Effects of Temperature and Chemical Speciation of Mineral Elements on PM10 Formation during Zhundong Coal Combustion
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Chemical Fractionation Analysis
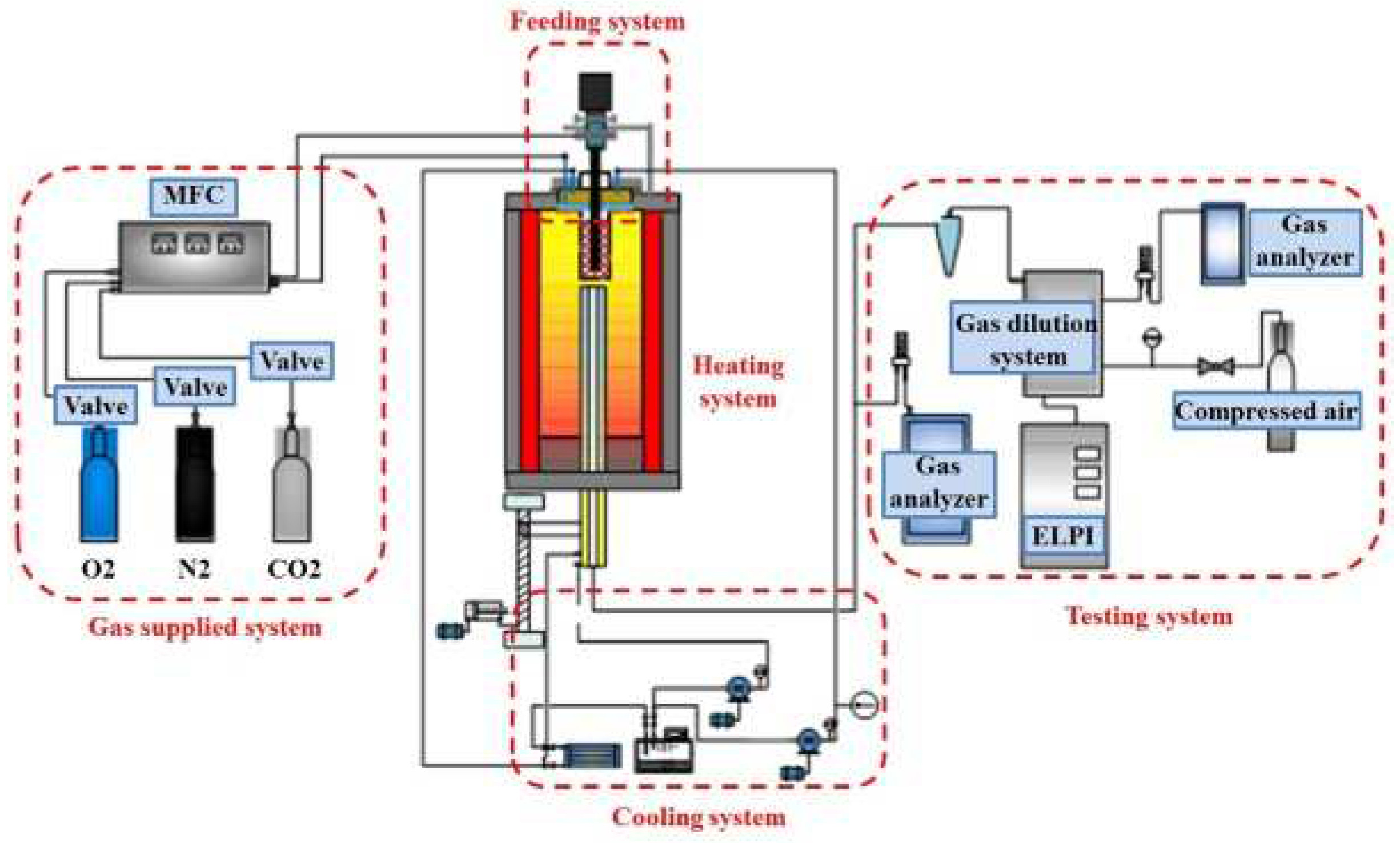
2.3. Sampling System
3. Results and Discussion
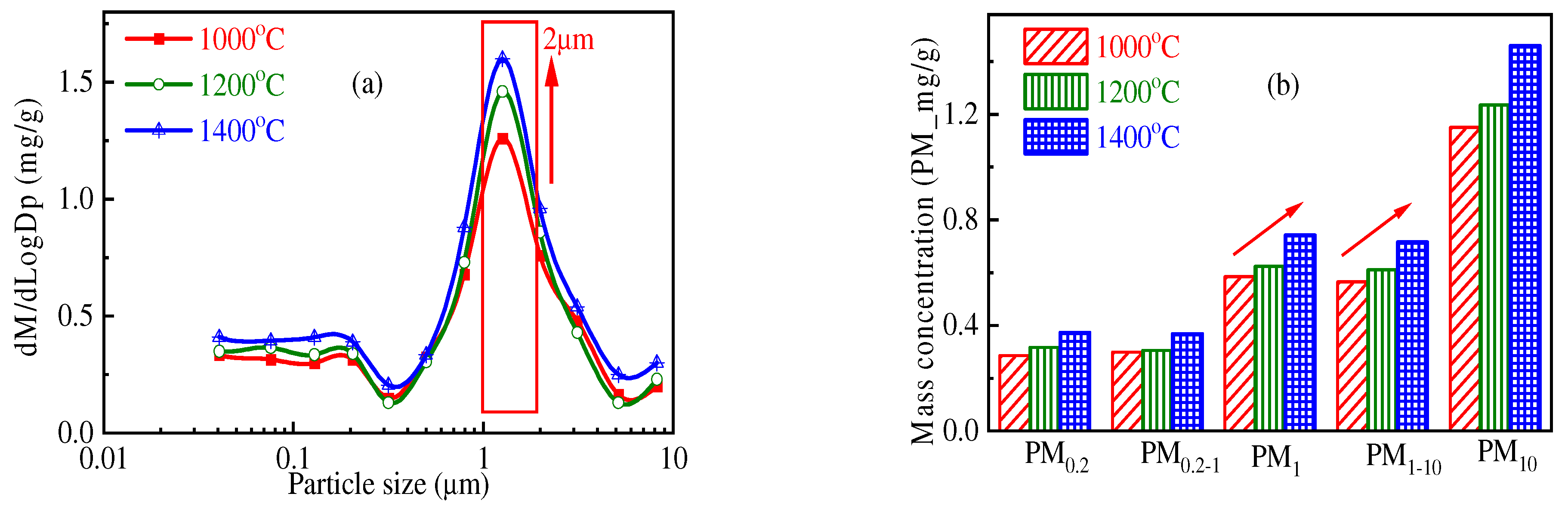
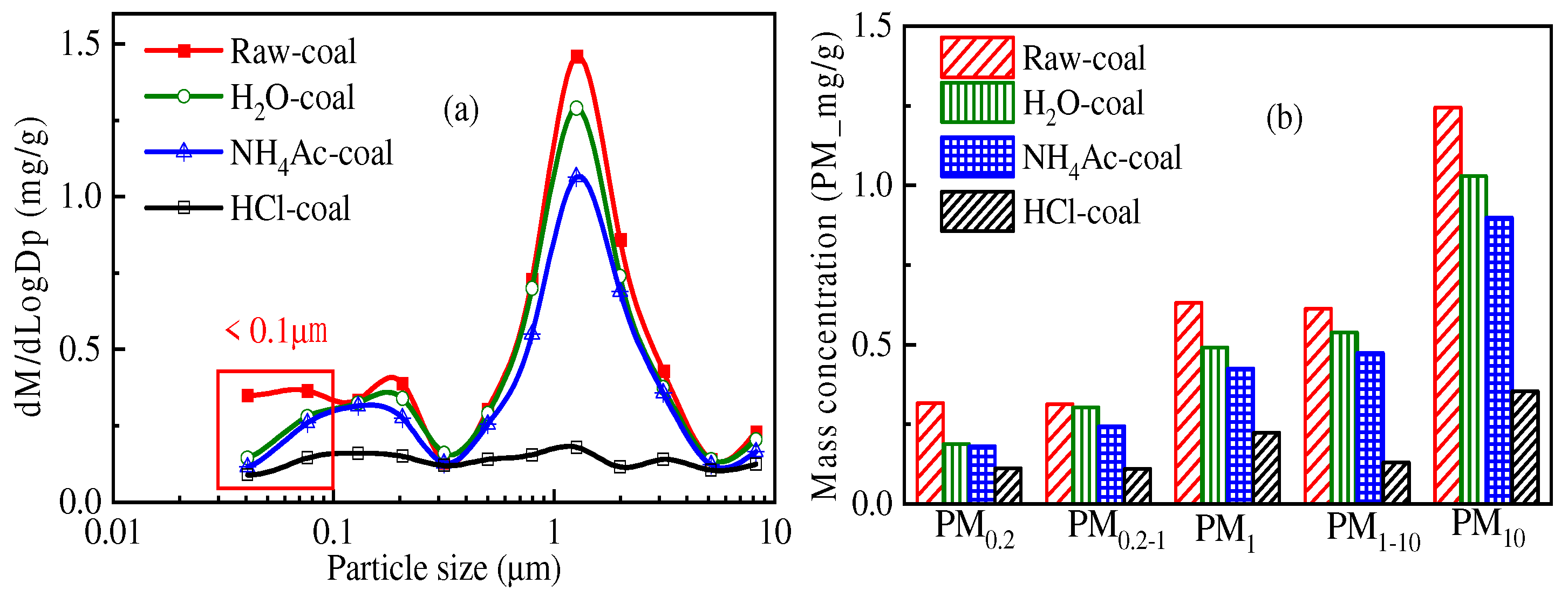
3.1. Impact of Temperature on PM10 Formation
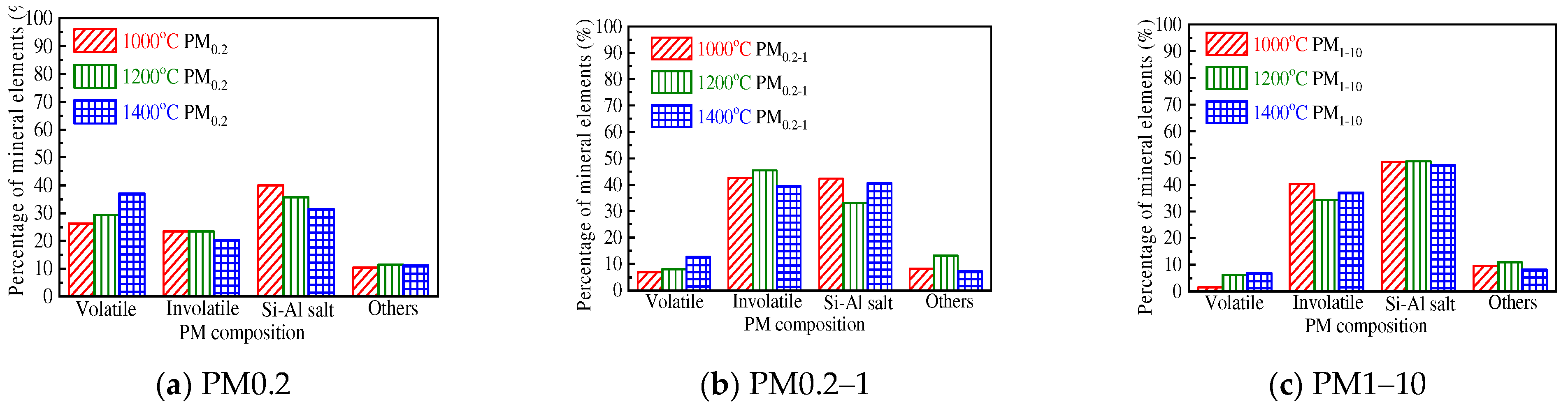
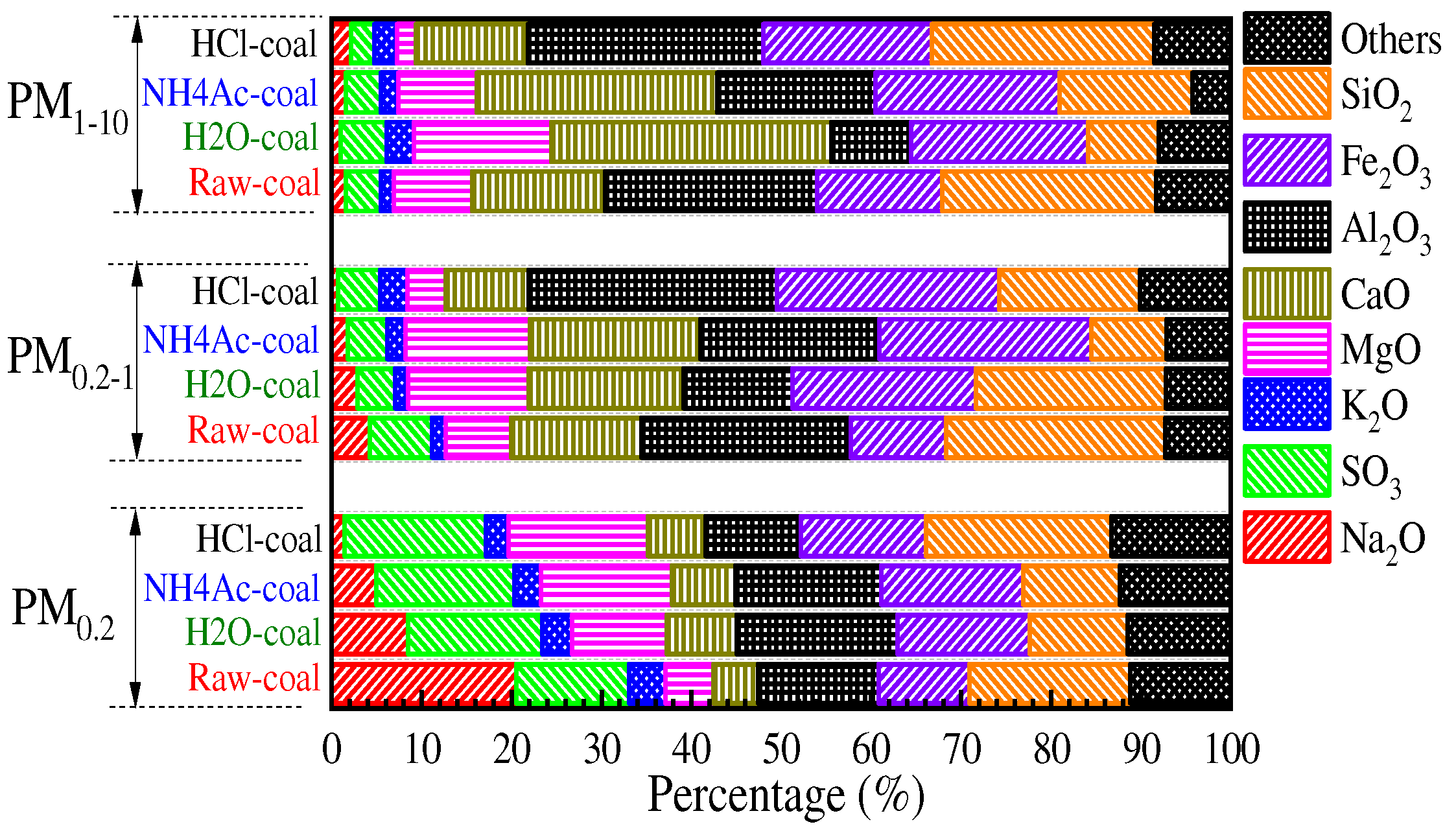
3.2. Effect of Chemical Speciation of Mineral Elements on PM10 Formation
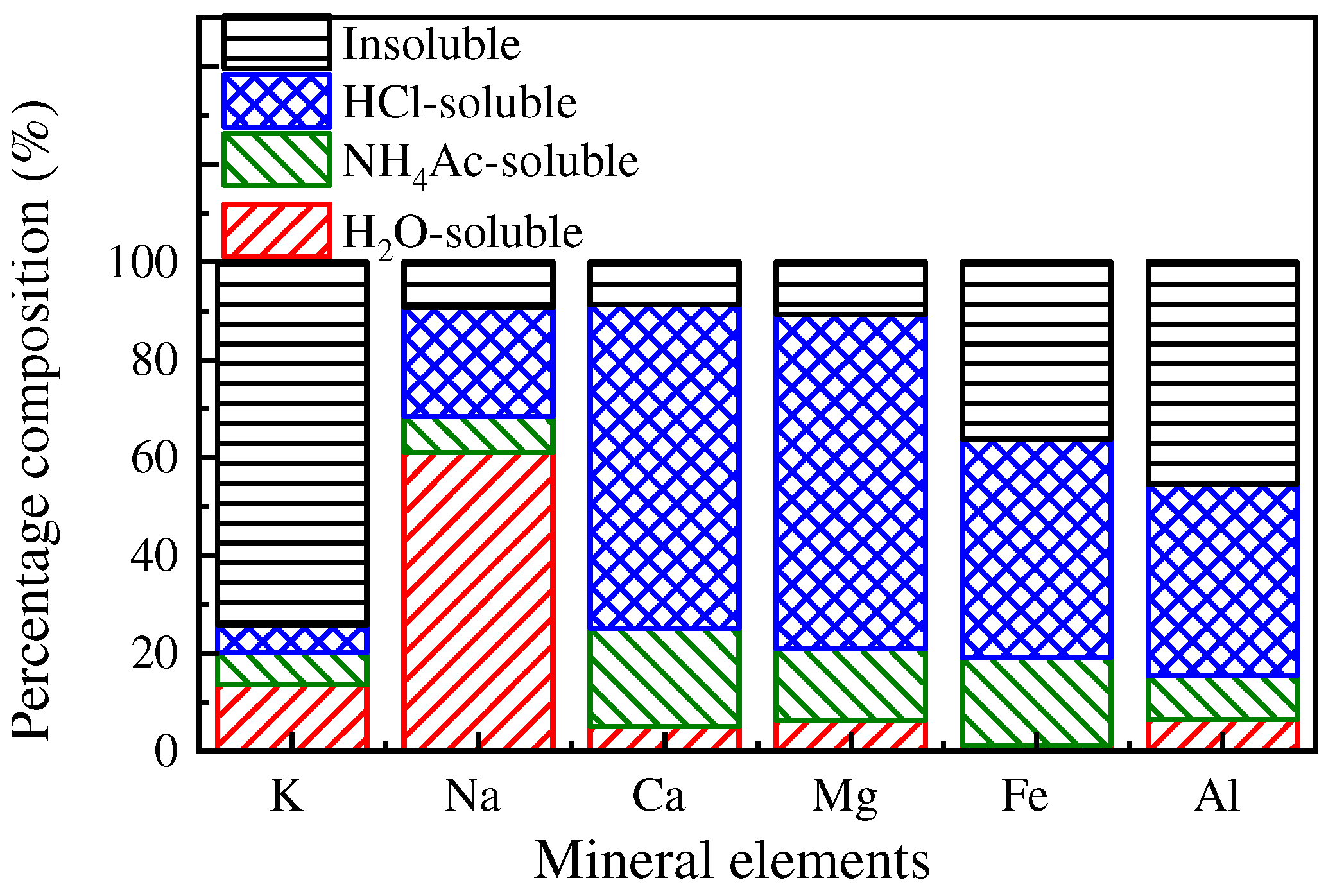
3.2.1. Chemical Speciation of Mineral Elements
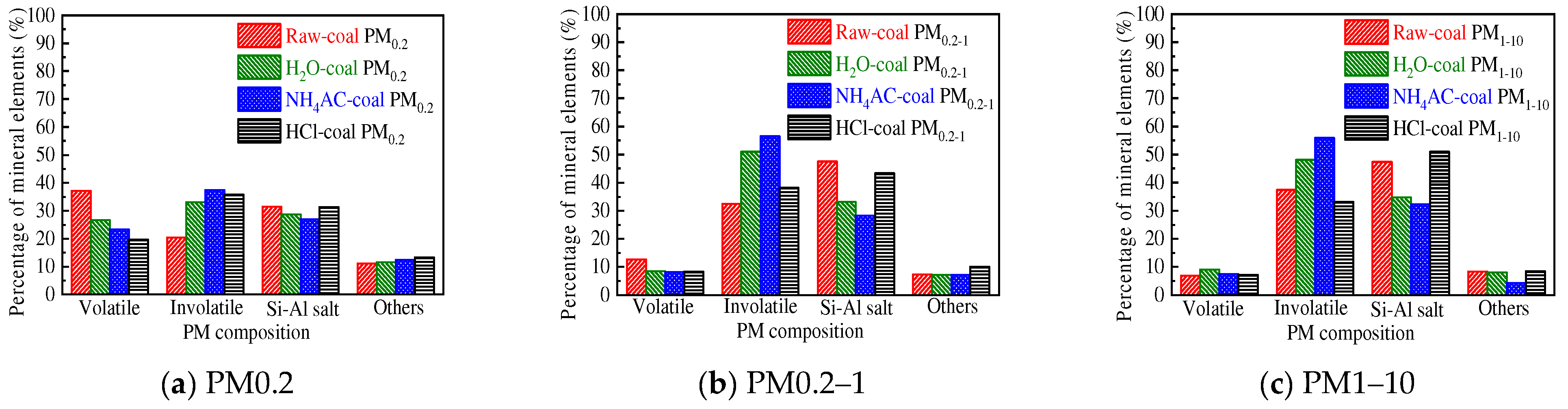
3.2.2. Distribution of the Primary Mineral in PM10
3.2.3. Speciation of Mineral Elements in PM10
4. Conclusions
- (1)
- When Zhundong pulverized coal with the same combustion atmosphere and particle size burns, the total amount of PM10 generated at high combustion temperature is generally more. At 1000 °C, 1200 °C and 1400 °C, the amount of PM10 generated by the unit mass of Zhundong pulverized coal is 1.15 mg/g, 1.23 mg/g and 1.46 mg/g, respectively. The reason may be that with the increase in combustion temperature, the steam concentration of mineral elements in coal during combustion is increased. When the temperature decreases, more mineral steam will condense on the surface of particles.
- (2)
- The mineral elements in raw coal are divided into water-soluble mineral elements, organically bound mineral elements, acid-soluble mineral elements and acid-insoluble mineral elements by chemical fractionation. Water-soluble salts play an important role in the formation of ultrafine particles (PM0.2). The organic Fe and Ca elements do not react with the aluminosilicate in the pulverized coal during the combustion of pulverized coal but evaporate into steam and distribute in the flue gas. When the flue gas temperature decreases, they condense and form on PM1, resulting in these two elements on PM1; Mg, Ca, and Fe react with aluminosilicate to form molten particles, which makes particles PM1–10 contain these three elements. Different fractionation methods will not greatly affect Si and Al in the PM1–10 combustion process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, Q.; Li, S.Q.; Xu, H.W.; Zhuo, J.K.; Song, Q. Studies on formation and control of combustion particulate matter in China: A review. Energy 2009, 34, 1296–1309. [Google Scholar] [CrossRef]
- Chung, W.S.; Chen, Q.; Osammor, O.; Nolan, A.; Zhang, X.H.; Sharifi, V.N.; Swithenbank, J. Characterisation of particulate matter on the receptor level in a city environment. Environ. Monit. Assess. 2012, 184, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.M.; Naik, V.; Leibensperger, E.M. Air Quality and Climate Connections. J. Air Waste Manag. Assoc. 2015, 65, 645–685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yan, Y.; Zhou, B.; Zhong, X.; Hu, X.; Zhang, L.; Huo, P.; Xiao, K.; Zhang, Y.; Zhang, Y. Insights into reactive oxygen species formation induced by water-soluble organic compounds and transition metals using spectroscopic method. J. Environ. Sci. 2023, 124, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.D.; Guo, D.W.; Zhang, Y.; Sun, S.Z.; Zhao, Y.J.; Shang, Q.; Sun, H.L.; Wu, J.Q.; Tan, H.P. Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem. Eng. J. 2021, 410, 127707. [Google Scholar] [CrossRef]
- Li, L.; Karatzos, S.; Saddler, J. The potential of forest-derived bioenergy to contribute to China's future energy and transportation fuel requirements. For. Chron. 2012, 88, 547–552. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.-P.; Zhang, C.; Tan, P.; Li, X.; Fang, Q.-Y.; Chen, G. Effects of hydrothermal upgrading on the physicochemical structure and gasification characteristics of Zhundong coal. Fuel Processing Technol. 2018, 172, 200–208. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2011, 94, 3–21. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, D.; Guo, J.; Ning, H. Hydrogenation properties of Mg17Al12 doped with alkaline-earth metal (Be, Ca, Sr and Ba). J. Alloys Compd. 2019, 774, 865–872. [Google Scholar] [CrossRef]
- Li, G.Y.; Wang, C.A.; Yan, Y.; Jin, X.; Liu, Y.H.; Che, D.F. Release and transformation of sodium during combustion of Zhundong coals. J. Energy Inst. 2016, 89, 48–56. [Google Scholar] [CrossRef]
- Feng, D.D.; Shang, Q.; Dong, H.M.; Zhang, Y.; Wang, Z.L.; Li, D.; Xie, M.; Wei, Q.Y.; Zhao, Y.J.; Sun, S.Z. Catalytic mechanism of Na on coal pyrolysis-derived carbon black formation: Experiment and DFT simulation. Fuel Process. Technol. 2021, 224, 107011. [Google Scholar] [CrossRef]
- Linak, W.P.; Miller, C.A.; Seames, W.S.; Wendt, J.O.L.; Ishinomori, T.; Endo, Y.; Miyamae, S. On trimodal particle size distributions in fly ash from pulverized-coal combustion. Proc. Combust. Inst. 2002, 29, 441–447. [Google Scholar] [CrossRef]
- Jia, Y.; Lighty, J.A.S. Ash Particulate Formation from Pulverized Coal under Oxy-Fuel Combustion Conditions. Environ. Sci. Technol. 2012, 46, 5214–5221. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, M.; Li, Z.; Zhang, Y.; Yang, H.; Sun, J.; Lyu, J. Influence of combustion temperature and coal types on alumina crystal phase formation of high-alumina coal ash. Proc. Combust. Inst. 2019, 37, 2919–2926. [Google Scholar] [CrossRef]
- Ke, X.; Li, D.; Zhang, M.; Jeon, C.-h.; Cai, R.; Cai, J.; Lyu, J.; Yang, H. Ash formation characteristics of two Indonesian coals and the change of ash properties with particle size. Fuel Process. Technol. 2019, 186, 73–80. [Google Scholar] [CrossRef]
- Buhre, B.J.P.; Hinkley, J.T.; Gupta, R.P.; Nelson, P.F.; Wall, T.F. Fine ash formation during combustion of pulverised coal–coal property impacts. Fuel 2006, 85, 185–193. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Kitano, K.; Takeda, S.; Tsurue, T. Influence of mineral and chemical-composition of coal ashes on their fusibility. Fuel Processing Technol. 1995, 45, 27–51. [Google Scholar] [CrossRef]
- Liu, X.; Xu, M.; Yao, H.; Yu, D.; Lv, D. Study on the effect of the occurrence of sodium elements in coal on the formation of submicron particles. J. Eng. Therm. Phys. 2009, 30, 1589–1592. [Google Scholar]
- Su, X.B.; Ding, R.; Zhuang, X.G. Characteristics of Dust in Coal Mines in Central North China and Its Research Significance. ACS Omega 2020, 5, 9233–9250. [Google Scholar] [CrossRef]
- Yu, D. Modal Identification and Formation Mechanism of Fine Particle in Coal Combustion; Huazhong University of Science and Technology: Huazhong, China, 2007. [Google Scholar]
- Feng, D.D.; Guo, D.W.; Shang, Q.; Zhao, Y.J.; Zhang, L.Y.; Guo, X.; Cheng, J.; Chang, G.Z.; Guo, Q.J.; Sun, S.Z. Mechanism of biochar-gas-tar-soot formation during pyrolysis of different biomass feedstocks: Effect of inherent metal species. Fuel 2021, 293, 120409. [Google Scholar] [CrossRef]
- Zhou, K. Study on the Generation Characteristics and Furnace Control of Fine Particulate Matter in Coal Combustion; Huazhong University of Science and Technology: Huazhong, China, 2011. [Google Scholar]
- Quann, R.J.; Sarofim, A.F. Vaporization of refractory oxides during pulverized coal combustion. Symp. Combust. 1982, 19, 1429–1440. [Google Scholar] [CrossRef]
- Sun, J.; Guo, Y.; Yang, Y.; Li, W.; Zhou, Y.; Zhang, J.; Liu, W.; Zhao, C. Mode investigation of CO2 sorption enhancement for titanium dioxide-decorated CaO-based pellets. Fuel 2019, 256, 116009. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, M.; Zhang, Y.; Lyu, J.; Yang, H. The effect of the secondary air injection on the gas–solid flow characteristics in the circulating fluidized bed. Chem. Eng. Res. Des. 2019, 141, 220–228. [Google Scholar] [CrossRef]
- Jordan, C.A.; Akay, G. Speciation and distribution of alkali, alkali earth metals and major ash forming elements during gasification of fuel cane bagasse. Fuel 2012, 91, 253–263. [Google Scholar] [CrossRef]
- Zevenhoven-Onderwater, M.; Backman, R.; Skrifvars, B.J.; Hupa, M. The ash chemistry in fluidised bed gasification of biomass fuels. Part I: Predicting the chemistry of melting ashes and ash–bed material interaction. Fuel 2001, 80, 1489–1502. [Google Scholar] [CrossRef]
- Pettersson, A.; Åmand, L.E.; Steenari, B.M. Chemical fractionation for the characterisation of fly ashes from co-combustion of biofuels using different methods for alkali reduction. Fuel 2009, 88, 1758–1772. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, D.; Zhang, Y.; Huang, Y.; Sun, S. Effect of pyrolysis temperature on char structure and chemical speciation of alkali and alkaline earth metallic species in biochar. Fuel Process. Technol. 2016, 141, 54–60. [Google Scholar] [CrossRef]
- Feng, D.D.; Guo, D.W.; Zhang, Y.; Sun, S.Z.; Zhao, Y.J.; Chang, G.Z.; Guo, Q.J.; Qin, Y.K. Adsorption-enrichment characterization of CO2 and dynamic retention of free NH3 in functionalized biochar with H2O/NH3 center dot H2O activation for promotion of new ammonia-based carbon capture. Chem. Eng. J. 2021, 409, 128193. [Google Scholar] [CrossRef]
- Feng, D.D.; Zhao, Y.J.; Zhang, Y.; Sun, S.Z.; Gao, J.M. Steam Gasification of Sawdust Biochar Influenced by Chemical Speciation of Alkali and Alkaline Earth Metallic Species. Energies 2018, 11, 205. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Lyu, J.; Liu, M.; Yue, G.; Liu, J. The Effect of Inner Secondary Air on the Flow Field of a Swirl Burner. In Proceedings of the 2nd International Conference on Smart Power & Internet Energy Systems (SPIES), Bangkok, Thailand, 15–18 September 2020; pp. 452–456. [Google Scholar]
- Lv, J.; Li, D. Effect of temperature on the characteristics of primary particles in pulverized coal combustion. Chin. J. Electr. Eng. 2007, 27, 24–29. [Google Scholar]
- Nguyen, C.B.; Scherer, J.; Hartwich, M.; Richter, A. The morphology evolution of char particles during conversion processes. Combust. Flame 2021, 226, 117–128. [Google Scholar] [CrossRef]
- Yaroshchuk, A. Evaporation-driven electrokinetic energy conversion: Critical review, parametric analysis and perspectives. Adv. Colloid Interface Sci. 2022, 305, 102708. [Google Scholar] [CrossRef]
- Hao, Z.; Xie, G.; Liu, X.; Tan, Q.; Wang, R. The precipitation behaviours and strengthening mechanism of a Cu-0.4 wt% Sc alloy. J. Mater. Sci. Technol. 2022, 98, 1–13. [Google Scholar] [CrossRef]
- Lu, Y.; Cao, R.; Huang, D.W.; Cui, Z.N.; Wang, Y.; Zhang, Y.F. Investigation on the ash characteristics and AAEM migration during co-combustion of Zhundong coal and shale char in a fixed bed. Fuel 2022, 327, 125214. [Google Scholar] [CrossRef]
- Lighty, J.S.; Veranth, J.M.; Sarofim, A.F. Combustion aerosols: Factors governing their size and composition and implications to human health. J. Air Waste Manag. Assoc. 2000, 50, 1619–1622. [Google Scholar] [CrossRef]
- Zhang, L.; Ninomiya, Y.; Yamashita, T. Formation of submicron particulate matter (PM 1) during coal combustion and influence of reaction temperature. Fuel 2006, 85, 1446–1457. [Google Scholar] [CrossRef]
- Wood, B.J.; Smythe, D.J.; Harrison, T. The condensation temperatures of the elements: A reappraisal. Am. Mineral. J. Earth Planet. Mater. 2019, 104, 844–856. [Google Scholar] [CrossRef]
- Wang, Y.B.; Tan, H.Z. Condensation of KCl(g) under varied temperature gradient. Fuel 2019, 237, 1141–1150. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, Q.; Feng, D.D.; Sun, H.L.; Wang, F.H.; Hu, Z.C.; Cheng, Z.Y.; Zhou, Z.J.; Zhao, Y.J.; Sun, S.Z. Interaction mechanism of in-situ catalytic coal H2O-gasification over biochar catalysts for H2O-H2-tar reforming and active sites conversion. Fuel Process. Technol. 2022, 233, 107307. [Google Scholar] [CrossRef]
- Feng, D.D.; Zhang, Y.; Zhao, Y.J.; Sun, S.Z.; Wu, J.Q.; Tan, H.P. Mechanism of in-situ dynamic catalysis and selective deactivation of H2O-activated biochar for biomass tar reforming. Fuel 2020, 279, 118450. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, H.; Liu, W.; Yang, Y.; Li, H. Incorporation of CaO into inert supports for enhanced CO2 capture: A review. Chem. Eng. J. 2020, 396, 125253. [Google Scholar] [CrossRef]
- Pi, S.; Zhang, Z.; He, D.; Qin, C.; Ran, J. Investigation of Y2O3/MgO-modified extrusion–spheronized CaO-based pellets for high-temperature CO2 capture. Asia-Pac. J. Chem. Eng. 2019, 14, e2366. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.Z.; Feng, D.D.; Gao, J.M.; Dong, L.H.; Zhao, Y.J.; Sun, S.Z.; Huang, Y.D.; Qin, Y.K. Functional Biochar Synergistic Solid/Liquid-Phase CO2 Capture: A Review. Energy Fuels 2022, 36, 2945–2970. [Google Scholar] [CrossRef]
- Feng, D.D.; Zhao, Y.J.; Zhang, Y.; Xu, H.H.; Zhang, L.Y.; Sun, S.Z. Catalytic mechanism of ion-exchanging alkali and alkaline earth metallic species on biochar reactivity during CO2/H2O gasification. Fuel 2018, 212, 523–532. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, H.; Sun, Y.; Song, Y. Performance analysis of Fe-N compounds based on valence electron structure. J. Alloys Compd. 2019, 779, 427–432. [Google Scholar] [CrossRef]
- Helble, J.J. Mechanisms of Ash Formation and Growth During Pulverized Coal Combustion. Ph.D. Thesis, Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA, 1987. [Google Scholar]

| Proximate Analysis (wt.%, ad. = Air Dry Basis) | Ultimate Analysis (wt.%, ad. = Air Dry Basis) | |||||||
|---|---|---|---|---|---|---|---|---|
| M(Moisture) | A(Ash) | V(Volatile) | FC(Fixed Carbon) | C | H | Odiff. | N | S |
| 9.63 | 5.5 | 40.3 | 44.57 | 61.4 | 4.41 | 17.69 | 0.89 | 0.48 |
| Ash Compositions (wt. %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | SO3 | MnO2 | K2O | P2O5 | Na2O |
| 30.56 | 29.85 | 10.56 | 8.64 | 4.42 | 0.22 | 7.66 | 0.31 | 0.6 | 0.12 | 6.02 |
| Coal | K | Na | Ca | Mg | Fe | Al | Si | Ash |
|---|---|---|---|---|---|---|---|---|
| Raw-Coal | 0.2 | 1.8 | 2.6 | 1.2 | 2.8 | 6.9 | 6.8 | 5.3 |
| H2O-Coal | 0.1 | 0.7 | 2.4 | 1.1 | 2.5 | 6.4 | 6.0 | 4.6 |
| NH4Ac-Coal | 0.1 | 0.6 | 1.9 | 0.8 | 2.3 | 5.8 | 5.3 | 3.9 |
| HCl-Coal | 0.1 | 0.1 | 0.2 | 0.1 | 1.0 | 3.1 | 2.0 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Zhao, Z.; Wang, S.; Zhang, Y.; Da, Y.; Dong, H.; Wen, J.; Du, Q.; Gao, J. Effects of Temperature and Chemical Speciation of Mineral Elements on PM10 Formation during Zhundong Coal Combustion. Energies 2023, 16, 310. https://doi.org/10.3390/en16010310
Sun Q, Zhao Z, Wang S, Zhang Y, Da Y, Dong H, Wen J, Du Q, Gao J. Effects of Temperature and Chemical Speciation of Mineral Elements on PM10 Formation during Zhundong Coal Combustion. Energies. 2023; 16(1):310. https://doi.org/10.3390/en16010310
Chicago/Turabian StyleSun, Qiaoqun, Zhiqi Zhao, Shizhang Wang, Yu Zhang, Yaodong Da, Heming Dong, Jiwang Wen, Qian Du, and Jianmin Gao. 2023. "Effects of Temperature and Chemical Speciation of Mineral Elements on PM10 Formation during Zhundong Coal Combustion" Energies 16, no. 1: 310. https://doi.org/10.3390/en16010310
APA StyleSun, Q., Zhao, Z., Wang, S., Zhang, Y., Da, Y., Dong, H., Wen, J., Du, Q., & Gao, J. (2023). Effects of Temperature and Chemical Speciation of Mineral Elements on PM10 Formation during Zhundong Coal Combustion. Energies, 16(1), 310. https://doi.org/10.3390/en16010310









