Abstract
Energy Intensive Industries (EII) are high users of energy and some of these facilities are extremely dependent on Natural Gas for processing heat production. In European countries, where Natural Gas is mostly imported from external producers, the increase in international Natural Gas prices is making it difficult for some industries to deliver the required financial results. Therefore, they are facing complex challenges that could cause their delocalization in regions with lower energy costs. European countries lack on-site Natural Gas resources and the plans to reduce greenhouse gas emissions in the industrial sector make it necessary to find an alternative. Many different processes cannot be electrified, and in these cases, synthetic methane is one of the solutions and also represents an opportunity to reduce external energy supply dependency. This study analyzes the current development of power-to-gas technological solutions that could be implemented in large industrial consumers to produce Synthetic Methane using Green Hydrogen as a raw source and using Renewable Energy electricity mainly produced with photovoltaic or wind energy. The study also reviews the triple bottom line impact and the current development status and associated costs for each key component of a power-to-gas plant and the requirements to be fulfilled in the coming years to develop a cost-competitive solution available for commercial use.
1. Introduction
In the international ongoing context, the pollution of the environment and its consequences are one of the biggest problems that today’s society has to deal with; therefore, a global and multidisciplinary approach is required to deal with the problems and propose valuable solutions. Global warming is accelerating and its effects are becoming more intense; therefore, a strong impact on the economy, society and health, among others, is expected. A key factor in this scenario is the use of fossil fuels that generate CO2 which contributes to global warming. The triple bottom line impact on energy firms is clear (see Refs. [1,2] for an extended review). Management practices oriented to sustainability are needed such as eco-innovations and the reconfiguration of firm energy sources. These practices enhance firm productivity and profits, and at the same time, contribute to society by providing more safe environments and affordable prices of electricity for citizens, which has a direct impact on entrepreneurship and allows public institutions to improve the standard of life for their respective countries and regions.
Natural Gas (NG) has become one of the most important energy sources in industries and many industrial sectors directly rely on NG for heat generation, Combined Heat and Power (CHP), or power generation, among others, with socio-economic consequences for the sector and society. Moreover, many of the electrical demands of energy-intensive industries are matched using NG in power plants. The International Energy Agency (IEA) indicates that the industry’s CO2 emissions are 40% of total CO2 emissions and some energy-intensive industries, such as concrete or steel greatly contribute to the emissions as, for example, the steel industry contributes 27% of the industry sector [1,3]. This is a critical sector that can be used as a key example for these types of industries that strongly rely on fossil fuels usage because the steel and iron industry is one of the largest consumers of energy and coal in the world. Steel production processes and manufacturing are mainly based on coal and, therefore, highly dependent on fossil fuels, which produce enormous amounts of CO2. The steel industry is the second largest consumer of energy of all industries and is recognized as one of the largest direct sources of CO2 emissions. According to [3] in relation to the Intergovernmental Panel on Climate Change (IPCC), the steel industry accounts for 4–5% of global CO2 emissions. Similar scenarios are presented for the following sectors: concrete, glass manufacturing, sanitary ware, paper, ceramics and aluminum, among others. An ongoing price surge for NG is causing a disruption in manufacturing and causing some factories to cease their production, temporarily or definitively, due to the increase in NG costs and CO2 emission taxes. Moreover, stronger requirements for emission reductions require a reduction in fossil fuel consumption in these sectors. Firms need to continuously re-adapt their processes to reduce their consumption and be beneficial to society through their environmental awareness. For example, in Europe, in order to reduce climate change effects and to be able to meet a series of objectives, the Emission Trading Scheme (ETS) forces the European Union to reduce Greenhouse Gases (GHG) by 95% in 2050 compared to 1995 [4]. To this end, it is imperative for intensive NG industries to analyze their production chains to detect opportunities for reducing CO2 emissions. The required reductions can be achieved in three different ways:
- reducing manufactured material demand
- increasing used materials recycling or reutilization
- using innovative steel production technologies
For many of these industries, recent innovations and development in CO2 capture and utilization technologies such as Capture Carbon Storage (CCS) and Capture Carbon Utilization (CCU) seem to be the only way to substantially reduce emissions. In addition, these measures must be combined with the use of technologies to improve the operation of the factories by using the Best Available Technologies (BAT). According to [2,5], the CCU is a real option that can be extensively used to obtain chemicals or fuels. A particular use of captured CO2 is the production of CH4 (methane) which can be used as fuel for direct use in industries as a substitute for NG [6]. This process is known as Power to Gas (PtG) and represents a key opportunity for both fossil fuels and associated emission reductions, especially if Renewable Energy (RE) sources are used for electricity production. The whole process can be combined with implementation of the BAT to develop an effective fast track for emissions reduction [7]. The economic and social implications of the technologies are not less important: external energy dependence and subsequent energy supply costs are becoming critical factors for some regions, for example, in European countries. The consequences of the energy price surge are not only economic but social as many of the traditional employment sectors related to the industry are in a real collapse risk. Therefore, the use of PtG systems represents a multifactorial tool to reduce energy dependence, energy supply costs and associated GHGs emissions. PtG systems can also be combined with Power-to-Power (PtP) technologies that consist of the production of hydrogen that can be stored and later combined with electricity for consumption. As a consequence, multiple implementation schemes are feasible for intensive energy consumption industries that combine hydrogen generation, conversion to gas using a PtG process, hydrogen storage for excess renewable electricity production, or PtP conversion [3,4]. The recent effects of the COVID international crisis pose a risk for manufacturing companies as they need to face important challenges, two of the most important being increased raw material costs and supply chain interruption. For European countries the situation in Energy Intensive Industries is more complex as they need to face the additional risks related to NG cost increases and severe disruption risks derived from the Russian invasion of Ukraine, and the resultant consequences that have limited Russian gas availability and produced a price surge. This new scenario makes it necessary to find alternative NG suppliers, and this unexpected event has revealed the complex situation of European industries if they have to face energy supply disruptions or severe price increases. As a consequence, in the short-term the strategy to face the challenge is focused on finding alternative NG suppliers but, in the mid-term, the question about energy supply security arises and makes necessary the development of energy alternatives that reduce external dependency. Green Hydrogen (GH) production is getting high attention in the European Union and several countries, mainly the ones with large solar resources such as Spain, Italy and Greece, among others, are accelerating their plans for massive GH production. During the last few years, hydrogen production using electrolysis has undergone important research efforts but its potential use has not been considered as a key energy source in Europe. The ongoing situation and future scenarios make necessary the development of fast-track scenarios to produce alternative industrial fuels for NG substitution. The objective of this paper is to analyze the ongoing development of the key technologies of the PtG systems and the intermediate equipment, such as carbon capture or hydrogen production systems, the potential uses in intensive energy consumption industries, and the economic and social implications. The PtG technology relies on different technologies that have been traditionally analyzed and developed as isolated systems and PtG generation makes necessary and effective integration of all of them to effectively develop hydrogen for the Synthetic Methane (SM) production system. For EII the required approach differs from traditional schemes as the SM production system can be installed in situ in the industrial facility aiming for a total or part NG self-supply. This study analyzes the key parameters of each separate technology and the potential integration schemes and their most important technical aspects (generation potential, energy requirements and costs, among others) to generate SM to substitute NG in industries. The scientific objectives and benefits are directly related to the proposal of an updated and clear approach to the direct use of hydrogen technology in industries as a way to effectively have an alternative to Natural Gas in the energy transition process. The particular case of the European Union is deeply analyzed due to the inherent problems in this region previously mentioned: intensive Natural Gas dependency and no internal market production.
The study is structured as follows: Section 2 focuses on the Power to Gas technology and a review of the state of the art and the available technologies are presented, with a particular focus on the potential applications for industrial sectors. The most important technologies involved in the whole process (hydrogen production, carbon capture, and mechanization) are presented. In Section 3 the application of the energy transition process in industrial facilities with large Natural Gas consumption is studied. Section 4 discusses the most important aspects of the technology and the particular implications and potentials in Europe, and Section 5 presents the most relevant conclusions of the research. The appendix section provides extended information about the involved technologies (Appendix A) and the most relevant ongoing projects in Europe (Appendix B).
1.1. Analysis Methodology
The objective of the analysis is to review and analyze the specific challenges of EII in terms of thermal energy supply, focusing on the NG demand and later analyzing the development of the different key technologies that integrate a PtG system and the most important key parameters of its combination to produce SM. The effects of the unexpected COVID crisis, which had deeply affected European industries are being aggravated by the high rise of NG costs and the real possibility of supply disruptions. Despite the interest in some technologies directly related to SM generation, many of the efforts were strictly focused on its development as a separate technology. For example, in hydrogen generation for final use or to explore the possibility of effective Carbon Capture systems that could make it easier to tackle GHG emissions reduction objectives. The methodology of this analysis is as follows: the technological development of each of the involved technologies required for SM generation is analyzed and the last developments in the field and the most important conclusions presented in the recent literature are analyzed. Secondly, the European EII (EEII) dependency on NG and the current supply options are presented. With the results of both analyses in mind, the feasibility of effective SM generation for NG substitution is presented and the currently available technologies, their potentials, limitations, future research requirements and challenges are presented. Finally, a technical, economic and socio-economic analysis is presented and the real potential (based on the recent scientific data) of SM generation for European EII is presented. Finally, to provide a full-scope vision, the socio-economic aspects are also studied using a triple-bottom-line approach.
1.2. Natural Gas and Energy Intensive Industries
From a technical point of view, EII are the industries that highly depend on energy supply for their manufacturing or operational processes [8,9]. Therefore, although these industries have a large variability in their activities, for these industrial sectors, energy costs represent one of the most important production costs and they are directly influenced by final energy costs. Several international organizations classify the industrial sector by three categories: non-manufacturing industries, non-energy intensive manufacturing and energy-intensive manufacturing industries [9]. In many countries and regions, in industrial processes that require extensive heat supply, NG is one of the most used energy sources. In these cases, the need to search for alternative energy sources is directly driven by both the need of decarbonization and the extensive external NG supply dependency that also makes them dependent on NG prices. Electrification can be a mid-term solution only for some industrial sectors in which case electricity prices are also a challenge for EII because in many electricity markets, such as European or British markets among others, the final electricity prices are highly dependent on NG costs. As a consequence, it is necessary to search for potential NG substitutes for the industrial combustion processes, and the production of methane using hydrogen as a raw source represents one of the only large, scalable alternatives that could be implemented without compromising or changing the industrial process itself. The introduction of alternative energy sources in industrial processes, mainly electrification objectives, are not feasible in many industrial processes because there are not technologies that could directly substitute combustion systems by electrical ones. Moreover, these additional process manufacturing changes will require additional investments that involve operational risks.
2. State of the Art of Power to Gas Technology
This section analyzes the state of the art of each of the involved technologies required for PtG systems; in particular, those focused on industrial large-scale integration.
The PtG can be related to different gas production systems that use electrical power as an energy source. The PtG technology objective of this study consists of a process aiming to produce synthetic CH4, so-called Synthetic Methane using H2 and CO2 as raw materials. The whole production process includes all the methods of obtaining and storing the elements involved in the reaction to obtain synthetic CH4 [5]. Therefore, the key processes can be divided into three main sub-processes:
- Carbon capture and storage
- Hydrogen production and storage
- Synthetic fuel production (CH4)
The capture of the CO2 is considered on-site, in the energy-intensive facility. This gas can be produced in the manufacturing process itself, for example in concrete production or as a combustion product, and hydrogen could be produced in the same location or transported using both a hydrogen transport network or pressurized transport, such as road transport [5]. The flowchart of the PtG process is simplified in Figure 1. The scope of this review is to analyze feasibility and the current technologies that allow the use of both on-site generated H2 and CO2 for Synthetic Methane production and the possibilities of using the technology as an energy storage technology. SM is an interesting alternative in many different industries because it can be directly used in systems that currently use NG as fuel.
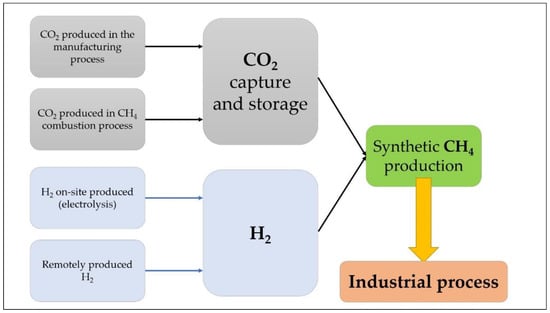
Figure 1.
Power to Gas technology (PtG).
Figure 2 presents the simplified flowchart for a PtP system. In this case, the electricity is used to produce H2 that can be effectively stored and later be reconverted again into electricity and self-consumed or injected into the grid. Therefore, it represents an effective alternative to conventional batteries and can provide a cost-effective solution that, as an additional advantage, does not extensively rely on raw materials, such as lithium or rare earth materials. The storage technology can be also effectively combined with a CHP system to produce electricity and reuse waste heat for industrial purposes.
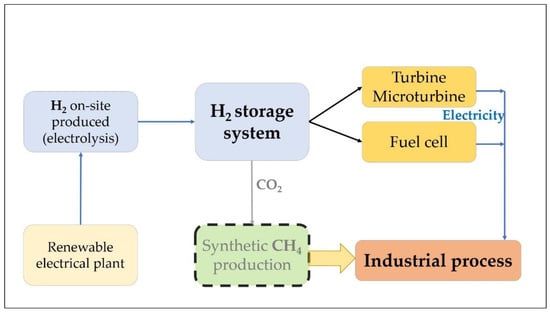
Figure 2.
Power to Power (PtP) system and possible integration of Combined Heat and Power.
2.1. Carbon Capture for Systems
CO2 capture systems for later methanization require an adequate source of CO2 that can range from about 1 MW in water treatment plants up to 1000 MW in coal-fired plants. The concentration of the gas in the flue gas stream depends on the process itself and the most common concentrations (% vol) are presented in Table 1 [10].

Table 1.
CO2 sources and concentration, adapted from [10].
CO2 capture technologies aim to recover carbon dioxide in different processes that, once captured, can be used in several areas such as the recovery of oil wells, the food industry and fertilizer production, etc.
The main methods of carbon dioxide capture are [7,10,11]:
- capture before combustion
- post-combustion capture
- capture in oxy-combustion processes
The method of capture before combustion is done by separating the fossil fuel into CO2 and H2 through three stages as presented below [12]:
- production of syngas, CO + H2
- conversion of CO to CO2
- effective separation of CO2 and H2
For the first stage, the methods are similar to the processes used to obtain hydrogen from fossil fuels and includes steam reforming, partial oxidation, autothermal reforming and gasification. To perform the second stage, a reaction process named “Water-Gas-Shift Reaction” is used and later, in stage 3, the separation of CO2 from H2 uses a gas separation technology such as absorption, adsorption, membranes and Cryogenic Distillation. The absorption of carbon dioxide can be chemical or physical; the chemical absorption process is carried out using amines, a type of chemical compound derived from ammonia. On the other hand, physical adsorption uses solvents with high affinity to capture acid gases that include CO2. Steam is required which makes the process energy intensive, and the method more expensive. The final separation of CO2 through the adsorption process is done using adsorbents such as activated carbon, zeolites and silicates that have the ability to attract particles on their surface. The process is not a selective process; therefore, carbon dioxide is adsorbed along with other gases. Also, membranes can be used for the gas separation process. Some of the materials that are used include materials that are metallic, ceramic and polymeric, etc. Cryogenic Distillation (CD) is a method of gas separation at low temperatures that is performed using the difference in the boiling points of gaseous components and its high cost makes it not suitable for large-scale PtG production [13,14].
The oxycombustion process is the capture of CO2 that begins with a combustion process using oxygen and recirculated gases (total oxycombustion) or air (so-called oxidizer). The resulting gas, which has a high percentage of CO2, is processed through a purification process where most nitrogen oxides and sulfur oxides are removed (NOx, SOx). In the particular case of this research where industrial furnaces are used (for example in cement plants), there is the associated problem due to combustion with oxygen because the temperature and heat distribution conditions inside the furnace would change and could, therefore, affect the manufacturing process. There is a lack of industrial-level tests about the effect of high levels of CO2 concentration inside the furnace and how reactions or CO2 products would change. To capture CO2 in post-combustion processes, the same methods applied before combustion are used: physical and chemical absorption, adsorption, membranes and carbonation-calcination. The carbonation-calcination technology is based on a reaction between CO2 and calcium oxide (CaO) in a gas stream followed by a reverse calcination reaction of calcium carbonate (CaCO3) in a concentrated atmosphere of CO2. For intensive energy consumption industries where the aim is reducing or eliminating the use of NG, only industrial-scale commercial systems could be effectively applied; chemical absorption of Monoethanolamine (MEA) is considered as a post-combustion carbon dioxide capture process that has a key advantage for existing industries because its implementation does not intervene in the manufacturing process and, therefore, the existing configuration of the plant is not modified [14].
The method consists of a reaction in the aqueous medium between the amine and the acid gas (CO2) and it is a very effective process of absorption, but it requires extensive energy usage because it is necessary to increase the temperature in the MEA regeneration column up to 100–140 °C. The last developments to reduce the cost per ton while avoiding CO2 are focused on the development of new absorbent substances that can reduce the energy contribution in the regeneration phase, although they are still in a preliminary phase because only small-scale studies and tests are currently carried out in pilot plants. Ammonia and amino acid salts have been tried or labeled as absorbent. In the case of ammonia, the temperature in the absorption tower drops, but the temperature in the regeneration tower increases. However, amino acid salts improve energy consumption, which worsens the process through the formation of solid precipitates [15]. From a commercial point of view, and because technology is ready to use only the chemical absorption by amines, will the one be available for implementation in industrial PtG plants, which is the objective of this work.
Thanks to the contact of these two flows, part of the CO2 in the gas flow is absorbed into the amine solution and later, the solution with the absorbed CO2, which is called the rich-loading solution, and is pumped to the top of the other column and the regenerator (stripper). In the regenerator, there is also a counter-current flow, the rich solution goes down the column, and the steam generated in the reboiler goes up. With the heat of the stripper steam, the chemical bonds between the CO2 and the solvent are broken and the CO2 is carried upwards thanks to the steam from the condenser. While the condensed steam is returned to the stripper as reflux, a flow of CO2 with high purity (around 99%) is obtained. At the bottom of the stripper, the amine solution called the lean-loading solution is recirculated to the top of the absorber and before entering the absorber, the lean-loading solution with a relatively high temperature (120 °C) is used to increase the temperature of the rich solution in order to recover some heat. The most significant indicator of this capture process is the ratio of CO2 that is captured to the input stream. In most processes, this value is set at 90% [12]. Specific values for the process, depending on the amine concentration, are shown in Table 2 [16].

Table 2.
CO2 capture system using amine with different concentrations, adapted from [17,18].
As shown in Table 1, in the base case (20% concentration) the energy consumption per ton of CO2 stored is 3.89 GJ, and to carry out the process it is necessary to provide 106 m3 of cooling water, and 20 m3 of amines per ton of CO2 that are required. For higher concentration values, such as 30% and 40% of MEA in solution, better results are obtained and a reduction in the energy needed to obtain a ton of CO2 is produced; however, on the other hand, it entails a greater consumption of amines. One additional system problem is the degradation of the absorbent agent results in strongly corrosive products. New research lines are focused on searching for an alternative to replacing 2-ethanolamine with tertiary, combined, sterically hindered amines, amino acid salts or ammonia. In the case of industrial systems with a not continuous operation system, one additional problem is the start-up time of the CO2 capture system which ranges between 45 min and 60 min [7].
2.2. Green Hydrogen Production Using Renewable Energy Driven Electrolizers
Green Hydrogen production using electrolysis consists of obtaining hydrogen and oxygen from water through the use of Renewable Energy-produced electricity. Considering the basic equation of the process, from one mole of water, one mole of hydrogen is obtained and half a mole of oxygen, meaning that if mass ratios are used, to obtain 1 kg of hydrogen it is required to use 9 kg of water, and 8 kg of oxygen is produced. At present, approximately 95% of the hydrogen produced comes from fossil fuels and the remaining 5% is a by-product that results from the production of chlorine through electrolysis which means that there is no significant production of hydrogen from renewable sources [13]. From a commercial point of view and aiming to provide a PtG solution from an industrial scale perspective, two types of electrolyzers that exist today are alkaline electrolyzers and Proton Membrane Exchange (PEM) electrolyzers. Solid Oxide Electrolyzers (SOE) present higher efficiencies than PEM or alkaline electrolyzers and operate at high temperatures of up to 1000 °C. This type of electrolyzer presents the potential of reducing energy demand and associated operating costs. Up to 40% of the energy required to produce hydrogen by steam electrolysis can be substituted by heat at up to 1000 °C, although new lab-scale developments work at about 700–800 °C and the electricity demand can be reduced by up to 25%. Therefore, it is expected to obtain better efficiencies with SOEs because its hydrogen production reaches up to 5.7 (Nm3H2)/h and its power up to 18 kW. However, these types of electrolyzers are under researched and under developed and are, therefore, not available for large-scale purposes in PtG applications. In applications for GH production, with intermittent or variable electricity generation, using RE continuous power changes results in heat loss and changes in cell temperature, which consequently produce ruptures in the membrane that reduce their lifetime.
The water electrolysis process is defined by two important parameters that characterize the process: cell efficiency and current density. The efficiency of the electrolysis cell is defined as the relation between the theoretical energy and the actual energy needed to stimulate the reaction [13]. The minimum potential to be applied for the reaction to occur under standard conditions (25 °C and 1 atm) is 1.23 V. An important part of the process involves catalysts that increase the speed of the reaction traditionally. The catalysts used are made of rare metals, but with technological advances new types of catalysts are appearing, for example, using nickel-based iron [19]. From an industrial point of view, alkaline and PEM electrolyzers are the solutions to be analyzed. If we compare the alkaline with the PEM in terms of efficiency and energy consumption, they are very similar. Alkaline has a higher production per hour of hydrogen, as well as a longer life cycle and lower economic cost. However, it is not so suitable for continuous energy changes, for which the PEM-type electrolyzer would perform better. According to the analysis performed by [20] the difference in investment between these two technologies is mainly on a small scale; if it is carried out on a larger scale, the final costs become very similar. PEM electrolyzers have the advantage of better adaptability to continuous changes in the power supply but their current price and state of development still make alkaline electrolyzers the most available commercial solution. Table 3 presents a comparison of both systems and, as shown, for a PtG system it can be considered that PEM-type electrolyzers have an efficiency of 70% and specific energy consumption of 55.6 kWhe/kgH2. Alkaline-type electrolyzers present a 70% efficiency and a specific energy consumption similar to that of the PEM type, the main difference being the production of hydrogen because alkaline electrolyzers have a capacity that is 25 times greater than PEM. More detailed information about electrolyzers is available in Appendix A.

Table 3.
Electrolyzers for Hydrogen production, adapted from [19].
The commercially available equipment range of PEM electrolyzers is about 150 kW and for alkaline electrolyzers, the rated power of the system ranges between 3 MW and 4 MW.
2.3. Methanization Technologies
The PtG core technology is the methanization process. Thermochemical methanization of CO2 is an exothermic catalytic reaction that takes place at temperatures between 200–500 °C, depending on the catalyst used. This is known as the Sabatier reaction after the chemist Paul Sabatier who discovered it. Taking into account the reactions involved in the process, the proportion of the methanization reactors is 5.5 kgCO2/kgH2 and 2 kg CH4/kgH2. The reaction at the industrial scale to produce Synthetic Methane is to combine the CO2 recovered in the industry with the H2 produced through electrolysis (using green energy) to obtain Synthetic Methane which is produced in two stages. The first stage consists of breaking down the CO2 molecules to obtain Carbon Monoxide (CO) and water through the use of a catalyst, and the second stage is the formation of Synthetic Methane. For the methanization reaction to occur under optimal conditions, an efficient catalyst is needed, and the most commonly used is the nickel catalyst. This catalyst reactor is cheap due to the abundance of metal but, on the other hand, they have the disadvantage that at high temperatures they are deactivated as a result of the sintering process (so-called thermal degradation phenomenon). Ruthenium catalysts (Ru) have been tested to be a more efficient system but the costs are very high and are increasing [21]. Temperature control is an important element in the design of the methanization reactor and the most commonly used reactor in this process is the fixed-bed reactor as a continuous CH4 flow is required and the fluidized-bed reactor presents a great advantage in terms of plant operation. The most adequate system for PtG systems is the isothermal fixed-bed reactor whose main advantage is presenting a constant temperature operation due to the use of a continuous cooling system throughout the reactor. The average heat efficiency of the methanization process is 80–85% and the excess heat, as a consequence of the exothermic process, can be used for any heat process requirements, or can be combined with the amine CO2 capture system that subsequently reduces energy demand for the process.
3. Analysis of Potential of Power to Gas Systems for Intensive Natural Gas Consumer Industrial Facilities
NG is one of the most important energy sources in the industrial sector and many industries extensively rely on this energy source for production. Figure 3a presents the energy consumption worldwide and Figure 3b presents the European data. Concrete manufacturing, steel industries, glass furnaces and hot rolled coil steel production are the most Natural as-intensive industries [22].
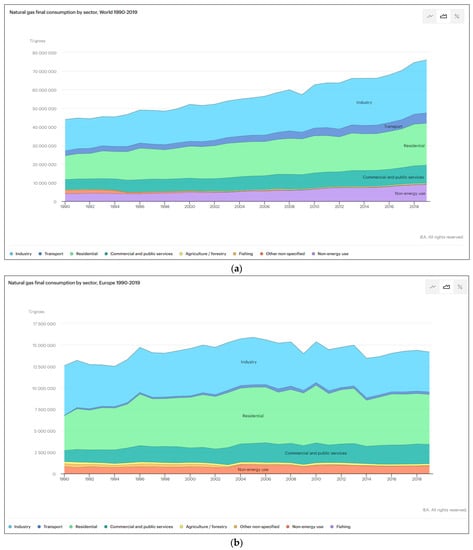
Figure 3.
Natural gas consumption by sector worldwide (a) and in Europe (b) [22,23].
European countries extensively rely on external countries for NG supply. For instance, in 2021 the EU imported up to 80% of its total required gas needs and the ongoing situation is getting worse as domestic production fell 50% in the last 10 years. Of the demand, 40% is consumed by the residential sector followed by industry and gas use for power generation. However, because of the combined effect of both the ongoing deindustrialization process in Europe and the increase in process energy efficiency, industry consumption has declined by 20% since 2000 [24]. Electrification of the economy is increasing the electrical demand and, despite the increase in Renewable Energy generation, in the same period, gas used for power generation has risen by 15%. Biogas represents up to 4% (the year 2020) of the whole total consumed gas. The ongoing situation in the NG markets is causing a rise in spot and futures prices that are producing a large increase in final costs for industries, and intensive consumers are more exposed to its effect. The supply crisis is suffering from a combined effect related to the COVID-19 supply disruption and the reduction of Russian supply due to the Ukraine–Russia war. As a consequence, the reference price in Europe, specifically Dutch TTF Gas (TTF) has multiplied and, therefore, all related industries are suffering strong inflation that has risen up to 45% for industrial products in Germany or 9.1% for the Harmonized Index of Consumer Prices (HICP) [25], Figure 4. The persistence of energy high prices can produce lasting damage in the industrial sector and definitively erode European competitiveness in the EII sector which will reduce its market share and definitely prompt the proprietary companies to relocate their plants to countries with lower energy costs. One of the most affected countries is Germany because it has a large manufacturing sector that also has high energy use in its industrial production, and it appears particularly vulnerable to the fallout from the energy crisis. To avoid these deindustrialization risks, European policymakers face difficulties as there are not possible NG alternative suppliers in the EU itself; therefore, alternative systems must be developed. In this scenario, SM is one of the most mature and promising technologies.
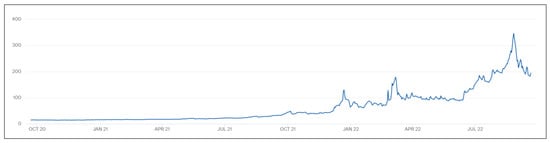
Figure 4.
Natural Gas TTF price evolution, adapted from [22,26].
3.1. Technical and Economic Parameters for Large-Scale P2G Plants
The EU has a plan to fully decarbonize the whole gas sector in 2050. The objective is quite ambitious because by 2020, the gas sector is supposed to be up to 25% of the total GHGs emissions in the EU. The reduction generation of coal and oil power plants has contributed to the increase of the NG share as new, combined cycle plants have been built. The roadmap to achieve this objective is not completely defined, but hydrogen and subsequently PtG technology will play a key role. Climate plans promoted by the European Commission and National Energy aim to install 80 GW of electrolyzers by 2030, and in that year Low Carbon Gases (LCG) should be about 15% of the total consumption. The EU Commission considers that the current cost of Synthetic Methane is at least three times that of conventional gas, although if the current NG prices continue to rise, this gap can be significantly reduced. On the other hand, the subsequent inflation is causing the cost of hydrogen production systems to not reduce their costs as fast as required; moreover, costs could be increased [27]. In this case, this paper analyzes the plants for onsite consumption and not for gas network-distributed gas. Different analyses prove that the gas can be directly injected into Europe’s existing natural-gas distribution networks if the Synthetic Methane has less than 2% volume of H2 and more than 97% volume of CH4.
3.1.1. Hydrogen Electrolysis and Storage Systems
The investment costs for a PtG system are still quite high for all the available technologies. The required electricity consumption per 1 kg of hydrogen is decreasing and for alkaline electrolyzers, the requirement is estimated to change from 53 kWhe/kg of H2 in 2015 to 50 kWhe/kg in 2030. If PEM electrolyzers are used, the cost will be reduced from 52 kWhe/kg of H2 in 2015 to 47 in 2030 [28]. This increase in efficiency will make electrolysis likely to become significantly cheaper and to be massively produced. Research shows that the costs will be reduced from 930 €/kWe in 2015 to 630 in 2020 and 580 €/kWe in 2030 for alkaline electrolyzers, and for PEM from 1570 €/kWe in 2015, to 1000 in 2020 and 760 €/kWe in 2030 [28,29]. The current development of both technologies makes the costs quite lower for alkaline electrolyzers in comparison with PEM. Recent analysis shows that the required investment in PEM systems is two times the costs in alkaline systems. Additionally, for an industrial PtG plant, the required investment in alkaline electrolysis equipment ranges from 600 €/kWe to 1000 €/kWe considering the efficiency of the electrolyzer of 80–92%, and a global 70% process efficiency due to the energy consumption associated to current control and gas compression. There are optimistic studies that suggest that PEM electrolysis will reduce its cost and could be acquired for less than 1000 €/kWe [17,18]. Thema et al. [18] conducted research for years up to 2050 and concluded that there is a potential for cost reductions for electrolysis to less than 500 €/kWe until 2050 while in 2030 costs are expected to be about 600 €/kWe for PEM and 700 €/kWe for alkaline electrolysis [30].
For the hydrogen storage stage, different methods have been studied for large-capacity plants [31] with the conclusion that only two systems could be considered as a real solution for PtG hydrogen storage: tanks with metal hydrides, and high-pressure gas tanks that will operate at 350 bar to 700 bar. In the case of hydrides tanks, they present the advantage of being stable at normal pressures and temperatures. In addition, they offer a cost reduction because no special pressure vessel is required. On the other hand, their price and weight are higher and they occupy more space, and their use in real conditions is still problematic. In this type of tank when the system is heated, the hydrogen is released gradually. Gahleitner [31] considers that for PtG systems high pressure hydrogen storage is the best available solution. Optimizing hydrogen storage technologies for later methanation has a high potential for final cost reductions. A study conducted by Gorre et al. [32] proved that the best system is an intermediate storage system with a capacity ranging from 100 kg to 3000 kg of H2 and that, in this case, the investment costs are about 490 €/kg. A service life of 20 years is estimated if hydrogen is stored in a medium-pressure tank with a rated pressure of 10 bar–30 bar. The generation technology for electricity used in the methanization process has a strong influence on the final cost of the storage system and its requirements. This is caused due to the intermittency of Renewable Energy generation that makes necessary hydrogen storage in order to compensate for the peaks and valleys in energy generation. This effect is most important in intermittent sources such as direct photovoltaic or wind farm-connected plants. There is a strong cost relationship between the electricity generation system, associated costs and hydrogen storage requirements. The literature shows that using a system of hydrogen storage results in increased flexibility of the electrolysis process and, therefore, the methanization phase can work on a stable basis and supply the required SNG for industrial processes. The lack of predictability is more important in wind energy as the periods and amounts of energy production can strongly vary and careful planning is required for the storage. Dealing with this problem now is becoming more important. There is a new discipline called Energy Meteorology that aims to perform high-resolution predictions of energy generation due to renewable sources (wind and solar) in order to secure the energy requirements and subsequent required energy storage [33].
Recent literature analysis such as that performed by Xiong et al. [34] shows that the most important aspects for the profitability of PtG systems are the initial investment costs and, subsequently, the development of synergies with other sectors related to hydrogen. For example, hydrogen-based transport or heat generation, among others, is key for the development of this technology. For long-distance or mass transit transport, H2 and CH4 can provide a replacement fuel for conventional fuels. In the case of Compressed Natural Gas (CNG), now used for urban buses or road transport, among others, SNG can be used as a direct substitute by using the currently built CNG recharging vehicle infrastructure [35]. H2, itself, can be used for transport uses in both direct combustion or fuel cell-driven vehicles, being that the use of fuel cells is the most promising and feasible solution.
3.1.2. Investment Costs for Industrial PtG Facilities
Different analyses have studied the total investment requirements for a commercial P2G plant at a large scale [36]. The total investment costs are distributed as shown in Table 4:

Table 4.
Cost for large-scale PtG plants, adapted from [36].
The large investment costs are directly related to electrolyzers; therefore, the reduction of this cost is a requirement if the technology aims to be extensively deployed as a real alternative to NG. In 2017, Zimicík [37] concluded that after analyzing the costs reported by German companies that sell and install this equipment, it is possible to consider that the price of a methanation fixed-bed adiabatic reactor and for an amine-based CO2 capture unit is about 20% of the price of the electrolyzer for each of them. Investments for electrolyzers that include all the auxiliary equipment and commissioning for a lifespan of 28 years can be estimated at 900 €/kWe, with a specific electricity consumption of 52 kWh/kgH2 [38] while the investment costs of methanation at 20 bar are about 400 €/kW for a rated power of 5 MW. This cost is considerably reduced for larger plants and can be 130 €/kW for 110 MW [39]. According to different studies, for an efficiency of 40% for electrolysis and 70% for methanization, the total costs increase as the output power of the plant decreases. For a large plant up to 50 MWe, the final costs are 1000 €/kWe, while for a small-scale plant (less than 10 MWe) the total cost will be 2000 €/kWe. Therefore, as presented before, the creation of synergic uses that could help build larger plants for multiple uses can be a cost-effective solution for small industries.
3.1.3. Triple Bottom Line Consequences of P2G Plants
As [1] shows, the quality of the environment is determined by the amount of energy used. Moreover, it is also determined by the type of energy used in line with the European Commission’s demands, which highlights that energy-related CO2 emissions need to be reduced to limit climate change as stated by IRENA; Global Renewables Outlook: Energy Transformation 2050 [40]. The technology presented in this research offers alternative security energy sources for firms and public organizations that enhance their profitability by reducing costs [41]. At the same time, these organizations reduce their CO2 emissions and improve the quality of the environment. Moreover, governments are working to promote energy challenges such as PtG Plants increasing their GDP and allowing firms and citizens of their countries to access reliable, affordable and abundant energy. This has a clear social impact because citizens can afford electricity costs and it is especially relevant for firms that are intensive in energy consumption. As we suggested, Trade-offs of Power to Gas energy are not limited to economic and environmental, it has a clear impact on social aspects like poverty, social equity, and health and social well-being [41].
3.2. Integration of Power to Power Systems in Industrial Facilities
Power to Power systems (PtP) represent an alternative configuration of PtG systems and can provide an effective large-scale energy storage solution for renewable electricity production in industrial uses. In a PtP system, hydrogen can be stored in an intermediate stage for later combustion in a Gas Turbine (GT) to produce electricity for self-consumption in the industry. Moreover, the process will produce alternative rejected heat that can be effectively used for process purposes or for low-grade heat applications such as district heating, domestic hot water production, or on-site industrial uses. This configuration can provide an effective solution for energy storage as an alternative to conventional battery-based schemes and act as a complementary alternative in combination with PtG systems. Table 5 shows the current state of the art of energy storage systems and their advantages and disadvantages [42].

Table 5.
Energy Storage technologies, adapted from [42].
After being stored the H2 should be combusted in a GT for electricity production with optional heat recovery (Combined Heat and Power). Different GT systems can be used for this purpose depending on the H2% volume of the combustion gas. Table 6 shows the current state of the art of this equipment that has an investment cost ranging from 400 €/kW to 900 €/kW installed.

Table 6.
Gas Turbines for hydrogen combustion, adapted from [43].
Small-scale microturbines, ranging from 30 kW to 200 kW, can provide an effective solution to be used in modular plants or small industries and now exist as commercial-ready equipment allowing 100% H2 volume combustion. Therefore, these systems can be used as an alternative to conventional industrial small-scale CHP solutions.
4. Discussion
The PtG system has proven to be a reliable, scalable, and feasible solution to provide locally available energy for intensive NG consumer industries. As shown in Figure 5, the energy dependency rate for NG in Europe is up to 90% and this external dependency rate varies depending on the EU country. Figure 6 presents the global energy flow Sankey diagram for the EU-27, depicting the vast external dependency for external NG production and imports. This figure differs from country to country and there are quite different situations among all the EU-27 countries (Figure 7a,b). For example, Norway (Figure 7a) is an NG producer and exporter, and their industrial consumption can be fed using locally produced energy; therefore, they are less exposed to global energy price variations and stock markets. On the other hand, countries such as Spain (Figure 7b) only rely on imports, and their industrial facilities are suffering a vast increase in energy costs without the possibility of finding a short-term alternative as they are completely exposed to the NG international markets situation. Most EU countries are in a similar situation including Germany, one of the most industrial countries in the region that is a worldwide reference manufacturer in some fields such as vehicles and machinery, among others.
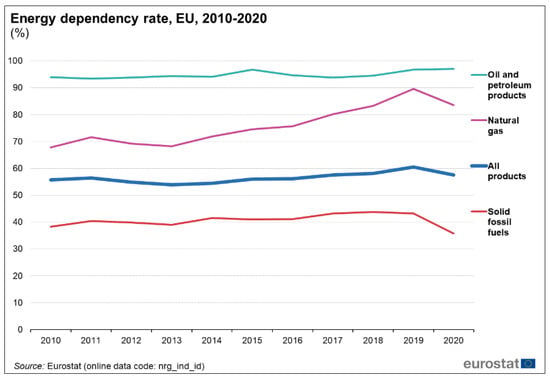
Figure 5.
European Union energy dependency rate by source [44].
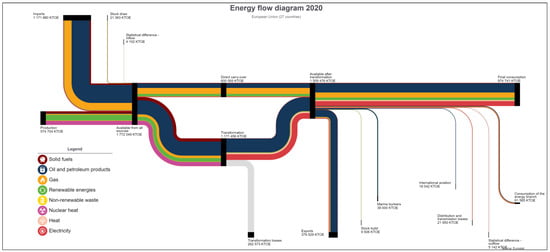
Figure 6.
Energy Sankey diagram EU-27 2020, [45].
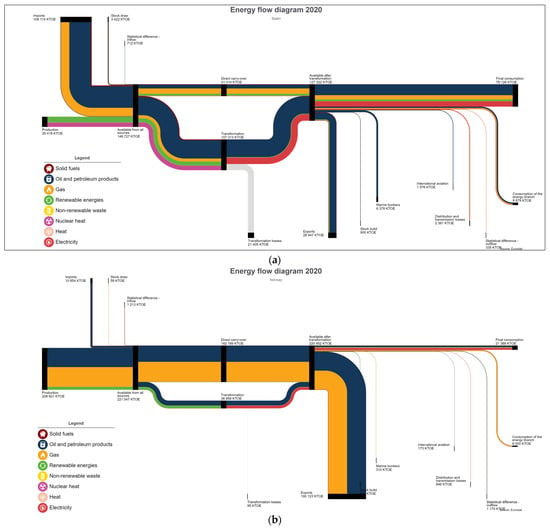
Figure 7.
(a) Energy Sankey diagram, Spain, 2020, [45]. (b) Energy Sankey diagram, Norway, 2019, [45].
PtG systems can provide a short-medium term solution for self-SM production reducing the external NG imports and, therefore, associated costs. Despite this, further research and development are required to deal with three big problems that currently exist: (1) high initial investment costs that make the solution not profitable, (2) lack of large-scale industrial solutions, and (3) lack of market-ready products and large-scale manufacturers able to provide commercial solutions in a relatively short time. This situation is aggravated by the raw material supply crisis and associated disruption because much of this equipment requires large quantities of these raw materials that are also mainly produced out of the EU. To develop a competitive technology, the hydrogen used in the PtG process should be Green Hydrogen obtained by water electrolysis. In addition, different research forecasts that the average production cost in 2020 of 5.09 €/kg will be reduced to a final cost of 2.12 €/kg, and is expected by the European Commission in 2030 [46], as a consequence of the renewable electricity production costs by this year. The European Green Deal has several timeframes, and in 2030 phase 1 will end and the second phase will take part in the year 2050, in which the cost of electrolyzers is projected to be 356.16 €/kW. The potential for industrial use of PtG technologies vastly depends on the renewable electricity potential in each country. Research carried out by G. Kakoulaki et al. in 2021 [47] calculated the potential of Renewable Energy (hydro, solar and wind energy) considering the annual electricity consumption for each country, and the calculated electricity demand required to match the requirements of hydrogen production using water electrolysis, with results showing that many of the EU countries have enough renewable electricity generation potential to cover their needs and, if required, to export to other countries.
For the particular case of Spain, one of the locations with a higher potential especially due to the high radiation levels and equivalent hours for PV systems, there are some regions that will require importing excess production from other locations. However, despite this, the country presents an excess potential of 1376 TWh as a whole. The International Renewable Energy Agency (IRENA) calculated in research published in 2019 that the price of wind energy-driven hydrogen production is 2.6 $/kgH2 and that Green Hydrogen production costs will match fossil fuels-produced hydrogen costs in 2024 and that in the following years this cost will be lower than in 2050. The cost will be reduced up to a Levelized Cost of Hydrogen (LCOH) of 2 $/kgH2 using PV energy and 1.5 $/kgH2 for wind energy, but that if the best-case scenario is achieved, the costs will be 1.2 $/kgH2 and 1 $/kgH2, respectively, for solar and wind energy.
The cement industry, as an example, presents high potential for these systems due to the high CO2 emissions associated with the production and the capacity of using this captured CO2 in the PtG process. According to the Guide to measurement methods and emission factors for the cement sector in Spain [48], 38% of total CO2 emissions are produced in the combustion process in the furnaces, and 62% is produced in the manufacturing process due to the calcination of calcium carbonate CaCO3 that is transformed into calcium oxide CaO and CO2. For a plant with an estimated hydrogen production capacity of 485 Nm3/h available as commercial equipment [49], the required power requirements will be 2.3 MW for hydrogen production of 43.6 kg/h. Considering the average costs presented in this review, the investment costs will be up to 2 M€ for the electrolyzer, about 0.18 M€ for the carbon capture system, 0.4 M€ for the methanization system and 1 M€ for an associated wind farm to produce wind energy. Only considering the investment costs (3 M€) and the average NG cost in the European markets, the investment presents a payback period higher than 50 months.
5. Conclusions
The increase in NG prices in the international markets and especially in European ones is causing the industries that extensively rely on this energy source (so-called Energy Intensive Industries, EII) such as glass, sanitary ware and cement, among others, to reduce their competitiveness and, in some cases, become unprofitable. The PtG technology can provide a reliable solution for the self-production of SM with the advantage of no directly associated CO2 emissions, which also makes it possible to contribute to the achievement of the climatic objectives, to reduce emissions and associated emission taxes. The technology requires the combination of four main systems: the hydrogen electrolysis system, the carbon capture system, the hydrogen storage system, and the methanization system. This implies that further development of each component is required to achieve a large-scale solution. In this study, the maturity and the current state of each of them are analyzed, and the mid-term forecasted development and associated costs and energy generation potential are studied. Different forecasts report that the price of hydrogen production will be reduced by up to 50% in the next years and that, combined with the estimated increase in NG costs, will make the investment more attractive and profitable. The reduction of renewable electricity generation, especially using wind and solar energy is a key parameter to reduce operational costs and initial investment costs related to the electricity generation plants. This study analyzes the key components of the technology, and the current situation from a technical, economic, social and environmental point of view, and remarks on the need to develop a fast-track roadmap to develop this technology if European countries aim to continue being an industrial producer in the international market. Our conclusions show that PtG can be an effective solution but only for some EII and that, although many of the required technological solutions are developed, the integration into large capacity PtG production plants requires extensive investment and research efforts. This study shows the vast external NG supply dependency of the EU as a whole, up to 90% and up to 100% in several countries, and how this fact is a handicap for industrial activity and particularly for EII future feasibility but also that, on the other hand, in the EU there exists a large and Renewable Energy generation potential that can be used to achieve the Green Hydrogen generation objectives and the subsequent SM production requirements. To this end, and due to the variability of energy requirements in EU countries, and Renewable Energy potential, NG suppliers and industrial activity, among others, only a unique European strategy considering all the countries can provide an effective solution.
Author Contributions
Conceptualization, D.B.-D.; methodology, D.B.-D.; validation, E.R.-A., E.A. and D.A.-M.; formal analysis, E.A. and D.B.-D.; investigation, D.B.-D.; resources, D.A.-M. and E.R.-A.; writing—original draft preparation, D.B.-D.; writing—review and editing, D.A.-M., E.R.-A. and E.A.; visualization, D.B.-D., E.A. and D.A.-M., E.A.; supervision, D.B.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| BAT | Best Available Technologies |
| CAES | Compressed Air Storage |
| CCS | Capture Carbon Storage |
| CCU | Capture Carbon Utilization |
| CD | Cryogenic Distillation |
| CHP | Combined Heat and Power |
| CNG | Compressed Natural Gas |
| CO | Carbon Monoxide |
| EII | Energy Intensive Industries |
| EEII | European Energy Intensive Industries |
| ETS | Emission Trading Scheme |
| EU | European Union |
| GHGs | Greenhouse Gases |
| GT | Gas Turbine |
| HG | Green Hydrogen |
| HICP | Harmonized Index of Consumer Prices |
| IPCC | Intergovernmental Panel on Climate Change |
| IRENA | International Renewable Energy Agency |
| LCG | Low Carbon Gases |
| LCOH | Levelized Cost of Hydrogen |
| MEA | Monoethanolamine |
| NG | Natural Gas |
| PEM | Proton Membrane Exchange Electrolyzer |
| PtG | Power to Gas |
| PtL | Power to Liquid Fuel |
| PtP | Power to Power |
| RE | Renewable Energy |
| SM | Synthetic Methane |
| SOE | Solid Oxide Electrolyzer |
| TTF | Dutch TTF Gas |
Appendix A
Table A1 presented below includes extended information of the available electrolyzers for Green Hydrogen production.

Table A1.
The most important characteristics of electrolyzers for Green Hydrogen production in P2G systems, adapted from [43,47,50].
Table A1.
The most important characteristics of electrolyzers for Green Hydrogen production in P2G systems, adapted from [43,47,50].
| Electrolyzer Type | Alkaline | PEM | SOE |
|---|---|---|---|
| Development status | Commercial | Commercial for small and medium scale (<300 kW) | Experimental development |
| Operation temperature | 40–90 °C | 20–100 °C | 700–1000 °C |
| Most important characteristics | Simple unipolar design of two metal electrodes in aqueous-electrolyte solution Dissociated gases cannot be mixed in order to avoid explosions Suitable for large-scale, high-load applications | Bipolar design to operate at high pressures The electrolyte is a solid membrane Can suffer vapor contamination in the system Allows working on partial load | Long service life with high rated efficiency Operation with water/water vapor Working at very high temperatures Needs continuous airflow Corrosion resistant |
| Anode reaction | |||
| Cathode reaction | |||
| Operating pressure | 1 bar–30 bar | 30 bar–80 bar | 1 bar |
| Electricity consumption (nominalconditions) | 51 kWh/kg | 55 kWh/kg–70 kWh/kg | 41 kWh/kg–40 kWh/kg |
| Current density | 0.5 A/cm2 | 2 A/cm2 | 2 A/cm2 |
| CAPEX | 750 €/kW | 1200 €/kW–2000 €/kW | 4500 €/kW–12,000 €/kW |
| OPEX | 32 €/(kg/day)/year | 58 €/(kg/day)/year | 225 €/(kg/day)/year–600 €/(kg/day)/year |
| Electrical efficiency | 63–70% | 56–60% | 74–81% |
| Average electricity consumption | 4.3 kWh/Nm3–5.5 kWh/Nm3 | 4.5 kWh/Nm3–5 kWh/Nm3 | 3.2 kWh/Nm3–3.7 kWh/Nm3 |
| Degradation rate (1000 operating hours) | 0.13% | 0.25% | 2.8–1.9% |
Appendix B
In this section, the most important Power to Gas and Power to Power projects currently developed or planned in Europe are listed below and they represent a good analysis of the current technological development of these systems.
- Robinson (Eigerøy, Norway): This project aims to decarbonize the islands by developing an intelligent, flexible and modular Energy Management System (EMS) to achieve better integration of Renewable Energy sources. It also aims to improve the current state of the use of biomass and wastewater by optimization and validation of innovative technologies in the field. Existing and emerging energy and storage technologies will be integrated across different energy vectors such as a small CHP unit based on a Gas Turbine and an anaerobic digester assisted by bioelectric systems to achieve the conversion of liquid waste into biomethane, or different technologies based on hydrogen (electrolyzers and storage). The study takes place in Eigerøy, but laboratory-level replication studies will be conducted for the island of Crete (Greece) and the Western Isles (Scotland). The project will have a total duration of 48 months starting in October 2020 and the budget is 8.37 M€ of which 7 M€ are funded by European funds and aims to greatly reduce CO2 emissions as well as the costs of generation and transport of energy [51].
- FLEXnCONFU (Ribatejo, Portugal): The project’s purpose is to demonstrate how greater flexibility and reuse can be achieved in the production of energy in combined cycle power plants in order to allow a more efficient and ecological operation of the energy market. The project considers the use of hydrogen and ammonia as storage elements. The most important objectives of the research are evaluating the combustion systems with unconventional fuels and developing numerical and experimental models in laboratories and studying the needs of flexibility and reference with other assets determining the economic and environmental basis of Power to Hydrogen and Power to Ammonia. The project involves countries such as Italy, the United Kingdom and Portugal. The hydrogen test will be carried out in the combined cycle plant of Ribatejo (Portugal) which is operated by the energy company EDP. The project began on April 6 2020 and has a duration of 48 months with financing of 10 M€ [52].
- Jupiter 1000 (Fos-sur-Mer, France): This finished project was the first PG project connected to the French NG transport network. This plant uses 100% Renewable Energy in order to generate Green Hydrogen through two electrolyzers, one PEM type and one alkaline with a total power of 1 MWe and the project includes the CO2 capture from a nearby factory so that through a methanation process SNG of renewable origin can be generated and mixed with hydrogen to be injected into the NG supply network. The project began in 2014 and ended in 2019 with an investment of 30 €M, of which 10 M€ were funded by the public entities ERDF (European Regional Development Fund) and ADEME (Agence De l’Environnement et de la Maîtrise de l’Énergie). The plant is designed to produce up to 25 Nm3/h of Synthetic Methane or 200 Nm3/h of hydrogen with an average production of 5 GWh over a period of 3 years [53].
- HyFlexPower (Saillat-sur-Vienne, Francia): It is the first demonstrating plant of a hydrogen-fired NG turbine integrated into one PtP system. The project was launched in May 2020 in the facilities of one specialized company in the production of recycled paper in France, and its purpose is to demonstrate that hydrogen itself can produce and store excess renewable electricity, and subsequently be used in CHP plants with high-power turbines that currently use NG. The project is based on a Siemens SGT-400 turbine that is adapted so that it can burn a mixture of NG and hydrogen fuel, and aims to be able to work with 100% hydrogen. The project is financially supported by the European Union through its Horizon 2020 program. The installation will generate a power of about 12 MWe, while producing the steam demanded by the process. The SGT-400 turbine operating entirely with hydrogen will mean savings of up to 65,000 tons of CO2 per year and the entire project will cost about 15.2 M€, of which 10.5 M€ will be funded by European Union funds in the Horizon 2020 program [54].
References
- Isiksal, A.Z.; Assi, A.F. Determinants of sustainable energy demand in the European economic area: Evidence from the PMG-ARDL model. Technol. Forecast. Soc. Chang. 2022, 183, 121901. [Google Scholar] [CrossRef]
- Rashidi, K.; Noorizadeh, A.; Kannan, D.; Cullinane, K. Applying the triple bottom line in sustainable supplier selection: A meta-review of the state-of-the-art. J. Clean. Prod. 2020, 269, 122001. [Google Scholar] [CrossRef]
- Quader, M.A.; Ahmed, S.; Ghazilla, R.A.R.; Ahmed, S.; Dahari, M. A comprehensive review on energy efficient CO2 breakthrough technologies for sustainable green iron and steel manufacturing. Renew. Sustain. Energy Rev. 2015, 50, 594–614. [Google Scholar] [CrossRef]
- Straka, P. A comprehensive study of Power-to-Gas technology: Technical implementations overview, economic assessments, methanation plant as auxiliary operation of lignite-fired power station. J. Clean. Prod. 2021, 127642, 311. [Google Scholar] [CrossRef]
- De Ras, K.; Van de Vijver, R.; Galvita, V.V.; Marin, G.B.; Van Geem, K.M. Carbon capture and utilization in the steel industry: Challenges and opportunities for chemical engineering. Curr. Opin. Chem. Eng. 2019, 26, 81–87. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona; Romeo, L.M. Avoidance of partial load operation at coal-fired power plants by storing nuclear power through power to gas. Int. J. Hydrog. Energy 2019, 44, 26063–26075. [Google Scholar] [CrossRef]
- Buchholz, O.S.; Van Der Ham, A.G.J.; Veneman, R.; Brilman, D.W.F.; Kersten, S.R.A. Power-to-Gas: Storing surplus electrical energy a design study. Energy Procedia 2014, 63, 7993–8009. [Google Scholar] [CrossRef]
- United Nations, DESA. United Nations. Available online: https://www.un.org/en/desa (accessed on 19 December 2022).
- Homepage—U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/index.php (accessed on 19 December 2022).
- Carbon Dioxide Capture and Storage—IPCC. Available online: https://www.ipcc.ch/report/carbon-dioxide-capture-and-storage/ (accessed on 19 October 2022).
- Stanger, R.; Wall, T.; Spörl, R.; Paneru, M.; Grathwohl, S.; Weidmann, M.; Scheffknecht, G.; McDonald, D.; Myöhänen, K.; Ritvanen, J.; et al. Oxyfuel combustion for CO2 capture in power plants. Int. J. Greenh. Gas Control 2015, 40, 55–125. [Google Scholar] [CrossRef]
- Romeo, L.M.; Bailera, M. Design configurations to achieve an effective CO2 use and mitigation through power to gas. J. CO2 Util. 2020, 101174, 39. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Kuramochi, T.; Ramírez, A.; Turkenburg, W.; Faaij, A. Comparative assessment of CO2 capture technologies for carbon-intensive industrial processes. Prog. Energy Combust. Sci. 2012, 38, 87–112. [Google Scholar] [CrossRef]
- Bailera, M.; Espatolero, S.; Lisbona; Romeo, L.M. Power to gas-electrochemical industry hybrid systems: A case study. Appl. Energy 2017, 202, 435–446. [Google Scholar] [CrossRef]
- Jung, J.; Jeong, Y.S.; Lim, Y.; Lee, C.S.; Han, C. Advanced CO2 Capture Process Using MEA Scrubbing: Configuration of a Split Flow and Phase Separation Heat Exchanger. Energy Procedia 2013, 37, 1778–1784. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renew. Sustain. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Fc Hyguide. Guidance Document for Performing Life Cycle Assessment (LCA) on Fuel Cells (FCs) and Hydrogen (H2) Technologies A Project Funded by Fuel Cell and Hydrogen–Joint Undertaking, EU. 2011. Available online: http://hytechcycling.eu/ (accessed on 19 October 2022).
- Burton, N.A.; Padilla, R.V.; Rose, A.; Habibullah, H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 2021, 110255, 135. [Google Scholar] [CrossRef]
- Li, S.; Tang, H.; Gong, D.; Ma, Z.; Liu, Y. Loading Ni/La2O3 on SiO2 for CO methanation from syngas. Catal. Today 2017, 297, 298–307. [Google Scholar] [CrossRef]
- Gas Market Report, Q4 2021—Analysis, IEA. Available online: https://www.iea.org/reports/gas-market-report-q4-2021 (accessed on 16 December 2021).
- Electricity—Fuels & Technologies, IEA. Available online: https://www.iea.org/fuels-and-technologies/electricity (accessed on 17 December 2021).
- Gas Factsheet|www.acer.europa.eu. Available online: https://www.acer.europa.eu/gas-factsheet (accessed on 19 October 2022).
- Bank, E.C. Measuring Inflation—HICP. European Central Bank. 7 February 2022. Available online: https://www.ecb.europa.eu/stats/macroeconomic_and_sectoral/hicp/html/index.en.html (accessed on 18 October 2022).
- Dutch TTF Gas Futures | ICE. Available online: https://www.theice.com/products/27996665/Dutch-TTF-Gas-Futures/data?marketId=5429405&span=3 (accessed on 18 October 2022).
- Natural Gas Price Assumptions, 2019–2025—Charts—Data & Statistics, IEA. Available online: https://www.iea.org/data-and-statistics/charts/natural-gas-price-assumptions-2019-2025 (accessed on 18 October 2022).
- Bertuccioli, L.; Chan, A.; Hart, D.; Lehner, F.; Madden, B.; Standen, E. Development of Water Electrolysis in the European Union. February 2014. Available online: https://www.h2knowledgecentre.com/content/researchpaper1120 (accessed on 19 October 2022).
- Al-Mufachi, N.A.; Shah, N. The role of hydrogen and fuel cell technology in providing security for the UK energy system. Energy Policy 2022, 171, 113286. [Google Scholar] [CrossRef]
- Böhm, H.; Zauner, A.; Rosenfeld, D.C.; Tichler, R. Projecting cost development for future large-scale power-to-gas implementations by scaling effects. Appl. Energy 2020, 264, 114780. [Google Scholar] [CrossRef]
- Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrog. Energy 2013, 38, 2039–2061. [Google Scholar] [CrossRef]
- Gorre, J.; Ruoss, F.; Karjunen, H.; Schaffert, J.; Tynjälä, T. Cost benefits of optimizing hydrogen storage and methanation capacities for Power-to-Gas plants in dynamic operation. Appl. Energy 2020, 113967, 257. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Gui, D.; Hua, Y.; Guo, P. Power system planning based on CSP-CHP system to integrate variable renewable energy. Energy 2021, 232, 121064. [Google Scholar] [CrossRef]
- Xiong, Y.; Tan, H.; Wang, Y.; Xu, W.; Mikulčić, H.; Duić, N. Pilot-scale study on water and latent heat recovery from flue gas using fluorine plastic heat exchangers. J. Clean. Prod. 2017, 161, 1416–1422. [Google Scholar] [CrossRef]
- Reiter, G.; Lindorfer, J. Global warming potential of hydrogen and methane production from renewable electricity via power-to-gas technology. Int. J. Life Cycle Assess 2015, 20, 477–489. [Google Scholar] [CrossRef]
- Aicher, T.; Iglesias-Gonzales, M.; Götz, M. Arbeitspaket 5: Betrachtungen Des Gesamtsystems im Hinblick auf Dynamik und Prozessintegration. Energ. Wasser Prax. 2014, 65, 51–55. [Google Scholar]
- Jakub, Z. Power to Gas. January 2017. Available online: https://dspace.cvut.cz/handle/10467/66840 (accessed on 19 October 2022).
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Graf, F.; Götz, M.; Henel, M.; Schaaf, T.; Tichler, R. Abschlussbericht techno- ökonomische studie von Power-to-Gas-Konzepten. DVGW Forschung–Deutscher Verein des Gas- und Wasserfaches e.V. Bonn 2014, 76–81. [Google Scholar]
- Global Renewables Outlook: Energy Transformation 2050. Available online: https://www.irena.org/publications/2020/Apr/Global-Renewables-Outlook-2020 (accessed on 19 October 2022).
- Liu, H.; Khan, I.; Zakari, A.; Alharthi, M. Roles of trilemma in the world energy sector and transition towards sustainable energy: A study of economic growth and the environment. Energy Policy 2022, 113238, 170. [Google Scholar] [CrossRef]
- Handbook of Energy Storage. Available online: https://link.springer.com/book/10.1007/978-3-662-55504-0 (accessed on 19 October 2022).
- Hydrogen: Energy Vector of a Decarbonised Economy, IREC. 5 November 2020. Available online: https://www.irec.cat/press-society/news/hydrogen-energy-vector-of-a-decarbonised-economy/ (accessed on 19 October 2022).
- Statistics Eurostat Electricity Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=File:Development_of_electricity_prices_for_non-household_consumers,_EU-27,_2008-2020_(EUR_per_kWh)_v4.png (accessed on 13 January 2021).
- Energy Flow Diagrams—Energy—Eurostat. Available online: https://ec.europa.eu/eurostat/web/energy/energy-flow-diagrams (accessed on 19 October 2022).
- Jäger-Waldau, A.; Kougias, I.; Taylor, N.; Thiel, C. How photovoltaics can contribute to GHG emission reductions of 55% in the EU by 2030. Renew. Sustain. Energy Rev. 2020, 126, 109836. [Google Scholar] [CrossRef]
- Kakoulaki, G.; Kougias, I.; Taylor, N.; Dolci, F.; Moya, J.; Jäger-Waldau, A. Green hydrogen in Europe—A regional assessment: Substituting existing production with electrolysis powered by renewables. Energy Convers. Manag. 2021, 113649, 228. [Google Scholar] [CrossRef]
- Nueva Guía de Métodos de Medición y Factores de Emisión del Sector Cementero|PRTR España. Available online: https://prtr-es.es/novedades/Nueva-Guia-Metodos-medicion-1412122019.html (accessed on 19 October 2022).
- Sahoo, N.; Kumar, A.; Samsher, S. Review on energy conservation and emission reduction approaches for cement industry. Environ. Dev. 2022, 44, 100767. [Google Scholar] [CrossRef]
- Ursua, A.L.M. Gandia, and Sanchis, Hydrogen Production from Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Admin, The Clean Energy Transition on Eigerøy—Norway, Robinson. 11 May 2021. Available online: https://www.robinson-h2020.eu/the-clean-energy-transition-on-eigeroy-norway/ (accessed on 19 October 2022).
- Home, FLEXnCONFU. Available online: https://flexnconfu.eu/ (accessed on 19 October 2022).
- Jupiter 1000—Power-to-Gas—Accueil, Jupiter1000. Available online: https://www.jupiter1000.eu (accessed on 19 October 2022).
- Home, Hyflexpower. Available online: https://www.hyflexpower.eu/ (accessed on 19 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).