Abstract
One viable option for meeting global energy demand is the creation of biofuels from plant species that demonstrate high biomass productivity and good energy characteristics. In this study, growth was evaluated using plant height (PH), the production of green (GB) and dry biomass (DB), and the energy quality of leaves, pods, and stems, considering apical and basal sections of maralfalfa plants at 28, 60, 90, and 140 days after applying a uniformity cut (AUC). The variables were analyzed with correlation tests and variance analyses (ANOVA) using a factorial array design; in addition, Tukey tests were performed. A steady increase in PH (72 to 239 cm) was found. The highest yield of stems was at 90 AUC (41,362 kg/ha) for GB and 140 days AUC (6331 kg/ha) for DB, and a high correlation was observed between PH and stem biomass production for both the GB (r = 0.91) and DB (r = 0.93). There was a strong correlation between higher heating value and DB from the apical stratum (r = 0.99) and the basal stratum (r = 0.97). Maralfalfa shows high biomass productivity and high energy production in short growth periods.
1. Introduction
Biomass is the only alternative energy source capable of supplying solid, liquid, and gaseous substitutes for fossil fuels [1,2]. For decades, wood has been the preferred source of biomass. However, public concerns about the environmental consequences of deforestation have forced researchers to look for different sources of biomass [3]. In addition, criticism suffered by first-generation biofuels (food-versus-fuel conflict) has led to the search for second-generation biofuels that do not have this controversy [4].
Perennial grasses such as maralfalfa (Cenchrus purpureus (Schumach.) Morrone) are native to Africa [3], and their use has expanded to tropical and subtropical areas around the world as feed for livestock [3,5,6]. However, due to their upright growth habit, energetic varieties, height, and rapid growth rate, they have drawn attention as feedstock for the biofuel industry [2,7]. Biomass from grasses can be transformed into heat for electric generators through direct combustion [2]. Using biomass from perennial crops is advantageous because it can also provide ecosystem services [2,8,9].
Grasses from the C. purpureus species have the world’s most significant biomass production potential [7]. Wild elephant grass dry matter yield (DMY) has ranged from 7 to 11 t ha−1 at the first harvest [1]. Previous research [10] has reported that maralfalfa reached its highest DMY (39.0 t ha−1) in a single harvest when allowed to regrow for 100 days in a temperate climate. In a study performed in a subhumid tropical climate, its DMY was 37.29 t ha−1 at 151 days [11]. However, this DMY could be considered the baseline for the C. purpureus species. Other reports have stated that the DMY can vary from 50 to 67 t ha−1 [12,13].
In recent years, biomass densification has extended; hence, the production of pellets and briquettes has increased [14,15,16]. In this case, biomass from maralfalfa is a promising alternative for producing pellets for residential and industrial use [17]. Biomass feedstocks intended for combustion should possess some quality characteristics, such as high calorific value, adequate carbon–nitrogen ratio, and low moisture, ash, and nitrogen content [18,19,20]. In this regard, the ash content of C. purpureus biomass is a characteristic with contrasting results. Some research has shown that its ash content varies from 3.1 to 4.7% [16,17], being less than 7%, and therefore, it complies with international standards [21]. However, a few studies have found ash contents of 10.51% and up to 17.50% [22,23].
High ash content in biomass is not desirable because of the creation of chemical compounds that, at elevated temperatures, are detrimental to metallic surfaces, and the buildup of residues will increase [24]. Leaf blades may increase ash accumulation in biomass compared to the high fiber portion from shoots [25]. Management of a biofuel crop (cutting age of different plant strata and components) could improve its biomass quality [12]. Hence, the objective of this study was to evaluate the productivity and quality of maralfalfa biomass obtained at different cutting dates, plant strata, and structures.
2. Materials and Methods
2.1. Characterization of the Study Area
The field study was carried out at 23°59′21″ N, 104°37′33″ W, at an altitude of 1880 m. The soil was predominantly loam (sandy or clay; Kastañozem), with a capacity for intermediate moisture retention, a medium depth, a slope of 0 to 2%, a pH of 7.9, and low organic matter content, phosphorus, and nitrogen. The climate of the studied region is temperate and semi-arid, with rainfall in the summer (BS1 Kw (w) (e)), an average annual temperature of 17.4 °C, and extreme temperature variations throughout the day and the year [26,27].
2.2. Agronomic Management
The biomass of maralfala was obtained from a plantation established in 2017. A uniformity cut was applied in July 2021 to control the phenological age of the plants. The experimental plot consisted of six rows, each 76 m long and 0.81 m apart. After the uniformity cut, fertilization occurred at a dose of 180-80-50 for nitrogen (N), phosphorus (P2O5), and potassium (K2O), respectively, by using urea (46-00-00, respectively), diammonium phosphate (18-46-00, respectively) and potassium chloride (00-00-60, respectively).
2.3. Variables Evaluated in the Field
From July to November, the morpho-physiological variables were evaluated monthly, such as the increase in plant height (PH) and accumulation of fresh (FB) and dry biomass (DB) at the level of strata (basal and apical) and organ (stem, leaf, and pod). In addition, the daily values of maximum and minimum temperatures were recorded [28,29], as was rainfall during the evaluation period. Then, the average (maximum and minimum) temperatures and accumulated rainfall were calculated in five-day periods (Figure 1).
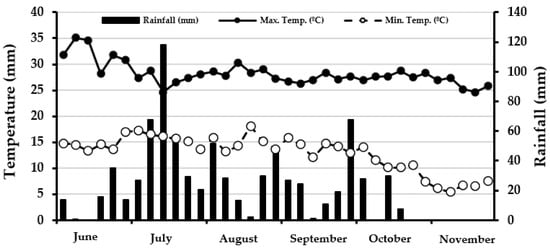
Figure 1.
Averages for the maximum and minimum temperatures and accumulated rainfall in five-day periods at INIFAP-CEVAG-Durango, México [28,29].
The mean value for the maximum temperature from June to November was 28 °C and the minimum 13 °C, while the accumulated precipitation reached 786 mm. However, the minimum temperature was maintained at acceptable levels for maralfalfa until October (>10 °C), and from the last week of that month to November, the minimum temperatures decreased to levels that will reduce the growth and development of some grasses (<10 °C) [30]. In addition, the accumulated rainfall decreased during October (<40 mm), influencing the observed response in biomass yield and quality.
2.4. Sampling Method
Four cluster biomass samplings were performed at 28, 60, 90, and 140 days after uniformity cutting (AUC). Three replicates were performed on each sampling date, and individual plots were established in sites without prior sampling in which the maralfalfa population also showed full competition among plants. First, each plant was divided into apical and basal strata, determined based on the fourth node of the dominant stem. Then, the stems and leaves were dissected in each stratum and separated into the blade (limbo) and foliar sheath. Each sample consisted of all the plants (6 to 7) within a sampling plot. The sampling plots consisted of two rows that were 2.00 m in length, with a separation of 0.81 m (3.24 m2).
Samples from each stratum and organ were placed in paper bags and weighed on a (± 0.01 g) scale to obtain the fresh weight. Then, they were introduced into a drying stove at 60 °C, where they were left for several days (5 to 10) until they reached a constant weight (dry weight). The data was used to estimate the yield of fresh and dry biomass per hectare (ha−1).
2.5. Proximate Analysis and Energy Characterization
The dried samples of maralfalfa were milled in an SM 300 apparatus at a particle size of 2 mm. The ground sample was passed through several sieves to separate the portion between the sieves of 40 and 60, which was later used in laboratory analyses. Chemical tests included immediate analyses in which moisture content was determined from each sample in an oven for four hours at 105 ± 2 °C, and the ash content was determined using the UNE-EN 14774-3 method [31]. The volatile compounds were determined based on EN 18123, and the fixed carbon was determined by calculating the difference from 100 with moisture content, ash, and volatile matter fraction values. In addition, the calorific value was evaluated using the calorimetric pump, following UNE-EN 14918.
2.6. Statistical Analysis
The data obtained were used to determine relationships between variables by using a correlation analysis, and a variance analysis (ANOVA) was also performed under a randomized complete design with a factorial arrangement with two strata (basal and apical), three organs (stem, leaf, and sheath), and three replications. When significant differences were observed between strata and among organs, a Tukey test (p ≤ 0.05) was applied for the multiple comparisons of the means. For the correlations, ANOVA, and mean comparison, the software SAS v. 9.4 was used [32].
3. Results and Discussion
3.1. Plant Height
The maralfalfa plants’ heights rose steadily as the number of days AUC increased. Thus, they grew from 72 cm at 28 days to 239 cm at 140 days AUC. The highest correlation was between plant height and biomass accumulation in the stems of the apical stratum, for both fresh (r = 0.91) and dry (r = 0.93) measurements. At the lower strata, there was a significant correlation between plant height and fresh biomass (r = 0.67) and dry biomass (r = 0.94) at the basal part of the stem. The rest of the plant structures showed lower correlations between plant heights and fresh and dry biomass accumulations. There was also a positive correlation between plant height and the calorific value in the apical stratum (r = 0.99) and basal stratum (r = 0.97). The height of a plant showed a relationship with variables related to the productivity and quality of biomass, with variations between strata and organs. These variations were correlated to the lignification of biomass in an acropetal direction [10,25].
3.2. Biomass in Apical Stratum
The plants showed a low accumulation of fresh biomass in the stems at the beginning of the sampling period, and this was surpassed by the leaves at the first two cutting dates, although it showed values similar to those obtained in the leaf sheaths from which its separation was difficult (Figure 2). From the third cutting age (90 days AUC), the biomass in the stems increased significantly, exceeding that of the leaves and sheaths. The highest value of fresh stem weight was recorded at an age of 90 days AUC (38,771 kg ha−1), and it then decreased to 29,167 kg ha−1 at the last sampling date performed at the age of 140 days AUC.
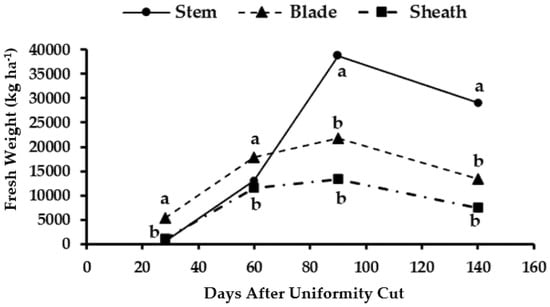
Figure 2.
Fresh biomass accumulation in different organs of the apical stratum of maralfalfa plants harvested at different cutting dates. The weight values with equal letters in each stratum of the plant were statistically similar (p ≤ 0.05).
The dry biomass accumulation in the apical stems was low at the first sampling dates compared to that of the other structures, and it was surpassed by leaves and pods at the first two cutting ages (Figure 3). Then, at the third cutting (90 days AUC), the weight was similar (5215 kg ha−1) between the stems and leaves, which exceeded that of the foliar sheaths (1944 kg/ha). Only at the fourth cutting age (140 days AUC) was the dry biomass higher in stems (5451 kg/ha), and so it exceeded those of the leaves (4362 kg ha−1) and sheaths (1461 kg ha−1) due to the senescence and fall of these plant organs during autumn. The highest dry stem weight (5451 kg ha−1) was at 140 days AUC due to the accumulation of photoassimilates that remobilized from the senescent leaves and sheaths. In addition, the apical stratum of the plants dominated the regular increase in leaf length [33].
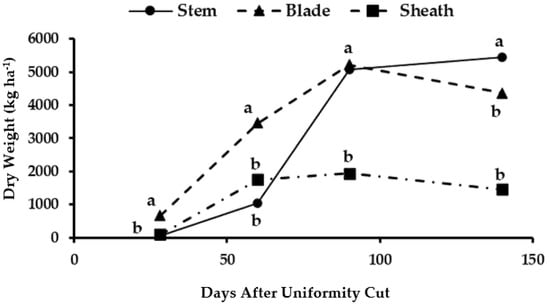
Figure 3.
Dry biomass accumulation in different organs of the apical stratum of maralfalfa plants harvested at different cutting dates. The weight values with equal letters in each stratum of the plant were statistically similar (p ≤ 0.05).
3.3. Biomass in the Basal Stratum
The biomass in the basal stems was significantly higher at 60 days AUC compared to those of the leaf blades and sheaths (Figure 4). The highest biomass yield of stems took place at 90 days AUC (41,362 kg ha−1), when decreased biomass levels occurred in the leaves and sheaths due to the senescence and loss of these organs in the basal stratum as the development of the maralfalfa progressed. The dry biomass of the basal stems (6331 kg ha−1) significantly exceeded that of the dry matter present in the leaves and sheaths of the basal part of the plant (Figure 5). In this stratum, the highest yield was observed at 140 days AUC due to the accumulation of photoassimilates from the apical leaves, which showed the highest photosynthetic activity. The lignification of the basal part of the stem was necessary for favoring the ascending growth of the plant [34].
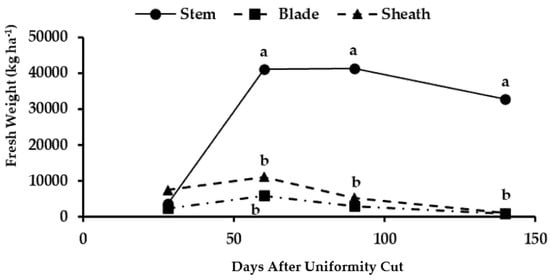
Figure 4.
Fresh biomass accumulation at different organs of the basal stratum of maralfalfa plants harvested at different cutting dates. The weight values with equal letters in each stratum of the plant were statistically similar (p ≤ 0.05).
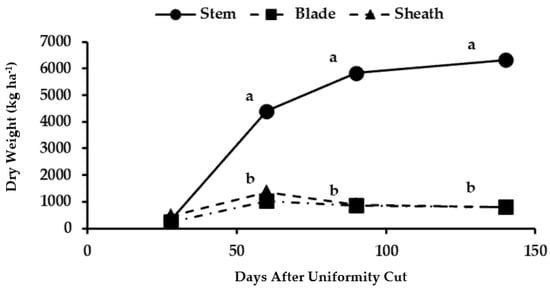
Figure 5.
Dry biomass accumulation at different organs of the basal stratum of maralfalfa plants harvested at different cutting dates. The weight values with equal letters in each stratum of the plant were statistically similar (p ≤ 0.05).
3.4. Total Biomass
The highest accumulation of fresh total biomass (123,379 kg ha−1) was at 90 days AUC (Figure 6), and a considerable decrease was observed at 140 days AUC. This decrease was because of the loss of moisture in plant tissues, which was due to the natural senescence of the leaves and basal sheaths, reducing their water content. In addition, some leaves and pods were detached from the plants and caused a reduction in total biomass at the age of 140 days AUC. For total dry biomass, the highest value (19,828 kg ha−1) was also recorded at the age of 90 days AUC, with the lowest value (19,206 kg ha−1) at the end of the sampling period (140 days AUC) because of the accumulation of photoassimilates that remobilized from the blades and senescent sheaths.
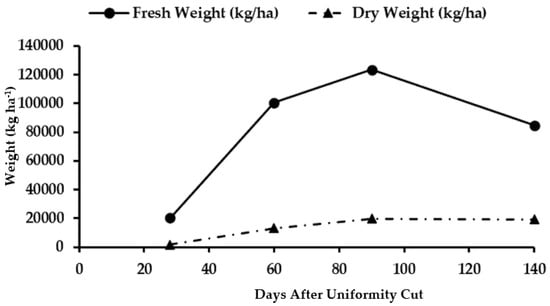
Figure 6.
Total fresh and dry biomass accumulation in maralfalfa plants harvested at different cutting dates.
3.5. Proximate Analysis
3.5.1. Volatile Material
The volatile material was higher in the stems. Values ranged from 66.0% to 72.7% in the apical stratum, while in the basal stratum, they varied between 66.9% and 70.8%. In the blades and sheaths, volatile material values ranged between 61.4% and 67.2% for all strata and ages (Table 1). At 60 days AUC, a significant statistical difference was observed for the interaction between strata and organ, with the highest value at 140 days AUC, which was related to the modification in the response of the organs between strata, which was primarily influenced by the effect of the changes in the phenological development and accumulation-remobilization of minerals and processed substances (photoassimilates). The above strengthened the suggestion to cut the maralfalfa for energy purposes after 140 days AUC, and separation of leaves and sheaths from the stems was necessary to reach values of volatile compounds according to the 69.4 to 85.4% intervals recorded for other biofuels, including wood pellets and briquettes [17,35,36]. Volatile compounds concentrate a high proportion of biomass calorific value [37]; therefore, maralfalfa may be an option for bioenergy generation.

Table 1.
Analysis of variance of proximal biomass analyses results for different strata and organs of maralfalfa (Cenchrus purpureus (Schumach.) Morrone) on different days after uniformity cut.
The leaf sheaths located in the apical parts showed constant increases in volatile compounds at all sampling dates (63.1% to 67.2%) (Table 2), whereas the leaves of the basal parts showed constant variation, reaching their maximum values at 140 days AUC (64.7%). The leaves and leaf sheaths showed low levels of volatile materials, which was related to their anatomy and low capacity to store processed substances. Volatile material values were lower than those reported in other studies on maralfalfa (72.3–77.9%) [38], primarily due to climatic differences in the evaluation period and the late start of our experiment, which influenced the lignification and other morpho-physiological processes influenced by the decreases in temperature and water availability recorded in Durango during autumn.

Table 2.
Average values of proximal biomass analyses in different strata and organs of maralfalfa (Cenchrus purpureus (Schumach.), Morrone) on different days after uniformity cut.
3.5.2. Ash
Statistical equality was observed for ash content at both 28 and 90 days AUC, while at 60 days, significant differences occurred (p ≤ 0.05) between the structures, and at 140 days AUC, between the plant strata (p ≤ 0.05) and organs (p ≤ 0.01) (Table 1 and Table 2). The ash content was higher in the stems, especially in the basal stratum of the plants (8.3 to 9.1%), although at the end of the evaluation period (140 days AUC), the stems of the apical strata also showed high ash levels (Table 2). The results confirm that in the basal parts, there is a constant accumulation of elaborated compounds, such as hemicellulose (15 to 30%), cellulose (40 to 60%), and lignin (10 to 30%) [36], due to the need to provide structural support to the plant, which was reflected in the high ash content in the basal strata of the stems, which gradually advanced in an acropetal direction.
The leaves and sheaths showed a variable ash level throughout the study period, with slight differences between strata and occasional significant superiority between plant organs. The recording of maximum values at the end of the evaluation period (140 days AUC) was due to the temporary accumulation of minerals and processed substances, their subsequent remobilization to other parts of the plant, and new culms in formation.
The results showed that leaves temporarily accumulate minerals and photoassimilates, which move to other plant organs and growing stems [39,40]. The ash content was in the ranges obtained in other studies on maralfalfa, in which values between 5.2 and 9.7% were recorded [38,41]. The high ash content limits the direct utility of maralfalfa biomass as a biofuel, although using it as pellets decreases ash formation (3.1%) [17].
3.5.3. Fixed Carbon
Fixed carbon was significantly lower in the stems of both strata, at all cutting ages, with values of 13.2 to 17.3% (Table 1 and Table 2), with the lowest levels occurring at the end of the study (13.2 to 13.9%). In contrast, the leaves and sheaths showed highly significant levels of fixed carbon, with values ranging between 18.5% and 24.4%. In the sample from 90 days AUC, it was observed that the fixed carbon registered significant differences for the stratum–organ interaction, which was related to the variation in the response of the organ between plant strata, caused primarily by the production of photoassimilates and their remobilization to accumulation structures and growing points. The fixed carbon levels were low, even at the highest point recorded in the stems of both strata (90 DCU), where values close to 25% were observed and located within the range established for wheat straw (18.3–29.9%) [42], and they overpassed those observed in maralfalfa for different cutting dates (16.7 to 19.2%) [17,38,41].
The leaves of both strata showed high levels of fixed carbon at 28 days AUC (from 20.6 to 20.9%). This content gradually increased between cutting dates, especially in the apical parts, where it reached values between 22.8 and 24.4%, primarily because this is where carbon fixation occurs for the photosynthetic process. In the case of basal blades, slightly higher fixed carbon values were observed in contrast to the apical ones, primarily due to the storage and remobilization of photoassimilates to the growing points of the apical parts and other growing stems in the same plant [39,40].
The sheaths of the basal parts showed high levels of fixed carbon compared with those of the apical parts, although, only at 60 days AUC, significant differences were registered between the plant strata (Table 1 and Table 2). The sheaths of the apical stratum showed fixed carbon reduction as the development of the plant progressed, and so the lowest value recorded in the last sampling performed was at 140 days AUC, with 19.3%. This response was also related to the constant removal of photoassimilates from the apical parts to other organs of the same stem and to other growing stems within the same plant.
3.5.4. Higher Heating Value
The higher calorific values were similar between plant strata and organs at 28 days AUC, with values between 14.8 and 16.2 MJ kg−1. In the subsequent cutting dates (60 and 90 days AUC), there were significant differences (p ≤ 0.01) between the strata and plant organs. The statistical differences were observed only between organs on the third cutting date (90 days AUC). Variations in composition of tissues in each organ are the cause of differences in calorific values. The stems showed gradual increases in the higher heating values, reaching the highest levels at 140 days AUC, being significantly higher (16.4 to 17.1 MJ kg−1) compared to the leaves (13.2 to 14.7 MJ kg−1) and sheaths (14.7 to 16.1 MJ kg−1). At that cutting date, the blades and sheaths of the apical stratum showed higher heating values than the basal stratum. The blades showed significantly lower levels of heating values in comparison to the stems and foliar sheaths. From the second cutting date (60 days AUC), the basal stems showed higher calorific values than the apical ones due to the higher levels of lignification [43]. The higher heating values were similar to those obtained for maralfalfa (16.6 MJ kg−1) in other studies previously carried out at the same site [17].
4. Conclusions
The height of maralfalfa plants positively correlates with biomass yield and quality. Total dry biomass yield reached its highest point at 90 days AUC. The highest dry biomass accumulation in both strata occurred in the stems at 140 days AUC. At the last sampling date, the stems recorded the highest volatile material concentrations and higher heating values compared to the leaf blades and sheaths. However, ash content was also highest in the stems. These findings indicate that the harvest of maralfalfa biomass for fuel purposes should occur at 140 days AUC. In addition, leaf blades and sheaths should be separated from the stems to reach higher heating values. Ash content limits the direct use of maralfalfa biomass, but further processing it into pellets and briquettes could be beneficial.
Author Contributions
Conceptualization, R.R.-S. and A.C.-P.; methodology, R.R.-S. and S.S.-E.; software, R.R.-S.; validation, R.R.-S., J.C.R.-S. and R.J.-O.; formal analysis, A.C.-P. and J.A.M.-G.; investigation, R.R.-S. and J.A.M.-G.; resources, J.C.R.-S. and A.C.-P.; data curation, R.R.-S. and J.A.M.-G.; writing—original draft preparation, R.R.-S.; writing—review and editing, A.C.-P. and P.A.D.-M.; visualization, R.R.-S.; supervision, R.R.-S.; project administration, R.R.-S.; funding acquisition, R.R.-S., J.C.R.-S., A.C.-P., R.J.-O., S.S.-E. and P.A.D.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We appreciate the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) and the Universidad Juárez del Estado de Durango (UJED) for providing the facilities for the use of installations, field plots, and laboratories. We are also very grateful to the reviewers and editors for their contributions to improving this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ohimain, E.I.; Kendabie, P.; Nwachukwu, R.E.S. Bioenergy potentials of elephant grass, Pennisetum purpureum Schumach. Annu. Res. Rev. Biol. 2014, 4, 2215–2227. [Google Scholar] [CrossRef]
- Danquah, J.A.; Roberts, C.; Appiah, M. Elephant Grass (Pennisetum purpureum): A Potential Source of Biomass for Power Generation in Ghana. Curr. J. Appl. Sci. Technol. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Montero-Lagunes, M.; Vinay-Vadillo, J.C.; Enríquez-Quiroz, J.F.; Jiménez-Montero, A.; Juárez-Lagunes, F.I.; Bolaños-Aguilar, E.D. Lignocellulosic biomass production in six cultivars of Cenchrus purpureus (Schumach.) Morrone in the tropics. Agro Product. 2022, 15. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.; Rout, P.K.; Dalai, A. Production of first and second generation biofuels: A comprehensive review. Renew. Sust. Energ. Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Kongkeitkajorn, M.B.; Sae-Kuay, C.; Reungsang, A. Evaluation of Napier Grass for Bioethanol Production through a Fermentation Process. Processes 2020, 8, 567. [Google Scholar] [CrossRef]
- Ventura-Ríos, J.; Honorato-Salazar, J.A.; Apolinar-Hidalgo, F.; Barrera-Martinez, I.; Aburto-Anell, J.; Huerta, H.V. Agronomic characterization of Taiwan grass [Cenchrus purpureus (Schumach.) Morrone] and evaluation of its potential to produce bioethanol in the warm sub-humid climate of Mexico. Trop. Grassl.-Forrajes Trop. 2022, 10, 22–31. [Google Scholar] [CrossRef]
- Reyes-Castro, S.; Enríquez-Quiroz, J.F.; Hernandez-Garay, A.C.; Esqueda-Esquivel, V.A.; Gutiérrez-Arenas, D.A. Rendimiento de seis cultivares de C. purpureus (Schumach.) Morrone con potencial para producción de bioetanol. Agro Product. 2018, 11, 56–62. [Google Scholar]
- Viana-Otero, M.V.; Siri-Prieto, G. Producción de biomasa de cultivos lignocelulósicos según el número de cortes. Agrociencia (Uruguay) 2018, 22, 13–23. [Google Scholar] [CrossRef]
- Capetillo-Burela, Á.; Zetina-Lezama, R.; Reynolds-Chávez, M.A.; Cadena-Zapata, M.; López-López, J.A.; Matilde-Hernández, C.; del Carmen, A.E. Elaboración de papel con seis variedades de Pennisetum purpureum Schumach en Veracruz, México. Rev. Iberoam. Bioecon. Cambio Clim. 2021, 7, 1644–1665. [Google Scholar] [CrossRef]
- Nava-Berumen, C.A.; Carrete-Carreón, F.O.; Rosales-Serna, R.; Reyes-Estrada, O.; Domínguez-Martínez, P.; Herrera-Torres, E. Rendimiento y calidad de forraje obtenido con el pasto maralfalfa cosechado a diferentes edades de rebrote en Durango, México. Investig. Cienc. Univ. Autónoma Aguascalientes 2021, 29, e3070. [Google Scholar] [CrossRef]
- Calzada-Marín, J.M.; Enríquez-Quiroz, J.F.; Hernández-Garay, A.; Ortega-Jiménez, E.; Mendoza-Pedroza, S.I. Análisis de crecimiento del pasto maralfalfa (Pennisetum sp.) en clima cálido subhúmedo. Rev. Mex. Cienc. Pecu. 2014, 5, 247–260. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-242014000200009&lng=es&tlng=es (accessed on 16 December 2022). [CrossRef]
- Favare, H.G.; Abreu, J.; Barros, L.; Silva, F.G.; Ferreira, L.M.; Barelli, M.A.; da Neto, I.M.; Cabral, C.E.; Peixoto, W.; da Campos, F.I.; et al. Effect of Elephant Grass Genotypes to Bioenergy Production. J. Exp. Agric. Int. 2019, 38, 1–11. [Google Scholar] [CrossRef]
- Wijitphan, S.; Lowilai, P. Effects of cutting interval on yields and nutritive values of King Napier grass (Pennisetum purpureum cv. King grass) under Irrigation Supply. KKU Res. J. 2011, 16, 215–224. [Google Scholar]
- Lajili, M.; Guizani, C.; Escudero-Sanz, F.J.; Jeguirim, M. Fast pyrolysis and steam gasification of pellets prepared from olive oil mill residues. Energy 2018, 150, 61–68. [Google Scholar] [CrossRef]
- Reid, W.V.; Ali, M.K.; Field, C.B. The future of bioenergy. Glob. Chang. Biol. 2020, 26, 274–286. [Google Scholar] [CrossRef]
- Bueno, A.M.; de Andrade, A.F.; Viçosi, K.A.; Flores, R.A.; Sette, C.R., Jr.; da Cunha, T.Q.G.; Santos, G.G. Does Nitrogen Application Improve Elephant Grass Yield and Energetic Characteristics of Biofuels? Bioenerg. Res. 2021, 14, 774–784. [Google Scholar] [CrossRef]
- Ríos-Saucedo, J.C.; Rosales-Serna, R.; Jiménez-Ocampo, R.; Domínguez-Martínez, P.A.; Carrillo-Parra, A.; Valenzuela-Nuñez, L.M. Calidad de pélets a partir de biomasa de ocho especies dendroenergéticas de crecimiento rápido. Agrociencia 2021, 55, 557–568. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Jaradat, A.A. Genetic resources of energy crops: Biological systems to combat climate change. Aust. J. Crop. Sci. 2010, 4, 309–323. [Google Scholar]
- Carrillo-Parra, A.; Ngangyo-Heya, M.; Colín-Urieta, S.; Foroughbakhch-Pournavab, R.; Rutiaga-Quiñones, J.G.; Correa-Méndez, F. Physical, mechanical and energy characterization of wood pellets obtained from three common tropical species. PeerJ 2018, 6, e5504. [Google Scholar] [CrossRef]
- Wibowo, S.; Laia, D.P.O.; Khotib, M.; Pari, G. Characterization of carbon pellets made from elephant grass (Pennisetum purpureum Scumach) mixed with Nyamplung Shell (Calophyllum inophyllum Linn.). J. Penelit. Hasil Hutan 2017, 35, 73–82. [Google Scholar] [CrossRef]
- Garcia, D.; Caraschi, J.; Ventorim, G.; Vieira, F.; Protásio, T. Comparative energy properties of torrefied pellets in relation to pine and elephant grass pellets. BioResources 2018, 13, 2898–2906. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Barnthaler, G. Chemical properties of solid biofuels–significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Paciulo, D.S.C.; Gomide, J.A.; Ribeiro, K.G. Nitrogen fertilization of elephant grass cv. Mott. 1. Forage yield and morphophysiological characteristics when reaching 80 and 120 cm in height. Braz. J. Anim. Sci. 1998, 27, 1069–1075. [Google Scholar]
- García, M.E. Modificaciones al Sistema de Clasificación Climática de Köppen (Para Adaptarlo a las Condiciones de la República Mexicana), 4th ed.; Enriqueta García de Miranda: Mexico City, Mexico, 1987; 217p. [Google Scholar]
- Medina, G.G.; Díaz, P.G.; López, H.J.; Ruíz CJ, A.; Marín, S.M. Estadísticas Climatológicas Básicas del Estado de Durango (Periodo 1961–2003). Libro Técnico Núm. 1. SAGARPA-INIFAP-CIRNOC-Campo Experimental Valle del Guadiana; SAGARPA-INIFAP-CIRNOC-Campo Esperimental Valle del Guadiana: Durango, Mexico, 2005; 224p. [Google Scholar]
- CONAGUA (Comisión Nacional del Agua); Dirección local Durango. Observaciones Meteorológicas 2020; INIFAP-CIRNOC-Campo Experimental Valle del Guadiana: Durango, Mexico, 2021; 3p. [Google Scholar]
- INIFAP (Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias); Estación local Durango (CEVAG). Datos Consultados Localmente 15/11/2021; INIFAP-CIRPAC-CE Centro Altos de Jalisco: Durango, Mexico, 2021; 5p. [Google Scholar]
- Ruiz, C.J.A.; Medina, G.G.; González, I.J.A.; Flores, H.E.L.; Ramírez, G.O.; Ortiz, C.T.; Byerly, K.F.M.; Martínez, R.A.P. Requerimientos Agroecológicos de los Cultivos. Libro Técnico Núm. 3. INIFAP-CIRPAC-CE Centro Altos de Jalisco; Tepatitlán de Morelos: Jalisco, Mexico, 2013; 564p. [Google Scholar]
- UNE-EN 14774-3; Determinación del Contenido de Humedad. Método de Secado en Estufa. Parte 3. Humedad de la Muestra para Análisis General. Asociación Española de Normalización (UNE): Madrid, Spain, 2010. Available online: http://www.aenor.es/aenor/normas/normas/fichanorma.asp?tipo=N&codigo=N0045728#.WH_EN7GZNsM (accessed on 1 February 2022).
- SAS Institute Inc. SAS/STAT® 12.3 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2013; Available online: https://support.sas.com/documentation/cdl/en/statug/66103/HTML/default/viewer.htm#titlepage.htm (accessed on 16 October 2022).
- Valašinaitė, S.; Šimkūnas, A.; Denisov, V. Leaf size regularities in Festuca pratensis from the systemic viewpoint. Plant Biosyst. 2013, 147, 629–637. [Google Scholar] [CrossRef]
- Emonet, A.; Hay, A. Development and diversity of lignin patterns. Plant Phys. 2022, 190, 31–43. [Google Scholar] [CrossRef]
- Ibarra, B.J.C.; Rueda, Y.J.O. Biomasa Para el Aprovechamiento Energético. In Una Revisión de la Caracterización y los Modelos por Descomposición Termoquímica; Research Group on Energy and Environment GIEMA: Bucaramanga, Colombia, 2017; 18p. [Google Scholar]
- Baray, G.M.R.; Porras DA, F.; Hoffmann HE, E.; Manjarrez, C.B.D. Tratamiento de la biomasa lignocelulósica mediante la pirólisis lenta y a baja temperatura para la producción de biocombustibles. Rev. Energías Renov. 2019, 3, 1–9. [Google Scholar]
- EB (Energía Biomasa). Energías Renovables; Secretaría de Energía: Buenos Aires, Argentina, 2008; 16p. [Google Scholar]
- Ventura, R.J.; Honorato Salazar, J.A.; Hernández Garay, A.; Aburto Anell, J.A.; Vaquera Huerta, H.; Enríquez Quiroz, J.F. Composición química y rendimiento de maralfalfa para producción de bioetanol de segunda generación. Rev. Mex. Cien. Agríc. 2017, 8, 215–221. [Google Scholar]
- Carvalho, D.D.; Irving, L.J.; Carnevalli, R.A.; Hodgson, J.; Matthew, C. Distribution of current photosynthate in two Guinea grass (Panicum maximum Jacq.) cultivars. J. Exp. Bot. 2006, 57, 2015–2024. [Google Scholar] [CrossRef]
- Oitate, H.; Noguchi, K.; Terashima, I.; Suzuki, A.A. Patterns of photoassimilate translocation to reproductive shoots from adjacent shoots in Camellia sasanqua by manipulation of sink-source balance between the shoots. J. Plant Res. 2011, 124, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.S.; Islam, S.N.; Afroze, S.; Bakar, M.S.A.; Sukri, R.S.; Rahman, S.; Azad, A.K. Evaluation of the bioenergy potential of invasive Pennisetum purpureum through pyrolysis and thermogravimetric analysis. Energy Ecol. Environ. 2020, 5, 118–133. [Google Scholar] [CrossRef]
- Ferro, D.T.; Soler, P.B.; Zanzi, R. Torrefacción de biomasa densificada. Tecnol. Química 2009, 29, 180–186. [Google Scholar]
- Portilla, L.J.P.; Figueiredo, R.; dos Santos, M.B.; Kiyota, E.; Sampaio, J.L.M.; Araujo, P.; Schimpl, F.C.; Dama, M.; Pauly, M.; Mazzafera, P. Deposition of lignin in four species of Saccharum. Sci. Rep. 2019, 9, 5877. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).