Study on the Adsorption Properties and Mechanisms of CO on Nickel Surfaces Based on Density Functional Theory
Abstract
1. Introduction
2. Computational Model and Parameter-Setting
3. Comparison of Adsorption Energies
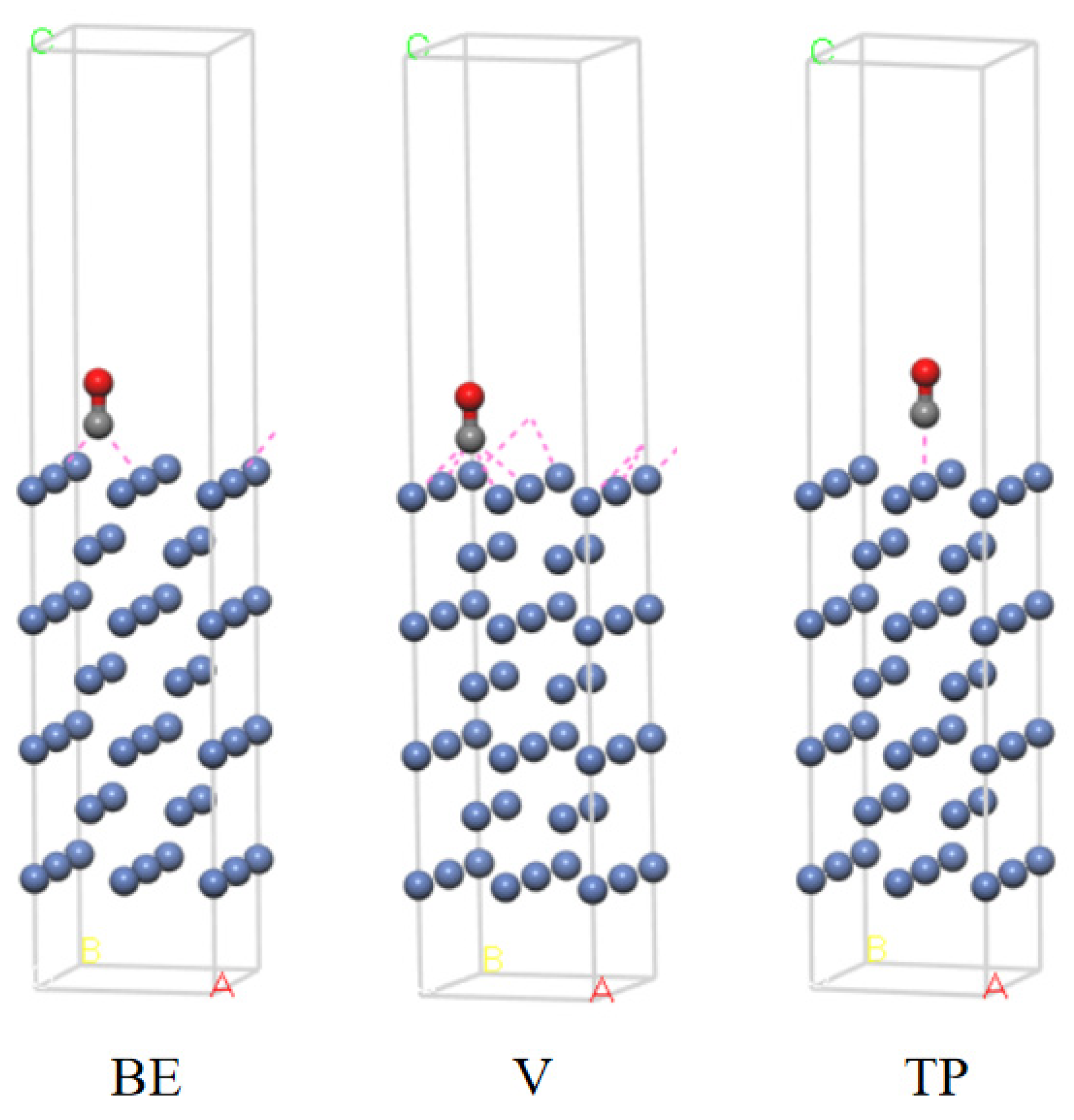
3.1. Exploration of the Adsorption Ability of a Ni (111) Crystal Surface
3.2. Exploration of the Adsorption Ability of a Ni (100) Crystal Surface
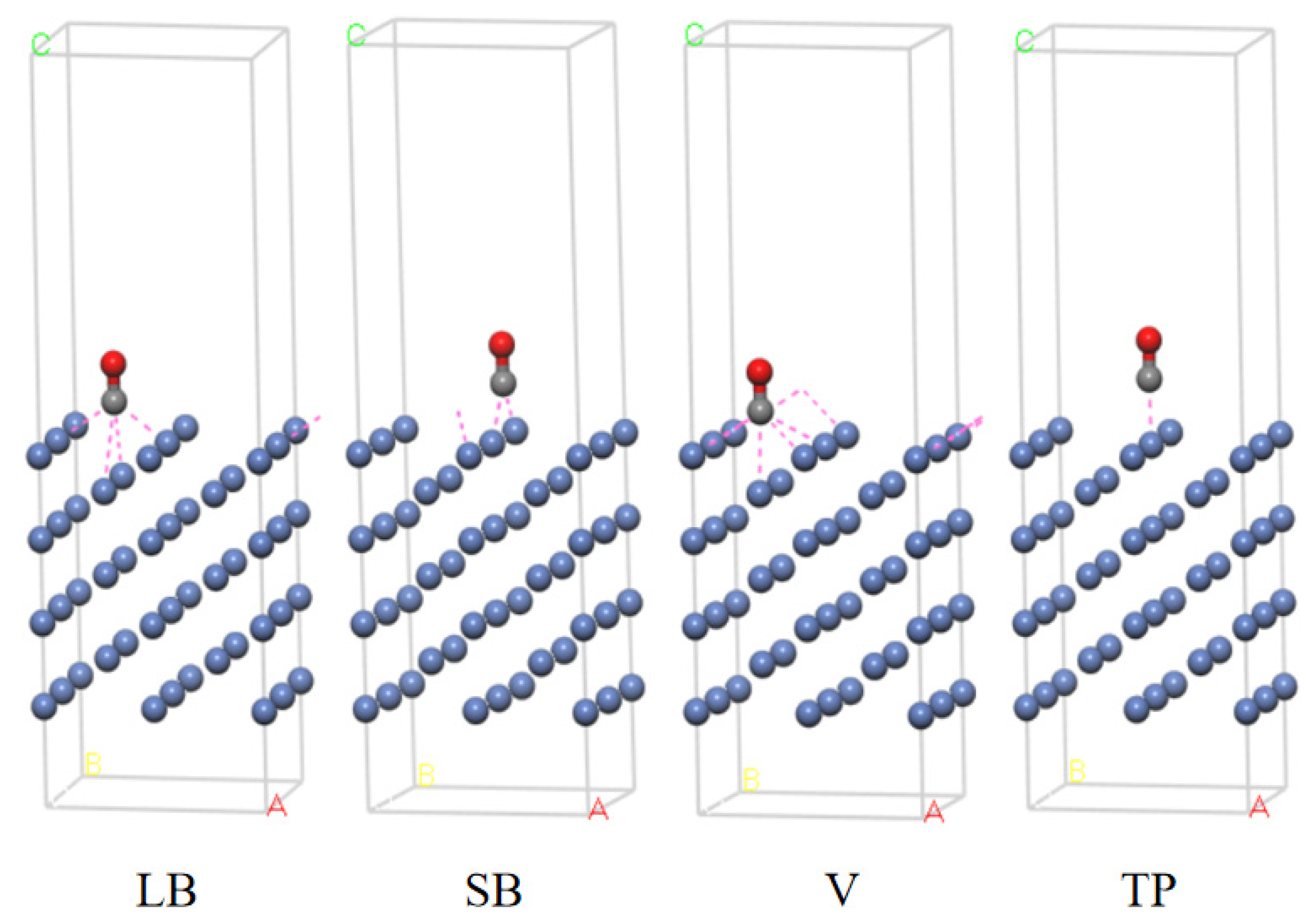
3.3. Exploration of the Adsorption Ability of a Ni (110) Crystal Surface
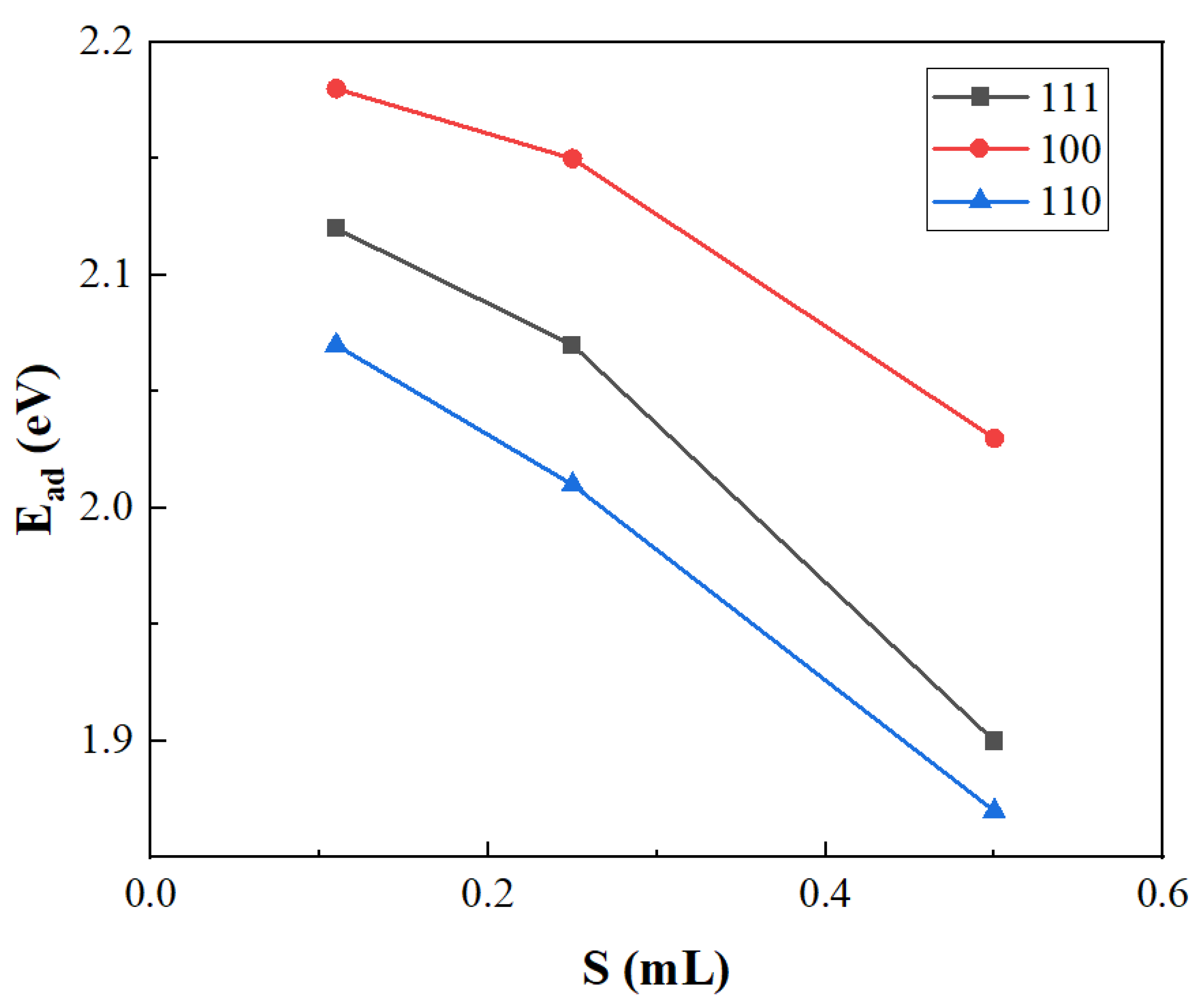
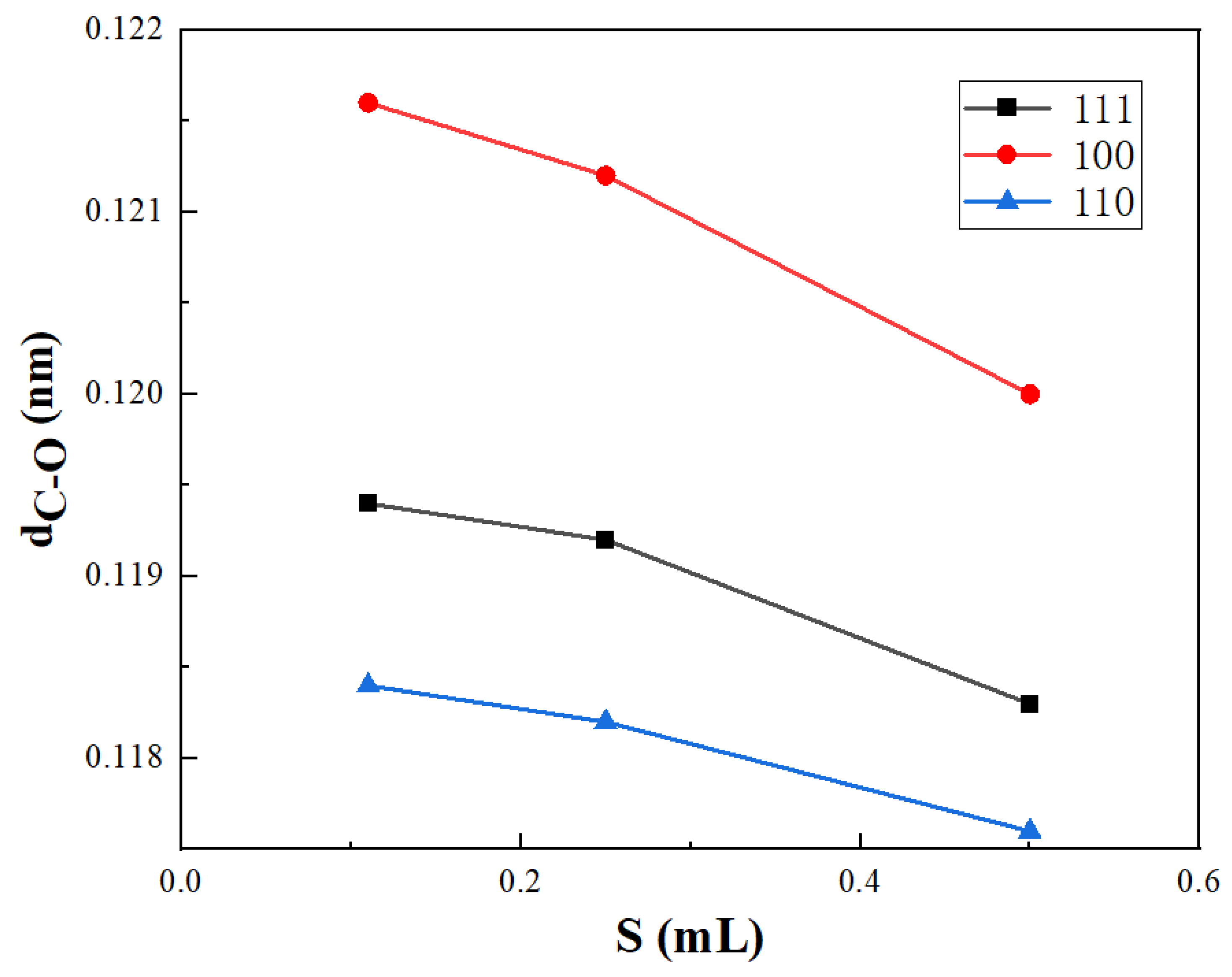
3.4. Influence of Coverage on CO Adsorption
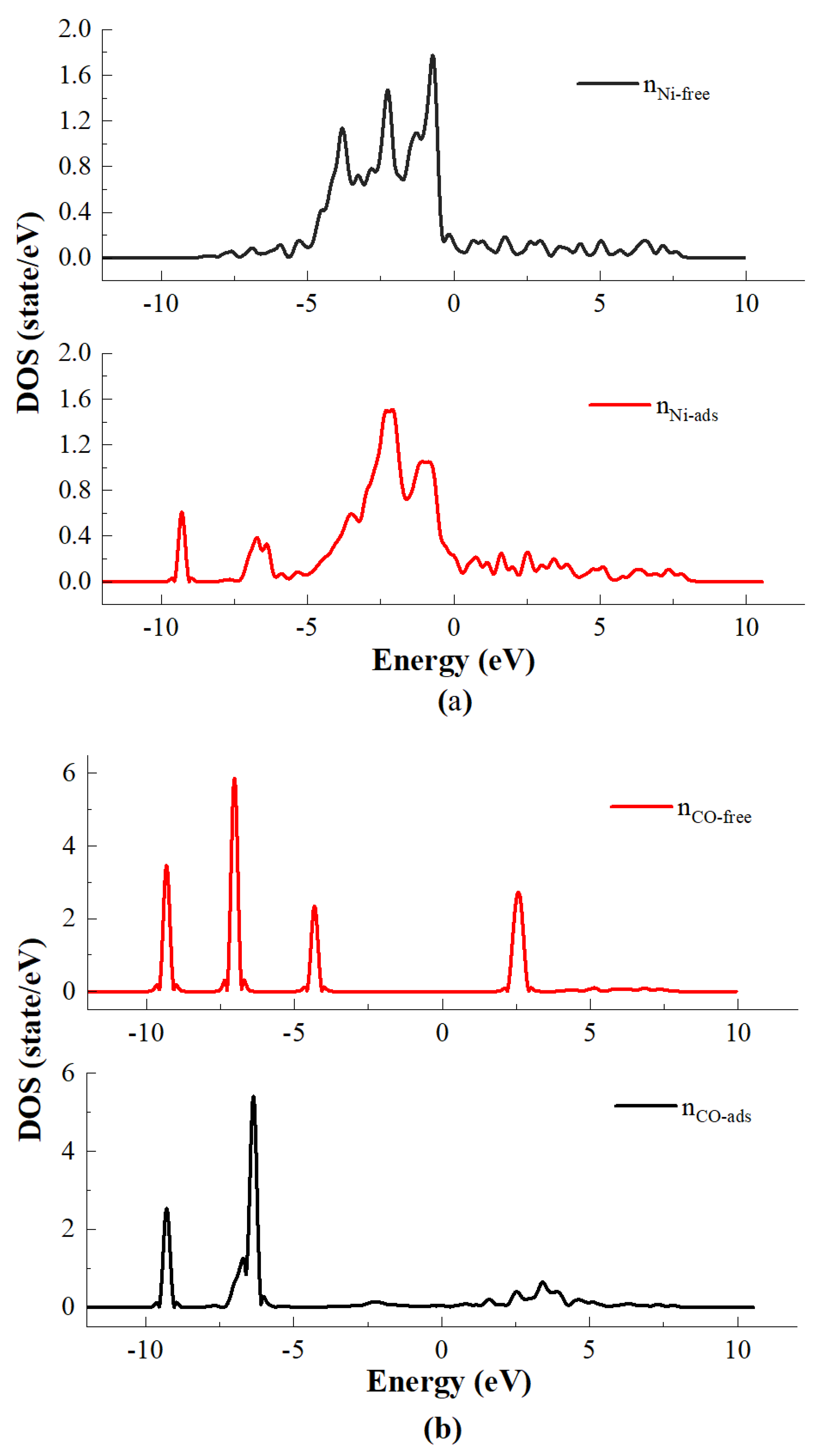
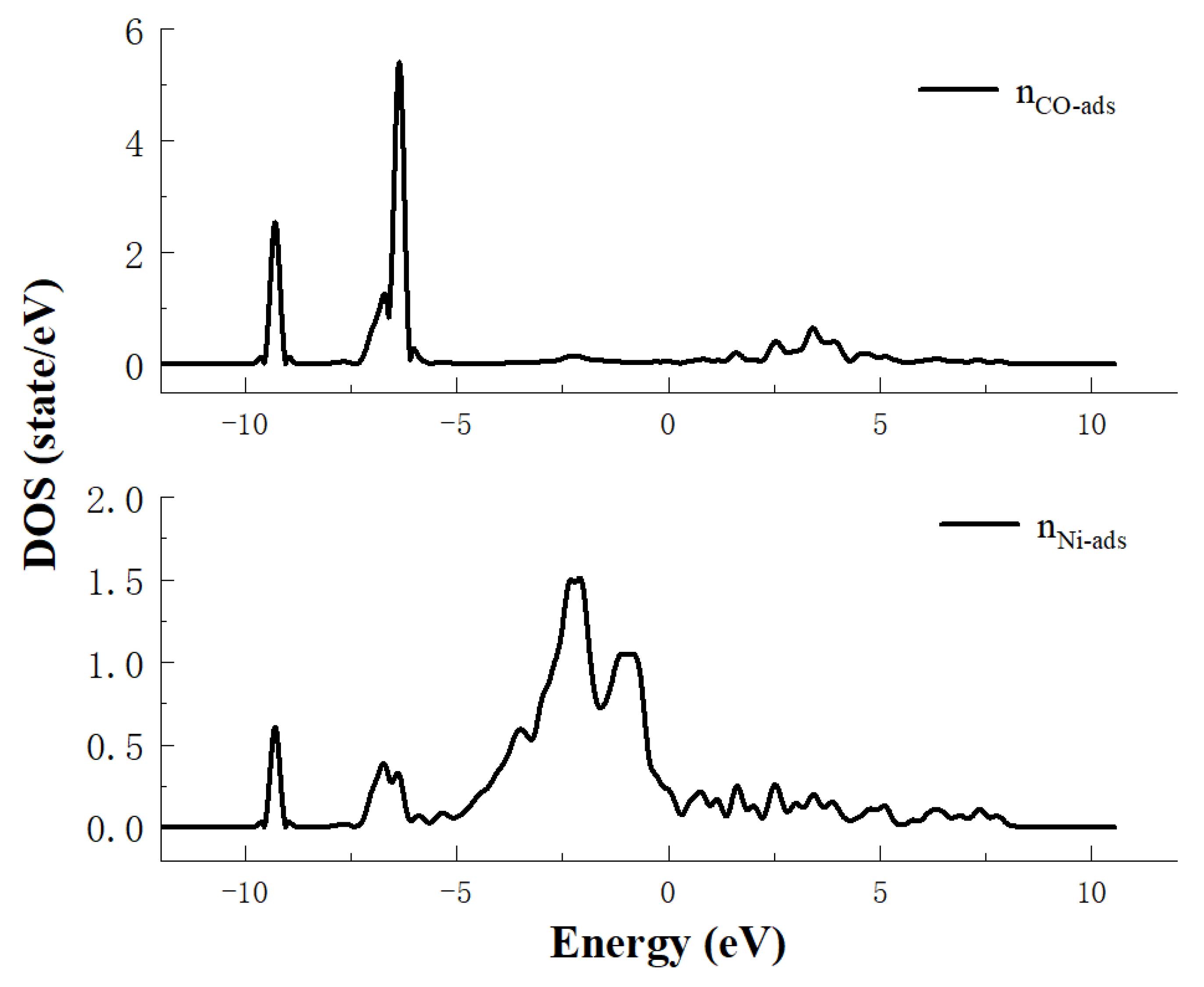
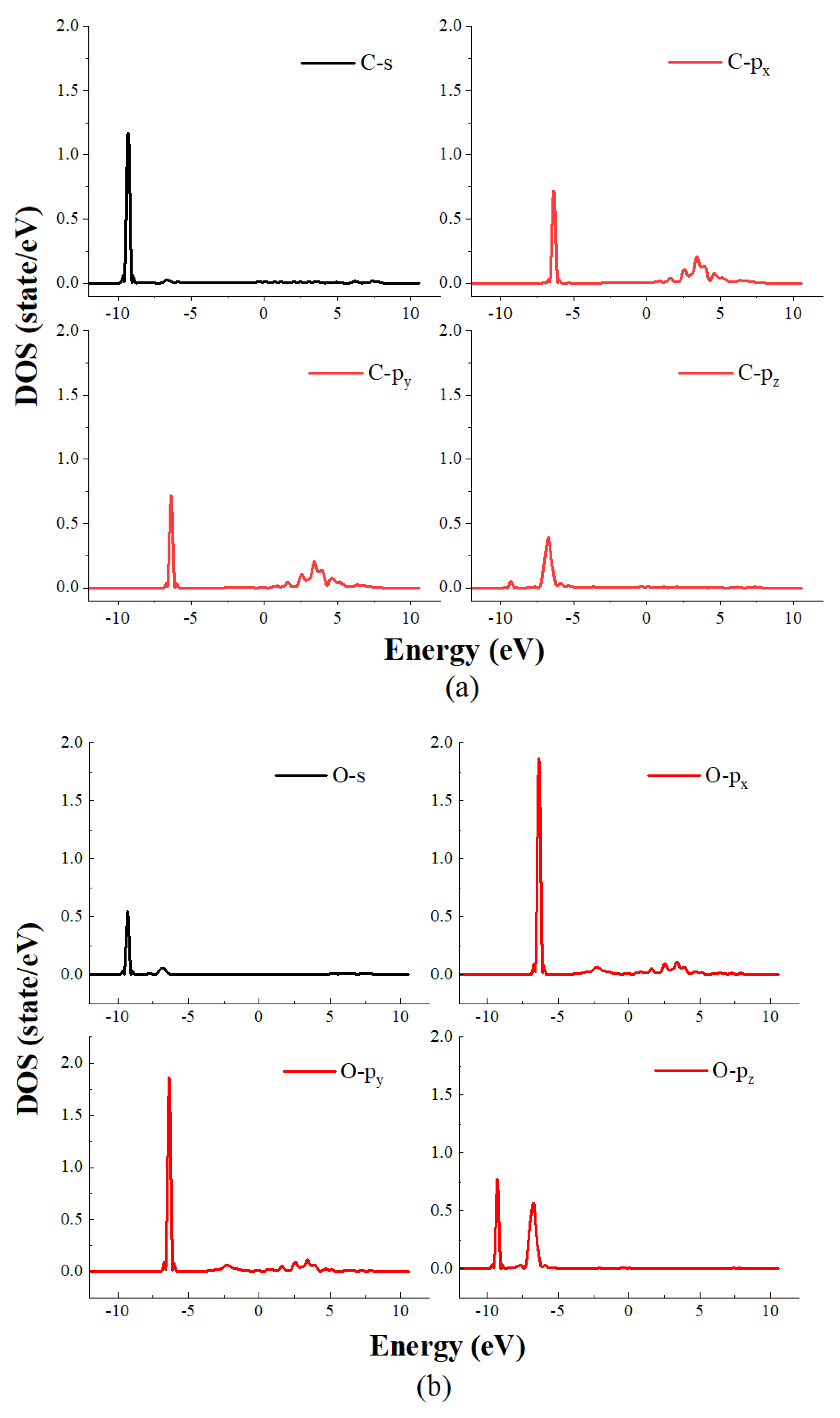
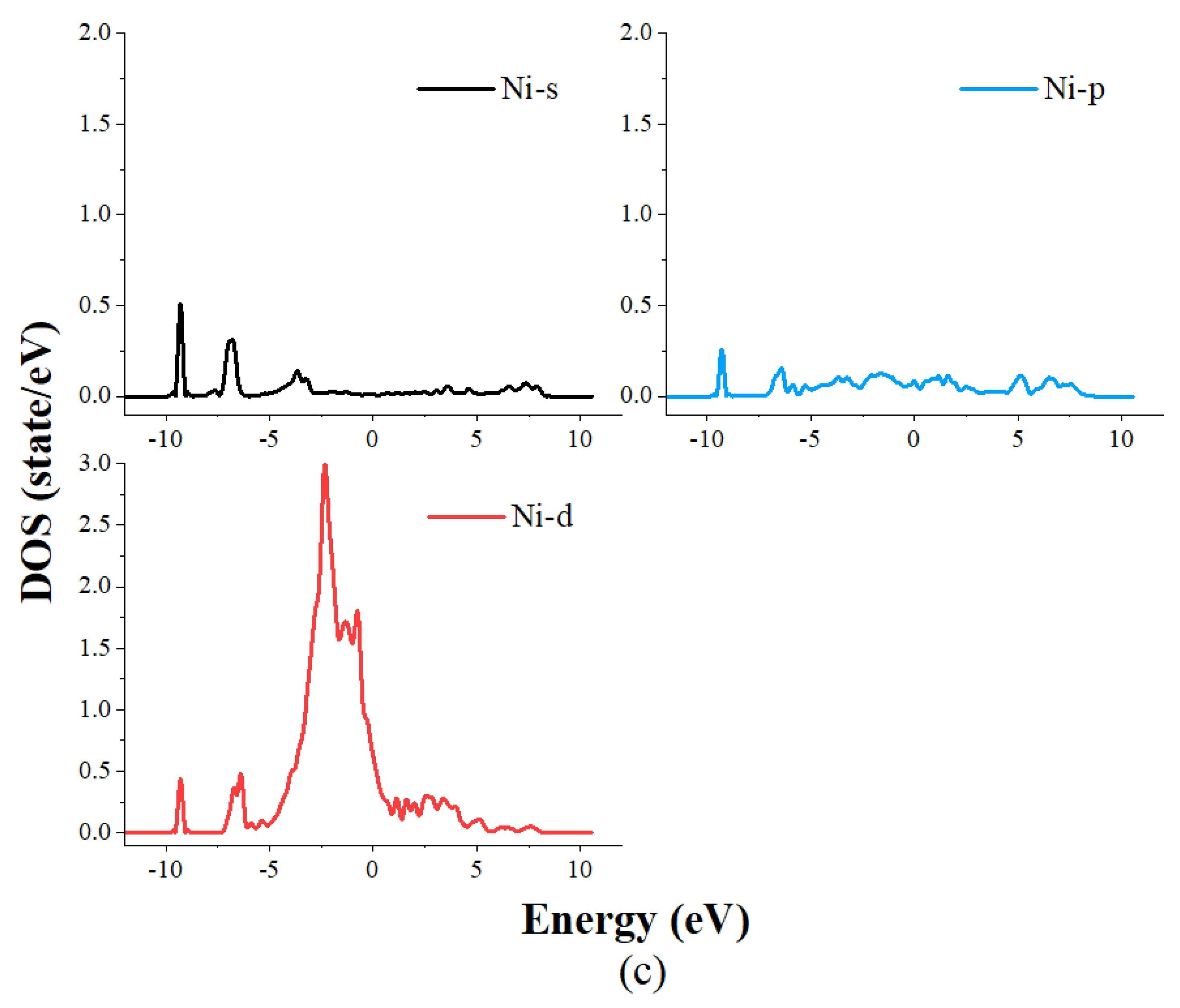
4. The Density of State Analysis
5. Conclusions
- By simulating and calculating the parameters of CO adsorption energy, the adsorption structure and the bond length on different crystal faces and different adsorption sites, we conclude that CO has the strongest energy at the valley position on the Ni (111) crystal surface under the four models. Similarly, the most stable adsorption position on the Ni (100) crystal surface is the valley position. Different from the former, the most substantial adsorption position on the Ni (110) crystal face under the four adsorption models is the short bridge site. CO is activated to varying degrees at all adsorption sites. At 0.11 mL, 0.25 mL and 0.5 mL, the preferential adsorption order of CO is (100) > (111) > (110).
- When a carbon monoxide molecule is adsorbed on Ni (111) and Ni (100) crystal surfaces, the length of the C-O bond will be longer with the increase in adsorption energy. However, there is no such linear relationship when it is adsorbed on a Ni (110) crystal surface. In addition, when located at the same adsorption position, the C-O bond length decreases with the increase in coverage, and the adsorption effect becomes weaker.
- The results of the density of states study show that the main reason for the adsorption of CO on the Ni crystal plane contains two parts: the first part is the hybridization of the s orbitals of carbon atoms and the s and pz orbitals of oxygen atoms with the s, p and d orbitals of nickel atoms in an energy range of −10 eV~−8 eV. The second part is the hybridization of the px and py orbitals of both carbon and oxygen atoms with the s and d orbitals of nickel atoms in an energy range of −7.5 eV~−6 eV.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Zhou, C.; Yue, W.; Yan, B.; Lin, Y.; Huang, A. Facile and green template-free synthesis of morphology-controllable Co3O4 catalysts for CO oxidation. Chem. Phys. Lett. 2020, 756, 137817. [Google Scholar] [CrossRef]
- Huan, W.; Li, J.; Ji, J.; Xing, M. In situ studies on ceria promoted cobalt oxide for CO oxidation. Chin. J. Catal. 2019, 40, 656–663. [Google Scholar] [CrossRef]
- Zheng, B.; de Beurs, K.M.; Owsley, B.C.; Henebry, G.M. Scaling relationship between CO pollution and population size over major US metropolitan statistical areas. Landsc. Urban Plan. 2019, 187, 191–198. [Google Scholar] [CrossRef]
- Lou, Z.; Yuan, D.; Zhang, F.; Wang, Y.; Li, Y.; Zhu, L. Fe3Si assisted Co3O4 nanorods: A case study of photothermal catalytic CO oxidation under ambient solar irradiation. Nano Energy 2019, 62, 653–659. [Google Scholar] [CrossRef]
- Rezaei, P.; Rezaei, M.; Meshkani, F. Low temperature CO oxidation over mesoporous iron and copper mixed oxides nanopowders synthesized by a simple one-pot solid-state method. Process Saf. Environ. Prot. 2018, 119, 379–388. [Google Scholar] [CrossRef]
- Ma, J.; Xu, X.; Zhao, C.; Yan, P. A review of atmospheric chemistry research in China: Photochemical smog, haze pollution, and gas-aerosol interactions. Adv. Atmos. Sci. 2012, 29, 1006–1026. [Google Scholar] [CrossRef]
- Gavrilă, C.; Ardelean, F.; Coman, A.; Burchiu, E. Statistical analysis for prognosis of a photochemical smog episode. Model. Civ. Environ. Eng. 2013, 9, 20–24. [Google Scholar] [CrossRef]
- Miyajima, K.; Mafune, F. Catalytic reactions of gas phase zirconium oxide clusters with NO and CO revealed by post heating. Chem. Phys. Lett. 2016, 660, 261–265. [Google Scholar] [CrossRef]
- Han, Y.-X.; Kong, C.; Hou, L.-J.; Chen, D.-P.; Wu, B.-W.; Geng, Z.-Y. Theoretical research on the catalytic reaction mechanism of N2O and CO over Ni-5 cluster. Comput. Theor. Chem. 2017, 1117, 12–19. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic oxidation of carbon monoxide over transition metal oxides. Chemcatchem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Tian, M. Catalytic oxidation of CO on noble metal-based catalysts. Environ. Sci. Pollut. Res. 2021, 28, 24847–24871. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.M.; Kodgire, P.; Dwivedi, A.H. Low temperature oxidation of carbon monoxide for heat recuperation: A green approach for energy production and a catalytic review. J. Clean. Prod. 2020, 245, 118838. [Google Scholar] [CrossRef]
- Ovcharov, M.L.; Granchak, V.M. Photocatalytic activation of carbon monoxide on semiconductors and derived nanocomposites: Basic principles and mechanisms: A review. Theor. Exp. Chem. 2019, 55, 173–200. [Google Scholar] [CrossRef]
- Rao, Y.; Wu, Y.; Dai, X.; Zhang, Y.W.; Qin, G.; Qi, W.; Li, S. A tale of two sites: Neighboring atomically dispersed Pt sites cooperatively remove trace H2 in CO-rich stream. Small 2022, 18, e2204611. [Google Scholar] [CrossRef]
- Huo, J.; Zhang, Y.-B.; Zou, W.-Y.; Hu, X.; Deng, Q.; Chen, D. Mini-review on an engineering approach towards the selection of transition metal complex-based catalysts for photocatalytic H2 production. Catal. Sci. Technol. 2019, 9, 2716–2727. [Google Scholar] [CrossRef]
- Tanaka, H.; Misono, M. Advances in designing perovskite catalysts. Curr. Opin. Solid State Mater. Sci. 2001, 5, 381–387. [Google Scholar] [CrossRef]
- Behr, A.; Henze, G.; Johnen, L.; Awungacha, C. Advances in thermomorphic liquid/liquid recycling of homogeneous transition metal catalysts. J. Mol. Catal. A Chem. 2008, 285, 20–28. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Catalytic mechanism of transition-metal compounds on Mg hydrogen sorption reaction. J. Phys. Chem. B 2006, 110, 11020–11024. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Akolekar, D.B. Adsorption of NO and CO over transition-metal-incorporated mesoporous catalytic materials. J. Colloid Interface Sci. 2005, 281, 171–178. [Google Scholar] [CrossRef]
- Canto, G.; Dzib, L.; Lanz, C.; Juan, A.; Brizuela, G.; Simonetti, S. Carbon monoxide adsorption on a nickel iron surface: Bonding and electronic structure computational study. Mol. Phys. 2012, 110, 113–120. [Google Scholar] [CrossRef]
- Yu, X.; Jin, L.; Zhao, C.; Liu, Z. High coverage CO adsorption on Fe6O6 cluster using GGA plus U. J. Clust. Sci. 2020, 31, 591–600. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, R.; Ling, L.; Wang, B. Insights into the effect of coverage on CO adsorption and dissociation over Rh(100) surface: A theoretical study. Appl. Surf. Sci. 2014, 320, 681–688. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.; Huang, W.; Zhou, S.; Hu, Z.; Wang, L. Density functional theory study of Ni segregation in CuNi(111) alloy with chemisorbed CO, O, or H. J. Phys. Chem. Solids 2022, 171, 111021. [Google Scholar] [CrossRef]
- Bai, Y.; Kirvassilis, D.; Xu, L.; Mavrikakis, M. Atomic and molecular adsorption on Ni(111). Surf. Sci. 2019, 679, 240–253. [Google Scholar] [CrossRef]
- Mitchell, G.E.; Gland, J.L.; White, J.M. Vibrational spectra of coadsorbed CO and H on Ni(100) and Ni(111). Surf. Sci. 1983, 131, 167–178. [Google Scholar] [CrossRef]
- Bromfield, T.C.; Ferre, D.C.; Niemantsverdriet, J.W. A DFT study of the adsorption and dissociation of CO on Fe(100): Influence of surface coverage on the nature of accessible adsorption states. Chemphyschem 2005, 6, 254–260. [Google Scholar] [CrossRef]
- Scheijen, F.J.E.; Niemantsverdriet, J.W.H.; Ferre, D.C. Density Functional theory study of CO adsorption and dissociation on molybdenum(100). J. Phys. Chem. C 2007, 111, 13473–13480. [Google Scholar] [CrossRef]
- Mahaffy, P.R.; Dignam, M.J. Carbon monoxide adsorption on Ni(110) studied by infrared ellipsometric spectroscopy. Surf. Sci. 1980, 97, 377–392. [Google Scholar] [CrossRef]
- Haq, S.; Love, J.G.; King, D.A. The adsorption of CO on Ni{110} and its interaction with hydrogen: A RAIRS study. Surf. Sci. 1992, 275, 170–184. [Google Scholar] [CrossRef]
- Sodeifian, G.; Razmimanesh, F. Diffusional interaction behavior of NSAIDs in lipid bilayer membrane using molecular dynamics (MD) simulation: Aspirin and Ibuprofen. J. Biomol. Struct. Dyn. 2019, 37, 1666–1684. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Chen, X.-S.; Lu, W. The study of oxygen and sulfur adsorption on the InSb (110) surface, using first-principle energy calculations. Acta Phys. Sin. 2013, 62, 206102. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Shu, X.; Wang, Y.; Ran, Q. Adsorption of organic molecules on mineral surfaces studied by first-principle calculations: A review. Adv. Colloid Interface Sci. 2018, 256, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Delley, B. Fast calculation of electrostatics in crystals and large molecules. J. Phys. Chem. 1996, 100, 6107–6110. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244. [Google Scholar] [CrossRef]
- Peng, H.; Xie, Y.; Nie, Y. Electronic Structure of Ni Metal. Mater. Rev. 2007, 4, 131–134. [Google Scholar]
- Shao, J.; Cheng, X.; Yang, X.; Zhang, F.; Ge, S. Calculations of bond dissociation energies and bond lengths of C-H, C-N, C-O, N-N. J. At. Mol. Phys. 2006, 23, 80–84. [Google Scholar]
- Xi, Y.-J.; Li, Y.; Wu, D.; Li, Z.-R. Theoretical study of the COLin complexes: Interaction between carbon monoxide and lithium clusters of different sizes. Comput. Theor. Chem. 2012, 994, 6–13. [Google Scholar] [CrossRef]
- Gross, L.; Mohn, F.; Moll, N.; Schuler, B.; Criado, A.; Guitian, E.; Pena, D.; Gourdon, A.; Meyer, G. Bond-Order Discrimination by Atomic Force Microscopy. Science 2012, 337, 1326–1329. [Google Scholar] [CrossRef]
- Ge, Q.; Jenkins, S.J.; King, D.A. Localisation of adsorbate-induced demagnetisation: CO chemisorbed on Ni{110}. Chem. Phys. Lett. 2000, 327, 125–130. [Google Scholar] [CrossRef]
- Eichler, A. CO adsorption on Ni(111)––A density functional theory study. Surf. Sci. 2003, 526, 332–340. [Google Scholar] [CrossRef]
- Inderwildi, O.R.; Lebiedz, D.; Deutschmann, O.; Warnatz, J. Coverage dependence of oxygen decomposition and surface diffusion on rhodium (111): A DFT study. J. Chem. Phys. 2005, 122, 034710. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Cao, D.B.; Li, Y.W.; Wang, J.G.; Jiao, H.J. Chemisorption of CO2 on nickel surfaces. J. Phys. Chem. B 2005, 109, 18956–18963. [Google Scholar] [CrossRef] [PubMed]
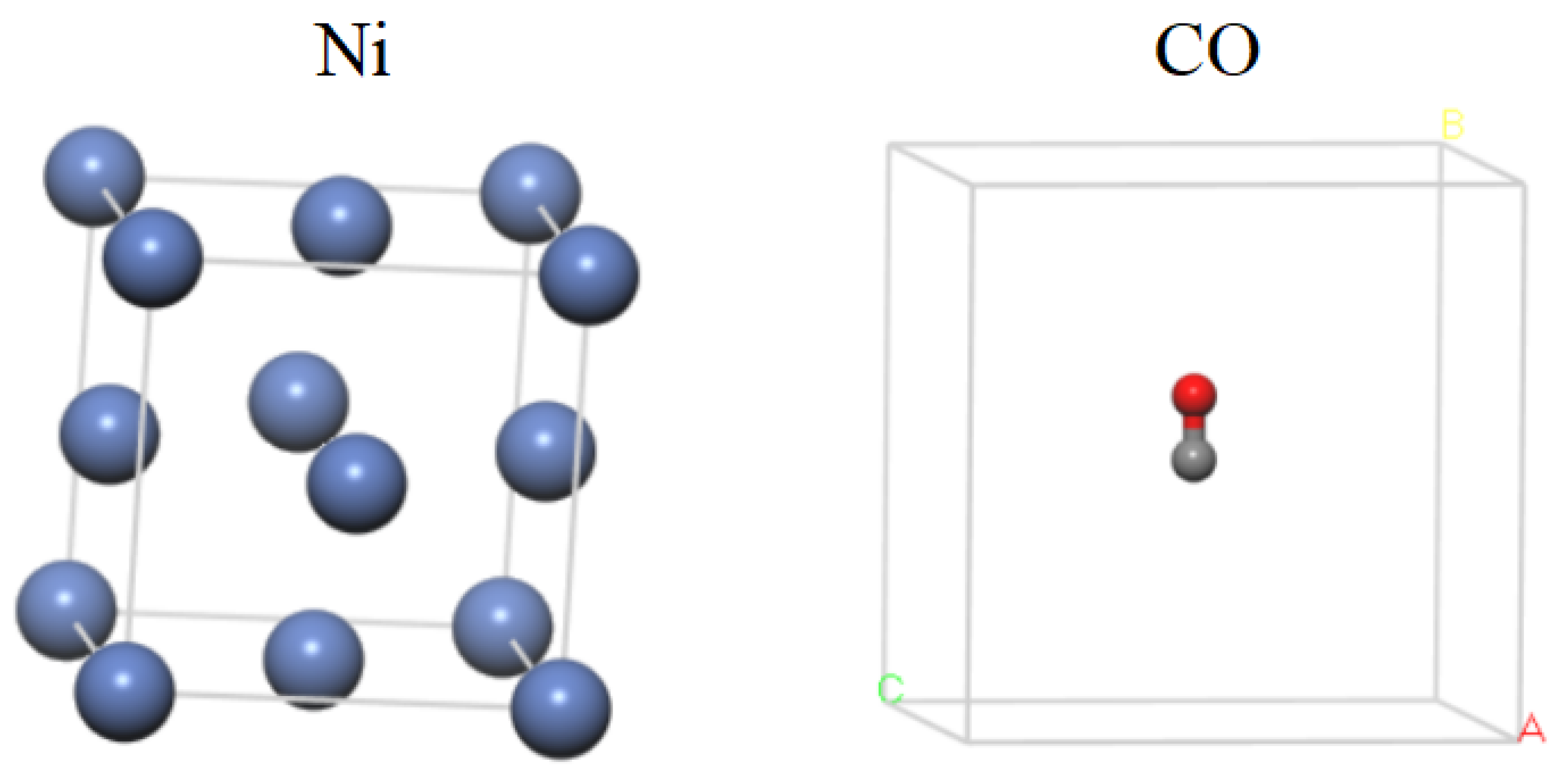

| Surface | Site | No. of Layers | ECO_ad (eV) | Ead (eV) | dC-O (nm) | dNi-C (nm) |
|---|---|---|---|---|---|---|
| CO | 0.114 | |||||
| Ni (111) | BE | 7 | −162.88 | 2.02 | 0.118 | 0.187 |
| V fcc | 7 | −162.91 | 2.05 | 0.119 | 0.194 | |
| V hcp | 7 | −162.93 | 2.07 | 0.119 | 0.194 | |
| TP | 7 | −162.47 | 1.61 | 0.116 | 0.173 | |
| Ni (100) | BE | 7 | −161.19 | 2.04 | 0.118 | 0.187 |
| V | 7 | −161.3 | 2.15 | 0.121 | 0.203 | |
| TP | 7 | −160.94 | 1.79 | 0.116 | 0.173 | |
| Ni (110) | LB | 7 | −157.51 | 1.82 | 0.125 | 0.197 |
| SB | 7 | −157.71 | 2.05 | 0.118 | 0.187 | |
| V | 7 | −157.45 | 1.76 | 0.121 | 0.198 | |
| TP | 7 | −157.48 | 1.79 | 0.116 | 0.174 |
| Scheme 111 | Energy (eV) |
|---|---|
| Ni (111) | −146.08 |
| Ni (100) | −144.37 |
| Ni (110) | −140.91 |
| CO | −14.78 |
| Surface | 111 | 100 | 110 |
|---|---|---|---|
| S (mL) | |||
| Energy (eV) | |||
| 0.11 | 2.12 | 2.18 | 2.07 |
| 0.25 | 2.07 | 2.15 | 2.01 |
| 0.5 | 1.90 | 2.03 | 1.87 |
| dC-O (nm) | |||
| 0.11 | 0.1194 | 0.1216 | 0.1184 |
| 0.25 | 0.1192 | 0.1212 | 0.1182 |
| 0.5 | 0.1183 | 0.1200 | 0.1176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Li, K.; Wang, F. Study on the Adsorption Properties and Mechanisms of CO on Nickel Surfaces Based on Density Functional Theory. Energies 2023, 16, 525. https://doi.org/10.3390/en16010525
Wang K, Li K, Wang F. Study on the Adsorption Properties and Mechanisms of CO on Nickel Surfaces Based on Density Functional Theory. Energies. 2023; 16(1):525. https://doi.org/10.3390/en16010525
Chicago/Turabian StyleWang, Kun, Kunlun Li, and Fuqing Wang. 2023. "Study on the Adsorption Properties and Mechanisms of CO on Nickel Surfaces Based on Density Functional Theory" Energies 16, no. 1: 525. https://doi.org/10.3390/en16010525
APA StyleWang, K., Li, K., & Wang, F. (2023). Study on the Adsorption Properties and Mechanisms of CO on Nickel Surfaces Based on Density Functional Theory. Energies, 16(1), 525. https://doi.org/10.3390/en16010525







