Reactivity Effects of Inorganic Content in Biomass Gasification: A Review
Abstract
1. Introduction
2. Materials and Methods
- (i)
- Effect of inherent inorganic content:
- Char gasification studies further classified into TGA and reactor studies;
- Biomass (raw/treated or washed) gasification studies.
- (ii)
- Effect of externally added inorganic content:
- Char gasification studies (including tar reforming);
- Biomass (raw/treated) gasification studies.
3. Results
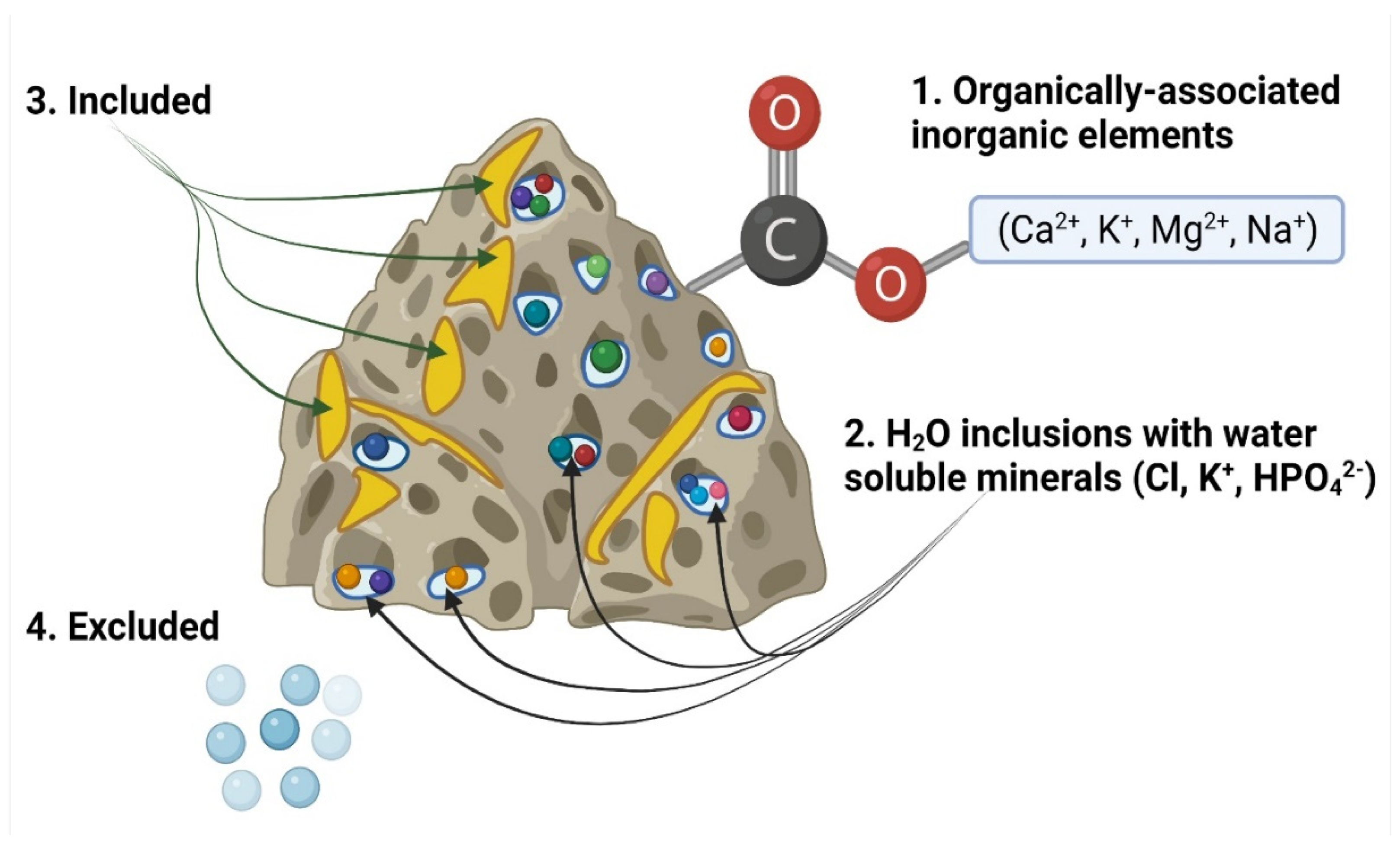
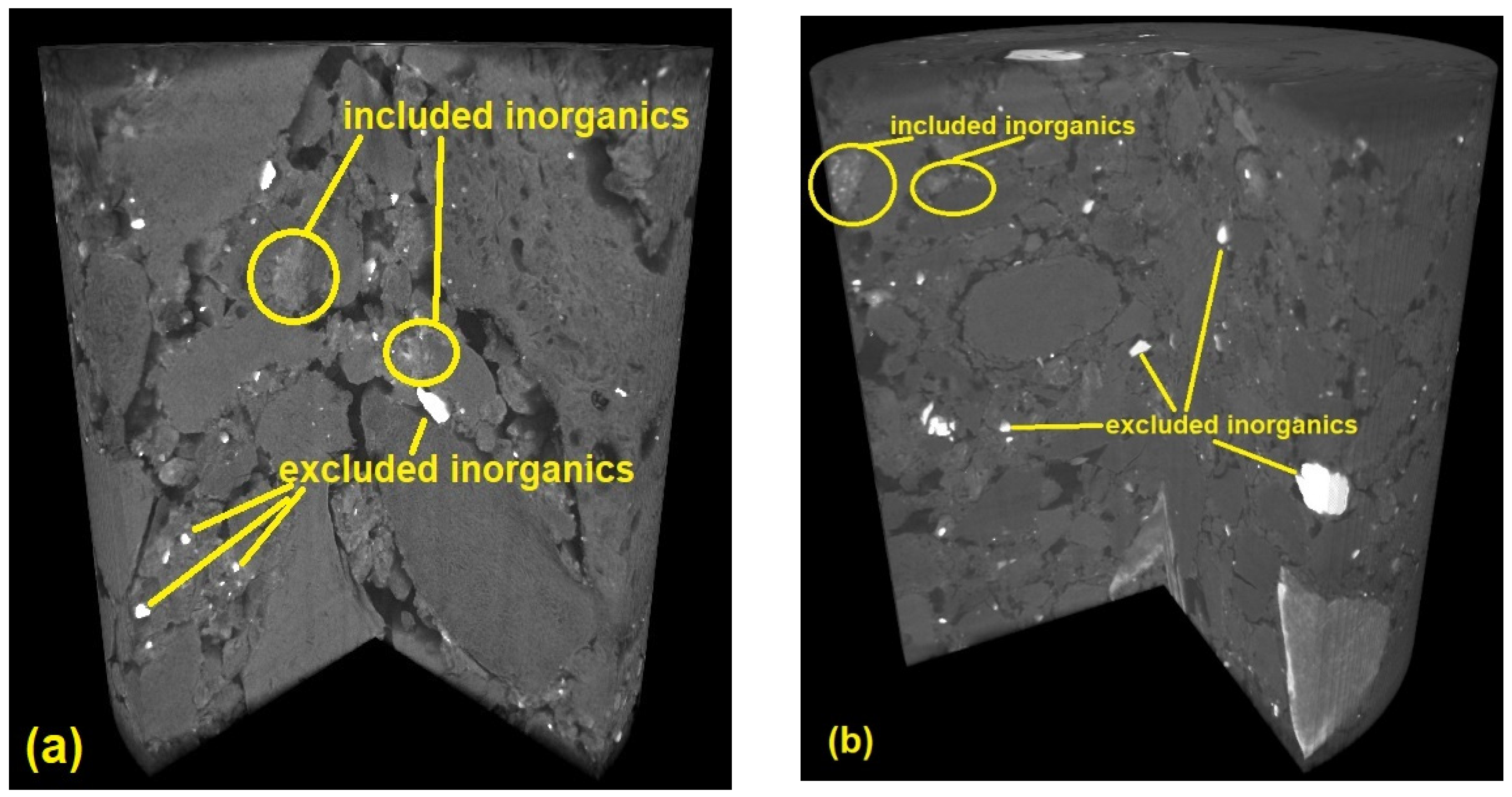
3.1. Inorganic Content
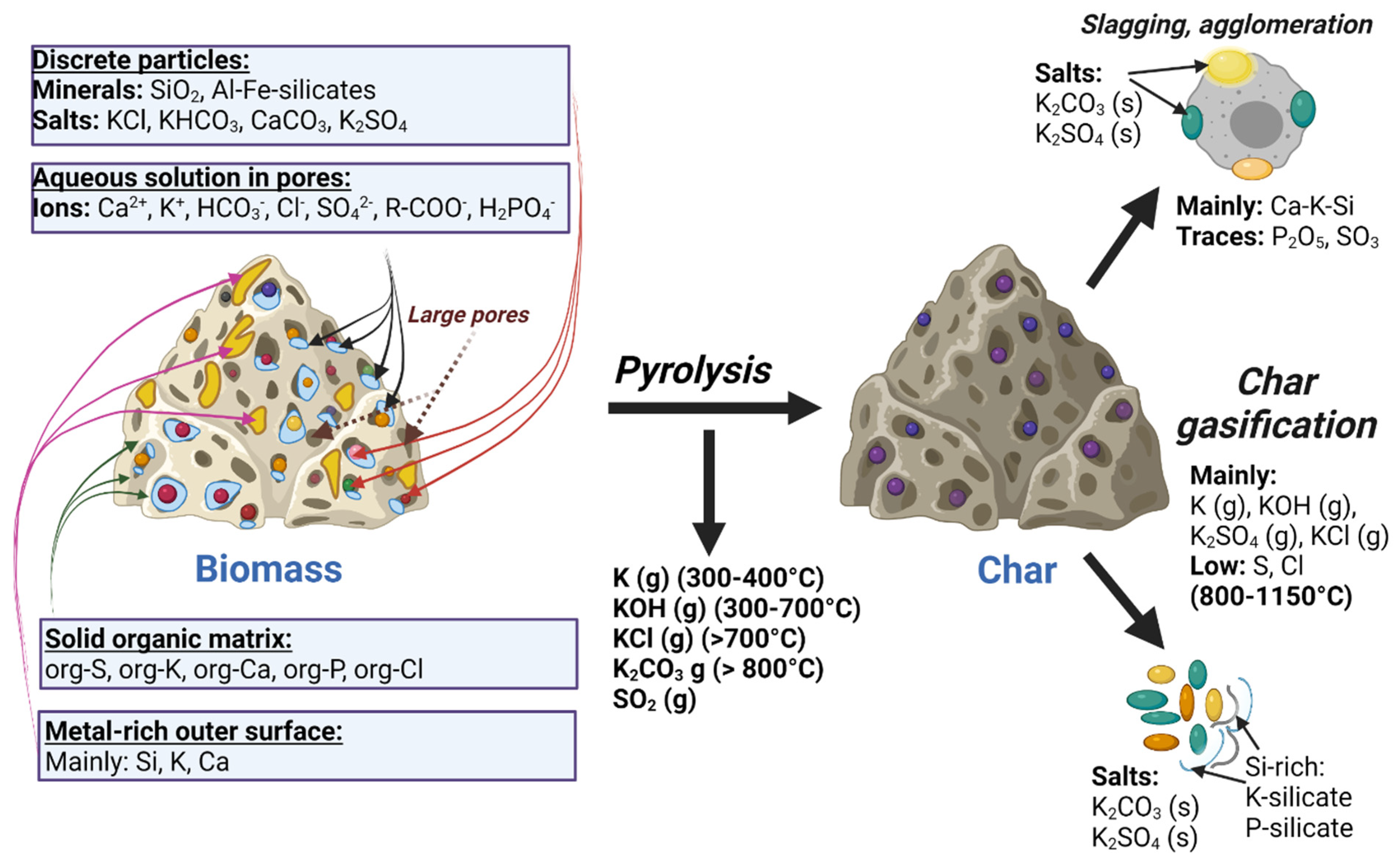
- Organically associated inorganic elements: Inorganic species in the lignocellulosic matrix of biomass feedstocks include cations such as Na+, K+, Ca2+, Mg2+, Fe3+, and Al3+ and non-metals such as organic Cl, S, and P attached with covalent bonds. Inorganic species in the organic phase are often associated with oxygen-containing functional groups such as carboxylic acids [26].
- Dissolved salts: These originate from the liquid phases inside plants, and include cations dissolved in plant fluids, i.e., K+, Na+, and Ca2+, and anions, i.e., SO42−, Cl−, and HPO42−.
- Included minerals: Discrete inorganic particles are located within the crystalline or non-crystalline lignocellulosic matrix. Typical minerals in wood are composed of Mg, Ca, and Si. The Ca element frequently appeared to be found in the form of calcium oxalate CaC2O4, and Si exists as silicic acid Si(OH)4, which provides strength to the plant tissue, often in herbaceous feedstock.
- Excluded minerals: These include inorganics set free from the organic structure, such as clay minerals in the form of quartz, feldspars, or aluminosilicates, which are rich in K, Na, Ca, and Fe elements. Feedstocks can also contain impurities which originate from the various contaminants or soil.
3.2. Characterization of Inorganic Content
3.3. Overview of the Effects of Inorganic Content in Pyrolysis
3.4. Effect of Inherent Inorganic Content on Char Gasification
| Ref. | Gasification System and Feedstock | Char Production | Main Findings |
|---|---|---|---|
| Gasification in TGA | |||
| [66] | TGA at 725, 750, and 800 °C; CO2: 60 mL/min; pH2O: 1.7, 3.2, 19.9, and 47.4 kPa in N2. Grapefruit skin | Fixed-bed quartz tube reactor at 700 °C for 2 h in N2 at 150 mL/min | Ea values for CO2 gasification: 200–250 kJ/mol; for steam gasification: 130–170 kJ/mol. K mostly contributed to the increased reactivity compared to Ca, Na, and Si. Decreasing the inorganic content of the char through washing reduced CO2 reactivity. |
| [81] | Macro TGA, 900 °C/min till 927 °C; 20% H2O in N2 (4 L/min) Beechwood | Refractory steel box swept with N2 in muffle furnace, at 2.6 and 12 °C/min, kept for 8 min at 900 °C | The ash content (mainly the elements Ca and K) in beechwood char was proportional to its initial apparent reactivity of gasification. Individual component effects could not be distinguished in the study. |
| [82] | TGA, 10 °C/ min till 600–1000 °C; 50% CO2 with argon. Pinewood, birchwood | Fixed-bed reactor at 500 °C for 150 min | The inorganic content of the chars was low, around 1%, and the decomposition kinetics for both chars revealed considerable similarities in CO2 environments. The activation energy (Ea) for the gasification step was 262–263 kJ/mol. |
| [60] | TGA, 24 °C/min to 750–900 °C, 1 atm using pH2O in N2 = 0 to 0.27 MPa. Woody biomass | TGA, <10 °C/min to 450 °C, held for 4 h in N2 flow of 0.05 L/min | The concentration of inorganic elements increased with the conversion, which increased their catalytic or inhibitor effect. K showed catalytic and Si showed inhibiting effects on the steam gasification of char. |
| [71] | TGA (800–1300 °C) and aerosol-based method (1100–1300 °C) using CO2 and H2O. Wood, straw, miscanthus | Tubular fixed-bed reactor at 600–800 °C in N2 (5 KW) | Straw and miscanthus chars had higher contents of alkali metals (K, Na), but showed lower reactivities than wood due to the high Si content in herbaceous feedstock and the formation of an inorganic layer on the outer surface. This can lead to a blockage that further hinders gas diffusion to the carbonaceous surface. |
| [72] | TGA, 800 °C, using a mixture of H2O/N2 (pH2O = 0.2 bar) flow of 0.05 L/min. Algal and lignocellulosic biomass | TGA, 24 °C/min to 450°C in N2 flow of 0.05 L/min for 1 h and then to 800 °C | Phosphorus (P) was included in the expression because of its higher percentage in algae. For feedstocks with K/(Si + P) > 1, the reaction rate remained constant during mostly the entire reaction and then it slightly increased at higher conversions. For feedstocks with K/(Si + P) < 1, the reaction rate decreased during the reaction. |
| [83] | TGA, CO2 flow of 200 mL/min isothermally at 900 °C for 40 min. Corn stalk, metasequoia pruning | TGA/DSC, 200 mL/min N2, 50 °C/min up to 900 °C, and maintained for 10 min | Torrefaction concentrated AAEMs in char, leading to higher CO2 gasification reactivity than the raw biomass. Torrefied chars were richer in active Ca, K, and Na than the original feedstocks. Char gasification, as the rate-determining step, can be improved by torrefaction. |
| [84] | TGA and Setaram TAG24 analyser, 1 atm, 700–1000 °C, using 75/25 vol.% N2/H2O. 12 biomass (hardwoods, softwoods) | Bubbling fluidized-bed reactor, 1000 °C in N2 flow of 1.365 L/min | K, Na, and Mg showed a positive effect, while Si, P, and Ca showed a negative effect on char reactivity. The activation energy for gasification ranged between 59 and 196 kJ/mol. |
| [85] | TGA isothermal process 700–800 °C for raw char and washed char, 7.6 mol% H2O in N2. Pine wood | Obtained from scrubber sediment tank of entrained-flow gasifier, 900–1150 °C, air; TGA at 950 °C in N2 for 3 h | For conversions < 70%, the char surface area is a predominant factor irrespective of the inorganic content. At conversions > 70%, the catalytic effect of K becomes predominant. |
| [86] | Macro-TGA 800 °C, 20 vol% H2O in N2 flow of 5 L/min. Rice husks, sunflower seed shells | Holder with 30–50 g sample in furnace heated at 10 °C/min to 450 °C, held for 1 h, in N2 flow of 1 L/min | For two biomasses, the inorganic composition affected gasification kinetics more than the physicochemical properties of the carbon matrix. Inorganics also affected the microporosity and the number of surface functions. |
| Gasification in reactor | |||
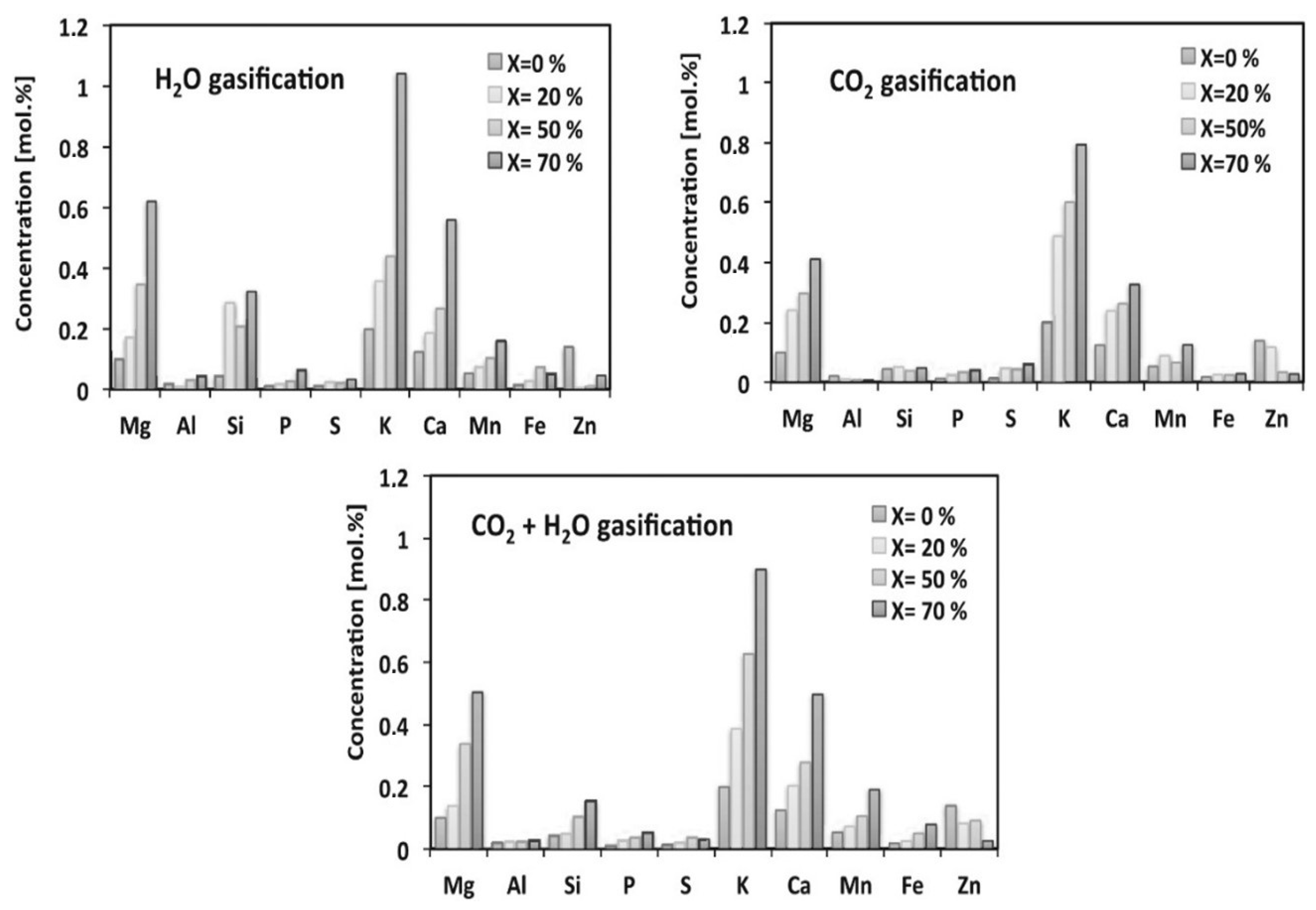
| [79] | Reactor diameter of 30 mm and a length of 500 mm (drop tube furnace) using CO2 or H2O and N2 at 900–1000 °C. Woody biomass, lawn grass | Obtained from pilot-scale entrained-flow gasifier (5 kW) using steam and oxygen | For H2O gasification, (K2O + Na2O) showed a stronger effect than it did for CO2 gasification. The CO2 gasification rates of different chars linearly depended on the concentrations of the predominant inorganic compounds (K and Ca). |
| [77] | Quartz fixed-bed reactor 8.2% vol. H2O in 3 L/min of argon, 750 °C. Mallee leaf, wood, bark | Quartz fixed-bed reactor at 10 °C/min to 750 °C, held for 15 min; char was later acid-treated | Acid treatment reduced AAEM content and the catalytic effect on gasification reactivity, which also depended on the char carbon structure and catalyst dispersion. AAEMs had an insignificant effect on the water–gas shift reaction. |
| [69] | 15 mm diameter quartz tube reactor; 27.3 °C/min at 850 °C, H2O-N2 or H2O-H2-N2 mixture at 0.2 L/min. Japanese bamboo, cedar | Horizontal screw-conveyor reactor 47 s, 500 °C, 5 to 5.5 °C/s and 1 atm | Acid washing of chars removed K and Na without changing Mg and Ca, and lowered the gasification reactivities, confirming the catalytic effect of K. |
| [87] | Lab-scale semi-batch reactor in H2O at 750–900 °C (0.2 bar). Food waste (dog food) | Lab-scale semi-batch reactor in argon at 900 °C for 1 h | As the conversion increased from 0.1 to 0.9, the reactivity increased mainly due to an increase in the pre-exponential factor, indicating an increased adsorption rate of the gasifying agent to the char surface. |
| [88] | Quartz fixed-bed reactor, fed at 900 °C, H2O, flow rate 200 mL/min, 2–25 min. Maize stalk, rice husk, cotton stalk | Quartz fixed-bed reactor, fed at 900 °C, N2, 10–300 s | Char gasification led to a further loss of 12–34% of the alkali metals. The Na concentration remained constant, but the H2O reactivity changed along with the dropping concentrations of Mg, Ca, and K. |
| [78] | Quartz fixed-bed reactor at 785 to 865 °C in 100% CO2 flow of 750 mL/min. Pinewood sawdust pellets and coal | Fixed-bed reactor at 6 °C/min to 900 °C, held for 3 h in N2 flow of 1.5 L/min | During co-pyrolysis, calcium in the biomass reacted with aluminosilicate in the coal to form catalytically inactive Ca2Al2SiO7 crystals, which lowered gasification reactivity. |
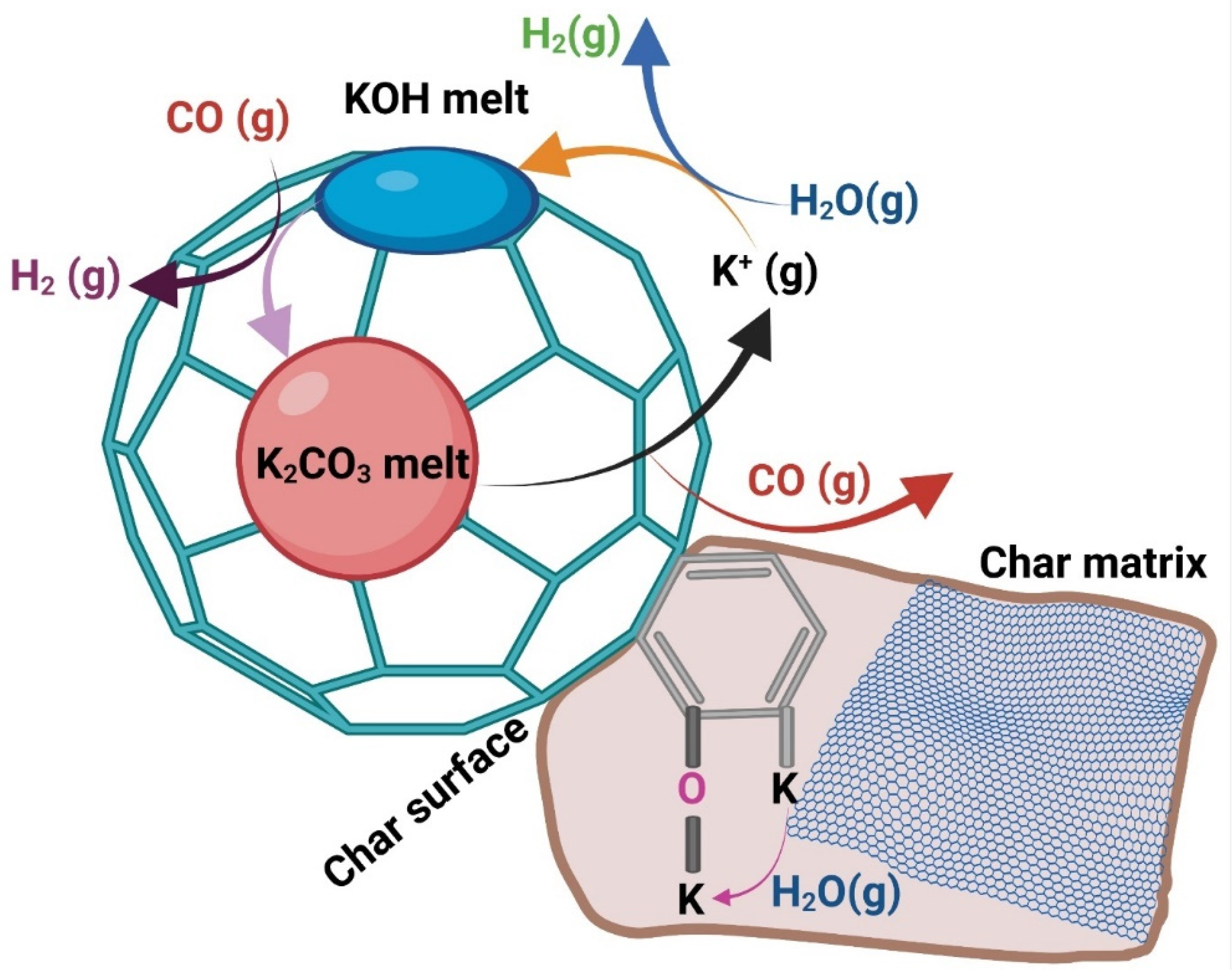
3.5. Mechanisms for Potassium, Calcium, and Silica Effects
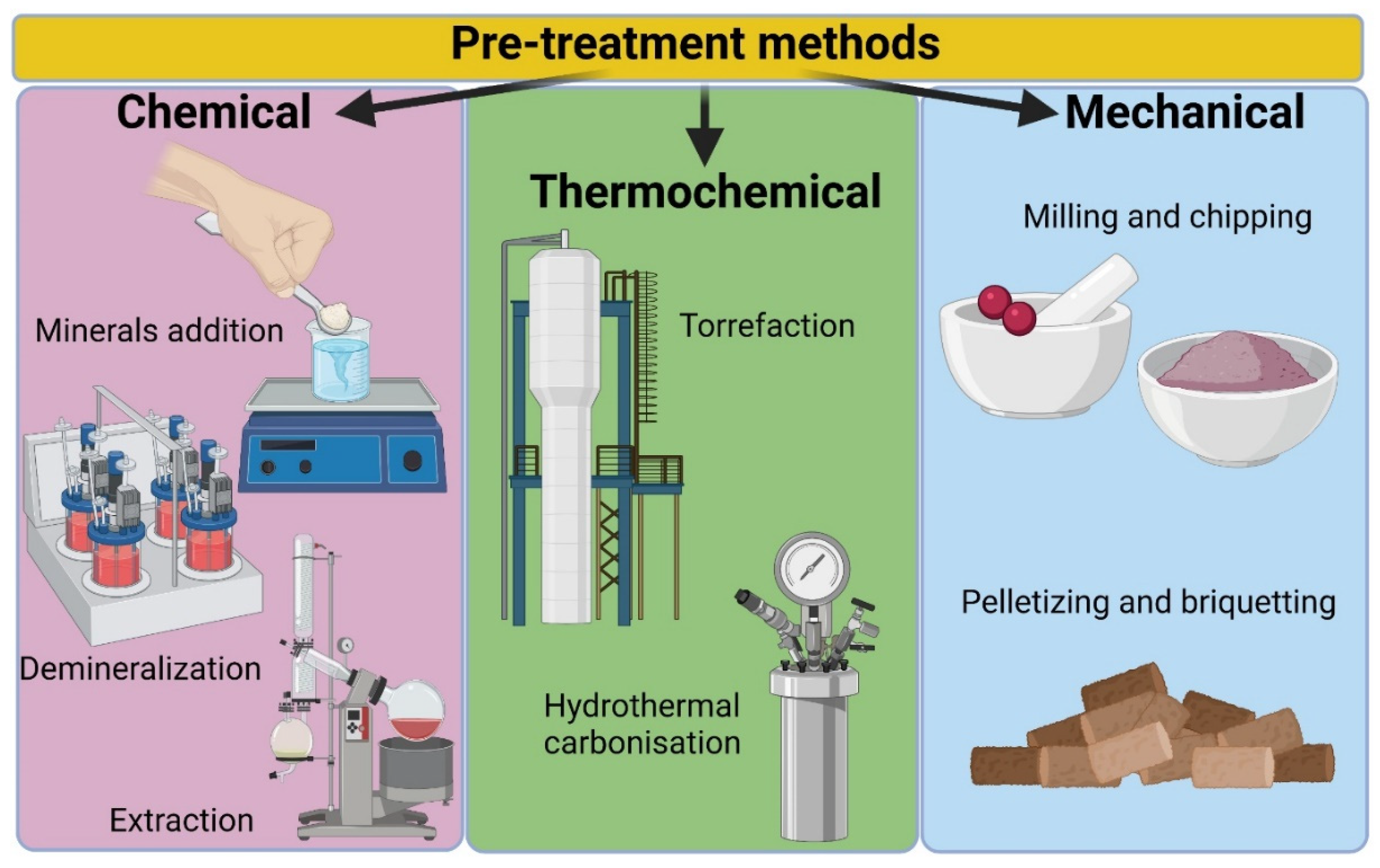
3.6. Pre-Treatment of Feedstocks
3.7. Effect of Inherent Inorganic Content on Biomass Gasification
| Ref. | Gasification System | Feedstock | Main Findings |
|---|---|---|---|
| [146] | Fluidized-bed reactor at 1 atm, 800 °C in air at ER ~0.3 | Bagasse and banana grass samples | Gas-phase inorganic species had K, Na, and Ca at concentration levels higher than specified for combustion turbine fuel, along with Si, Fe, P, and Cl. Bed material composition influenced inorganic retention. |
| [147] | Pressurized TGA, 800–900 °C; 1, 5, 10 bar, 100%, 70%, 30% CO2 and H2O | Barks of pine, spruce, birch, aspen | Reactivity increased at high temperature, but ash sintering was observed. The formation of silicates resulted in reduced catalytic activity, the formation of less reactive products, and high sintering tendencies. |
| [119] | TGA, heated at 15 °C/min from 25 to 1200 °C in CO2 flow of 100 mL/min | Woody and agricultural biomass. 1 M H2SO4 wash | Interaction between organic components (cellulose/lignin) and AAEMs influences reactivity. Higher cellulose contents probably prolong gasification time and elevate the peak temperature during CO2 gasification. Acid-washed biomass showed lower peak values. |
| [141] | Spout-fluidized-bed reactor, 900 °C in steam flow of 125 mL/min | Rice straw and rice husk (dried and pulverized) | Solid AAEMs existing in chars promoted heterogeneous reactions (char gasification), gaseous AAEMs vapored from biomass promoted homogeneous reactions (water–gas shift, reforming reactions). |
| [144] | Fixed-bed downdraft gasifier of 10–15 kg/h feed, 800 °C in air at ER ~0.2 to 0.35 | Garden waste (GW) (dry leaf litter) pellets | Higher ER increased the combustion zone temperature, which increased clinker formation. Wood with much lower ash contents showed better syngas quality than GW pellets, which confirms the dependance of reactivity on factors other than ash content. |
| [143] | Fluidized-bed reactor, 20 °C/min to 750–850 °C in 15% to 90% H2O in N2, total flow 11.7 L/min for 1 to 3 h | Oil palm shells (OPS), coconut shells (CS), and bamboo guadua (BG) | CO and CO2 desorption of gasification chars followed the order of CS < BG < OPS, for which the respective K/(Si + P) values of the raw biomass were 3.9, 0.2, and 0.17. For K/(Si + P) > 1, char AAEM (especially K) resulted in a higher surface area and O-containing functional groups. |
| [148] | Fixed-bed reactor in 1 kW furnace, 600, 700, 800 °C in different ratios of CO2 and N2 | Pine sawdust raw and mixed with CaO | CaO absorbed CO2 and decreased the overall production of CO2 but increased the production of H2. The highest H2/CO ratio was detected at 700 °C for a 2:1 ratio of N2:CO2. |
| [142] | 100 kWth dual fluidized-bed (DFB) steam gasification system | Softwood, chicken manure, mixture of two | Bed material and fuel ash both have a catalytic effect on gasification, as reported for the water–gas shift reaction. Pure K-Feldspar and olivine showed lower catalytic activity. High limestone led to a positive WGS equilibrium deviation through the catalysis of WGS and other reactions. |
3.8. Effect of Externally Added Inorganic Content on Char/Biomass Gasification
| Ref. | Gasification System and Feedstock | Char Production | Main Findings |
|---|---|---|---|
| Impregnated in biomass | |||
| [151] | Fluidized-bed reactor, 2.4 atm, 700 °C H2O/C (mol) = 1.1 Poplar wood mixed with 30% dry wood ash | TGA, 1 atm, 700–800 °C in N2 | The addition of alkali-rich ash to wood reduced tar and methane content and increased syngas production. However, alkali-rich ash can also form particle agglomerates. |
| [152] | TGA, 800 °C in CO2 flow of 40 mL/min Wood, waste wood impregnated with 2 wt.% metal (Na, K, Ca, Mg, Cu, Pb, and Zn) nitrate solution | Fixed-bed reactor with pre-pyrolysis at 600 °C at 2.5 h and post-pyrolysis at 900 °C for 20 min in argon | A high catalytic activity was observed during the early gasification stage, but it was reduced in the next stage due to sintering. All heavy metal nitrate salts lowered the charcoal reactivity over the entire process. |
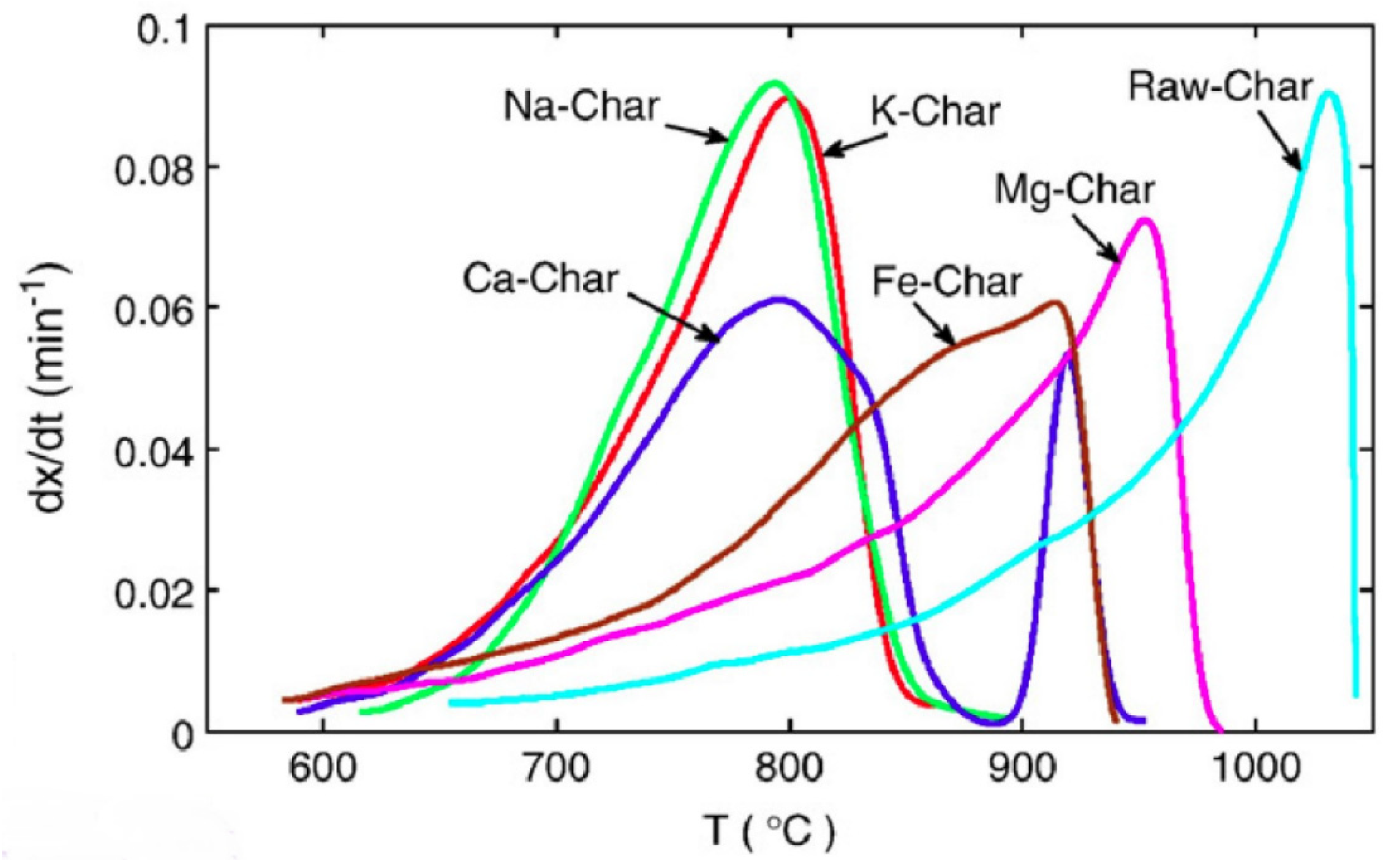
| [92] | TGA, 1 atm, 10 °C/min up to 1150 °C in CO2 flow of 400 mL/min Chinese fir with metal loading 0.04 g/g | Quartz tube reactor, 5 °C/min until 550 °C, and held for 60 min in N2 flow of 400 mL/min Wash: water | Catalytic effects on the CO2 reactivity of char in the sequence of the most influencial to the least significant: K > Na > Ca > Fe > Mg. Ca tends to agglomerate and deactivate at high temperatures. |
| [32] | TGA, 1 atm, 10 °C/min to 950 °C, using CO2 flow of 25 mL/min Municipal solid waste, undigested sewage sludge, paper External agent: CaSO4 and various alkali bicarbonates (5–20% w/w) | TGA, 1 atm, 10 °C/min to 950 °C, in He flow | The difference in catalytic activities is due to different initial and intermediate compositions during gasification, and the different molecular sizes and mobility on the carbon surface. For waste paper gasification, activity: Li2CO3 > K2CO3 > CaCO3 > Rb2CO3 > CaSO4 > Cs2CO3 > Na2CO3 For sewage sludge gasification, activity: CaCO3 > Na2CO3 > Li2CO3 > Rb2CO3 > CaSO4 > Cs2CO3 > K2CO3. |
| [156] | TGA, 1 atm, 10 °C/min to 700 °C, in steam flow of 600 mL/min Pine wood and wheat straw External agent: Various salts of K, NaOH, CaO, Fe2O3 | TGA, 1 atm, 10 °C/min to 700 °C, in N2 flow of 600 mL/min | The enhancement of the gasification rate depends on the amount of ash as well as its distribution among and inside the char particles. For a metal/carbon ratio of 0.05 in wood, the catalytic activity trend was KNO3 > KHCO3 ≈ K2CO3 ≈ KOH > NaOH > CaO > K2HPO4 > KBr > KCl > no additive > Fe2O3. |
| [75] | Bubbling fluidized-bed reactor, 20% CO2 and 80% N2, gas velocity 0.2 m/s Raw and acid-washed birch wood External agent: Ca and K nitrate | Bubbling fluidized-bed reactor, using N2, gas velocity 0.2 m/s External agent: Ca and K nitrate solutions | Leached wood showed poor reactivity upon doping compared to doped char. An unreactive coke layer developed over potassium-doped biomass chars, prohibiting K from the catalysis of char during gasification. Ca proved to be the primary active element in the gasification of birchwood. |
| [64] | Macro-TGA, 750–900 °C under CO2 Pine sawdust External agent: 0.1 and 1 M K+/Na+ of salt solutions (K2CO3, Na2CO3, NaOH and NaCl) | Macro-TGA 600 °C for 4–5 min with the flow of CO2 at 5 L/min, cooled down to room temperature by N2 | High metal loading altered char morphology. Reactivity was attributed to the combined effect of both organometallic bonds and alkali species, which were well dispersed on the surface. The enlarged surface area due to swelling and gasification temperature, and the final composition of alkali ions in chars, affect the reactivity. |
| Impregnated in char | |||
| [154] | TGA, 1 atm, 15 °C/min up to 800 °C in pCO2 = 0.1 MPa and pH2O = 0.05 MPa in the argon flow of 450 mL/min, isothermal Wet coffee grounds | Infrared furnace, 600 °C/min to 900 °C, in argon flow of 200 mL/min for 1 min Ca loading during oil-slurry dewatering | Calcium loading up to 3 wt.% led to strong Ca dispersion into a biomass matrix and increased the reactivity, but with higher than 3 wt.% it led to poor dispersion with a significant decrease in the external surface area of the catalyst. |
| [161] | TGA, 1 atm, 10 °C/min to 850–950 °C, using CO2 (20–80%) Japanese cypress | TGA, 9 °C/min to 850 °C, for 2 h in N2 Wash: 3M HCl External agent: K2CO3 and Ca(OH)2 | The increase in CO2 concentration increased the gasification rate of char at higher temperatures above 900 °C. At temperatures ≤ 850 °C, the gasification rate decreased at 80% CO2 due to the inhibition effect of CO on alkali metal catalysts of carbon and CO2. |
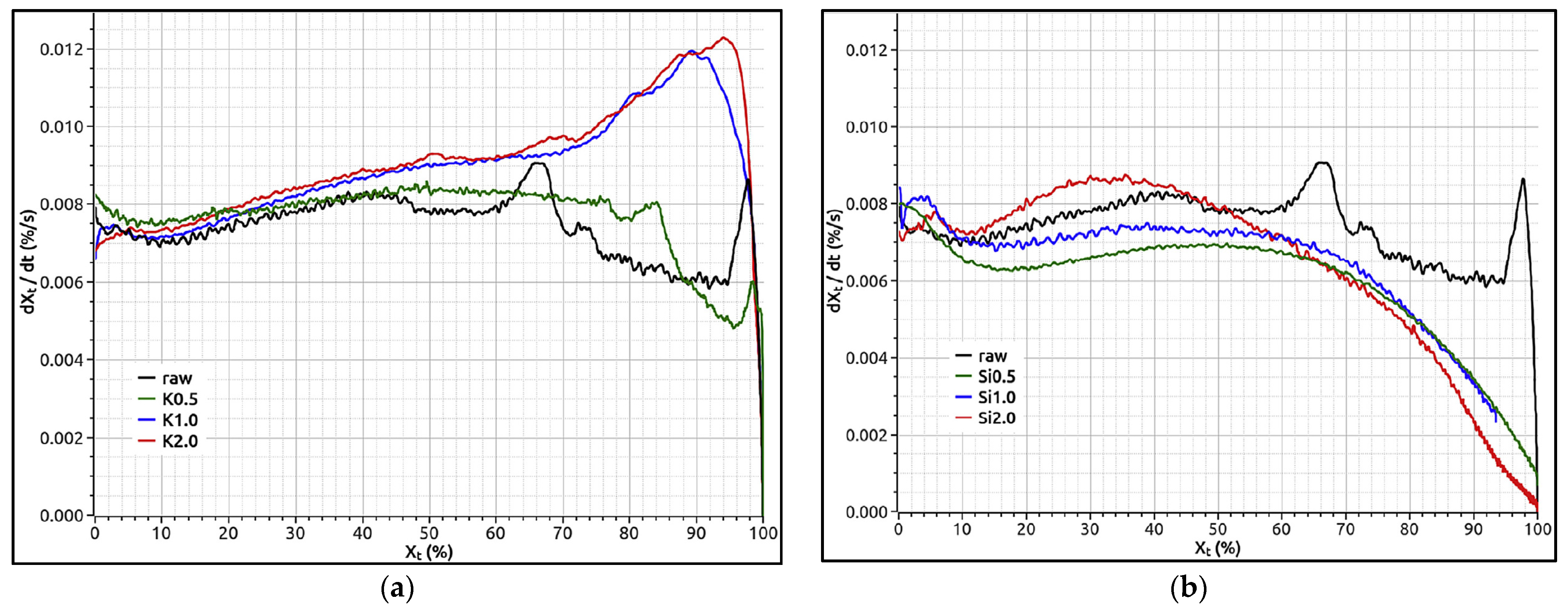
| [159] | TGA, 1 atm, 800 °C, using 80% N2 and 20% CO2 at 12 L/h Beechwood | TGA, 10 °C/min to 800 °C, in 25 L/h Ar External agent: KNO3 and SiO2 | For impregnated char samples with a K/Si mass ratio from 0.2 to 3.8, the reactivity was same for all the samples until 60% conversion. Later, the effects of K and Si became evident, but for K/Si > 3, an acceleration in the gasification reaction was found at conversions ≥ 90%. |
| [68] | TGA 700–950 °C in CO2 flow of 150 mL/min Jackfruit, mango, raintree, and eucalyptus leaf litter | Fixed-bed reactor at 10 °C/min to 800 °C in N2 Wash: Water External agent: K2CO3 (0–40% w/w) | Char with a higher alkali index shows higher gasification reactivity. A modified random pore model with parameters incorporating the effect of inorganic species fitted the data for all biomass chars. The external addition of K2CO3 up to 20% loading (w/w) significantly increased the gasification rate. |
3.9. Kinetics Models
4. Challenge and Future Research
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Biomass Pyrolysis Reactions [166] | |||
| Primary reaction | Secondary reaction | ||
| Homogeneous (tar cracking) | Heterogeneous (tar–char reaction) | ||
| Raw biomass → Char (cp) + Tar (tr) + Non-condensable gas (gp) | Tar (tr) → Char (cs1) + Non-condensable gas (gs1) | Tar (tr) + Char (cp, cs1, cs2) → Char (cs2) + Non-condensable gas (gs2) | |
| Biomass Gasification Reactions [167] | |||
| No. | Description | Equation | ΔH (KJ/mole) |
| 1 | Drying and Devolatilization | >0 | |
| 2 | Partial oxidation | −111 | |
| 3 | Complete oxidation | −394 | |
| 4 | Boudouard reaction/CO2 gasification | +173 | |
| 5 | Water gas reaction/Steam gasification | +131 | |
| 6 | Hydrogen gasification | −75 | |
| 7 | CO oxidation | −283 | |
| 8 | H2 oxidation | −242 | |
| 9 | Methane oxidation | −283 | |
| 10 | Water gas shift reaction | −42 | |
| 11 | Methanation reaction | −88 | |
| 12 | reactions | +200 to 300 | |
Appendix B
| Types | Residence Time | Temperature (°C) | Heating Rate | Desired Products |
|---|---|---|---|---|
| Slow | days | 400 | Very low | Charcoal |
| Intermediate | 5–30 min | 600 | Low | Char, bio-oil, gas |
| Fast | <2 s | 500 | Very high | Bio-oil |
| Flash | <1 s | 1000 | High | Bio-oil, chemicals, gas |
| Vacuum | 2–30 s | 400 | Medium | Bio-oil |
| Hydro-pyrolysis | <10 s | <500 | High | Bio-oil |
| Under pressure | 2–30 s | 400 | High | Bio-oil |
Appendix C
| Ref. | Equipment and Feedstock | Operating Conditions | Main Findings |
|---|---|---|---|
| Inherent inorganic content | |||
| [8] | Fluidized-bed reactor Switchgrass | 500 °C, N2, vapour residence time < 0.4 s, 0.34 mm particle size | Alkali metals in the char contribute to the mineral content of the bio-oil. For chlorine, nitrogen, and sulfur, there is only partial sequestration in the char particles. |
| [9] | Bubbling fluidized-bed reactor Willow, reed canary grass, switchgrass, wheat straw, and low lignin-containing grasses | 150 g/h feed, 500 °C, N2, vapor residence time 0.4–1.5 s | Total liquid yield (wt.%) increased with an increase in lignin, while the ash and alkali metal content decreased. Alkali metals lowered biomass degradation temperatures. Ash dominated over the lignin effect on pyrolysis yields, but lignin governed the higher-molecular-weight compounds in bio-oil. |
| [166] | Fixed-bed reactor Raw rice straw (RS), water-washed rice straw (WRS), and acid-washed rice straw (ARS) | 2.2 g sample heated in argon (300 mL/min) at 10 °C/min to (275–725 °C) | Internal AAEMs and the changed organic matter influence the pyrolysis product distribution. Internal AAEMs act as catalysts for the decomposition of hemicellulose, cellulose, and lignin in raw RS. |
| [88] | Quartz fixed-bed reactor Maize stalk, rice husk, cotton stalk | At 900 °C the feedstock is pushed into the furnace, N2, 10–300 s | Over half of the alkali metals (K, Na) were released during pyrolysis at 900 °C. Maize stalk char had a larger pore volume and superficial area than rice husks and cotton stalk char samples, indicating that the distinct reactivity of chars depends on the composition and distribution of the alkali metals released and those left in the char matrix. |
| [57] | Fluidized-bed reactor Pine wood (raw and acid-washed) | 150 g biomass fed during 30–40 min, 530 °C, vapor residence time 1.6–1.9 s, feed particle average size of 1 mm | Acid washing reduced the yields of lignin-derived water insoluble content and guaiacol, due to the effect of mineral content on the decomposition behaviour of the lignin. |
| Impregnated inorganic content | |||
| [168] | Fixed-bed reactor and TGA 13 woody and herbaceous biomasses (raw and impregnated with metal chloride salt) | Reactor: 500 °C, 10–25 g sample in a batch reactor TGA: 50 °C/min, N2 | Devolatilization rate, volatiles yield, and the initial decomposition temperature increased upon demineralization for most biomasses. However, rice husk, groundnut shell, and coir pith showed different behaviour because of a high potassium (and/or zinc) content in combination with a high lignin content. |
| [121] | TGA Cellulose, hemicellulose, and lignin (raw and mixed with metal oxides and carbonates) | ∼20 mg sample heated at 10 °C/min up to 900 °C and kept for 3 min, N2 flow 120 mL/min | The addition of K2CO3 inhibited hemicellulose pyrolysis but enhanced cellulose pyrolysis significantly by shifting its peak to a lower temperature. The assumed addition of K2CO3 changes the chemical structure of hemicellulose or the decomposition steps of cellulose. |
| [169] | TGA Pine wood, cotton stalk, fir (raw and treated with sodium-based catalysts, TiO2 and HZSM-5) | 10 mg sample heated at 10 °C/min, N2 flow 100 mL/min | The devolatilization temperature was reduced with the increasing basicity of sodium-containing species. Sodium catalysts caused pyrolysis to be more exothermic and promoted char formation. TiO2 and HZSM-5 increased the pyrolysis temperature of cotton stalk because the basic minerals were deactivated by the acidic nature of the catalysts. |
| [56] | TGA and analytical Py-GC/MS Poplar wood (demineralized with HF and impregnated with K, Ca, and Mg) | 10 mg sample heated at 10 °C/min up to 700 °C | An increase in the potassium content of biomass increased char from 10.5 wt.% to 19.6 wt.% at 550 °C, and lowered the temperature of the maximum degradation rate from 367 °C to 333 °C. An increase in magnesium content increased the maximum degradation rate from 1.21 wt.%/°C to 1.43 wt.%/°C. K promoted the low-molecular-weight compounds and C6 and C2C6 lignin derivatives but suppressed levoglucosan. |
References
- Bruhn, T.; Naims, H.; Olfe-Kräutlein, B. Separating the debate on CO2 utilisation from carbon capture and storage. Environ. Sci. Policy 2016, 60, 38–43. [Google Scholar] [CrossRef]
- Thengane, S.K.; Bandyopadhyay, S. Biochar mines: Panacea to climate change and energy crisis? Clean Technol. Environ. Policy 2019, 22, 5–10. [Google Scholar] [CrossRef]
- Sanchez, D.L.; Kammen, D.M. A commercialization strategy for carbon-negative energy. Nat. Energy 2016, 1, 15002. [Google Scholar] [CrossRef]
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Sikarwar, V.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Agblevor, F.A.; Besler, S. Inorganic Compounds in Biomass Feedstocks. 1. Effect on the Quality of Fast Pyrolysis Oils. Energy Fuels 1996, 10, 293–298. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Donnison, I.; Yates, N.; Jones, J.M. The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability. Fuel 2008, 87, 1230–1240. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part II. Ash fusion and ash formation mechanisms of biomass types. Fuel 2013, 117, 152–183. [Google Scholar] [CrossRef]
- Herman, A.P.; Yusup, S.; Shahbaz, M.; Patrick, D.O. Bottom Ash Characterization and its Catalytic Potential in Biomass Gasification. Procedia Eng. 2016, 148, 432–436. [Google Scholar] [CrossRef]
- James, A.K.; Thring, R.W.; Helle, S.; Ghuman, H.S. Ash Management Review—Applications of Biomass Bottom Ash. Energies 2012, 5, 3856–3873. [Google Scholar] [CrossRef]
- Liu, Q. Pretreatment and Posttreatment Approaches for Reducing Biomass Inorganic Impurities during Gasification. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2017. [Google Scholar]
- Risnes, H.; Fjellerup, J.; Henriksen, U.; Moilanen, A.; Norby, P.; Papadakis, K.; Posselt, D.; Sørensen, L.H. Calcium addition in straw gasification. Fuel 2003, 82, 641–651. [Google Scholar] [CrossRef]
- Di Blasi, C. Combustion and gasification rates of lignocellulosic chars. Prog. Energy Combust. Sci. 2009, 35, 121–140. [Google Scholar] [CrossRef]
- Dayton, D. A Review of the Literature on Catalytic Biomass Tar Destruction; National Renewable Energy Laboratory: Golden, CO, USA, 2002.
- Gómez-Barea, A.; Ollero, P.; Leckner, B. Optimization of char and tar conversion in fluidized bed biomass gasifiers. Fuel 2013, 103, 42–52. [Google Scholar] [CrossRef]
- Min, F.; Zhang, M.; Zhang, Y.; Cao, Y.; Pan, W.-P. An experimental investigation into the gasification reactivity and structure of agricultural waste chars. J. Anal. Appl. Pyrolysis 2011, 92, 250–257. [Google Scholar] [CrossRef]
- Kannan, M.P.; Richards, G.N. Gasification of biomass chars in carbon dioxide: Dependence of gasification rate on the indigenous metal content. Fuel 1990, 69, 747–753. [Google Scholar] [CrossRef]
- Okumura, Y.; Hanaoka, T.; Sakanishi, K. Effect of pyrolysis conditions on gasification reactivity of woody biomass-derived char. Proc. Combust. Inst. 2009, 32, 2013–2020. [Google Scholar] [CrossRef]
- Giudicianni, P.; Gargiulo, V.; Grottola, C.M.; Alfè, M.; Ferreiro, A.I.; Mendes, M.A.A.; Fagnano, M.; Ragucci, R. Inherent Metal Elements in Biomass Pyrolysis: A Review. Energy Fuels 2021, 35, 5407–5478. [Google Scholar] [CrossRef]
- Leijenhorst, E.J.; Wolters, W.; Van De Beld, L.; Prins, W. Inorganic element transfer from biomass to fast pyrolysis oil: Review and experiments. Fuel Process. Technol. 2016, 149, 96–111. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Song, Y.-C.; Li, W.-Y.; Feng, J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel 2017, 208, 377–409. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2011, 94, 1–33. [Google Scholar] [CrossRef]
- Dahou, T.; Defoort, F.; Nguyen, H.N.; Bennici, S.; Jeguirim, M.; Dupont, C. Biomass steam gasification kinetics: Relative impact of char physical properties vs. inorganic composition. Biomass-Convers. Biorefinery 2020, 1–16. [Google Scholar] [CrossRef]
- Frandsen, F.J. Ash Formation, Deposition and Corrosion when Utilizing Straw for Heat and Power Production; DTU Chemical Engineering: Lyngby, Denmark, 2010. [Google Scholar]
- De Jong, W.; Van Ommen, J.R. Biomass as a Sustainable Energy Source for the Future: Fundamentals of Conversion Processes; John Wiley & Sons, Inc.: New York, NY, USA, 2014; pp. 1–582. [Google Scholar] [CrossRef]
- Kleinhans, U.; Wieland, C.; Frandsen, F.J.; Spliethoff, H. Ash formation and deposition in coal and biomass fired combustion systems: Progress and challenges in the field of ash particle sticking and rebound behavior. Prog. Energy Combust. Sci. 2018, 68, 65–168. [Google Scholar] [CrossRef]
- Devi, T.G.; Kannan, M.P.; Abduraheem, V. Jump in the air gasification rate of potassium-doped cellulosic chars. Fuel Process. Technol. 2010, 91, 1826–1831. [Google Scholar] [CrossRef]
- Surup, G.R.; Hunt, A.J.; Attard, T.; Budarin, V.L.; Forsberg, F.; Arshadi, M.; Abdelsayed, V.; Shekhawat, D.; Trubetskaya, A. The effect of wood composition and supercritical CO2 extraction on charcoal production in ferroalloy industries. Energy 2019, 193, 116696. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Budarin, V.; Arshadi, M.; Magalhães, D.; Kazanç, F.; Hunt, A.J. Supercritical extraction of biomass as an effective pretreatment step for the char yield control in pyrolysis. Renew. Energy 2021, 170, 107–117. [Google Scholar] [CrossRef]
- Lestander, T.A.; Lindeberg, J.; Eriksson, D.; Bergsten, U. Prediction of Pinus sylvestris clear-wood properties using NIR spectroscopy and biorthogonal partial least squares regression. Can. J. For. Res. 2008, 38, 2052–2062. [Google Scholar] [CrossRef]
- Christensen, K.A.; Livbjerg, H. A Field Study of Submicron Particles from the Combustion of Straw. Aerosol Sci. Technol. 1996, 25, 185–199. [Google Scholar] [CrossRef]
- Trubetskaya, A. Fast Pyrolysis of Biomass at HIGH Temperatures. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2016. [Google Scholar]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Zevenhoven, M.; Yrjas, P.; Skrifvars, B.-J.; Hupa, M. Characterization of Ash-Forming Matter in Various Solid Fuels by Selective Leaching and Its Implications for Fluidized-Bed Combustion. Energy Fuels 2012, 26, 6366–6386. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Leahy, J.J.; Yazhenskikh, E.; Müller, M.; Layden, P.; Johnson, R.; Ståhl, K.; Monaghan, R.F. Characterization of woodstove briquettes from torrefied biomass and coal. Energy 2019, 171, 853–865. [Google Scholar] [CrossRef]
- Llorente, M.J.F.; García, J.E.C. Concentration of elements in woody and herbaceous biomass as a function of the dry ashing temperature. Fuel 2006, 85, 1273–1279. [Google Scholar] [CrossRef]
- Tolvanen, H. Advanced Solid Fuel Characterization for Reactivity and Physical Property Comparison. Ph.D. Thesis, Tampere University of Technology, Tampere, Finland, 2016. [Google Scholar]
- Yao, M.; Wang, D.; Zhao, M. Element Analysis Based on Energy-Dispersive X-ray Fluorescence. Adv. Mater. Sci. Eng. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Vamvuka, D.; Karouki, E.; Sfakiotakis, S.; Salatino, P. Gasification of Waste Biomass Chars by Carbon Dioxide via Thermogravimetry—Effect of Catalysts. Combust. Sci. Technol. 2012, 184, 64–77. [Google Scholar] [CrossRef]
- Limbeck, A.; Galler, P.; Bonta, M.; Bauer, G.; Nischkauer, W.; Vanhaecke, F. Recent advances in quantitative LA-ICP-MS analysis: Challenges and solutions in the life sciences and environmental chemistry ABC Highlights: Authored by Rising Stars and Top Experts. Anal. Bioanal. Chem. 2015, 407, 6593–6617. [Google Scholar] [CrossRef]
- McComb, J.Q.; Rogers, C.; Han, F.X.; Tchounwou, P.B. Rapid Screening of Heavy Metals and Trace Elements in Environmental Samples Using Portable X-ray Fluorescence Spectrometer, A Comparative Study. Water Air Soil Pollut. 2014, 225, 1–10. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd ed.; Wiley: New York, NY, USA, 1974; p. 992. [Google Scholar]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Surup, G.R.; Nielsen, H.K.; Großarth, M.; Deike, R.; Van den Bulcke, J.; Kibleur, P.; Müller, M.; Ziegner, M.; Yazhenskikh, E. Effect of operating conditions and feedstock composition on the properties of manganese oxide or quartz charcoal pellets for the use in ferroalloy industries. Energy 2020, 193, 116736. [Google Scholar] [CrossRef]
- Turpeinon, T. Analysis of Microtomographic Images of Porous Heterogeneous Materials; University of Jyväskylä: Jyväskylä, Finland, 2015. [Google Scholar]
- Vanderesse, N.; Maire, E.; Chabod, A.; Buffière, J.-Y. Microtomographic study and finite element analysis of the porosity harmfulness in a cast aluminium alloy. Int. J. Fatigue 2011, 33, 1514–1525. [Google Scholar] [CrossRef]
- Guntoro, P.I.; Ghorbani, Y.; Koch, P.-H.; Rosenkranz, J. X-ray Microcomputed Tomography (µCT) for Mineral Characterization: A Review of Data Analysis Methods. Minerals 2019, 9, 183. [Google Scholar] [CrossRef]
- Bohlke, J.K.; Irwin, J. Laser microprobe analyses of Cl, Br, I, and K in fluid inclusions: Implications for sources of salinity in some ancient hydrothermal fluids. Geochim. Cosmochim. Acta 1992, 56, 203–225. [Google Scholar] [CrossRef]
- Lanzetta, M.; Di Blasi, C. Pyrolysis kinetics of wheat and cornstraw. J. Anal. Appl. Pyrolysis 1998, 44, 181–192. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Kuppens, T.; Van Dael, M.; Vanreppelen, K.; Carleer, R.; Yperman, J.; Schreurs, S.; Van Passel, S. Techno-economic assessment of pyrolysis char production and application a review. Chem. Eng. Trans. 2014, 37, 67–72. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Grottola, C.M.; Giudicianni, P.; Pindozzi, S.; Stanzione, F.; Faugno, S.; Fagnano, M.; Fiorentino, N.; Ragucci, R. Steam assisted slow pyrolysis of contaminated biomasses: Effect of plant parts and process temperature on heavy metals fate. Waste Manag. 2019, 85, 232–241. [Google Scholar] [CrossRef]
- Eom, I.-Y.; Kim, J.-Y.; Kim, T.-S.; Lee, S.-M.; Choi, D.; Choi, I.-G.; Choi, J.-W. Effect of essential inorganic metals on primary thermal degradation of lignocellulosic biomass. Bioresour. Technol. 2012, 104, 687–694. [Google Scholar] [CrossRef]
- Oudenhoven, S.R.G.; Westerhof, R.J.M.; Aldenkamp, N.; Brilman, D.W.F.; Kersten, S.R.A. Demineralization of wood using wood-derived acid: Towards a selective pyrolysis process for fuel and chemicals production. J. Anal. Appl. Pyrolysis 2013, 103, 112–118. [Google Scholar] [CrossRef]
- Ranzi, E.; Cuoci, A.; Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Pierucci, S.; Sommariva, S. Chemical Kinetics of Biomass Pyrolysis. Energy Fuels 2008, 22, 4292–4300. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Surup, G.; Shapiro, A.; Bates, R.B. Modeling the influence of potassium content and heating rate on biomass pyrolysis. Appl. Energy 2017, 194, 199–211. [Google Scholar] [CrossRef]
- Dupont, C.; Nocquet, T.; Da Costa, J.A.; Verne-Tournon, C. Kinetic modelling of steam gasification of various woody biomass chars: Influence of inorganic elements. Bioresour. Technol. 2011, 102, 9743–9748. [Google Scholar] [CrossRef] [PubMed]
- Guizani, C.; Jeguirim, M.; Gadiou, R.; Escudero Sanz, F.J.; Salvador, S. Biomass char gasification by H2O, CO2 and their mixture: Evolution of chemical, textural and structural properties of the chars. Energy 2016, 112, 133–145. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Ling, Z.; Song, Y. Some new insights into the synergy occurring during char gasification in CO2/H2O mixtures. Fuel 2020, 268, 117307. [Google Scholar] [CrossRef]
- DeGroot, W.F.; Kannan, M.P.; Richards, G.N.; Theander, O. Gasification of agricultural residues (biomass): Influence of inorganic constituents. J. Agric. Food Chem. 1990, 38, 320–323. [Google Scholar] [CrossRef]
- Kirtania, K.; Axelsson, J.; Matsakas, L.; Christakopoulos, P.; Umeki, K.; Furusjö, E. Kinetic study of catalytic gasification of wood char impregnated with different alkali salts. Energy 2017, 118, 1055–1065. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, L.; Guan, J.; Xiong, Q.; Zhang, S.; Jin, X. A Review on Biomass Gasification: Effect of Main Parameters on Char Generation and Reaction. Energy Fuels 2020, 34, 13438–13455. [Google Scholar] [CrossRef]
- Marquez-Montesinos, F.; Cordero, T.; Rodríguez-Mirasol, J.; Rodríguez, J. CO2 and steam gasification of a grapefruit skin char. Fuel 2002, 81, 423–429. [Google Scholar] [CrossRef]
- Dupont, C.; Boissonnet, G.; Seiler, J.-M.; Gauthier-Maradei, P.; Schweich, D. Study about the kinetic processes of biomass steam gasification. Fuel 2007, 86, 32–40. [Google Scholar] [CrossRef]
- Gupta, A.; Thengane, S.K.; Mahajani, S. CO2 gasification of char from lignocellulosic garden waste: Experimental and kinetic study. Bioresour. Technol. 2018, 263, 180–191. [Google Scholar] [CrossRef]
- Kajita, M.; Kimura, T.; Norinaga, K.; Li, C.-Z.; Hayashi, J.-I. Catalytic and Noncatalytic Mechanisms in Steam Gasification of Char from the Pyrolysis of Biomass. Energy Fuels 2010, 24, 108–116. [Google Scholar] [CrossRef]
- Zhu, W.; Song, W.; Lin, W. Catalytic gasification of char from co-pyrolysis of coal and biomass. Fuel Process. Technol. 2008, 89, 890–896. [Google Scholar] [CrossRef]
- Lin, L.; Strand, M. Investigation of the intrinsic CO2 gasification kinetics of biomass char at medium to high temperatures. Appl. Energy 2013, 109, 220–228. [Google Scholar] [CrossRef]
- Hognon, C.; Dupont, C.; Grateau, M.; Delrue, F. Comparison of steam gasification reactivity of algal and lignocellulosic biomass: Influence of inorganic elements. Bioresour. Technol. 2014, 164, 347–353. [Google Scholar] [CrossRef]
- Kirnbauer, F.; Wilk, V.; Kitzler, H.; Kern, S.; Hofbauer, H. The positive effects of bed material coating on tar reduction in a dual fluidized bed gasifier. Fuel 2012, 95, 553–562. [Google Scholar] [CrossRef]
- Kuba, M.; Kraft, S.; Kirnbauer, F.; Maierhans, F.; Hofbauer, H. Influence of controlled handling of solid inorganic materials and design changes on the product gas quality in dual fluid bed gasification of woody biomass. Appl. Energy 2018, 210, 230–240. [Google Scholar] [CrossRef]
- Kramb, J.; Gómez-barea, A.; Demartini, N.; Romar, H.; Rama, T.; Doddapaneni, K.C.; Konttinen, J. The effects of calcium and potassium on CO2 gasification of birch wood in a fluidized bed. Fuel 2017, 196, 398–407. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, P.A.; Dam-Johansen, K. Transformation and Release to the Gas Phase of Cl, K, and S during Combustion of Annual Biomass. Energy Fuels 2004, 18, 1385–1399. [Google Scholar] [CrossRef]
- Yip, K.; Tian, F.; Hayashi, J.-I.; Wu, H. Effect of Alkali and Alkaline Earth Metallic Species on Biochar Reactivity and Syngas Compositions during Steam Gasification†. Energy Fuels 2010, 24, 173–181. [Google Scholar] [CrossRef]
- Ellis, N.; Masnadi, M.S.; Roberts, D.G.; Kochanek, M.A.; Ilyushechkin, A.Y. Mineral matter interactions during co-pyrolysis of coal and biomass and their impact on intrinsic char co-gasification reactivity. Chem. Eng. J. 2015, 279, 402–408. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takeno, K.; Ichinose, T.; Ogi, T.; Nakanishi, M. Gasification reaction kinetics on biomass char obtained as a by-product of gasification in an entrained-flow gasifier with steam and oxygen at 900–1000 °C. Fuel 2009, 88, 519–527. [Google Scholar] [CrossRef]
- Johansen, J.M.; Jakobsen, J.G.; Frandsen, F.J.; Glarborg, P. Release of K, Cl, and S during Pyrolysis and Combustion of High-Chlorine Biomass. Energy Fuels 2011, 25, 4961–4971. [Google Scholar] [CrossRef]
- Mermoud, F.; Salvador, S.; Vandesteene, L.; Golfier, F. Influence of the pyrolysis heating rate on the steam gasification rate of large wood char particles. Fuel 2006, 85, 1473–1482. [Google Scholar] [CrossRef]
- Khalil, R.; Várhegyi, G.; Jäschke, S.; Grønli, M.G.; Hustad, J. CO2 Gasification of Biomass Chars: A Kinetic Study. Energy Fuels 2008, 23, 94–100. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, P.; Liu, R. Synergistic combination of biomass torrefaction and co-gasification: Reactivity studies. Bioresour. Technol. 2017, 245, 225–233. [Google Scholar] [CrossRef]
- González-Vázquez, M.D.P.; García, R.; Gil, M.; Pevida, C.; Rubiera, F. Unconventional biomass fuels for steam gasification: Kinetic analysis and effect of ash composition on reactivity. Energy 2018, 155, 426–437. [Google Scholar] [CrossRef]
- Ding, S.; Kantarelis, E.; Engvall, K. Effects of Porous Structure Development and Ash on the Steam Gasification Reactivity of Biochar Residues from a Commercial Gasifier at Different Temperatures. Energies 2020, 13, 5004. [Google Scholar] [CrossRef]
- Dahou, T.; Defoort, F.; Khiari, B.; Labaki, M.; Dupont, C.; Jeguirim, M. Role of inorganics on the biomass char gasification reactivity: A review involving reaction mechanisms and kinetics models. Renew. Sustain. Energy Rev. 2020, 135, 110136. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Gupta, A.K. Kinetics of woodchips char gasification with steam and carbon dioxide. Appl. Energy 2011, 88, 1613–1619. [Google Scholar] [CrossRef]
- Long, J.; Song, H.; Jun, X.; Sheng, S.; Lun-Shi, S.; Kai, X.; Yao, Y. Release characteristics of alkali and alkaline earth metallic species during biomass pyrolysis and steam gasification process. Bioresour. Technol. 2012, 116, 278–284. [Google Scholar] [CrossRef]
- Mckee, D.W. Gasification of graphite in CO2 and water vapor- the catalytic effect of metal salts. Carbon N. Y. 1982, 20, 59–66. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Cerfontain, M.B.; Kapteijn, F. Mechanism of the potassium catalysed gasification of carbon in CO2. Fuel 1984, 63, 1043–1047. [Google Scholar] [CrossRef]
- Masnadi, M.S.; Grace, J.R.; Bi, X.T.; Ellis, N.; Lim, C.J.; Butler, J.W. Biomass/coal steam co-gasification integrated with in-situ CO2 capture. Energy 2015, 83, 326–336. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, X.; Wu, C.; Wang, C.; Xie, J.; Zhou, Z.; Ma, L.; Li, H. Effects of metal catalysts on CO2 gasification reactivity of biomass char. Biotechnol. Adv. 2009, 27, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Miura, K.; Xu, J.-J.; Watanabe, A.; Masukami, H. Relation between the gasification rate of carbons supporting alkali metal salts and the amount of oxygen trapped by the metal. Fuel 1986, 65, 489–494. [Google Scholar] [CrossRef]
- Duman, G.; Uddin, M.A.; Yanik, J. The effect of char properties on gasification reactivity. Fuel Process. Technol. 2014, 118, 32–40. [Google Scholar] [CrossRef]
- Strandberg, A.; Holmgren, P.; Wagner, D.R.; Molinder, R.; Wiinikka, H.; Umeki, K.; Broström, M. Effects of Pyrolysis Conditions and Ash Formation on Gasification Rates of Biomass Char. Energy Fuels 2017, 31, 6507–6514. [Google Scholar] [CrossRef]
- Strandberg, A. Fuel Conversion and Ash Formation Interactions A Thermochemical Study on Lignocellulosic Biomass. Ph.D. Thesis, Umeå University, Umeå, Sweden, 2018. [Google Scholar]
- Trubetskaya, A.; Jensen, P.A.; Jensen, A.D.; Steibel, M.; Spliethoff, H.; Glarborg, P.; Larsen, F.H. Comparison of high temperature chars of wheat straw and rice husk with respect to chemistry, morphology and reactivity. Biomass-Bioenergy 2016, 86, 76–87. [Google Scholar] [CrossRef]
- Novaković, A.; van Lith, S.C.; Frandsen, F.J.; Jensen, P.A.; Holgersen, L.B. Release of Potassium from the Systems K−Ca−Si and K−Ca−P. Energy Fuels 2009, 23, 3423–3428. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Q.; Yao, Q.; Yang, R. Influence of the Atmosphere on the Transformation of Alkali and Alkaline Earth Metallic Species during Rice Straw Thermal Conversion. Energy Fuels 2012, 26, 1892–1899. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Gong, Y.; Ding, L.; Wang, J.; Yu, G. A review of the effects of alkali and alkaline earth metal species on biomass gasification. Fuel Process. Technol. 2021, 214, 106723. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Larsen, F.H.; Shchukarev, A.; Ståhl, K.; Umeki, K. Potassium and soot interaction in fast biomass pyrolysis at high temperatures. Fuel 2018, 225, 89–94. [Google Scholar] [CrossRef]
- Vilches, T.B.; Maric, J.; Knutsson, P.; Rosenfeld, D.C.; Thunman, H.; Seemann, M. Bed material as a catalyst for char gasification: The case of ash-coated olivine activated by K and S addition. Fuel 2018, 224, 85–93. [Google Scholar] [CrossRef]
- Larsson, A.; Kuba, M.; Vilches, T.B.; Seemann, M.; Hofbauer, H.; Thunman, H. Steam gasification of biomass—Typical gas quality and operational strategies derived from industrial-scale plants. Fuel Process. Technol. 2020, 212, 106609. [Google Scholar] [CrossRef]
- Kuba, M.; Fürsatz, K.; Janisch, D.; Aziaba, K.; Chlebda, D.; Łojewska, J.; Forsberg, F.; Umeki, K.; Hofbauer, H. Surface characterization of ash-layered olivine from fluidized bed biomass gasification. Biomass-Convers. Biorefinery 2020, 11, 29–38. [Google Scholar] [CrossRef]
- Kryca, J.; Priščák, J.; Łojewska, J.; Kuba, M.; Hofbauer, H. Apparent kinetics of the water-gas-shift reaction in biomass gasification using ash-layered olivine as catalyst. Chem. Eng. J. 2018, 346, 113–119. [Google Scholar] [CrossRef]
- Benedikt, F.; Fuchs, J.; Schmid, J.C.; Müller, S.; Hofbauer, H. Advanced dual fluidized bed steam gasification of wood and lignite with calcite as bed material. Korean J. Chem. Eng. 2017, 34, 2548–2558. [Google Scholar] [CrossRef]
- Kuba, M.; Skoglund, N.; Öhman, M.; Hofbauer, H. A review on bed material particle layer formation and its positive influence on the performance of thermo-chemical biomass conversion in fluidized beds. Fuel 2021, 291, 120214. [Google Scholar] [CrossRef]
- Namkung, H.; Park, J.-H.; Lee, Y.-J.; Song, G.-S.; Choi, J.W.; Park, S.-J.; Kim, S.; Liu, J.; Choi, Y.-C. Performance evaluation of biomass pretreated by demineralization and torrefaction for ash deposition and PM emissions in the combustion experiments. Fuel 2021, 292, 120379. [Google Scholar] [CrossRef]
- Zhao, D.; Dai, Y.; Chen, K.; Sun, Y.; Yang, F.; Chen, K. Effect of potassium inorganic and organic salts on the pyrolysis kinetics of cigarette paper. J. Anal. Appl. Pyrolysis 2013, 102, 114–123. [Google Scholar] [CrossRef]
- Davidsson, K.O.; Korsgren, J.G.; Pettersson, J.B.C. The effects of fuel washing techniques on alkali release from biomass. Fuel 2002, 81, 137–142. [Google Scholar] [CrossRef]
- Arnold, R.A.; Hill, J.M. Catalysts for gasification: A review. Sustain. Energy Fuels 2019, 3, 656–672. [Google Scholar] [CrossRef]
- Liu, X.; Bi, X.T. Removal of inorganic constituents from pine barks and switchgrass. Fuel Process. Technol. 2011, 92, 1273–1279. [Google Scholar] [CrossRef]
- Mayer, Z.A.; Apfelbacher, A.; Hornung, A. Effect of sample preparation on the thermal degradation of metal-added biomass. J. Anal. Appl. Pyrolysis 2012, 94, 170–176. [Google Scholar] [CrossRef]
- Fierro, V.; Torné-Fernández, V.; Celzard, A.; Montané, D. Influence of the demineralisation on the chemical activation of Kraft lignin with orthophosphoric acid. J. Hazard. Mater. 2007, 149, 126–133. [Google Scholar] [CrossRef]
- Keown, D.M.; Hayashi, J.-I.; Li, C.-Z. Effects of volatile–char interactions on the volatilisation of alkali and alkaline earth metallic species during the pyrolysis of biomass. Fuel 2008, 87, 1187–1194. [Google Scholar] [CrossRef]
- Das, P.; Ganesh, A.; Wangikar, P. Influence of pretreatment for deashing of sugarcane bagasse on pyrolysis products. Biomass- Bioenergy 2004, 27, 445–457. [Google Scholar] [CrossRef]
- Mourant, D.; Wang, Z.; He, M.; Wang, X.S.; Garcia-Perez, M.; Ling, K.; Li, C.-Z. Mallee wood fast pyrolysis: Effects of alkali and alkaline earth metallic species on the yield and composition of bio-oil. Fuel 2011, 90, 2915–2922. [Google Scholar] [CrossRef]
- Tan, H.; Wang, S.-R. Experimental study of the effect of acid-washing pretreatment on biomass pyrolysis. J. Fuel Chem. Technol. 2009, 37, 668–672. [Google Scholar] [CrossRef]
- Lv, D.; Xu, M.; Liu, X.; Zhan, Z.; Li, Z.; Yao, H. Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. Fuel Process. Technol. 2010, 91, 903–909. [Google Scholar] [CrossRef]
- Eom, I.-Y.; Kim, K.-H.; Kim, J.-Y.; Lee, S.-M.; Yeo, H.-M.; Choi, I.-G.; Choi, J.-W. Characterization of primary thermal degradation features of lignocellulosic biomass after removal of inorganic metals by diverse solvents. Bioresour. Technol. 2010, 102, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Jakab, E.; Sebestyén, Z.; May, Z.; Réczey, K. The effect of alkaline pretreatment on the thermal decomposition of hemp. J. Therm. Anal. 2010, 105, 1061–1069. [Google Scholar] [CrossRef]
- Jensen, A.; Dam-Johansen, K.; Wójtowicz, M.A.; Serio, M.A. TG-FTIR Study of the Influence of Potassium Chloride on Wheat Straw Pyrolysis. Energy Fuels 1998, 12, 929–938. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Jensen, P.A.; Jensen, A.D.; Llamas, A.D.G.; Umeki, K.; Glarborg, P. Effect of fast pyrolysis conditions on biomass solid residues at high temperatures. Fuel Process. Technol. 2016, 143, 118–129. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Budarin, V.; Arshadi, M.; Grams, J.; Hunt, A.J. Supercritical extraction of biomass Extraction of Biomass—A Green and Sustainable Method to Control the Pyrolysis Product Distirbution. ACS Sustain. Chem. Eng. 2021, 9, 5278–5287. [Google Scholar] [CrossRef]
- Schneider, C.; Walker, S.; Phounglamcheik, A.; Umeki, K.; Kolb, T. Effect of calcium dispersion and graphitization during high-temperature pyrolysis of beech wood char on the gasification rate with CO2. Fuel 2020, 283, 118826. [Google Scholar] [CrossRef]
- Bajcar, M.; Zagula, G.; Saletnik, B.; Tarapatskyy, M.; Puchalski, C. Relationship between Torrefaction Parameters and Physicochemical Properties of Torrefied Products Obtained from Selected Plant Biomass. Energies 2018, 11, 2919. [Google Scholar] [CrossRef]
- Li, T.; Geier, M.T.; Wang, L.; Shaddix, C.R.; Ku, X.; Matas Güell, B.; Lovås, T. Effect of Torrefaction on Physical Properties and Conversion Behavior of High Heating Rate Char of Forest Residue. Energy Fuels 2014, 29, 177–184. [Google Scholar] [CrossRef]
- Shoulaifar, T.K.; DeMartini, N.; Zevenhoven, M.; Verhoeff, F.; Kiel, J.; Hupa, M. Ash-Forming Matter in Torrefied Birch Wood: Changes in Chemical Association. Energy Fuels 2013, 27, 5684–5690. [Google Scholar] [CrossRef]
- Barta-Rajnai, E.; Jakab, E.; Sebestyén, Z.; May, Z.; Barta, Z.; Wang, L.; Skreiberg, Ø.; Grønli, M.; Bozi, J.; Czégény, Z. Comprehensive Compositional Study of Torrefied Wood and Herbaceous Materials by Chemical Analysis and Thermoanalytical Methods. Energy Fuels 2016, 30, 8019–8030. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C.; Galgano, A.; Broström, M. Effects of the Torrefaction Conditions on the Fixed-Bed Pyrolysis of Norway Spruce. Energy Fuels 2014, 28, 5882–5891. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Leahy, J.J.; Grams, J.; Kwapinska, M.; Gallagher, P.; Johnson, R.; Monaghan, R.F.D. The effect of particle size, temperature and residence time on the yields and reactivity of olive stones from torrefaction. Renew. Energy 2020, 160, 998–1011. [Google Scholar] [CrossRef]
- Surup, G.R.; Timko, M.T.; Leahy, J.J.; Trubetskaya, A. Hydrothermal carbonization of olive wastes to produce renewable, binder-free pellets for use as metallurgical reducing agents. Renew. Energy 2020, 155, 347–357. [Google Scholar] [CrossRef]
- Mosqueda, A.; Medrano, K.; Gonzales, H.; Yoshikawa, K. Effect of hydrothermal and washing treatment of banana leaves on co-gasification reactivity with coal. Energy Procedia 2019, 158, 785–791. [Google Scholar] [CrossRef]
- Hedler, A.; Klaumünzer, S.L.; Wesch, W. Amorphous silicon exhibits a glass transition. Nat. Mater. 2004, 3, 804–809. [Google Scholar] [CrossRef]
- Momeni, M.; Yin, C.; Kær, S.K.; Hansen, T.B.; Jensen, P.A.; Glarborg, P. Experimental Study on Effects of Particle Shape and Operating Conditions on Combustion Characteristics of Single Biomass Particles. Energy Fuels 2012, 27, 507–514. [Google Scholar] [CrossRef]
- Lu, H. Experimental and Modeling Investigations of Biomass Particle Combustion. Ph.D. Thesis, Brigham Young University, Provo, UT, USA, 2006. [Google Scholar]
- Lu, H.; Ip, E.; Scott, J.; Foster, P.; Vickers, M.; Baxter, L.L. Effects of particle shape and size on devolatilization of biomass particle. Fuel 2010, 89, 1156–1168. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Perlmutter, D.D. A random pore model for fluid-solid reactions: I. Isothermal, kinetic control. AIChE J. 1980, 26, 379–386. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Wang, Y.; Su, S.; Sun, L.; Xu, B.; He, L.; Xiang, J. Catalytic effects of inherent alkali and alkaline earth metallic species on steam gasification of biomass. Int. J. Hydrogen Energy 2015, 40, 15460–15469. [Google Scholar] [CrossRef]
- María, L.; Millán, R.; Emiro, F.; Vargas, S.; Nzihou, A. Steam gasification behavior of tropical agrowaste: A new modeling approach based on the inorganic composition. Fuel 2018, 235, 45–53. [Google Scholar] [CrossRef]
- Fürsatz, K.; Fuchs, J.; Benedikt, F.; Kuba, M.; Hofbauer, H. Effect of biomass fuel ash and bed material on the product gas composition in DFB steam gasification. Energy 2020, 219, 119650. [Google Scholar] [CrossRef]
- Millán, L.M.R.; Vargas, F.E.S.; Nzihou, A. Catalytic Effect of Inorganic Elements on Steam Gasification Biochar Properties from Agrowastes. Energy Fuels 2019, 33, 8666–8675. [Google Scholar] [CrossRef]
- Siddiqui, H.; Thengane, S.K.; Sharma, S.; Mahajani, S.M. Revamping downdraft gasifier to minimize clinker formation for high-ash garden waste as feedstock. Bioresour. Technol. 2018, 266, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Thengane, S.K.; Gupta, A.; Mahajani, S.M. Co-gasification of high ash biomass and high ash coal in downdraft gasifier. Bioresour. Technol. 2018, 273, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Turn, S.Q.; Kinoshita, C.M.; Ishimura, D.M.; Zhou, J. The fate of inorganic constituents of biomass in fluidized bed gasification. Fuel 1998, 77, 135–146. [Google Scholar] [CrossRef]
- Moilanen, A.; Nasrullah, M.; Kurkela, E. The effect of biomass feedstock type and process parameters on achieving the total carbon conversion in the large scale fluidized bed gasification of biomass. Environ. Prog. Sustain. Energy 2009, 28, 355–359. [Google Scholar] [CrossRef]
- Gao, N.; Śliz, M.; Quan, C.; Bieniek, A.; Magdziarz, A. Biomass CO2 gasification with CaO looping for syngas production in a fixed-bed reactor. Renew. Energy 2020, 167, 652–661. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B.; Sharrock, P. A review of catalysts for the gasification of biomass char, with some reference to coal. Energy 2013, 58, 305–317. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Hauserman, W. High-yield hydrogen production by catalytic gasification of coal or biomass. Int. J. Hydrogen Energy 1994, 19, 413–419. [Google Scholar] [CrossRef][Green Version]
- Struis, R.P.W.J.; Von Scala, C.; Stucki, S.; Prins, R. Gasification reactivity of charcoal with CO2. Part I: Conversion and structural phenomena. Chem. Eng. Sci. 2002, 57, 3581–3592. [Google Scholar] [CrossRef]
- Moilanen, A.; Nasrullah, M. Gasification Reactivity and Ash Sintering Behaviour of Biomass Feedstocks; VTT Publications: Espoo, Finland, 1982. [Google Scholar]
- Zhang, Y.; Ashizawa, M.; Kajitani, S. Calcium loading during the dewatering of wet biomass in kerosene and catalytic activity for subsequent char gasification. Fuel 2008, 87, 3024–3030. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Y.; Zhang, Y.; Sun, S. Effects of H2O and CO2 on the homogeneous conversion and heterogeneous reforming of biomass tar over biochar. Int. J. Hydrogen Energy 2017, 42, 13070–13084. [Google Scholar] [CrossRef]
- Nanou, P.; Murillo, H.E.G.; van Swaaij, W.P.; van Rossum, G.; Kersten, S.R. Intrinsic reactivity of biomass-derived char under steam gasification conditions-potential of wood ash as catalyst. Chem. Eng. J. 2013, 217, 289–299. [Google Scholar] [CrossRef]
- Lee, D.; Kung, K.; Ratti, C. Mapping the Waste Handling Dynamics in Mombasa Using Mobile Phone GPS. In Proceedings of the 14th International Conference on Computers in Urban Planning and Urban Management, Cambridge, MA, USA, 7–10 July 2015. [Google Scholar]
- Seemen, A.; Atong, D.; Sricharoenchaikul, V. Effect of Silica and Alumina Ratio on Bed Agglomeration during Fluidized Bed Gasification of Rice Straw. In Proceedings of the 2018 2nd International Conference on Green Energy and Applications (ICGEA), Singapore, 24–26 March 2018. [Google Scholar]
- Bouraoui, Z.; Jeguirim, M.; Limousy, L.; Dupont, C.; Gadiou, R. CO2 gasification of woody biomass chars: The influence of K and Si on char reactivity. C. R. Chim. 2016, 19, 457–465. [Google Scholar] [CrossRef]
- Baxter, L.L.; Miles, T.R.; Miles, T.R.; Jenkins, B.M.; Milne, T.; Dayton, D.; Bryers, R.W.; Oden, L.L. The behavior of inorganic material in biomass-fired power boilers: Field and laboratory experiences. Fuel Process. Technol. 1998, 54, 47–78. [Google Scholar] [CrossRef]
- Mitsuoka, K.; Hayashi, S.; Amano, H.; Kayahara, K.; Sasaoaka, E.; Uddin, A. Gasification of woody biomass char with CO2: The catalytic effects of K and Ca species on char gasification reactivity. Fuel Process. Technol. 2011, 92, 26–31. [Google Scholar] [CrossRef]
- López-González, D.; Fernandez-Lopez, M.; Valverde, J.L.; Sanchez-Silva, L. Gasification of lignocellulosic biomass char obtained from pyrolysis: Kinetic and evolved gas analyses. Energy 2014, 71, 456–467. [Google Scholar] [CrossRef]
- Lahijani, P.; Alimuddin, Z.; Rahman, A.; Mohammadi, M. CO2 gasification reactivity of biomass char: Catalytic influence of alkali, alkaline earth and transition metal salts. Bioresour. Technol. 2013, 144, 288–295. [Google Scholar] [CrossRef]
- Boström, D.; Skoglund, N.; Grimm, A.; Boman, C.; Öhman, M.; Broström, M.; Backman, R. Ash Transformation Chemistry during Combustion of Biomass. Energy Fuels 2011, 26, 85–93. [Google Scholar] [CrossRef]
- Skoglund, N. Ash Chemistry and Fuel Design Focusing on Combustion of Phosphorus-Rich Biomass. Ph.D. Thesis, Umeå University, Umeå, Sweden, 2014. [Google Scholar]
- Shi, L.; Yu, S.; Wang, F.C.; Wang, J. Pyrolytic characteristics of rice straw and its constituents catalyzed by internal alkali and alkali earth metals. Fuel 2012, 96, 586–594. [Google Scholar] [CrossRef]
- Gomez-Barea, A.; Leckner, B. Modeling of biomass gasification in fluidized bed. Prog. Energy Combust. Sci. 2010, 36, 444–509. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Influence of mineral matter on biomass pyrolysis characteristics. Fuel 1995, 74, 1812–1822. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Chen, M.; Min, F.; Zhang, S.; Ren, Z.; Yan, Y. Catalytic effects of six inorganic compounds on pyrolysis of three kinds of biomass. Thermochim. Acta 2006, 444, 110–114. [Google Scholar] [CrossRef]
| Group | Organic Forms | Inorganic Forms | Dominant Forms |
|---|---|---|---|
| Alkali metals (K, Na) | Oxalate | Cation in liquid matter (Na+, K+), KNO3, NaCl, KCl, NaNO3 | Often in ionic salt forms |
| Alkaline earth metals (Ca, Mg) | Carbonates, oxalates | Cation in liquid matter (Ca2+, Mg2+), Ca3(PO4)2, CaCl2, Mg3(PO4)2, MgCl2 | Mg and Ca form structures with counterions of organic origin |
| Transition metals (Zn, Fe, Cd, Cu, Cr, Ni, Mn, Co) | Fe-chelates, Mn-carbohydrate | Cation in fluid matter (Mn2+, Fe2+, Cr3+), metallic structures, iron oxide | Often in small (<2 μm) crystal structures which are introduced by harvesting and/or pre-/post-treatment |
| Post-transition metals (Pb, Al) | - | Aluminium hydroxide (Al(OH)3), kaolinite | Highly variable and typically present in inorganic forms from different processing |
| Non-metals (S, P) | Covalently bound proteins, amino acids | Sulfite (SO32−), sulfate anion (SO42−), and phosphate anion (PO43−) | Differs with feedstock type |
| Fuel | Pinewood | Beechwood [18] | Wheat Straw [18] | Alfalfa Straw [18] | Rice Husk [20] | Miscan-Thus [21] | Olive Stones [22] | Anthra-Cite [22] | Poultry Litter [23] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Needles [13] | Bark [19] | Branches [19] | Cones [19] | Stumps [19] | Stem [18] | ||||||||||
| Small | Large | ||||||||||||||
| Proximate and ultimate analysis (% on dry basis) | |||||||||||||||
| Moisture | 5.6 | 7.4 | 8 | 6.8 | 7.5 | 5.7 | 5.1 | 4.5 | 5.5 | 5.2 | 4.5 | 27.0 | 15.5 | 2.7 | 22.1 |
| Ash | 2.3 | 2.3 | 0.5 | 1 | 0.5 | 0.1 | 0.3 | 1.4 | 4.1 | 7.4 | 21.7 | 3.4 | 0.8 | 9.9 | 17.5 |
| Volatiles | 78.8 | 76.7 | 70.9 | 79.9 | 78.3 | 86.5 | 86.6 | 79.4 | 77.5 | 75.9 | 64.3 | 77.8 | 76 | 15.8 | 73.7 |
| HHV | 21.3 | 19.5 | 21.3 | 20.9 | 20 | 21.2 | 21.6 | 20.2 | 18.8 | 19.7 | 15.5 | 14.1 | 20.3 | 32.2 | 19.7 |
| LHV | 20 | 18.2 | 20.1 | 19.6 | 18.8 | 19.8 | 20.2 | 19 | 17.5 | 16.9 | 14.5 | 12.5 | 18.8 | 31.5 | 13.5 |
| C | 51.8 | 49.5 | 54.5 | 51.4 | 51.1 | 52.3 | 53.1 | 50.7 | 46.6 | 42.5 | 37.8 | 48.5 | 44.8 | 72.3 | 35.1 |
| H | 6.3 | 5.6 | 5.4 | 5.9 | 5.5 | 6 | 6.5 | 5.9 | 6.1 | 6.7 | 4.7 | 6.0 | 5.8 | 2.9 | 4.1 |
| O | 38.2 | 42 | 39.4 | 41.2 | 42.6 | 41.5 | 40 | 41.9 | 42.5 | 43.1 | 0.3 | 41.6 | 48.3 | 13.2 | 30.8 |
| N | 1.4 | 0.6 | 0.2 | 0.5 | 0.3 | 0.1 | 0.06 | 0.13 | 0.6 | 0.3 | 35.5 | 0.5 | 0.2 | 1.0 | 4.2 |
| S | 0.1 | 0.04 | 0.02 | 0.03 | 0.02 | <0.01 | <0.01 | 0.02 | 0.1 | 0.03 | 0.03 | 0.07 | 0.1 | 0.7 | 0.6 |
| Ash compositional analysis (mg kg−1 on dry basis) | |||||||||||||||
| Cl | 0.02 | 0.01 | <0.01 | <0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.1 | 0.5 | 0.05 | 0.3 | 0.01 | 0.03 | 0.5 |
| Al | 250 | 550 | 250 | 150 | 150 | 40 | 10 | 10 | 150 | 600 | 70 | 270 | 100 | 12,000 | 5500 |
| Ca | 2450 | 4700 | 1200 | 1300 | 250 | 500 | 600 | 2000 | 2500 | 12,900 | 750 | 1100 | 1650 | 3500 | 7800 |
| Fe | 70 | 60 | 60 | 60 | 20 | 30 | 20 | 10 | 200 | - | 80 | 270 | 70 | 7200 | 400 |
| K | 5600 | 35 | 800 | 2000 | 2000 | 200 | 200 | 3600 | 11,000 | 28,000 | 2500 | 7900 | 1600 | 2000 | 1600 |
| Mg | 750 | 90 | 200 | 400 | 350 | 100 | 100 | 600 | 750 | 1400 | 400 | 540 | 150 | 350 | 2000 |
| Na | 25 | 10 | 10 | <10 | 210 | <10 | 30 | 100 | 150 | 1000 | 70 | 340 | 300 | 2000 | 2000 |
| P | 1500 | 75 | 150 | 400 | 350 | <10 | 6 | 150 | 550 | 1900 | 600 | 740 | 100 | 800 | 6800 |
| Si | 400 | 15 | 350 | 400 | 350 | 150 | 50 | 200 | 8500 | 2000 | 9850 | 6200 | 1800 | 41,000 | 9200 |
| Ti | 4 | 1 | 2 | 6 | 7 | 1 | 2 | 8 | 10 | 3 | 5 | 13 | 10 | 700 | 40 |
| Ref. | Model | System, Gasifying Agent, Feedstock |
|---|---|---|
| [150] | where b is a constant of dimension [time−1] and p is a dimensionless power law constant | TGA, CO2, Fir charcoal |
| [60] | TGA, H2O, woody biomass | |
| [67,68] | where c is a dimensionless constant, and p is a dimensionless power law constant | TGA, H2O, CO2, wood, herbaceous biomass |
| [162] | where ka is the activation constant, | TGA-MS, H2O, Eucalyptus wood, fir wood, pine bark, lignin, cellulose, hemicellulose |
| [141] | where P is the steam partial pressure, n is the theoretical model reaction order, and k1 is the coefficient dependent on the ratio K/(Si + P) | TGA, H2O, coconut shells, oil palm shells, bamboo guadua |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trubetskaya, A. Reactivity Effects of Inorganic Content in Biomass Gasification: A Review. Energies 2022, 15, 3137. https://doi.org/10.3390/en15093137
Trubetskaya A. Reactivity Effects of Inorganic Content in Biomass Gasification: A Review. Energies. 2022; 15(9):3137. https://doi.org/10.3390/en15093137
Chicago/Turabian StyleTrubetskaya, Anna. 2022. "Reactivity Effects of Inorganic Content in Biomass Gasification: A Review" Energies 15, no. 9: 3137. https://doi.org/10.3390/en15093137
APA StyleTrubetskaya, A. (2022). Reactivity Effects of Inorganic Content in Biomass Gasification: A Review. Energies, 15(9), 3137. https://doi.org/10.3390/en15093137