Mechanical Response of Geopolymer Foams to Heating—Managing Coal Gangue in Fire-Resistant Materials Technology
Abstract
:1. Introduction
2. Materials and Methods
3. Results
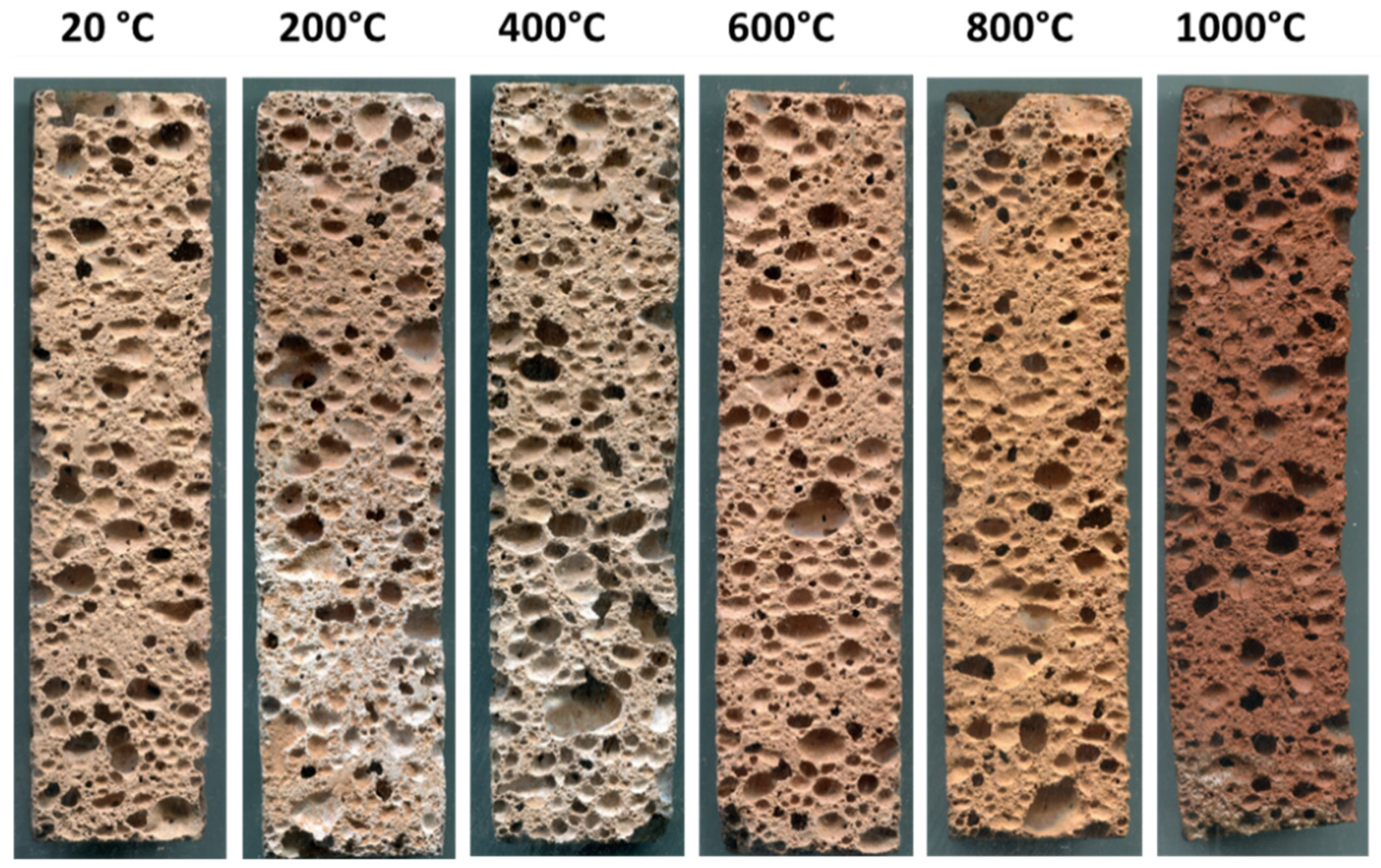
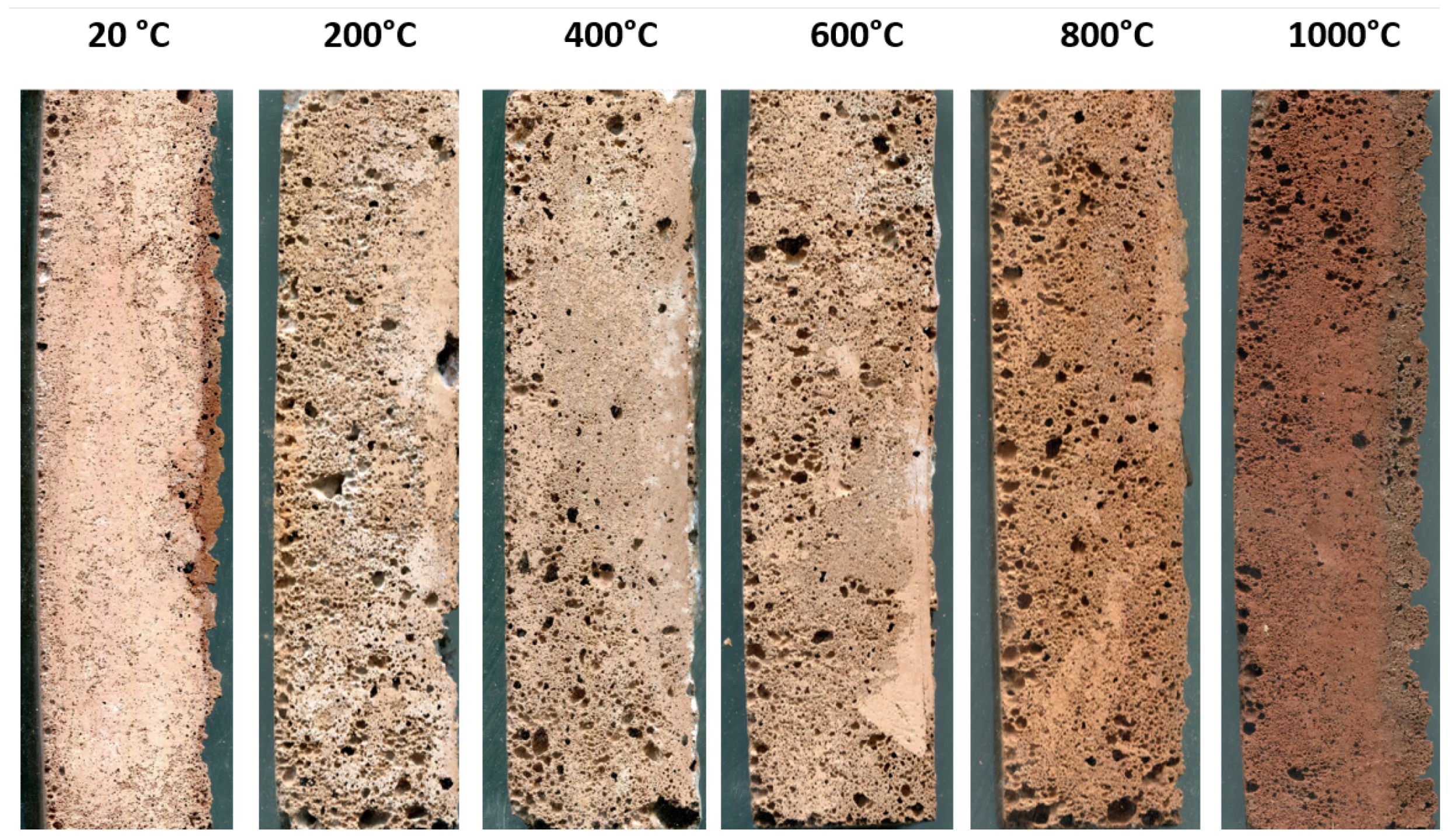
3.1. Visual Aspect and Colour Change of the Heated Foams
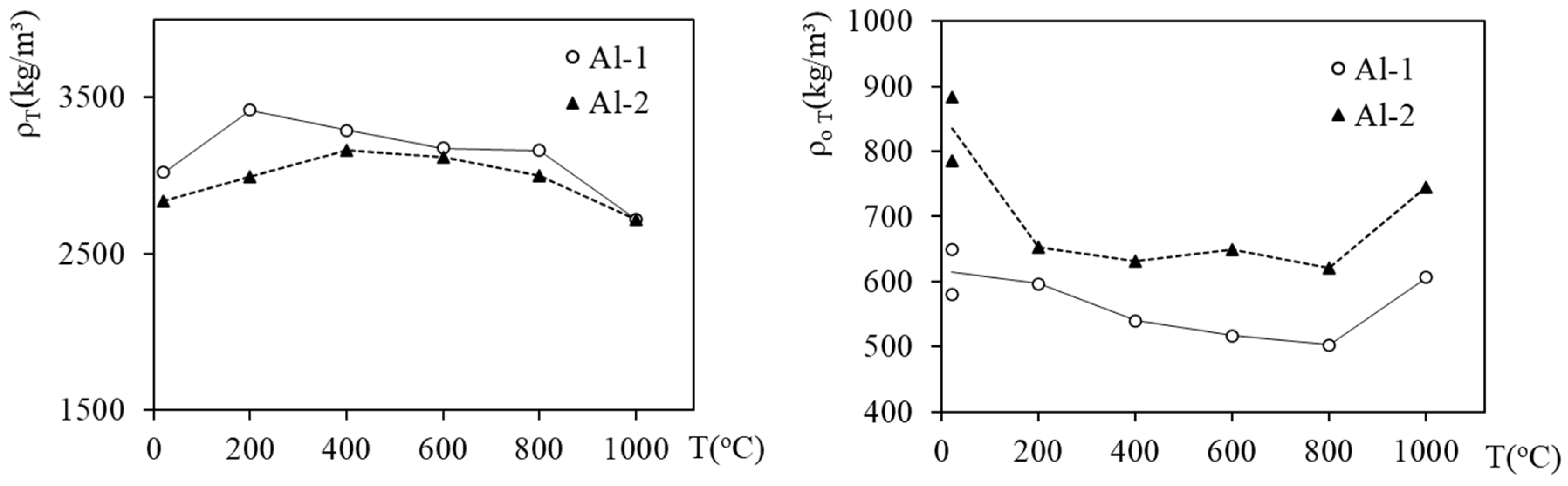
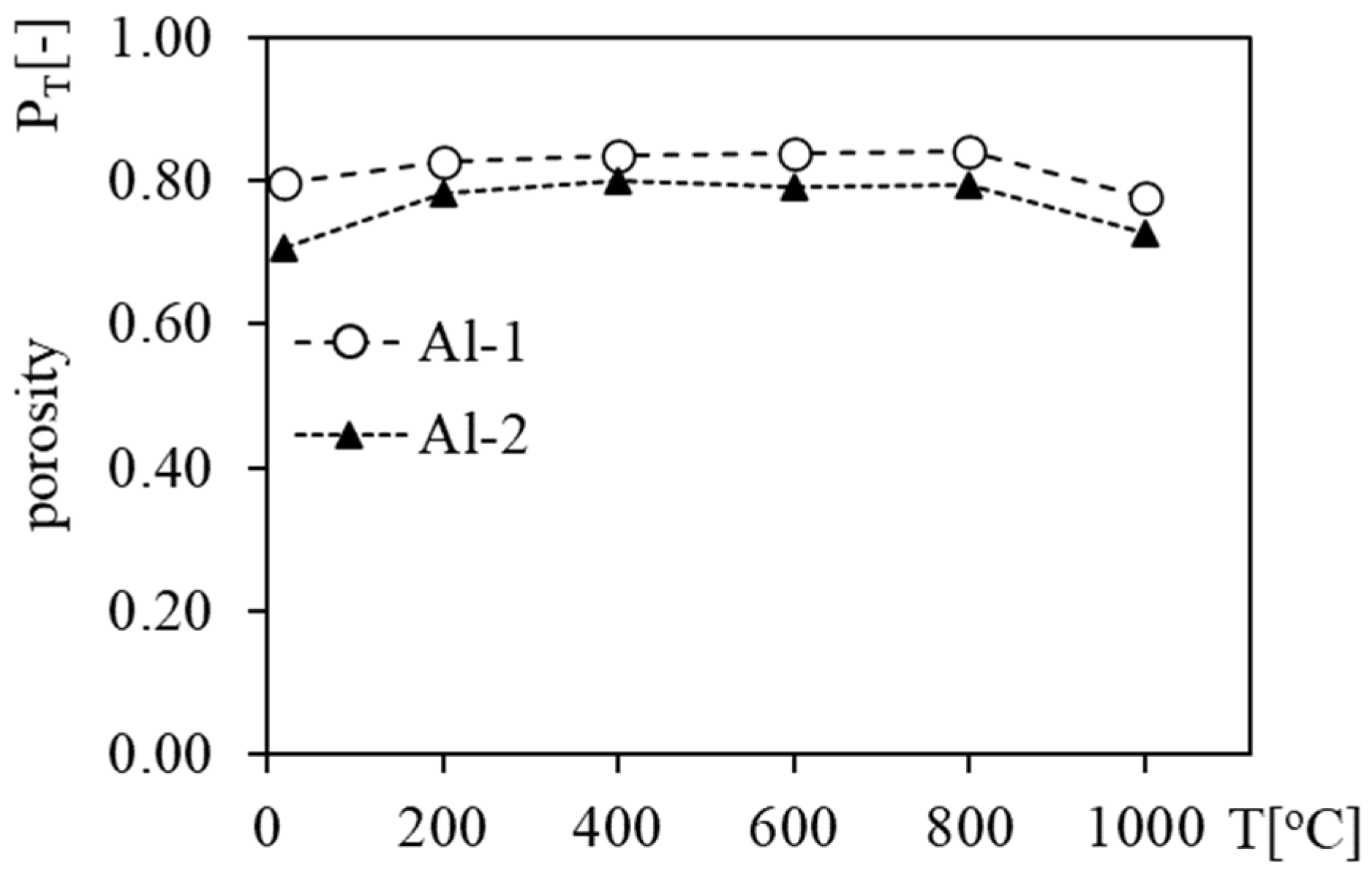
3.2. Apparent Density, Density and Porosity of Heated Foams
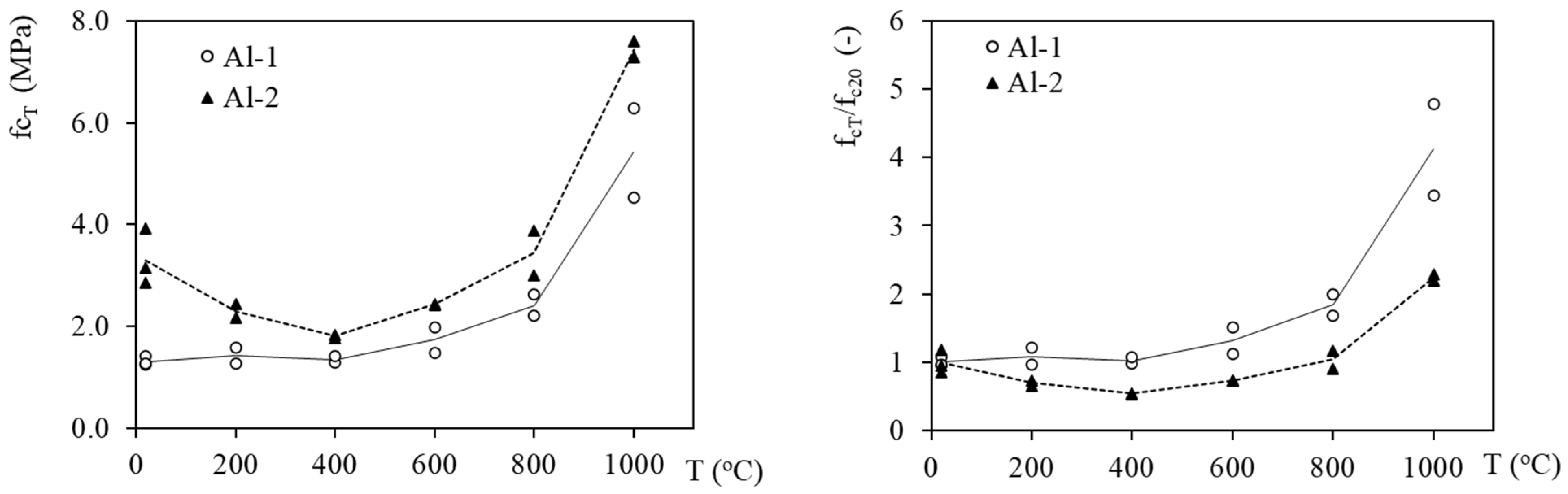
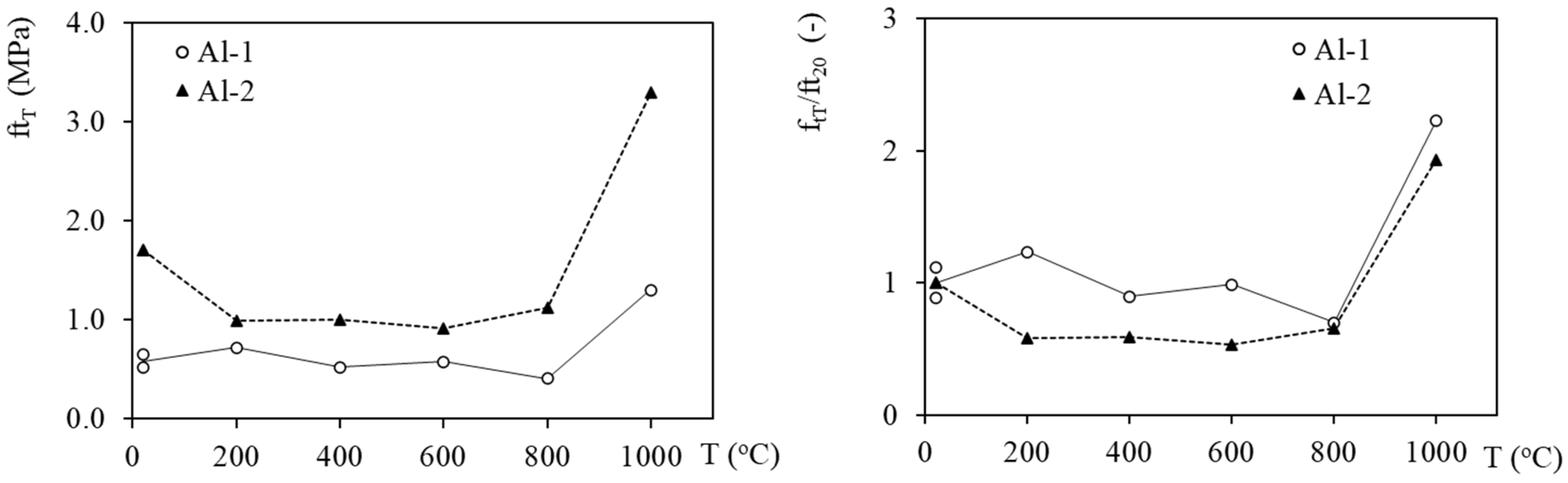
3.3. Mechanical Properties Evolution with Temperature
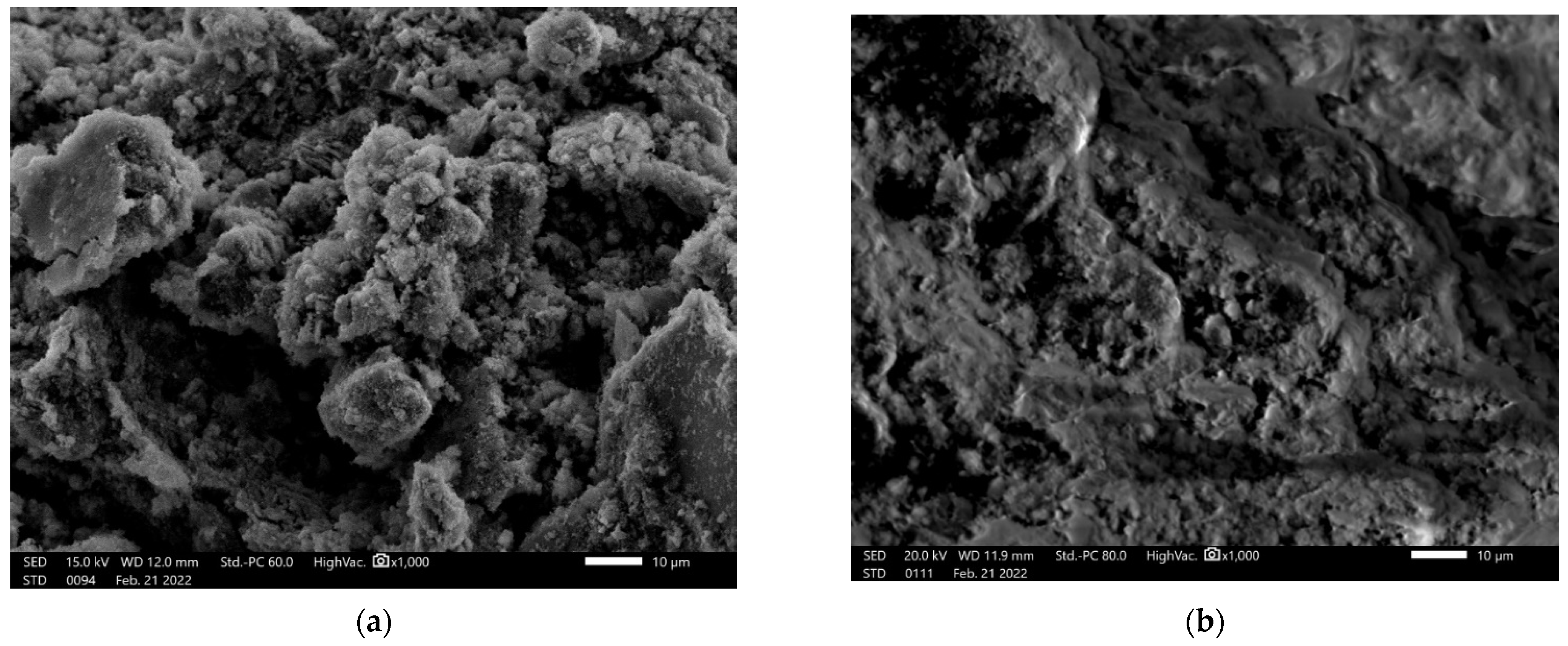
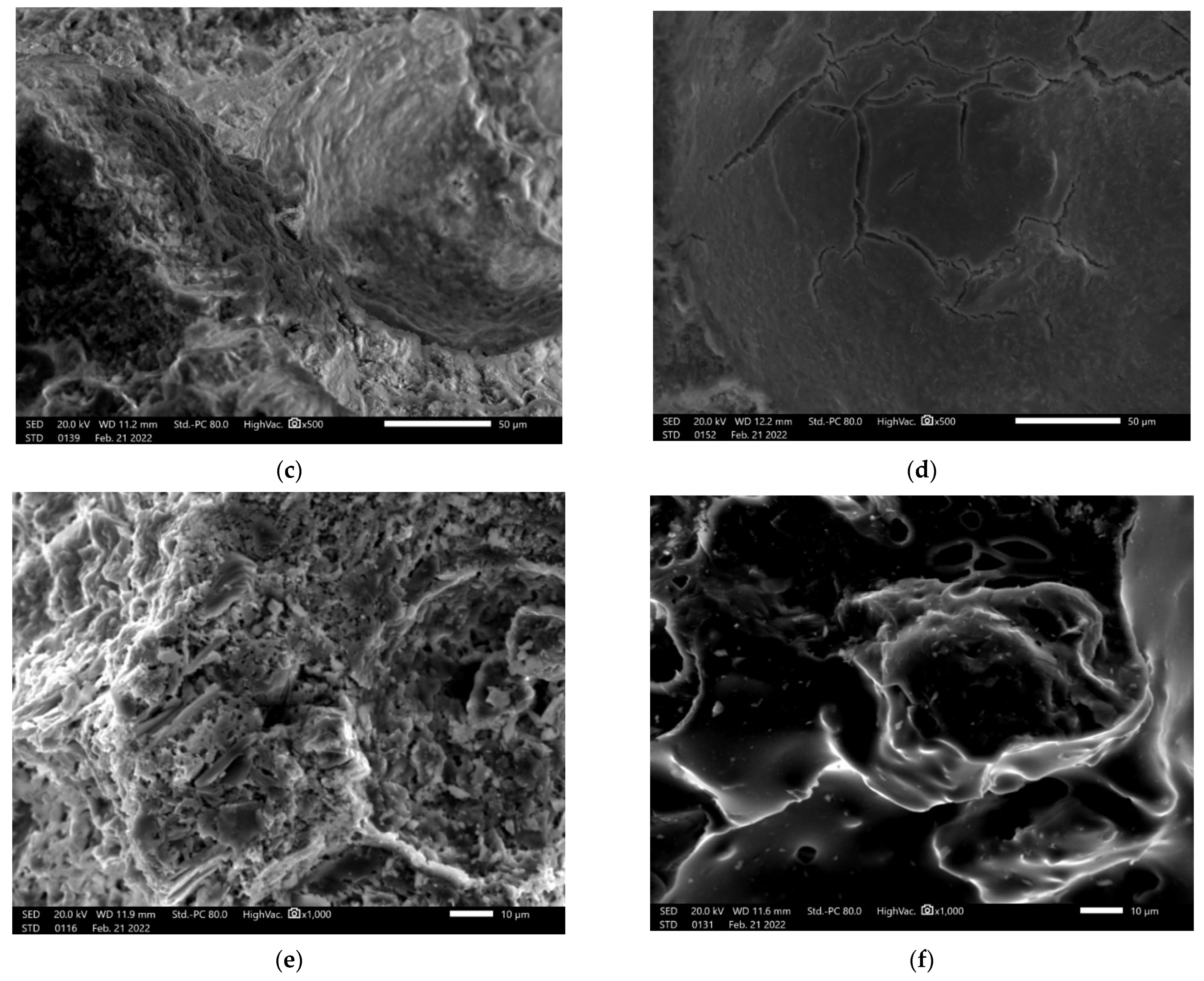
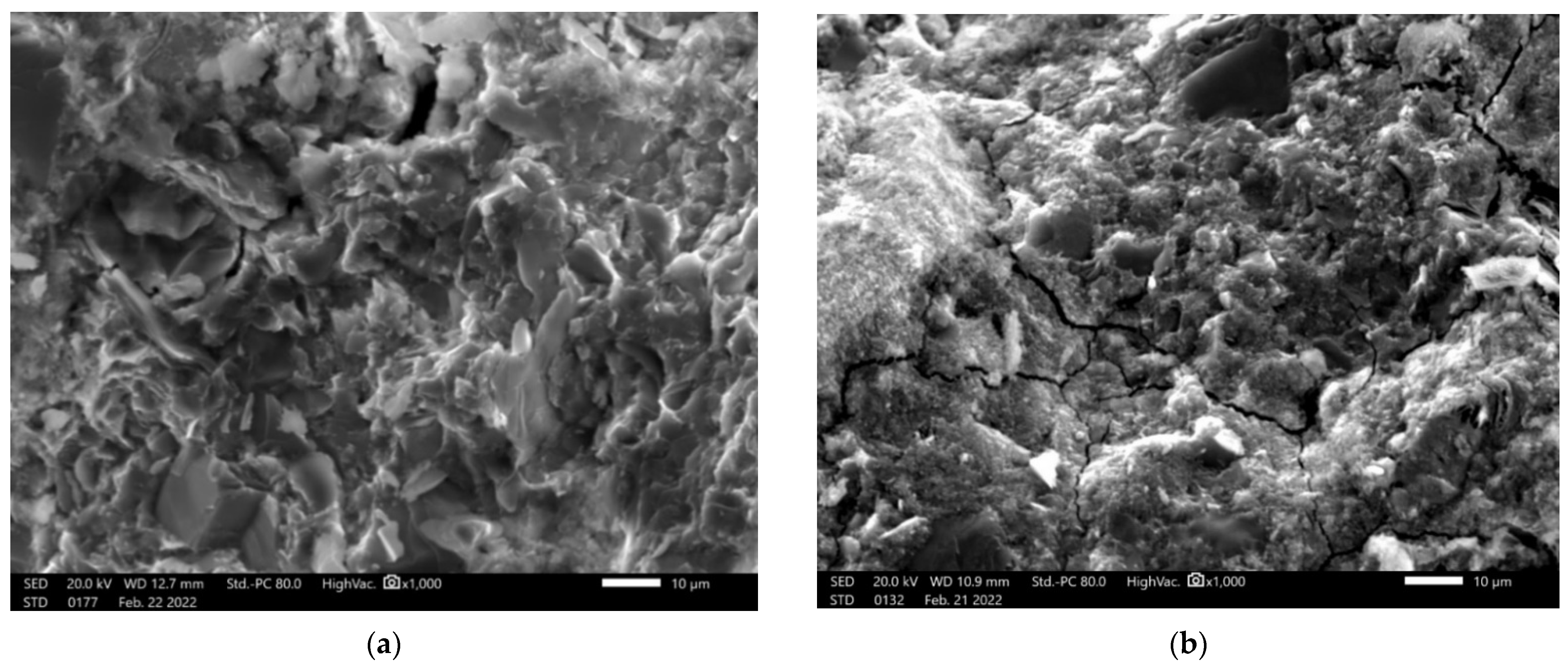
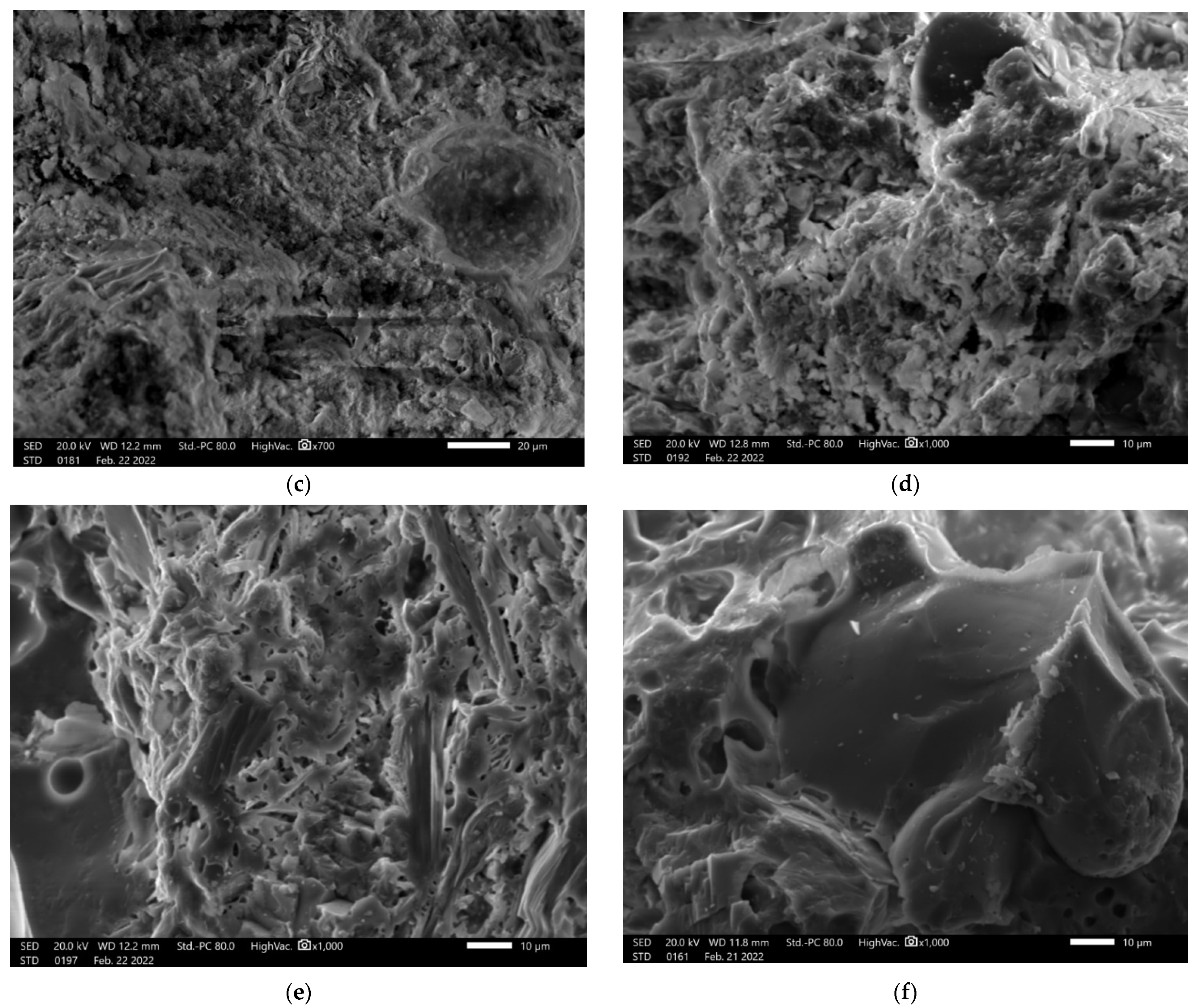
3.4. Microstructure Investigation with the Use of a Scanning Electron Microscope (SEM) and Chemical Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. 30 Years of Successes and Failures in Geopolymer Applications. Market Trends and Potential Breakthroughs. In Proceedings of the Geopolymer 2002 Conference, Melbourne, VIC, Australia, 28–29 October 2002; Available online: https://www.geopolymer.org/wp-content/uploads/30YearsGEOP.pdf (accessed on 1 March 2022).
- Łach, M. Geopolymer Foams—Will They Ever Become a Viable Alternative to Popular Insulation Materials?—A Critical Opinion. Materials 2021, 14, 3568. [Google Scholar] [CrossRef] [PubMed]
- Amran, M.; Huang, S.S.; Debbarma, S.; Rashid, R.S.M. Fire resistance of geopolymer concrete: A critical review. Constr. Build. Mater. 2022, 324, 126722. [Google Scholar] [CrossRef]
- Łach, M.; Korniejenko, K.; Mikuła, J. Thermal Insulation and Thermally Resistant Materials Made of Geopolymer Foams. Procedia Eng. 2016, 151, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Korniejenko, K.; Figiela, B.; Ziejewska, C.; Marczyk, J.; Bazan, P.; Hebda, M.; Choińska, M.; Lin, W.-T. Fracture Behavior of Long Fiber Reinforced Geopolymer Composites at Different Operating Temperatures. Materials 2022, 15, 482. [Google Scholar] [CrossRef] [PubMed]
- Lahoti, L.; Hai Tan, K.; Yang, E.H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Kozub, B.; Bazan, P.; Gailitis, R.; Korniejenko, K.; Mierzwiński, D. Foamed Geopolymer Composites with the Addition of Glass Wool Waste. Materials 2021, 14, 4978. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Le, V.S.; Louda, P.; Tran, H.N.; Nguyen, P.D.; Bakalova, T.; Ewa Buczkowska, K.; Dufkova, I. Study on Temperature-Dependent Properties and Fire Resistance of Metakaolin-Based Geopolymer Foams. Polymers 2020, 12, 2994. [Google Scholar] [CrossRef]
- Shaikh, F.; Haque, S. Behaviour of Carbon and Basalt Fibres Reinforced Fly Ash Geopolymer at Elevated Temperatures. Int. J. Concr. Struct. Mater. 2018, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-Y.; Hao, X.; Fan, W. Experimental Study on High Temperature Properties of Carbon Fiber Sheets Strengthened Concrete Cylinders Using Geopolymer as Adhesive. Procedia Eng. 2016, 135, 47–55. [Google Scholar] [CrossRef]
- Behera, P.; Baheti, V.; Militky, J.; Naeem, S. Microstructure and mechanical properties of carbon microfiber reinforced geopolymers at elevated temperatures. Constr. Build. Mater. 2018, 160, 733–743. [Google Scholar] [CrossRef]
- Behera, P.; Baheti, V.; Militky, J.; Louda, P. Elevated temperature properties of basalt microfibril filled geopolymer composites. Constr. Build. Mater. 2018, 163, 850–860. [Google Scholar] [CrossRef]
- Celik, A.; Yilmaz, K.; Canpolat, O.; Al-Mashhadani, M.M.; Aygörmez, Y.; Uysal, M. High-temperature behavior and mechanical characteristics of boron waste additive metakaolin based geopolymer composites reinforced with synthetic fibers. Constr. Build. Mater. 2018, 187, 1190–1203. [Google Scholar] [CrossRef]
- Alomayri, T.; Shaikh, F.; Low, I.M. Characterisation of cotton fibre-reinforced geopolymer composites. Compos. Part B Eng. 2013, 50, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Alomayri, T.; Shaikh, F.; Low, I. Mechanical and thermal properties of ambient cured cotton fabric-reinforced fly ash-based geopolymer composites. Ceram. Int. 2014, 40, 14019–14028. [Google Scholar] [CrossRef]
- Constancio Trindade, A.C.; Alcamand, H.A.; Ribeiro Borges, P.H.; de Andrade Silva, F. Influence of Elevated Temperatures on the Mechanical Behavior of Jute-Textile-Reinforced Geopolymers. J. Ceram. Sci. Technol. 2017, 8, 389–398. [Google Scholar]
- Tayeh, B.A.; Zeyad, A.M.; Agwa, I.S.; Amin, M. Effect of elevated temperatures on mechanical properties of lightweight geopolymer concrete. Case Stud. Constr. Mater. 2021, 15, e00673. [Google Scholar] [CrossRef]
- Nodehi, M. A comparative review on foam-based versus lightweight aggregate-based alkali-activated materials and geopolymer. Innov. Infrastruct. Solut. 2021, 6, 231. [Google Scholar] [CrossRef]
- Ali, M.F.; Natrajan, M.M.V. A Review of Geopolymer Composite Thermal Properties. IOP Conf. Ser. Earth Environ. Sci. 2021, 822, 012051. [Google Scholar] [CrossRef]
- Cheng-Yong, H.; Yun-Ming, L.; Al Bakri Abdullah, M.M.; Hussin, K. Thermal Resistance Variations of Fly Ash Geopolymers: Foaming Responses. Sci. Rep. 2017, 7, 45355. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Progress, current thinking and challenges in geopolymer foam concrete technology. Cem. Concr. Compos. 2021, 116, 103886. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concrete. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Duan, P.; Song, L.; Yan, C.; Ren, D.; Li, Z. Novel thermal insulating and lightweight composites from metakaolin geopolymer and polystyrene particles. Ceram. Int. 2017, 43, 5115–5120. [Google Scholar] [CrossRef]
- Teng, N.H.; Cheng-Yong, H.; Yun-Ming, L.; Al Bakri Abdullah, M.M.; Hussin, K. The Mechanical Properties and Thermal Resistance of Fly Ash Geopolymer Foams. Solid State Phenom. 2018, 281, 175–181. [Google Scholar] [CrossRef]
- Le, V.S.; Szczypinski, M.M.; Hájková, P.; Kovacic, V.; Bakalova, T.; Volesky, L.; Hiep, L.C.; Louda, P. Mechanical properties of geopolymer foam at high temperature. Sci. Eng. Compos. Mater. 2020, 27, 129–138. [Google Scholar] [CrossRef]
- PN-EN 1008:2017. Mixing Water for Concrete—Specification for Sampling, Testing and Assessing the Suitability of Water, Including Water Recovered from Processes in the Concrete Industry, As Mixing Water for Concrete. Available online: https://standards.iteh.ai/catalog/standards/cen/8ab1a0fa-b727-48f7-9b31-739eba3732ca/en-1008-2002 (accessed on 25 March 2022). (In Polish).
- Kastiukas, G.; Zhou, X.; Wan, K.T.; Castro-Gomes, J. Lightweight alkali-activated material from mining and glass waste by chemical and physical Foaming. J. Mater. Civ. Eng. 2019, 31, 04018397. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Marczyk, J.; Ziejewska, C.; Hordyńska, N.; Mikuła, J.; Hebda, M. Optimal design of pH-neutral geopolymer foams for their use in ecological plant cultivation systems. Materials 2019, 12, 2999. [Google Scholar] [CrossRef] [Green Version]
- Schneider, U.; Felicetti, R.; Debicki, G.; Diederichs, U.; Franssen, J.M.; Jumppanen, U.M.; Khoury, G.A.; Leonovich, S.; Millard, A.; Morris, W.A.; et al. Recommendation of RILEM TC 200-HTC: Mechanical concrete properties at high temperatures-modelling and applications: PGeneral presentation. Mater. Struct. Constr 2007, 40, 841–853. [Google Scholar] [CrossRef] [Green Version]
- Hager, I.; Sitarz, M.; Mróz, K. Fly-ash based geopolymer mortar for high-temperature application—Effect of slag addition. J. Clean. Prod. 2021, 316, 128168. [Google Scholar] [CrossRef]
- Dudek, M.; Sitarz, M. Analysis of changes in the microstructure of geopolymer mortar after exposure to high temperatures. Materials 2020, 13, 4263. [Google Scholar] [CrossRef]
- Hager, I.; Sitarz, M.; Mróz, K. Behaviour of fly ash geopolymer at high temperature. In Proceedings of the 2020 5th International Conference on Smart and Sustainable Technologies (SpliTech), Split, Croatia, 23–26 September 2020. [Google Scholar]
- Hager, I. Colour change in heated concrete. Fire Technol. 2014, 50, 945–958. [Google Scholar] [CrossRef] [Green Version]
- Pantongsuk, T.; Kittisayarm, P.; Muenglue, N.; Benjawan, S.; Thavorniti, P.; Tippayasam, C.; Nilpairach, S.; Heness, G.; Chaysuwan, D. Effect of hydrogen peroxide and bagasse ash additions on thermal conductivity and thermal resistance of geopolymer foams. Mater. Today Commun. 2021, 26, 102149. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Factors affecting the performance of metakaolin geopolymers exposed to elevated temperatures. J. Mater. Sci. 2008, 43, 824–831. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Badanoiu, A.; Balanoiu, M.; Mathias, A.; Voicu, G. Alkali Activated Mortars with Intumescent Properties. Rev. Chim. 2019, 70, 431–437. [Google Scholar] [CrossRef]
- Luo, Y.; Li, S.H.; Klima, K.M.; Brouwers, H.J.H.; Yu, O. Degradation mechanism of hybrid fly ash/slag based geopolymers exposed to elevated temperatures. Cem. Concr. Res. 2022, 151, 106649. [Google Scholar] [CrossRef]
- Liu, H.; Jing, W.; Qin, L.; Duan, P.; Zhang, Z.; Guo, R.; Li, W. Thermal stability of geopolymer modified by different silicon source materials prepared from solid wastes. Constr. Build. Mater. 2022, 315, 125709. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Tan, K.H.; Yang, E.H. Effect of alkali cation type on strength endurance of fly ash geopolymers subject to high temperature exposure. Mater. Des. 2018, 154, 8–19. [Google Scholar] [CrossRef]
- Kumara, R.; Miyaoka, H.; Shinzato, K.; Ichikawa, T. Analysis of sodium generation by sodium oxide decomposition on corrosion resistance materials: A new approach towards sodium redox water-splitting cycle. RSC Adv. 2021, 11, 21017–21022. [Google Scholar] [CrossRef]
- Obonyo, E.A.; Kamseu, E.; Lemougna, P.N.; Tchamba, A.B.; Melo, U.C.; Leonelli, C. A Sustainable Approach for the Geopolymerization of Natural Iron-Rich Aluminosilicate Materials. Sustainability 2014, 6, 5535–5553. [Google Scholar] [CrossRef] [Green Version]
- Zawrah, M.F.; Sadek, H.E.H.; Ngida, R.E.A.; Abo Sawan, S.E.; El-Kheshen, A.A. Effect of low-rate firing on physico-mechanical properties of unfoamed and foamed geopolymers prepared from waste clays. Ceram. Int. 2022, 48, 11330–11337. [Google Scholar] [CrossRef]
- Choi, Y.C.; Park, B. Effects of high-temperature exposure on fractal dimension of fly-ash-based geopolymer composites. J. Mater. Res. Technol. 2020, 9, 7655–7668. [Google Scholar] [CrossRef]
- Klima, K.M.; Schollbach, K.; Brouwers, H.J.H.; Yu, O. Thermal and fire resistance of Class F fly ash based geopolymers—A review. Constr. Build. Mater. 2022, 323, 126529. [Google Scholar] [CrossRef]
- Cilla, M.S.; de Mello Innocentini, M.D.; Morelli, M.R.; Colombo, P. Geopolymer foams obtained by the saponification/peroxide/gelcasting combined route using different soap foam precursors. J. Am. Ceram. Soc. 2017, 100, 3440–3450. [Google Scholar] [CrossRef]
- Abo Sawan, S.E.; Zawrah, M.F.; Khattab, R.M.; Abdel-Shafi, A.A. In-situ formation of geopolymer foams through addition of silica fume: Preparation and sinterability. Mater. Chem. Phys. 2020, 239, 121998. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, F.; Feng, X.; Kong, J.; Wang, B.; Yang, J. Effect of high temperature on the mechanical properties of hierarchical porous cenosphere/geopolymer composite foams. Int. J. Appl. Ceram. Technol. 2021, 18, 817–829. [Google Scholar] [CrossRef]
- Bai, C.; Li, H.; Bernardo, E.; Colombo, P. Waste-to-resource preparation of glass-containing foams from geopolymers. Ceram. Int. 2019, 45, 7196–7202. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, M.C.; Hon, M.H. Phase transformation and growth of mullite in kaolin ceramics. J. Eur. Ceram. Soc. 2004, 24, 2389–2397. [Google Scholar] [CrossRef]
- Luhar, S.; Nicolaides, D.; Luhar, I. Fire Resistance Behaviour of Geopolymer Concrete: An Overview. Buildings 2021, 11, 82. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
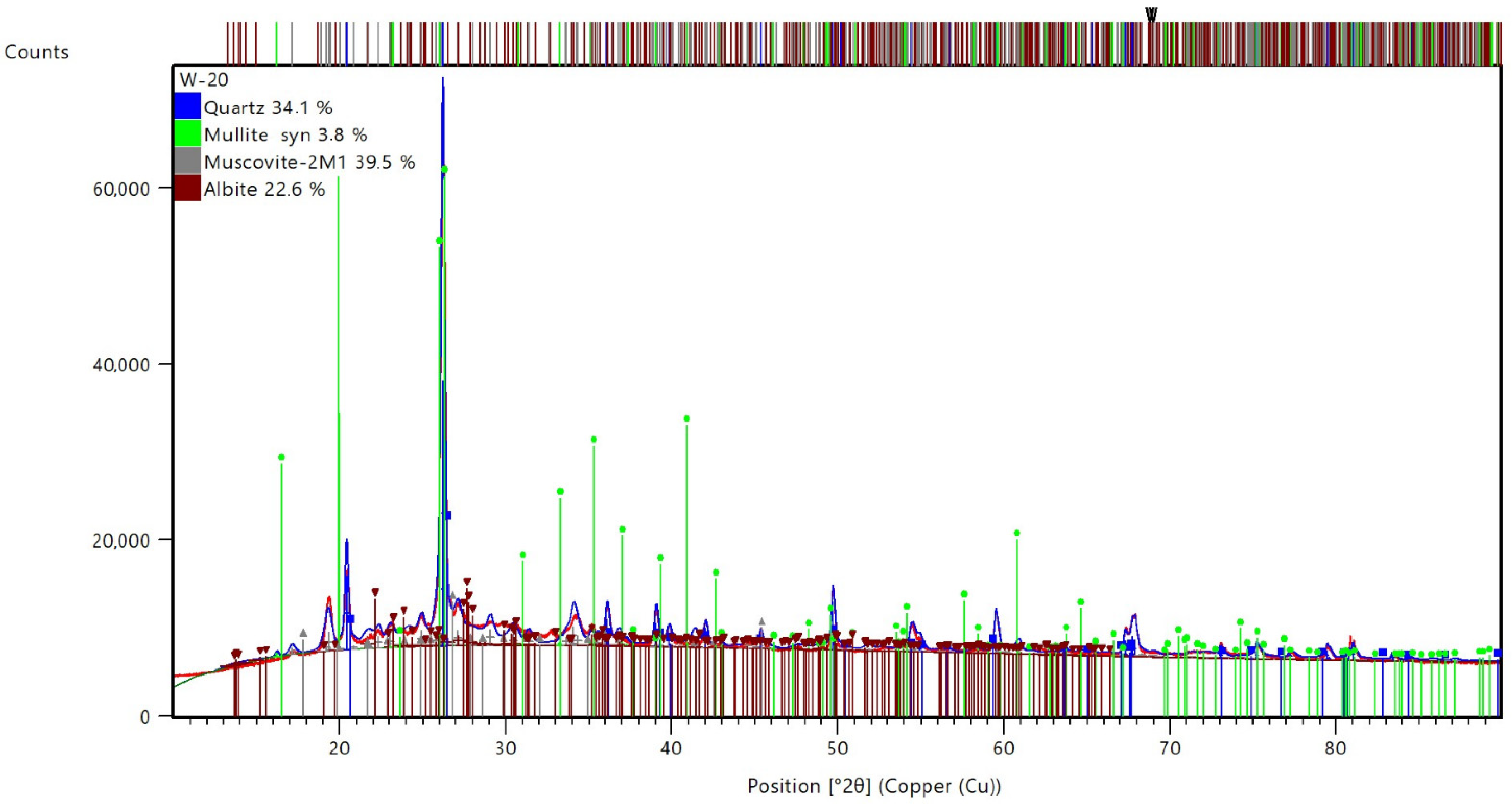
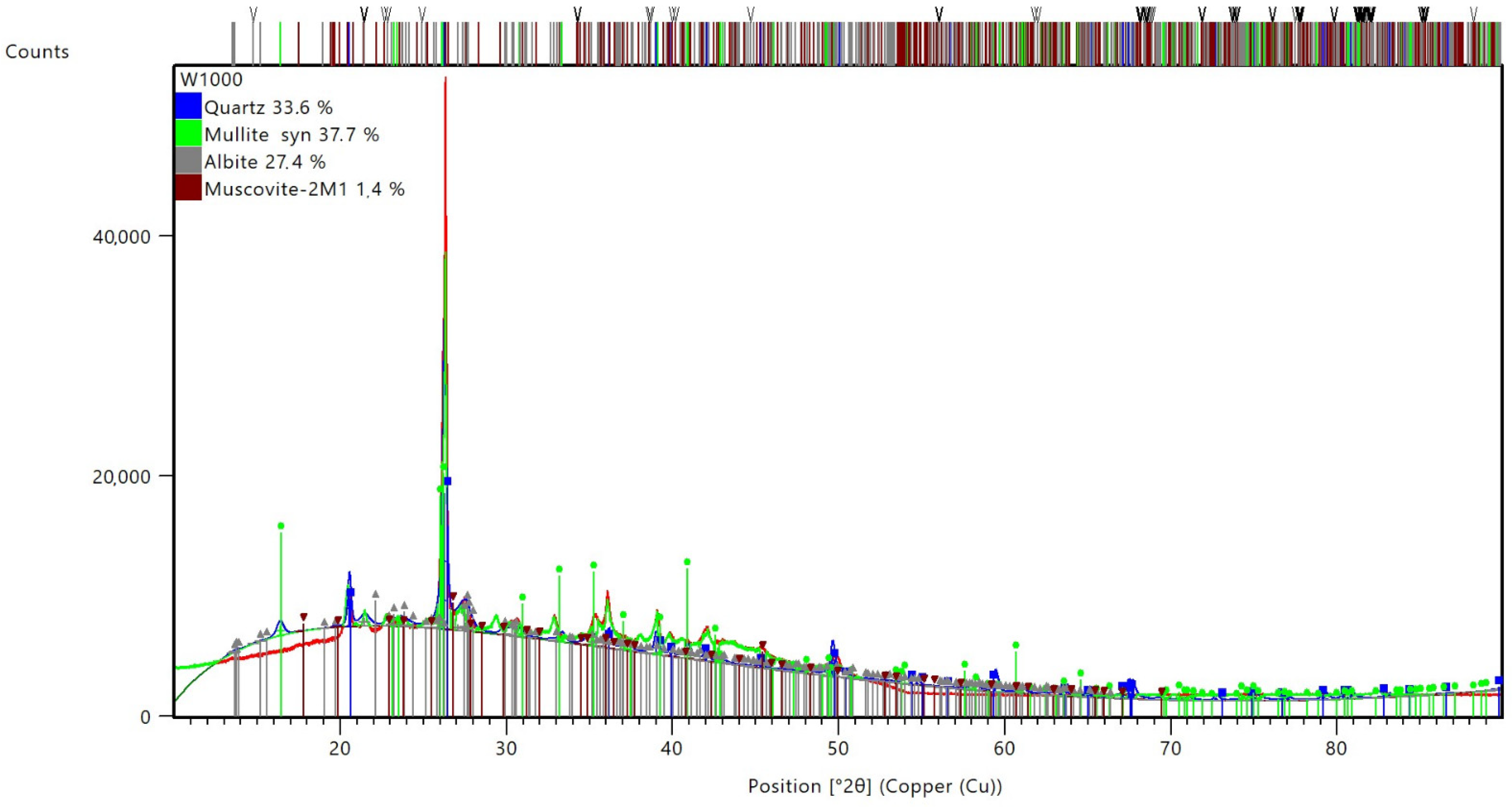
| Sample | Identified Mineral | Percentage [%] | |
|---|---|---|---|
| Name | Chemical Formula | ||
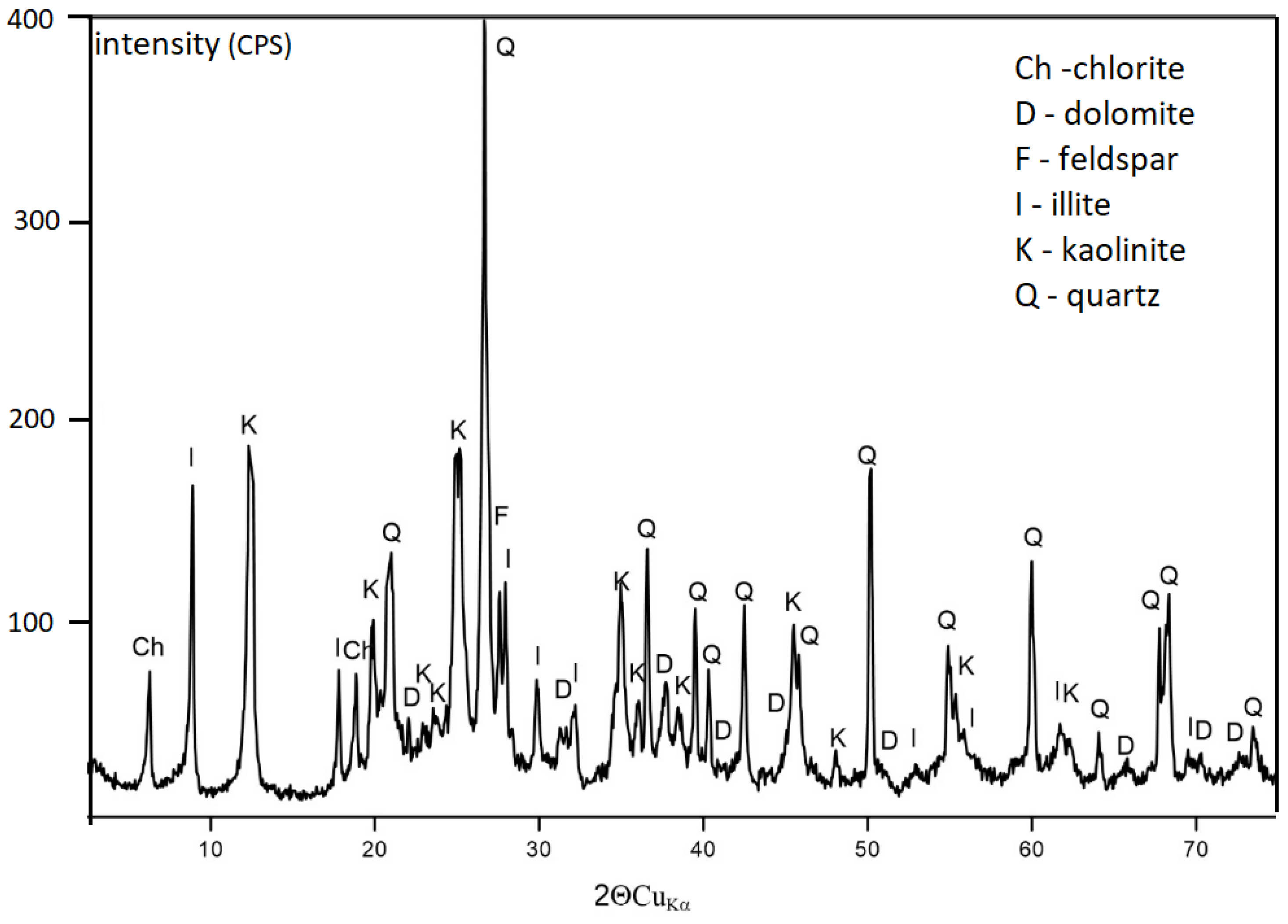
| coal gangue from Wieczorek mine | quartz | SiO2 | 50.0 |
| muscovite-2M1 | KAl2(Si3Al)O10(OH,F)2 | 7.5 | |
| kaolinite-1Ad | Al2Si2O5(OH)4 | 28.2 | |
| illite-2M1 | (KH3O)Al2Si3AlO10(OH)2 | 14.3 | |
| Compound Formula | Conc. (%) |
|---|---|
| SiO2 | 63.925 |
| Al2O3 | 23.839 |
| Fe2O3 | 4.699 |
| K2O | 2.561 |
| MgO | 1.864 |
| TiO2 | 0.986 |
| Na2O | 0.813 |
| SO3 | 0.429 |
| CaO | 0.233 |
| P2O5 | 0.115 |
| Cr2O3 | 0.113 |
| MnO | 0.056 |
| CeO2 | 0.055 |
| NiO | 0.037 |
| ZrO2 | 0.028 |
| BaO | 0.026 |
| Co3O4 | 0.014 |
| Rb2O | 0.012 |
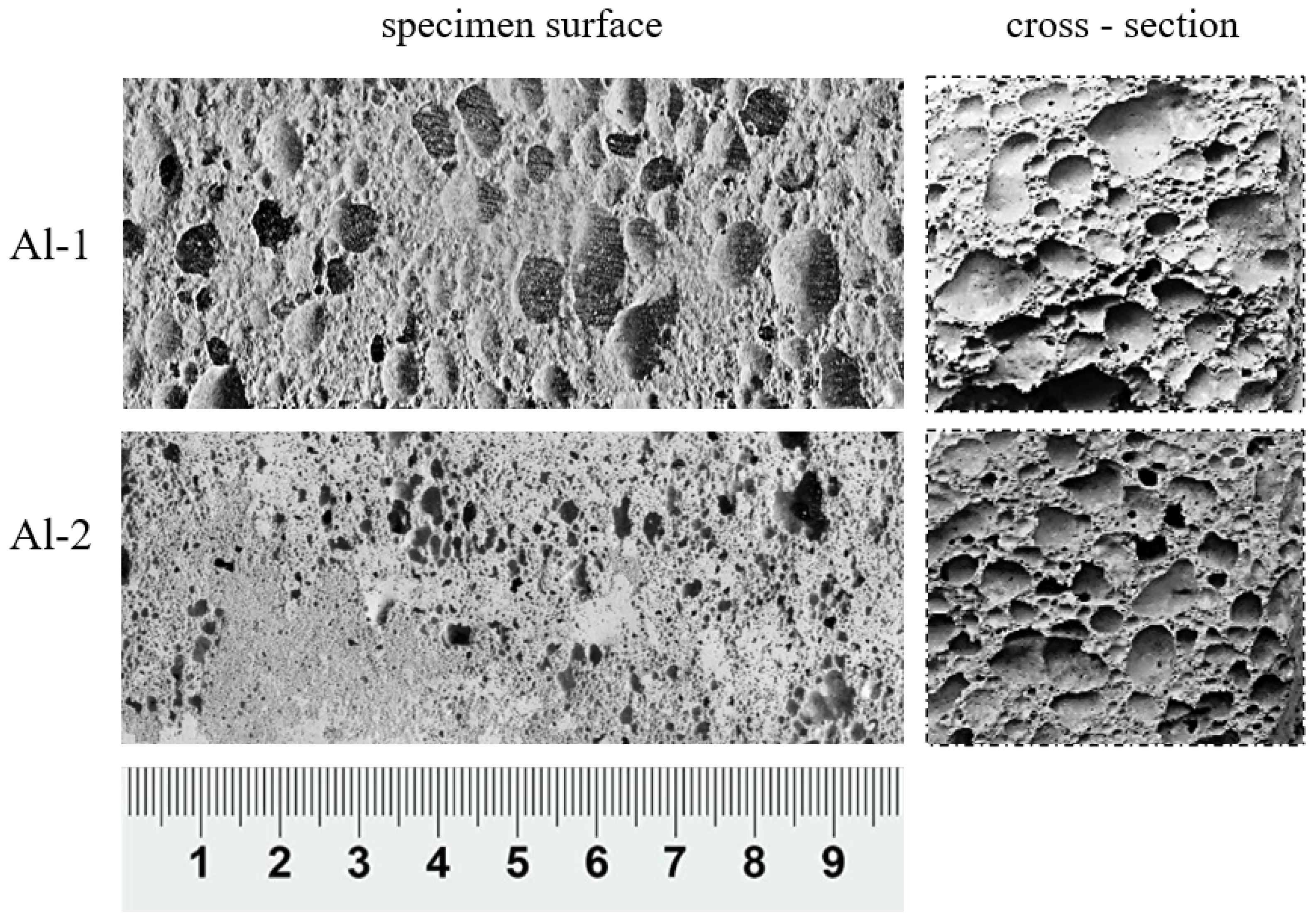
| Sample | Precursor | Foaming Agent | Stabiliser | |
|---|---|---|---|---|
| Calcinated Wieczorek Coal Gangue | Aluminium Powder | 36% Hydrogen Peroxide | Hydroxyethyl Cellulose | |
| Al-1 | 98.8% | 0.15% | 0.75% | 0.3% |
| Al-2 | 98.7% | 0.30% | 0.75% | 0.3% |
| Oxide | 20 °C | 200 °C | 400 °C | 600 °C | 800 °C | 1000 °C |
|---|---|---|---|---|---|---|
| Na2O | 44.86 ± 0.17 | 23.20 ± 0.11 | 15.14 ± 0.11 | 12.39 ± 0.10 | 13.16 ± 0.10 | 10.13 ± 1.08 |
| MgO | 0.75 ± 0.04 | 1.39 ± 0.04 | 1.40 ± 0.04 | 1.37 ± 0.04 | 2.18 ± 0.04 | 1.81 ± 0.04 |
| Al2O3 | 15.22 ± 0.13 | 18.54 ± 0.12 | 19.65 ± 0.12 | 20.29 ± 0.13 | 20.38 ± 0.13 | 23.86 ± 1.53 |
| SiO2 | 36.84 ± 0.21 | 50.27 ± 0.21 | 58.36 ± 0.23 | 59.74 ± 0.25 | 55.02 ± 0.22 | 55.01 ± 2.58 |
| K2O | 2.34 ± 0.05 | 2.21 ± 0.04 | 1.88 ± 0.03 | 2.23 ± 0.04 | 2.21 ± 0.04 | 2.65 ± 0.46 |
| FeO | 4.41 ± 0.20 | 4.39 ± 0.10 | 3.58 ± 0.07 | 3.97 ± 0.08 | 6.43 ± 0.09 | 6.35 ± 1.21 |
| Oxide | 20 °C | 200 °C | 400 °C | 600 °C | 800 °C | 1000 °C |
|---|---|---|---|---|---|---|
| Na2O | 17.97 ± 0.11 | 12.57 ± 0.17 | 7.55 ± 0.10 | 13.27 ± 0.24 | 9.90 ± 0.12 | 10.09 ± 0.18 |
| MgO | 1.33 ± 0.03 | 1.74 ± 0.07 | 1.91 ± 0.05 | 1.11 ± 0.08 | 1.19 ± 0.05 | 1.12 ± 0.07 |
| Al2O3 | 17.11 ± 0.11 | 19.57 ± 0.21 | 20.51 ± 0.16 | 18.88 ± 0.27 | 23.20 ± 0.18 | 23.08 ± 0.25 |
| SiO2 | 56.41 ± 0.21 | 56.38 ± 0.39 | 61.62 ± 0.30 | 52.30 ± 0.49 | 56.99 ± 0.31 | 53.01 ± 0.43 |
| K2O | 1.68 ± 0.03 | 2.83 ± 0.07 | 3.37 ± 0.06 | 2.66 ± 0.09 | 4.10 ± 0.07 | 4.55 ± 0.10 |
| TiO2 | 0.99 ± 0.03 | --- | --- | 1.31 ± 0.09 | --- | --- |
| FeO | 4.51 ± 0.07 | 6.92 ± 0.17 | 5.03 ± 0.11 | 10.47 ± 0.27 | 4.62 ± 0.11 | 8.17 ± 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitarz, M.; Figiela, B.; Łach, M.; Korniejenko, K.; Mróz, K.; Castro-Gomes, J.; Hager, I. Mechanical Response of Geopolymer Foams to Heating—Managing Coal Gangue in Fire-Resistant Materials Technology. Energies 2022, 15, 3363. https://doi.org/10.3390/en15093363
Sitarz M, Figiela B, Łach M, Korniejenko K, Mróz K, Castro-Gomes J, Hager I. Mechanical Response of Geopolymer Foams to Heating—Managing Coal Gangue in Fire-Resistant Materials Technology. Energies. 2022; 15(9):3363. https://doi.org/10.3390/en15093363
Chicago/Turabian StyleSitarz, Mateusz, Beata Figiela, Michał Łach, Kinga Korniejenko, Katarzyna Mróz, João Castro-Gomes, and Izabela Hager. 2022. "Mechanical Response of Geopolymer Foams to Heating—Managing Coal Gangue in Fire-Resistant Materials Technology" Energies 15, no. 9: 3363. https://doi.org/10.3390/en15093363
APA StyleSitarz, M., Figiela, B., Łach, M., Korniejenko, K., Mróz, K., Castro-Gomes, J., & Hager, I. (2022). Mechanical Response of Geopolymer Foams to Heating—Managing Coal Gangue in Fire-Resistant Materials Technology. Energies, 15(9), 3363. https://doi.org/10.3390/en15093363











