Large Scale Microalgae Biofuel Technology—Development Perspectives in Light of the Barriers and Limitations
Abstract
1. Introduction and background
2. Technological Complexity
3. Process Optimization
4. Research Scale-up and Reliable LCA
5. Monitoring, Measurement and Control Systems
6. Genetic Engineering
7. Symbiotic Systems
8. Incentives, Subsidies, and Law Regulations
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, J.; Wang, Z.; Chen, G. Biological Characteristics of Energy Conversion in Carbon Fixation by Microalgae. Renew. Sustain. Energy Rev. 2021, 152, 111661. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A Review of High Value-Added Molecules Production by Microalgae in Light of the Classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J.; Dudek, M.; Świca, I.; Rudnicka, A. The Cultivation of Lipid-Rich Microalgae Biomass as Anaerobic Digestate Valorization Technology—A Pilot-Scale Study. Processes 2020, 8, 517. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M. Sustainable Production of Monoraphidium Microalgae Biomass as a Source of Bioenergy. Energies 2020, 13, 5975. [Google Scholar] [CrossRef]
- Barros, R.; Raposo, S.; Morais, E.G.; Rodrigues, B.; Afonso, V.; Gonçalves, P.; Marques, J.; Cerqueira, P.R.; Varela, J.; Teixeira, M.R.; et al. Biogas Production from Microalgal Biomass Produced in the Tertiary Treatment of Urban Wastewater: Assessment of Seasonal Variations. Energies 2022, 15, 5713. [Google Scholar] [CrossRef]
- Dębowski, M.; Dudek, M.; Nowicka, A.; Quattrocelli, P.; Kazimierowicz, J.; Zieliński, M. Suitability of Pre-Digested Dairy Effluent for Mixotrophic Cultivation of the Hydrogen-Producing Microalgae Tetraselmis Subcordiformis. Environ. Technol. 2022. [Google Scholar] [CrossRef]
- Kosowska-Golachowska, M.; Luckos, A. Direct Combustion of Microalgae Biomass to Generate Bioelectricity. In 3rd Generation Biofuels: Disruptive Technologies to Enable Commercial Production; Woodhead Publishing: Sawston, UK, 2022; pp. 665–698. [Google Scholar] [CrossRef]
- Sung, Y.J.; Lee, J.S.; Yoon, H.K.; Ko, H.; Sim, S.J. Outdoor Cultivation of Microalgae in a Coal-Fired Power Plant for Conversion of Flue Gas CO2 into Microalgal Direct Combustion Fuels. Syst. Microbiol. Biomanuf. 2021, 1, 90–99. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Kalra, R.; Gaur, S.; Goel, M. Microalgae Bioremediation: A Perspective towards Wastewater Treatment along with Industrial Carotenoids Production. J. Water Process Eng. 2021, 40, 101794. [Google Scholar] [CrossRef]
- Rajagopal, R.; Mousavi, S.E.; Goyette, B.; Adhikary, S. Coupling of Microalgae Cultivation with Anaerobic Digestion of Poultry Wastes: Toward Sustainable Value Added Bioproducts. Bioengineering 2021, 8, 57. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sahoo, D.; Pandey, A. Resource Recovery through Bioremediation of Wastewaters and Waste Carbon by Microalgae: A Circular Bioeconomy Approach. Environ. Sci. Pollut. Res. 2021, 28, 58837–58856. [Google Scholar] [CrossRef]
- de Faria Ferreira Carraro, C.; Loures, C.C.A.; de Castro, J.A. Microalgae Technique for Bioremediation Treatment of Cassava Wastewater. Water. Air. Soil Pollut. 2021, 232, 1–13. [Google Scholar] [CrossRef]
- Dębowski, M.; Krzemieniewski, M.; Zieliński, M.; Kazimierowicz, J. Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept. Atmosphere 2021, 12, 1031. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Outflow from a Biogas Plant as a Medium for Microalgae Biomass Cultivation—Pilot Scale Study and Technical Concept of a Large-Scale Installation. Energies 2022, 15, 2912. [Google Scholar] [CrossRef]
- Behera, B.; Selvam, S.M.; Paramasivan, B. Research Trends and Market Opportunities of Microalgal Biorefinery Technologies from Circular Bioeconomy Perspectives. Bioresour. Technol. 2022, 351, 127038. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Shukla, R.; Singh, N.; Hemansi; Saini, J.K. Next Generation Biofuels from Macroalgae: Prospects and Challenges. Microb. Biotechnol. Renew. Sustain. Energy 2022, 55–75. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Dhaka, V.; Ariyadasa, T.U.; Malik, A. Exploring Algal Technologies for a Circular Bio-Based Economy in Rural Sector. J. Clean. Prod. 2022, 354, 131653. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Hasselström, L.; Gröndahl, F. Payments for Nutrient Uptake in the Blue Bioeconomy—When to Be Careful and When to Go for It. Mar. Pollut. Bull. 2021, 167, 112321. [Google Scholar] [CrossRef]
- European Commission Directorate-General for Maritime Affairs and Fisheries Joint Research Centre. The EU Blue Economy Report 2019—Publications Office of the EU. 2019. Available online: https://prod5.assets-cdn.io/event/3769/assets/8442090163-fc038d4d6f.pdf (accessed on 14 October 2022).
- Abomohra, A.E.F.; Almutairi, A.W. A Close-Loop Integrated Approach for Microalgae Cultivation and Efficient Utilization of Agar-Free Seaweed Residues for Enhanced Biofuel Recovery. Bioresour. Technol. 2020, 317, 124027. [Google Scholar] [CrossRef]
- Singh, A.; Ummalyma, S.B. Bioremediation and Biomass Production of Microalgae Cultivation in River Water Contaminated with Pharmaceutical Effluent. Bioresour. Technol. 2020, 307, 123233. [Google Scholar] [CrossRef] [PubMed]
- Culaba, A.B.; Ubando, A.T.; Ching, P.M.L.; Chen, W.H.; Chang, J.S. Biofuel from Microalgae: Sustainable Pathways. Sustainability 2020, 12, 8009. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O.; Imanah, O.E.; Ndibe, H. Biofuels from Microalgae Biomass: A Review of Conversion Processes and Procedures. Arab. J. Chem. 2022, 15, 103591. [Google Scholar] [CrossRef]
- Ganesan, R.; Manigandan, S.; Samuel, M.S.; Shanmuganathan, R.; Brindhadevi, K.; Lan Chi, N.T.; Duc, P.A.; Pugazhendhi, A. A Review on Prospective Production of Biofuel from Microalgae. Biotechnol. Reports 2020, 27, e00509. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Agrawal, K.; Upadhyaya, H.M.; Gupta, V.K.; Verma, P. Microalgae Conversion to Alternative Energy, Operating Environment and Economic Footprint: An Influential Approach towards Energy Conversion, and Management. Energy Convers. Manag. 2022, 269, 116118. [Google Scholar] [CrossRef]
- Kasani, A.A.; Esmaeili, A.; Golzary, A. Software Tools for Microalgae Biorefineries: Cultivation, Separation, Conversion Process Integration, Modeling, and Optimization. Algal Res. 2022, 61, 102597. [Google Scholar] [CrossRef]
- Yadav, G.; Dubey, B.K.; Sen, R. A Comparative Life Cycle Assessment of Microalgae Production by CO2 Sequestration from Flue Gas in Outdoor Raceway Ponds under Batch and Semi-Continuous Regime. J. Clean. Prod. 2020, 258, 120703. [Google Scholar] [CrossRef]
- Su, B.; Lin, Y.; Wang, J.; Quan, X.; Chang, Z.; Rui, C. Sewage Treatment System for Improving Energy Efficiency Based on Particle Swarm Optimization Algorithm. Energy Rep. 2022, 8, 8701–8708. [Google Scholar] [CrossRef]
- Sun, H.; Huang, K.; Zhang, X.; Ren, H.; Ye, L. Stable Isotope Probing Reveals Specific Assimilating Bacteria of Refractory Organic Compounds in Activated Sludge. Water Res. 2022, 212, 118105. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Pecorini, I.; Iannelli, R. Multilinear Regression Model for Biogas Production Prediction from Dry Anaerobic Digestion of OFMSW. Sustainability 2022, 14, 4393. [Google Scholar] [CrossRef]
- Burch, T.R.; Firnstahl, A.D.; Spencer, S.K.; Larson, R.A.; Borchardt, M.A. Fate and Seasonality of Antimicrobial Resistance Genes during Full-Scale Anaerobic Digestion of Cattle Manure across Seven Livestock Production Facilities. J. Environ. Qual. 2022, 51, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, R.; Muñoz, R.; Polanco, M.; Díaz, I.; Susmozas, A.; Moreno, A.D.; Guirado, M.; Carreras, N.; Ballesteros, M. Biogas from Anaerobic Digestion as an Energy Vector: Current Upgrading Development. Energies 2021, 14, 2742. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Two-Stage Cultivation of Microalgae for Production of High-Value Compounds and Biofuels: A Review. Algal Res. 2021, 57, 102353. [Google Scholar] [CrossRef]
- Ioannou, I.; D’Angelo, S.C.; Galán-Martín, Á.; Pozo, C.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Process Modelling and Life Cycle Assessment Coupled with Experimental Work to Shape the Future Sustainable Production of Chemicals and Fuels. React. Chem. Eng. 2021, 6, 1179–1194. [Google Scholar] [CrossRef]
- Guo, B.; Ringwood, J.V. A Review of Wave Energy Technology from a Research and Commercial Perspective. IET Renew. Power Gener. 2021, 15, 3065–3090. [Google Scholar] [CrossRef]
- Sun, Y.; Bi, K.; Yin, S. Measuring and Integrating Risk Management into Green Innovation Practices for Green Manufacturing under the Global Value Chain. Sustainability 2020, 12, 545. [Google Scholar] [CrossRef]
- Brutyan, M.M. Foresight of Microalgae Usage for the Production of Third-Generation Biofuel. Indian J. Sci. Technol. 2017, 10, 1–10. [Google Scholar] [CrossRef]
- Severo, I.A.; dos Santos, A.M.; Deprá, M.C.; Barin, J.S.; Jacob-Lopes, E. Microalgae Photobioreactors Integrated into Combustion Processes: A Patent-Based Analysis to Map Technological Trends. Algal Res. 2021, 60, 102529. [Google Scholar] [CrossRef]
- Assunção, J.; Malcata, F.X. Enclosed “Non-Conventional” Photobioreactors for Microalga Production: A Review. Algal Res. 2020, 52, 102107. [Google Scholar] [CrossRef]
- Rumin, J.; Nicolau, E.; de Oliveira, R.G.; Fuentes-Grünewald, C.; Picot, L. Analysis of Scientific Research Driving Microalgae Market Opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Factories 2018, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Balbaud, F.; Cabet, C.; Cornet, S.; Dai, Y.; Gan, J.; Hernández Mayoral, M.; Hernández, R.; Jianu, A.; Malerba, L.; Maloy, S.A.; et al. A NEA Review on Innovative Structural Materials Solutions, Including Advanced Manufacturing Processes for Nuclear Applications Based on Technology Readiness Assessment. Nucl. Mater. Energy 2021, 27, 101006. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Nanda, S.; Castello, D.; Dalai, A.K.; Kozinski, J.A. Modeling and Process Optimization of Hydrothermal Gasification for Hydrogen Production: A Comprehensive Review. J. Supercrit. Fluids 2021, 173, 105199. [Google Scholar] [CrossRef]
- Rodríguez-Rangel, H.; Arias, D.M.; Morales-Rosales, L.A.; Gonzalez-Huitron, V.; Partida, M.V.; García, J. Machine Learning Methods Modeling Carbohydrate-Enriched Cyanobacteria Biomass Production in Wastewater Treatment Systems. Energies 2022, 15, 2500. [Google Scholar] [CrossRef]
- Tourigny, D.S.; Goldberg, A.P.; Karr, J.R. Simulating Single-Cell Metabolism Using a Stochastic Flux-Balance Analysis Algorithm. Biophys. J. 2021, 120, 5231–5242. [Google Scholar] [CrossRef]
- Kujawska, N.; Talbierz, S.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Optimizing Docosahexaenoic Acid (DHA) Production by Schizochytrium Sp. Grown on Waste Glycerol. Energies 2021, 14, 1685. [Google Scholar] [CrossRef]
- Zielińska, M.; Rusanowska, P.; Zieliński, M.; Dudek, M.; Kazimierowicz, J.; Quattrocelli, P.; Dębowski, M. Liquid Fraction of Digestate Pretreated with Membrane Filtration for Cultivation of Chlorella Vulgaris. Waste Manag. 2022, 146, 1–10. [Google Scholar] [CrossRef]
- Pourmirza, Z.; Hosseini, S.H.R.; Walker, S.; Giaouris, D.; Taylor, P. The Landscape and Roadmap of the Research and Innovation Infrastructures in Energy: A Review of the Case Study of the UK. Sustainability 2022, 14, 7197. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent Advances in Scaling-up of Non-Conventional Extraction Techniques: Learning from Successes and Failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.N.; Sanchez, A.; Rocha, G.J.M.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering Aspects of Hydrothermal Pretreatment: From Batch to Continuous Operation, Scale-up and Pilot Reactor under Biorefinery Concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Sustainability Analysis of Microalgae Production Systems: A Review on Resource with Unexploited High-Value Reserves. Environ. Sci. Technol. 2018, 52, 14031–14049. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of Microalgae in Adjusted Wastewater to Enhance Biofuel Production and Reduce Environmental Impact: Pyrolysis Performances and Life Cycle Assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Li, G.; Hao, Y.; Yang, T.; Xiao, W.; Pan, M.; Huo, S.; Lyu, T. Enhancing Bioenergy Production from the Raw and Defatted Microalgal Biomass Using Wastewater as the Cultivation Medium. Bioengineering 2022, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Mendieta, O.; Castro, L.; Vera, E.; Rodríguez, J.; Escalante, H. Toward the Adoption of Anaerobic Digestion Technology through Low-Cost Biodigesters: A Case Study of Non-Centrifugal Cane Sugar Producers in Colombia. Water 2021, 13, 2566. [Google Scholar] [CrossRef]
- Ubando, A.T.; Anderson S. Ng, E.; Chen, W.H.; Culaba, A.B.; Kwon, E.E. Life Cycle Assessment of Microalgal Biorefinery: A State-of-the-Art Review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef] [PubMed]
- Saranya, G.; Ramachandra, T.V. Life Cycle Assessment of Biodiesel from Estuarine Microalgae. Energy Convers. Manag. X 2020, 8, 100065. [Google Scholar] [CrossRef]
- Sangma, C.B.K.; Chalie-u, R. Life Cycle Assessment of Wastewater Treatment by Microalgae. Valorization Microalgal Biomass Wastewater Treat. 2023, 137–178. [Google Scholar] [CrossRef]
- Juliano, P.; Reyes-De-Corcuera, J.I. Food Engineering Innovations across the Food Supply Chain: Debrief and Learnings from the ICEF13 Congress and the Future of Food Engineering. Food Eng. Innov. Across Food Supply Chain 2022, 431–476. [Google Scholar] [CrossRef]
- Kuo, C.M.; Sun, Y.L.; Lin, C.H.; Lin, C.H.; Wu, H.T.; Lin, C.S. Cultivation and Biorefinery of Microalgae (Chlorella sp.) for Producing Biofuels and Other Byproducts: A Review. Sustainability 2021, 13, 13480. [Google Scholar] [CrossRef]
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal Biomass Valorization for Biofuel Production and Carbon Sequestration: A Review. Environ. Chem. Lett. 2022, 20, 2797–2851. [Google Scholar] [CrossRef]
- Rivera, D.R.T.; Ubando, A.T.; Chen, W.H.; Culaba, A.B. Energy Balance of Torrefied Microalgal Biomass with Production Upscale Approached by Life Cycle Assessment. J. Environ. Manag. 2021, 294, 112992. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Teng, S.Y.; How, B.S.; Nam, K.J.; Heo, S.K.; Máša, V.; Stehlík, P.; Yoo, C.K. From Microalgae to Bioenergy: Identifying Optimally Integrated Biorefinery Pathways and Harvest Scheduling under Uncertainties in Predicted Climate. Renew. Sustain. Energy Rev. 2022, 168, 112865. [Google Scholar] [CrossRef]
- Chamkalani, A.; Zendehboudi, S.; Rezaei, N.; Hawboldt, K. A Critical Review on Life Cycle Analysis of Algae Biodiesel: Current Challenges and Future Prospects. Renew. Sustain. Energy Rev. 2020, 134, 110143. [Google Scholar] [CrossRef]
- Mu, D.; Xin, C.; Zhou, W. Life Cycle Assessment and Techno-Economic Analysis of Algal Biofuel Production. Microalgae Cultiv. Biofuels Prod. 2020, 281–292. [Google Scholar] [CrossRef]
- Ideris, F.; Zamri, M.F.M.A.; Shamsuddin, A.H.; Nomanbhay, S.; Kusumo, F.; Fattah, I.M.R.; Mahlia, T.M.I. Progress on Conventional and Advanced Techniques of In Situ Transesterification of Microalgae Lipids for Biodiesel Production. Energies 2022, 15, 7190. [Google Scholar] [CrossRef]
- Jouannais, P.; Hindersin, S.; Löhn, S.; Pizzol, M. Stochastic LCA Model of Upscaling the Production of Microalgal Compounds. Environ. Sci. Technol. 2022, 56, 10454–10464. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae—A Green Multi-Product Biorefinery for Future Industrial Prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Kapoor, S.; Singh, M.; Srivastava, A.; Chavali, M.; Chandrasekhar, K.; Verma, P. Extraction and Characterization of Microalgae-Derived Phenolics for Pharmaceutical Applications: A Systematic Review. J. Basic Microbiol. 2022, 62, 1044–1063. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Ki, M.R.; Pack, S.P. Recent Progress in Flocculation, Dewatering, and Drying Technologies for Microalgae Utilization: Scalable and Low-Cost Harvesting Process Development. Bioresour. Technol. 2022, 344, 126404. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Badawi, M.; Mohanakrishna, G.; Aminabhavi, T.M. Valorization of Micro-Algae Biomass for the Development of Green Biorefinery: Perspectives on Techno-Economic Analysis and the Way towards Sustainability. Chem. Eng. J. 2023, 453, 139754. [Google Scholar] [CrossRef]
- Wicker, R.J.; Kumar, G.; Khan, E.; Bhatnagar, A. Emergent Green Technologies for Cost-Effective Valorization of Microalgal Biomass to Renewable Fuel Products under a Biorefinery Scheme. Chem. Eng. J. 2021, 415, 128932. [Google Scholar] [CrossRef]
- Klin, M.; Pniewski, F.; Latała, A. Multistage Optimization of Growth and Physiological Condition of Brackish Green Microalgae with the Use of Natural Waters. Aquaculture 2023, 562, 738820. [Google Scholar] [CrossRef]
- Rashid, T.U.; Salem, K.S.; Islam, M.M.; Zaman, A.; Khan, M.N.; Luna, I.Z.; Haque, P.; Rahman, M.M. Recent developments of microbial fuel cell as sustainable bio-energy sources. In Microbial Catalysts; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2019; Volume 2, pp. 57–115. [Google Scholar]
- Ranglová, K.; Lakatos, G.E.; Manoel, J.A.C.; Grivalský, T.; Estrella, F.S.; Fernández, F.G.A.; Molnár, Z.; Ördög, V.; Masojídek, J. Growth, biostimulant and biopesticide activity of the MACC-1 Chlorella strain cultivated outdoors in inorganic medium and wastewater. Algal Res. 2021, 53, 102136. [Google Scholar] [CrossRef]
- Kujawska, N.; Talbierz, S.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Cultivation Method Effect on Schizochytrium Sp. Biomass Growth and Docosahexaenoic Acid (DHA) Production with the Use of Waste Glycerol as a Source of Organic Carbon. Energies 2021, 14, 2952. [Google Scholar] [CrossRef]
- Sundui, B.; Ramirez Calderon, O.A.; Abdeldayem, O.M.; Lázaro-Gil, J.; Rene, E.R.; Sambuu, U. Applications of Machine Learning Algorithms for Biological Wastewater Treatment: Updates and Perspectives. Clean Technol. Environ. Policy 2021, 23, 127–143. [Google Scholar] [CrossRef]
- Lim, H.R.; Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Ho, S.H.; Show, P.L. Smart Microalgae Farming with Internet-of-Things for Sustainable Agriculture. Biotechnol. Adv. 2022, 57, 107931. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Chuka-Ogwude, D.; Vadiveloo, A.; Ogbonna, J.C. Process Control Strategies Applied to Microalgae-Based Biofuel Production. In 3rd Generation Biofuels: Disruptive Technologies to Enable Commercial Production; Woodhead Publishing: Sawston, UK, 2022; pp. 105–134. [Google Scholar] [CrossRef]
- Rodríguez-Miranda, E.; Guzmán, J.L.; Berenguel, M.; Acién, F.G.; Visioli, A. Diurnal and Nocturnal PH Control in Microalgae Raceway Reactors by Combining Classical and Event-Based Control Approaches. Water Sci. Technol. 2020, 82, 1155–1165. [Google Scholar] [CrossRef]
- EKİN, İ. Types of Microalgae Cultivation Photobioreactors and Production Process of Microalgal Biodiesel as Alternative Fuel. Acta Biol. Turc. 2020, 33, 114–131. [Google Scholar]
- Masojídek, J.; Ranglová, K.; Lakatos, G.E.; Benavides, A.M.S.; Torzillo, G. Variables Governing Photosynthesis and Growth in Microalgae Mass Cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Seop Lee, J.; Joon Sung, Y.; Jun Sim, S. Kinetic Analysis of Microalgae Cultivation Utilizing 3D-Printed Real-Time Monitoring System Reveals Potential of Biological CO2 Conversion. Bioresour. Technol. 2022, 364, 128014. [Google Scholar] [CrossRef]
- Sirohi, R.; Kumar Pandey, A.; Ranganathan, P.; Singh, S.; Udayan, A.; Kumar Awasthi, M.; Hoang, A.T.; Chilakamarry, C.R.; Kim, S.H.; Sim, S.J. Design and Applications of Photobioreactors—A Review. Bioresour. Technol. 2022, 349, 126858. [Google Scholar] [CrossRef] [PubMed]
- Balkanlı, N.E.; Işıldak, İ.; İnan, B.; Özer, T.; Özçimen, D. Monitoring Microalgal Growth of Chlorella Minutissima with a New All Solid-State Contact Nitrate Selective Sensor. Biotechnol. Prog. 2022, 38, e3247. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Geranpayehvaghei, M.; Ejeian, F.; Razmjou, A.; Asadnia, M. Recent Advances in Polymeric Nanostructured Ion Selective Membranes for Biomedical Applications. Talanta 2021, 235, 122815. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.L.; Bernard, O. Modeling the Influence of Temperature, Light Intensity and Oxygen Concentration on Microalgal Growth Rate. Processes 2021, 9, 496. [Google Scholar] [CrossRef]
- Rossi, S.; Casagli, F.; Mantovani, M.; Mezzanotte, V.; Ficara, E. Selection of Photosynthesis and Respiration Models to Assess the Effect of Environmental Conditions on Mixed Microalgae Consortia Grown on Wastewater. Bioresour. Technol. 2020, 305, 122995. [Google Scholar] [CrossRef]
- Havlik, I.; Beutel, S.; Scheper, T.; Reardon, K.F. On-Line Monitoring of Biological Parameters in Microalgal Bioprocesses Using Optical Methods. Energies 2022, 15, 875. [Google Scholar] [CrossRef]
- Lacour, T.; Larivière, J.; Ferland, J.; Morin, P.I.; Grondin, P.L.; Donaher, N.; Cockshutt, A.; Campbell, D.A.; Babin, M. Photoacclimation of the Polar Diatom Chaetoceros Neogracilis at Low Temperature. PLoS ONE 2022, 17, e0272822. [Google Scholar] [CrossRef]
- Vázquez, V.; León, P.; Gordillo, F.J.L.; Jiménez, C.; Concepción, I.; Mackenzie, K.; Bresnan, E.; Segovia, M. High-CO2 Levels Rather than Acidification Restrict Emiliania Huxleyi Growth and Performance. Microb. Ecol. 2022, 1–17. [Google Scholar] [CrossRef]
- Salgueiro, J.L.; Pérez, L.; Sanchez, Á.; Cancela, Á.; Míguez, C. Microalgal Biomass Quantification from the Non-Invasive Technique of Image Processing through Red–Green–Blue (RGB) Analysis. J. Appl. Phycol. 2022, 34, 871–881. [Google Scholar] [CrossRef]
- Zahedi, A.; Labbafi, S. Optimization of Biomass Growth for a Novel Quadruple Renewable Geothermal/Hydro/Biomass/Solar Hybrid System. Fuel 2021, 306, 121694. [Google Scholar] [CrossRef]
- Sá, M.; Bertinetto, C.G.; Ferrer-Ledo, N.; Jansen, J.J.; Wijffels, R.; Crespo, J.G.; Barbosa, M.; Galinha, C.F. Fluorescence Spectroscopy and Chemometrics for Simultaneous Monitoring of Cell Concentration, Chlorophyll and Fatty Acids in Nannochloropsis Oceanica. Sci. Rep. 2020, 10, 7688. [Google Scholar] [CrossRef]
- Divya Kuravi, S.; Venkata Mohan, S. Mixotrophic Cultivation of Monoraphidium Sp. In Dairy Wastewater Using Flat-Panel Photobioreactor and Photosynthetic Performance. Bioresour. Technol. 2022, 348, 126671. [Google Scholar] [CrossRef]
- Salam, A. Internet of Things in Sustainable Energy Systems. Internet Things Sustain. Community Dev. 2020, 183–216. [Google Scholar] [CrossRef]
- Paul, A.; Jeyaraj, R. Internet of Things: A Primer. Hum. Behav. Emerg. Technol. 2019, 1, 37–47. [Google Scholar] [CrossRef]
- Wang, K.; Khoo, K.S.; Leong, H.Y.; Nagarajan, D.; Chew, K.W.; Ting, H.Y.; Selvarajoo, A.; Chang, J.S.; Show, P.L. How Does the Internet of Things (IoT) Help in Microalgae Biorefinery? Biotechnol. Adv. 2022, 54, 107819. [Google Scholar] [CrossRef]
- Issac, K.; Pranay, G.; Bharanidharan, N.; Rajaguru, H. A Study on Real World Implementation and Future Trends of Internet of Things. In Proceedings of the 2020 Fourth International Conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC), Palladam, India, 7–9 October 2020; pp. 357–361. [Google Scholar] [CrossRef]
- Lombardi, M.; Pascale, F.; Santaniello, D. Internet of Things: A General Overview between Architectures, Protocols and Applications. Information 2021, 12, 87. [Google Scholar] [CrossRef]
- Thiviyanathan, V.A.; Lim, H.R.; Tham, P.E.; Show, P.L. How Far Has the Development for Industrial Internet of Things (IoT) in Microalgae? Microalgae Environ. Biotechnol. 2022, 145–174. [Google Scholar] [CrossRef]
- Srivastava, R.K. Bio-Energy Production by Contribution of Effective and Suitable Microbial System. Mater. Sci. Energy Technol. 2019, 2, 308–318. [Google Scholar] [CrossRef]
- Fayyaz, M.; Chew, K.W.; Show, P.L.; Ling, T.C.; Ng, I.S.; Chang, J.S. Genetic Engineering of Microalgae for Enhanced Biorefinery Capabilities. Biotechnol. Adv. 2020, 43, 107554. [Google Scholar] [CrossRef]
- Barati, B.; Zeng, K.; Baeyens, J.; Wang, S.; Addy, M.; Gan, S.Y.; El-Fatah Abomohra, A. Recent Progress in Genetically Modified Microalgae for Enhanced Carbon Dioxide Sequestration. Biomass Bioenergy 2021, 145, 105927. [Google Scholar] [CrossRef]
- Xie, Y.; Khoo, K.S.; Chew, K.W.; Devadas, V.V.; Phang, S.J.; Lim, H.R.; Rajendran, S.; Show, P.L. Advancement of Renewable Energy Technologies via Artificial and Microalgae Photosynthesis. Bioresour. Technol. 2022, 363, 127830. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.; Jaiswal, K.K.; Vlaskin, M.S.; Nanda, M.; Tripathi, M.K.; Kumar, S. Microalgae with a Truncated Light-Harvesting Antenna to Maximize Photosynthetic Efficiency and Biomass Productivity: Recent Advances and Current Challenges. Process Biochem. 2021, 104, 83–91. [Google Scholar] [CrossRef]
- Fajardo, C.; De Donato, M.; Carrasco, R.; Martínez-Rodríguez, G.; Mancera, J.M.; Fernández-Acero, F.J. Advances and Challenges in Genetic Engineering of Microalgae. Rev. Aquac. 2020, 12, 365–381. [Google Scholar] [CrossRef]
- Trovão, M.; Schüler, L.M.; Machado, A.; Bombo, G.; Navalho, S.; Barros, A.; Pereira, H.; Silva, J.; Freitas, F.; Varela, J. Random Mutagenesis as a Promising Tool for Microalgal Strain Improvement towards Industrial Production. Mar. Drugs 2022, 20, 440. [Google Scholar] [CrossRef] [PubMed]
- Godbole, V.; Pal, M.K.; Gautam, P. A Critical Perspective on the Scope of Interdisciplinary Approaches Used in Fourth-Generation Biofuel Production. Algal Res. 2021, 58, 102436. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, X.; Cui, Y.; Hu, X.; Qian, J.; Wang, F.; Kubar, A.A.; Xu, L.; Huo, S. Weak Magnetic Field Intervention on Outdoor Production of Oil-Rich Filamentous Microalgae: Influence of Seasonal Changes. Bioresour. Technol. 2022, 348, 126707. [Google Scholar] [CrossRef]
- Severo, I.A.; Siqueira, S.F.; Deprá, M.C.; Maroneze, M.M.; Zepka, L.Q.; Jacob-Lopes, E. Biodiesel Facilities: What Can We Address to Make Biorefineries Commercially Competitive? Renew. Sustain. Energy Rev. 2019, 112, 686–705. [Google Scholar] [CrossRef]
- Sadatshojaei, E.; Wood, D.A.; Mowla, D. Third Generation of Biofuels Exploiting Microalgae. Nanotechnol. Life Sci. 2020, 575–588. [Google Scholar] [CrossRef]
- Sebesta, J.; Xiong, W.; Guarnieri, M.T.; Yu, J. Biocontainment of Genetically Engineered Algae. Front. Plant Sci. 2022, 13, 839446. [Google Scholar] [CrossRef]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Intisar, M.; Hussain, A.; Bilal, M.; Iqbal, H.M.N. Genetically Engineered Microorganisms for Environmental Remediation. Chemosphere 2023, 310, 136751. [Google Scholar] [CrossRef]
- Jasni, J.; Yasin, M.; Haiza, N.; Ruslan, N.; Nurul, C.; Che, A.; Arisht, S.N.; Bajunaid Hariz, H.; Sobri Takriff, M.; Sajab, M.S. Strategies of Utilising Agriculture Wastewater for Microalgae Cultivation and Its Possible Applications: A Review Microalgal Biomass Harvesting for Subsequence Production of Valuable Products View Project MISC SMAFEG Research Project View Project Strategi. ASM Sci. J. 2021, 16, 2021. [Google Scholar] [CrossRef]
- Wicker, R.J.; Kwon, E.; Khan, E.; Kumar, V.; Bhatnagar, A. The Potential of Mixed-Species Biofilms to Address Remaining Challenges for Economically-Feasible Microalgal Biorefineries: A Review. Chem. Eng. J. 2023, 451, 138481. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. The Promising Way to Treat Wastewater by Microalgae: Approaches, Mechanisms, Applications and Challenges. J. Water Process Eng. 2022, 49, 103012. [Google Scholar] [CrossRef]
- Chu, R.; Li, S.; Zhu, L.; Yin, Z.; Hu, D.; Liu, C.; Mo, F. A Review on Co-Cultivation of Microalgae with Filamentous Fungi: Efficient Harvesting, Wastewater Treatment and Biofuel Production. Renew. Sustain. Energy Rev. 2021, 139, 110689. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent Advances Biodegradation and Biosorption of Organic Compounds from Wastewater: Microalgae-Bacteria Consortium—A Review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A Review on Algal-Bacterial Symbiotic System for Effective Treatment of Wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Harun, M.R.; Lau, S.Y.; Sewu, D.D.; Danquah, M.K. Microalgal Biomass Generation via Electroflotation: A Cost-Effective Dewatering Technology. Appl. Sci. 2020, 10, 9053. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-Bacterial Consortia: From Interspecies Interactions to Biotechnological Applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- dos Santos, V.Z.; Vieira, K.R.; Nass, P.P.; Zepka, L.Q.; Jacob-Lopes, E. Application of Microalgae Consortia/Cocultures in Wastewater Treatment. Recent Adv. Microb. Degrad. 2021, 131–154. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.M.; Jung, S.; Park, Y.K.; Tsang, Y.F.; Lin, K.Y.A.; Choi, Y.E.; Kwon, E.E. Biodiesel from Microalgae: Recent Progress and Key Challenges. Prog. Energy Combust. Sci. 2022, 93, 101020. [Google Scholar] [CrossRef]
- Nayana, K.; Arunkumar, K. Heterotrophic Microalgal Production System Via Utilization of Wastewater in Microalgal Production. Micro-algae Next-generation Feed. Biorefineries 2022, 177–191. [Google Scholar] [CrossRef]
- Ray, A.; Nayak, M.; Ghosh, A. A Review on Co-Culturing of Microalgae: A Greener Strategy towards Sustainable Biofuels Production. Sci. Total Environ. 2022, 802, 149765. [Google Scholar] [CrossRef]
- Vieira de Mendonça, H.; Assemany, P.; Abreu, M.; Couto, E.; Maciel, A.M.; Duarte, R.L.; Barbosa dos Santos, M.G.; Reis, A. Microalgae in a Global World: New Solutions for Old Problems? Renew. Energy 2021, 165, 842–862. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Wang, Q. Microalgal Biofuels in China: The Past, Progress and Prospects. GCB Bioenergy 2020, 12, 1044–1065. [Google Scholar] [CrossRef]
- Allnutt, F.C.T. Promising Future Products from Microalgae for Commercial Applications. Sustain. Downstr. Process. Microalgae Ind. Appl. 2019, 39–68. [Google Scholar] [CrossRef]
- Johansson, P.O.; Kriström, B. Welfare Evaluation of Subsidies to Renewable Energy in General Equilibrium: Theory and Application. Energy Econ. 2019, 83, 144–155. [Google Scholar] [CrossRef]
- Bashir, M.F.; MA, B.; Bashir, M.A.; Radulescu, M.; Shahzad, U. Investigating the Role of Environmental Taxes and Regulations for Renewable Energy Consumption: Evidence from Developed Economies. Econ. Res. 2021, 35, 1262–1284. [Google Scholar] [CrossRef]
- Mahata, C.; Das, P.; Khan, S.; Thaher, M.I.A.; Abdul Quadir, M.; Annamalai, S.N.; Al Jabri, H. The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F). Ferment. 2022, 8, 316. [Google Scholar] [CrossRef]
- Strapasson, A.; Woods, J.; Meessen, J.; Mwabonje, O.; Baudry, G.; Mbuk, K. EU Land Use Futures: Modelling Food, Bioenergy and Carbon Dynamics. Energy Strateg. Rev. 2020, 31, 100545. [Google Scholar] [CrossRef]
- Vigani, M. Bioeconomy of Microalgae-Based Fuels. In 3rd Generation Biofuels: Disruptive Technologies to Enable Commercial Production; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Malik, S.; Shahid, A.; Betenbaugh, M.J.; Liu, C.G.; Mehmood, M.A. A Novel Wastewater-Derived Cascading Algal Biorefinery Route for Complete Valorization of the Biomass to Biodiesel and Value-Added Bioproducts. Energy Convers. Manag. 2022, 256, 115360. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Raj, T.; Ramanaiah, S.V.; Kumar, G.; Banu, J.R.; Varjani, S.; Sharma, P.; Pandey, A.; Kumar, S.; Kim, S.H. Algae Biorefinery: A Promising Approach to Promote Microalgae Industry and Waste Utilization. J. Biotechnol. 2022, 345, 1–16. [Google Scholar] [CrossRef]
- Business Climate Efforts and Their Importance for Competitiveness and Development-Perspectives from Poland and the Baltics. Available online: https://www.teraz-srodowisko.pl/media/pdf/aktualnosci/5953-business-climate-report-Poland-Baltic-countries.pdf (accessed on 20 October 2022).
- ORLEN Group. Integrated Report. 2017. Available online: https://raportzintegrowany2017.orlen.pl/ (accessed on 22 October 2022).
- Mikielewicz, D.; Kosowski, K.; Tucki, K.; Piwowarski, M.; Stepien, R.; Orynycz, O.; Włodarski, W. Gas Turbine Cycle with External Combustion Chamber for Prosumer and Distributed Energy Systems. Energies 2019, 12, 3501. [Google Scholar] [CrossRef]
- PKN ORLEN Invests in Biofuels of the Future. Available online: https://www.orlen.pl/pl (accessed on 22 October 2022).
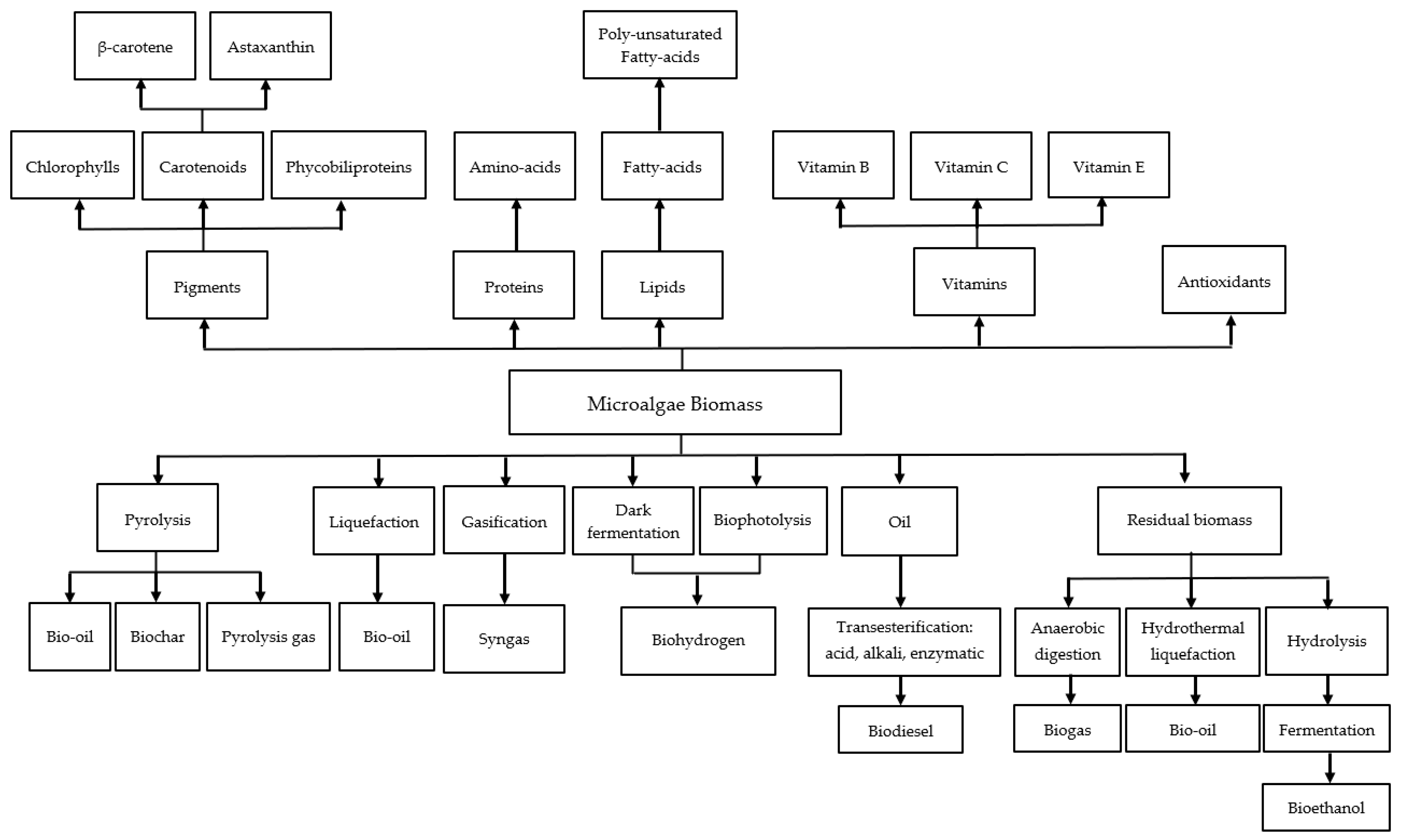
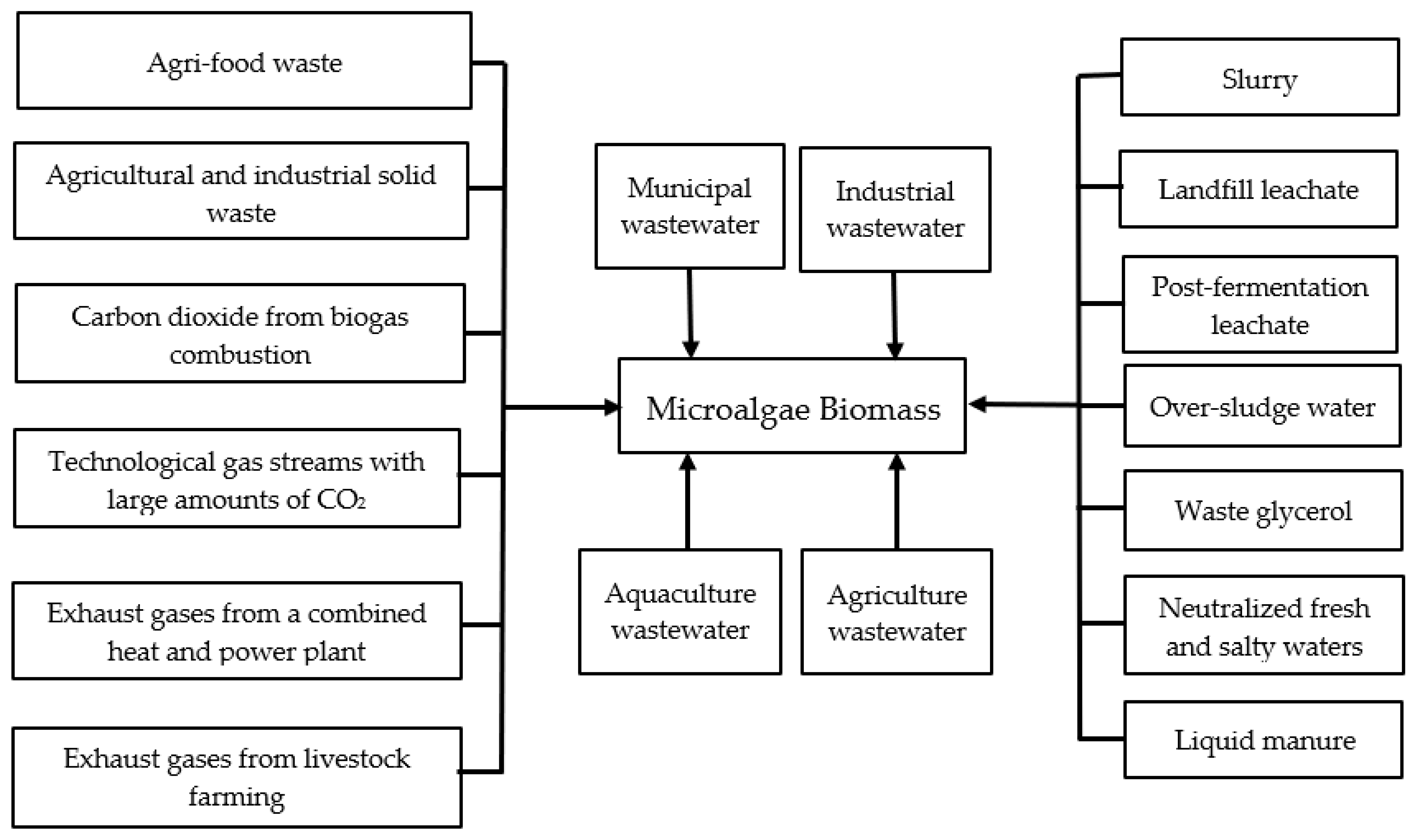
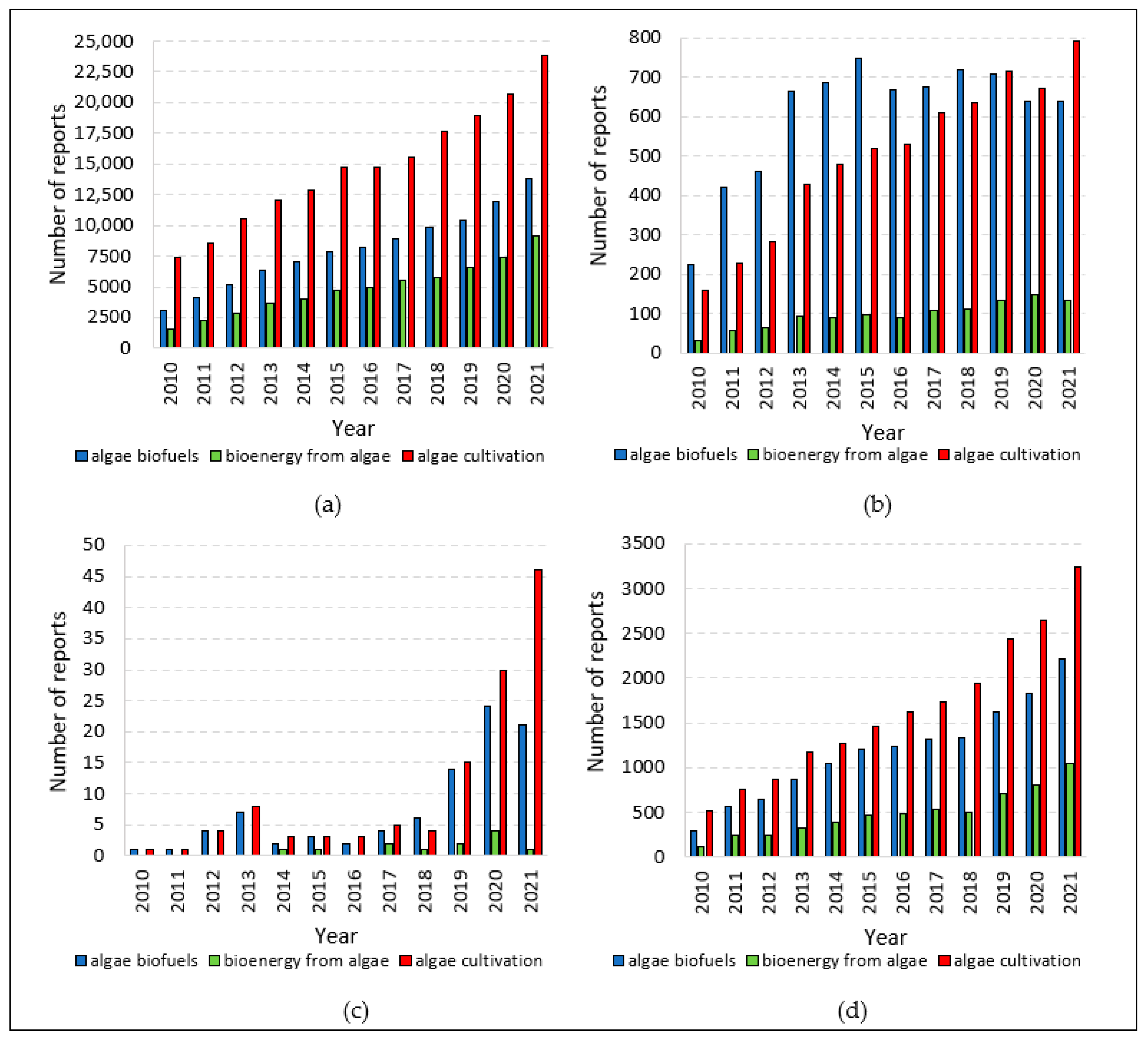
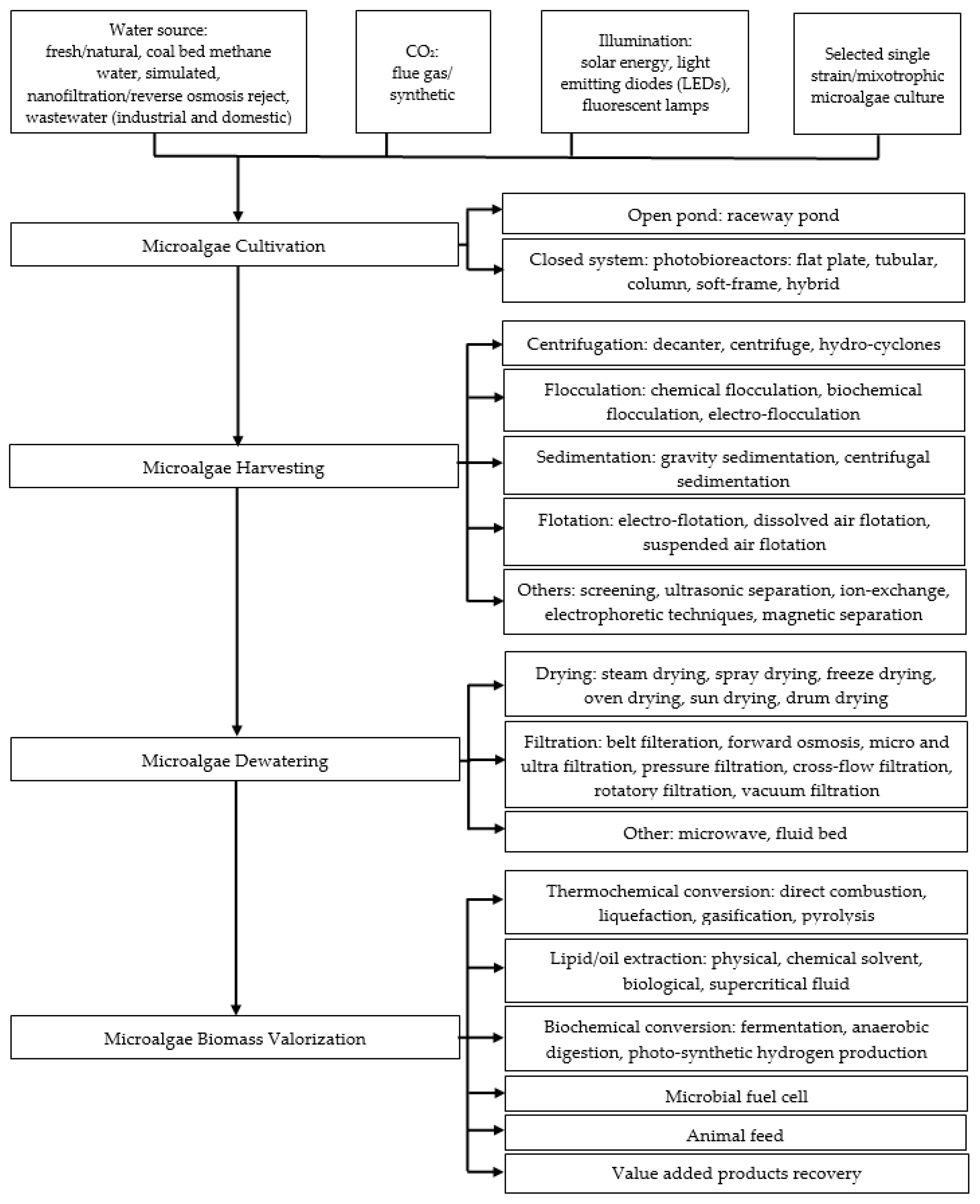
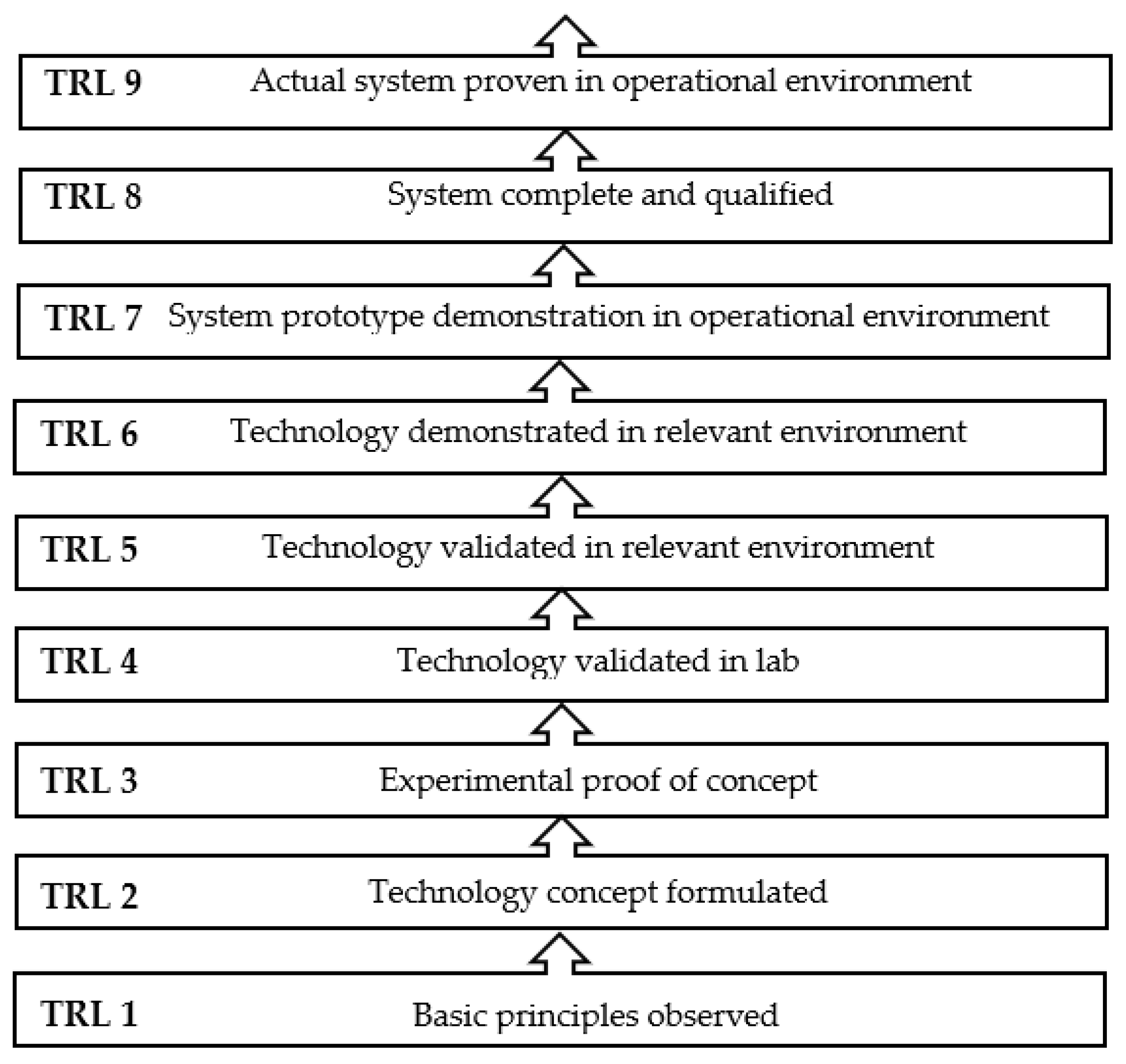

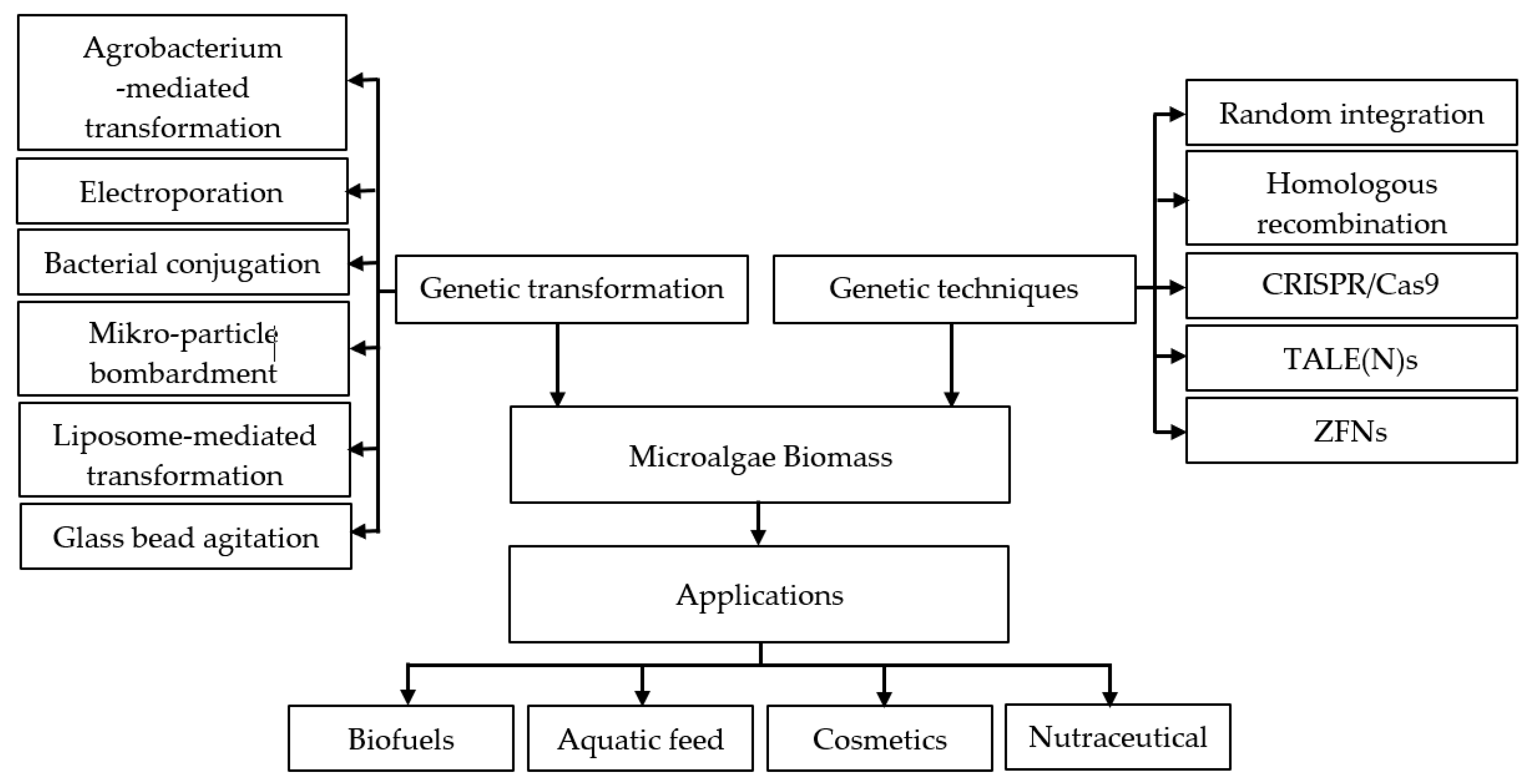
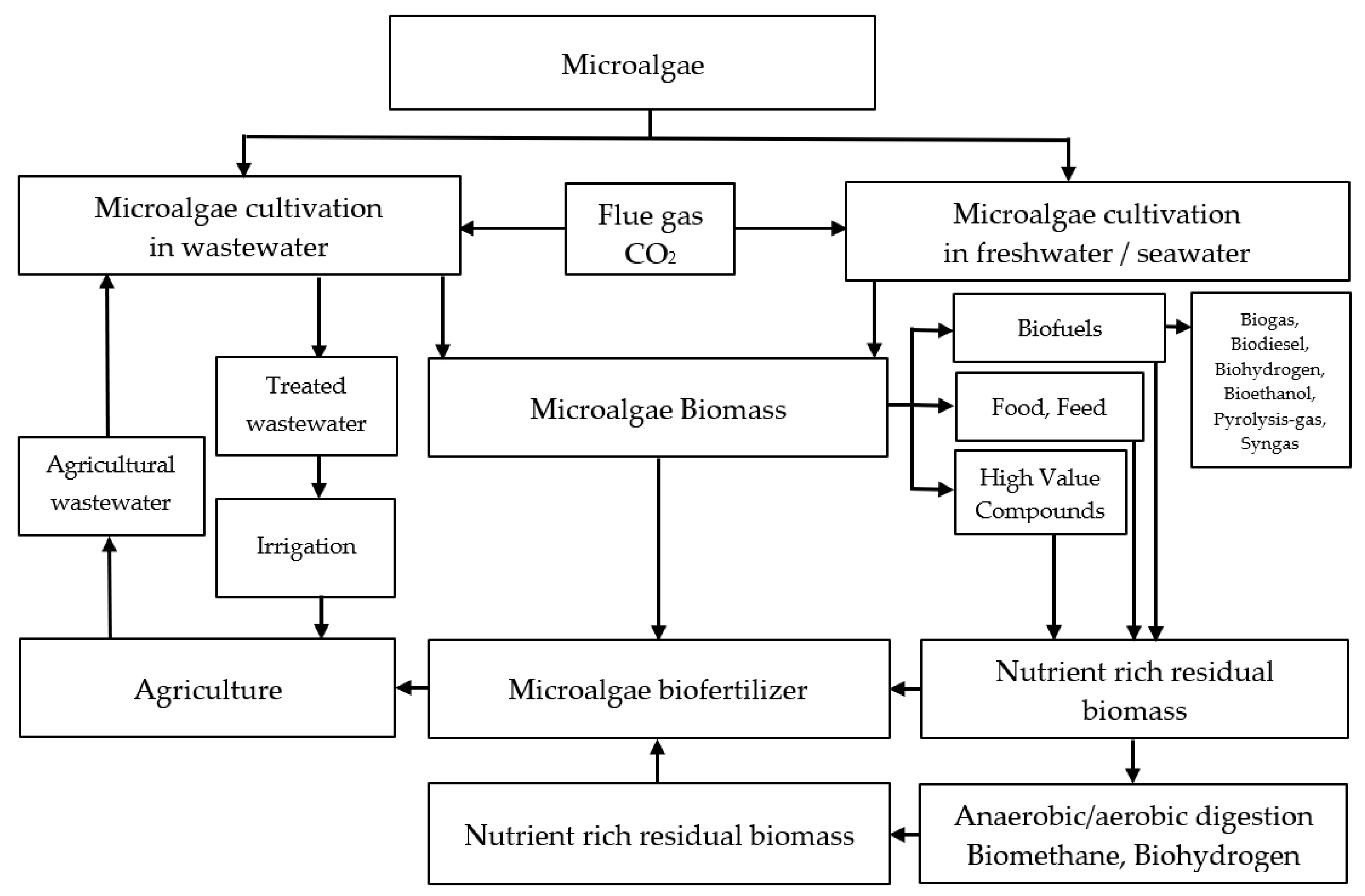
| Monitored Parameters | Sensors | Control Options/Monitoring Method | Acceptable Range or Off-Line/On-Line | Comment | Refs. |
| pH | Optical pH sensor; pH glass electrode | CO2 injection | 7–10 | Value out of range: growth rate decrease | [80,81] |
| Temperature | Thermoelement | Water bath; Water spraying; Heat exchanger; Shading | 15–35 °C | High: culture death; Low: slow growth | [80,82] |
| Light density | Quantum sensor: flat cosine, fiberoptic spherical, PAR dosimeter, integration solarimeter | Culture density; PBR design; mixing | 10–250 μM/m2/s (optimal); 0–2000 μM/m2/s (actual) | High: PE sinks at PFD > 250, Photoinhibition at >1500; Low: slow growth | [80,83] |
| Mixing | None | Agitation intensity; Gas addition rate | Re < 6000–10,000 | High: mechanical damage to cells; Low CO2 limitation/O2 inhibition | [84,85] |
| Inorganic nutrients | Colorimetric assays; UV spectroscopy; Ion-selective electrodes | Nutrient addition | Varies with nutrient | Low: growth limitation, lipid or starch accumulation | [86,87] |
| O2 | Paramagnetic and polarometric analyzer | - | Depends on O2 and mixing intensity | High: growth rate decrease | [88,89] |
| CO2 | Mass spectrometer; IR analyzer | CO2 feed | >0.15% | Low: growth rate decreases | [80,90] |
| pCO2 (liquid phase) | IR analyzer + flow meter; pCO2 electrode | CO2 injection | >0.1 kPa | Low: growth rate decreases below 0.1 kPa | [80,90] |
| Cell number concentration; Cell mass concentration; Cell morphology; Population composition | Microscope + CCD | ISM | On-line | Image-analysis software is critical | [91,92] |
| Cell mass concentration | CCD camera | Color analysis | Off-line | Data analysis software is critical | [93,94] |
| Cell mass concentration | OD sensor; Turbidity sensor | OD, turbidity | Off-line and on-line | Wavelength choice depends upon pigments | [80,90] |
| Protein; Lipid; Carbohydrate content | ATR flow system; Fiberoptic probe | IR radiation (MIR, NIR, FTIR) | Off-line and on-line | Data analysis software is critical | [80,90] |
| Lipid content; Cell size | Flow cytometer | FC | Off-line and on-line | Sample processing necessary for lipids and starch | [80,90] |
| Pigments; Fatty acids | Spectrophotometer | Absorbance spectrum | Off-line | Fatty acid levels may be estimated by correlation to pigment ratio | [90,95] |
| Pigments; Lipids; Photosynthetic efficiency; Quantum yield | Pulse amplitude modulated fluorometer | Fluorometry | Off-line and on-line | PAM identifies stress leading to lipid production onset | [90,96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Świca, I.; Kazimierowicz, J.; Zieliński, M. Large Scale Microalgae Biofuel Technology—Development Perspectives in Light of the Barriers and Limitations. Energies 2023, 16, 81. https://doi.org/10.3390/en16010081
Dębowski M, Świca I, Kazimierowicz J, Zieliński M. Large Scale Microalgae Biofuel Technology—Development Perspectives in Light of the Barriers and Limitations. Energies. 2023; 16(1):81. https://doi.org/10.3390/en16010081
Chicago/Turabian StyleDębowski, Marcin, Izabela Świca, Joanna Kazimierowicz, and Marcin Zieliński. 2023. "Large Scale Microalgae Biofuel Technology—Development Perspectives in Light of the Barriers and Limitations" Energies 16, no. 1: 81. https://doi.org/10.3390/en16010081
APA StyleDębowski, M., Świca, I., Kazimierowicz, J., & Zieliński, M. (2023). Large Scale Microalgae Biofuel Technology—Development Perspectives in Light of the Barriers and Limitations. Energies, 16(1), 81. https://doi.org/10.3390/en16010081









