An Experimental and a Kinetic Modelling Study of Ethanol/Acetone/Ethyl Acetate Mixtures
Abstract
:1. Introduction
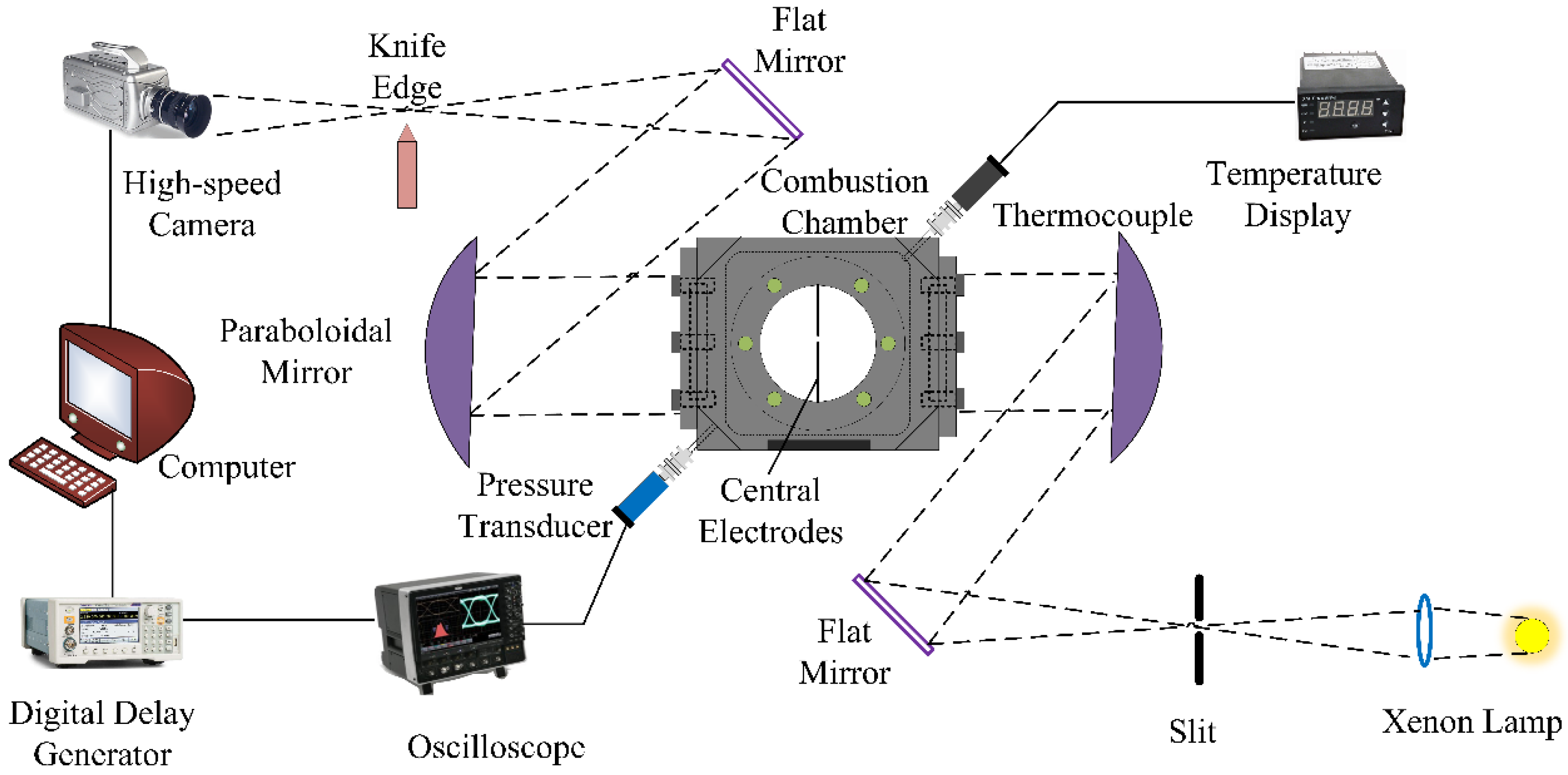
2. Experimental Set-Up
3. Data Processing
4. Results and Discussion
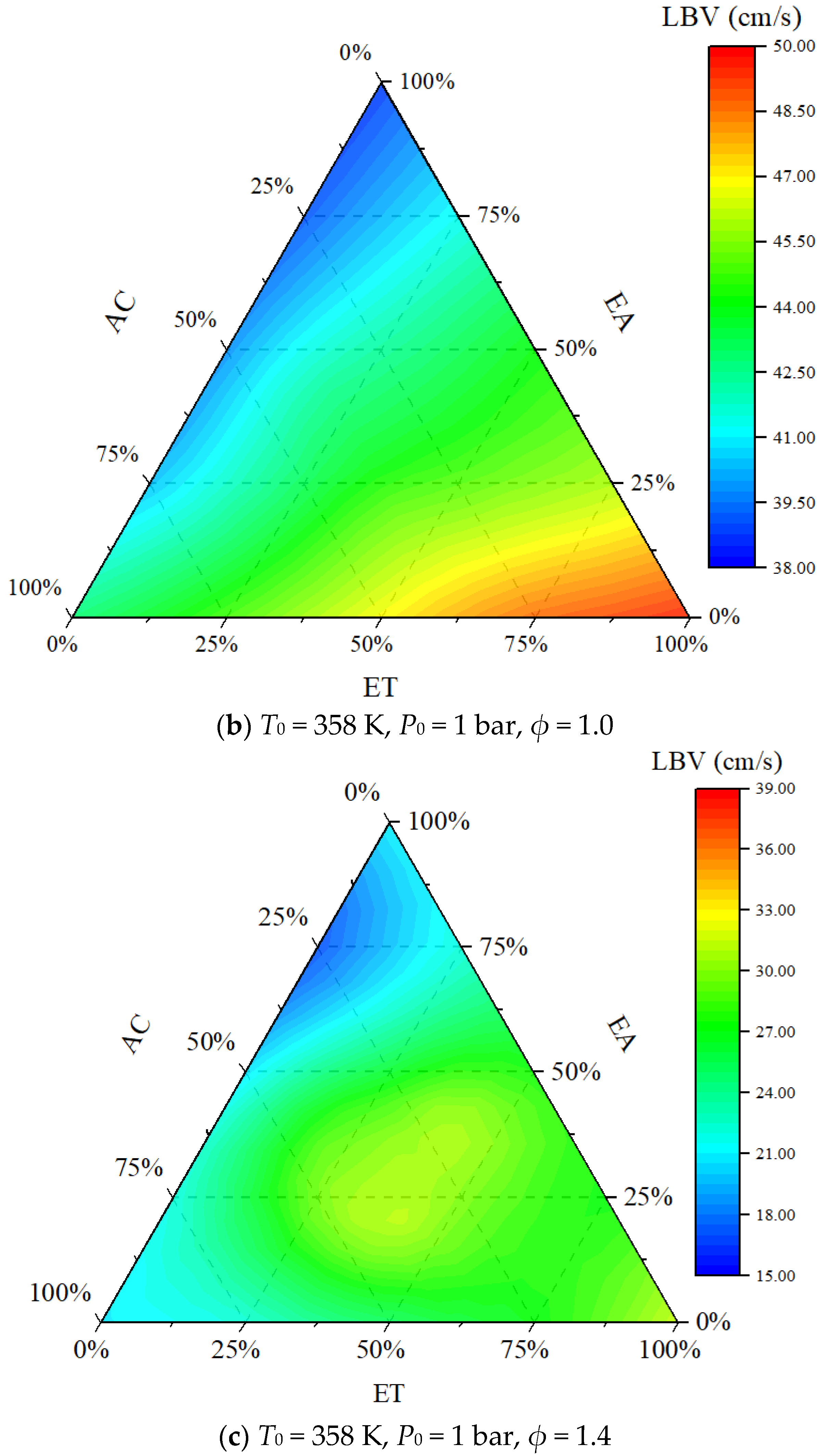
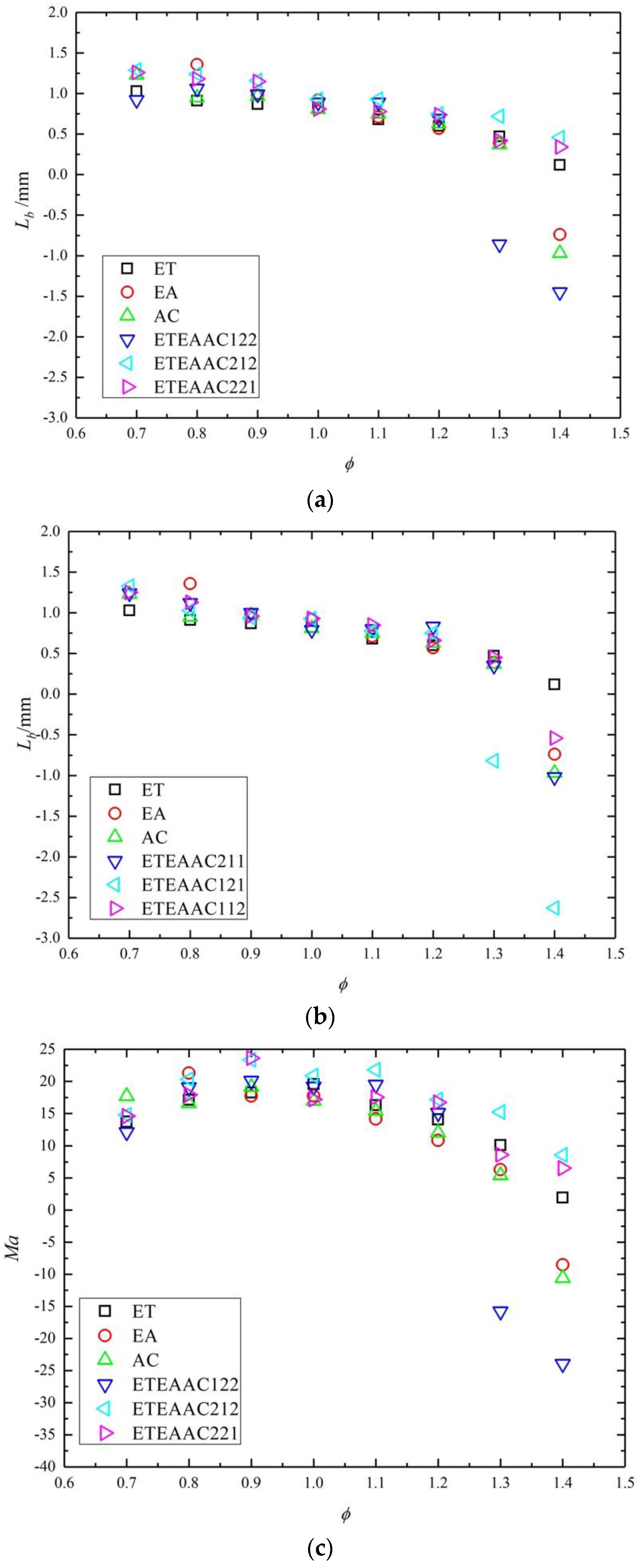
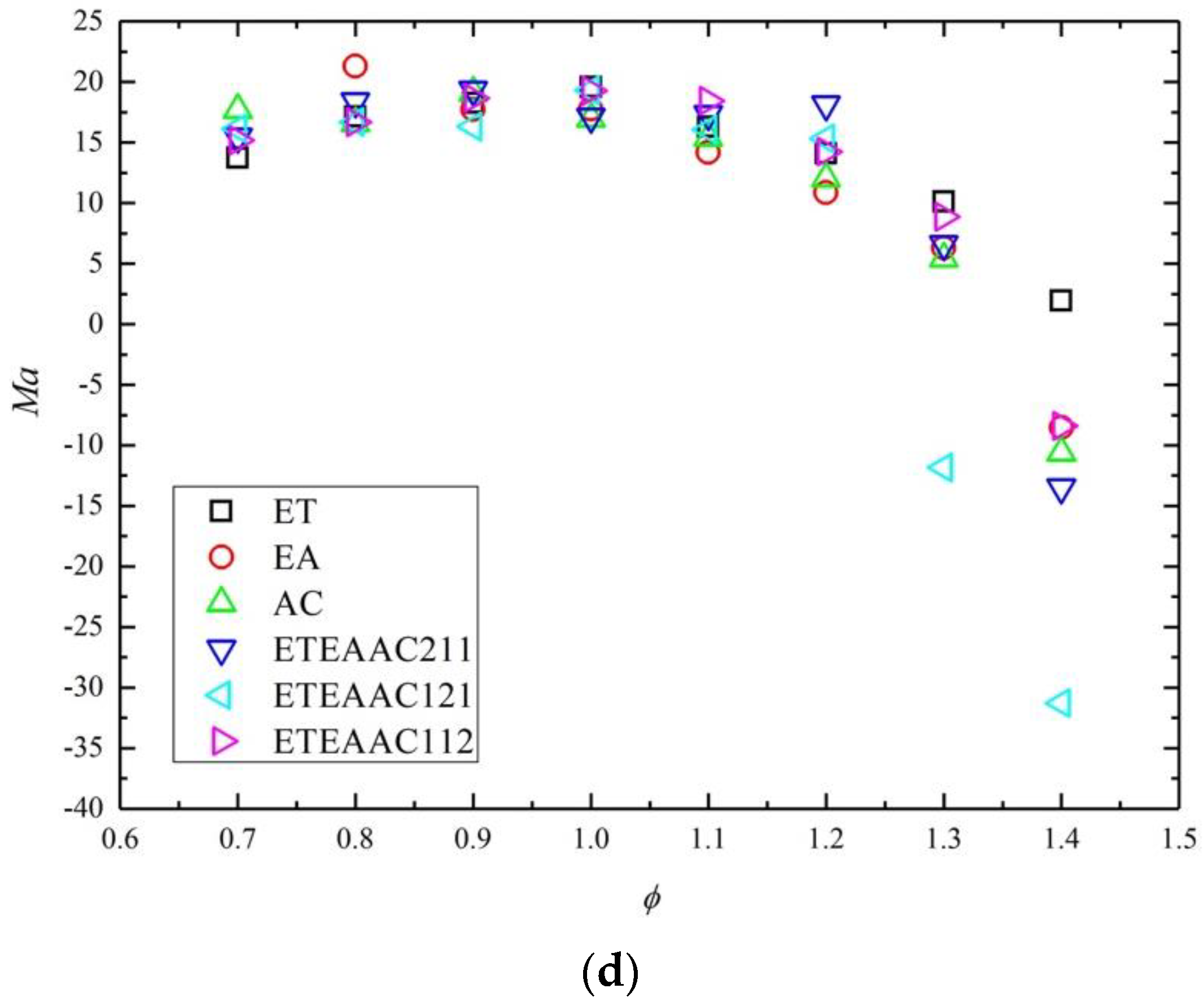
4.1. Flame Topography
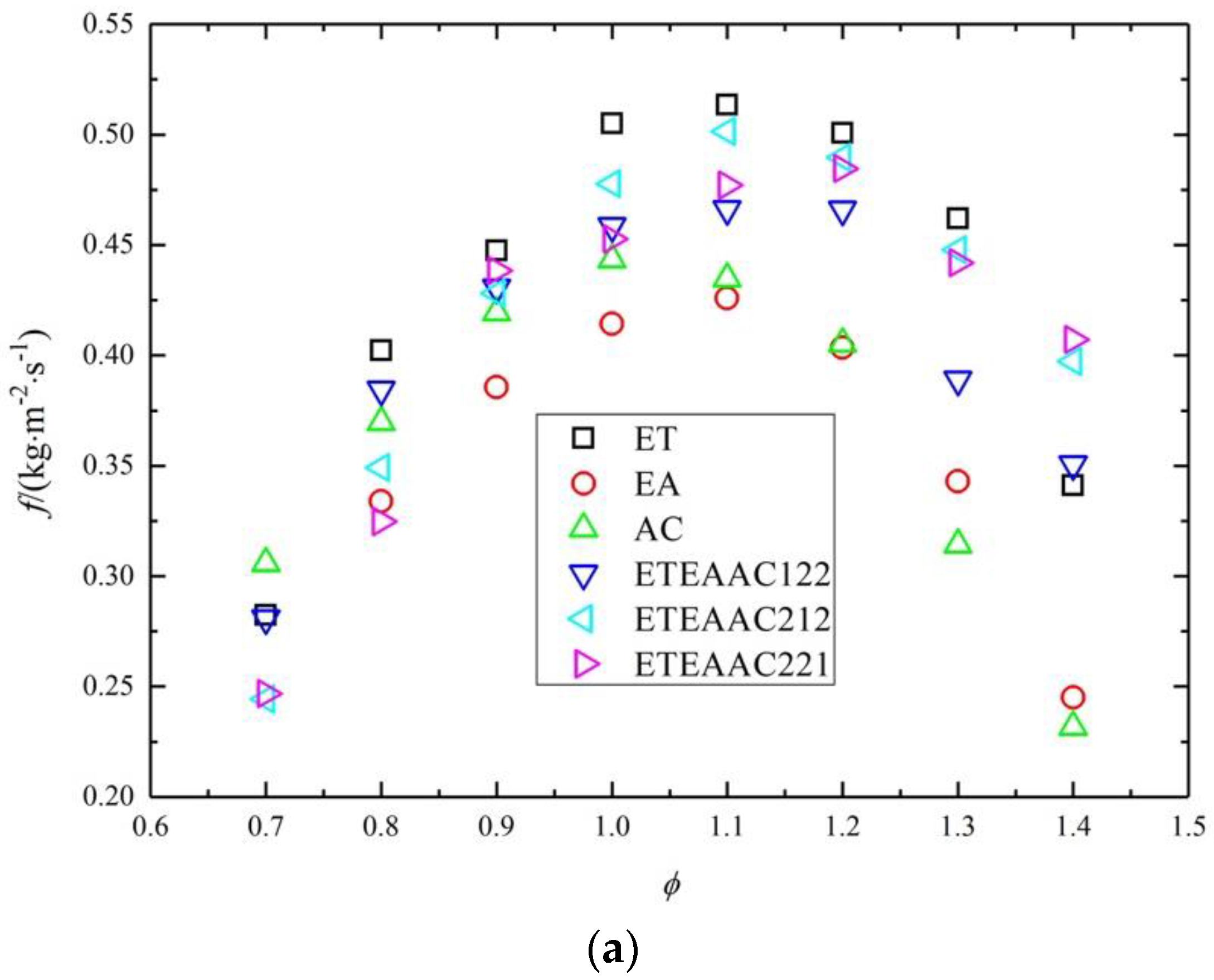
4.2. Combustion Characteristics
4.3. Mechanism Reduction
5. Conclusions
- The order of the promoting effect of the concentrations of ET, AC, and EA on the laminar burning velocity is ET > AC > EA.
- With the change of the equivalence ratio, the Markstein number presents a parabolic trend.
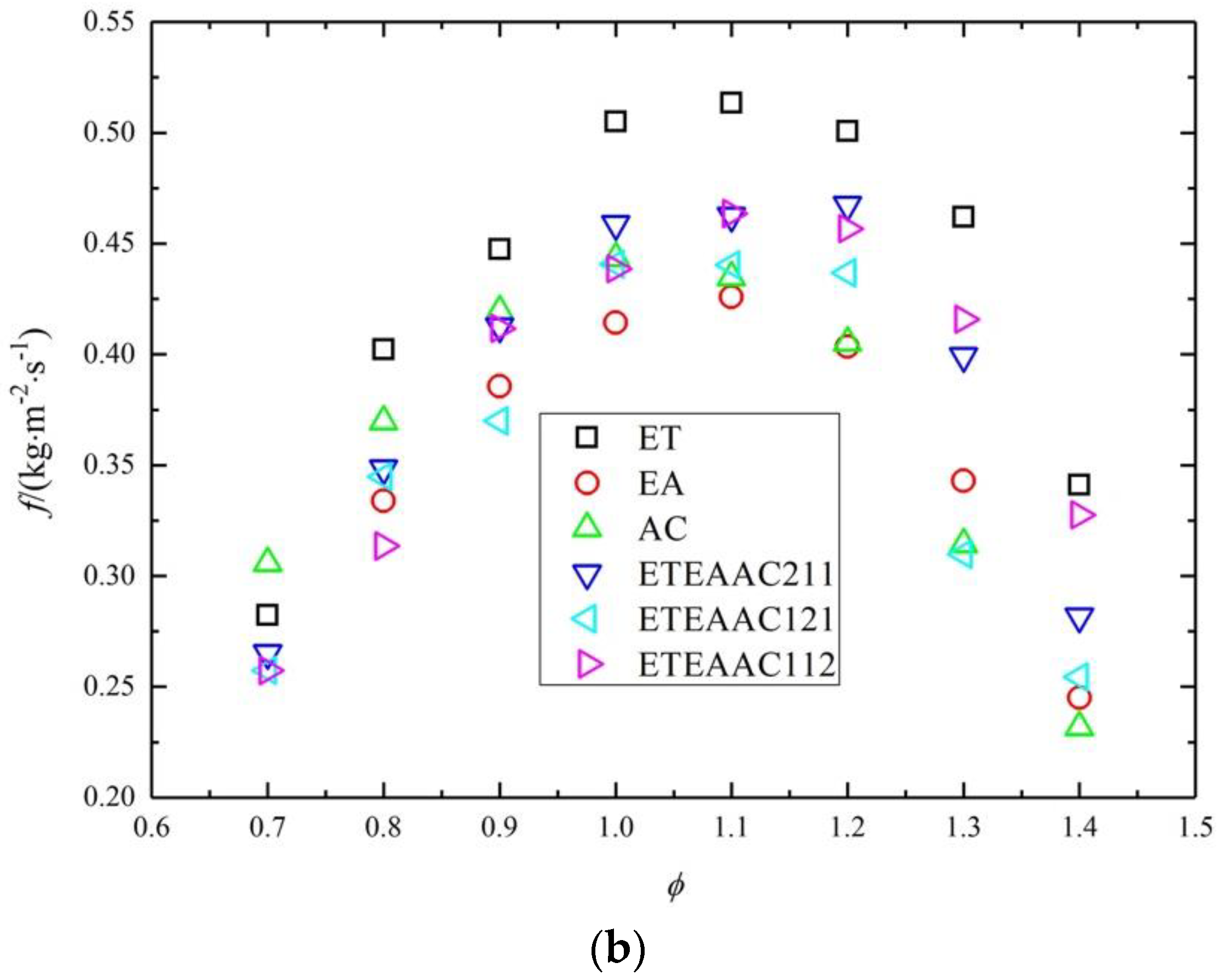
- The maximum laminar burning flux appears at the equivalence ratio of 1.0–1.2, and the laminar burning flux of ETEAAC ternary fuel is closer to that of pure fuels on the lean side.
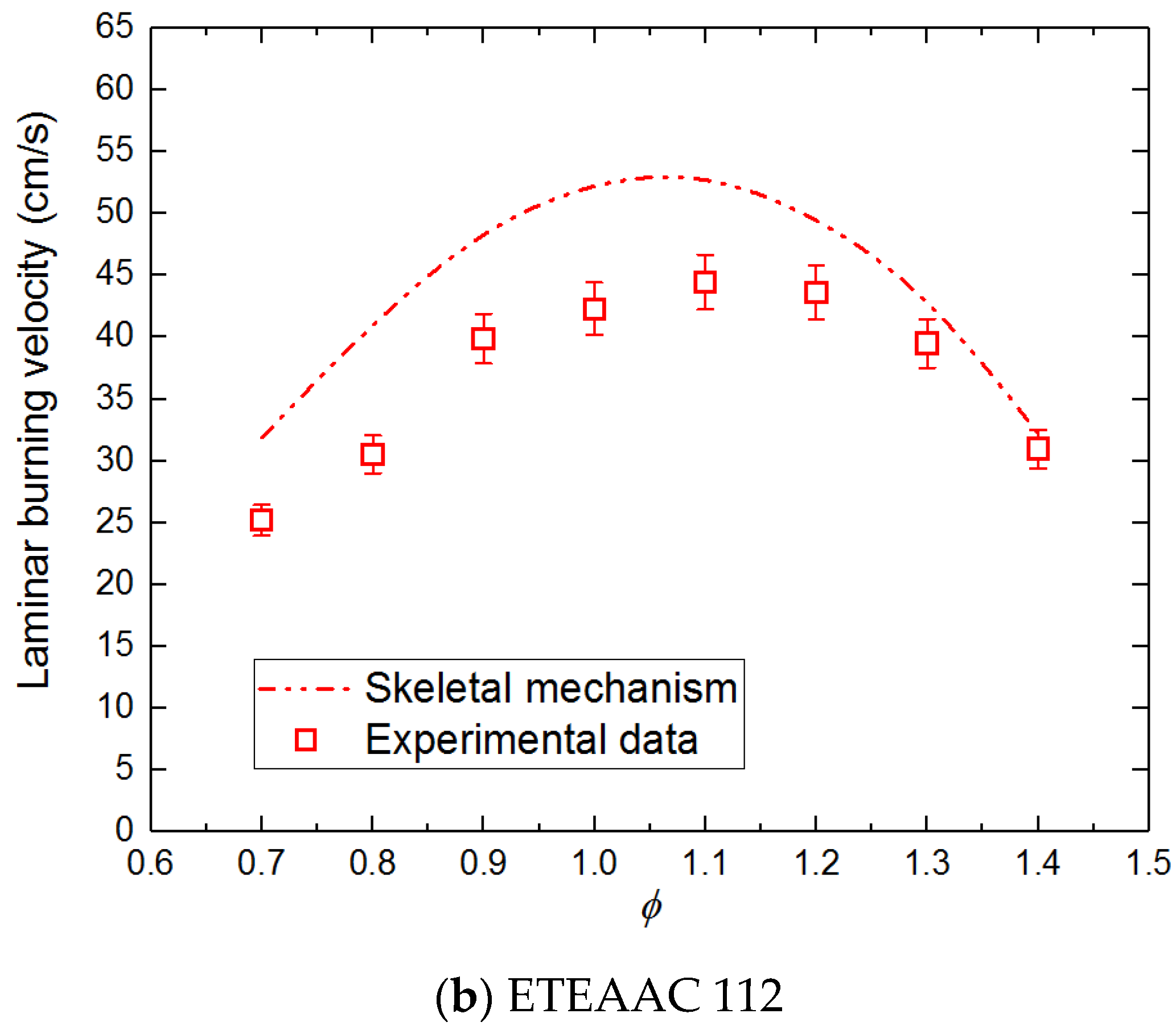
- The experimental and the simulation results of laminar burning velocities have a good consistency, and the relative deviation of ETEAAC 112 is approximately 17.5%.
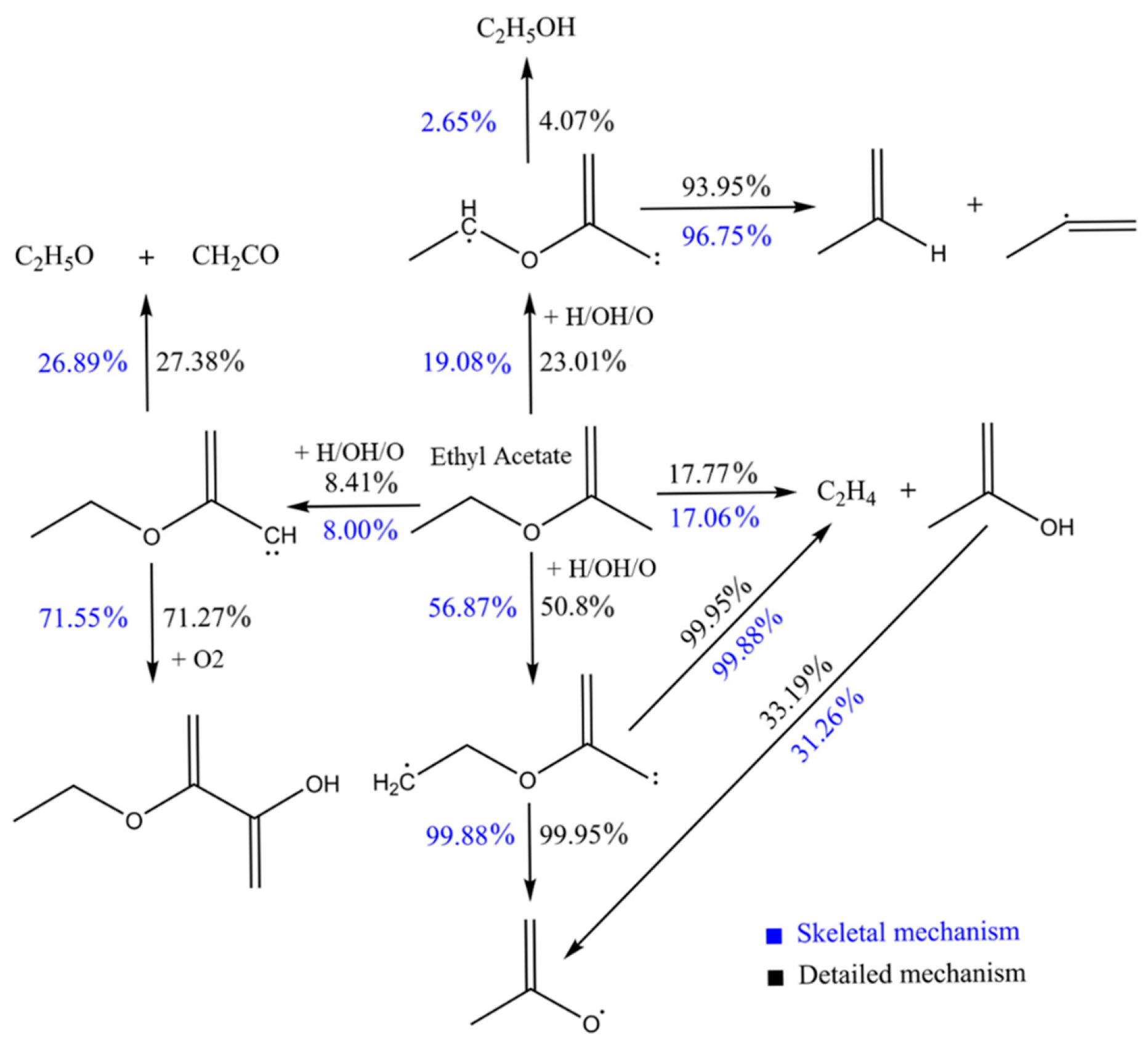
- The reaction pathways show that H-abstraction reactions are significant for EA consumption; SC2H4OCOC and PC2H4OCOC are important species for EA combustion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Polverino, P.; Arsie, I.; Pianese, C. Optimal energy management for hybrid electric vehicles based on dynamic programming and receding horizon. Energies 2021, 14, 3502. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, D.; Huang, Z. Spray properties of alternative fuels: A comparative analysis of ethanol-gasoline blends and gasoline. Fuel 2007, 86, 1645–1650. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, D.; Lapuerta, M.; German, L. Progress in the use of biobutanol blends in diesel engines. Energies 2021, 14, 3215. [Google Scholar] [CrossRef]
- Kohse-Hoeinghaus, K.; Osswald, P.; Cool, T.A.; Kasper, T.; Hansen, N.; Qi, F.; Westbrook, C.K.; Westmoreland, P.R. Biofuel combustion chemistry: From ethanol to biodiesel. Angew. Chem. Int. Edit. 2010, 49, 3572–3597. [Google Scholar] [CrossRef]
- Celik, M.B. Experimental determination of suitable ethanol-gasoline blend rate at high compression ratio for gasoline engine. Appl. Therm. Eng. 2008, 28, 396–404. [Google Scholar] [CrossRef]
- Iodice, P.; Amoresano, A.; Langella, G. A review on the effects of ethanol/gasoline fuel blends on NOx emissions in spark-ignition engines. Biofuel Res. J. BRJ 2021, 8, 1465–1480. [Google Scholar] [CrossRef]
- Oxenham, L.; Wang, Y. A study of the impact of methanol, ethanol and the miller cycle on a gasoline engine. Energies 2021, 14, 4847. [Google Scholar] [CrossRef]
- Iodice, P.; Cardone, M. Ethanol/Gasoline Blends as alternative fuel in last generation spark-ignition engines: A review on co and hc engine out emissions. Energies 2021, 14, 4034. [Google Scholar] [CrossRef]
- Cooper, S.P.; Gregoire, C.M.; Mohr, D.J.; Mathieu, O.; Alturaifi, S.A.; Petersen, E.L. An experimental kinetics study of isopropanol pyrolysis and oxidation behind reflected shock waves. Energies 2021, 14, 6808. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, X.; Huang, Y.; Shi, W.; Guo, Z.; Li, Z.; Du, Y.; Jin, Z.; Li, D.; Wang, T.; et al. Experimental study on combustion and emission of an SI engine with ethanol /gasoline combined injection and EGR. J. Clean. Prod. 2022, 331, 129903. [Google Scholar] [CrossRef]
- Elfasakhany, A. Dual and ternary biofuel blends for desalination process: Emissions and heat recovered assessment. Energies 2021, 14, 61. [Google Scholar] [CrossRef]
- Dhanarasu, M.; RameshKumar, K.A.; Maadeswaran, P. Effect of acetone as an oxygenated additive with used sunflower oil biodiesel on performance, combustion and emission in diesel engine. Environ. Technol. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dinesha, P.; Mohan, S.; Kumar, S. Experimental investigation of SI engine characteristics using Acetone-Butanol-Ethanol (ABE)—gasoline blends and optimization using particle swarm optimization. Int. J. Hydrogen Energy 2021, 47, 5692–5708. [Google Scholar] [CrossRef]
- Sekularac, N.; Fang, X.H.; Shankar, V.; Baker, S.J.; Leach, F.C.P.; Davy, M.H. Development of a laminar burning velocity empirical correlation for combustion of iso-octane/ethanol blends in air. Fuel 2022, 307, 121880. [Google Scholar] [CrossRef]
- Katoch, A.; Millan-Merino, A.; Kumar, S. Measurement of laminar burning velocity of ethanol-air mixtures at elevated temperatures. Fuel 2018, 231, 37–44. [Google Scholar] [CrossRef]
- Aghsaee, M.; Nativel, D.; Bozkurt, M.; Fikri, M.; Chaumeix, N.; Schulz, C. Experimental study of the kinetics of ethanol pyrolysis and oxidation behind reflected shock waves and in laminar flames. Proc. Combust. Inst. 2015, 35, 393–400. [Google Scholar] [CrossRef]
- Knorsch, T.; Zackel, A.; Mamaikin, D.; Zigan, L.; Wensing, M. Comparison of different gasoline alternative fuels in terms of laminar burning velocity at increased gas temperatures and exhaust gas recirculation rates. Energy Fuel 2014, 28, 1446–1452. [Google Scholar] [CrossRef]
- Sileghem, L.; Alekseev, V.A.; Vancoillie, J.; Nilsson, E.J.K.; Verhelst, S.; Konnov, A.A. Laminar burning velocities of primary reference fuels and simple alcohols. Fuel 2014, 115, 32–40. [Google Scholar] [CrossRef]
- Dirrenberger, P.; Glaude, P.A.; Bounaceur, R.; Le Gall, H.; Da Cruz, A.P.; Konnov, A.A.; Battin-Leclerc, F. Laminar burning velocity of gasolines with addition of ethanol. Fuel 2014, 115, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Lee, T.H.; Wu, H.; Pei, J.; Wu, W.; Liu, F.; Zhang, C. Experimental and kinetic studies on laminar flame characteristics of acetone-butanol-ethanol (ABE) and toluene reference fuel (TRF) blends at atmospheric pressure. Fuel 2018, 232, 755–768. [Google Scholar] [CrossRef]
- Nilsson, E.J.K.; de Goey, L.P.H.; Konnov, A.A. Laminar burning velocities of acetone in air at room and elevated temperatures. Fuel 2013, 105, 496–502. [Google Scholar] [CrossRef]
- Zhang, S.; Lee, T.H.; Wu, H.; Pei, J.; Wu, W.; Liu, F. Experimental and kinetical study of component volumetric effects on laminar flame speed of Acetone-Butanol-Ethanol (ABE). Energy Fuel 2018, 32, 6278–6292. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, S.; Cheng, Y.; Huang, Z.; Tang, C.; Zhang, J. A comparative study of n-propanol, propanal, acetone, and propane combustion in laminar flames. Proc. Combust. Inst. 2015, 35, 795–801. [Google Scholar] [CrossRef]
- Ahmed, A.; Pitz, W.J.; Cavallotti, C.; Mehl, M.; Lokachari, N.; Nilsson, E.J.K.; Wang, J.; Konnov, A.A.; Wagnon, S.W.; Chen, B.; et al. Small ester combustion chemistry: Computational kinetics and experimental study of methyl acetate and ethyl acetate. Proc. Combust. Inst. 2019, 37, 419–428. [Google Scholar] [CrossRef]
- Wang, Y.L.; Lee, D.J.; Westbrook, C.K.; Egolfopoulos, F.N.; Tsotsis, T.T. Oxidation of small alkyl esters in flames. Combust. Flame 2014, 161, 810–817. [Google Scholar] [CrossRef]
- Osipova, K.N.; Dmitriev, A.M.; Shmakov, A.G.; Korobeinichev, O.P.; Minaev, S.S.; Knyazkov, D.A. Combustion of ethyl acetate: The experimental study of flame structure and validation of chemical kinetic mechanisms. Mendeleev Commun. 2019, 29, 690–692. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, K.; Li, X.; Zhong, A.; Oppong, F.; Wang, H.; Wu, S.; Zhou, W.; Wang, C. Laminar burning characteristics of two rice-husk-derived biofuels. Energy Fuel 2018, 32, 9872–9882. [Google Scholar] [CrossRef]
- Xu, C.; Zhong, A.; Li, X.; Wang, C.; Sahu, A.; Xu, H.; Lattimore, T.; Zhou, K.; Huang, Y. Laminar burning characteristics of upgraded biomass pyrolysis fuel derived from rice husk at elevated pressures and temperatures. Fuel 2017, 210, 249–261. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Oppong, F.; Li, X.; Zhou, K.; Zhou, W.; Wu, S.; Wang, C. Determination of laminar burning characteristics of a surrogate for a pyrolysis fuel using constant volume method. Energy 2020, 190, 116315. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; Zhang, B.; Liao, H.; He, W.; Wei, L. Experimental and numerical study on laminar premixed flame characteristics of 2-ethylfuran. Combust. Flame 2021, 234, 111631. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Z.; Hou, X.; He, X.; Sjöberg, M.; Vuilleumier, D.; Liu, C.; Liu, F. Measurements of laminar flame speeds and flame instability analysis of E30-air premixed flames at elevated temperatures and pressures. Fuel 2020, 259, 116223. [Google Scholar] [CrossRef]
- Kelley, A.P.; Law, C.K. Nonlinear effects in the extraction of laminar flame speeds from expanding spherical flames. Combust. Flame 2009, 156, 1844–1851. [Google Scholar] [CrossRef]
- Luo, Z.; Oppong, F.; Wang, H.; Li, X.; Xu, C.; Wang, C. Investigating the laminar burning velocity of 2-methylfuran. Fuel 2018, 234, 1469–1480. [Google Scholar]
- Liu, Y.; Liu, W.; Liao, H.; Zhou, W.; Xu, C. An experimental and kinetic modelling study on laminar premixed flame characteristics of ethanol/acetone mixtures. Energies 2021, 14, 6713. [Google Scholar] [CrossRef]
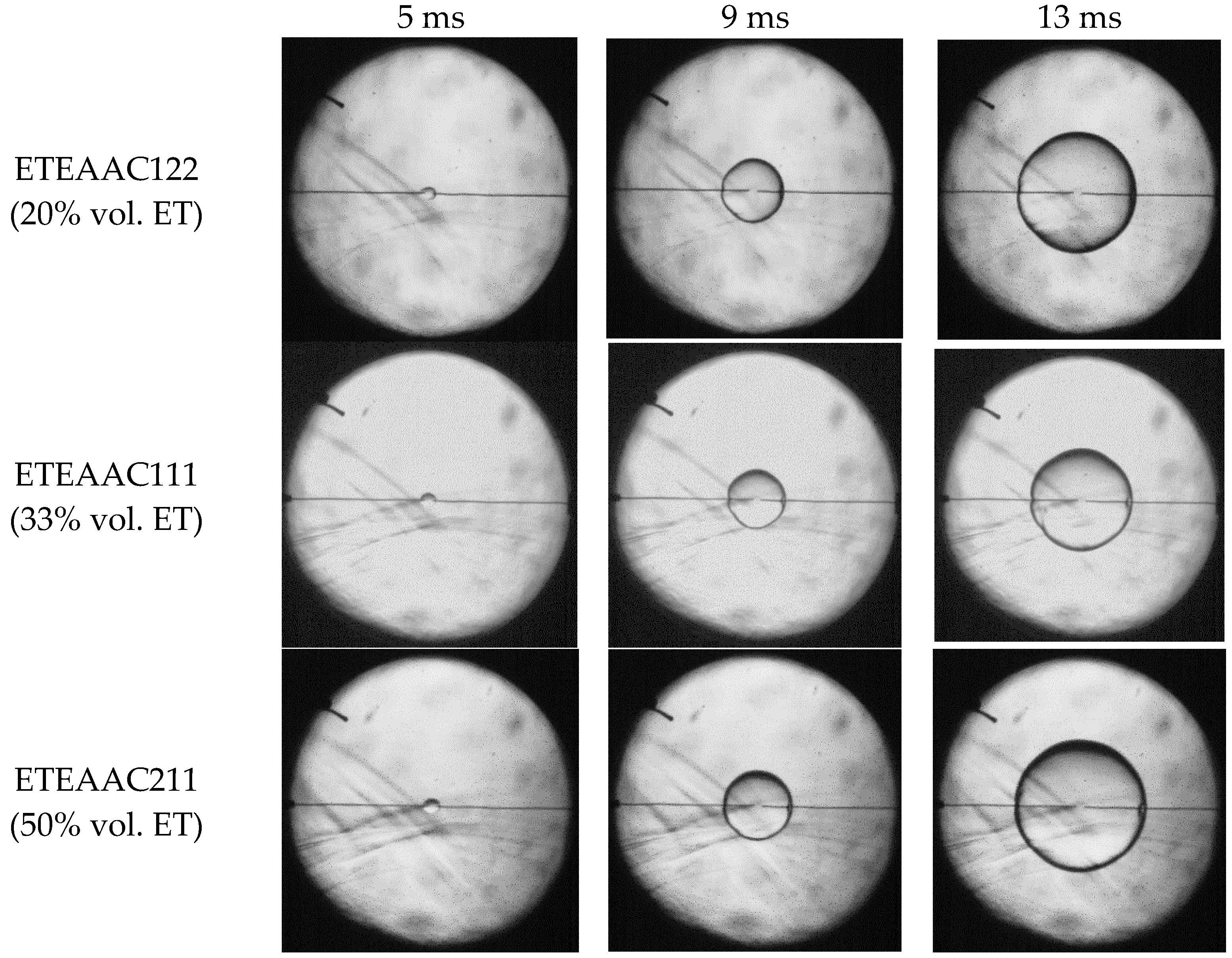
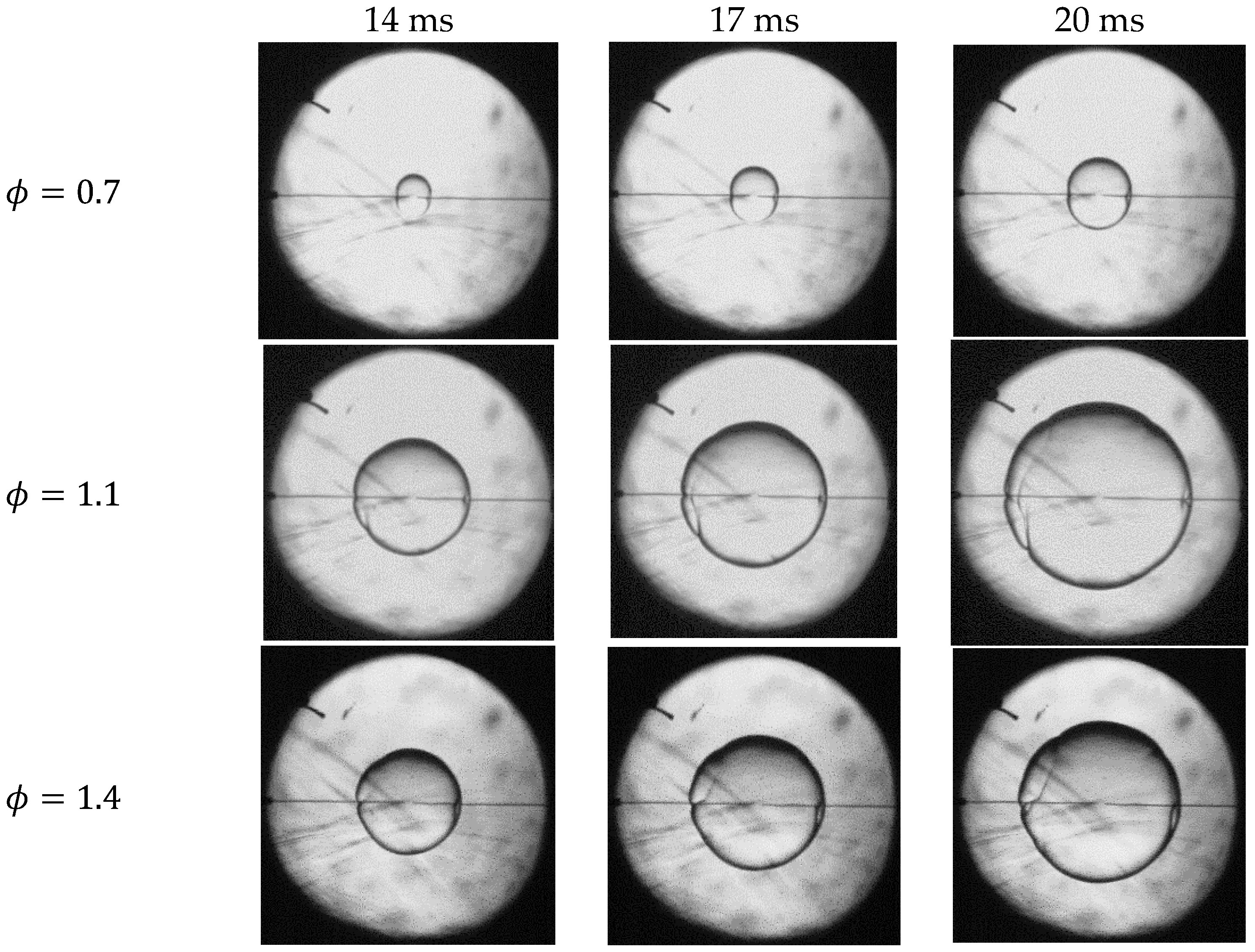
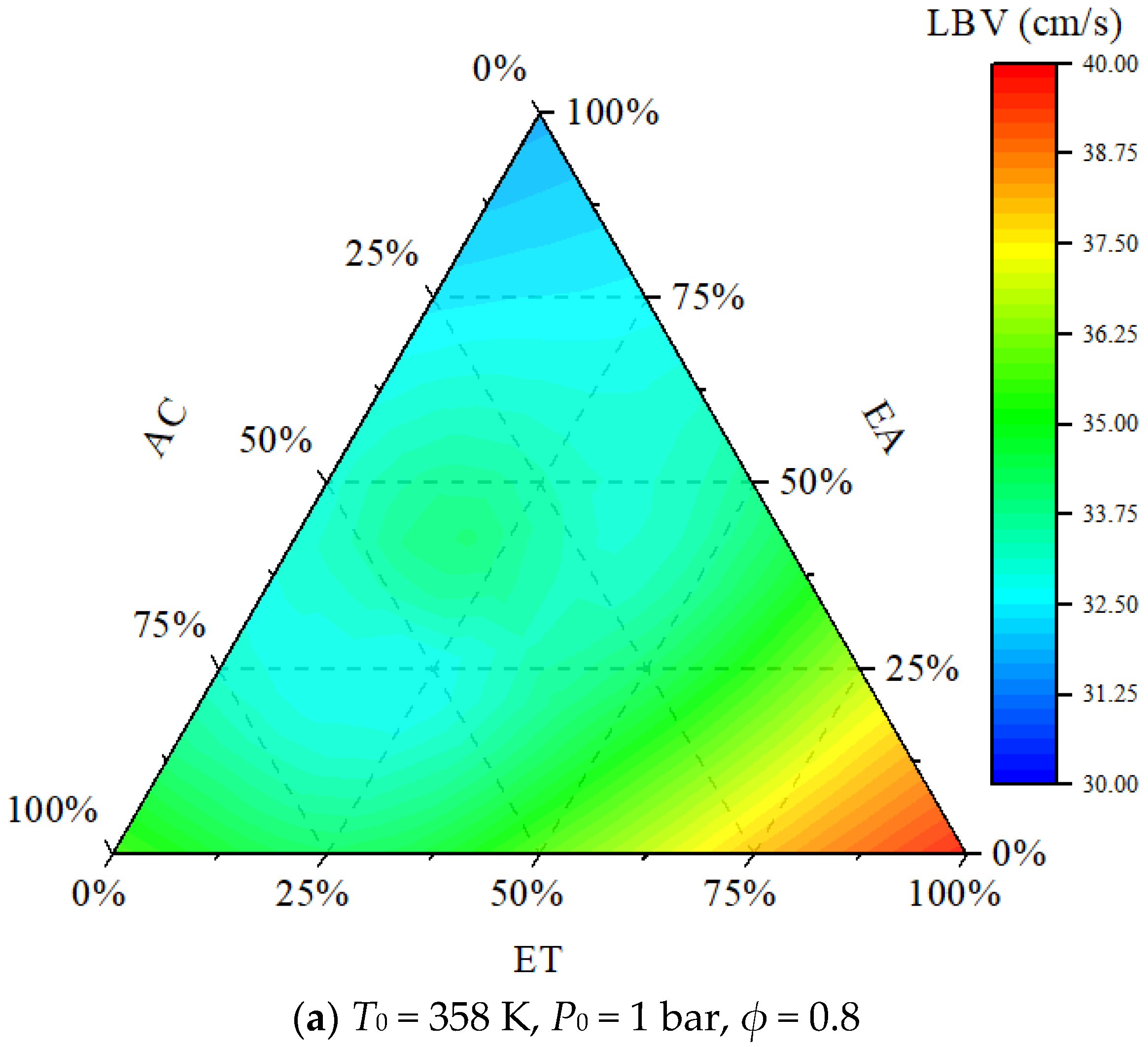



| Fuel Group | Ethanol (ET)/Vol. | Acetone (AC)/Vol. | Ethyl Acetate (EA)/Vol. |
|---|---|---|---|
| ET | 100% | - | - |
| AC | - | 100% | - |
| EA | - | - | 100% |
| ETAC13 | 25% | 75% | - |
| ETAC11 | 50% | 50% | - |
| ETAC31 | 75% | 25% | - |
| ETEA13 | 25% | - | 75% |
| ETEA11 | 50% | - | 50% |
| ETEA31 | 75% | - | 25% |
| ACEA13 | - | 25% | 75% |
| ACEA11 | - | 50% | 50% |
| ACEA31 | - | 75% | 25% |
| ETEAAC211 | 50% | 25% | 25% |
| ETEAAC121 | 25% | 25% | 50% |
| ETEAAC112 | 25% | 50% | 25% |
| ETEAAC221 | 40% | 20% | 40% |
| ETEAAC212 | 40% | 40% | 20% |
| ETEAAC122 | 20% | 40% | 40% |
| ETEAAC111 | 33.33% | 33.33% | 33.34% |
| Species | Reaction | Rate of Production (Mole/cm3·s) | Reaction Flux (%) |
|---|---|---|---|
| EA | EA + H = PC2H4OCOC + H2 | −7.80 × 10−6 | 44.39% |
| EA = C2H4 + CH3COOH | −3.12 × 10−6 | 17.77% | |
| EA + OH = SC2H4OCOC + H2O | −2.07 × 10−6 | 11.79% | |
| EA + H = SC2H4OCOC + H2 | −1.52 × 10−6 | 8.68% | |
| EA + OH = PC2H4OCOC + H2O | −9.72 × 10−7 | 5.53% | |
| EA + OH = C2H5OCOCH + H2O | −5.60 × 10−7 | 3.19% | |
| EA + H = C2H5OCOCH + H2 | −5.28 × 10−7 | 3.00% | |
| EA + O = SC2H4OCOC + OH | −4.47 × 10−7 | 2.54% | |
| EA + O = C2H5OCOCH + OH | −3.90 × 10−7 | 2.22% | |
| EA + O = PC2H4OCOC + OH | −1.54 × 10−7 | 0.88% | |
| PC2H4OCOC | PC2H4OCOC = C2H4 + CH3CO2 | −8.92 × 10−6 | 99.95% |
| PC2H4OCOC => C2H5OCOCH | −2.35 × 10−9 | 0.03% | |
| H + VINACET = PC2H4OCOC | −1.62 × 10−9 | 0.02% | |
| SC2H4OCOC | SC2H4OCOC = CH3CHO + CH3CO | −3.81 × 10−6 | 93.95% |
| SC2H4OCOC => C2H5OCOCH | −1.65 × 10−7 | 4.07% | |
| H + VINACET = SC2H4OCOC | −3.75 × 10−8 | 0.93% | |
| SC2H4OCOC + O2 <=> VINCET + HO2 | −3.42 × 10−8 | 0.84% | |
| SC2H4OCOC + H = EA | −8.78 × 10−9 | 0.22% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, W.; Liao, H.; Ashan, H.; Zhou, W.; Xu, C. An Experimental and a Kinetic Modelling Study of Ethanol/Acetone/Ethyl Acetate Mixtures. Energies 2022, 15, 2992. https://doi.org/10.3390/en15092992
Liu Y, Liu W, Liao H, Ashan H, Zhou W, Xu C. An Experimental and a Kinetic Modelling Study of Ethanol/Acetone/Ethyl Acetate Mixtures. Energies. 2022; 15(9):2992. https://doi.org/10.3390/en15092992
Chicago/Turabian StyleLiu, Yangxun, Weinan Liu, Huihong Liao, Hasier Ashan, Wenhua Zhou, and Cangsu Xu. 2022. "An Experimental and a Kinetic Modelling Study of Ethanol/Acetone/Ethyl Acetate Mixtures" Energies 15, no. 9: 2992. https://doi.org/10.3390/en15092992
APA StyleLiu, Y., Liu, W., Liao, H., Ashan, H., Zhou, W., & Xu, C. (2022). An Experimental and a Kinetic Modelling Study of Ethanol/Acetone/Ethyl Acetate Mixtures. Energies, 15(9), 2992. https://doi.org/10.3390/en15092992





