Efficient Plasma Technology for the Production of Green Hydrogen from Ethanol and Water
Abstract
1. Introduction
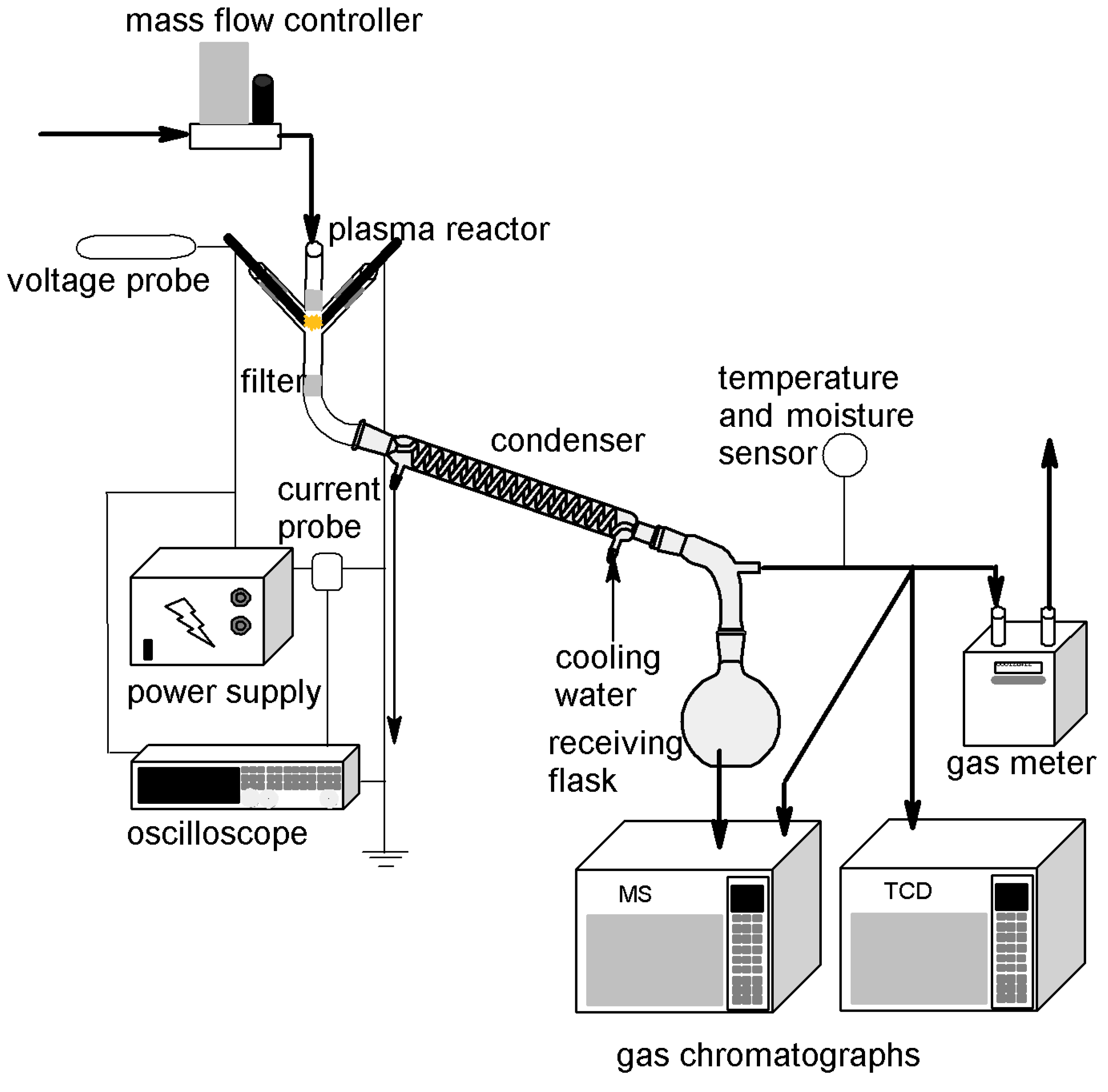
2. Materials and Methods
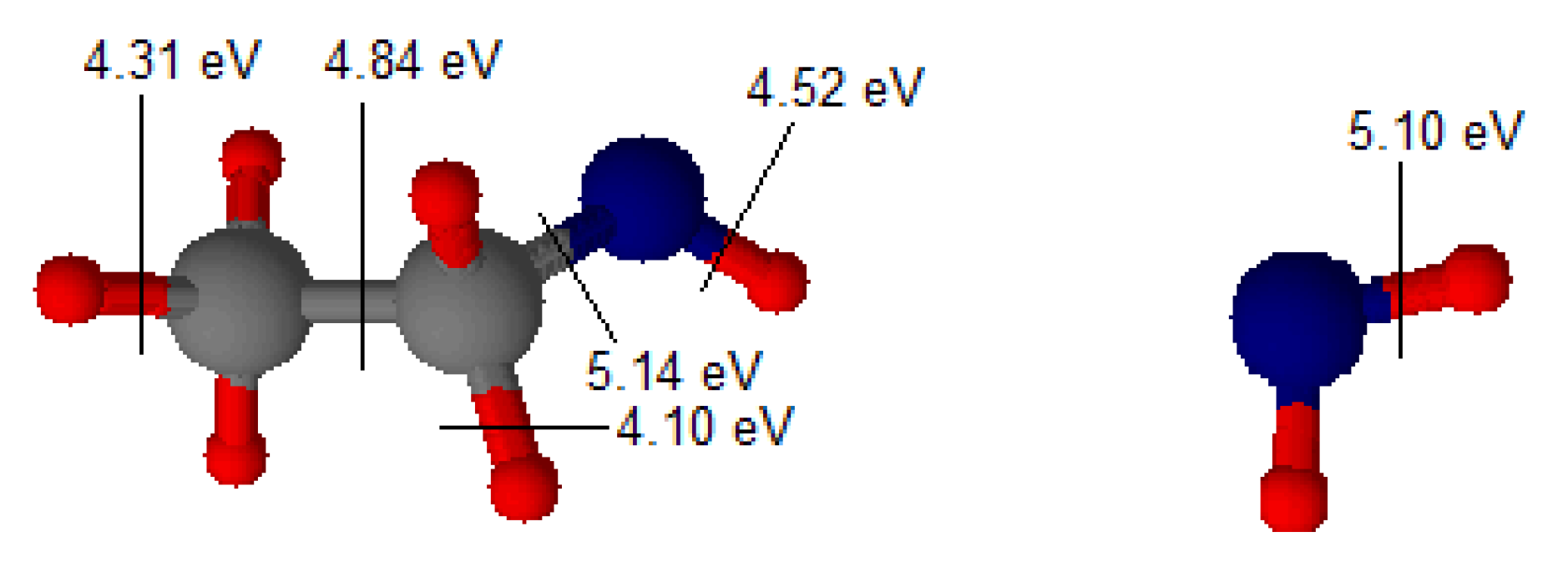
3. Results and Discussion
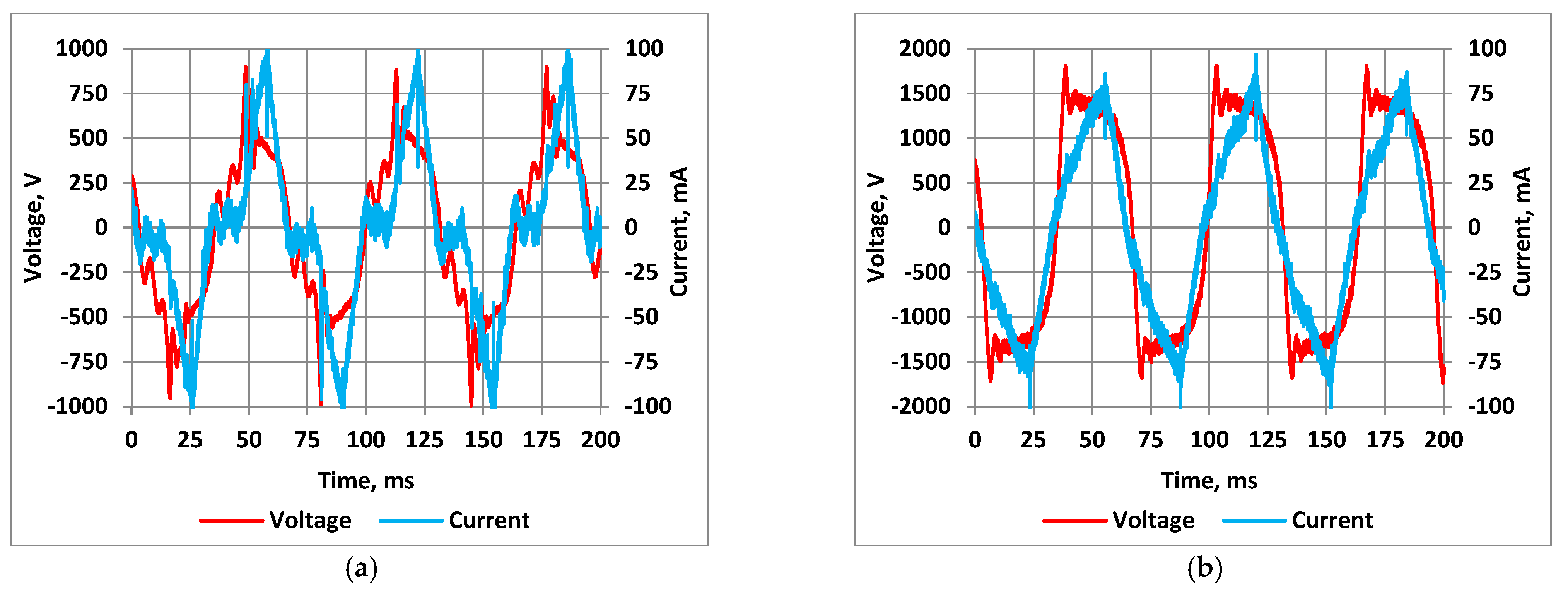
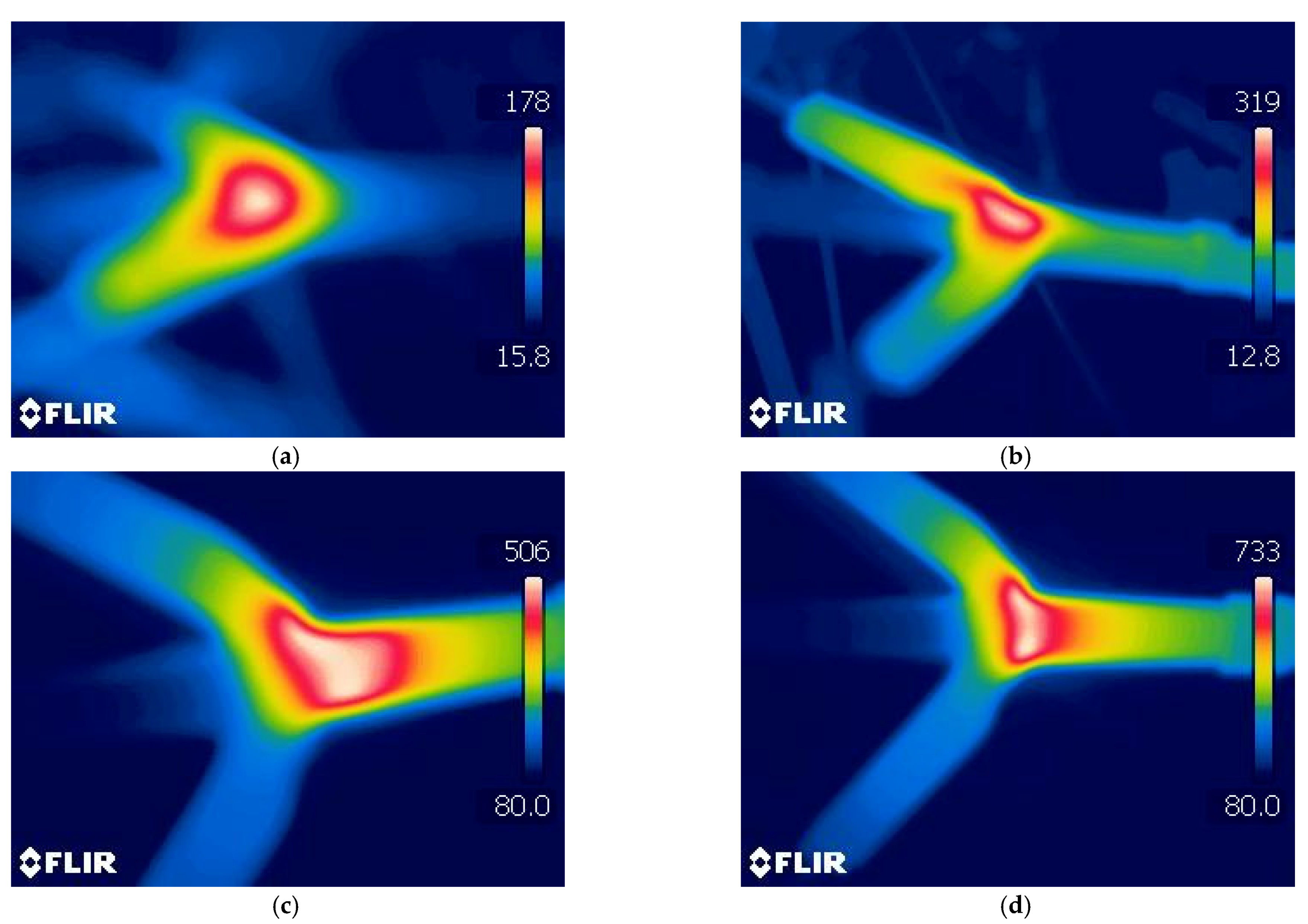
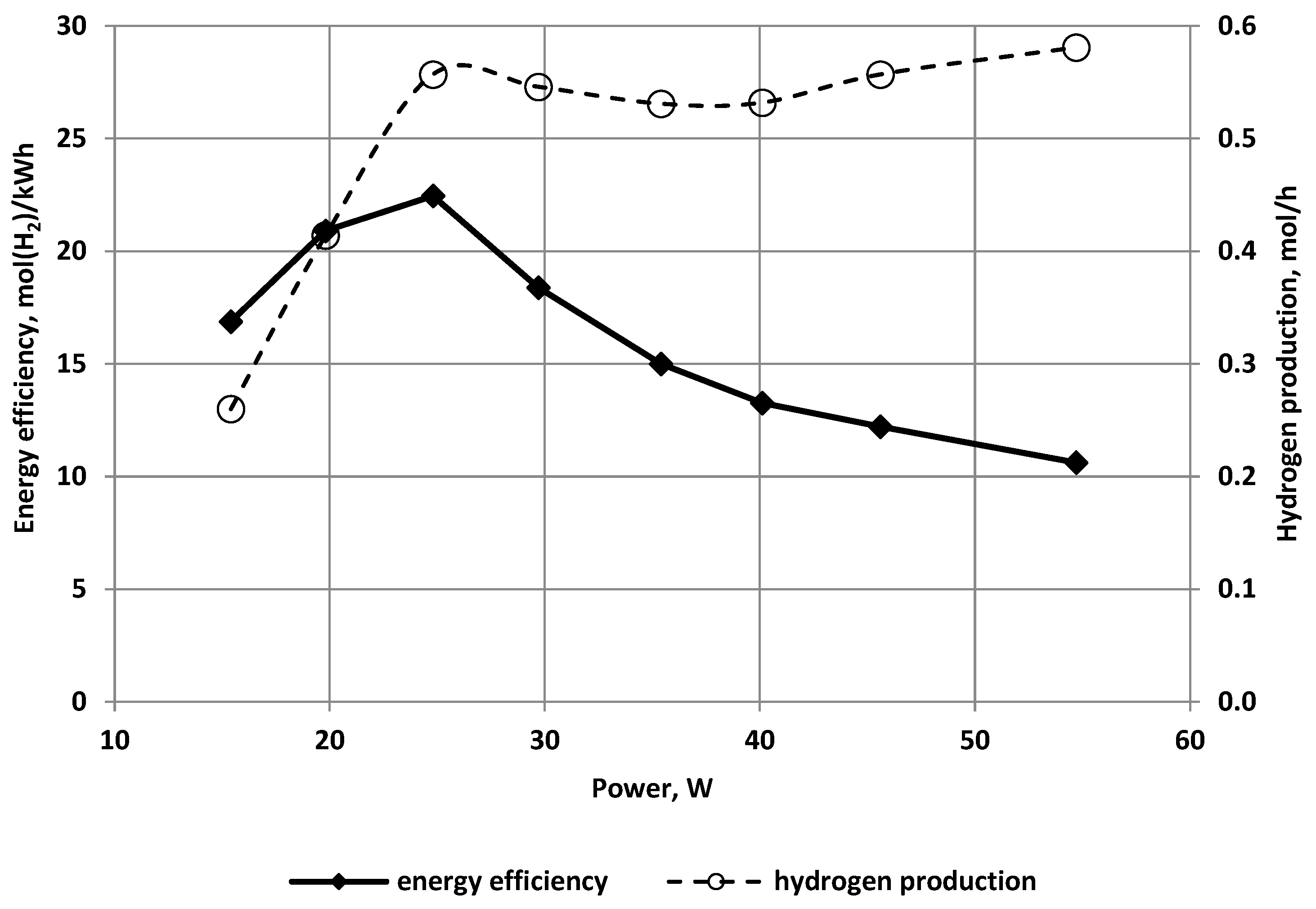
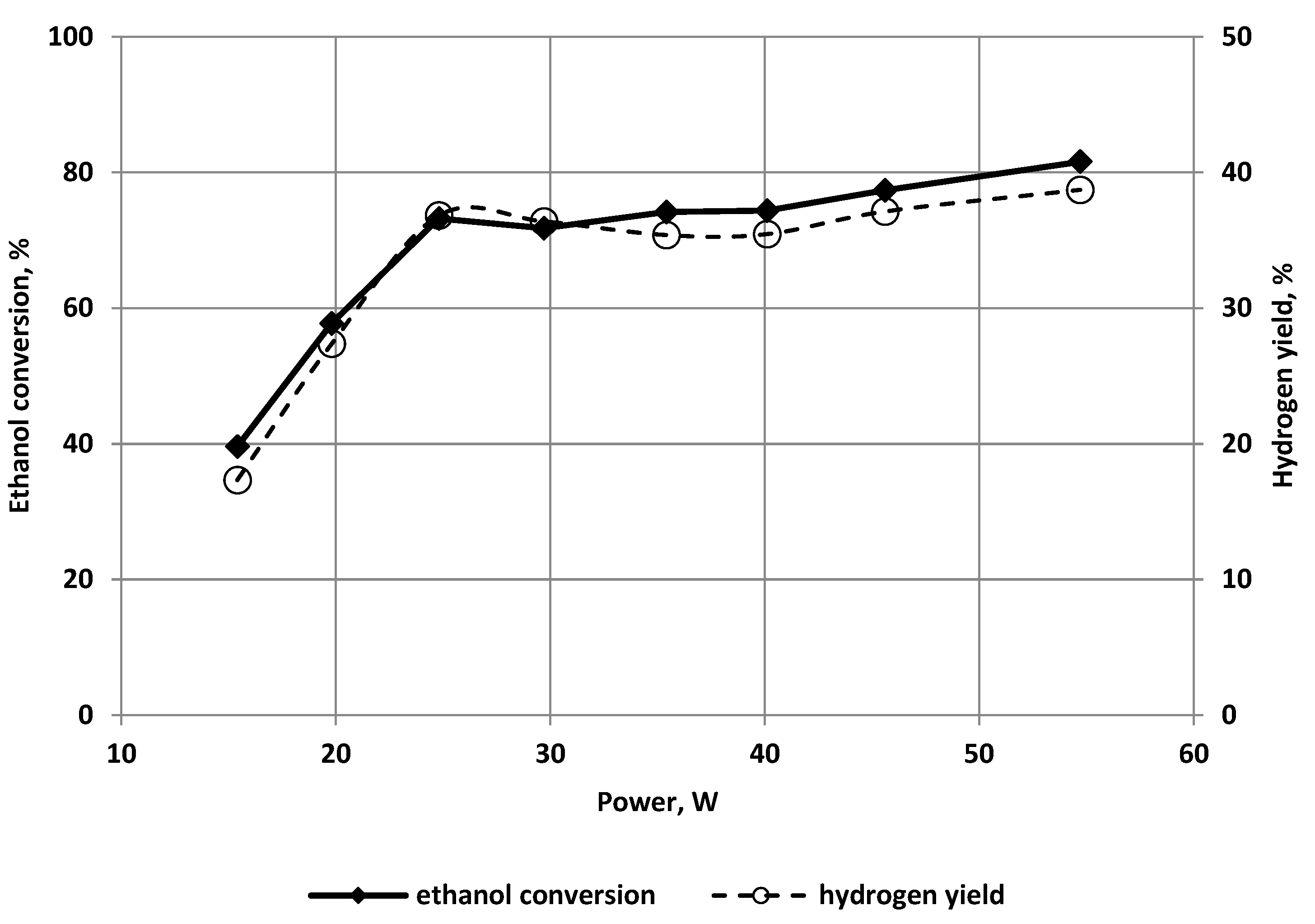
3.1. The Effect of the Discharge Power
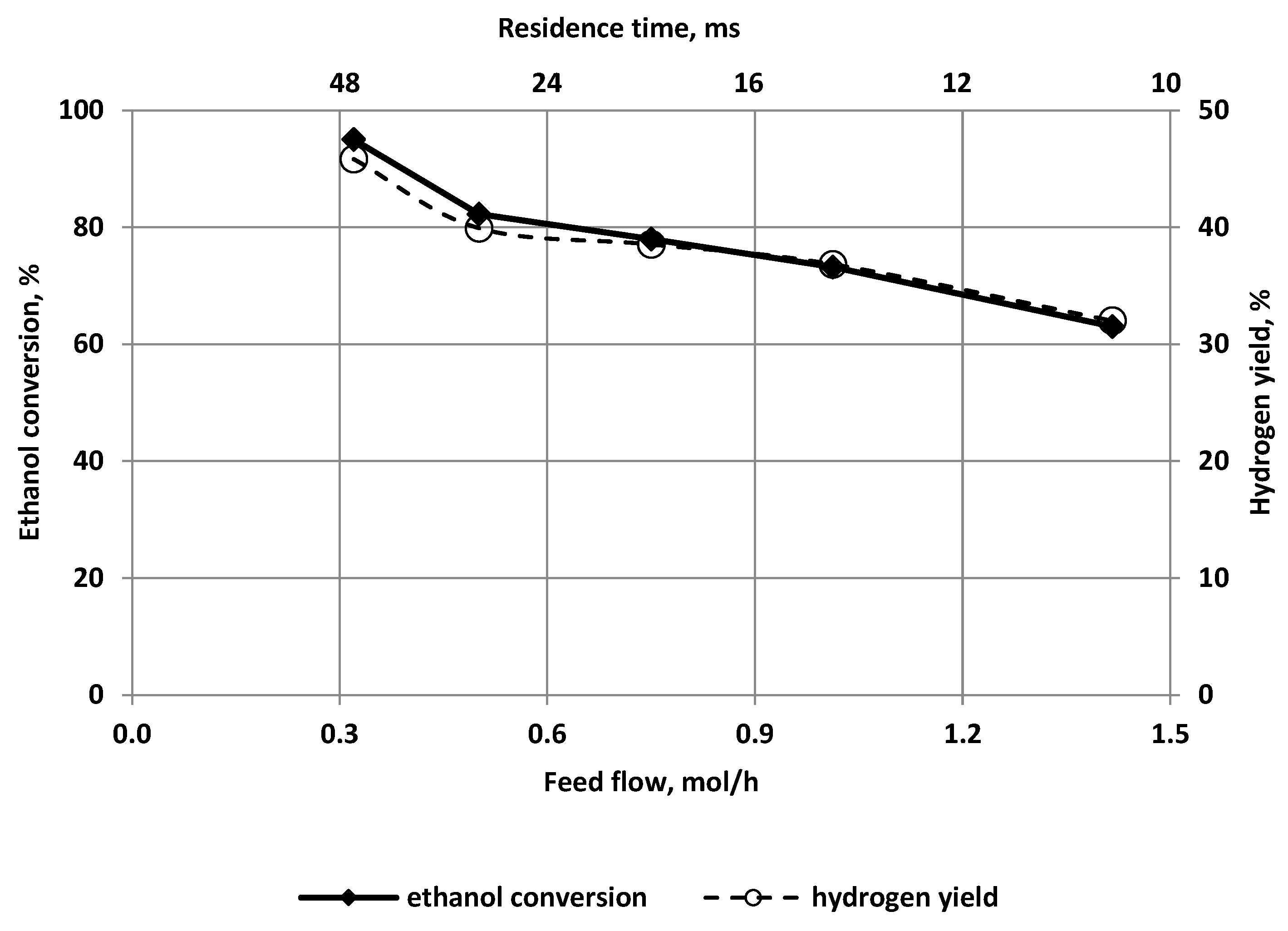
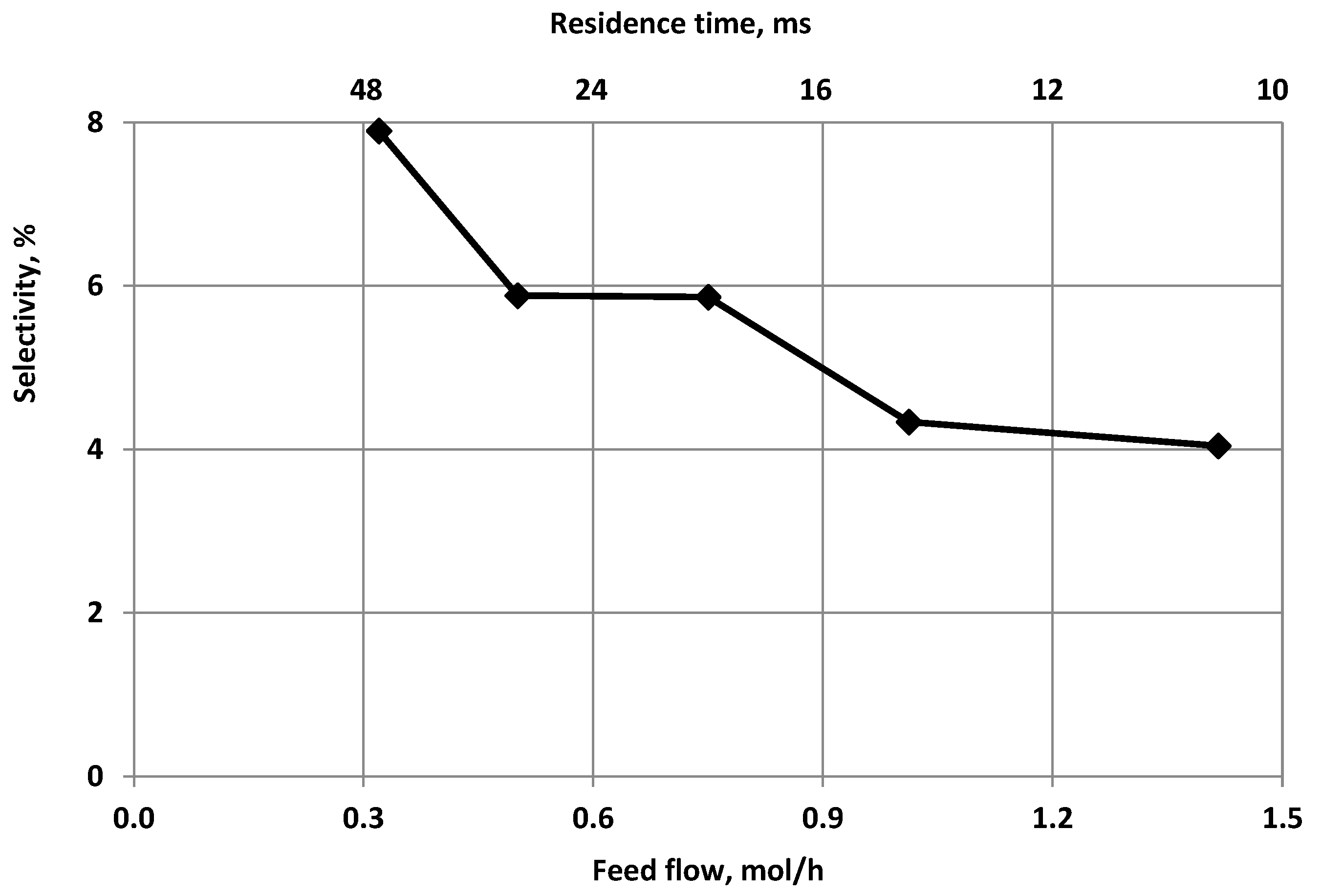
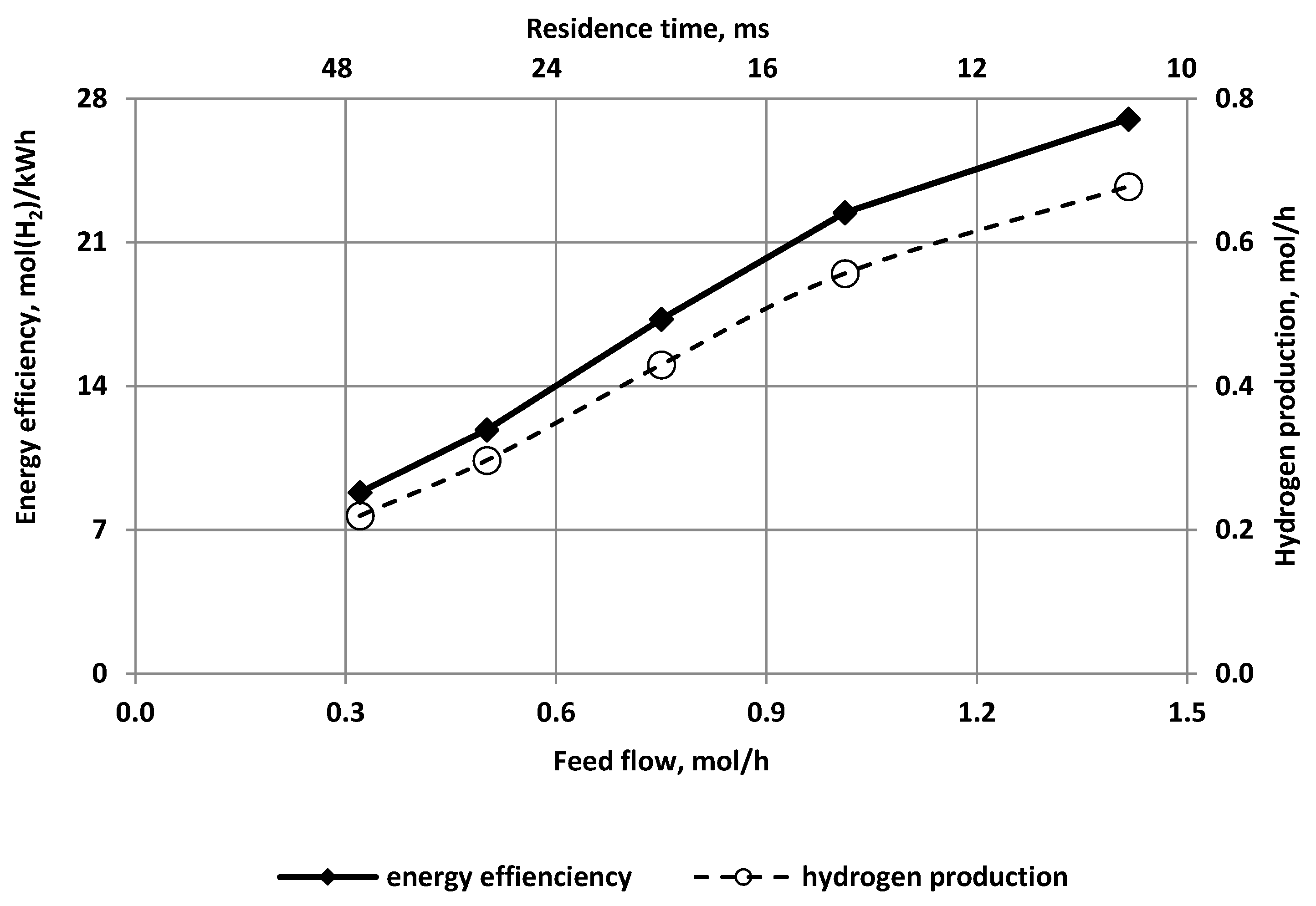
3.2. The Effect of the Feed Flow
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jozwiak, L.; Balcerzak, J.; Tyczkowski, J. Plasma-Deposited Ru-Based Thin Films for Photoelectrochemical Water Splitting. Catalysts 2020, 10, 278. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H.; Tyczkowski, J.; Jarota, A.; Abramczyk, H. Hydrogen production in liquid water by femtosecond laser-induced plasma. Appl. Energy 2019, 247, 24–31. [Google Scholar] [CrossRef]
- Burlica, R.; Hnatiuc, B.; Hnatiuc, E.; Ursachi, M. Effect of electrical current on H2/H2O2 generation in non-thermal plasma gliding arc reactors. Environ. Eng. Manag. J. 2011, 10, 579–583. [Google Scholar] [CrossRef]
- Dey, G.R.; Zode, S.D.; Namboodiri, V. Application of plasma for efficient H2 production: A realism of copper electrode in single dielectric barrier discharge reactor. Phys. Plasmas 2018, 25, 103508. [Google Scholar] [CrossRef]
- Kirkpatrick, M.J.; Locke, B.R. Hydrogen, Oxygen, and Hydrogen Peroxide Formation in Aqueous Phase Pulsed Corona Electrical Discharge. Ind. Eng. Chem. Res. 2005, 44, 4243–4248. [Google Scholar] [CrossRef]
- García, R.; Gil, M.; Rubiera, F.; Chen, D.; Pevida, C. Renewable hydrogen production from biogas by sorption enhanced steam reforming (SESR): A parametric study. Energy 2020, 218, 119491. [Google Scholar] [CrossRef]
- Kim, H.J.; Chun, Y.N. Conversion of Biogas to Renewable Energy by Microwave Reforming. Energies 2020, 13, 4093. [Google Scholar] [CrossRef]
- Czylkowski, D.; Hrycak, B.; Jasiński, M.; Dors, M.; Mizeraczyk, J. Hydrogen production by conversion of ethanol injected into a microwave plasma. Eur. Phys. J. D 2017, 71, 321. [Google Scholar] [CrossRef][Green Version]
- Baránková, H.; Bardos, L.; Bardos, A. Non-Conventional Atmospheric Pressure Plasma Sources for Production of Hydrogen. MRS Adv. 2018, 3, 921–929. [Google Scholar] [CrossRef]
- Bardos, L.; Baránková, H.; Bardos, A. Production of Hydrogen-Rich Synthesis Gas by Pulsed Atmospheric Plasma Submerged in Mixture of Water with Ethanol. Plasma Chem. Plasma Process. 2016, 37, 115–123. [Google Scholar] [CrossRef]
- Zhu, T.; Sun, B.; Zhu, X.; Wang, L.; Xin, Y.; Liu, J. Mechanism analysis of hydrogen production by microwave discharge in ethanol liquid. J. Anal. Appl. Pyrolysis 2021, 156, 105111. [Google Scholar] [CrossRef]
- Wang, B.; Ge, W.; Lü, Y.; Yan, W. H2 production by ethanol decomposition with a gliding arc discharge plasma reactor. Front. Chem. Sci. Eng. 2013, 7, 145–153. [Google Scholar] [CrossRef]
- Du, C.; Ma, D.; Wu, J.; Lin, Y.; Xiao, W.; Ruan, J.; Huang, D. Plasma-catalysis reforming for H2 production from ethanol. Int. J. Hydrogen Energy 2015, 40, 15398–15410. [Google Scholar] [CrossRef]
- Wang, B.; Lü, Y.; Zhang, X.; Hu, S. Hydrogen generation from steam reforming of ethanol in dielectric barrier discharge. J. Nat. Gas Chem. 2011, 20, 151–154. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, Ł.; Młotek, M.; Krawczyk, K. Hydrogen production from ethanol using dielectric barrier discharge. Energy 2019, 174, 261–268. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, Ł.; Młotek, M.; Falkowski, P.; Krawczyk, K. Hydrogen production from ethanol using a special multi-segment plasma-catalytic reactor. J. Energy Inst. 2021, 95, 179–186. [Google Scholar] [CrossRef]
- Hu, Y.; Li, G.; Yang, Y.; Gao, X.; Lu, Z. Hydrogen generation from hydro-ethanol reforming by DBD-plasma. Int. J. Hydrogen Energy 2012, 37, 1044–1047. [Google Scholar] [CrossRef]
- Zhu, X.; Hoang, T.; Lobban, L.L.; Mallinson, R.G. Low CO content hydrogen production from bioethanol using a combined plasma reforming–catalytic water gas shift reactor. Appl. Catal. B Environ. 2010, 94, 311–317. [Google Scholar] [CrossRef]
- Xin, Y.; Sun, B.; Zhu, X.; Yan, Z.; Zhao, X.; Sun, X. Hydrogen production from ethanol decomposition by pulsed discharge with needle-net configurations. Appl. Energy 2017, 206, 126–133. [Google Scholar] [CrossRef]
- Burlica, R.; Shih, K.-Y.; Hnatiuc, B.; Locke, B.R. Hydrogen Generation by Pulsed Gliding Arc Discharge Plasma with Sprays of Alcohol Solutions. Ind. Eng. Chem. Res. 2011, 50, 9466–9470. [Google Scholar] [CrossRef]
- Chernyak, V.Y.; Iukhymenko, V.V.; Iukhymenko, K.V.; Oberemok, Y.A.; Tretiakov, D.D.; Fedirchyk, I.I. Plasmochemical Synthesis of Optically Active Substances. IEEE Trans. Plasma Sci. 2021, 49, 1050–1054. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, Ł.; Jóźwik, P.; Młotek, M.; Krawczyk, K. Plasma-Catalytic Process of Hydrogen Production from Mixture of Methanol and Water. Catalysts 2021, 11, 864. [Google Scholar] [CrossRef]
- Panda, N.R.; Sahu, D. Enhanced hydrogen generation efficiency of methanol using dielectric barrier discharge plasma methodology and conducting sea water as an electrode. Heliyon 2020, 6, e04717. [Google Scholar] [CrossRef] [PubMed]
- Tsymbalyuk, A.N.; Levko, D.S.; Chernyak, V.Y.; Martysh, E.V.; Nedybalyuk, O.A.; Solomenko, E.V. Influence of the gas mixture temperature on the efficiency of synthesis gas production from ethanol in a nonequilibrium plasma. Tech. Phys. 2013, 58, 1138–1143. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Meloni, E.; Ricca, A. Influence of Catalytic Formulation and Operative Conditions on Coke Deposition over CeO2-SiO2 Based Catalysts for Ethanol Reforming. Energies 2017, 10, 1030. [Google Scholar] [CrossRef]
- Matus, E.; Sukhova, O.; Ismagilov, I.; Kerzhentsev, M.; Stonkus, O.; Ismagilov, Z. Hydrogen Production through Autothermal Reforming of Ethanol: Enhancement of Ni Catalyst Performance via Promotion. Energies 2021, 14, 5176. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Jóźwik, P.; Młotek, M.; Krawczyk, K. A Promising Cobalt Catalyst for Hydrogen Production. Catalysts 2022, 12, 278. [Google Scholar] [CrossRef]
- Saksono, N.; Batubara, T.; Bismo, S. Hydrogen production by plasma electrolysis reactor of KOH-ethanol solution. IOP Conf. Ser. Mater. Sci. Eng. 2016, 162, 012010. [Google Scholar] [CrossRef]
- Saksono, N.; Sasiang, J.; Rosalina, C.D.; Budikania, T. Hydrogen Generation by Koh-Ethanol Plasma Electrolysis Using Double Compartement Reactor. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012011. [Google Scholar] [CrossRef]
- Nedybaliuk, O.A.; Chernyak, V.Y.; Fedirchyk, I.I.; Demchina, V.P.; Popkov, V.S.; Bogaenko, M.V.; Iukhymenko, V.V.; Klochok, N.V.; Martysh, E.V.; Bortyshevsky, V.A.; et al. Plasma-Catalytic Reforming of Biofuels and Diesel Fuel. IEEE Trans. Plasma Sci. 2017, 45, 1803–1811. [Google Scholar] [CrossRef]
- Tande, L.; Resendiz-Mora, E.; Dupont, V. Bioh2, Heat and Power from Palm Empty Fruit Bunch via Pyrolysis-Autothermal Reforming: Plant Simulation, Experiments, and CO2 Mitigation. Energies 2021, 14, 4767. [Google Scholar] [CrossRef]
- Bo, Z.; Yan, J.; Li, X.; Chi, Y.; Cen, K. Nitrogen dioxide formation in the gliding arc discharge-assisted decomposition of volatile organic compounds. J. Hazard. Mater. 2009, 166, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Benstaali, B.; Boubert, P.; Cheron, B.G.; Addou, A.; Brisset, J.L. Density and Rotational Temperature Measurements of the OH° and NO° Radicals Produced by a Gliding Arc in Humid Air. Plasma Chem. Plasma Process. 2002, 22, 553–571. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Schmidt-Szałowski, K.; Nogal, Ł.; Kuca, B.; Krawczyk, K.; Młotek, M. Decomposition of carbon tetrachloride in the reactor of dielectric barrier discharge with different power supplies. Eur. Phys. J. Appl. Phys. 2013, 61, 24324. [Google Scholar] [CrossRef]
- Burlica, R.; Finney, W.C.; Locke, B.R. Effects of the Voltage and Current Waveforms and Discharge Power on Hydrogen Peroxide Formation in Water-Spray Gliding Arc Reactors. IEEE Trans. Ind. Appl. 2013, 49, 1098–1103. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Krawczyk, K.; Młotek, M.; Schmidt-Szałowski, K.; Nogal, Ł.; Kuca, B. A comparison of carbon tetrachloride decomposition using spark and barrier discharges. Open Chem. 2014, 13, 509–516. [Google Scholar] [CrossRef]
- Krawczyk, K.; Jodzis, S.; Lamenta, A.; Kostka, K.; Ulejczyk, B.; Schmidt-Szalowski, K. Carbon Tetrachloride Decomposition by Pulsed Spark Discharges in Oxidative and Nonoxidative Conditions. IEEE Trans. Plasma Sci. 2011, 39, 3203–3210. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, Ł.; Młotek, M.; Krawczyk, K. Enhanced production of hydrogen from methanol using spark discharge generated in a small portable reactor. Energy Rep. 2021, 8, 183–191. [Google Scholar] [CrossRef]
- Dvonč, L.; Janda, M. Study of Transient Spark Discharge Properties Using Kinetic Modeling. IEEE Trans. Plasma Sci. 2015, 43, 2562–2570. [Google Scholar] [CrossRef]
- Janda, M.; Martišovitš, V.; Hensel, K.; Dvonč, L.; Machala, Z. Measurement of the electron density in Transient Spark discharge. Plasma Sources Sci. Technol. 2014, 23, 065016. [Google Scholar] [CrossRef]
- Oguri, T.; Shimamura, K.; Shibuta, Y.; Shimojo, F.; Yamaguchi, S. Bond dissociation mechanism of ethanol during carbon nanotube synthesis via alcohol catalytic CVD technique: Ab initio molecular dynamics simulation. Chem. Phys. Lett. 2014, 595–596, 185–191. [Google Scholar] [CrossRef]
- Matus, M.H.; Nguyen, M.T.; Dixon, D.A. Theoretical Prediction of the Heats of Formation of C2H5O•Radicals Derived from Ethanol and of the Kinetics of β-C−C Scission in the Ethoxy Radical. J. Phys. Chem. A 2006, 111, 113–126. [Google Scholar] [CrossRef]
- Maksyutenko, P.; Rizzo, T.R.; Boyarkin, O.V. A direct measurement of the dissociation energy of water. J. Chem. Phys. 2006, 125, 181101. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, B.; Brey, J.J.; Viera, I.G.; Gonzales-Elipe, A.R.; Cortino, J.; Roco, V.J. Hydrogen production by reforming of hydrocarbons and alcohols in a dielectric barrier discharge. J. Power Sources 2007, 169, 140–143. [Google Scholar] [CrossRef]
- Sahu, D. Degradation of Industrial Phenolic Wastewater Using Dielectric Barrier Discharge Plasma Technique. Russ. J. Appl. Chem. 2020, 93, 905–915. [Google Scholar] [CrossRef]
| Power, W | Concentration, % | |||||||
|---|---|---|---|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | C2H2 | C2H4 | C2H5OH | H2O | |
| 15.4 | 57.1 | 23.3 | 4.3 | 3.2 | 3.1 | 2.0 | 5.0 | 2.0 |
| 19.8 | 57.9 | 23.6 | 4.3 | 3.8 | 1.4 | 1.9 | 5.1 | 2.0 |
| 24.8 | 57.2 | 23.4 | 4.4 | 4.3 | 1.2 | 1.1 | 5.2 | 2.1 |
| 29.7 | 58.2 | 24.3 | 4.0 | 3.5 | 1.4 | 1.1 | 5.3 | 2.1 |
| 35.4 | 57.5 | 23.9 | 3.8 | 3.4 | 1.9 | 1.9 | 5.4 | 2.2 |
| 40.1 | 57.7 | 24.0 | 3.8 | 3.4 | 1.9 | 1.9 | 5.1 | 2.1 |
| 45.6 | 57.7 | 24.0 | 3.7 | 3.5 | 1.9 | 1.9 | 5.2 | 2.1 |
| 54.7 | 57.5 | 23.9 | 3.8 | 3.4 | 2.0 | 2.0 | 5.3 | 2.1 |
| Feed Flow Rate mol/h | Residence Time, ms | Concentration, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | C2H2 | C2H4 | C2H5OH | H2O | ||
| 0.32 | 45 | 61.5 | 21.0 | 4.2 | 6.1 | 2.0 | 2.0 | 1.2 | 1.9 |
| 0.50 | 29 | 58.9 | 21.5 | 4.2 | 5.4 | 1.7 | 1.8 | 4.4 | 2.0 |
| 0.75 | 19 | 58.6 | 22.6 | 4.2 | 4.7 | 1.4 | 1.5 | 5.0 | 2.0 |
| 1.01 | 14 | 58.2 | 23.4 | 4.4 | 4.3 | 1.2 | 1.1 | 5.2 | 2.1 |
| 1.42 | 10 | 58.0 | 24.3 | 4.3 | 3.7 | 1.1 | 1.0 | 5.4 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulejczyk, B.; Nogal, Ł.; Młotek, M.; Krawczyk, K. Efficient Plasma Technology for the Production of Green Hydrogen from Ethanol and Water. Energies 2022, 15, 2777. https://doi.org/10.3390/en15082777
Ulejczyk B, Nogal Ł, Młotek M, Krawczyk K. Efficient Plasma Technology for the Production of Green Hydrogen from Ethanol and Water. Energies. 2022; 15(8):2777. https://doi.org/10.3390/en15082777
Chicago/Turabian StyleUlejczyk, Bogdan, Łukasz Nogal, Michał Młotek, and Krzysztof Krawczyk. 2022. "Efficient Plasma Technology for the Production of Green Hydrogen from Ethanol and Water" Energies 15, no. 8: 2777. https://doi.org/10.3390/en15082777
APA StyleUlejczyk, B., Nogal, Ł., Młotek, M., & Krawczyk, K. (2022). Efficient Plasma Technology for the Production of Green Hydrogen from Ethanol and Water. Energies, 15(8), 2777. https://doi.org/10.3390/en15082777






