A Review of the Impact of Hydrogen Integration in Natural Gas Distribution Networks and Electric Smart Grids
Abstract
:1. Introduction
2. Hydrogen Storage Technologies
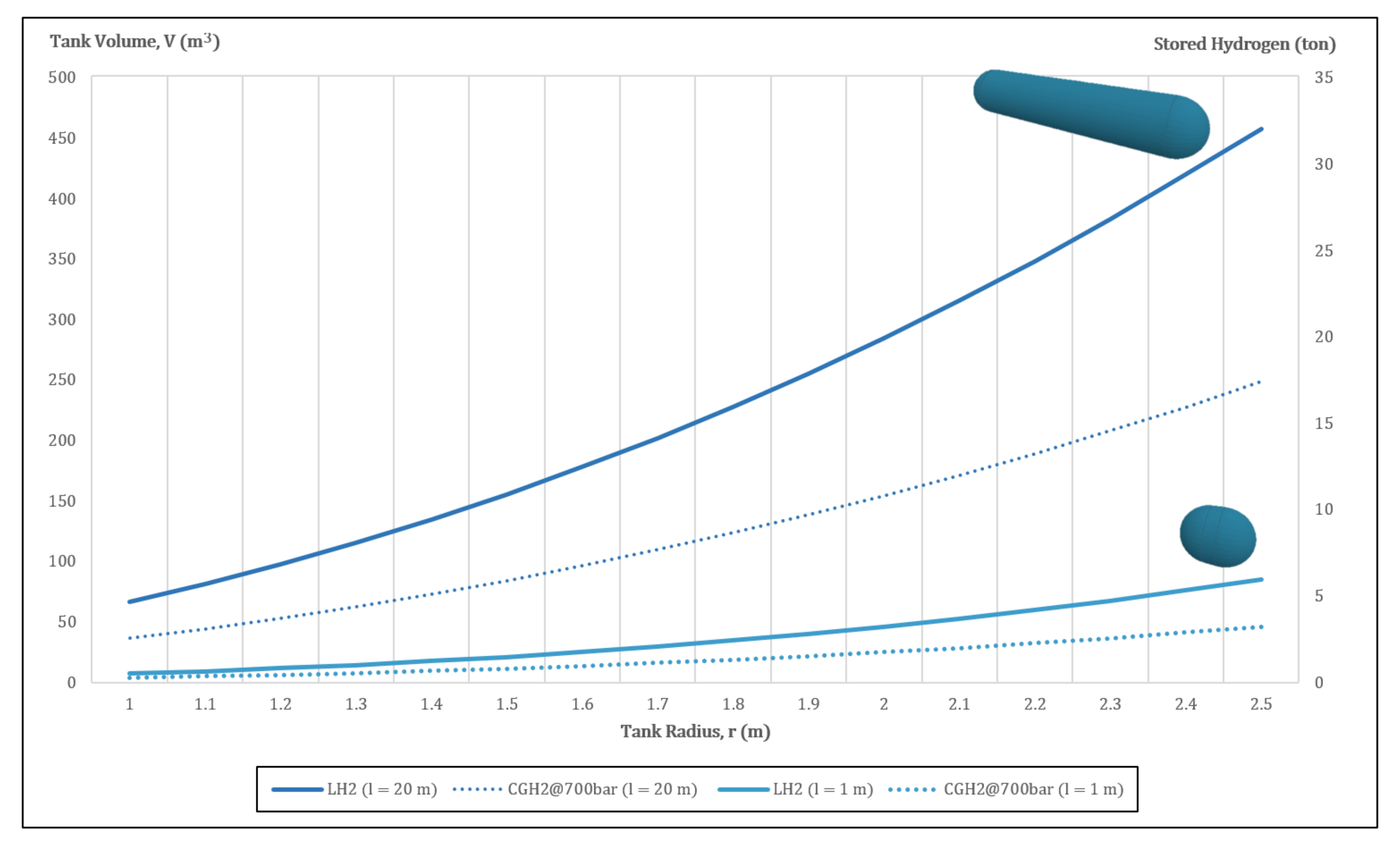
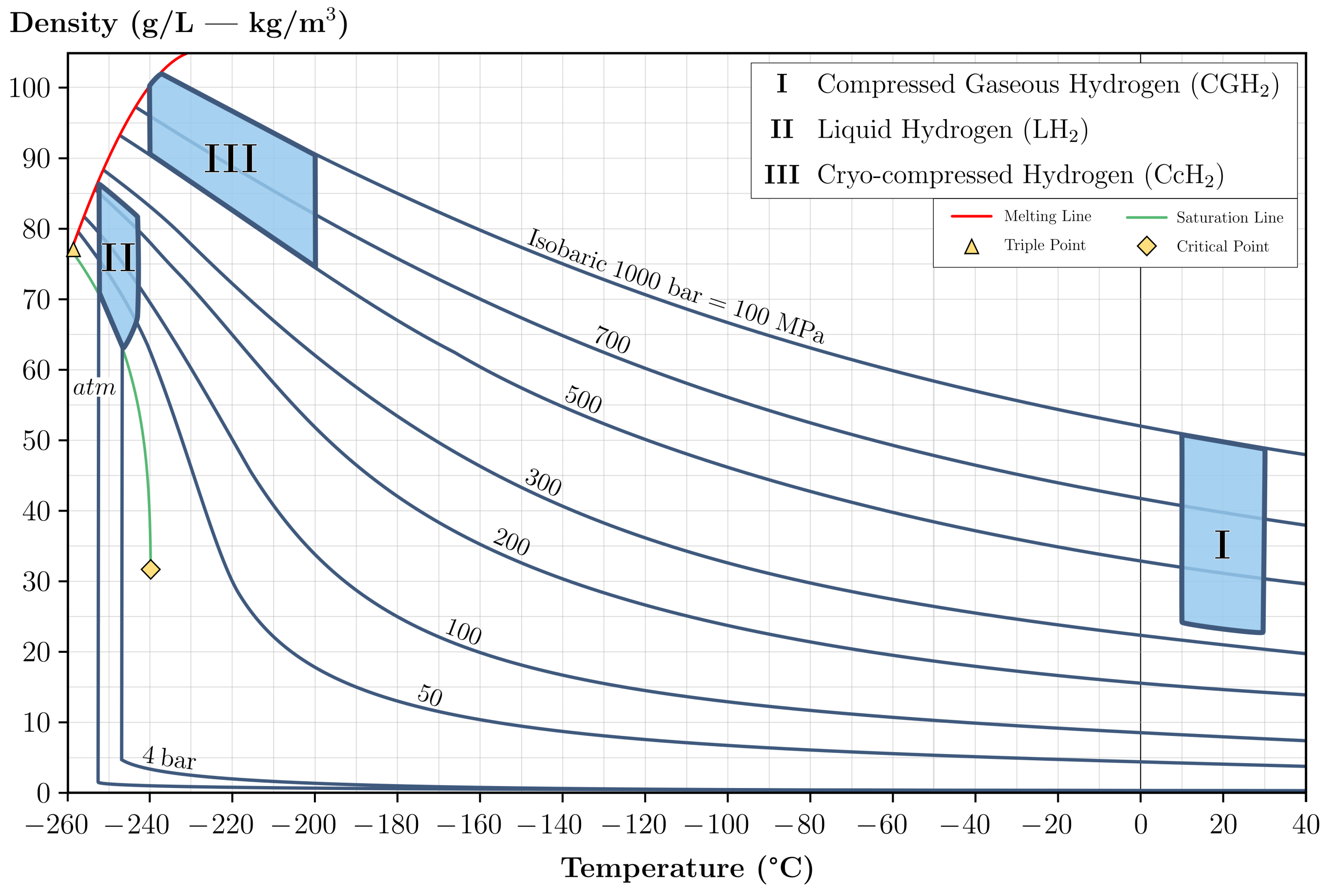
2.1. Compressed Gaseous Hydrogen
2.2. Liquid Hydrogen
- Vacuum insulation. While a perfect vacuum may seem to be the best solution to eliminate the boil-off effect, it is also extremely difficult to achieve; as demonstrated by A. Colozza [28], peripheral equipment and venting devices are required to actively maintain the vacuum region. A vacuum chamber also raises issues about the thickness of the tank walls—they must be wide enough to withstand the buckling effect caused by external ambient pressure; however, as Millis et al. [29] reiterated, large walls in conjunction with additional stiffeners (required in-between the vacuum jacket shell) will inevitably increase the overall weight of the tank.
- Foam insulation. Despite not having insulation levels as good as those of vacuum chambers, foam insulation is a passive technique, easier and cheaper to implement. Usually, these foams are very light, have low density, and low thermal conductivity; in his PhD thesis, I. Cumalioglu [30] studied how these are applied, sandwiched between two metal plaques, in order to improve structural stability and to better protect against external forces and impacts. Another great advantage of foam insulation over vacuum-jacketed insulation is its resistance to catastrophic failure; a pierced vacuum chamber would probably cause the overall collapse of the insulation layer, whereas foam insulation would not [26].
- Multi-layer insulation. This method can be seen more as a compliment the previous two rather than as an alternative—although it is also possible to implement it as a stand-alone. Multi-layer insulation systems are commonly a set of reflective foil made of thermal radiation shields aligned perpendicularly to the direction of the heat flux; they are usually wrapped over the external layer of the tank in order to impede radiation heat transfer. These radiation shields mostly consist of alternate layers of thin aluminum foil and an insulating material such as fiberglass or polyester [8]. While additional layers may improve radiation heat insulation, it also increases heat transfer by conduction; to cite Khandelwal and his co-authors [12], the optimal recommended number of layers is then between 60 and 100. Naturally, more layers will also add to the overall weight of the tank. Another drawback of multi-layer insulation sheets is their sensitivity to pressure gradients during manufacturing, as studied by Allideris and Janin [16]; this leads to the need for a very specialized type of production, which is more expensive.Further attention needs to be given to damage use accumulation resultant from external loads such as impacts during operation or unpredictable accidents, which can lead to fiber breaks, delamination, and matrix cracking [8].
2.3. Cryo-Compressed Hydrogen
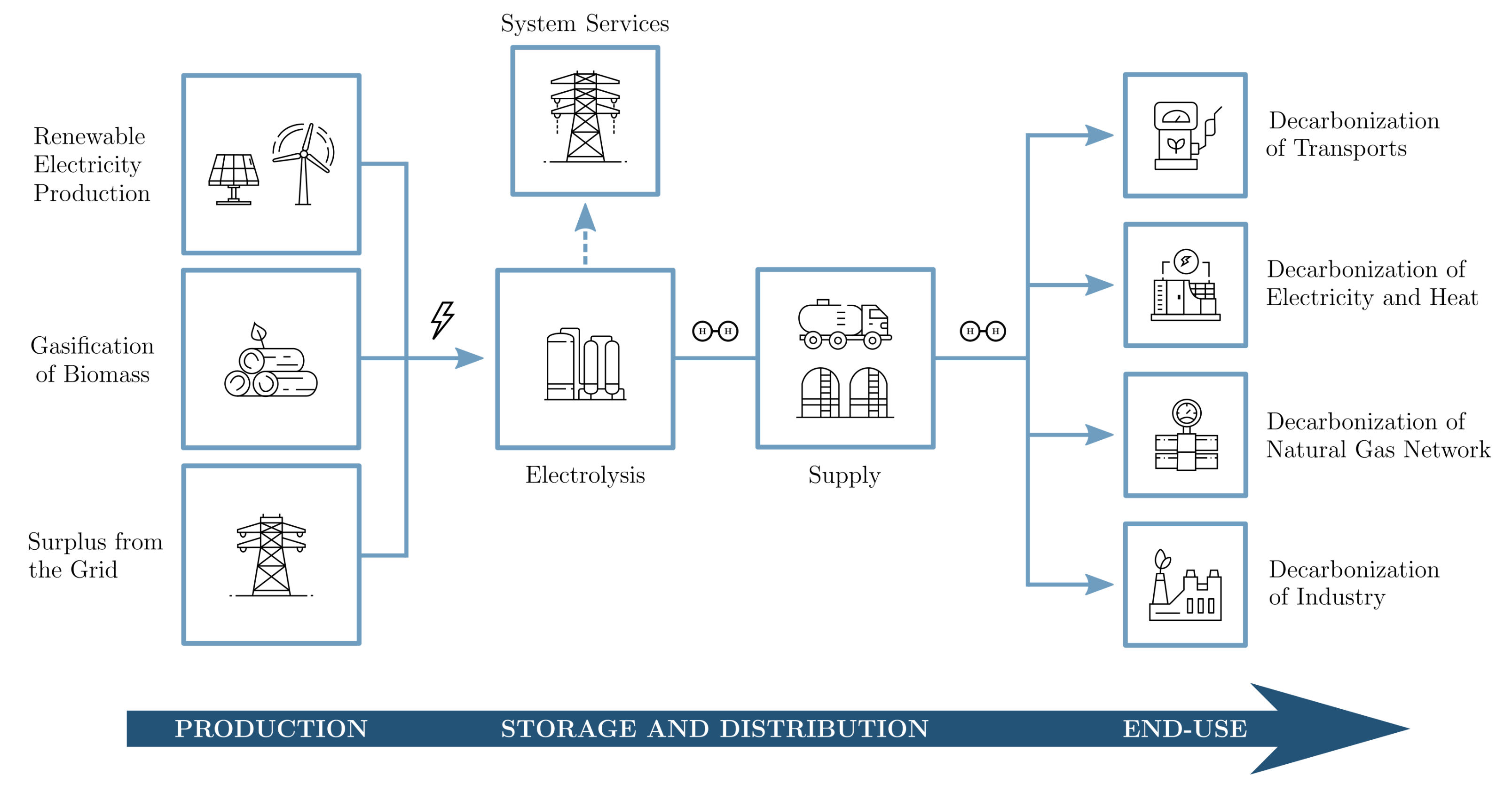
3. Hydrogen Integration in Power-to-Gas Networks
3.1. Evaluation of Gas Transmission Systems
3.2. Evaluation of Gas Storage Methods
3.3. Evaluation of Gas Distribution Systems
3.4. Evaluation of End-Use Applications
4. Hydrogen Integration in Power-to-Power Smart Grids
4.1. Hydrogen Application in Microgrids
4.2. Hydrogen Economy in Smart Grids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CcH | Cryo-compressed Hydrogen |
| CGH | Compressed Gaseous Hydrogen |
| CNG | Compressed Natural Gas |
| DSM | Demand Side Management |
| H | Hydrogen |
| HCV | Higher Calorific Value |
| LH | Liquid Hydrogen |
| NG | Natural Gas |
| NGI | Natural Gas Infrastructure |
| P2G | Power-to-Gas |
| P2P | Power-to-Power |
| ZEH2E | Zero-Energy Hydrogen Economy |
References
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Commission, E. Hydrogen Strategy for a Climate-Neutral Europe. 2020. Available online: https://ec.europa.eu/energy/sites/ener/files/hydrogen_strategy.pdf (accessed on 16 February 2022).
- Vidas, L.; Castro, R. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with a Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Legault, M. The First Commercial Type V Composite Pressure Vessel. 2012. Available online: https://www.compositesworld.com/articles/next-generation-pressure-vessels (accessed on 13 January 2022).
- Fradkov, A.; Troitskii, V. Liquefier with two-stage conversion to obtain 98 per cent parahydrogen. Cryogenics 1965, 5, 136–137. [Google Scholar] [CrossRef]
- Granath, B. Liquid Hydrogen—The Fuel of Choice for Space Exploration. 2015. Available online: https://www.nasa.gov/content/liquid-hydrogen-the-fuel-of-choice-for-space-exploration (accessed on 8 December 2021).
- Khandelwal, B.; Karakurt, A.; Sekaran, P.R.; Sethi, V.; Singh, R. Hydrogen powered aircraft: The future of air transport. Prog. Aerosp. Sci. 2013, 60, 45–59. [Google Scholar] [CrossRef]
- Kawasaki. Kawasaki Hydrogen Road. Available online: https://global.kawasaki.com/en/hydrogen/ (accessed on 8 December 2021).
- Qiu, Y.; Yang, H.; Tong, L.; Wang, L. Research Progress of Cryogenic Materials for Storage and Transportation of Liquid Hydrogen. Metals 2021, 11, 1101. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Wei, J.; Lan, Y.; Yang, S.; Jin, T. Evaluation and prediction of the safe distance in liquid hydrogen spill accident. Process Saf. Environ. Prot. 2021, 146, 1–8. [Google Scholar] [CrossRef]
- Allideris, L.; Janin, F. Fuel System Components-Mechanical Tank Design Trade-Off, Technical Report, Task Technical Report 3.6.2.2, Cryoplane Project. 2002.
- Mital, S.K.; Gyekenyesi, J.Z.; Arnold, S.M.; Sullivan, R.M.; Manderscheid, J.M.; Murthy, P.L. Review of Current State of the Art and Key Design Issues with Potential Solutions for Liquid Hydrogen Cryogenic Storage Tank Structures for Aircraft Applications; Aerospace Engineering Papers; NASA: Washington, DC, USA, 2006. [Google Scholar]
- Brewer, G.D. Hydrogen Aircraft Technology; Routledge: London, UK, 2017. [Google Scholar]
- Haberbusch, M.S.; Stochl, R.J.; Culler, A.J. Thermally optimized zero boil-off densified cryogen storage system for space. Cryogenics 2004, 44, 485–491. [Google Scholar] [CrossRef]
- ToolBox, E. Fuels—Higher and Lower Calorific Values. Available online: https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html (accessed on 6 January 2022).
- Sherif, S.; Zeytinoglu, N.; Veziroǧlu, T. Liquid hydrogen: Potential, problems, and a proposed research program. Int. J. Hydrogen Energy 1997, 22, 683–688. [Google Scholar] [CrossRef]
- Yang, C.; Ogden, J. Determining the lowest-cost hydrogen delivery mode. Int. J. Hydrogen Energy 2007, 32, 268–286. [Google Scholar] [CrossRef] [Green Version]
- Notardonato, W.U.; Swanger, A.M.; Fesmire, J.E.; Jumper, K.M.; Johnson, W.L.; Tomsik, T.M. Zero boil-off methods for large-scale liquid hydrogen tanks using integrated refrigeration and storage. IOP Conf. Ser. Mater. Sci. Eng. 2017, 278, 012012. [Google Scholar] [CrossRef]
- Wiley, J. Front Matter. In Hydrogen Storage Technologies; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. I–IX. [Google Scholar] [CrossRef]
- Ho, S.H.; Rahman, M.M. Nozzle injection displacement mixing in a zero boil-off hydrogen storage tank. Int. J. Hydrogen Energy 2008, 33, 878–888. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Reijerkerk, J. Potential of cryogenic hydrogen storage in vehicles. In Alternative fuels; Linde AG: Hoellriegelskreuth, Germany, 2004. [Google Scholar]
- Colozza, A.J.; Kohout, L. Hydrogen Storage for Aircraft Applications Overview; National Aeronautics and Space Administration, Glenn Research Center: Cleveland, OH, USA, 2002. [Google Scholar]
- Millis, M.G.; Tornabene, R.T.; Jurns, J.M.; Guynn, M.D.; Tomsik, T.M.; VanOverbeke, T.J. Hydrogen Fuel System Design Trades for High-Altitude Long-Endurance Remotely-Operated Aircraft. 2009. Available online: https://ntrs.nasa.gov/api/citations/20090013674/downloads/20090013674.pdf (accessed on 16 January 2022).
- Cumalioglu, I. Modeling and Simulation of a High Pressure Hydrogen Storage Tank with Dynamic Wall. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 2005. [Google Scholar]
- Kamiya, S.; Nishimura, M.; Harada, E. Study on Introduction of CO2 Free Energy to Japan with Liquid Hydrogen. Phys. Procedia 2015, 67, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Elberry, A.M.; Thakur, J.; Santasalo-Aarnio, A.; Larmi, M. Large-scale compressed hydrogen storage as part of renewable electricity storage systems. Int. J. Hydrogen Energy 2021, 46, 15671–15690. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Zhang, J.; Akiyama, E.; Wang, Y.; Song, X. Review of hydrogen embrittlement in metals: Hydrogen diffusion, hydrogen characterization, hydrogen embrittlement mechanism and prevention. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 759–773. [Google Scholar] [CrossRef]
- Petitpas, G.; Aceves, S. Modeling of sudden hydrogen expansion from cryogenic pressure vessel failure. Int. J. Hydrogen Energy 2013, 38, 8190–8198. [Google Scholar] [CrossRef] [Green Version]
- El-Eskandarany, M.S. Solid-state hydrogen storage nanomaterials for fuel cell applications. In Mechanical Alloying, 3rd ed.; William Andrew Publishing: Norwich, NY, USA, 2020; pp. 229–261. [Google Scholar]
- Aceves, S.M.; Espinosa-Loza, F.; Ledesma-Orozco, E.; Ross, T.O.; Weisberg, A.H.; Brunner, T.C.; Kircher, O. High-density automotive hydrogen storage with cryogenic capable pressure vessels. Int. J. Hydrogen Energy 2010, 35, 1219–1226. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Hua, T.; Peng, J.K.; Lasher, S.; McKenney, K.; Sinha, J.; Gardiner, M. Technical assessment of cryo-compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Energy 2010, 35, 4171–4184. [Google Scholar] [CrossRef]
- Sdanghi, G.; Maranzana, G.; Celzarda, A.; Fierro, V. Review of the current technologies and performances of hydrogen compression for stationary and automotive applications. Renew. Sustain. Energy Rev. 2019, 102, 150–170. [Google Scholar] [CrossRef]
- Moreno-Blanco, J.; Petitpas, G.; Espinosa-Loza, F.; Elizalde-Blancas, F.; Martinez-Frias, J.; Aceves, S.M. The storage performance of automotive cryo-compressed hydrogen vessels. Int. J. Hydrogen Energy 2019, 44, 16841–16851. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Bonadio, L. Fuels—Liquid hydrogen storage. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009; pp. 421–439. [Google Scholar]
- Stetson, N.; McWhorter, S.; Ahn, C. 1—Introduction to hydrogen storage. In Compendium of Hydrogen Energy; Gupta, R.B., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2016; pp. 3–25. [Google Scholar] [CrossRef]
- Baetcke, L.; Kaltschmitt, M. Chapter 5—Hydrogen Storage for Mobile Application: Technologies and Their Assessment. In Hydrogen Supply Chains; Azzaro-Pantel, C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 167–206. [Google Scholar] [CrossRef]
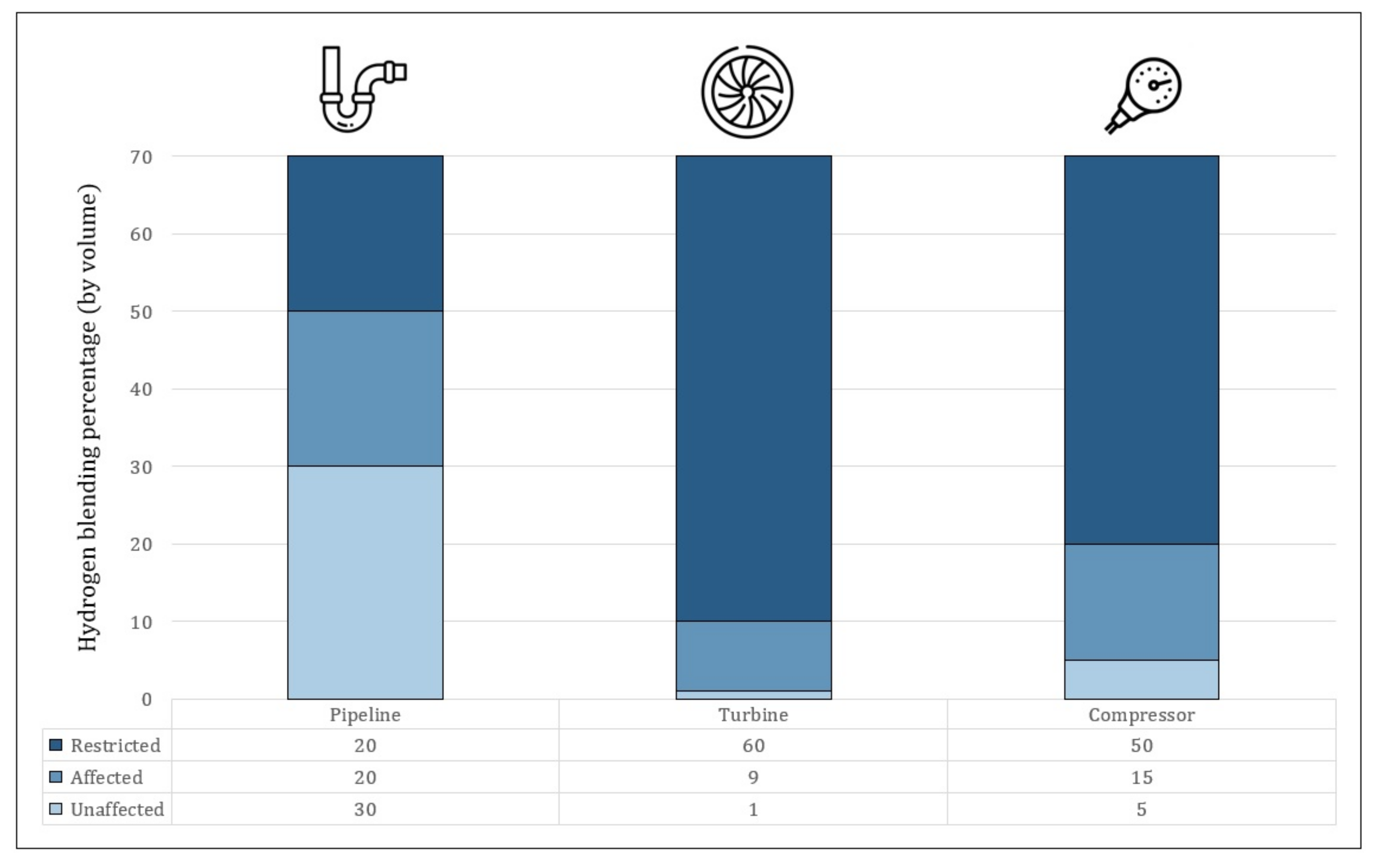
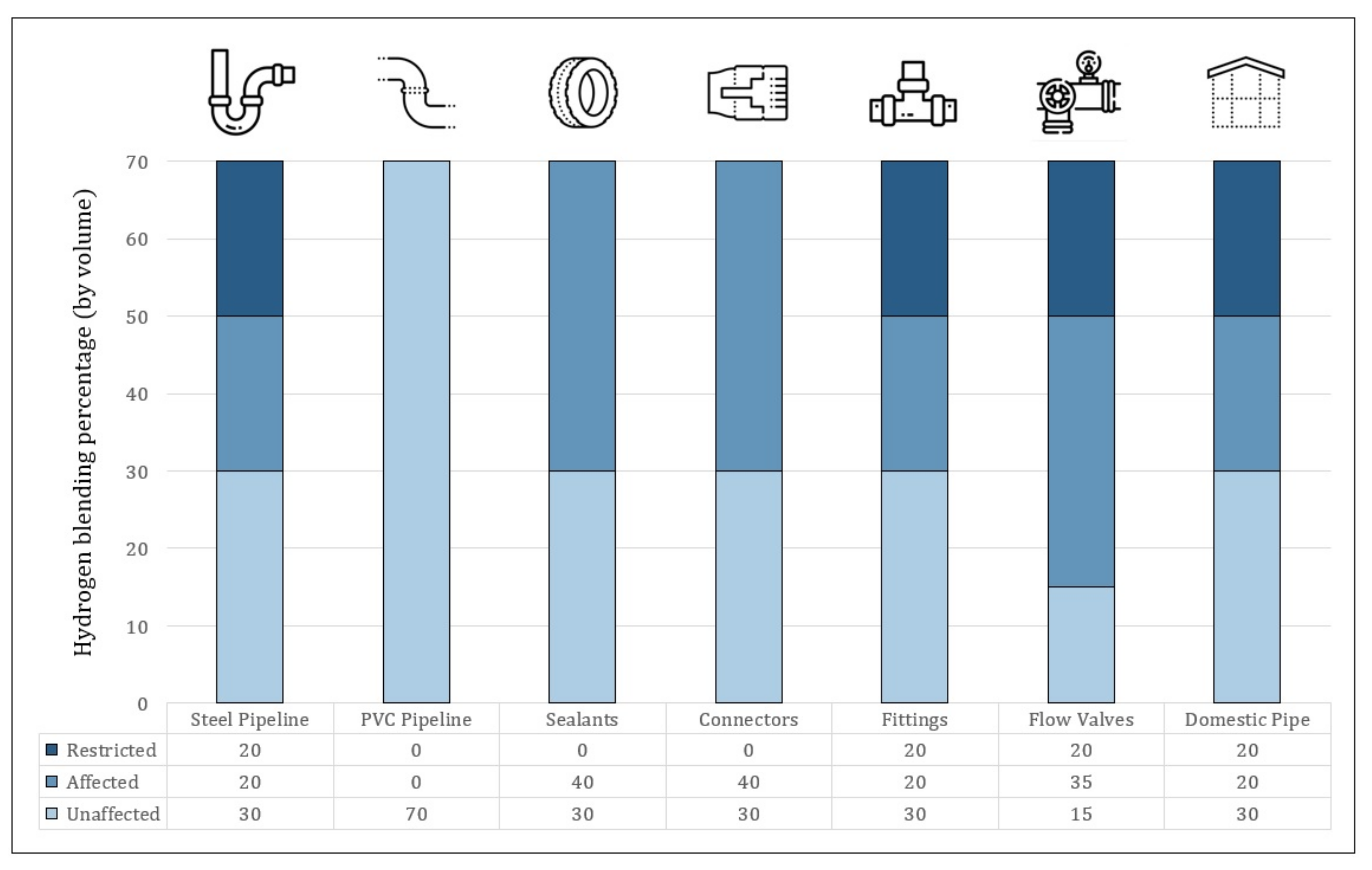
- Gondal, I.A. Hydrogen integration in power-to-gas networks. Int. J. Hydrogen Energy 2019, 44, 1803–1815. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues. 2013. Available online: https://www.energy.gov/sites/default/files/2014/03/f11/blending_h2_nat_gas_pipeline.pdf (accessed on 16 February 2022).
- Jaffe, A.M.; Ogden, J. Is Natural Gas the Transition Fuel for Hydrogen? 2014. Available online: https://www.cfr.org/blog/natural-gas-transition-fuel-hydrogen (accessed on 20 February 2022).
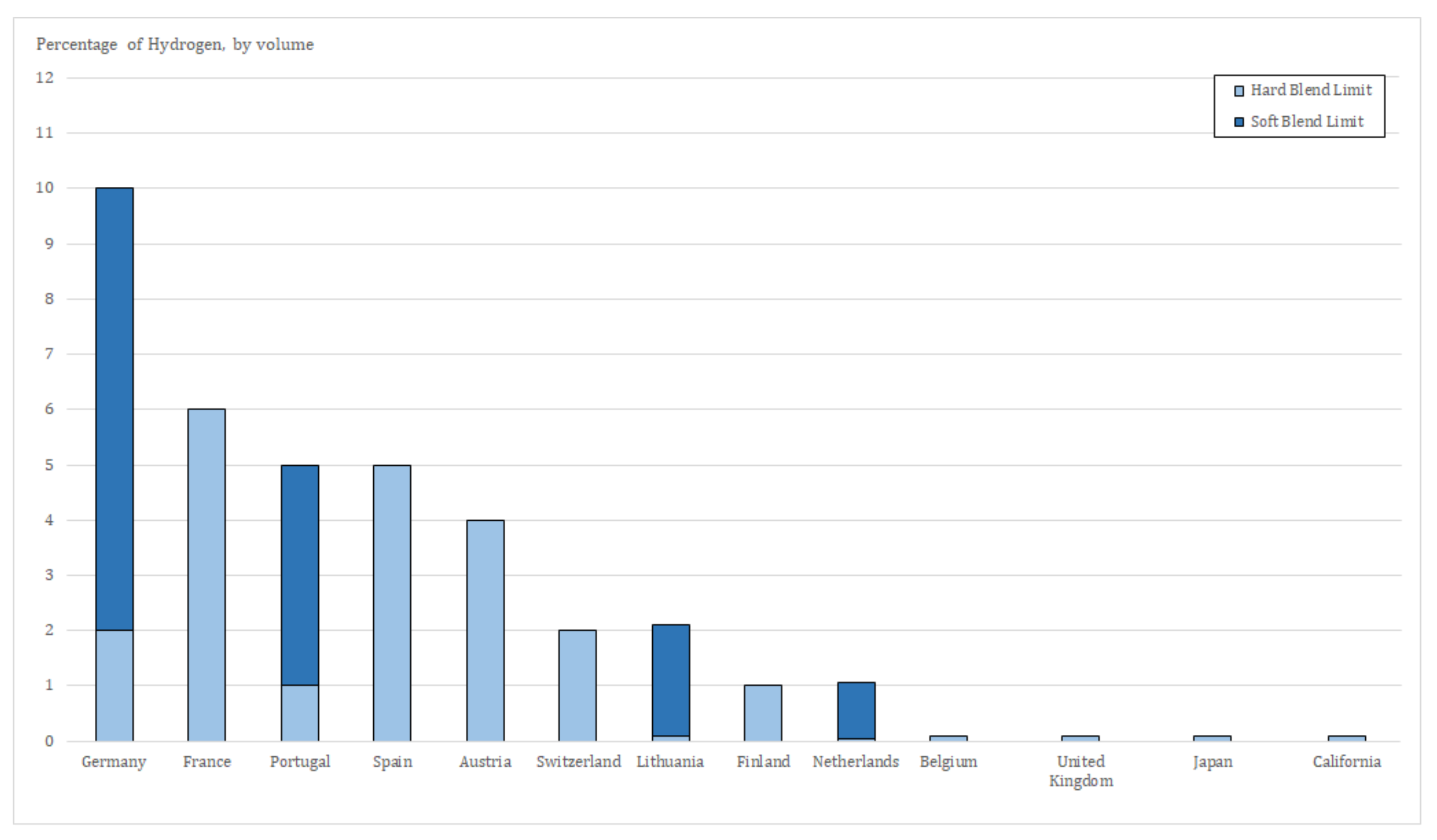
- Agency, I.E. Current Limits on Hydrogen Blending in Natural Gas Networks and Gas Demand per Capita in Selected Locations. Available online: https://www.iea.org/data-and-statistics/charts/current-limits-on-hydrogen-blending-in-natural-gas-networks-and-gas-demand-per-capita-in-selected-locations (accessed on 7 February 2022).
- EN-H2—Estratégia Nacional para o Hidrogénio. 2020. Available online: https://participa.pt/contents/consultationdocument/Estrate%CC%81gia%20Nacional%20para%20o%20Hidroge%CC%81nio%20DRAFT%20publicac%CC%A7ao.pdf (accessed on 20 November 2021).
- Qadrdan, M.; Abeysekera, M.; Chaudry, M.; Wu, J.; Jenkins, N. Role of power-to-gas in an integrated gas and electricity system in Great Britain. Int. J. Hydrogen Energy 2015, 40, 5763–5775. [Google Scholar] [CrossRef]
- Korb, B.; Kawauchi, S.; Wachtmeister, G. Influence of hydrogen addition on the operating range, emissions and efficiency in lean burn natural gas engines at high specific loads. Fuel 2016, 164, 410–418. [Google Scholar] [CrossRef]
- Mukherjee, U.; Elsholkami, M.; Walker, S.; Fowler, M.; Elkamel, A.; Hajimiragha, A. Optimal sizing of an electrolytic hydrogen production system using an existing natural gas infrastructure. Int. J. Hydrogen Energy 2015, 40, 9760–9772. [Google Scholar] [CrossRef]
- Lo Basso, G.; de Santoli, L.; Albo, A.; Nastasi, B. H2NG (hydrogen-natural gas mixtures) effects on energy performances of a condensing micro-CHP (combined heat and power) for residential applications: An expeditious assessment of water condensation and experimental analysis. Energy 2015, 84, 397–418. [Google Scholar] [CrossRef]
- labidine Messaoudani, Z.; Rigas, F.; Binti Hamid, M.D.; Che Hassan, C.R. Hazards, safety and knowledge gaps on hydrogen transmission via natural gas grid: A critical review. Int. J. Hydrogen Energy 2016, 41, 17511–17525. [Google Scholar] [CrossRef]
- de Vries, H.; Mokhov, A.V.; Levinsky, H.B. The impact of natural gas/hydrogen mixtures on the performance of end-use equipment: Interchangeability analysis for domestic appliances. Appl. Energy 2017, 208, 1007–1019. [Google Scholar] [CrossRef]
- Müller-Syring, G.; Henel, M.; Köppel, W.; Mlaker, H.; Sterner, M.; Höcher, T. Entwicklung von Modularen Konzepten zur Erzeugung, Speicherung und Einspeisung von Wasserstoff und Methan ins Erdgasnetz; DVGW Deutscher Verein des Gas-und Wasserfaches eV: Bonn, Germany, 2013. [Google Scholar]
- Taamallah, S.; Vogiatzaki, K.; Alzahrani, F.; Mokheimer, E.; Habib, M.; Ghoniem, A. Fuel flexibility, stability and emissions in premixed hydrogen-rich gas turbine combustion: Technology, fundamentals, and numerical simulations. Appl. Energy 2015, 154, 1020–1047. [Google Scholar] [CrossRef]
- Bolobov, V.I.; Latipov, I.U.; Popov, G.G.; Buslaev, G.V.; Martynenko, Y.V. Estimation of the Influence of Compressed Hydrogen on the Mechanical Properties of Pipeline Steels. Energies 2021, 14, 6085. [Google Scholar] [CrossRef]
- Power, M. MHPS Successfully Tests Large-Scale High-Efficiency Gas Turbine Fueled by 30% Hydrogen Mix. Available online: https://power.mhi.com/news/20180119.html (accessed on 6 January 2022).
- Gondal, I. 12—Hydrogen transportation by pipelines. In Compendium of Hydrogen Energy; Gupta, R.B., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2016; pp. 301–322. [Google Scholar] [CrossRef]
- Preuster, P.; Alekseev, A.; Wasserscheid, P. Hydrogen Storage Technologies for Future Energy Systems. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 445–471. [Google Scholar] [CrossRef]
- Ogden, J.; Jaffe, A.M.; Scheitrum, D.; McDonald, Z.; Miller, M. Natural gas as a bridge to hydrogen transportation fuel: Insights from the literature. Energy Policy 2018, 115, 317–329. [Google Scholar] [CrossRef]
- Chaczykowski, M.; Zarodkiewicz, P. Simulation of natural gas quality distribution for pipeline systems. Energy 2017, 134, 681–698. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Thian, T.C.; Othman, M.R. Evaluation of hydrogen concentration effect on the natural gas properties and flow performance. Int. J. Hydrogen Energy 2021, 46, 974–983. [Google Scholar] [CrossRef]
- Durbin, D.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Gas cylinders—High Pressure Cylinders for the On-Board Storage of Natural Gas as a Fuel for Automotive Vehicles. ISO 11439:2013; For Standardization, I.O. 2019. Available online: https://www.iso.org/standard/44755.html (accessed on 26 January 2022).
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Lois, E.; Keating, E.; Gupta, A. Fuels. In Encyclopedia of Physical Science and Technology, (Third Edition), 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 275–314. [Google Scholar] [CrossRef]
- Gondal, I.A. Infrastructure Analysis for Producing Hydrogen from Renewable Resources in Pakistan. Ph.D. Thesis, University of Engineering & Technology, Taxila, Pakistan, 2012. [Google Scholar]
- Donohoe, N.; Heufer, A.; Metcalfe, W.K.; Curran, H.J.; Davis, M.L.; Mathieu, O.; Plichta, D.; Morones, A.; Petersen, E.L.; Güthe, F. Ignition delay times, laminar flame speeds, and mechanism validation for natural gas/hydrogen blends at elevated pressures. Combust. Flame 2014, 161, 1432–1443. [Google Scholar] [CrossRef] [Green Version]
- Effects of Hydrogen Addition on the Flame Speeds of Natural Gas Blends Under Uniform Turbulent Conditions, Volume 4A: Combustion, Fuels and Emissions, Turbo Expo: Power for Land, Sea, and Air. Montreal, QC, Canada, 15-19 June 2015; 2015. Available online: https://asmedigitalcollection.asme.org/GT/proceedings-abstract/GT2015/56680/V04AT04A066/237014 (accessed on 25 January 2022). [CrossRef] [Green Version]
- Sofianopoulos, A.; Assanis, D.N.; Mamalis, S. Effects of Hydrogen Addition on Automotive Lean-Burn Natural Gas Engines: Critical Review. J. Energy Eng. 2016, 142, E4015010. [Google Scholar] [CrossRef]
- Alirahmi, S.M.; Assareh, E.; Chitsaz, A.; Ghazanfari Holagh, S.; Jalilinasrabady, S. Electrolyzer-fuel cell combination for grid peak load management in a geothermal power plant: Power to hydrogen and hydrogen to power conversion. Int. J. Hydrogen Energy 2021, 46, 25650–25665. [Google Scholar] [CrossRef]
- Okundamiya, M. Size optimization of a hybrid photovoltaic/fuel cell grid connected power system including hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 30539–30546. [Google Scholar] [CrossRef]
- Song, P.; Sui, Y.; Shan, T.; Hou, J.; Wang, X. Assessment of hydrogen supply solutions for hydrogen fueling station: A Shanghai case study. Int. J. Hydrogen Energy 2020, 45, 32884–32898. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A review on the role, cost and value of hydrogen energy systems for deep decarbonisation. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Lin, R.H.; Zhao, Y.Y.; Wu, B.D. Toward a hydrogen society: Hydrogen and smart grid integration. Int. J. Hydrogen Energy 2020, 45, 20164–20175. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Eltigani, D.; Masri, S. Challenges of integrating renewable energy sources to smart grids: A review. Renew. Sustain. Energy Rev. 2015, 52, 770–780. [Google Scholar] [CrossRef]
- Li, Z. Notice of Retraction: Comparison and Analysis of Dispatching Protocols in China. In Proceedings of the 2010 Asia-Pacific Power and Energy Engineering Conference, Chengdu, China, 28–31 March 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Kolhe, M. Smart Grid: Charting a New Energy Future: Research, Development and Demonstration. Electr. J. 2012, 25, 88–93. [Google Scholar] [CrossRef]
- Guney, M.S.; Tepe, Y. Classification and assessment of energy storage systems. Renew. Sustain. Energy Rev. 2017, 75, 1187–1197. [Google Scholar] [CrossRef]
- Hakimi, S.M.; Moghaddas-Tafreshi, S.M. Optimal Planning of a Smart Microgrid Including Demand Response and Intermittent Renewable Energy Resources. IEEE Trans. Smart Grid 2014, 5, 2889–2900. [Google Scholar] [CrossRef]
- Bornapour, M.; Hooshmand, R.A.; Parastegari, M. An efficient scenario-based stochastic programming method for optimal scheduling of CHP-PEMFC, WT, PV and hydrogen storage units in micro grids. Renew. Energy 2019, 130, 1049–1066. [Google Scholar] [CrossRef]
- Zhang, L.; Gari, N.; Hmurcik, L.V. Energy management in a microgrid with distributed energy resources. Energy Convers. Manag. 2014, 78, 297–305. [Google Scholar] [CrossRef]
- Li, B.; Roche, R.; Paire, D.; Miraoui, A. Sizing of a stand-alone microgrid considering electric power, cooling/heating, hydrogen loads and hydrogen storage degradation. Appl. Energy 2017, 205, 1244–1259. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xiang, J. The performance of a grid-tied microgrid with hydrogen storage and a hydrogen fuel cell stack. Energy Convers. Manag. 2014, 87, 421–427. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Riveros-Godoy, G.; Torres-Contreras, S.; Garcia-Perez, T.; Albornoz-Vintimilla, E. Production and use of electrolytic hydrogen in Ecuador towards a low carbon economy. Energy 2014, 64, 626–631. [Google Scholar] [CrossRef]
- Hajimiragha, A.; Fowler, M.W.; Cañizares, C.A. Hydrogen economy transition in Ontario—Canada considering the electricity grid constraints. Int. J. Hydrogen Energy 2009, 34, 5275–5293. [Google Scholar] [CrossRef]
- Brandon, N.; Kurban, Z. Clean energy and the hydrogen economy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160400. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrogen Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef] [Green Version]
- Alanne, K.; Cao, S. Zero-energy hydrogen economy (ZEH2E) for buildings and communities including personal mobility. Renew. Sustain. Energy Rev. 2017, 71, 697–711. [Google Scholar] [CrossRef]
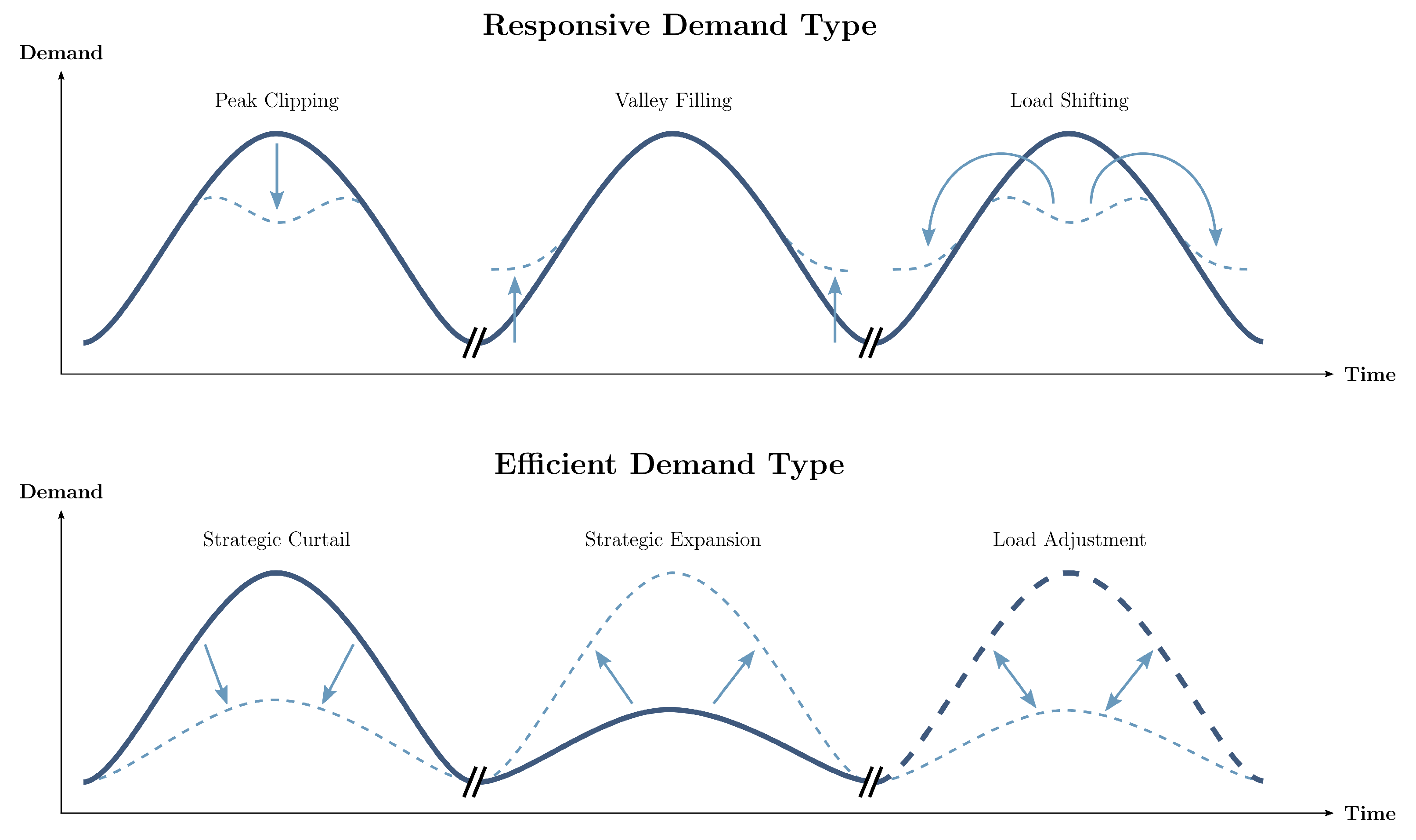
- Islam, N.; Xu, Z.; Gao, Y.; Hussain, M.; Cheng, P. Demand Side Management for Smart Grid Based on Smart Home Appliances with Renewable Energy Sources and an Energy Storage System. Math. Probl. Eng. 2020, 2020, 9545439. [Google Scholar] [CrossRef]
- Diamantoulakis, P.D.; Kapinas, V.M.; Karagiannidis, G.K. Big Data Analytics for Dynamic Energy Management in Smart Grids. Big Data Res. 2015, 2, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Li, H.; Maharjan, S.; Zhang, Y.; Guo, S. Green Energy Scheduling for Demand Side Management in the Smart Grid. IEEE Trans. Green Commun. Netw. 2018, 2, 596–611. [Google Scholar] [CrossRef]
- Goulden, M.; Bedwell, B.; Rennick-Egglestone, S.; Rodden, T.; Spence, A. Smart grids, smart users? The role of the user in demand side management. Energy Res. Soc. Sci. 2014, 2, 21–29. [Google Scholar] [CrossRef]
- Finn, P.; Fitzpatrick, C. Demand side management of industrial electricity consumption: Promoting the use of renewable energy through real-time pricing. Appl. Energy 2014, 113, 11–21. [Google Scholar] [CrossRef]
- You, S.; Hu, J.; Zong, Y.; Lin, J. Value assessment of hydrogen-based electrical energy storage in view of electricity spot market. J. Mod. Power Syst. Clean Energy 2016, 4, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Wang, X.; Pinson, P.; Wang, X. A Local Energy Market for Electricity and Hydrogen. IEEE Trans. Power Syst. 2018, 33, 3898–3908. [Google Scholar] [CrossRef] [Green Version]
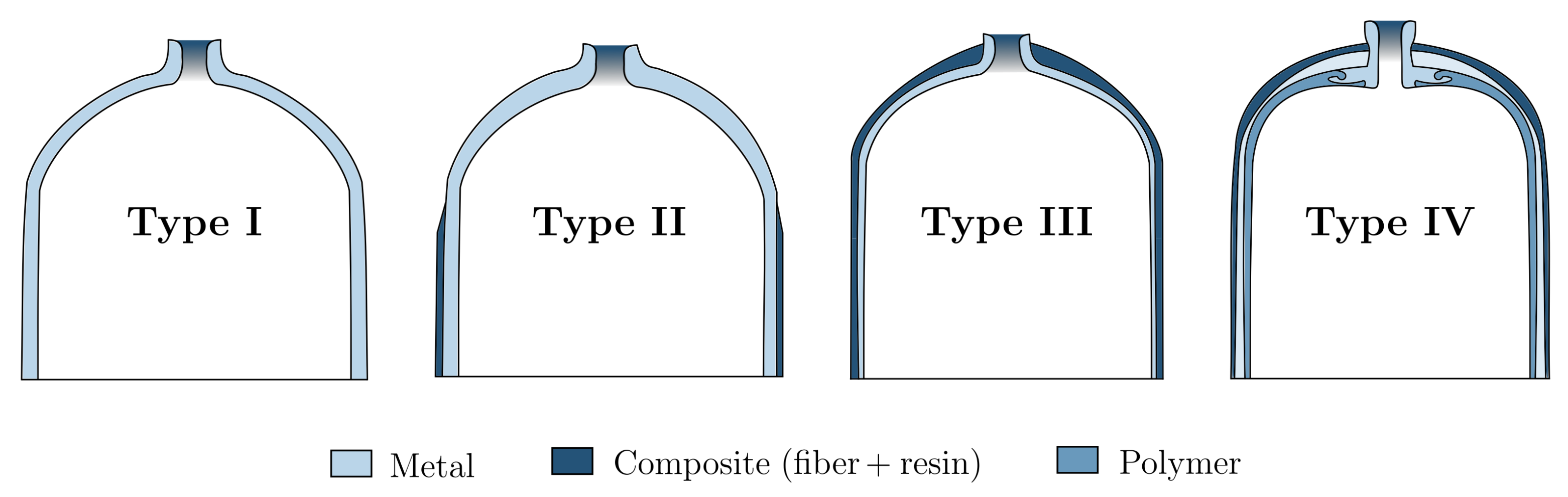





| Vessel Type | Type I | Type II | Type III | Type IV |
|---|---|---|---|---|
| Material Composition | ||||
| Core structure | Metal | Metal | Composite | Composite |
| Top liner | Metal | Metal | Metal | Polymer |
| Exterior wrapping | n.a. | Composite | Composite | Composite |
| Physical Properties | ||||
| Service pressure (MPa) | 50 | n.a. | 45 | 100 |
| Weight ratio (kg/L-H) | 1.50 | 0.90 | 0.40 | 0.25 |
| Miscellaneous | ||||
| Weight performance | – | 0 | + | ++ |
| Cost performance | ++ | + | 0 | – |
| Main application | Industry | Stationary | Portable | Portable |
| Storage Method | CGH | LH | CCH |
|---|---|---|---|
| Operational Parameters | |||
| Service pressure | Very High | Ambient | Very High |
| Service temperature | Ambient | Very Low | Very Low |
| Hydrogen density | Moderate | High | Very High |
| System Characteristics | |||
| Compression process | Required | Not Required | Required |
| Liquefaction process | Not Required | Required | Not Required |
| Thermal insulation | Not Required | Required | Required |
| Tank cost | Moderate | High | Moderate |
| Technology Features | |||
| Boil-off effect | Moderate | Very High | High |
| Hydrogen permeation | High | Moderate | High |
| Cooling capacity | n.a. | Very High | Very High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidas, L.; Castro, R.; Pires, A. A Review of the Impact of Hydrogen Integration in Natural Gas Distribution Networks and Electric Smart Grids. Energies 2022, 15, 3160. https://doi.org/10.3390/en15093160
Vidas L, Castro R, Pires A. A Review of the Impact of Hydrogen Integration in Natural Gas Distribution Networks and Electric Smart Grids. Energies. 2022; 15(9):3160. https://doi.org/10.3390/en15093160
Chicago/Turabian StyleVidas, Leonardo, Rui Castro, and Armando Pires. 2022. "A Review of the Impact of Hydrogen Integration in Natural Gas Distribution Networks and Electric Smart Grids" Energies 15, no. 9: 3160. https://doi.org/10.3390/en15093160
APA StyleVidas, L., Castro, R., & Pires, A. (2022). A Review of the Impact of Hydrogen Integration in Natural Gas Distribution Networks and Electric Smart Grids. Energies, 15(9), 3160. https://doi.org/10.3390/en15093160








