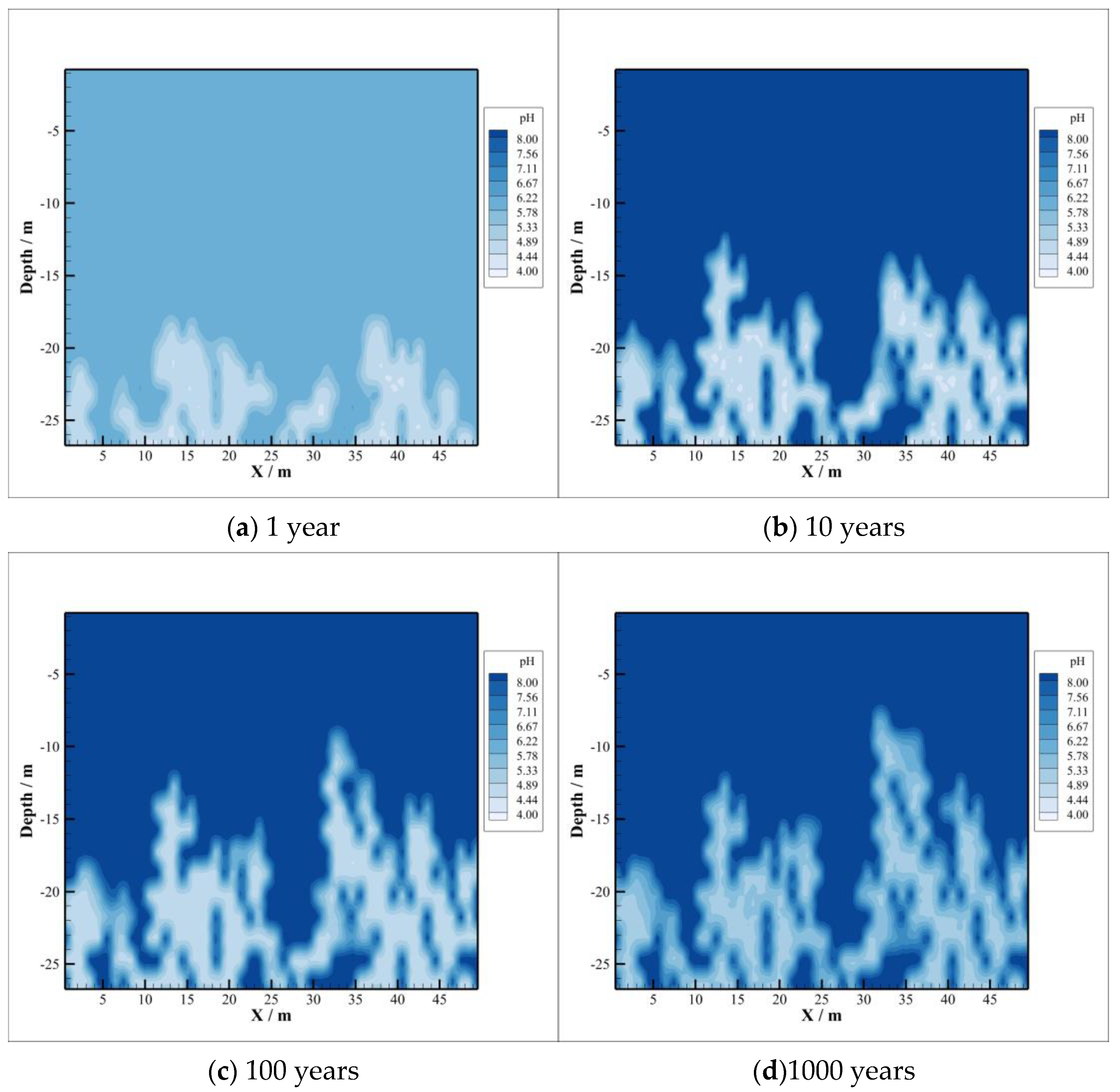
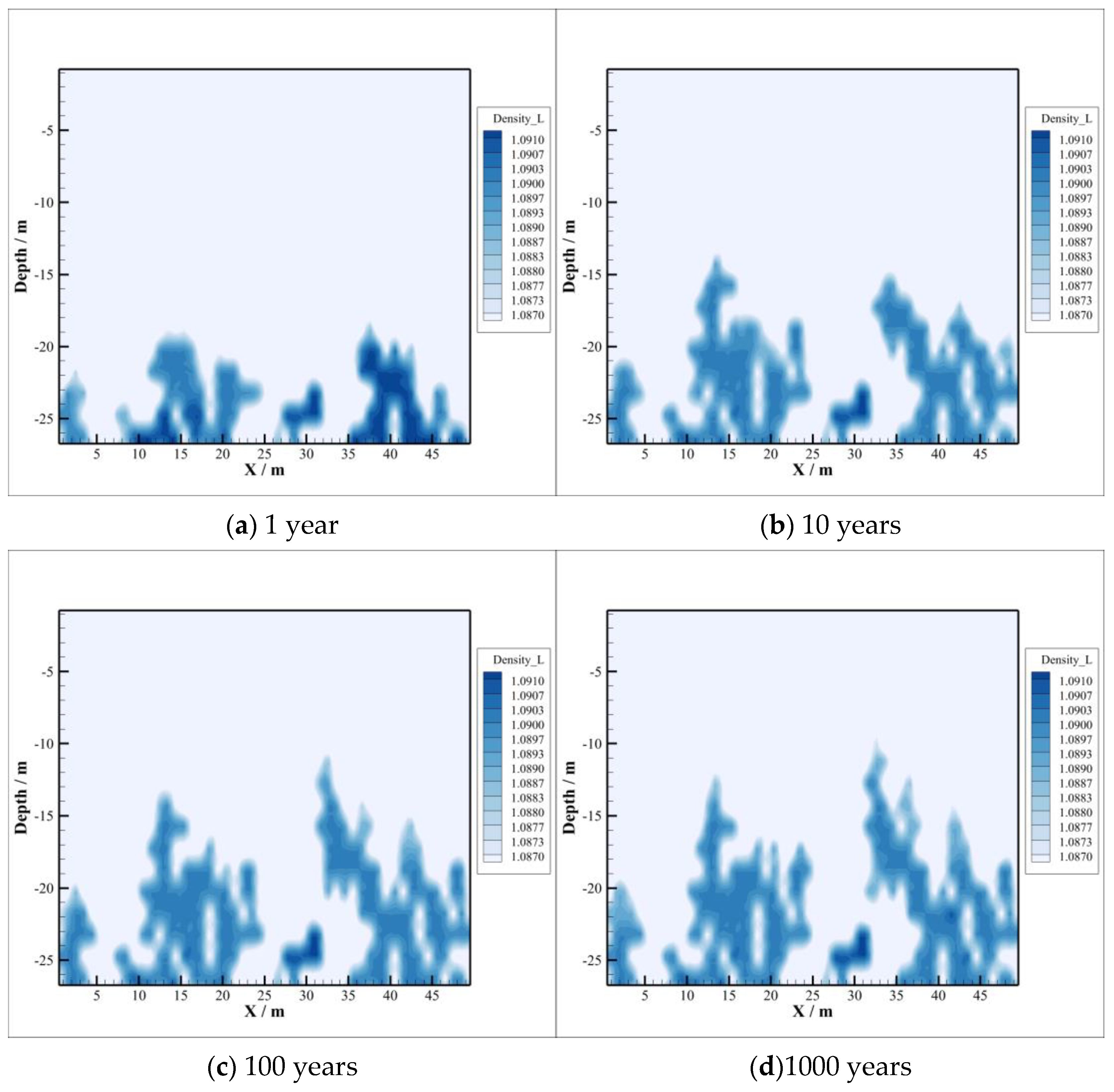
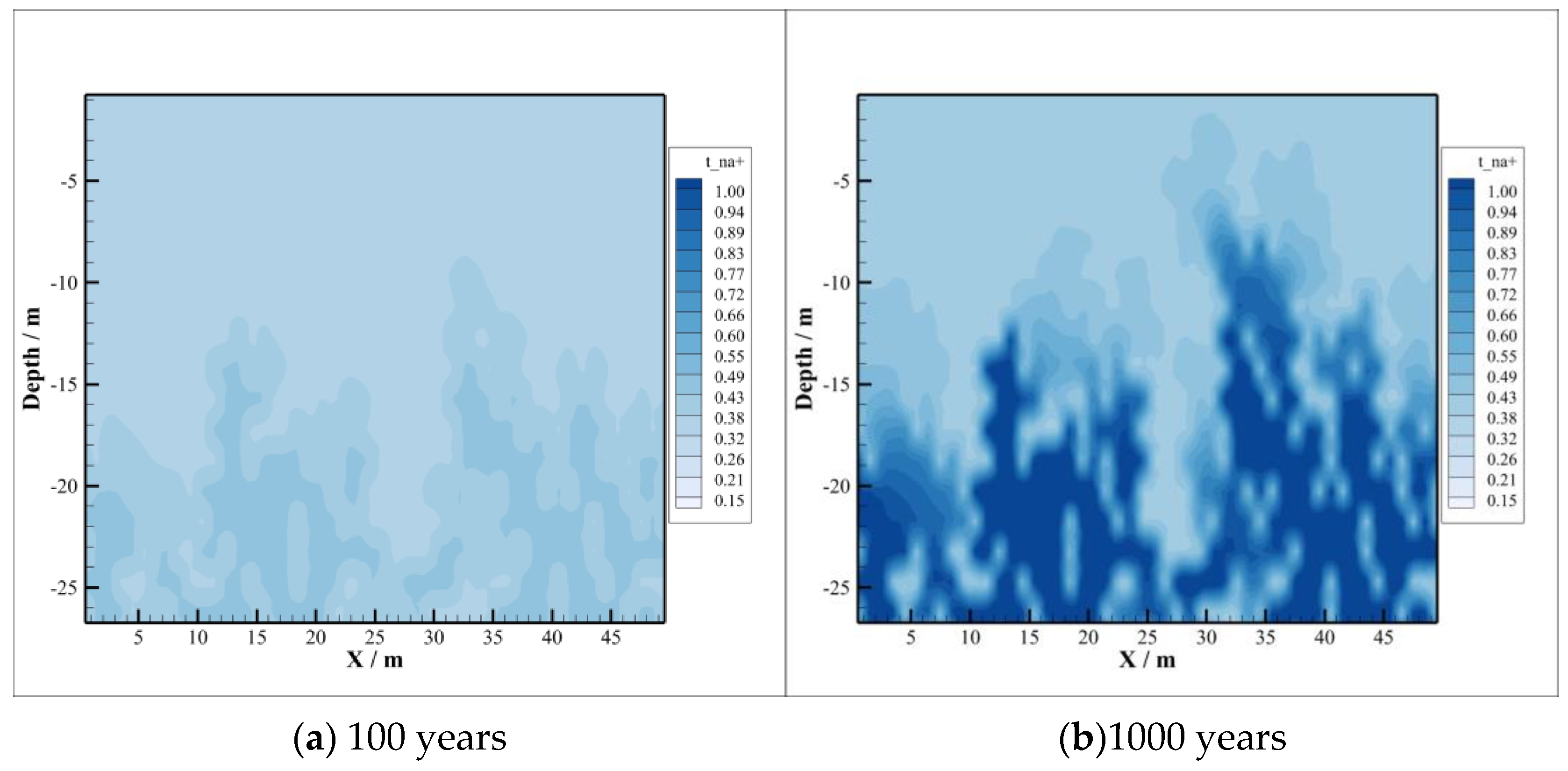
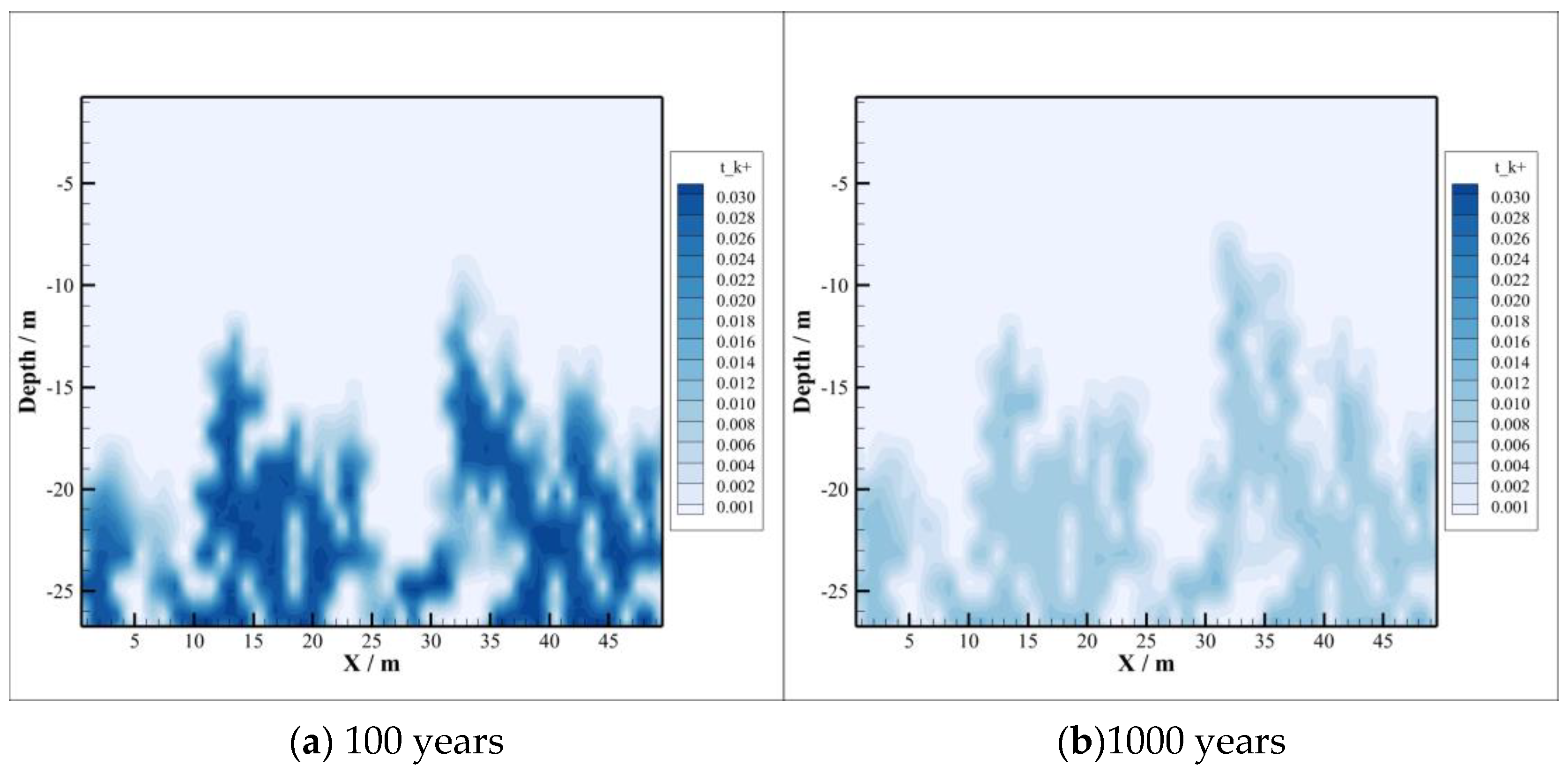
4.2. CO2 Dissolution and Concentration Changes of Brine Components
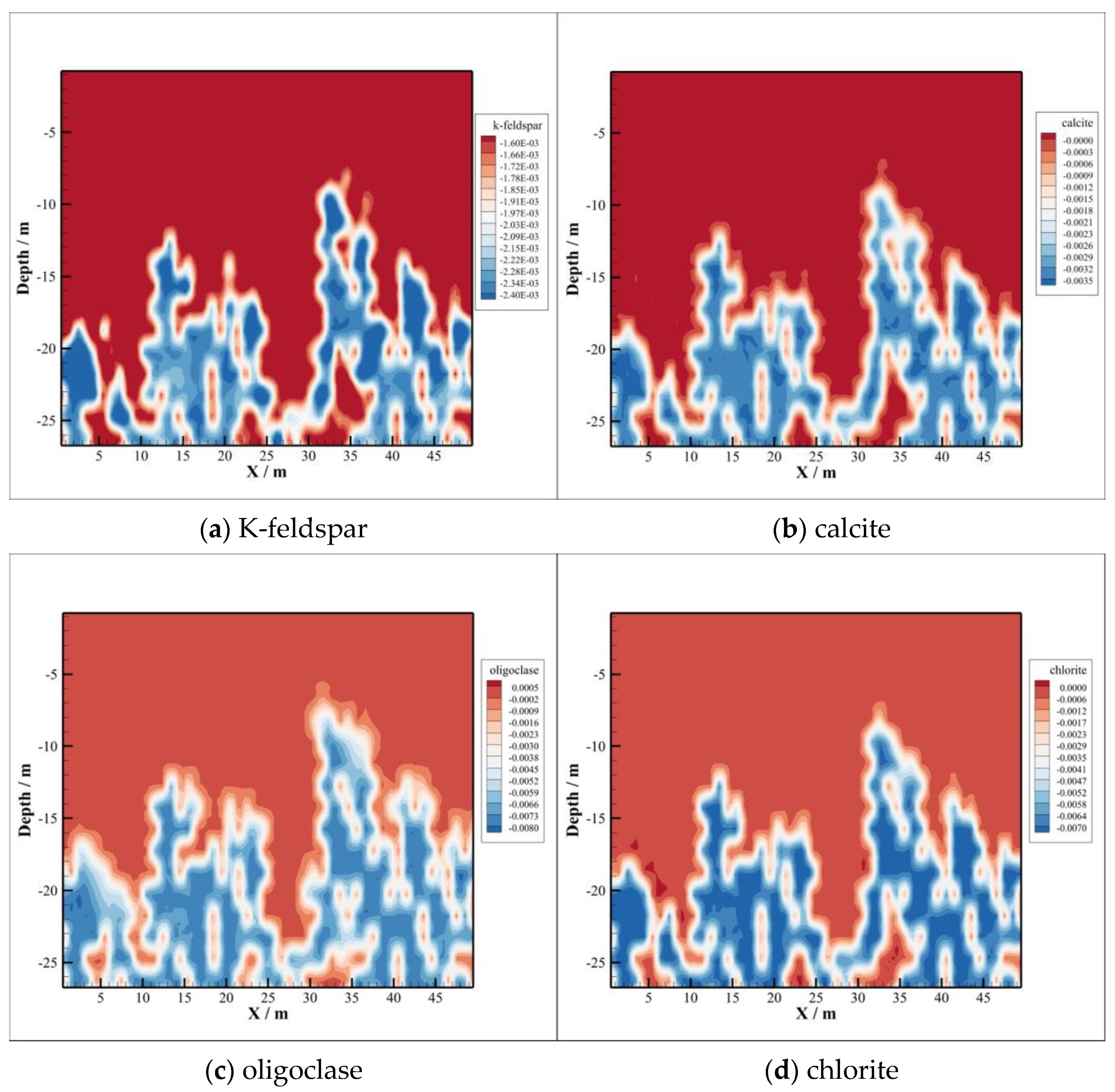
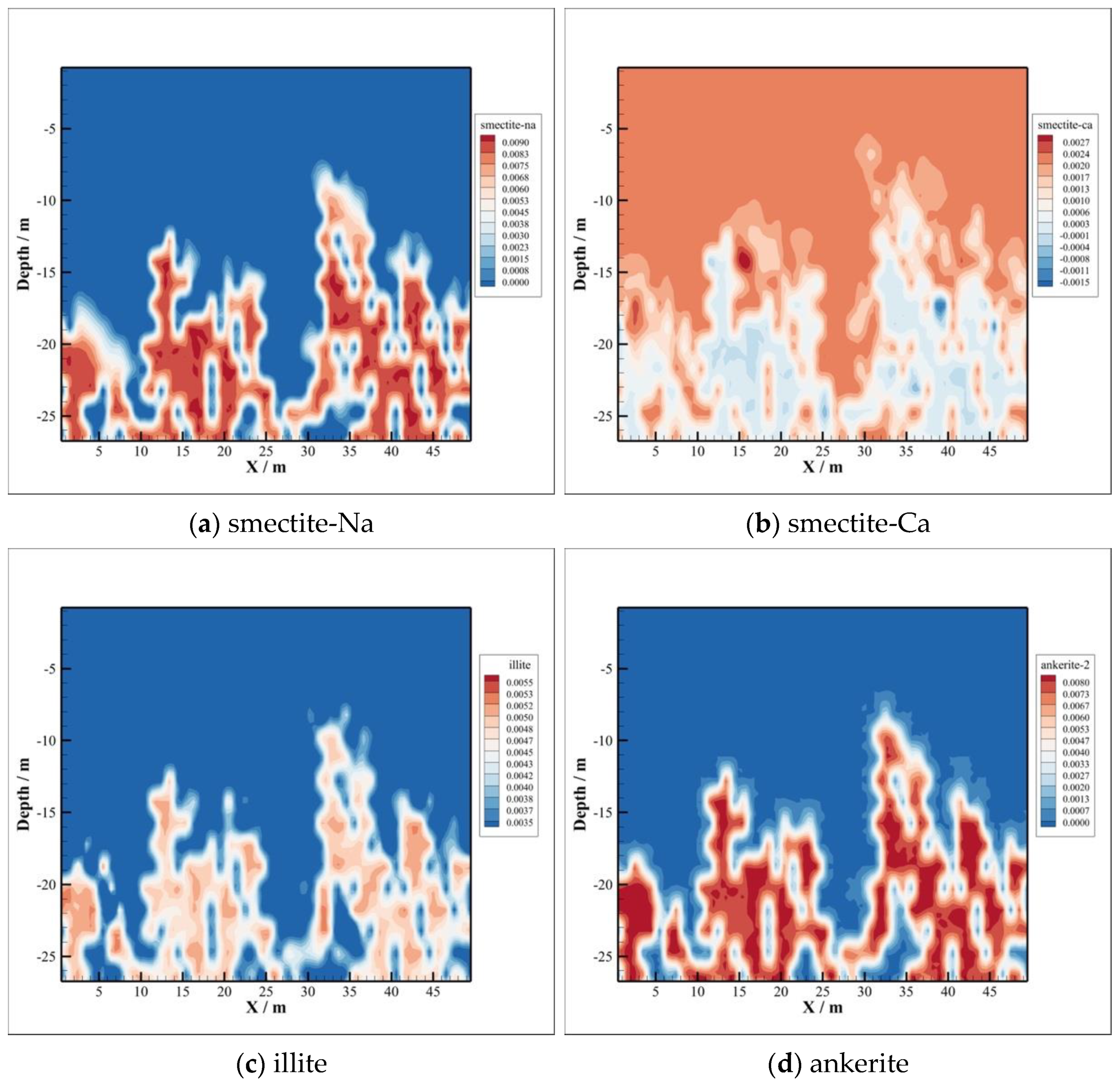
4.3. Minerals Transformation
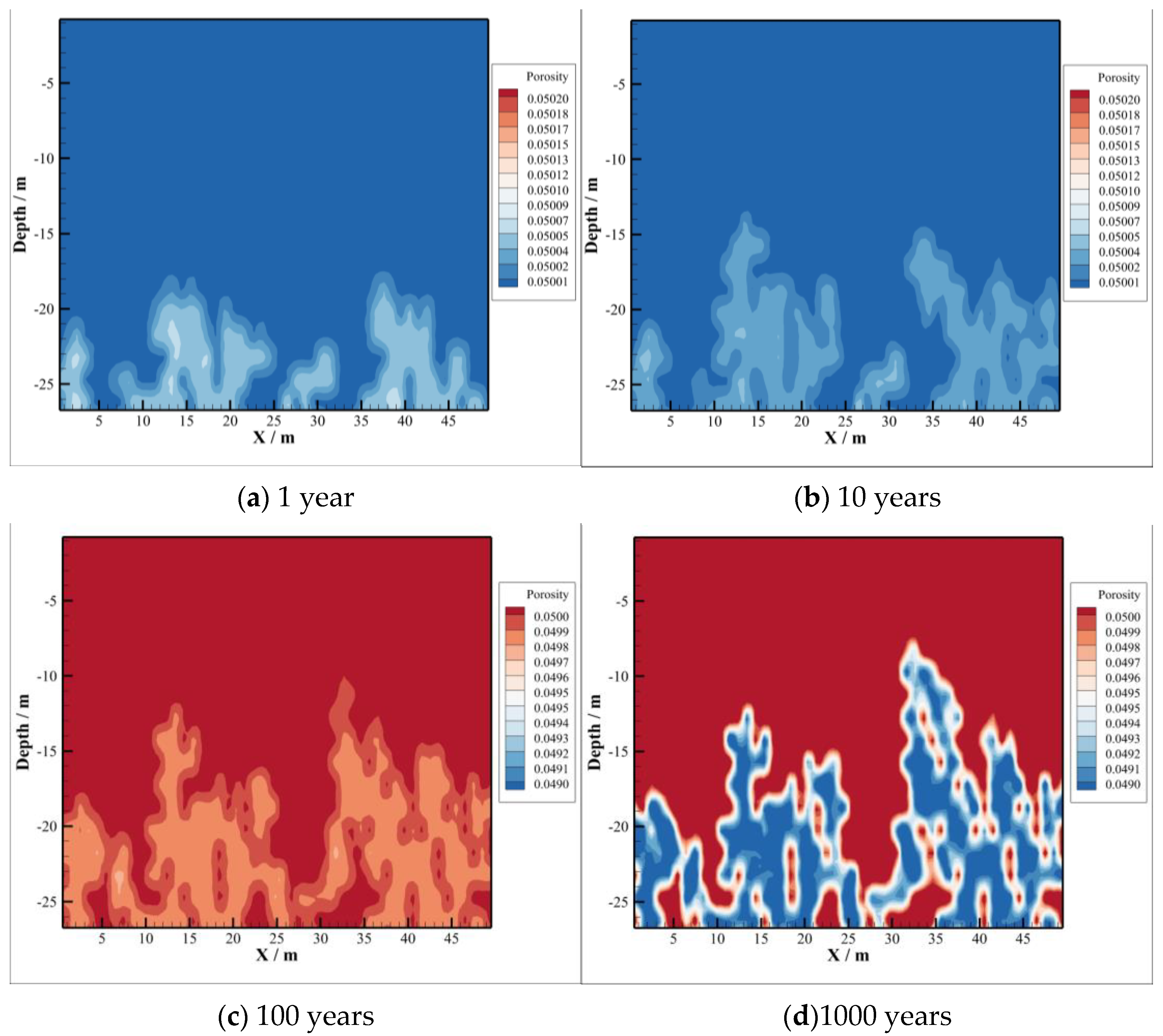
4.4. Porosity and Permeability of Caprock
5. Conclusions
- The heterogeneity of the caprock promotes the intrusion of CO2 into the caprock to a certain extent and accelerates the transport process of CO2 in the caprock. However, when CO2 migrates to a certain distance in the caprock, the vertical migration process slows down obviously. Simulation results show that the vertical transport distance of CO2 is almost unchanged during the period of 100 to 1000 years, about 20 m, but the horizontal transport distance increases.
- With the intrusion of CO2 gas into the caprock, part of the CO2 will be dissolved, which will significantly reduce the pH value of the brine in the caprock. However, the distribution of the dissolved CO2 in the caprock is smaller than the range of pH value change, which means that the ion transport range exceeds the range of CO2 dissolution, which can promote the CO2–brine–rock reaction in a larger range. On the one hand, CO2 dissolved in formation water can promote ion transport, which will promote the CO2–brine–rock reaction. On the other hand, the CO2–brine–rock reaction caused by the change in ion concentration in turn limits the ion transport distance. Therefore, the transport distance of CO2 almost does not change after a certain time.
- The CO2–brine–rock reaction during CO2 intrusion into the caprock can significantly change the changes in porosity and permeability. In the early stage of CO2 sequestration, due to the decrease in formation water pH value, the type of CO2–brine–rock reaction is mainly a dissolution reaction, so the porosity and permeability show an increasing trend. However, with the passage of time, the porosity and permeability with CO2 intrusion into the caprock decreased significantly, which means that the reaction type changed to precipitation, thus enhancing the sealing ability of the caprock.
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Special Report on Carbon Dioxide Capture and Storage: Technical Sum-Mary; IPCC: Geneva, Switzerland, 2005. [Google Scholar]
- Naderi, S.; Simjoo, M. Numerical study of Low Salinity Water Alternating CO2 injection for enhancing oil recovery in a sandstone reservoir: Coupled geochemical and fluid flow modeling. J. Pet. Sci. Eng. 2019, 173, 279–286. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, B.; Shang, J.; Gao, K.; Pu, W.; Xu, X.; Wood, C.; Sun, L. Alterations of geochemical properties of a tight sandstone reservoir caused by supercritical CO2-brine-rock interactions in CO2-EOR and geosequestration. J. CO2 Util. 2018, 28, 408–418. [Google Scholar] [CrossRef]
- Chen, Y.; Sari, A.; Zeng, L.; Saeedi, A.; Xie, Q. Geochemical insights for CO2 huff-n-puff process in shale oil reservoirs. J. Mol. Liq. 2020, 307, 112992. [Google Scholar] [CrossRef]
- Oldenburg, C.M.; Pruess, K.; Benson, S.M. Process Modeling of CO2 Injection into Natural Gas Reservoirs for Carbon Sequestration and Enhanced Gas Recovery. Energy Fuels 2000, 15, 293–298. [Google Scholar] [CrossRef]
- Spycher, N.; Pruess, K. A Phase-Partitioning Model for CO2–Brine Mixtures at Elevated Temperatures and Pressures: Application to CO2-Enhanced Geothermal Systems. Transp. Porous Media 2010, 82, 173–196. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.; Park, K. Comparison of nitrogen and carbon dioxide as cushion gas for underground gas storage reservoir. Geosystem Eng. 2015, 18, 163–167. [Google Scholar] [CrossRef]
- Oldenburg, C.M. Carbon Dioxide as Cushion Gas for Natural Gas Storage. Energy Fuels 2003, 17, 240–246. [Google Scholar] [CrossRef]
- Kühn, M.; Nakaten, N.; Streibel, M.; Kempka, T. CO2 Geological Storage and Utilization for a Carbon Neutral “Power-to-gas-to-power” Cycle to Even Out Fluctuations of Renewable Energy Provision. Energy Procedia 2014, 63, 8044–8049. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2–rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Gaus, I.; Azaroual, M.; Czernichowski-Lauriol, I. Reactive transport modelling of the impact of CO2 injection on the clayey cap rock at Sleipner (North Sea). Chem. Geol. 2005, 217, 319–337. [Google Scholar] [CrossRef]
- Doughty, C. Modeling geologic storage of carbon dioxide: Comparison of non-hysteretic and hysteretic characteristic curves. Energy Convers. Manag. 2007, 48, 1768–1781. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y. CO2 modelling in a deep saline aquifer: A predictive uncertainty analysis using design of experiment. Environ. Sci. Technol. 2011, 45, 3504–3510. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Stauffer, P.H.; Dai, Z.; Jiao, Z.; Surdam, R.C. Simulation of industrial-scale CO2 storage: Multi-scale heterogeneity and its impacts on storage capacity, injectivity and leakage. Int. J. Greenh. Gas Control 2012, 10, 397–418. [Google Scholar] [CrossRef]
- Gherardi, F.; Xu, T.; Pruess, K. Numerical modeling of self-limiting and self-enhancing caprock alteration induced by CO2 storage in a depleted gas reservoir. Chem. Geol. 2007, 244, 103–129. [Google Scholar] [CrossRef]
- Xu, T.; Sonnenthal, E.; Spycher, N.; Pruess, K. TOUGHREACT-A simulation program for non-isothermal multiphase reactive geochemical transport in variably saturated geologic media: Applications to geothermal injectivity and CO2 geological sequestration. Comput. Geosci. 2006, 32, 145–165. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, L.; Tian, H. Reactive transport modeling for CO2 geological sequestration. J. Pet. Sci. Eng. 2011, 78, 765–777. [Google Scholar] [CrossRef]
- Sahimi, M. Flow and Transport in Porous Media and Fractured Rock: From Classical Methods to Modern Approaches; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Pruess, K.; Oldenburg, C.M.; Moridis, G.J. TOUGH2 User’s Guide, Version 2; Lawrence Berkeley Laboratory Report LBL-43134; Lawrence Berkeley Laboratory: Berkeley, CA, USA, 1999.
- Pruess, K.; Zhang, K. Numerical Modeling Studies of the Dissolution-Diffusion-Convection Process during CO2 Storage in Saline Aquifers; Technical Report LBNL-1243E; Lawrence Berkeley Laboratory: Berkeley, CA, USA, 2008.
- Wu, X. China’s First Large-Scale Exploration of Carbon Dioxide Capture and Geological Storage, 1st ed.; Science Press: Beijing, China, 2013. [Google Scholar]
- Li, Q.; Liu, G.; Liu, X.; Li, X. Application of a health, safety, and environmental screening and ranking framework to the Shenhua CCS project. Int. J. Greenh. Gas Control 2013, 17, 504–514. [Google Scholar] [CrossRef]
- Tian, H. Impacts of CO2-Brine-Rock Interaction on the Caprock Sealing Efficiency: A Case Study of Shiqianfeng Formation Mudstone Caprock in Ordos Basin. Ph.D. Thesis, Jilin University, Changchun, China, 2014. [Google Scholar]




| Parameters | Reservoir | Caprock |
|---|---|---|
| depth (m) | 1 | 27 |
| temperature (°C) | 55 | 55 |
| pressure (bar) | 195 | 165 |
| rock density (kg/m3) | 2400 | 2500 |
| porosity (%) | 12.0 | 5.0 |
| horizontal permeability (m2) | 5.47 × 10−15 | 0.08 × 10−15 |
| vertical permeability (m2) | 5.47 × 10−15 | 0.08 × 10−15 |
| relative permeability of liquid phase | ||
| relative permeability of gas phase | ||
| capillary pressure | , , | |
| 0.200 | 0.200 | |
| (Pa) | ||
| (Pa) | ||
| No. | Minerals | Reservoir | Caprock |
|---|---|---|---|
| 1 | quartz | 53.70 | 11.28 |
| 2 | K-feldspar | - | 13.81 |
| 3 | oligoclase | 27.64 | 7.20 |
| 4 | calcite | - | 1.00 |
| 5 | smectite-Ca | 2.72 | 19.21 |
| 7 | smectite-Na | 1.65 | 19.16 |
| 8 | illite | 11.20 | 17.70 |
| 9 | kaolinite | 2.07 | 5.24 |
| 10 | chlorite | - | 5.40 |
| 11 | hematite | 1.02 | - |
| 12 | dolomite | - | - |
| 13 | albite | - | - |
| 14 | anorthite | - | - |
| 15 | magnesite | - | - |
| 16 | ankerite | - | - |
| 17 | dawsonite | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Lv, Y.; Liu, B. Numerical Simulation of CO2 Migration and Geochemical Reactions in Shihezi Formation Caprock, China. Energies 2023, 16, 92. https://doi.org/10.3390/en16010092
Li Z, Lv Y, Liu B. Numerical Simulation of CO2 Migration and Geochemical Reactions in Shihezi Formation Caprock, China. Energies. 2023; 16(1):92. https://doi.org/10.3390/en16010092
Chicago/Turabian StyleLi, Zhuo, Yanfang Lv, and Bin Liu. 2023. "Numerical Simulation of CO2 Migration and Geochemical Reactions in Shihezi Formation Caprock, China" Energies 16, no. 1: 92. https://doi.org/10.3390/en16010092
APA StyleLi, Z., Lv, Y., & Liu, B. (2023). Numerical Simulation of CO2 Migration and Geochemical Reactions in Shihezi Formation Caprock, China. Energies, 16(1), 92. https://doi.org/10.3390/en16010092









