A New Adsorption Equation for Nano-Porous Shale Rocks and Its Application in Pore Size Distribution Analysis
Abstract
:1. Introduction
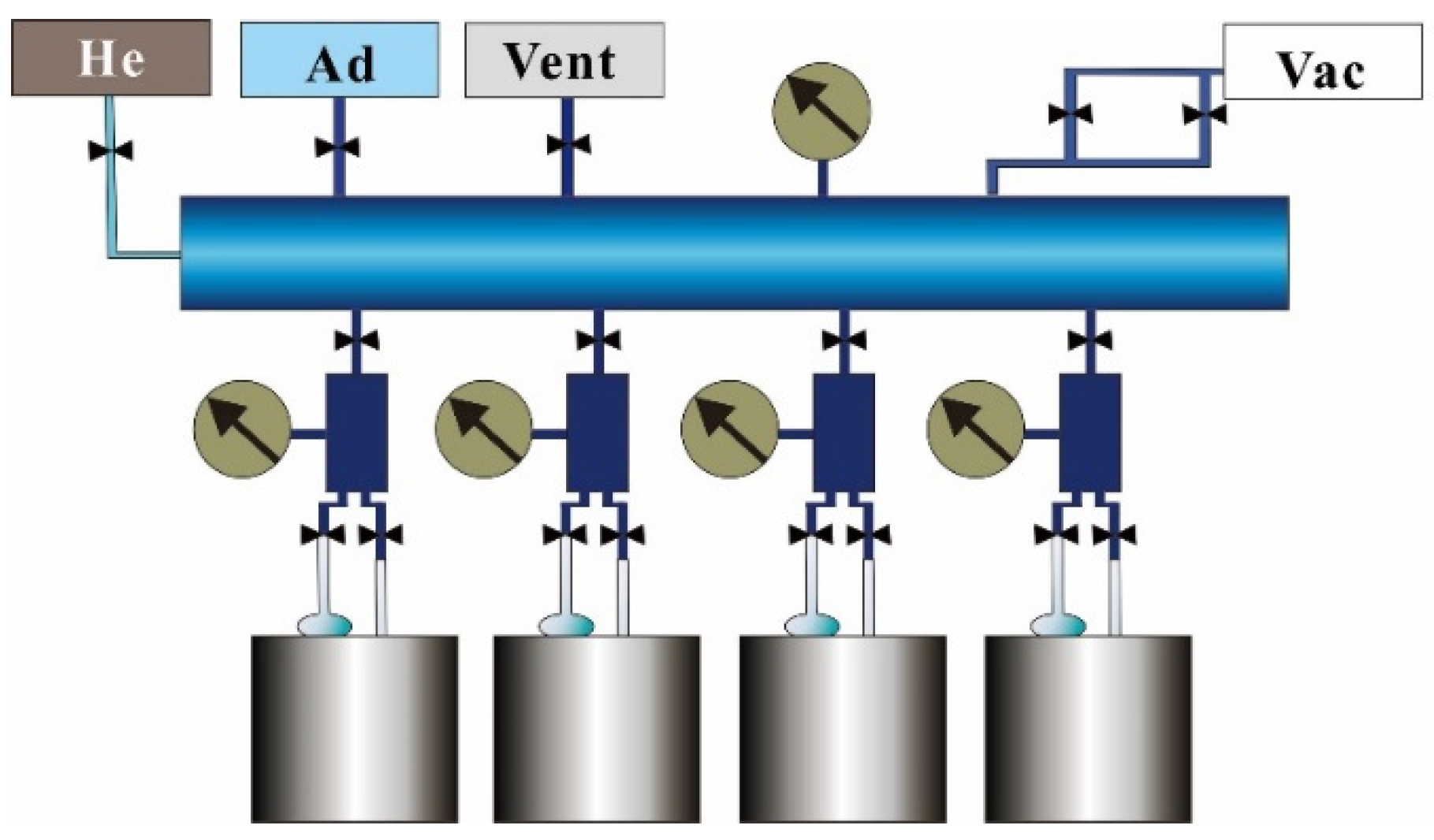
2. Materials and Methods
3. Results
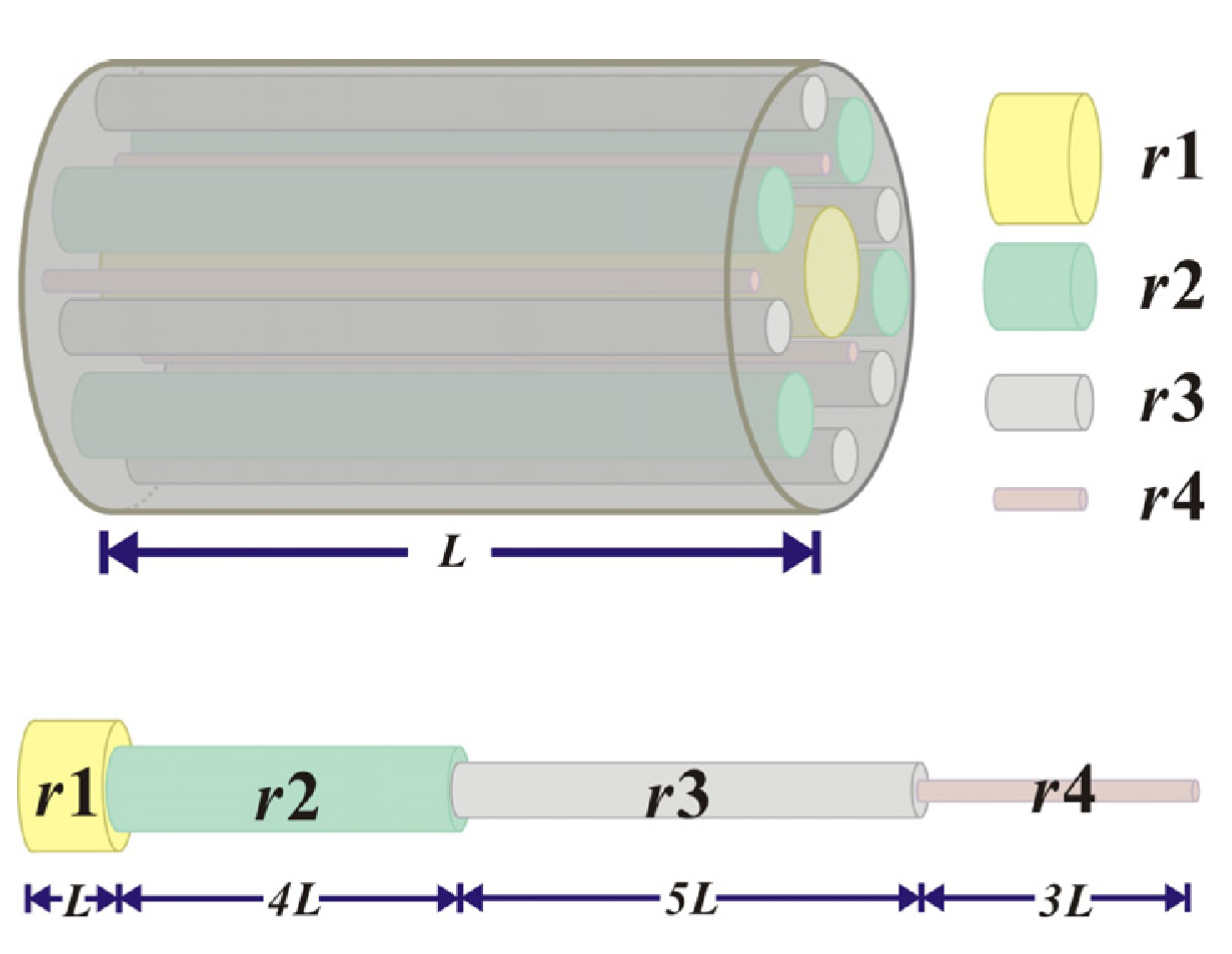
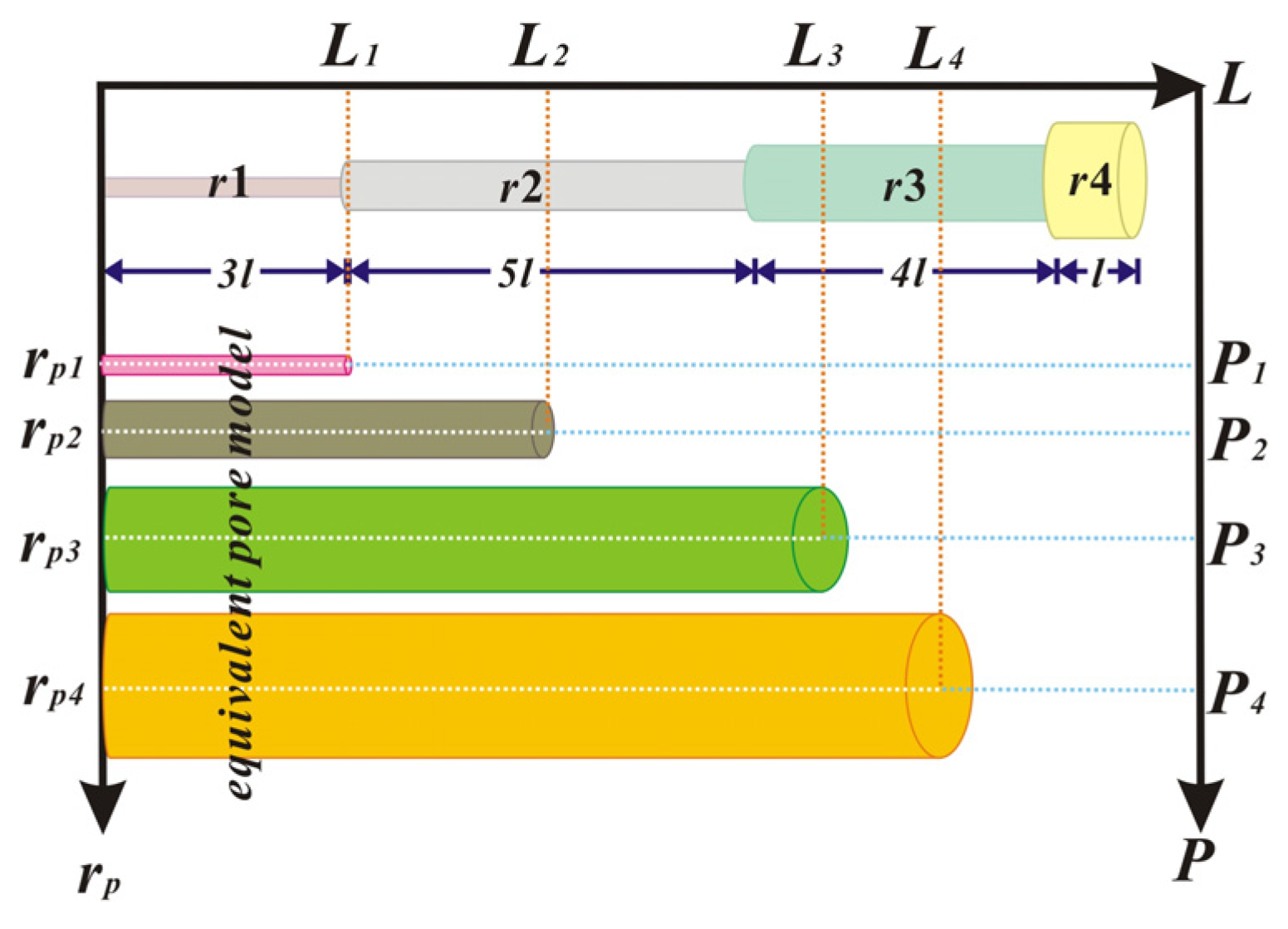
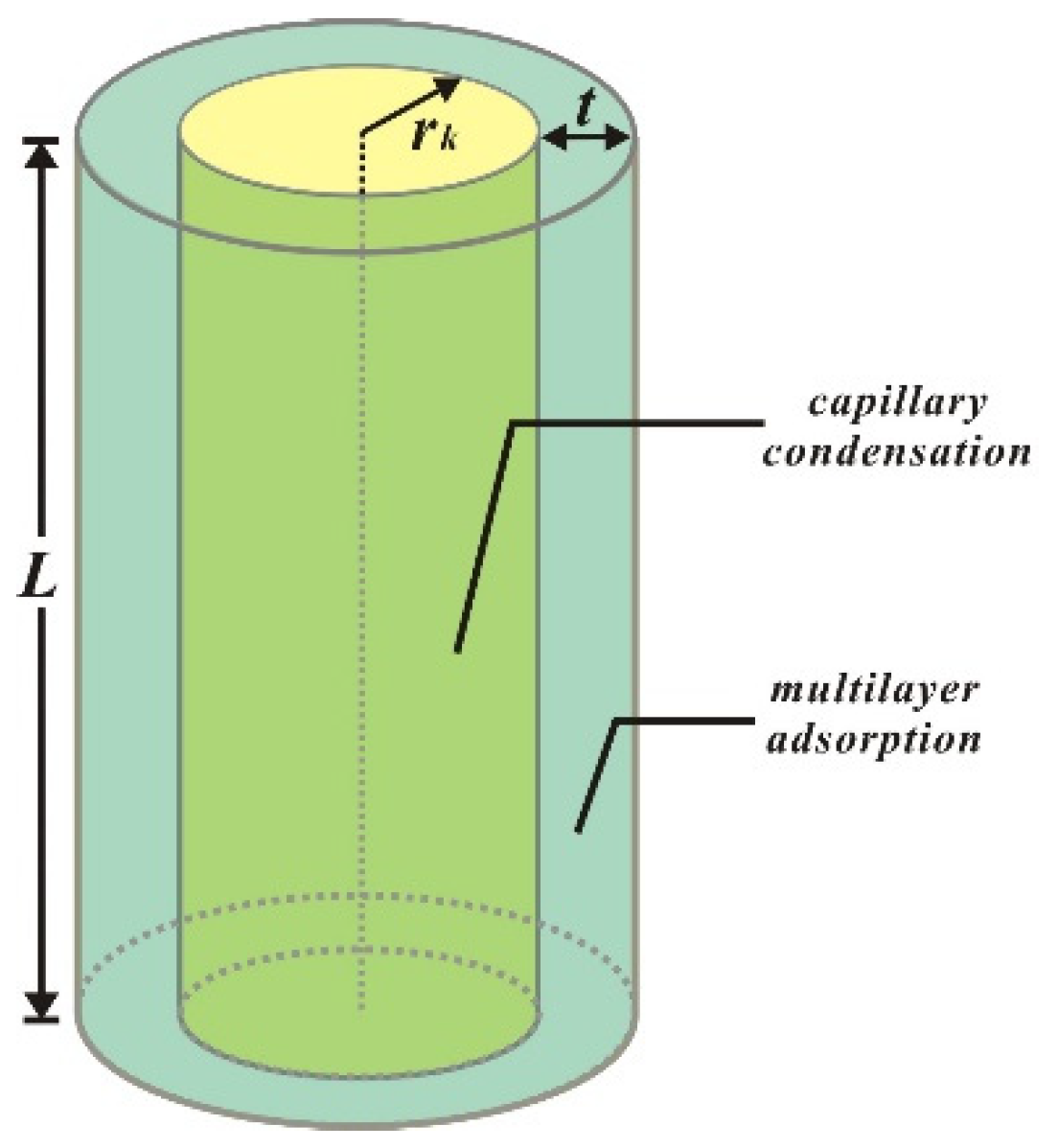
3.1. Adsorption Volume Constitution in Cylindrical Pores
3.2. Construction of Adsorption Equation
4. Discussion
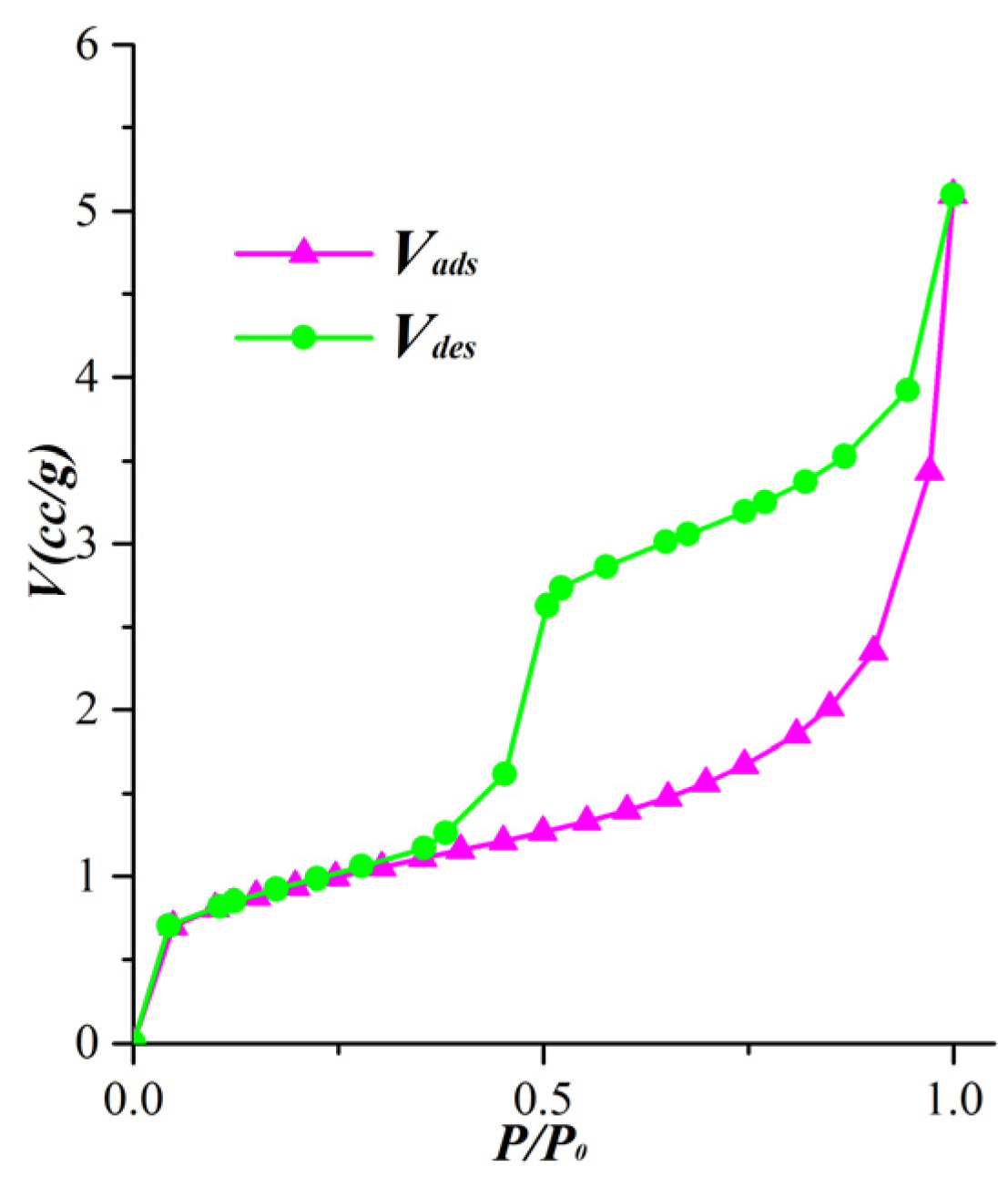
4.1. Adsorption Isotherm Confirmed by Adsorption Equation
4.2. Pore Size Distribution Derived from Adsorption Equation
5. Conclusions
- 1
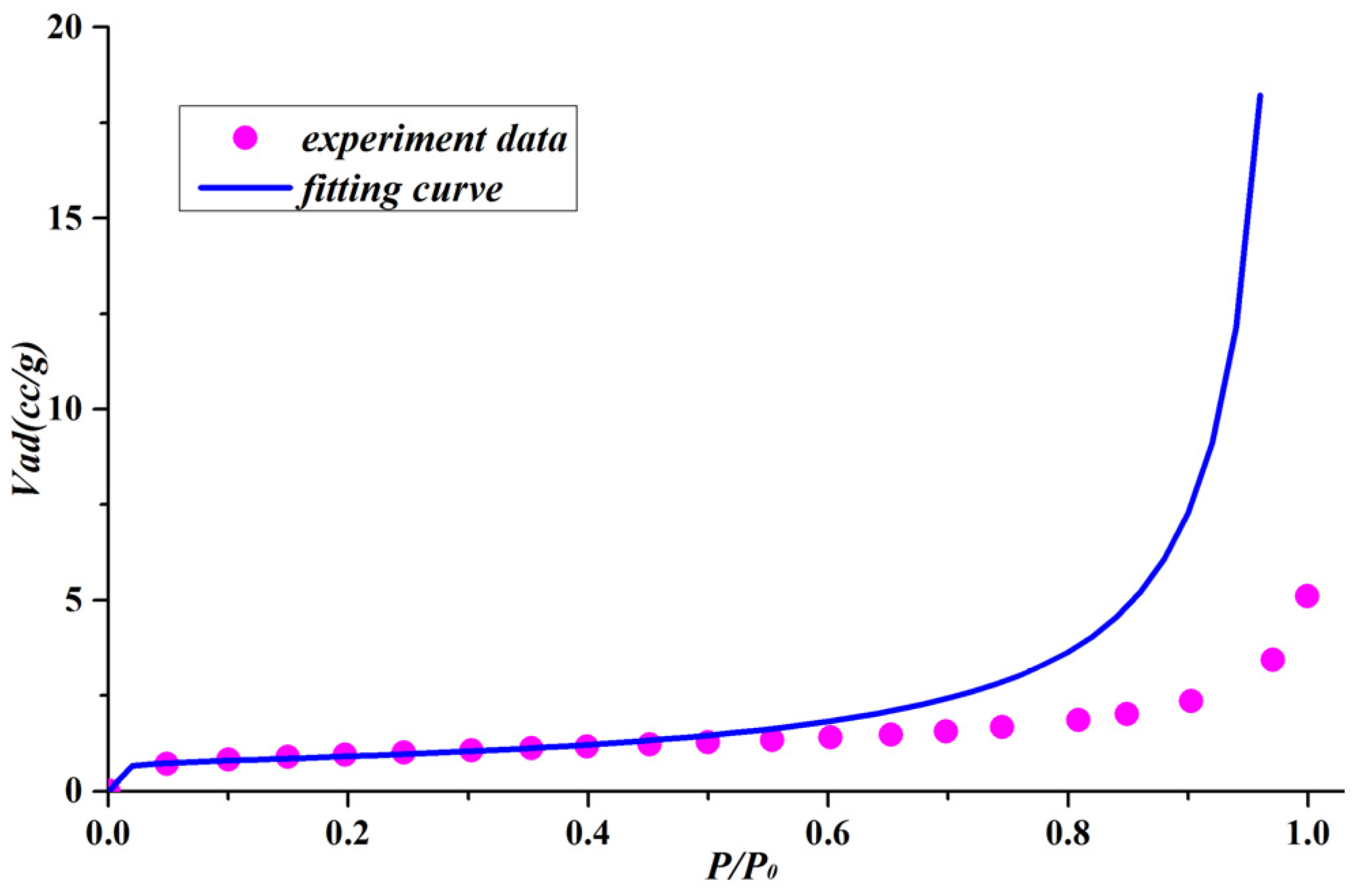
- We propose a cylindrical pore model by simplifying complex pore networks as tubes with different radii. The model considers that the adsorption processes of nitrogen in shale nanopores are monolayer adsorption, multilayer adsorption, and capillary condensation. In addition, a new adsorption equation was derived from the cylindrical pore model by regarding the total adsorption volume as consisting of the inner capillary volume and the multilayer adsorption volume. The fitting results revealed that the adsorption equation is able to properly depict the experimental data for shales with cylindrical pores and slit pores.
- 2
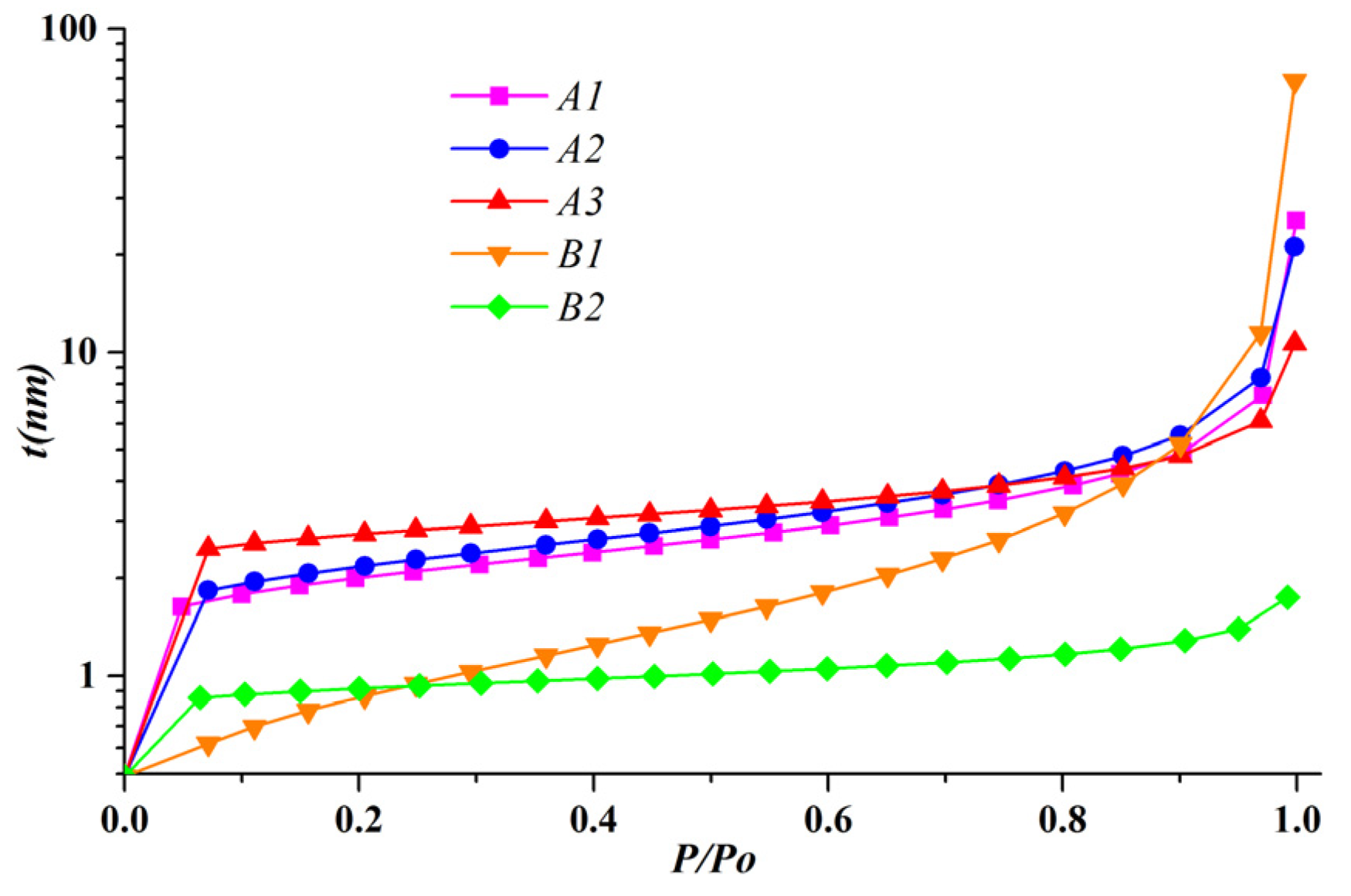
- The t-curves derived from the parameters of the adsorption equation demonstrate the multilayer adsorption behavior in shale nanopores. When the relative pressure is less than 0.9, the microporous shale shows a steadily increasing t-curve because of the micropore filling. However, the inflection points at relative pressure around 0.1 indicate the saturated monolayer adsorption in other samples. When the relative pressure is greater than 0.9, the adsorption on the external interface results in a steep section on the.
- 3
- For the cylindrical pore model, the pore volume is proportional to the inner capillary volume. When the capillary condensation volume at any relative pressure is calculated by the new adsorption isotherm, the pore size distributions of the shales can be determined successfully. In summary, it is a time-saving method to evaluate the pore volume and the dominant pore size.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javadpour, F.; Fisher, D.; Unsworth, M. Nanoscale Gas Flow in Shale Gas Sediments. J. Can. Pet. Technol. 2007, 46, 55–61. [Google Scholar] [CrossRef]
- Pang, Y.; He, Y.; Chen, S. An innovative method to characterize sorption-induced kerogen swelling in organic-rich shales. Fuel 2019, 254, 115629. [Google Scholar] [CrossRef]
- Ross, D.J.; Bustin, R.M. Characterizing the shale gas resource potential of Devonian–Mississippian strata in the Western Canada sedimentary basin: Application of an integrated formation evaluation. AAPG Bull. 2008, 92, 87–125. [Google Scholar] [CrossRef]
- Pang, Y.; Chen, S.; Soliman, M.Y.; Morse, S.M.; Hu, X. Evaluation of Matrix Swelling Behavior in Shale Induced by Methane Sorption under Triaxial Stress and Strain Conditions. Energy Fuels 2019, 33, 4986–5000. [Google Scholar] [CrossRef]
- Yaalon, D.H. Mineral Composition of the Average Shale. Clay Miner. 1962, 5, 31–36. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, H. Formation and enrichment mode of Jiaoshiba shale gas field, Sichuan Basin. Pet. Explor. Dev. 2014, 41, 31–40. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Clarkson, C.; Solano, N.; Bustin, R.; Bustin, A.; Chalmers, G.; He, L.; Melnichenko, Y.; Radliński, A.; Blach, T. Pore structure characterization of North American shale gas reservoirs using USANS/SANS, gas adsorption, and mercury intrusion. Fuel 2013, 103, 606–616. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Y.; Wang, H.; Liu, H.; Wei, W.; Fang, J. Shale gas reservoir characterisation: A typical case in the southern Sichuan Basin of China. Energy 2011, 36, 6609–6616. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, Y.; Li, P.; Yan, C.; Pang, Y.; Zheng, L.; Deng, H.; Zhou, W.; Meng, X. Study on Shale Adsorption Equation Based on Monolayer Adsorption, Multilayer Adsorption, and Capillary Condensation. J. Chem. 2017, 2017, 1496463. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. part 2: Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.; Ockrent, C. Studies in electrocapillarity. III. J. Phys. Chem. 1930, 34, 2841–2859. [Google Scholar] [CrossRef]
- Markham, E.C.; Benton, A.F. The adsorption of gas mixtures by silica. J. Am. Chem. Soc. 1931, 53, 497–507. [Google Scholar] [CrossRef]
- Piñeiro, Á.; Brocos, P.; Amigo, A.; Gracia-Fadrique, J.; Lemus, M.G. Extended Langmuir isotherm for binary liquid mixtures. Langmuir 2001, 17, 4261–4266. [Google Scholar] [CrossRef]
- Besedová, E.; Bobok, D. Single-component and Binary Adsorption Equilibrium of 1,2 Dichloroethane and 1,2 Dichloropropane on Activated Carbon. Pet. Coal 2005, 47, 47–54. [Google Scholar]
- Jain, J.S.; Snoeyink, V.L. Adsorption from bisolute systems on active carbon. J. Water Pollut. Control. Fed. 1973, 45, 2463–2479. [Google Scholar]
- Ho, Y.; McKay, G. Correlative biosorption equilibria model for a binary batch system. Chem. Eng. Sci. 2000, 55, 817–825. [Google Scholar] [CrossRef]
- Toth, J. State equation of the solid-gas interface layers. Acta Chim. Hung. 1971, 69, 311–328. [Google Scholar]
- Terzyk, A.P.; Chatłas, J.; Gauden, P.A.; Rychlicki, G.; Kowalczyk, P. Developing the solution analogue of the Toth adsorption isotherm equation. J. Colloid Interface Sci. 2003, 266, 473–476. [Google Scholar] [CrossRef]
- Jaroniec, M.; Töth, J. Adsorption of gas mixtures on heterogeneous solid surfaces: I. Extension of Tóth isotherm on adsorption from gas mixtures. Colloid Polym. Sci. 1976, 254, 643–649. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Yao, C. Extended and improved Langmuir equation for correlating adsorption equilibrium data. Sep. Purif. Technol. 2000, 19, 237–242. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Wang, G.; Wang, K.; Ren, T. Improved analytic methods for coal surface area and pore size distribution determination using 77 K nitrogen adsorption experiment. Int. J. Min. Sci. Technol. 2014, 24, 329–334. [Google Scholar] [CrossRef]
- Aranovich, G.L.; Donohue, M.D. A New Approach to Analysis of Multilayer Adsorption. J. Colloid Interface Sci. 1995, 173, 515–520. [Google Scholar] [CrossRef]
- Hill, T.L. Theory of Multimolecular Adsorption from a Mixture of Gases. J. Chem. Phys. 1946, 14, 268–275. [Google Scholar] [CrossRef]
- Bussey, B.W. Multicomponent Gas Adsorption of Ideal Mixtures. Ind. Eng. Chem. Fundam. 1966, 5, 103–106. [Google Scholar] [CrossRef]
- Tiren, G. The Comparison of Different BET-Type Mixed Adsorption Equations with Some Experimental Results. J. Chem. Ind. Eng. 1984, 1, 80–84. [Google Scholar]
- Tiren, G. The extended BET adsortion theory in mixture solution. Chemistry 1984, 9, 1–7. [Google Scholar]
- Fisher, L.; Israelachvili, J.N. Direct experimental verification of the Kelvin equation for capillary condensation. Nature 1979, 277, 548–549. [Google Scholar] [CrossRef]
- Fisher, L.; Gamble, R.A.; Middlehurst, J. The Kelvin equation and the capillary condensation of water. Nature 1981, 290, 575–576. [Google Scholar] [CrossRef]
- Wang, R.; Sang, S.; Zhu, D.; Liu, S.; Yu, K. Pore characteristics and controlling factors of the Lower Cambrian Hetang Formation shale in Northeast Jiangxi, China. Energy Explor. Exploit. 2017, 36, 43–65. [Google Scholar] [CrossRef]
- Voigt, E.M.; Tomlinson, R.H. The determination of pore size distribution and surface area from ADSO. Can. J. Chem. 1955, 33, 215–231. [Google Scholar] [CrossRef] [Green Version]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Wang, Y.; Do, D.; Nicholson, D. Study of heat of adsorption across the capillary condensation in cylindrical pores. Colloids Surf. A Physicochem. Eng. Asp. 2011, 380, 66–78. [Google Scholar] [CrossRef]
- Broekhof, J.C.; van Beek, W.P. Scanning studies on capillary condensation and evaporation of nitrogen. Part 1—Apparatus and calculation method. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1979, 75, 36–41. [Google Scholar] [CrossRef]
- Fan, C.; Do, D.D.; Nicholson, D. Condensation and Evaporation in Capillaries with Nonuniform Cross Sections. Ind. Eng. Chem. Res. 2013, 52, 14304–14314. [Google Scholar] [CrossRef]
- Vishnyakov, A.; Neimark, A.V. Monte Carlo Simulation Test of Pore Blocking Effects. Langmuir 2003, 19, 3240–3247. [Google Scholar] [CrossRef]
- Cohan, L.H. Sorption Hysteresis and the Vapor Pressure of Concave Surfaces. J. Am. Chem. Soc. 1938, 60, 433–435. [Google Scholar] [CrossRef]
- Zsigmondy, R. Structure of silicic acid gels and theory of dehydration. Z. Anorg. Chem. 1911, 71, 356–377. [Google Scholar] [CrossRef] [Green Version]
- Taylor’s, E.K.H. A Treatise on Physical Chemistry; Van Nostrand: New York, NY, USA, 1931; p. 1661. [Google Scholar]
- Nguyen, P.T.; Do, D.; Nicholson, D. Simulation study of hysteresis of argon adsorption in a conical pore and a constricted cylindrical pore. J. Colloid Interface Sci. 2013, 396, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Marconi, U.M.B.; Tarazona, P. Capillary condensation and adsorption in cylindrical and slit-like pores. J. Chem. Soc. Faraday Trans. 1986, 82, 1763–1787. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Ciach, A.; Neimark, A.V. Adsorption-Induced Deformation of Microporous Carbons: Pore Size Distribution Effect. Langmuir 2008, 24, 6603–6608. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Furmaniak, S.; Harris, P.J.; Gauden, P.A.; Włoch, J.; Kowalczyk, P.; Rychlicki, G. How realistic is the pore size distribution calculated from adsorption isotherms if activated carbon is composed of fullerene-like fragments? Phys. Chem. Chem. Phys. 2007, 9, 5919–5927. [Google Scholar] [CrossRef]
- de Oliveira, J.C.A.; López, R.H.; Toso, J.P.; Lucena, S., Jr.; Azevedo, D.; Zgrablich, G. On the influence of heterogeneity of graphene sheets in the determination of the pore size distribution of activated carbons. Adsorption 2011, 17, 845–851. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Horváth, G.; Kawazoe, K. Method for the calculation of effective pore size distribution in molecular sieve carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.S.; Yang, R.T. Improved Horvath-Kawazoe Equations Including Spherical Pore Models for Calculating Micropore Size Distribution. Chem. Eng. Sci. 1994, 49, 2599–2609. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T. Corrected Horváth-Kawazoe equations for pore-size distribution. AIChE J. 2000, 46, 734–750. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Lin, M. A Novel Method for Analyzing Pore Size Distribution of Complex Geometry Shaped Porous Shale. Mater. Sci. Forum 2020, 1003, 134–143. [Google Scholar]
- Hashemipour, H.; Haghighatju, F.; Esmaeilzadeh, F. Estimation of the dimension of micropores and mesopores in single walled carbon nanotubes using the method Horvath and Kawazoe, Saito and Foley and BJH equations. Micro Nano Lett. 2016, 12, 1–5. [Google Scholar]
- Yang, W.; He, S.; Iglauer, S.; Guo, X.; Zhai, G.; Zhou, Z.; Dong, T.; Tao, Z.; Wei, S. Porosity characteristics of different lithofacies in marine shale: A case study of Neoproterozoic Sinian Doushantuo formation in Yichang area, China. J. Pet. Sci. Eng. 2019, 187, 106856. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Gong, D.; Tan, J.; Liu, Y.; Wang, D.; Li, P.; Tong, Z.; Niu, J. Gas-bearing property of the Lower Cambrian Niutitang Formation shale and its influencing factors: A case study from the Cengong block, northern Guizhou Province, South China. Mar. Pet. Geol. 2020, 120, 104556. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, C.; Li, X.; Zhang, K.; Yuan, Y.; Zang, X.; Cui, W.; Liu, S.; Jiang, Z. Organic matter pores structure and evolution in shales based on the he ion microscopy (HIM): A case study from the Triassic Yanchang, Lower Silurian Longmaxi and Lower Cambrian Niutitang shales in China. J. Nat. Gas Sci. Eng. 2020, 84, 103682. [Google Scholar] [CrossRef]
- Halsey, G. Physical adsorption on non-uniform surfaces. J. Chem. Phys. 1948, 16, 931–937. [Google Scholar] [CrossRef]
- Grosman, A.; Ortega, C. Capillary Condensation in Porous Materials. Hysteresis and Interaction Mechanism without Pore Blocking/Percolation Process. Langmuir 2008, 24, 3977–3986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Boer, J.H.; Lippens, B.C. Studies on pore systems in catalysts II. The shapes of pores in aluminum oxide systems. J. Catal. 1964, 3, 38–43. [Google Scholar] [CrossRef]
- Derjaguin, B. A theory of capillary condensation in the pores of sorbents and of other capillary phenomena taking into account the disjoining action of polymolecular liquid films. Prog. Surf. Sci. 1992, 40, 46–61. [Google Scholar] [CrossRef]
- Dietrich, S. Phase Transitions and Critical Phenomena; Academic Press: Cambridge, MA, USA, 1988; Volume 12, p. 1. [Google Scholar]
- Wallacher, D.; Künzner, N.; Kovalev, D.; Knorr, N.; Knorr, K. Capillary Condensation in Linear Mesopores of Different Shape. Phys. Rev. Lett. 2004, 92, 195704. [Google Scholar] [CrossRef]
- Morishige, K.; Tateishi, N. Adsorption hysteresis in ink-bottle pore. J. Chem. Phys. 2003, 119, 2301–2306. [Google Scholar] [CrossRef]
- Morishige, K.; Kittaka, S. Kinetics of Capillary Condensation of Water in Mesoporous Carbon: Nucleation and Meniscus Growth. J. Phys. Chem. C 2015, 119, 18287–18292. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
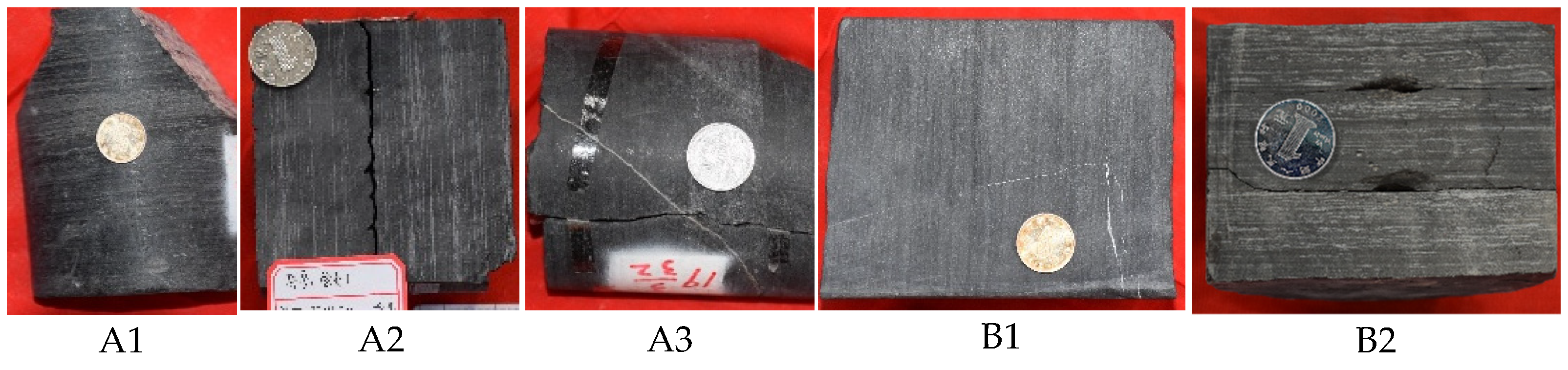

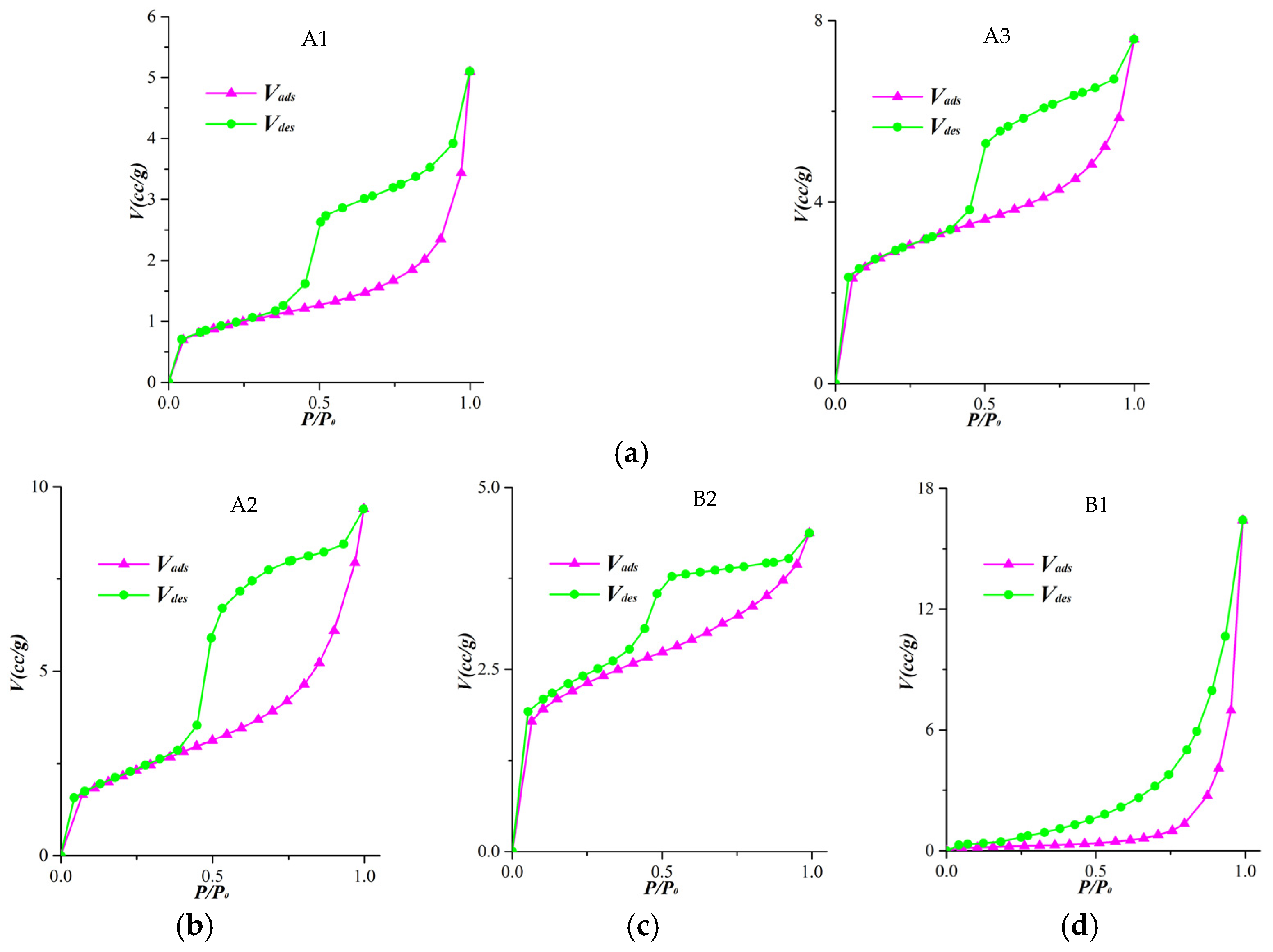
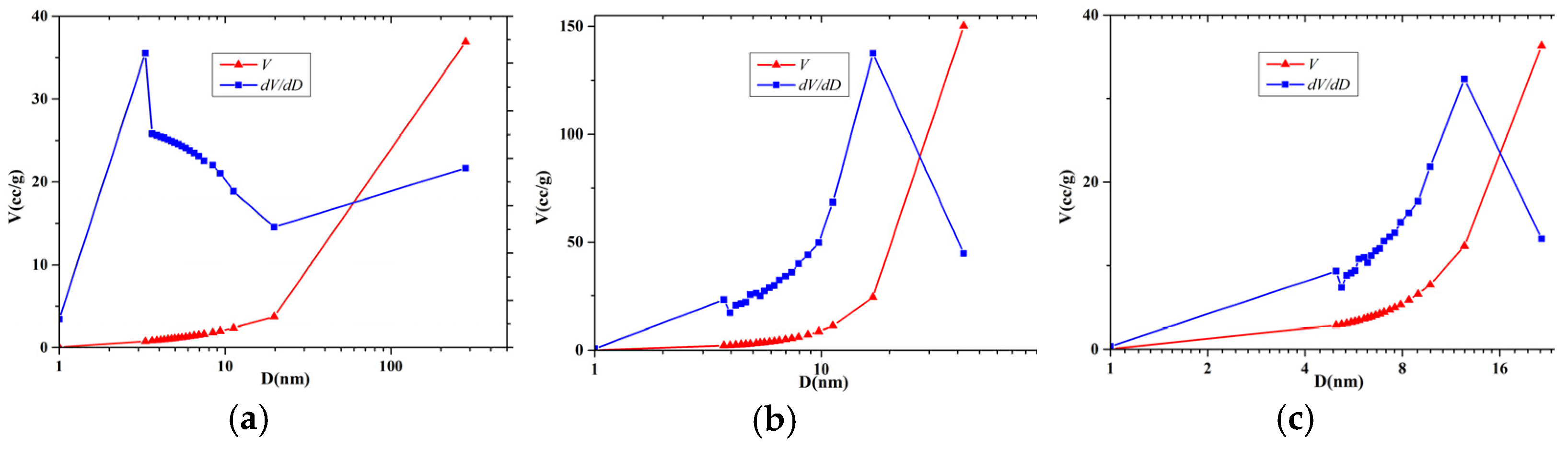
| Sample ID | Basin | Lithology | Formation |
|---|---|---|---|
| A1 | Sichuan basin | Dark-black shale | Niutitang |
| A2 | Ordos basin | Dark-black shale | Yanchang7 |
| A3 | Sichuan basin | Black shale | Doushantuo |
| B1 | Ordos basin | Pale-black shale | Yanchang7 |
| B2 | Sichuan basin | Pale-black shale | Niutitang |
| Sample | Vm | A | B | C | R-Square |
|---|---|---|---|---|---|
| A1 | 0.16835 | 6.61549 | 0.32524 | 9.53452 | 0.98719 |
| A2 | 0.365124 | 7.235891 | 0.34235 | 9.45812 | 0.95000 |
| A3 | 0.38336 | 8.52541 | 0.20451 | 9.82451 | 0.97331 |
| B1 | 0.20359 | 3.31599 | 0.65949 | 8.85840 | 0.76594 |
| B2 | 0.90222 | 2.74084 | 0.12231 | 8.79350 | 0.73911 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Chen, Q.; Yan, C.; Chen, H.; He, Y.; He, Y. A New Adsorption Equation for Nano-Porous Shale Rocks and Its Application in Pore Size Distribution Analysis. Energies 2022, 15, 3199. https://doi.org/10.3390/en15093199
Tian Y, Chen Q, Yan C, Chen H, He Y, He Y. A New Adsorption Equation for Nano-Porous Shale Rocks and Its Application in Pore Size Distribution Analysis. Energies. 2022; 15(9):3199. https://doi.org/10.3390/en15093199
Chicago/Turabian StyleTian, Yuanyuan, Qing Chen, Changhui Yan, Hongde Chen, Yanqing He, and Yufeng He. 2022. "A New Adsorption Equation for Nano-Porous Shale Rocks and Its Application in Pore Size Distribution Analysis" Energies 15, no. 9: 3199. https://doi.org/10.3390/en15093199
APA StyleTian, Y., Chen, Q., Yan, C., Chen, H., He, Y., & He, Y. (2022). A New Adsorption Equation for Nano-Porous Shale Rocks and Its Application in Pore Size Distribution Analysis. Energies, 15(9), 3199. https://doi.org/10.3390/en15093199





