Modeling of Multi-Layer Phase Change Material in a Triplex Tube under Various Thermal Boundary Conditions
Abstract
:1. Introduction
2. Research Methodology
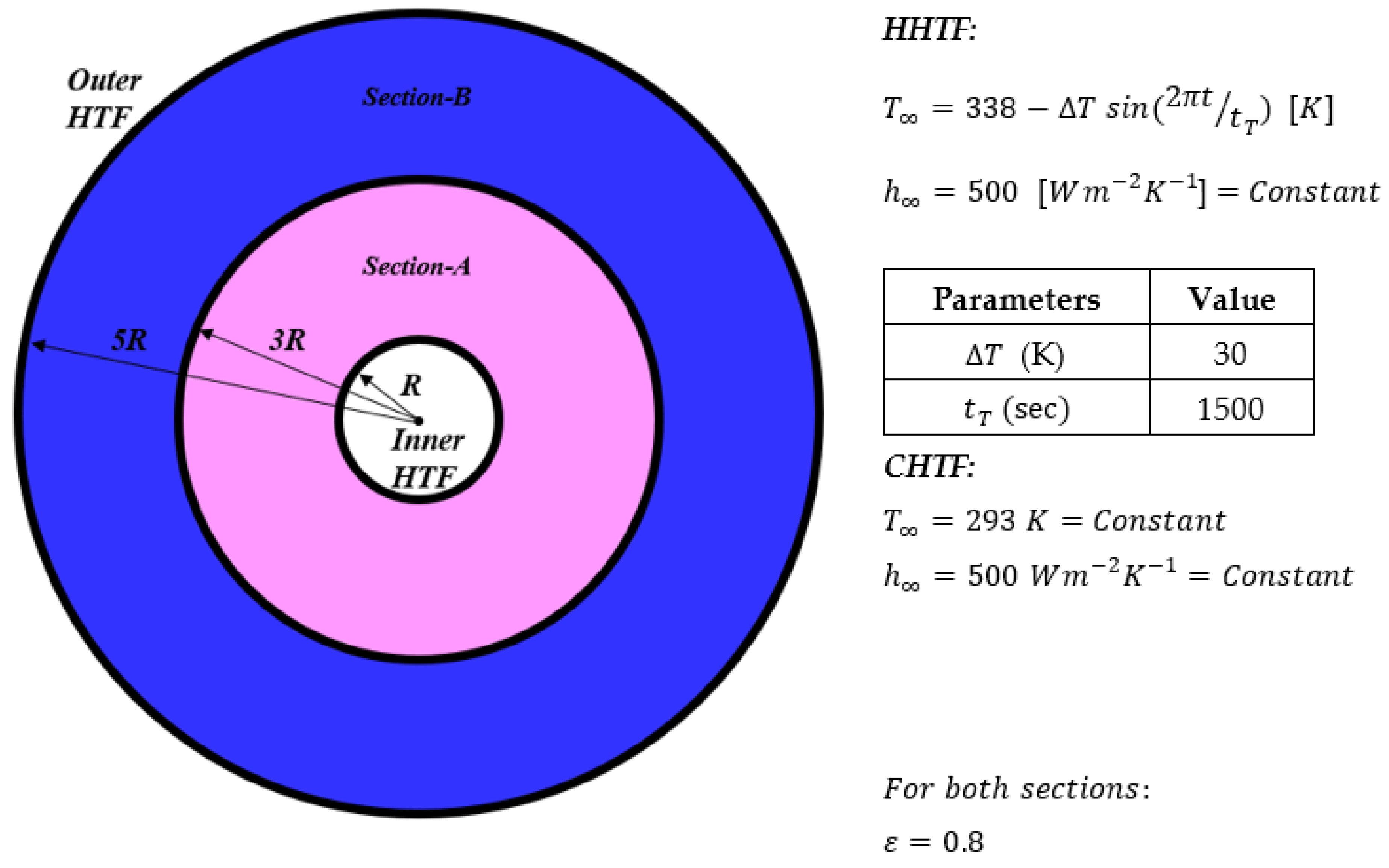
2.1. Physical Model and Boundary Conditions
- (1)
- Two-dimensional fluid flow is employed as incompressible, laminar and unsteady, with simultaneous solidification and melting processes including free convection in the liquid phase;
- (2)
- To initially be solid, the temperature of the whole system was chosen to be lower than the melting temperature of each PCM at t = 0 s, and higher than those for initially liquid cases;
- (3)
- Thermo-physical properties of each PCM are assumed to be independent of temperature except the density in liquid phase;
- (4)
- The Boussinesq approximation is assumed for density variation in the liquid phase [20];
- (5)
- Thermal resistance of aluminum walls cannot be ignored because their thickness is considerable, and conductive heat transfers through walls;
- (6)
- The Rayleigh number, defined as Ra = (gβ(∆T)r3)⁄αυ, is set at a fixed value of 106. ΔT stands for the amplitude of bulk HHTF temperature, g is the gravitational acceleration, r is the radius of PCM container, β is thermal expansion coefficient, and υ and α are the kinematic viscosity and thermal diffusivity, respectively;
- (7)
- Super-cooling effects and viscous dissipation are negligible;
- (8)
- Volume change in PCMs due to phase change is insignificant;
- (9)
- In annulus walls, no slip boundary conditions are employed;
- (10)
- To simulate the phase change process, the enthalpy method is employed;
- (11)
- In order to evaluate the flow within the porous matrix, Brinkman–Forchheimer-extended Darcy model is used [23];
- (12)
- The PCM is saturated in homogeneous and isotropic porous matrix.
2.2. Governing Equations
2.3. Numerical Model
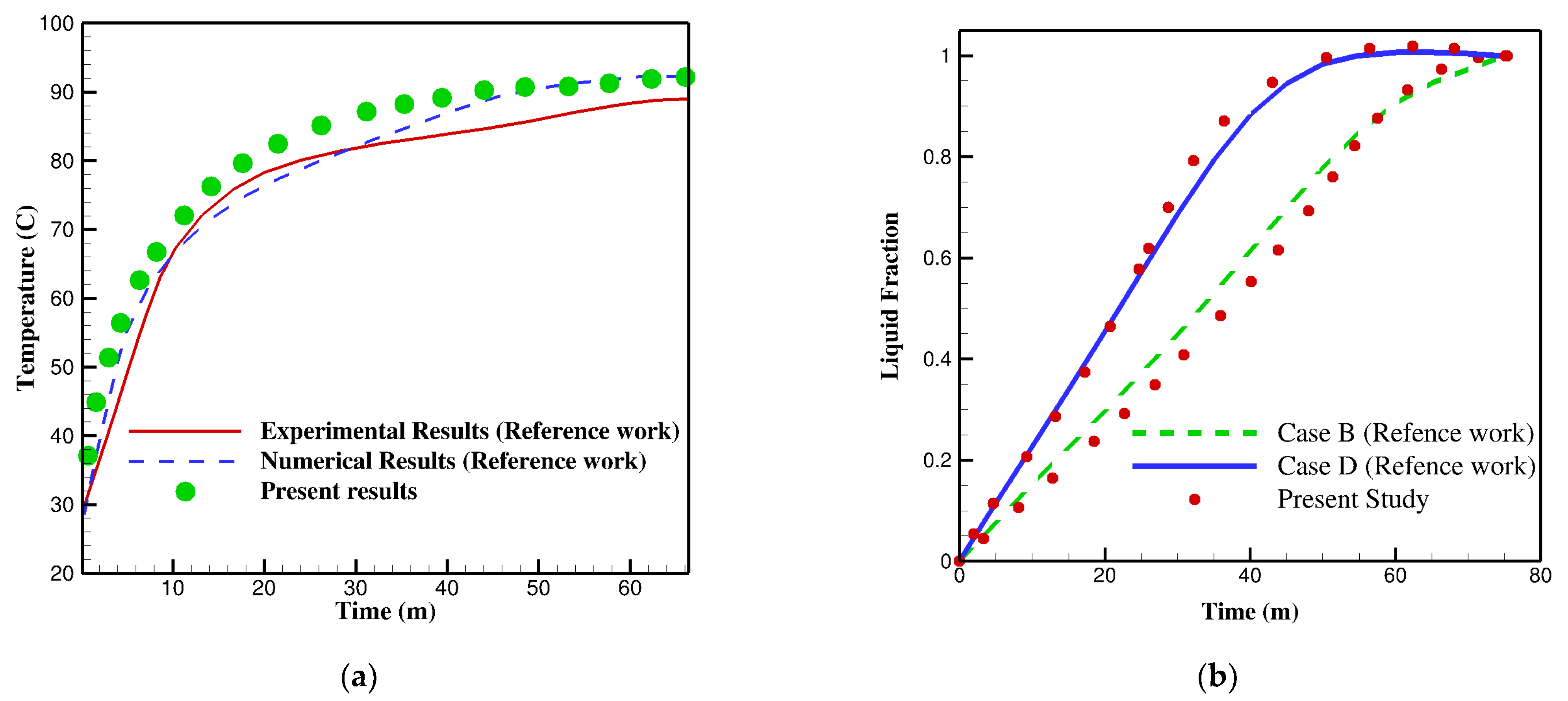
2.4. Validation
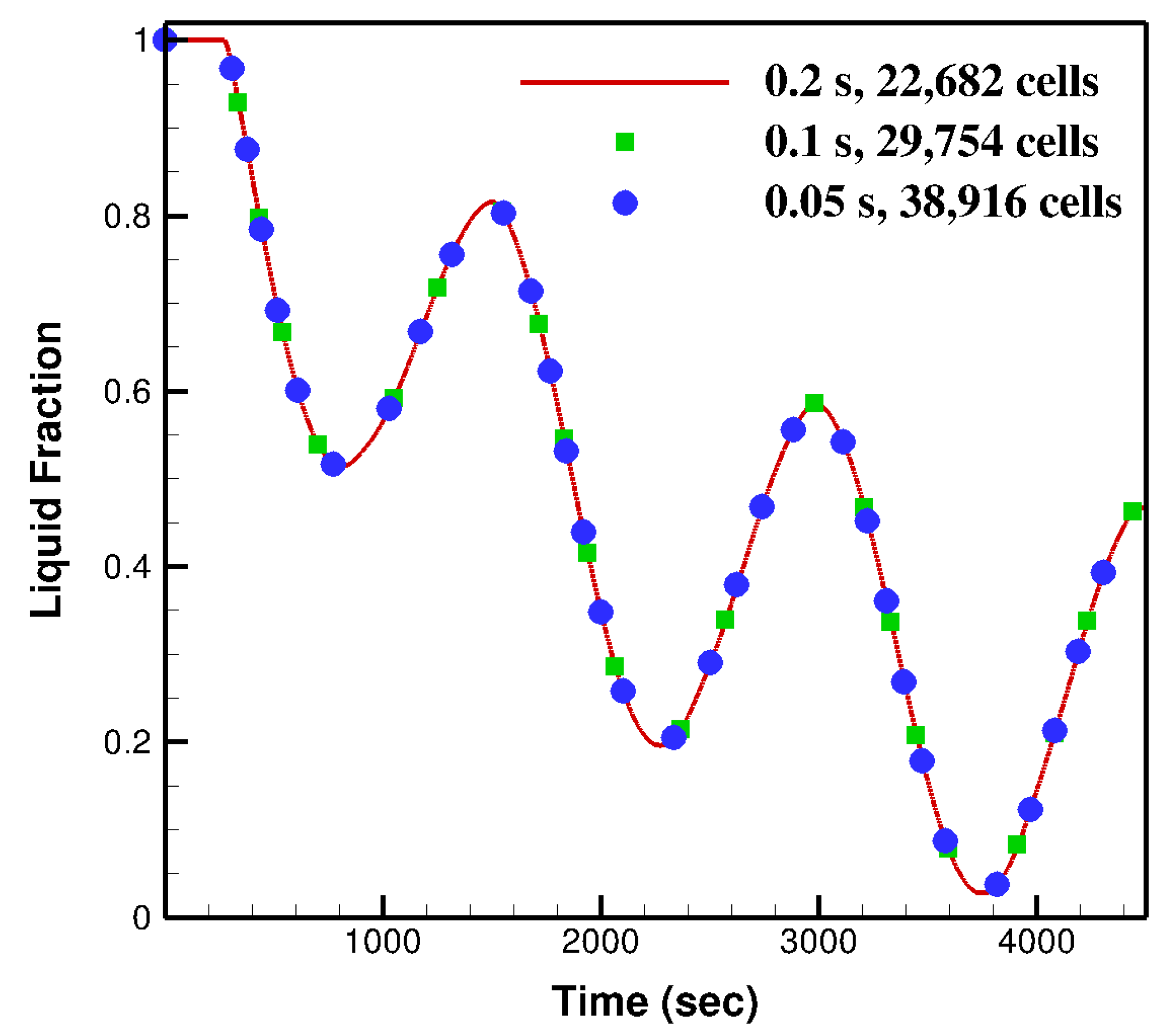
2.5. Mesh and Time Step Independency
3. Results and Discussion
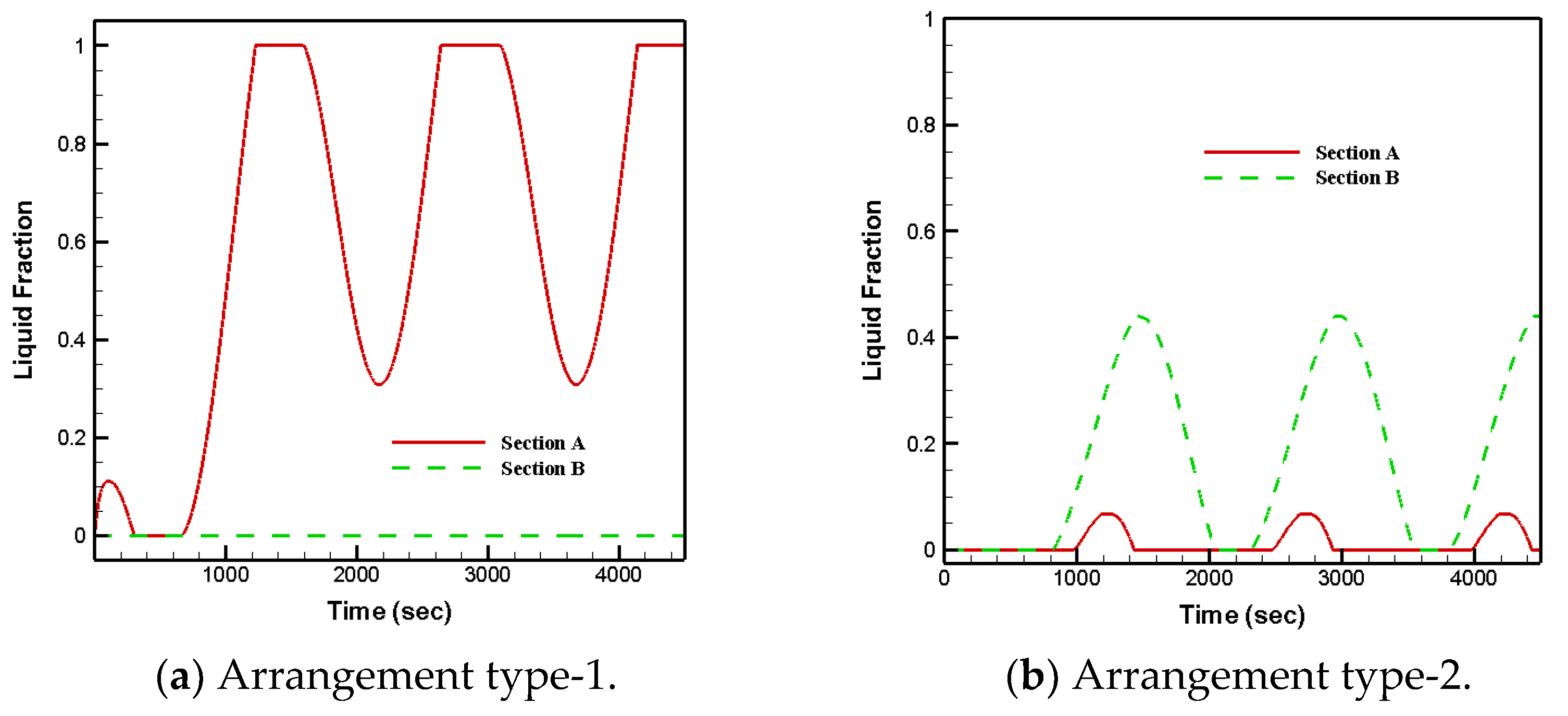
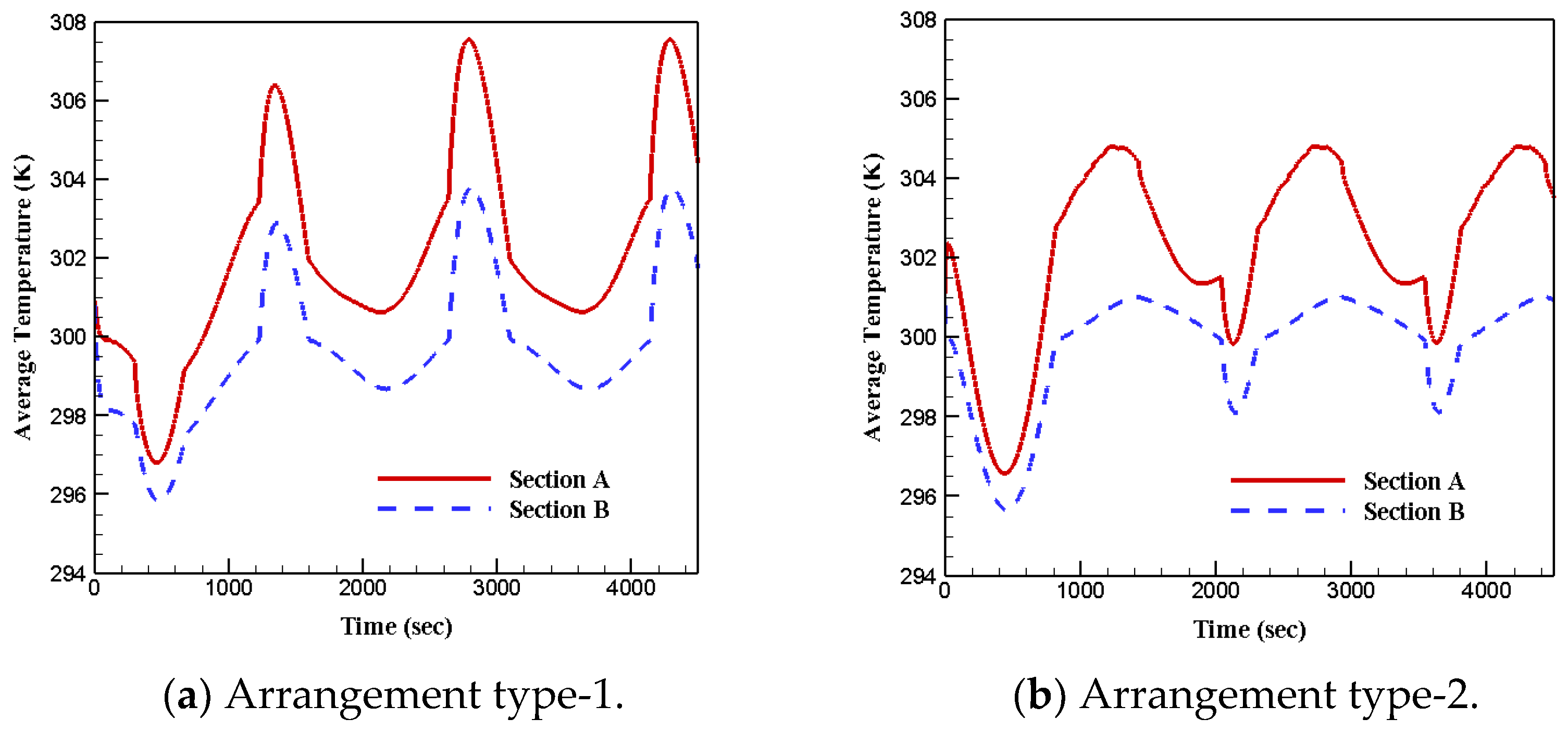
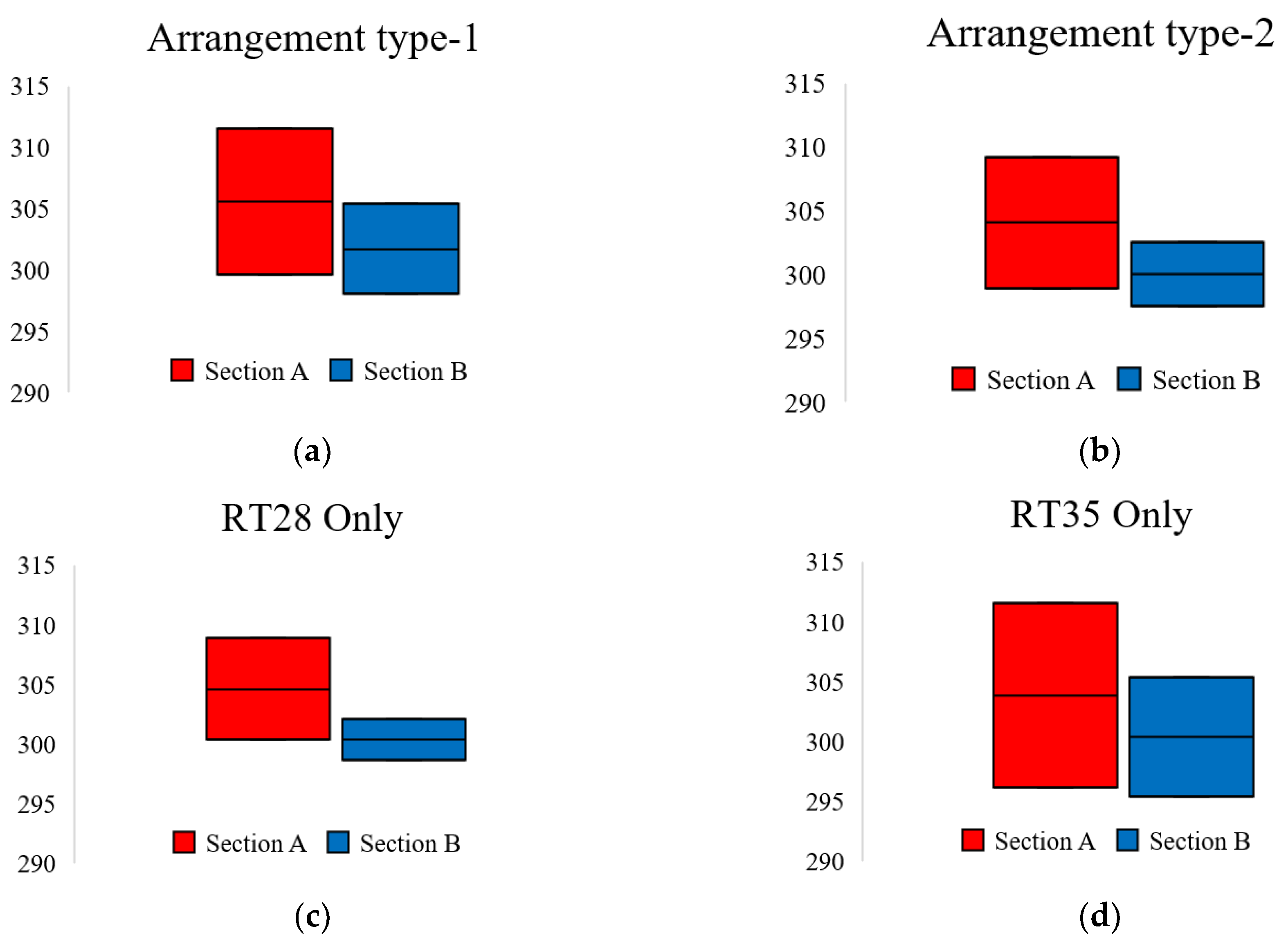
3.1. PCM Arrangements
3.2. Effects of Employing Double-Layer PCM
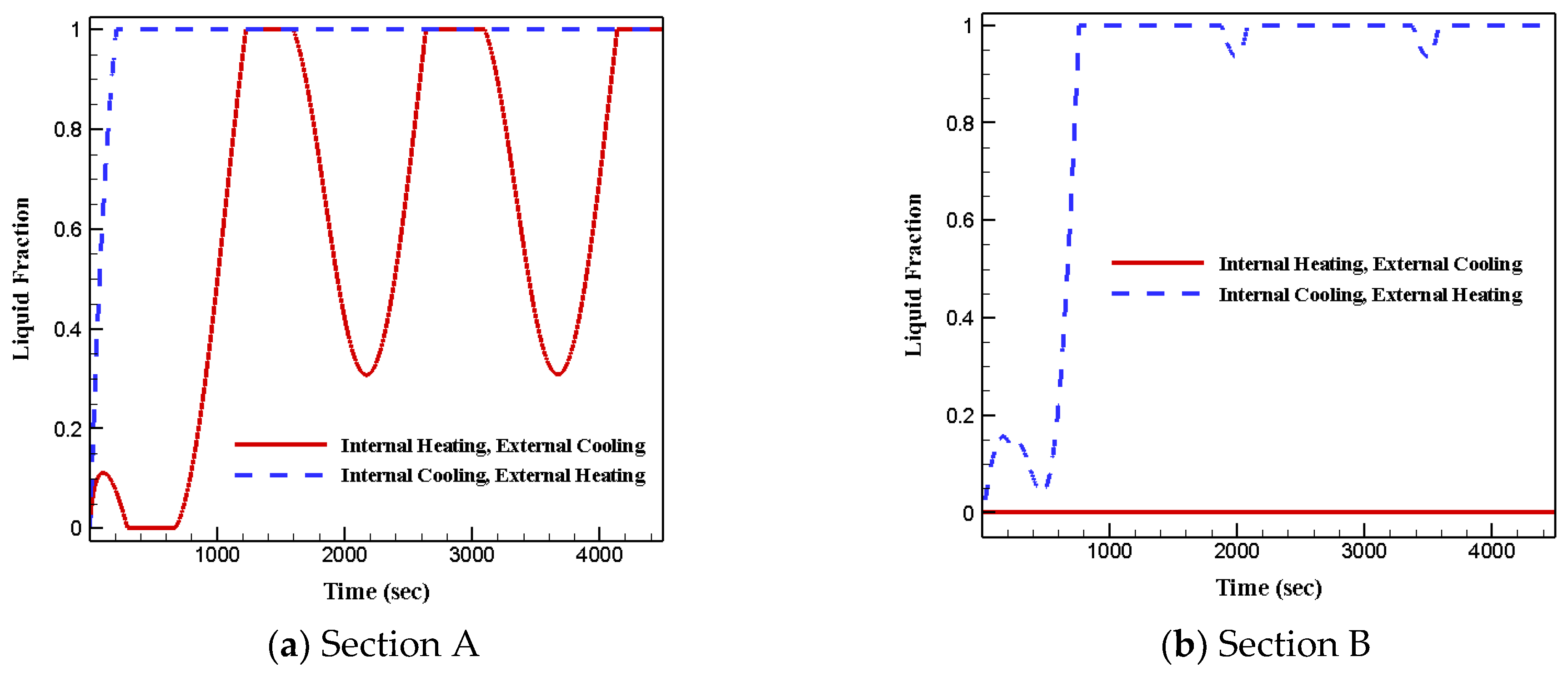
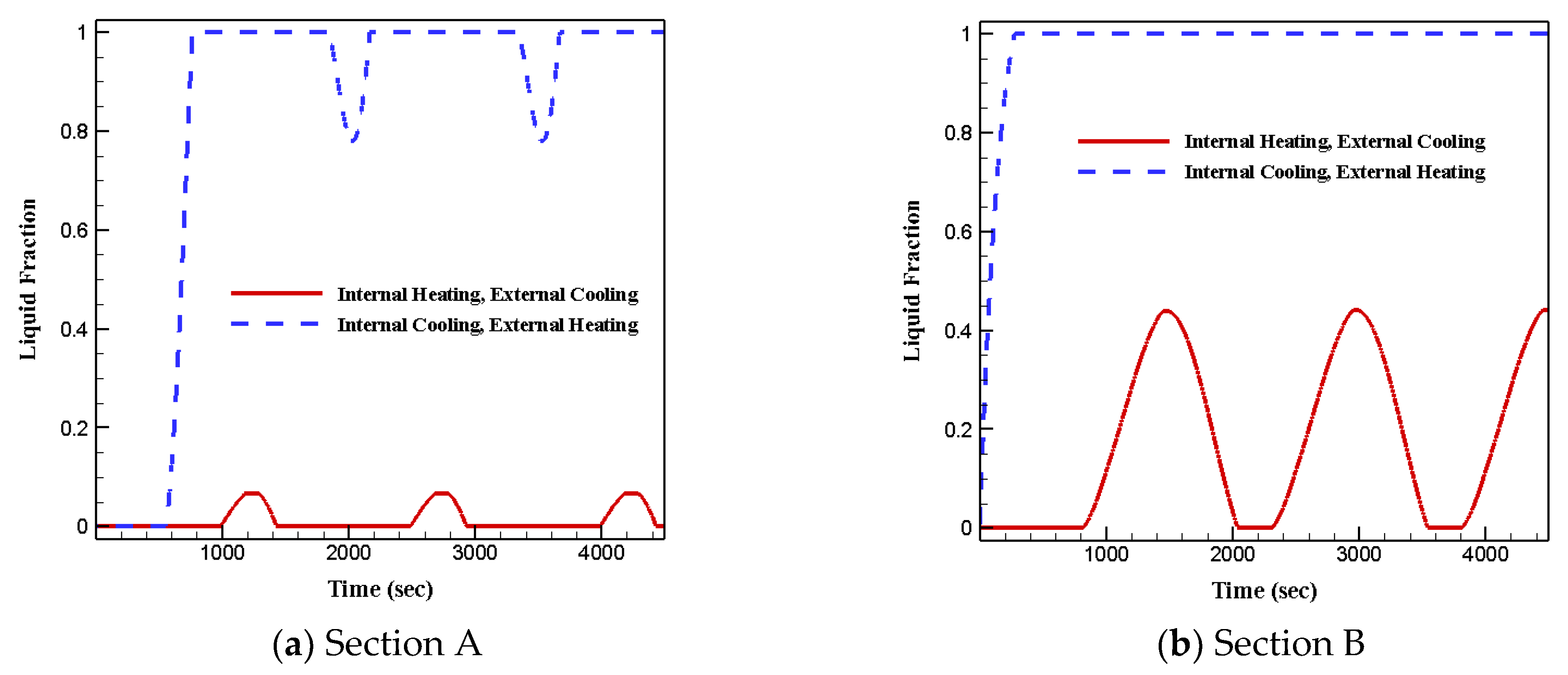
3.3. Effects of Various Boundary Conditions
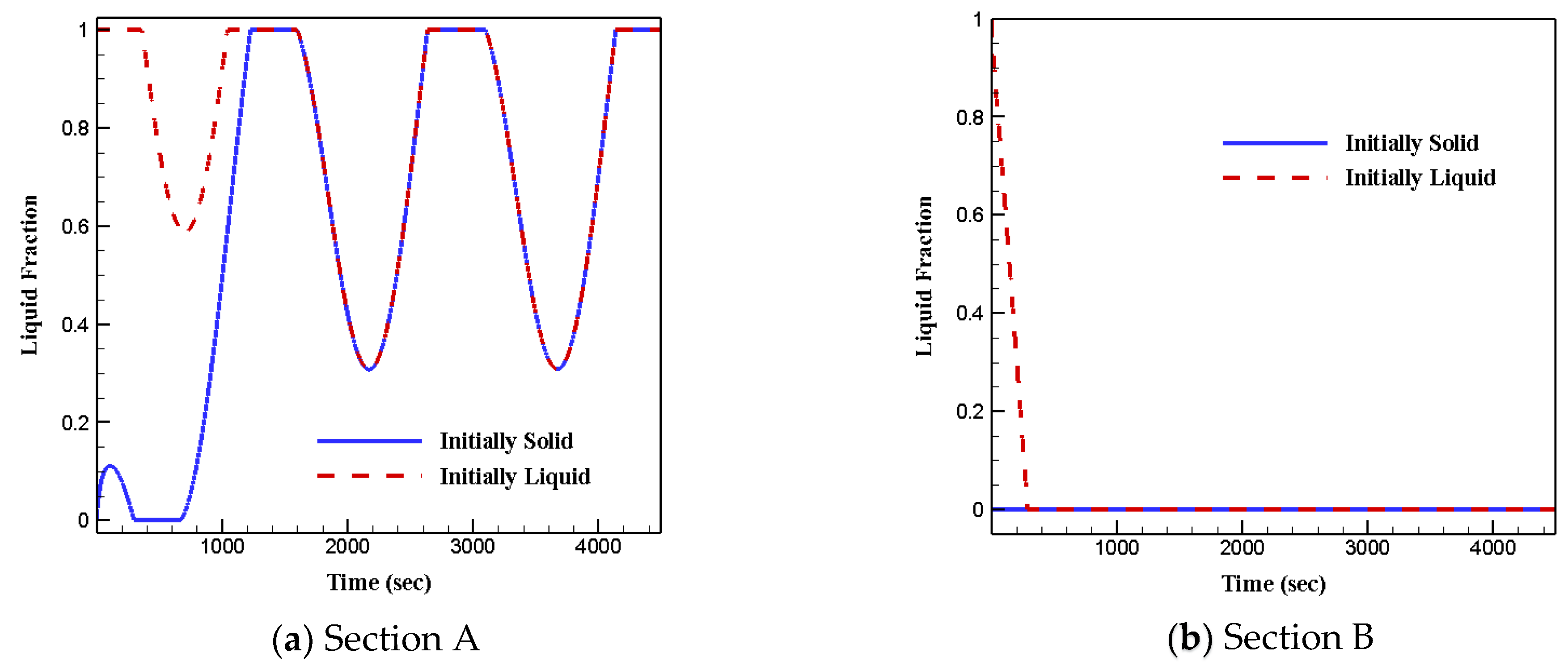
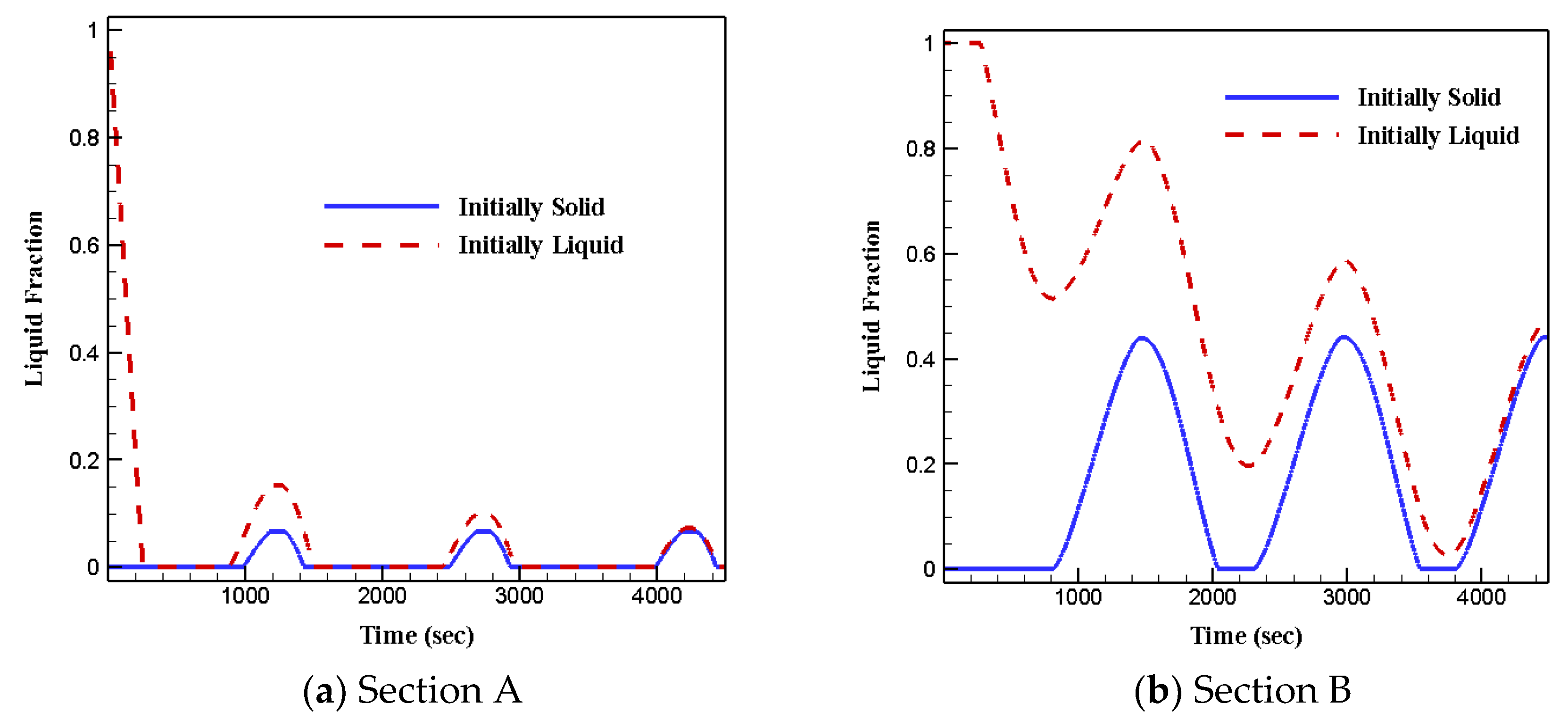
3.4. Effects of Initial Status
4. Conclusions
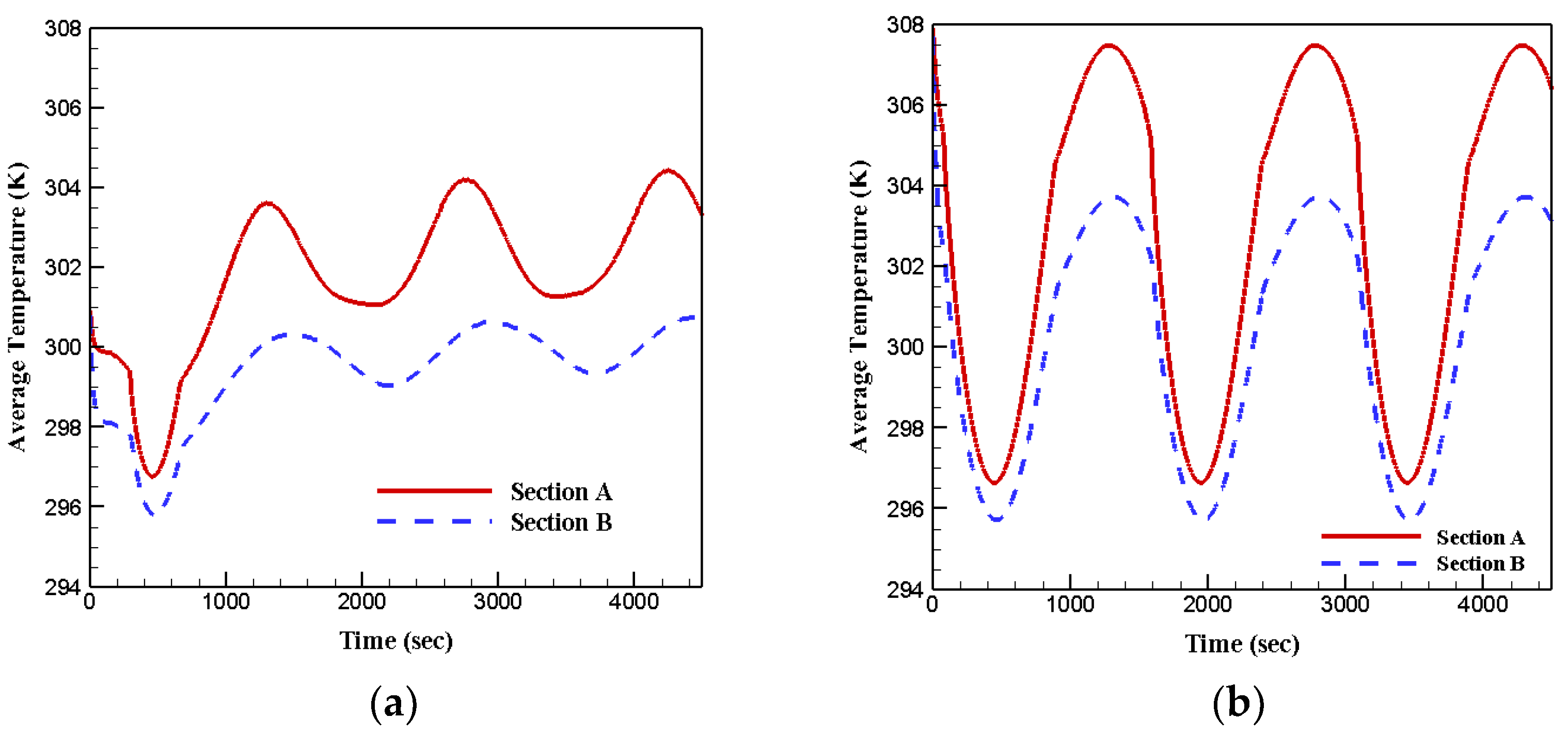
- The amplitude of locally average temperature oscillation for case 3 was the lowest, whereas case 4 fluctuated with the widest amplitude among the four cases;
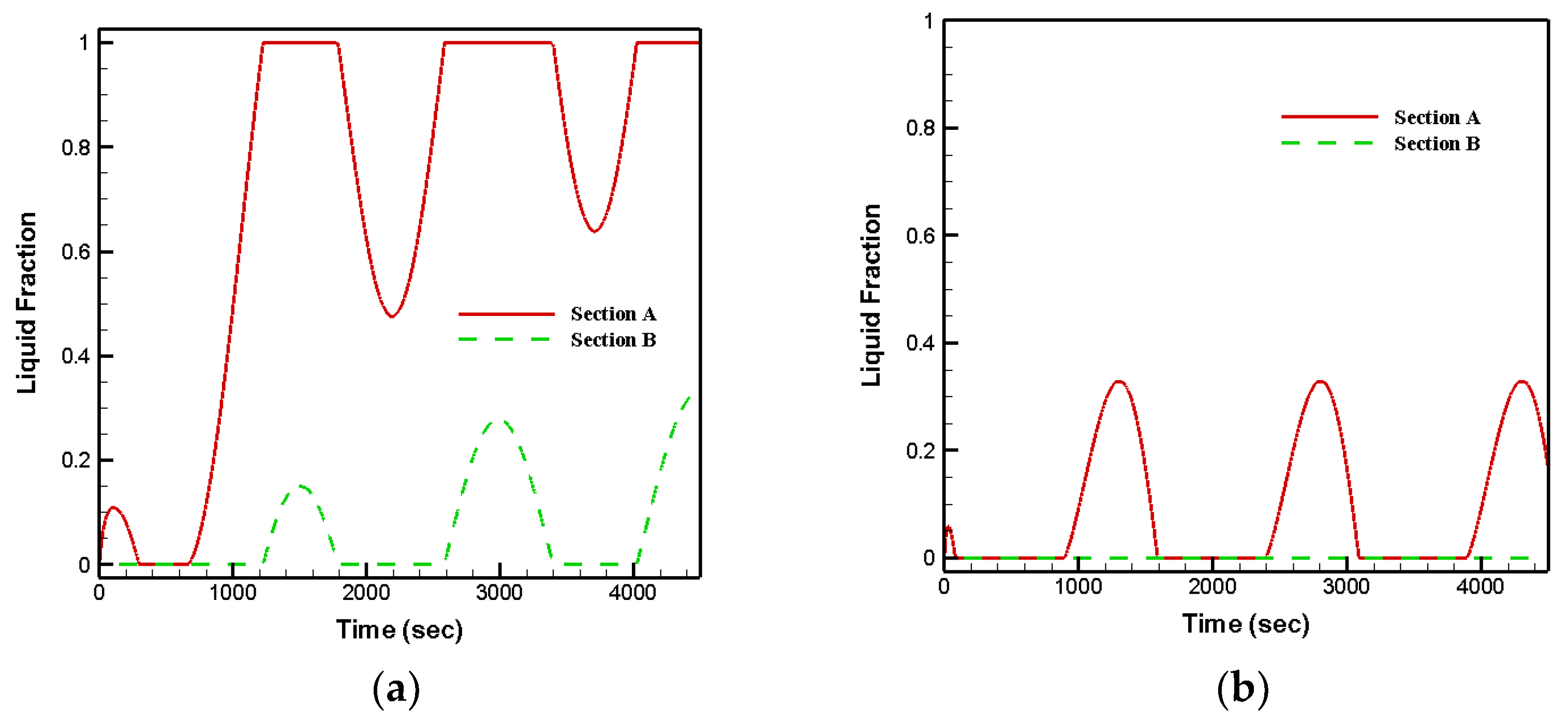
- Arrangement type-2 and that fully filled with RT28 led to solid–liquid phase changes in both sections;
- Arrangement type-1 and that fully filled with RT35 led to the samples remaining at solid phase for the entire period in section B;
- Under the first boundary condition, the temperature of section A spanned wider than section B for all four cases;
- Under the first boundary condition, the lowest temperature was seen in section B and the highest was captured in section A;
- Under the second boundary condition, most of the phase change materials in both sections stayed in liquid phase due to the extended heat transfer surface;
- The initial phase status of phase change materials does not affect heat transfer and fluid flow features.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahiraei, M.; Khosravi, R.; Heshmatian, S. Assessment and optimization of hydrothermal characteristics for a non-Newtonian nanofluid flow within miniaturized concentric-tube heat exchanger considering designer’s viewpoint. Appl. Therm. Eng. 2017, 123, 266–276. [Google Scholar] [CrossRef]
- Bahiraei, M.; Naghibzadeh, S.M.; Jamshidmofid, M. Efficacy of an eco-friendly nanofluid in a miniature heat exchanger regarding to arrangement of silver nanoparticles. Energy Convers. Manag. 2017, 144, 224–234. [Google Scholar] [CrossRef]
- Bahiraei, M. Particle migration in nanofluids: A critical review. Int. J. Therm. Sci. 2016, 109, 90–113. [Google Scholar] [CrossRef]
- Mirzaei, P.A.; Haghighat, F. Modeling of phase change materials for applications in whole building simulation. Renew. Sustain. Energy Rev. 2012, 16, 5355–5362. [Google Scholar] [CrossRef]
- El-Sawi, A.; Haghighat, F.; Akbari, H. Assessing long-term performance of centralized thermal energy storage system. Appl. Therm. Eng. 2014, 62, 313–321. [Google Scholar] [CrossRef]
- Joybari, M.M.; Haghighat, F.; Moffat, J.; Sra, P. Heat and cold storage using phase change materials in domestic refrigeration systems: The state-of-the-art review. Energy Build. 2015, 106, 111–124. [Google Scholar] [CrossRef]
- Bastani, A.; Haghighat, F. Expanding Heisler chart to characterize heat transfer phenomena in a building envelope integrated with phase change materials. Energy Build. 2015, 106, 164–174. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhanced thermal properties of SiO2 nanocomposite for solar thermal energy storage applications. Int. J. Heat Mass Transf. 2015, 84, 898–902. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.B.; Lin, C.H.; He, Y.L. Preparation and thermal properties characterization of carbonate salt/carbon nanomaterial composite phase change material. Energy Convers. Manag. 2015, 97, 103–110. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, C.; Liu, J.; Fang, X.; Zhang, Z. Highly stable graphite nanoparticle-dispersed phase change emulsions with little supercooling and high thermal conductivity for cold energy storage. Appl. Energy 2017, 188, 97–106. [Google Scholar] [CrossRef]
- Erek, A.; İlken, Z.; Acar, M.A. Experimental and numerical investigation of thermal energy storage with a finned tube. Int. J. Energy Res. 2005, 29, 283–301. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Zhang, P.; Li, M. Preparation and thermal characterization of paraffin/metal foam composite phase change material. Appl. Energy 2013, 112, 1357–1366. [Google Scholar] [CrossRef]
- Wei, L.C.; Malen, J.A. Amplified charge and discharge rates in phase change materials for energy storage using spatially-enhanced thermal conductivity. Appl. Energy 2016, 181, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, F.; Li, Z.; Tang, Y.; Yu, B.; Yuan, W. Experimental investigation on the thermal performance of a heat sink filled with porous metal fiber sintered felt/paraffin composite phase change material. Appl. Energy 2016, 176, 221–232. [Google Scholar] [CrossRef]
- Pasupathy, A.; Velraj, R. Effect of double layer phase change material in building roof for year round thermal management. Energy Build. 2008, 40, 193–203. [Google Scholar] [CrossRef]
- Hamza, H.; Hanchi, N.; Abouelkhayrat, B.; Lahjomri, J.; Oubarra, A. Location and Thickness Effect of Two Phase Change Materials Between Layers of Roof on Energy Consumption for Air-Conditioned Room. J. Therm. Sci. Eng. Appl. 2016, 8, 021009. [Google Scholar] [CrossRef]
- Kanzawa, A. Thermal performance of a heat storage module using PCM’s with different melting temperatures: Mathematical modeling. ASME J. Energy Resour. Technol. 1989, 111, 152–157. [Google Scholar]
- Mosaffa, A.H.; Ferreira, C.I.; Talati, F.; Rosen, M.A. Thermal performance of a multiple PCM thermal storage unit for free cooling. Energy Convers. Manag. 2013, 67, 1–7. [Google Scholar] [CrossRef]
- Sefidan, A.M.; Sojoudi, A.; Saha, S.C.; Cholette, M. Multi-layer PCM solidification in a finned triplex tube considering natural convection. Appl. Therm. Eng. 2017, 123, 901–916. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Zhao, C.Y. A numerical investigation of heat transfer in phase change materials (PCMs) embedded in porous metals. Energy 2011, 36, 5539–5546. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.Y.; Lu, W.; Tian, Y. Heat transfer enhancement for thermal energy storage using metal foams embedded within phase change materials (PCMs). Sol. Energy 2010, 84, 1402–1412. [Google Scholar] [CrossRef] [Green Version]
- Sefidan, A.M.; Taghilou, M.; Mohammadpour, M.; Sojoudi, A. Effects of different parameters on the discharging of double-layer PCM through the porous channel. Appl. Therm. Eng. 2017, 123, 592–602. [Google Scholar] [CrossRef]
- Mesalhy, O.; Lafdi, K.; Elgafy, A.; Bowman, K. Numerical study for enhancing the thermal conductivity of phase change material (PCM) storage using high thermal conductivity porous matrix. Energy Convers. Manag. 2005, 46, 847–867. [Google Scholar] [CrossRef]
- Taghilou, M.; Sefidan, A.M.; Sojoudi, A.; Saha, S.C. Solid-liquid phase change investigation through a double pipe heat exchanger dealing with time-dependent boundary conditions. Appl. Therm. Eng. 2017, 128, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Ismail, K.A.; Maria das Graças, E. Melting of PCM around a horizontal cylinder with constant surface temperature. Int. J. Therm. Sci. 2003, 42, 1145–1152. [Google Scholar] [CrossRef]
- Atal, A.; Wang, Y.; Harsha, M.; Sengupta, S. Effect of porosity of conducting matrix on a phase change energy storage device. Int. J. Heat Mass Transf. 2016, 93, 9–16. [Google Scholar] [CrossRef]
- Taghilou, M.; Talati, F. Numerical investigation on the natural convection effects in the melting process of PCM in a finned container using lattice Boltzmann method. Int. J. Refrig. 2016, 70, 157–170. [Google Scholar] [CrossRef]
- Talati, F.; Taghilou, M. Lattice Boltzmann application on the PCM solidification within a rectangular finned container. Appl. Therm. Eng. 2015, 83, 108–120. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, Q.; Zheng, Z.J.; He, Y.L. Evaluation and optimization of melting performance for a latent heat thermal energy storage unit partially filled with porous media. Appl. Energy 2017, 193, 84–95. [Google Scholar] [CrossRef]
- Calmidi, V.V.; Mahajan, R.L. Forced convection in high porosity metal foams. Trans.-Am. Soc. Mech. Eng. J. Heat Transf. 2000, 122, 557–565. [Google Scholar] [CrossRef]
- Calmidi, V.V. Transport Phenomena in High Porosity Fibrous Metal Foams; University of Colorado at Boulde: Boulder, CO, USA, 1998. [Google Scholar]
- Al-Abidi, A.A.; Mat, S.; Sopian, K.; Sulaiman, M.Y.; Mohammad, A.T. Internal and external fin heat transfer enhancement technique for latent heat thermal energy storage in triplex tube heat exchangers. Appl. Therm. Eng. 2013, 53, 147–156. [Google Scholar] [CrossRef]
| Cases | Section A | Section B | Initial Status of Section A | Initial Status of Section B | Inner HTF | Outer HTF |
|---|---|---|---|---|---|---|
| 1 | RT28 | RT35 | Solid | Solid | Hot | Cold |
| 2 | RT35 | RT28 | Solid | Solid | Hot | Cold |
| 3 | RT28 | Solid | Solid | Hot | Cold | |
| 4 | RT35 | Solid | Solid | Hot | Cold | |
| 5 | RT28 | RT35 | Liquid | Liquid | Hot | Cold |
| 6 | RT35 | RT28 | Liquid | Liquid | Hot | Cold |
| 7 | RT28 | RT35 | Solid | Solid | Cold | Hot |
| 8 | RT35 | RT28 | Solid | Solid | Cold | Hot |
| Thermo-Physical Properties | RT 28 | RT 35 | Aluminum |
|---|---|---|---|
| Density () | 810 | 820 | 2719 |
| () | 1900 | 2100 | 871 |
| 0.2 | 0.2 | 202.4 | |
| 0.0025 | 0.0027 | - | |
| 245,000 | 157,000 | - | |
| 301 | 308 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sefidan, A.M.; Sangari, M.E.; Sellier, M.; Khan, M.I.H.; Saha, S.C. Modeling of Multi-Layer Phase Change Material in a Triplex Tube under Various Thermal Boundary Conditions. Energies 2022, 15, 3465. https://doi.org/10.3390/en15093465
Sefidan AM, Sangari ME, Sellier M, Khan MIH, Saha SC. Modeling of Multi-Layer Phase Change Material in a Triplex Tube under Various Thermal Boundary Conditions. Energies. 2022; 15(9):3465. https://doi.org/10.3390/en15093465
Chicago/Turabian StyleSefidan, Ali M., Mehdi E. Sangari, Mathieu Sellier, Md. Imran Hossen Khan, and Suvash C. Saha. 2022. "Modeling of Multi-Layer Phase Change Material in a Triplex Tube under Various Thermal Boundary Conditions" Energies 15, no. 9: 3465. https://doi.org/10.3390/en15093465
APA StyleSefidan, A. M., Sangari, M. E., Sellier, M., Khan, M. I. H., & Saha, S. C. (2022). Modeling of Multi-Layer Phase Change Material in a Triplex Tube under Various Thermal Boundary Conditions. Energies, 15(9), 3465. https://doi.org/10.3390/en15093465








