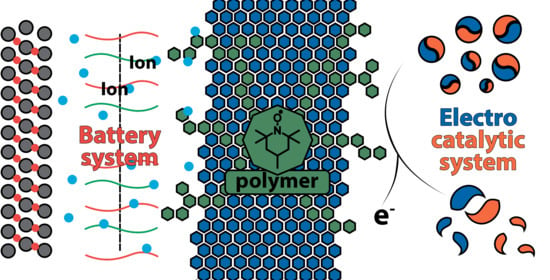
Key Features of TEMPO-Containing Polymers for Energy Storage and Catalytic Systems
Abstract
1. Introduction
2. Electroactive Materials Based on TEMPO
2.1. Synthetic Approaches
2.1.1. Non-Conductive Backbones
2.1.2. Conductive Backbones
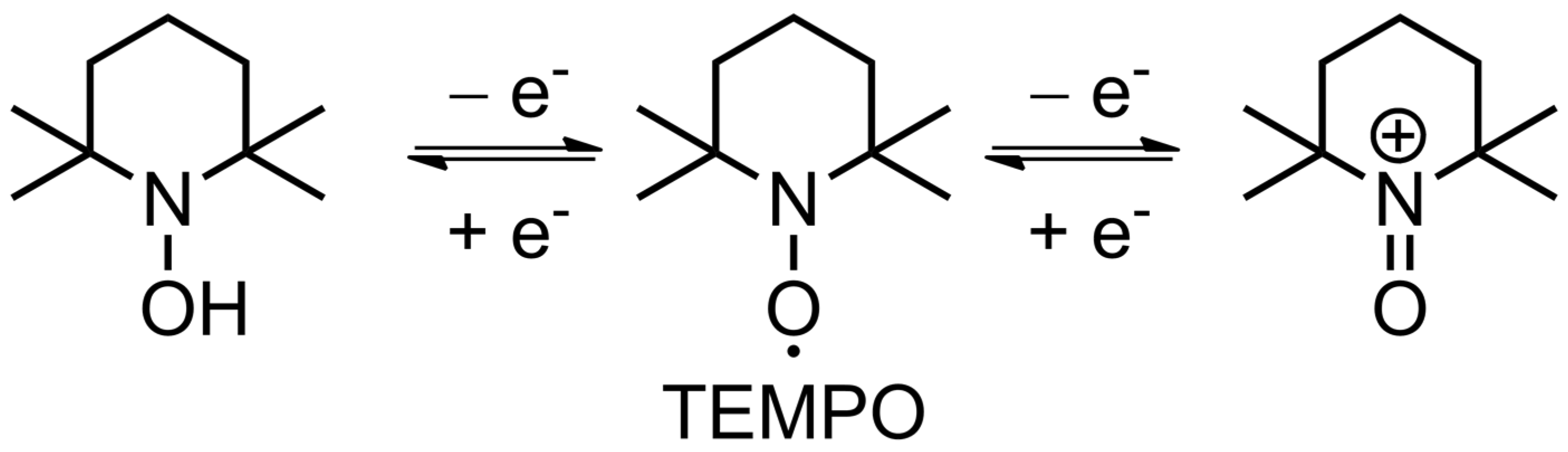
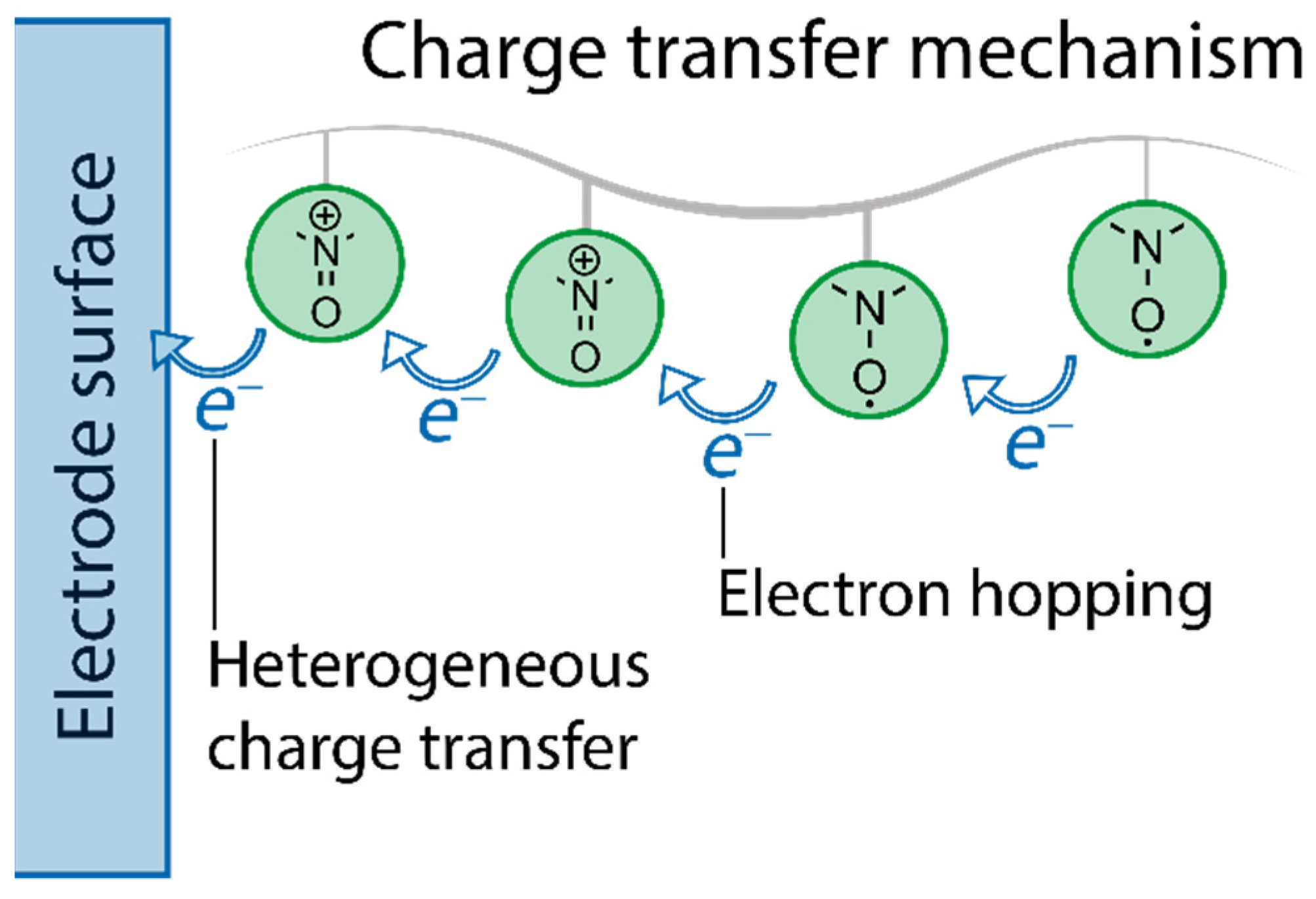
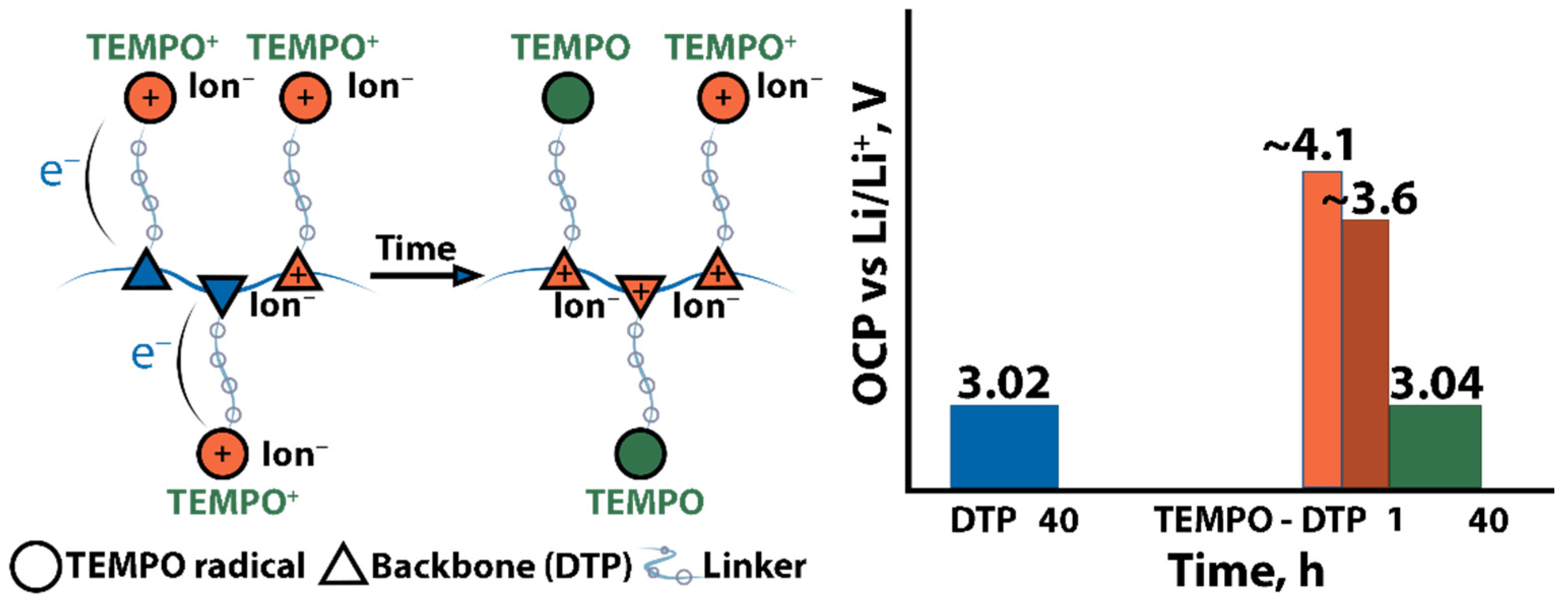
2.2. Fundamental Electrochemistry of TEMPO Transformations
3. Application of TEMPO-Containing Polymers in Energy Storage Devices
3.1. Batteries
3.1.1. Radical Polymers
| No. | Material Structure | Electrode Composition (Active:Conductive:Binder) / % | Negative Electrode | Electrolyte | Output Voltage vs. Li+ / V | Capacity Retention / % of Initial Capacity | Current / C | Capacity per Electrode Mass (Polymer mass) / mAh g−1 | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 |  | 80:15:5 | Li | 1 M LiPF6 / EC:DEC | 3.5 | n/a | 1 | 83(104) | [76] |
| 2 | 20:70:10 | Li | 1 M LiPF6 / EC:DEC | 3.5 | n/a | 0.6 | 23(114) | [20] | |
| 3 | 100:0:0 | Zn | 0.1 M NH4Cl / H2O | 1.7 vs. Zn/Zn2+ | 75 after 1000 cycles | 60 | 131(131) | [70] | |
| 4 | 100:0:0 | Zn | 0.1 M NaCl / H2O | 0.75 vs. Ag/AgCl | 75 after 1000 cycles | 60 | 131(131) | [46] | |
| 5 | 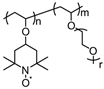 | 10:60:30 | Li | 1 M LiBETI / EC:DEC | 3.55 | n/a | 1 | 10(103) r = 1 | [77] |
| 6 |  | 10:60:30 | Li | 1 M LiBETI / EC:DEC | 3.55 | n/a | 1 | 11(113) r = 3 | [77] |
| 7 | 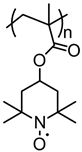 | 25:75:10 | Li | 1 M LiClO4 / EC:DEC | 3.6 | 96 after 370 cycles | 10 | 23(94) | [91] |
| 8 | 100:0:0 | Pt | 0.1 M TBAClO4 / AN | 0.4 vs. Ag/AgNO3 | 90 after 50 cycles | 10 | 97(97) | [92] | |
| 9 | 100:0:0 | Li | 0.1 M LiTFSI / EC:DEC | 3.65 | 97 after 100 cycles | 20 | 94(94) | [93] | |
| 10 | 30:60:10 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 80 after 500 cycles | 1 | 40(133) | [94] | |
| 11 | 100:0:0 | Li | 1 M LiPF6 / EC:DMC | 3.6/2.9 | 88 after 100 cycles | 0.1 | 221(221) | [95] | |
| 12 | 10:80:10 | Li | 1 M LiPF6 / EC:DMC:DEC | 3.6/2.9 | 45 after 20,000 cycles | 1 | 22(222) | [96] | |
| 13 | 80:10:10 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 77 after 300 cycles | 1 | 182(228) | [96] | |
| 14 | 100:0:0 | Li | 1 M LiPF6 / EC:DMC:DEC | 3.65 | 87 after 200 cycles | 0.5 | 94(94) | [97] | |
| 15 | 100:0:0 | Li | 1 M LiPF6 / EC:DEC | 3.6 | 72 after 150 cycles | 0.5 | 111(111) | [98] | |
| 16 | 30:60:10 | Li | 1 M LiPF6 / EC:PC:DEC | 3.6 | 88 after 100 cycles | 1 | 27(90) | [99] | |
| 17 | 90:10:0 | Li | 1 M LiPF6 / EC:PC:DEC | 3.6 | 85 after 1200 cycles | 10 | 49(55) | [100] | |
| 18 | 80:20:0 | Li | 1 M LiTFSI / EC:DEC:DMC | 3.7 | 70 after 80 cycles | 1 | 12(15) | [101] | |
| 19 | 56:29:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | 96 after 1000 cycles | 1 | 61(103) | [17] | |
| 20 | 60:30:10 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 99 after 10 cycles | 50 | 65(109) | [102] | |
| 21 | 40:50:10 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 90 after 100 cycles | 1 | 44(111) | [103] | |
| 22 | 60:30:10 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 88 after 200 cycles | 1 | 47(79) | [104] | |
| 23 | 80:10:10 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 90 after 50 cycles | 1 | 88(110) | [105] | |
| 24 | 50:30:20 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 91 after 150 cycles | 10 | 56(111) | [106] | |
| 25 | 25:65:10 | Li | 1 M LiPF6 / DMC | 3.6 | 99 after 300 cycles | 1 | 17(67) | [107] | |
| 26 | 40:50:10 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 84 after 100 cycles | 30 | 44(111) | [108] | |
| 27 | 10:80:10 | Pt | 0.1 M TBABF4 / AN | 3.6 | 99 after 1000 cycles | 10 | 8(77) | [109] | |
| 28 | 50:45:5 | Graphite | 1 M LiPF6 / EC:DEC | 3.5 | 82 after 100 cycles | 1 | 40(80) | [110] | |
| 29 | 50:45:5 | Li | 1 M LiPF6 / EC:DEC | 3.6 | 92 after 100 cycles | 1 | 55(110) | [22] | |
| 30 | 60:35:5 | Carbon | 1 M Pyr14TFSI / PC | 0.7 vs. Ag/Ag+ | n/a | 0.2 | 58(96) | [111] | |
| 31 | 77:15:8 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 98 after 100 cycles | 1 | 60(78) | [112] | |
| 32 | 10:80:10 | Li | 1 M LiPF6 / EC:DEC | 3.5 | 88 after 500 cycles | 0.1 | 8(77) | [43] | |
| 33 | 40:50:10 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 85 after 400 cycles | 10 | 44(110) | [113] | |
| 34 | 27:46:27 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 95 after 200 cycles | 2 | 26(97) | [18] | |
| 35 | 50:50:0 | Li | 1 M LiPF6 / DMC | 3.6 | 82 after 100 cycles | 1 | 125(250) | [114] | |
| 36 | 100:0:0 | Carbon | 1 M LiClO4 / H2O | 0.6 vs. Ag/AgCl | 55 after 1000 cycles | 1 | 83(83) | [115] | |
| 37 | 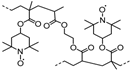 | 70:21:9 | Li | 1 M LiPF6 / EC:DMC | 3.6 | 95 after 500 cycles | 1 | 73(104) | [116] |
| 38 | 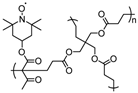 | 100:0:0 | Pt | 0.5 M (n-C4H9)4NClO4 / AN | 0.8 vs. Ag/AgCl | n/a | 1 | 55(55) | [26] |
| 39 |  | 10:80:10 | Li | 1 M LiPF6 / EC:DEC:EMC | 3.6 | 83 after 2000 cycles | 10 | 9(90) | [31] |
| 40 | 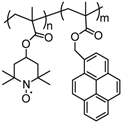 | 30:60:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | 87 after 170 cycles | 0.16 | 33(110) | [78] |
| 41 | 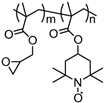 | 100:0:0 | Pt | 0.1 M TBAClO4 / AN | 0.8 vs. Ag/AgCl | 99 after 500 cycles | 100 | 70(70) | [45] |
| 42 | 30:60:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | 95 after 50 cycles | 0.1 | 31(104) | [117] | |
| 43 |  | 10:80:10 | Li | 0.1 M LiClO4 / EC:DEC | 3.67 | 85 after 100 cycles | 5 | 2(20) | [67] |
| 44 | 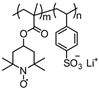 | 100:0:0 | Graphite | 0.05 M LiPF6 / DMF:DEC | 3.9 | n/a | 10 | 39(39) | [79] |
| 45 | 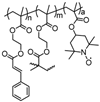 | 10:80:10 | Li | 1 M LiPF6 / EC:DMC:EMC | 3.6 | 90 after 1000 cycles | 10 | 7(65) | [80] |
| 46 |  | 100:0:0 | Carbon | 0.1 M TBAClO4 / EC:DEC | 0.8 vs. Ag/AgCl | 90 after 100 cycles | 1 | 103(103) | [28] |
| 47 |  | 10:80:10 | Li | 1 M LiPF6 / EC:DMC | 3.62 | 98 after 50 cycles | 1 | 4 (41) | [81] |
| 48 |  | 10:80:10 | Pt | 0.1 M TBAClO4 / AN | 0.7 vs. Ag/AgCl | 90 after 1000 cycles | 60 | 7(74) | [118] |
| 49 | 90:10:0 | Pt | 0.1 M TBAClO4 / AN | 0.7 vs. Ag/AgCl | n/a | 10 | 53(59) | [118] | |
| 50 | 30:60:10 | Li | 1 M LiPF6 / EC:DEC | 3.5 | 99 after 1000 cycles | 10 | ~23(77) | [24] | |
| 51 | 90:10:0 | Li | 1 M LiPF6 / EC:DEC | 3.5 | 70 after 200 cycles | 1 | 72(80) | [27] | |
| 52 | 30:60:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | n/a | 1 | 24(80) | [119] | |
| 53 | 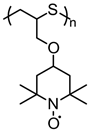 | 95:5:0 | Pt | 0.5 M (n-C4H9)4NClO4 / AN | 0.2 vs. Fc/Fc+ | n/a | 10 | 83(87) | [82] |
| 54 | 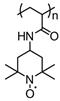 | 100:0:0 | Organic PAQE | 3 M NaCl / H2O | 0.7 vs. Ag/AgCl | n/a | 18 | 60(60) | [120] |
| 55 | 100:0:0 | Polyviologen | 0.1 M NaBF4 / H2O | 0.7 vs. Ag/AgCl | 80 after 2000 cycles | 60 | 110(110) | [19] | |
| 56 | 80:10:10 | Li | 1 M LiPF6 / EC:DMC | n/a | 63 after 100 cycles | 1 | 64(80) | [29] | |
| 57 | 100:0:0 | Pt | 0.1 M NaBF4 / H2O | 0.7 vs. Ag/AgCl | 97 after 1000 cycles | 60 | 114(114) | [121] | |
| 58 |  | 80:10:10 | Li | 1 M LiPF6 / EC:DMC | n/a | 95 after 100 cycles | 1 | 76(96) | [29] |
| 59 |  | 100:0:0 | Li | 1 M LiPF6 / EC:DEC | 3.6 | 99 after 1000 cycles | 10 | 106(106) | [61] |
| 60 | 10:80:10 | Organic | 1 M TBAClO4 -TBAOH / AN | 0.66 | 80 after 250 cycles | 360 | 3(32) | [122] | |
| 61 | 10:80:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | 90 after 500 cycles | 1 | 11(109) | [123] | |
| 62 | 30:60:10 | Li | 1 M LiTFSI/EMITFSI | 3.5 | 99 after 100 cycles | 1 | 22(74) | [124] | |
| 63 | 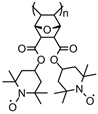 | 10:80:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | 90 after 100 cycles | 1 | 11(107) | [25] |
| 64 | 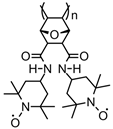 | 10:80:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | n/a | 1 | 10(93) | [25] |
| 65 | 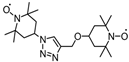 | 40:50:10 | Li | 1 M LiPF6 / EC:DMC:PC | 3.6 | 80 after 200 cycles | 0.5 | 47(117) | [83] |
| 66 | 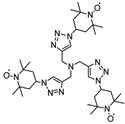 | 40:50:10 | Li | 1 M LiPF6 / EC:DMC:PC | 3.6 | 96 after 200 cycles | 0.5 | 35(87) | [83] |
| 67 |  | 20:70:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | Stable after 80 cycles | 0.25 | 6.2(31) | [21] |
| 68 | 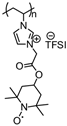 | 100:0:0 | Li | 1 M LiTFSI / EC:DEC:DMC | 3.62 | 89 after 100 cycles | 1 | 170(170) | [23] |
| 69 |  | 50:50:0 | Li | 1 M LiTFSI / EC:DEC:DMC | n/a | 92 after 3600 cycles | 60 | 35(69) | [84] |
| 70 |  | 30:60:10 | Li | 1 M LiPF6 / EC:DMC | 3.5 | 76 after 300 cycles | 1 | 18(59) | [85] |
| 71 |  | 30:60:10 | Li | 1 M LiPF6 / EC:DMC | 3.5 | 99 after 50 cycles | 1 | 24(80) | [85] |
| 72 | 40:50:10 | Li | 0.5 M LiTFSI / EMImTFSI | 3.45 | 98 after 30 cycles | 1 | 32(80) | [86] | |
| 73 | 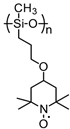 | 20:70:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | n/a | 0.5 | 9(46) | [87] |
| 74 | 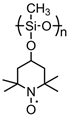 | 20:70:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | 99 after 100 cycles | 0.5 | 18(89) | [87] |
| 75 | TEMPO-DNA molecule | 10:80:10 | Li | 1 M LiPF6 / EC:DEC | 3.6/2.8 | 90 after 100 cycles | 1 | 6(60) | [125] |
| 76 | TEMPO-cellulose | 10:80:10 | Li | 1 M LiPF6 / EC:DEC | 3.6/2.8 | 93 after 110 cycles | 1 | 6(61) | [32] |
| 77 | TEMPO-Carbon | 80:15:5 | Naphtalimide | 1 M TBABF4 / PC | 0.9 vs. Ag/Ag+ | 70 after 50 cycles | 0.1 | 6(8) | [126] |
| 78 | TEMPO-functionalized graphene | 80:10:10 | Li | 1 M LiPF6 / EC:DMC | 1.4 | n/a | 0.6 | 874(1093) | [127] |
| 79 | TEMPO- functionalized graphite nanoplatelet | 100:0:0 | Li | 1 M LiPF6 / EC:DEC:DMC | n/a | n/a | 1 | 450(450) | [128] |
| 80 | TEMPO / hollow carbon spheres composite | 80:10:10 | Li | 1 M LiPF6 / EC:DEC:DMC | 3.7 | 82 after 200 cycles | 0.5 | 264(330) | [88] |
| 81 | TEMPO-based six-arm star | 40:50:10 | Li | 1 M LiPF6 / EC:DEC | 3.6/2.9 | n/a | 1 | 13(32) | [89] |
| 82 | 20:70:10 | Li | 1 M LiPF6 / EC:DEC | 3.6 | n/a | 1 | 23(115) | [90] |
3.1.2. Redox-Conducting Polymers
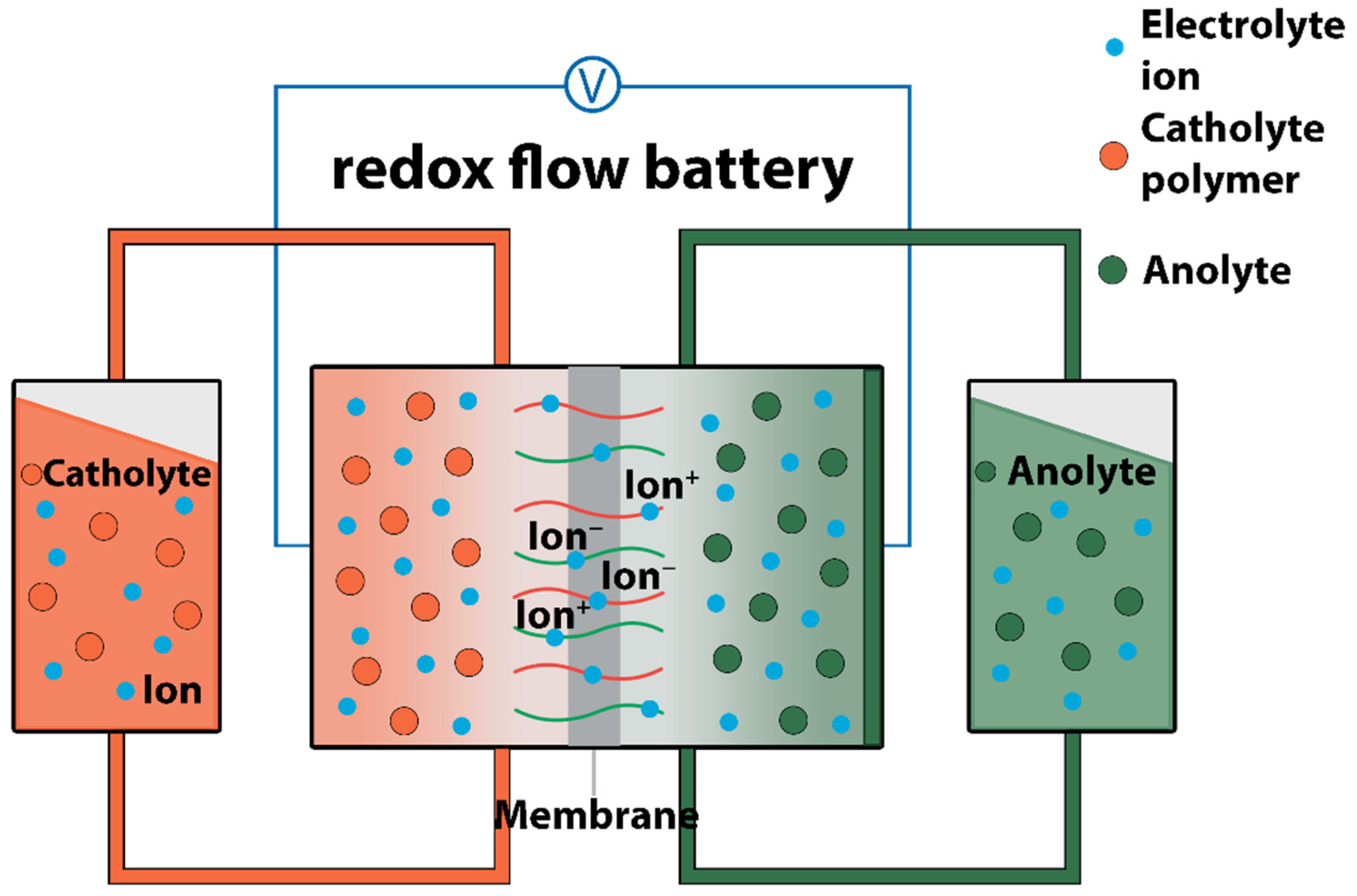
3.2. Redox Flow Batteries
3.3. Catalytic Mediators
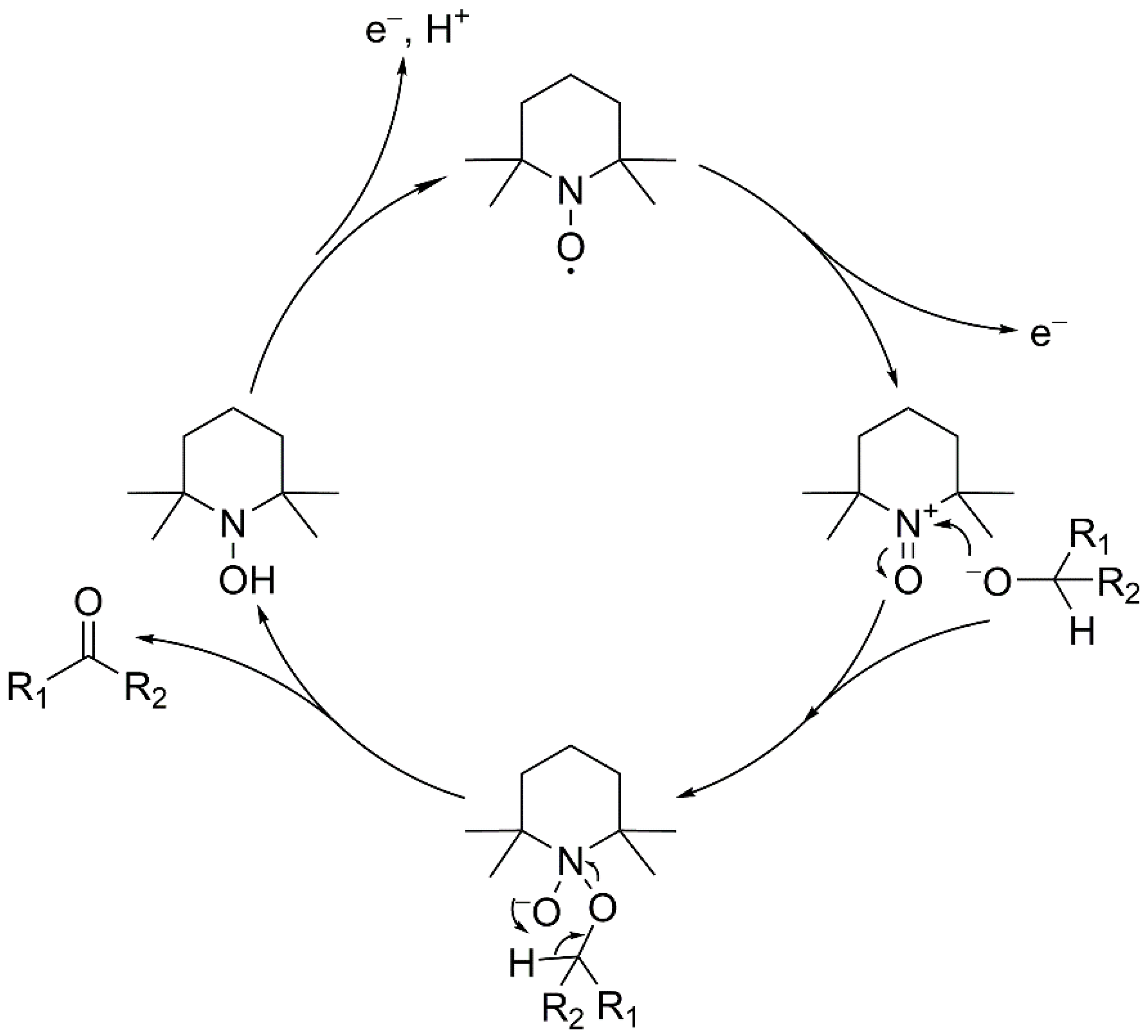
3.3.1. Oxidation of Alcohols
3.3.2. Oxidation of Amines
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Banza Lubaba Nkulu, C.; Casas, L.; Haufroid, V.; De Putter, T.; Saenen, N.D.; Kayembe-Kitenge, T.; Musa Obadia, P.; Kyanika Wa Mukoma, D.; Lunda Ilunga, J.M.; Nawrot, T.S.; et al. Sustainability of artisanal mining of cobalt in DR Congo. Nat. Sustain. 2018, 1, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Poizot, P.; Gaubicher, J.; Renault, S.; Dubois, L.; Liang, Y.; Yao, Y. Opportunities and Challenges for Organic Electrodes in Electrochemical Energy Storage. Chem. Rev. 2020, 120, 6490–6557. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, K.; Yamauchi, Y.; Oyaizu, K.; Jia, Z. Nitroxide radical polymers for emerging plastic energy storage and organic electronics: Fundamentals, materials, and applications. Mater. Horiz. 2021, 8, 803–829. [Google Scholar] [CrossRef] [PubMed]
- Esser, B.; Dolhem, F.; Becuwe, M.; Poizot, P.; Vlad, A.; Brandell, D. A perspective on organic electrode materials and technologies for next generation batteries. J. Power Sources 2021, 482, 228814. [Google Scholar] [CrossRef]
- Goujon, N.; Casado, N.; Patil, N.; Marcilla, R.; Mecerreyes, D. Organic batteries based on just redox polymers. Prog. Polym. Sci. 2021, 122, 101449. [Google Scholar] [CrossRef]
- Rohland, P.; Schröter, E.; Nolte, O.; Newkome, G.R.; Hager, M.D.; Schubert, U.S. Redox-active polymers: The magic key towards energy storage—A polymer design guideline progress in polymer science. Prog. Polym. Sci. 2022, 125, 101474. [Google Scholar] [CrossRef]
- Tan, Y.; Hsu, S.N.; Tahir, H.; Dou, L.; Savoie, B.M.; Boudouris, B.W. Electronic and Spintronic Open-Shell Macromolecules, Quo Vadis? J. Am. Chem. Soc. 2022, 144, 626–647. [Google Scholar] [CrossRef]
- Hansen, K.A.; Blinco, J.P. Nitroxide radical polymers—A versatile material class for high-tech applications. Polym. Chem. 2018, 9, 1479–1516. [Google Scholar] [CrossRef]
- Muench, S.; Wild, A.; Friebe, C.; Haupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef]
- Janoschka, T.; Hager, M.D.; Schubert, U.S. Powering up the Future: Radical Polymers for Battery Applications. Adv. Mater. 2012, 24, 6397–6409. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhuo, S.; Li, Z.; Wang, C. Redox polymers for rechargeable metal-ion batteries. EnergyChem 2020, 2, 100030. [Google Scholar] [CrossRef]
- Kim, K.; Baldaguez Medina, P.; Elbert, J.; Kayiwa, E.; Cusick, R.D.; Men, Y.; Su, X. Molecular Tuning of Redox-Copolymers for Selective Electrochemical Remediation. Adv. Funct. Mater. 2020, 30, 2004635. [Google Scholar] [CrossRef]
- Mohebbati, N.; Prudlik, A.; Scherkus, A.; Gudkova, A.; Francke, R. TEMPO-Modified Polymethacrylates as Mediators in Electrosynthesis—Redox Behavior and Electrocatalytic Activity toward Alcohol Substrates. ChemElectroChem 2021, 8, 3837–3843. [Google Scholar] [CrossRef]
- Yang, S.G.; Park, H.J.; Kim, J.W.; Jung, J.M.; Kim, M.J.; Jegal, H.G.; Kim, I.S.; Kang, M.J.; Wee, G.; Yang, H.Y.; et al. Mito-TEMPO improves development competence by reducing superoxide in preimplantation porcine embryos. Sci. Rep. 2018, 8, 10130. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Kumar, R.; Bharati, S. Mito-TEMPO, a mitochondria-targeted antioxidant, prevents N-nitrosodiethylamine-induced hepatocarcinogenesis in mice. Free Radic. Biol. Med. 2019, 136, 76–86. [Google Scholar] [CrossRef]
- Komaba, S.; Tanaka, T.; Ozeki, T.; Taki, T.; Watanabe, H.; Tachikawa, H. Fast redox of composite electrode of nitroxide radical polymer and carbon with polyacrylate binder. J. Power Sources 2010, 195, 6212–6217. [Google Scholar] [CrossRef]
- Bugnon, L.; Morton, C.J.H.; Novak, P.; Vetter, J.; Nesvadba, P. Synthesis of Poly(4-methacryloyloxy-TEMPO) via Group-Transfer Polymerization and Its Evaluation in Organic Radical Battery. Chem. Mater. 2007, 19, 2910–2914. [Google Scholar] [CrossRef]
- Koshika, K.; Chikushi, N.; Sano, N.; Oyaizu, K.; Nishide, H. A TEMPO-substituted polyacrylamide as a new cathode material: An organic rechargeable device composed of polymer electrodes and aqueous electrolyte. Green Chem. 2010, 12, 1573–1575. [Google Scholar] [CrossRef]
- Suguro, M.; Iwasa, S.; Kusachi, Y.; Morioka, Y.; Nakahara, K. Cationic Polymerization of Poly(vinyl ether) Bearing a TEMPO Radical: A New Cathode-Active Material for Organic Radical Batteries. Macromol. Rapid Commun. 2007, 28, 1929–1933. [Google Scholar] [CrossRef]
- Sertkol, S.B.; Sinirlioglu, D.; Esat, B.; Muftuoglu, A.E. A novel cathode material based on polystyrene with pendant TEMPO moieties obtained via click reaction and its use in rechargeable batteries. J. Polym. Res. 2015, 22, 136. [Google Scholar] [CrossRef]
- Nakahara, K.; Iriyama, J.; Iwasa, S.; Suguro, M.; Satoh, M.; Cairns, E.J. Cell properties for modified PTMA cathodes of organic radical batteries. J. Power Sources 2007, 165, 398–402. [Google Scholar] [CrossRef]
- Aqil, M.; Ouhib, F.; Aqil, A.; El Idrissi, A.; Detrembleur, C.; Jérôme, C. Polymer ionic liquid bearing radicals as an active material for organic batteries with ultrafast charge-discharge rate. Eur. Polym. J. 2018, 106, 242–248. [Google Scholar] [CrossRef]
- Suga, T.; Yoshimura, K.; Nishide, H. Nitroxide-Substituted Polyether as a New Material for Batteries. Macromol. Symp. 2006, 245–246, 416–422. [Google Scholar] [CrossRef]
- Qu, J.; Katsumata, T.; Satoh, M.; Wada, J.; Masuda, T. Poly(7-oxanorbornenes) carrying 2,2,6,6-tetramethylpiperidine-1-oxy (TEMPO) radicals: Synthesis and charge/discharge properties. Polymer 2009, 50, 391–396. [Google Scholar] [CrossRef]
- Ibe, T.; Frings, R.B.; Lachowicz, A.; Kyo, S.; Nishide, H. Nitroxide polymer networks formed by Michael addition: On site-cured electrode-active organic coating. Chem. Commun. 2010, 46, 3475–3477. [Google Scholar] [CrossRef]
- Sukegawa, T.; Sato, K.; Oyaizu, K.; Nishide, H. Efficient charge transport of a radical polyether/SWCNT composite electrode for an organic radical battery with high charge-storage density. RSC Adv. 2015, 5, 15448–15452. [Google Scholar] [CrossRef]
- Sukegawa, T.; Masuko, I.; Oyaizu, K.; Nishide, H. Expanding the Dimensionality of Polymers Populated with Organic Robust Radicals toward Flow Cell Application: Synthesis of TEMPO-Crowded Bottlebrush Polymers Using Anionic Polymerization and ROMP. Macromolecules 2014, 47, 8611–8617. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, T.; Tuo, H.; Zhang, W. Synthesis of a TEMPO-Substituted Polyacrylamide Bearing a Sulfonate Sodium Pendant and Its Properties in an Organic Radical Battery. Polymers 2019, 11, 2076. [Google Scholar] [CrossRef]
- Hickey, D.P.; Milton, R.D.; Chen, D.; Sigman, M.S.; Minteer, S.D. TEMPO-Modified Linear Poly(ethylenimine) for Immobilization-Enhanced Electrocatalytic Oxidation of Alcohols. ACS Catal. 2015, 5, 5519–5524. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Liu, X.; Fan, X.; Bai, B.; Yang, K.; Liang, Z.; Zhang, Z.; Mai, K. Long-Life and High-Power Binder-Free Cathode Based on One-Step Synthesis of Radical Polymers with Multi-Pendant Groups. Macromol. Rapid Commun. 2018, 39, 1800195. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Khan, F.Z.; Satoh, M.; Wada, J.; Hayashi, H.; Mizoguchi, K.; Masuda, T. Synthesis and charge/discharge properties of cellulose derivatives carrying free radicals. Polymer 2008, 49, 1490–1496. [Google Scholar] [CrossRef]
- Aydın, M.; Esat, B.; Kılıç, Ç.; Köse, M.E.; Ata, A.; Yılmaz, F. A polythiophene derivative bearing TEMPO as a cathode material for rechargeable batteries. Eur. Polym. J. 2011, 47, 2283–2294. [Google Scholar] [CrossRef]
- Xu, L.; Ji, L.; Wang, G.; Zhang, C.; Su, C. A novel nitroxide radical polymer-containing conductive polyaniline as molecular skeleton: Its synthesis and electrochemical properties as organic cathode. Ionics 2016, 22, 1377–1385. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, A.M.; McMillan, S.R.; Harmon, N.J.; Flatté, M.E.; Fuchs, G.D.; Ober, C.K. Charge Transport in Conjugated Polymers with Pendent Stable Radical Groups. Chem. Mater. 2018, 30, 4799–4807. [Google Scholar] [CrossRef]
- Qu, J.; Fujii, T.; Katsumata, T.; Suzuki, Y.; Shiotsuki, M.; Sanda, F.; Satoh, M.; Wada, J.; Masuda, T. Helical polyacetylenes carrying 2,2,6,6-tetramethyl-1-piperidinyloxy and 2,2,5,5-tetramethyl-1-pyrrolidinyloxy moieties: Their synthesis, properties, and function. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 5431–5445. [Google Scholar] [CrossRef]
- Xu, L.; Yang, F.; Su, C.; Ji, L.; Zhang, C. Synthesis and properties of novel TEMPO-contained polypyrrole derivatives as the cathode material of organic radical battery. Electrochim. Acta 2014, 130, 148–155. [Google Scholar] [CrossRef]
- Yi, J.; Tang, D.; Song, D.; Wu, X.; Shen, Z.; Li, M. Selective oxidation of benzyl alcohol on poly(4-(3-(pyrrol-1-yl)propionamido)-2,2,6,6-tetramethylpiperidin-1-yloxy) electrode. J. Solid State Electrochem. 2015, 19, 2291–2297. [Google Scholar] [CrossRef]
- Li, F.; Gore, D.N.; Wang, S.; Lutkenhaus, J.L. Unusual Internal Electron Transfer in Conjugated Radical Polymers. Angew. Chem. 2017, 56, 9856–9859. [Google Scholar] [CrossRef]
- Schwartz, P.O.; Pejic, M.; Wachtler, M.; Bäuerle, P. Synthesis and characterization of electroactive PEDOT-TEMPO polymers as potential cathode materials in rechargeable batteries. Synth. Met. 2018, 243, 51–57. [Google Scholar] [CrossRef]
- Su, C.; Yang, F.; Xu, L.; Zhu, X.; He, H.; Zhang, C. Radical Polymer Containing a Polytriphenylamine Backbone: Its Synthesis and Electrochemical Performance as the Cathode of Lithium-Ion Batteries. Chempluschem 2015, 80, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagin, A.A.; Lukyanov, D.A.; Kulikov, I.R.; Panjwani, N.A.; Alekseeva, E.A.; Behrends, J.; Levin, O.V. The Fast and the Capacious: A [Ni(Salen)]-TEMPO Redox-Conducting Polymer for Organic Batteries. Batter. Supercaps 2021, 4, 336–346. [Google Scholar] [CrossRef]
- Nakahara, K.; Iwasa, S.; Satoh, M.; Morioka, Y.; Iriyama, J.; Suguro, M.; Hasegawa, E. Rechargeable batteries with organic radical cathodes. Chem. Phys. Lett. 2002, 359, 351–354. [Google Scholar] [CrossRef]
- Nevers, D.R.; Brushett, F.R.; Wheeler, D.R. Engineering radical polymer electrodes for electrochemical energy storage. J. Power Sources 2017, 352, 226–244. [Google Scholar] [CrossRef]
- Takahashi, K.; Korolev, K.; Tsuji, K.; Oyaizu, K.; Nishide, H.; Bryuzgin, E.; Navrotskiy, A.; Novakov, I. Facile grafting-onto-preparation of block copolymers of TEMPO and glycidyl methacrylates on an oxide substrate as an electrode-active layer. Polymer 2015, 68, 310–314. [Google Scholar] [CrossRef]
- Koshika, K.; Sano, N.; Oyaizu, K.; Nishide, H. An ultrafast chargeable polymer electrode based on the combination of nitroxide radical and aqueous electrolyte. Chem. Commun. 2009, 7, 836–838. [Google Scholar] [CrossRef]
- Nishide, H.; Suga, T. Organic Radical Battery. Electrochem. Soc. Interface 2005, 14, 32–36. [Google Scholar] [CrossRef]
- Wang, S.; Easley, A.D.; Thakur, R.M.; Ma, T.; Yun, J.; Zhang, Y.; Ober, C.K.; Lutkenhaus, J.L. Quantifying internal charge transfer and mixed ion-electron transfer in conjugated radical polymers. Chem. Sci. 2020, 11, 9962–9970. [Google Scholar] [CrossRef]
- Kemper, T.W.; Larsen, R.E.; Gennett, T. Relationship between Molecular Structure and Electron Transfer in a Polymeric Nitroxyl-Radical Energy Storage Material. J. Phys. Chem. C 2014, 118, 17213–17220. [Google Scholar] [CrossRef]
- Oyaizu, K.; Ando, Y.; Konishi, H.; Nishide, H. Nernstian Adsorbate-like Bulk Layer of Organic Radical Polymers for High-Density Charge Storage Purposes. J. Am. Chem. Soc. 2008, 130, 14459–14461. [Google Scholar] [CrossRef]
- Suga, T.; Pu, Y.J.; Oyaizu, K.; Nishide, H. Electron-Transfer Kinetics of Nitroxide Radicals as an Electrode-Active Material. Bull. Chem. Soc. Jpn. 2004, 77, 2203–2204. [Google Scholar] [CrossRef]
- Nakahara, K.; Oyaizu, K.; Nishide, H. Organic Radical Battery Approaching Practical Use. Chem. Lett. 2011, 40, 222–227. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Studer, A. Encyclopedia of Radicals in Chemistry, Biology, and Materials; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2012; Volume 2. [Google Scholar]
- Grampp, G.; Rasmussen, K. Solvent dynamical effects on the electron self-exchange rate of the TEMPO/TEMPO+ couple (TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxy radical) Part I. ESR-linebroadening measurements at T = 298 K. Phys. Chem. Chem. Phys. 2002, 4, 5546–5549. [Google Scholar] [CrossRef]
- Martin, H.J.; Hughes, B.K.; Braunecker, W.A.; Gennett, T.; Dadmun, M.D. The impact of radical loading and oxidation on the conformation of organic radical polymers by small angle neutron scattering. J. Mater. Chem. A 2018, 6, 15659–15667. [Google Scholar] [CrossRef]
- Katsumata, T.; Satoh, M.; Wada, J.; Shiotsuki, M.; Sanda, F.; Masuda, T. Polyacetylene and Polynorbornene Derivatives Carrying TEMPO. Synthesis and Properties as Organic Radical Battery Materials. Macromol. Rapid Commun. 2006, 27, 1206–1211. [Google Scholar] [CrossRef]
- Lutkenhaus, J. A radical advance for conducting polymers. Science 2018, 359, 1334–1335. [Google Scholar] [CrossRef]
- Joo, Y.; Agarkar, V.; Sung, S.H.; Savoie, B.M.; Boudouris, B.W. A nonconjugated radical polymer glass with high electrical conductivity. Science 2018, 359, 1391–1395. [Google Scholar] [CrossRef]
- Oka, K.; Nishide, H. Chapter 4 Radical Polymers for Rechargeable Batteries. In Redox Polymers for Energy and Nanomedicine; The Royal Society of Chemistry: London, UK, 2021; pp. 137–165. [Google Scholar]
- Tokue, H.; Oyaizu, K.; Sukegawa, T.; Nishide, H. TEMPO/Viologen Electrochemical Heterojunction for Diffusion-Controlled Redox Mediation: A Highly Rectifying Bilayer-Sandwiched Device Based on Cross-Reaction at the Interface between Dissimilar Redox Polymers. ACS Appl. Mater. Interfaces 2014, 6, 4043–4049. [Google Scholar] [CrossRef]
- Suga, T.; Konishi, H.; Nishide, H. Photocrosslinked nitroxide polymer cathode-active materials for application in an organic-based paper battery. Chem. Commun. 2007, 17, 1730–1732. [Google Scholar] [CrossRef]
- Ou, Y.; Zhang, Y.; Xiong, Y.; Hu, Z.; Dong, L. Three-dimensional porous radical polymer/reduced graphene oxide composite with two-electron redox reactions as high-performance cathode for lithium-ion batteries. Eur. Polym. J. 2021, 143, 110191. [Google Scholar] [CrossRef]
- Stolze, C.; Janoschka, T.; Flauder, S.; Müller, F.A.; Hager, M.D.; Schubert, U.S. Investigation of Ice-Templated Porous Electrodes for Application in Organic Batteries. ACS Appl. Mater. Interfaces 2016, 8, 23614–23623. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured Conjugated Polymers: Toward High-Performance Organic Electrodes for Rechargeable Batteries. ACS Energy Lett. 2017, 2, 1985–1996. [Google Scholar] [CrossRef]
- Nakahara, K.; Oyaizu, K.; Nishide, H. Electrolyte anion-assisted charge transportation in poly(oxoammonium cation/nitroxyl radical) redox gels. J. Mater. Chem. 2012, 22, 13669–13673. [Google Scholar] [CrossRef]
- Chae, I.S.; Koyano, M.; Oyaizu, K.; Nishide, H. Self-doping inspired zwitterionic pendant design of radical polymers toward a rocking-chair-type organic cathode-active material. J. Mater. Chem. A 2013, 1, 1326–1333. [Google Scholar] [CrossRef]
- Tokue, H.; Murata, T.; Agatsuma, H.; Nishide, H.; Oyaizu, K. Charge–Discharge with Rocking-Chair-Type Li+ Migration Characteristics in a Zwitterionic Radical Copolymer Composed of TEMPO and Trifluoromethanesulfonylimide with Carbonate Electrolytes for a High-Rate Li-Ion Battery. Macromolecules 2017, 50, 1950–1958. [Google Scholar] [CrossRef]
- Suga, T.; Sakata, M.; Aoki, K.; Nishide, H. Synthesis of Pendant Radical- and Ion-Containing Block Copolymers via Ring-Opening Metathesis Polymerization for Organic Resistive Memory. ACS Macro Lett. 2014, 3, 703–707. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Easley, A.D.; Lutkenhaus, J.L. Real-time insight into the doping mechanism of redox-active organic radical polymers. Nat. Mater. 2019, 18, 69–75. [Google Scholar] [CrossRef]
- Koshika, K.; Sano, N.; Oyaizu, K.; Nishide, H. An Aqueous, Electrolyte-Type, Rechargeable Device Utilizing a Hydrophilic Radical Polymer-Cathode. Macromol. Chem. Phys. 2009, 210, 1989–1995. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Kwon, S.R.; Lutkenhaus, J.L. Electropolymerized Polythiophenes Bearing Pendant Nitroxide Radicals. ACS Macro Lett. 2016, 5, 337–341. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, K.; Monteiro, M.J.; Jia, Z. Conjugated Nitroxide Radical Polymers: Synthesis and Application in Flexible Energy Storage Devices. ACS Appl. Mater. Interfaces 2019, 11, 7096–7103. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Perera, K.; Wang, Z.; Mei, J.; Boudouris, B.W. Impact of open-shell loading on mass transport and doping in conjugated radical polymers. J. Polym. Sci. 2021, 59, 2771–2782. [Google Scholar] [CrossRef]
- Li, F.; Wang, S.; Zhang, Y.; Lutkenhaus, J.L. Electrochemical Energy Storage in Poly(dithieno[3,2-b:2′,3′-d]pyrrole) Bearing Pendant Nitroxide Radicals. Chem. Mater. 2018, 30, 5169–5174. [Google Scholar] [CrossRef]
- Suguro, M.; Iwasa, S.; Nakahara, K. Fabrication of a Practical and Polymer-Rich Organic Radical Polymer Electrode and Its Rate Dependence. Macromol. Rapid Commun. 2008, 29, 1635–1639. [Google Scholar] [CrossRef]
- Suguro, M.; Iwasa, S.; Nakahara, K. Effect of Ethylene Oxide Structures in TEMPO Polymers on High Rate Discharge Properties. Electrochem. Solid-State Lett. 2009, 12, A194. [Google Scholar] [CrossRef]
- Hergué, N.; Ernould, B.; Minoia, A.; Lazzaroni, R.; Gohy, J.F.; Dubois, P.; Coulembier, O. Improving the Performance of Batteries by Using Multi-Pyrene PTMA Structures. Batter. Supercaps 2018, 1, 102–109. [Google Scholar] [CrossRef]
- Chae, I.S.; Koyano, M.; Sukegawa, T.; Oyaizu, K.; Nishide, H. Redox equilibrium of a zwitterionic radical polymer in a non-aqueous electrolyte as a novel Li+ host material in a Li-ion battery. J. Mater. Chem. A 2013, 1, 9608–9611. [Google Scholar] [CrossRef]
- Qin, H.; Liu, X.; Huang, J.; Liang, H.; Zhang, Z.; Lu, J. Design and Synthesis of a Facile Solution-Processing and Ultrastable Crosslinkable Branched Nitroxide Polymer. Macromol. Chem. Phys. 2019, 220, 1900068. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Li, L.; Lu, G.; Zhang, S.; Gu, L.; Xia, Y.; Huang, X. Polyallene with pendant nitroxyl radicals. Polymer 2008, 49, 3393–3398. [Google Scholar] [CrossRef]
- Hatakeyama-Sato, K.; Wakamatsu, H.; Matsumoto, S.; Sadakuni, K.; Matsuoka, K.; Nagatsuka, T.; Oyaizu, K. TEMPO-Substituted Poly(ethylene sulfide) for Solid-State Electro-Chemical Charge Storage. Macromol. Rapid Commun. 2021, 42, 2000607. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, Y.; Monteiro, M.J.; Jia, Z. Triazole-enabled small TEMPO cathodes for lithium-organic batteries. Energy Storage Mater. 2021, 35, 122–129. [Google Scholar] [CrossRef]
- Aqil, M.; Aqil, A.; Ouhib, F.; El Idrissi, A.; Dahbi, M.; Detrembleur, C.; Jérôme, C. Nitroxide TEMPO-containing PILs: Kinetics study and electrochemical characterizations. Eur. Polym. J. 2021, 152, 110453. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.K.; Cheruvally, G.; Choi, J.W.; Ahn, J.H.; Chauhan, G.S.; Song, C.E. Electrochemical properties of new organic radical materials for lithium secondary batteries. J. Power Sources 2008, 184, 503–507. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.K. Organic di-radical rechargeable battery with an ionic liquid-based gel polymer electrolyte. Korean J. Chem. Eng. 2016, 33, 858–861. [Google Scholar] [CrossRef]
- Suguro, M.; Mori, A.; Iwasa, S.; Nakahara, K.; Nakano, K. Syntheses and Electrochemical Properties of TEMPO Radical Substituted Silicones: Active Material for Organic Radical Batteries. Macromol. Chem. Phys. 2009, 210, 1402–1407. [Google Scholar] [CrossRef]
- Lu, C.; Pan, G.; Mao, Z.; Shi, L.; Huang, Q.; Tian, W.; Hu, Y.; Wu, H.; Wang, Z.; Sun, K. Multiradical-stabilized hollow carbon spheres as a pressure-resistant cathode for fast lithium/sodium storage with excellent performance. J. Mater. Chem. A 2020, 8, 8875–8882. [Google Scholar] [CrossRef]
- Gopinath, A.; Sultan Nasar, A. Electroactive six arm star poly(2,2,6,6-tetramethylpiperidinyloxy methacrylate): Synthesis and application as cathode material for rechargeable Li-ion batteries. Polymer 2019, 178, 121601. [Google Scholar] [CrossRef]
- Aydin, M.; Gorur, M.; Yilmaz, F. Phosphazene-cored star polymer bearing redox-active side groups as a cathode-active material in Li-ion batteries. React. Funct. Polym. 2016, 102, 11–19. [Google Scholar] [CrossRef]
- Lin, H.C.; Li, C.C.; Lee, J.T. Nitroxide polymer brushes grafted onto silica nanoparticles as cathodes for organic radical batteries. J. Power Sources 2011, 196, 8098–8103. [Google Scholar] [CrossRef]
- Wang, Y.H.; Hung, M.K.; Lin, C.H.; Lin, H.C.; Lee, J.T. Patterned nitroxide polymer brushes for thin-film cathodes in organic radical batteries. Chem. Commun. 2011, 47, 1249–1251. [Google Scholar] [CrossRef]
- Hung, M.K.; Wang, Y.H.; Lin, C.H.; Lin, H.C.; Lee, J.T. Synthesis and electrochemical behaviour of nitroxidepolymer brush thin-film electrodes for organic radical batteries. J. Mater. Chem. 2012, 22, 1570–1577. [Google Scholar] [CrossRef]
- Liu, C.M.; Chen, J.; Wang, F.Q.; Yi, B.L. Improvement of electrochemical properties of PTMA cathode by using carbon blacks with high specific surface area. Russ. J. Electrochem. 2012, 48, 1052–1057. [Google Scholar] [CrossRef][Green Version]
- Kim, T.S.; Lim, J.E.; Oh, M.S.; Kim, J.K. Carbon conductor- and binder-free organic electrode for flexible organic rechargeable batteries with high energy density. J. Power Sources 2017, 361, 15–20. [Google Scholar] [CrossRef]
- Guo, W.; Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Superior radical polymer cathode material with a two-electron process redox reaction promoted by graphene. Energy Environ. Sci. 2012, 5, 5221–5225. [Google Scholar] [CrossRef]
- Ernould, B.; Devos, M.; Bourgeois, J.P.; Rolland, J.; Vlad, A.; Gohy, J.F. Grafting of a redox polymer onto carbon nanotubes for high capacity battery materials. J. Mater. Chem. A 2015, 3, 8832–8839. [Google Scholar] [CrossRef]
- Ernould, B.; Bertrand, O.; Minoia, A.; Lazzaroni, R.; Vlad, A.; Gohy, J.F. Electroactive polymer/carbon nanotube hybrid materials for energy storage synthesized via a “grafting to” approach. RSC Adv. 2017, 7, 17301–17310. [Google Scholar] [CrossRef]
- Lin, C.H.; Lee, J.T.; Yang, D.R.; Chen, H.W.; Wu, S.T. Nitroxide radical polymer/carbon-nanotube-array electrodes with improved C-rate performance in organic radical batteries. RSC Adv. 2015, 5, 33044–33048. [Google Scholar] [CrossRef]
- Vlad, A.; Rolland, J.; Hauffman, G.; Ernould, B.; Gohy, J.F. Melt-Polymerization of TEMPO Methacrylates with Nano Carbons Enables Superior Battery Materials. ChemSusChem 2015, 8, 1692–1696. [Google Scholar] [CrossRef]
- Hauffman, G.; Maguin, Q.; Bourgeois, J.P.; Vlad, A.; Gohy, J.F. Micellar Cathodes from Self-Assembled Nitroxide-Containing Block Copolymers in Battery Electrolytes. Macromol. Rapid Commun. 2014, 35, 228–233. [Google Scholar] [CrossRef]
- Kim, J.K. Micro-fibrous organic radical electrode to improve the electrochemical properties of organic rechargeable batteries. J. Power Sources 2013, 242, 683–686. [Google Scholar] [CrossRef]
- Kim, J.K.; Cheruvally, G.; Choi, J.W.; Ahn, J.H.; Lee, S.H.; Choi, D.S.; Song, C.E. Effect of radical polymer cathode thickness on the electrochemical performance of organic radical battery. Solid State Ion. 2007, 178, 1546–1551. [Google Scholar] [CrossRef]
- Kim, J.K.; Cheruvally, G.; Ahn, J.H.; Seo, Y.G.; Choi, D.S.; Lee, S.H.; Song, C.E. Organic radical battery with PTMA cathode: Effect of PTMA content on electrochemical properties. J. Ind. Eng. Chem. 2008, 14, 371–376. [Google Scholar] [CrossRef]
- Kim, Y.; Jo, C.; Lee, J.; Lee, C.W.; Yoon, S. An ordered nanocomposite of organic radical polymer and mesocellular carbon foam as cathode material in lithium ion batteries. J. Mater. Chem. 2012, 22, 1453–1458. [Google Scholar] [CrossRef]
- Kim, J.K.; Scheers, J.; Ahn, J.H.; Johansson, P.; Matic, A.; Jacobsson, P. Nano-fibrous polymer films for organic rechargeable batteries. J. Mater. Chem. A 2013, 1, 2426–2430. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, Y.; Wang, L.; Fan, J.; Monteiro, M.J.; Jia, Z. The impact of the molecular weight on the electrochemical properties of poly(TEMPO methacrylate). Polym. Chem. 2017, 8, 1815–1823. [Google Scholar] [CrossRef]
- Kim, J.K.; Cheruvally, G.; Choi, J.W.; Ahn, J.H.; Choi, D.S.; Song, C.E. Rechargeable Organic Radical Battery with Electrospun, Fibrous Membrane-Based Polymer Electrolyte. J. Electrochem. Soc. 2007, 154, A839. [Google Scholar] [CrossRef]
- Nishide, H.; Iwasa, S.; Pu, Y.J.; Suga, T.; Nakahara, K.; Satoh, M. Organic radical battery: Nitroxide polymers as a cathode-active material. Electrochim. Acta 2004, 50, 827–831. [Google Scholar] [CrossRef]
- Nakahara, K.; Iriyama, J.; Iwasa, S.; Suguro, M.; Satoh, M.; Cairns, E.J. Al-laminated film packaged organic radical battery for high-power applications. J. Power Sources 2007, 163, 1110–1113. [Google Scholar] [CrossRef]
- Muench, S.; Gerlach, P.; Burges, R.; Strumpf, M.; Hoeppener, S.; Wild, A.; Lex-Balducci, A.; Balducci, A.; Brendel, J.C.; Schubert, U.S. Emulsion Polymerizations for a Sustainable Preparation of Efficient TEMPO-Based Electrodes. ChemSusChem 2021, 14, 449–455. [Google Scholar] [CrossRef]
- Deng, L.F.; Li, X.H.; Xiao, L.X.; Zhang, Y.H. Synthesis and electrochemical properties of polyradical cathode material for lithium second batteries. J. Cent. South Univ. Technol. 2003, 10, 190–194. [Google Scholar] [CrossRef]
- Kim, J.K.; Ahn, J.H.; Cheruvally, G.; Chauhan, G.S.; Choi, J.W.; Kim, D.S.; Ahn, H.J.; Lee, S.H.; Song, C.E. Electrochemical properties of rechargeable organic radical battery with PTMA cathode. Met. Mater. Int. 2009, 15, 77–82. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, Y.; Wang, L.; Monteiro, M.J.; Jia, Z. Pyrene-Functionalized PTMA by NRC for Greater π–π Stacking with rGO and Enhanced Electrochemical Properties. ACS Appl. Mater. Interfaces 2017, 9, 34900–34908. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagin, A.A.; Vlasov, P.S.; Konev, A.S.; Yang, P.; Grechishnikova, G.A.; Levin, O.V. Novel highly conductive cathode material based on stable-radical organic framework and polymerized nickel complex for electrochemical energy storage devices. Electrochim. Acta 2019, 295, 1075–1084. [Google Scholar] [CrossRef]
- Iwasa, S.; Nishi, T.; Sato, H.; Nakamura, S. Flexibility and High-Rate Discharge Properties of Organic Radical Batteries with Gel-State Electrodes. J. Electrochem. Soc. 2017, 164, A884–A888. [Google Scholar] [CrossRef]
- Wang, S.; Park, A.M.G.; Flouda, P.; Easley, A.D.; Li, F.; Ma, T.; Fuchs, G.D.; Lutkenhaus, J.L. Solution-Processable Thermally Crosslinked Organic Radical Polymer Battery Cathodes. ChemSusChem 2020, 13, 2371–2378. [Google Scholar] [CrossRef]
- Sato, K.; Sukegawa, T.; Oyaizu, K.; Nishide, H. Synthesis of Poly(TEMPO-Substituted Glycidyl Ether) by Utilizing t-BuOK/18-Crown-6 for an Organic Cathode-Active Material. Macromol. Symp. 2015, 351, 90–96. [Google Scholar] [CrossRef]
- Oyaizu, K.; Suga, T.; Yoshimura, K.; Nishide, H. Synthesis and Characterization of Radical-Bearing Polyethers as an Electrode-Active Material for Organic Secondary Batteries. Macromolecules 2008, 41, 6646–6652. [Google Scholar] [CrossRef]
- Hatakeyama-Sato, K.; Wakamatsu, H.; Yamagishi, K.; Fujie, T.; Takeoka, S.; Oyaizu, K.; Nishide, H. Ultrathin and Stretchable Rechargeable Devices with Organic Polymer Nanosheets Conformable to Skin Surface. Small 2019, 15, 1805296. [Google Scholar] [CrossRef]
- Chikushi, N.; Yamada, H.; Oyaizu, K.; Nishide, H. TEMPO-substituted polyacrylamide for an aqueous electrolyte-typed and organic-based rechargeable device. Sci. China Chem. 2012, 55, 822–829. [Google Scholar] [CrossRef]
- Suga, T.; Ohshiro, H.; Sugita, S.; Oyaizu, K.; Nishide, H. Emerging N-Type Redox-Active Radical Polymer for a Totally Organic Polymer-Based Rechargeable Battery. Adv. Mater. 2009, 21, 1627–1630. [Google Scholar] [CrossRef]
- Katsumata, T.; Qu, J.; Shiotsuki, M.; Satoh, M.; Wada, J.; Igarashi, J.; Mizoguchi, K.; Masuda, T. Synthesis, Characterization, and Charge/Discharge Properties of Polynorbornenes Carrying 2,2,6,6-Tetramethylpiperidine-1-oxy Radicals at High Density. Macromolecules 2008, 41, 1175–1183. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, Y.; Gao, L.; Xu, G.; Xie, J. Electrochemical Performance of Organic Radical Cathode with Ionic Liquid Based Electrolyte. J. Electrochem. Soc. 2011, 158, A291. [Google Scholar] [CrossRef]
- Qu, J.; Morita, R.; Satoh, M.; Wada, J.; Terakura, F.; Mizoguchi, K.; Ogata, N.; Masuda, T. Synthesis and Properties of DNA Complexes Containing 2,2,6,6-Tetramethyl-1-piperidinoxy (TEMPO) Moieties as Organic Radical Battery Materials. Chem.–A Eur. J. 2008, 14, 3250–3259. [Google Scholar] [CrossRef] [PubMed]
- Lebègue, E.; Brousse, T.; Gaubicher, J.; Retoux, R.; Cougnon, C. Toward fully organic rechargeable charge storage devices based on carbon electrodes grafted with redox molecules. J. Mater. Chem. A 2014, 2, 8599–8602. [Google Scholar] [CrossRef]
- Du, Z.; Ai, W.; Xie, L.; Huang, W. Organic radical functionalized graphene as a superior anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 9164–9168. [Google Scholar] [CrossRef]
- Aqil, M.; Dahbi, M.; Saadoune, I.; Idrissi, A.E.; Aqil, A.; Jérôme, C. Functionalized Graphite Nanoplatelet by Nitroxide Radical PILs as Anode Materials for Li-ion Battery. In Proceedings of the 2019 7th International Renewable and Sustainable Energy Conference (IRSEC), Agadir, Morocco, 27–30 November 2019; pp. 1–4. [Google Scholar]
- Guo, X.; Facchetti, A. The journey of conducting polymers from discovery to application. Nat. Mater. 2020, 19, 922–928. [Google Scholar] [CrossRef]
- Inzelt, G. Conducting Polymers a New Era in Electrochemistry; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2012. [Google Scholar]
- MacDiarmid, A.G.; Mammone, R.J.; Kaner, R.B.; Porter, L. The concept of ‘doping’ of conducting polymers: The role of reduction potentials. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1997, 314, 3–15. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH) x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Qu, J.; Katsumata, T.; Satoh, M.; Wada, J.; Igarashi, J.; Mizoguchi, K.; Masuda, T. Synthesis and charge/discharge properties of polyacetylenes carrying 2,2,6,6-tetramethyl-1-piperidinoxy radicals. Chemistry 2007, 13, 7965–7973. [Google Scholar] [CrossRef]
- Bahceci, S.; Esat, B. A polyacetylene derivative with pendant TEMPO group as cathode material for rechargeable batteries. J. Power Sources 2013, 242, 33–40. [Google Scholar] [CrossRef]
- Qu, J.; Katsumata, T.; Satoh, M.; Wada, J.; Masuda, T. Synthesis and Properties of Polyacetylene and Polynorbornene Derivatives Carrying 2,2,5,5-Tetramethyl-1-pyrrolidinyloxy Moieties. Macromolecules 2007, 40, 3136–3144. [Google Scholar] [CrossRef]
- Xu, L.; Guo, P.; He, H.; Zhou, N.; Ma, J.; Wang, G.; Zhang, C.; Su, C. Preparation of TEMPO-contained pyrrole copolymer by in situ electrochemical polymerization and its electrochemical performances as cathode of lithium ion batteries. Ionics 2017, 23, 1375–1382. [Google Scholar] [CrossRef]
- Xu, L.H.; Yang, F.; Su, C.; Zhang, C. Research of Properties on Li-Ion Batteries Based on a Polypyrrole Derivative Bearing TEMPO as a Cathode Material. Adv. Mater. Res. 2014, 936, 447–451. [Google Scholar] [CrossRef]
- Oyaizu, K.; Tatsuhira, H.; Nishide, H. Facile charge transport and storage by a TEMPO-populated redox mediating polymer integrated with polyaniline as electrical conducting path. Polym. J. 2014, 47, 212–219. [Google Scholar] [CrossRef]
- Schon, T.B.; McAllister, B.T.; Li, P.F.; Seferos, D.S. The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wei, Z.; Xu, T.; Zhang, Y.; Xiong, C.; Dong, L. Polytriphenylamine derivative with enhanced electrochemical performance as the organic cathode material for rechargeable batteries. Polymer 2017, 130, 135–142. [Google Scholar] [CrossRef]
- Aydin, M.; Esat, B. A polythiophene derivative bearing two electroactive groups per monomer as a cathode material for rechargeable batteries. J. Solid State Electrochem. 2015, 19, 2275–2281. [Google Scholar] [CrossRef]
- Casado, N.; Hernandez, G.; Veloso, A.; Devaraj, S.; Mecerreyes, D.; Armand, M. PEDOT Radical Polymer with Synergetic Redox and Electrical Properties. ACS Macro Lett. 2016, 5, 59–64. [Google Scholar] [CrossRef]
- Assumma, L.; Kervella, Y.; Mouesca, J.M.; Mendez, M.; Maurel, V.; Dubois, L.; Gutel, T.; Sadki, S. A New Conducting Copolymer Bearing Electro-Active Nitroxide Groups as Organic Electrode Materials for Batteries. ChemSusChem 2020, 13, 2419–2427. [Google Scholar] [CrossRef]
- Hagemann, T.; Winsberg, J.; Grube, M.; Nischang, I.; Janoschka, T.; Martin, N.; Hager, M.D.; Schubert, U.S. An aqueous all-organic redox-flow battery employing a (2,2,6,6-tetramethylpiperidin-1-yl)oxyl-containing polymer as catholyte and dimethyl viologen dichloride as anolyte. J. Power Sources 2018, 378, 546–554. [Google Scholar] [CrossRef]
- Hagemann, T.; Strumpf, M.; Schröter, E.; Stolze, C.; Grube, M.; Nischang, I.; Hager, M.D.; Schubert, U.S. (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl-Containing Zwitterionic Polymer as Catholyte Species for High-Capacity Aqueous Polymer Redox Flow Batteries. Chem. Mater. 2019, 31, 7987–7999. [Google Scholar] [CrossRef]
- Janoschka, T.; Martin, N.; Martin, U.; Friebe, C.; Morgenstern, S.; Hiller, H.; Hager, M.D.; Schubert, U.S. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 2015, 527, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Janoschka, T.; Morgenstern, S.; Hiller, H.; Friebe, C.; Wolkersdörfer, K.; Häupler, B.; Hager, M.D.; Schubert, U.S. Synthesis and characterization of TEMPO- and viologen-polymers for water-based redox-flow batteries. Polym. Chem. 2015, 6, 7801–7811. [Google Scholar] [CrossRef]
- Winsberg, J.; Janoschka, T.; Morgenstern, S.; Hagemann, T.; Muench, S.; Hauffman, G.; Gohy, J.F.; Hager, M.D.; Schubert, U.S. Poly(TEMPO)/Zinc Hybrid-Flow Battery: A Novel, “Green,” High Voltage, and Safe Energy Storage System. Adv. Mater. 2016, 28, 2238–2243. [Google Scholar] [CrossRef] [PubMed]
- Kozhunova, E.Y.; Gvozdik, N.A.; Motyakin, M.V.; Vyshivannaya, O.V.; Stevenson, K.J.; Itkis, D.M.; Chertovich, A.V. Redox-Active Aqueous Microgels for Energy Storage Applications. J. Phys. Chem. Lett. 2020, 11, 10561–10565. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama-Sato, K.; Nagano, T.; Noguchi, S.; Sugai, Y.; Du, J.; Nishide, H.; Oyaizu, K. Hydrophilic Organic Redox-Active Polymer Nanoparticles for Higher Energy Density Flow Batteries. ACS Appl. Polym. Mater. 2019, 1, 188–196. [Google Scholar] [CrossRef]
- Winsberg, J.; Muench, S.; Hagemann, T.; Morgenstern, S.; Janoschka, T.; Billing, M.; Schacher, F.H.; Hauffman, G.; Gohy, J.F.; Hoeppener, S.; et al. Polymer/zinc hybrid-flow battery using block copolymer micelles featuring a TEMPO corona as catholyte. Polym. Chem. 2016, 7, 1711–1718. [Google Scholar] [CrossRef]
- Beejapur, H.A.; Zhang, Q.; Hu, K.; Zhu, L.; Wang, J.; Ye, Z. TEMPO in Chemical Transformations: From Homogeneous to Heterogeneous. ACS Catal. 2019, 9, 2777–2830. [Google Scholar] [CrossRef]
- Schille, B.; Giltzau, N.O.; Francke, R. On the Use of Polyelectrolytes and Polymediators in Organic Electrosynthesis. Angew. Chem. Int. Ed. 2018, 57, 422–426. [Google Scholar] [CrossRef]
- Macazo, F.C.; Hickey, D.P.; Abdellaoui, S.; Sigman, M.S.; Minteer, S.D. Polymer-immobilized, hybrid multi-catalyst architecture for enhanced electrochemical oxidation of glycerol. Chem. Commun. 2017, 53, 10310–10313. [Google Scholar] [CrossRef]
- Franco, J.H.; Neto, S.A.; Hickey, D.P.; Minteer, S.D.; de Andrade, A.R. Hybrid catalyst cascade architecture enhancement for complete ethanol electrochemical oxidation. Biosens. Bioelectron. 2018, 121, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Ma, J.Q.; Yi, J.M.; Shen, Z.L.; Zhong, Y.J.; Ma, C.A.; Li, M.C. Electrochemical Polymerization of Pyrrole Containing TEMPO Side Chain on Pt Electrode and Its Electrochemical Activity. Electrochim. Acta 2014, 130, 412–417. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Song, D.; Shen, Z.; Li, M.; Ma, C. Characterization and Electrocatalytic Activity of Poly(4-thienylacetyl-oxy-2,2,6,6-tetramethylpiperidin-1-yloxy) Prepared by Electrochemical Polymerization. ECS Electrochem. Lett. 2014, 3, H12–H15. [Google Scholar] [CrossRef]
- Niu, P.; Cai, Y.; Guo, M.; Shen, Z.; Li, M. Preparation and electrochemical performance of TEMPO-modified polyterthiophene electrode obtained by electropolymerization. Electrochem. Commun. 2020, 110, 106623. [Google Scholar] [CrossRef]
- Niu, P.; Huang, H.; Zhao, L.; Zhang, C.; Shen, Z.; Li, M. Preparation of poly(carbazole-TEMPO) electrode and its electrochemical performance. J. Electroanal. Chem. 2021, 894, 115352. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Chiba, S.; Anzai, J.I. Amperometric determination of optically active 1-phenylethanol using chiral nitroxyl radical-modified polypyrrole films prepared by electrochemical polymerization. J. Electroanal. Chem. 2004, 566, 257–262. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Ono, T.; Tsunoda, M.; Takahashi, S.; Obata, T.; Sone, T. Voltammetric Behavior of Mediator-Modified Electrode by Electrochemical Polymerization of Nitroxyl Radical Precursor Containing Pyrrole Side Chain. Heterocycles 2010, 81, 2771–2780. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Tsunoda, M.; Ono, T. Voltammetric Behavior of Mediator-Modified Electrode by Electrochemical Copolymerization of Nitroxyl Radical Precursor Containing Pyrrole Side Chain and Thiophenes. Heterocycles 2011, 83, 2517–2524. [Google Scholar] [CrossRef]
- Ono, T.; Sato, K.; Shimizu, S.; Yoshida, K.; Dairaku, T.; Suzuki, Y.; Kashiwagi, Y. Electrocatalytic Oxidation of Carbohydrates Mediated by Nitroxyl Radical-modified Electrodes in Aqueous Solution. Electroanalysis 2018, 30, 24–26. [Google Scholar] [CrossRef]
- Place, S.D.; Kavanagh, P. Electroactivity of PIPO nitroxide radical polymer films. Electrochim. Acta 2021, 392, 139044. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Kurashima, F.; Kikuchi, C.; Anzai, J.I.; Osa, T.; Bobbin, J.M. Electrocatalytic Oxidation of Amines to Nitriles on a TEMPO-modified Graphite Felt Electrode (TEMPO = 2,2,6,6-tetramethylpiperidin-1-yloxyl). J. Chin. Chem. Soc. 1998, 45, 135–138. [Google Scholar] [CrossRef]
- MacCorquodale, F.; Crayston, J.A.; Walton, J.C.; Worsfold, D.J. Synthesis and electrochemical characterisation of poly(tempoacrylate). Tetrahedron Lett. 1990, 31, 771–774. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Takamori, Y.; Yoshida, K.; Ono, T. Electrocatalytic Oxidation of Amines on a Mediator-Modified Electrode by Electrochemical Copolymerization of Nitroxyl Radical Precursor Containing Pyrrole Side Chain and Bithiophene. Electroanalysis 2013, 25, 2575–2577. [Google Scholar] [CrossRef]


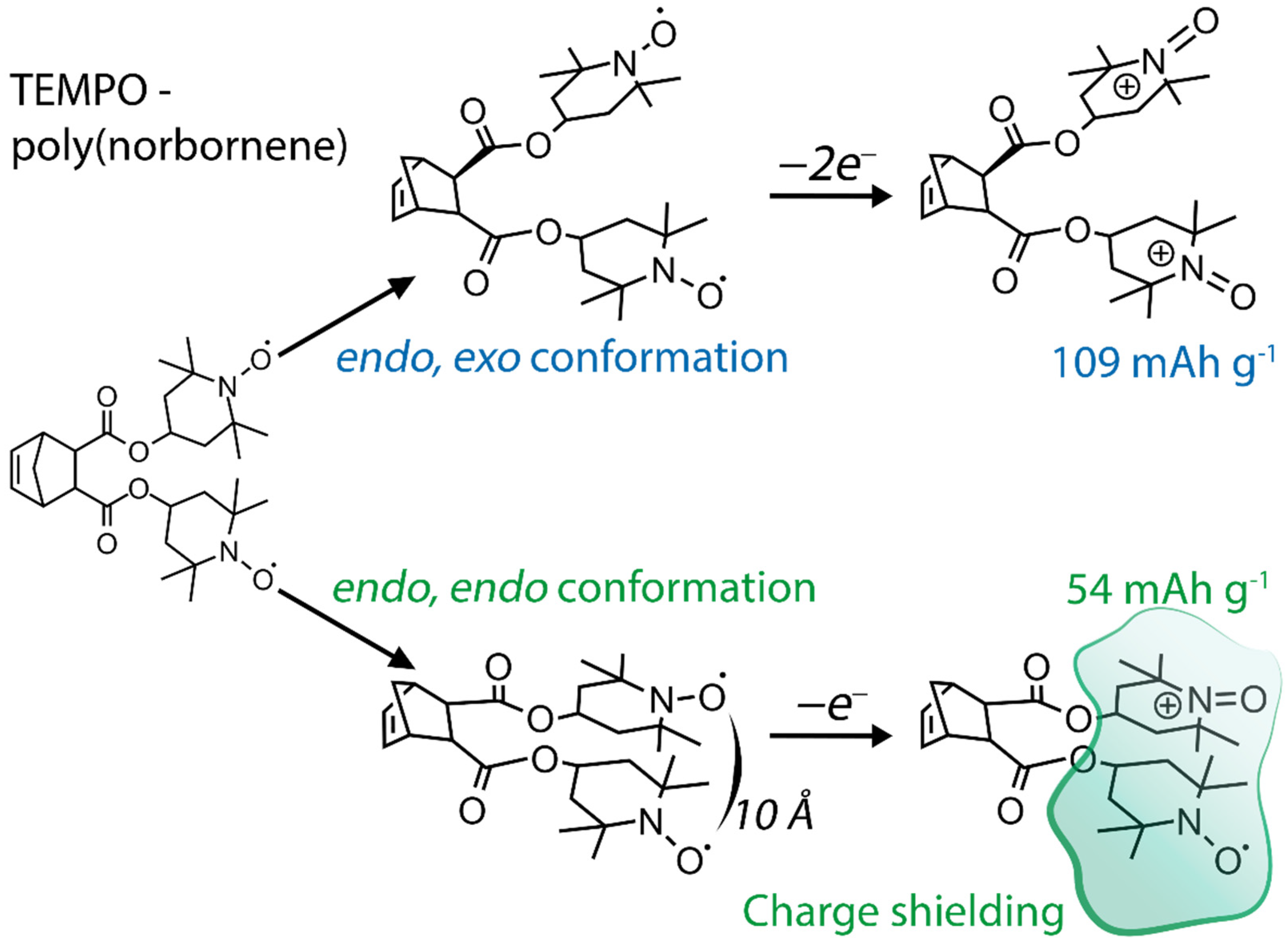
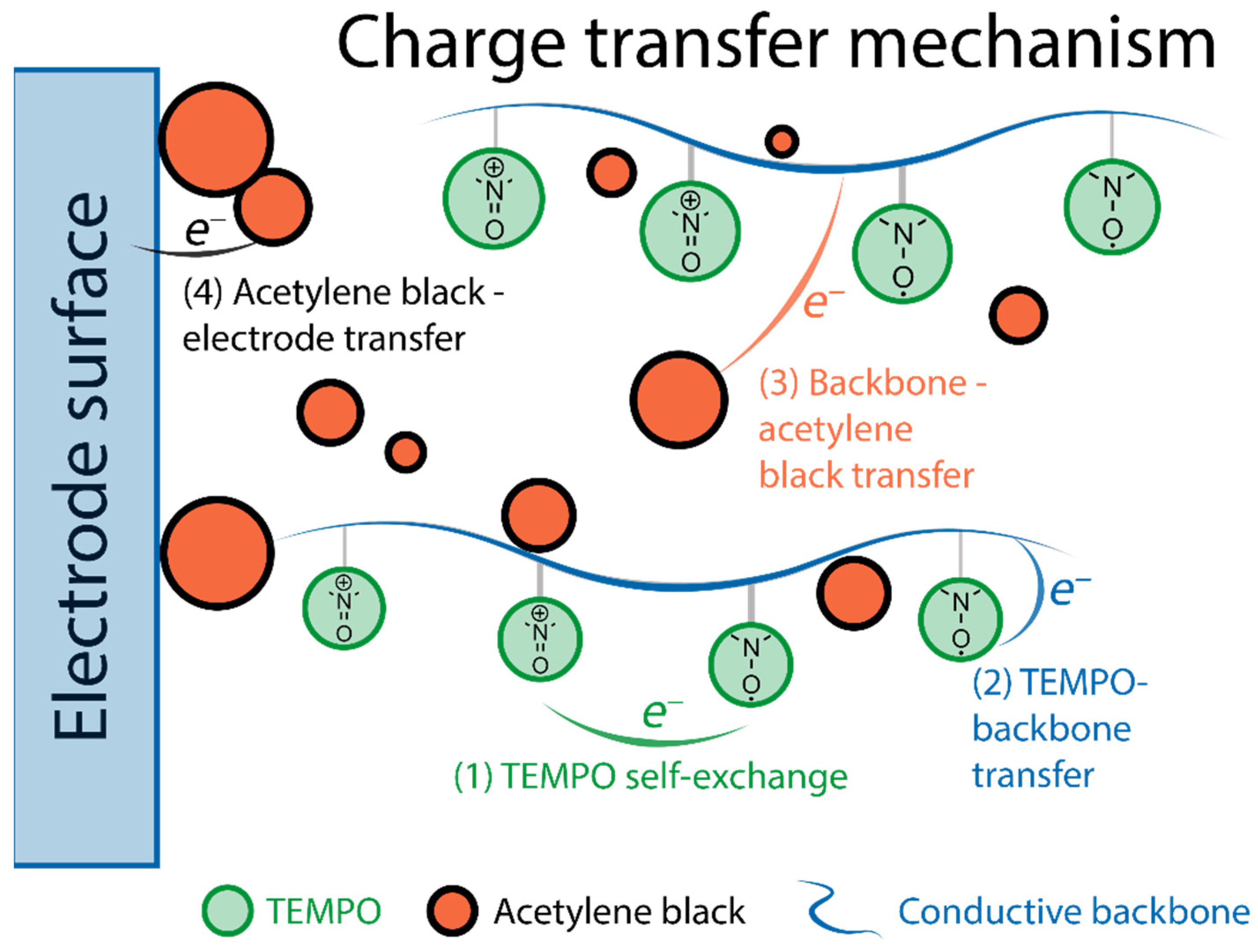
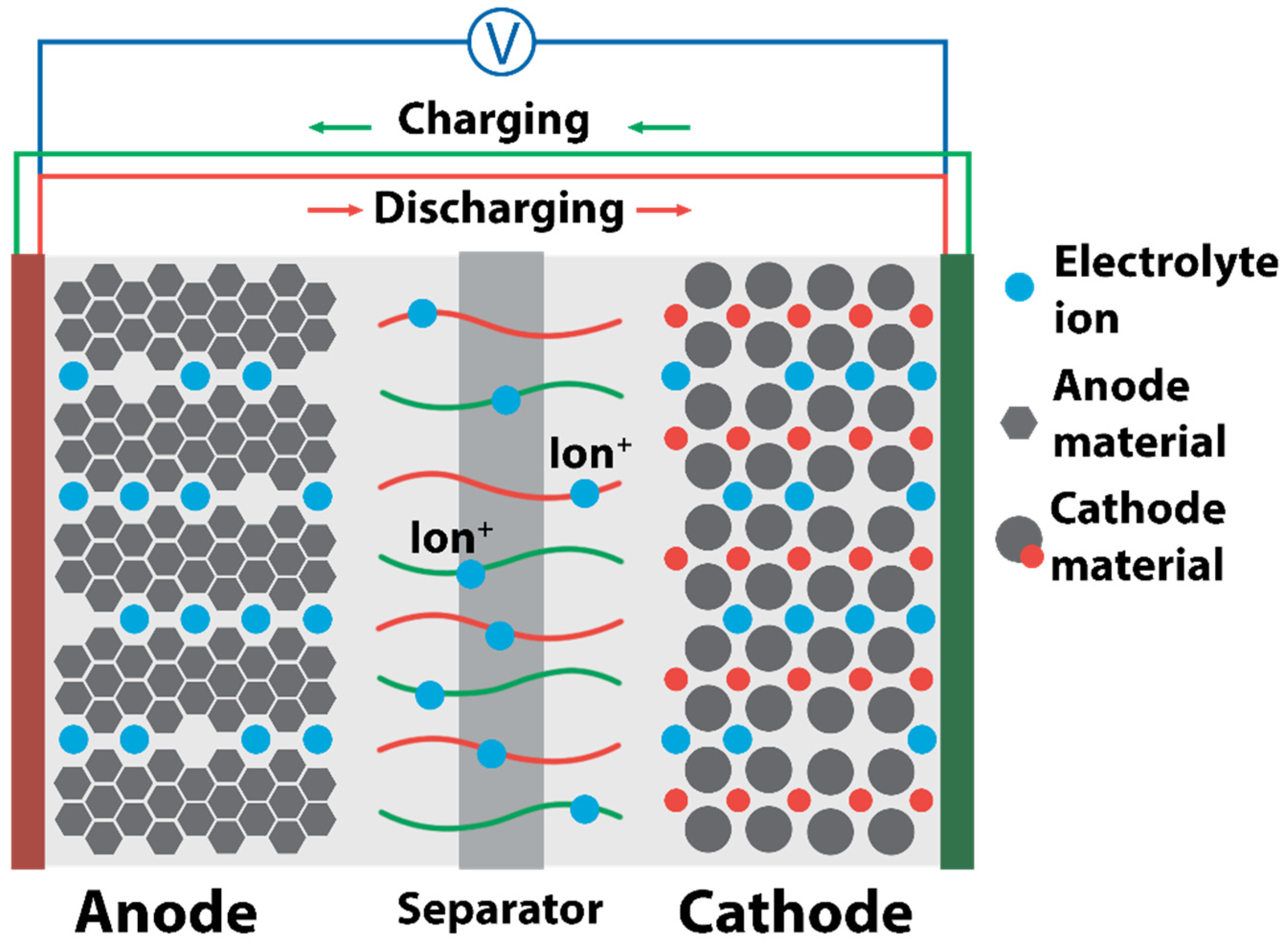
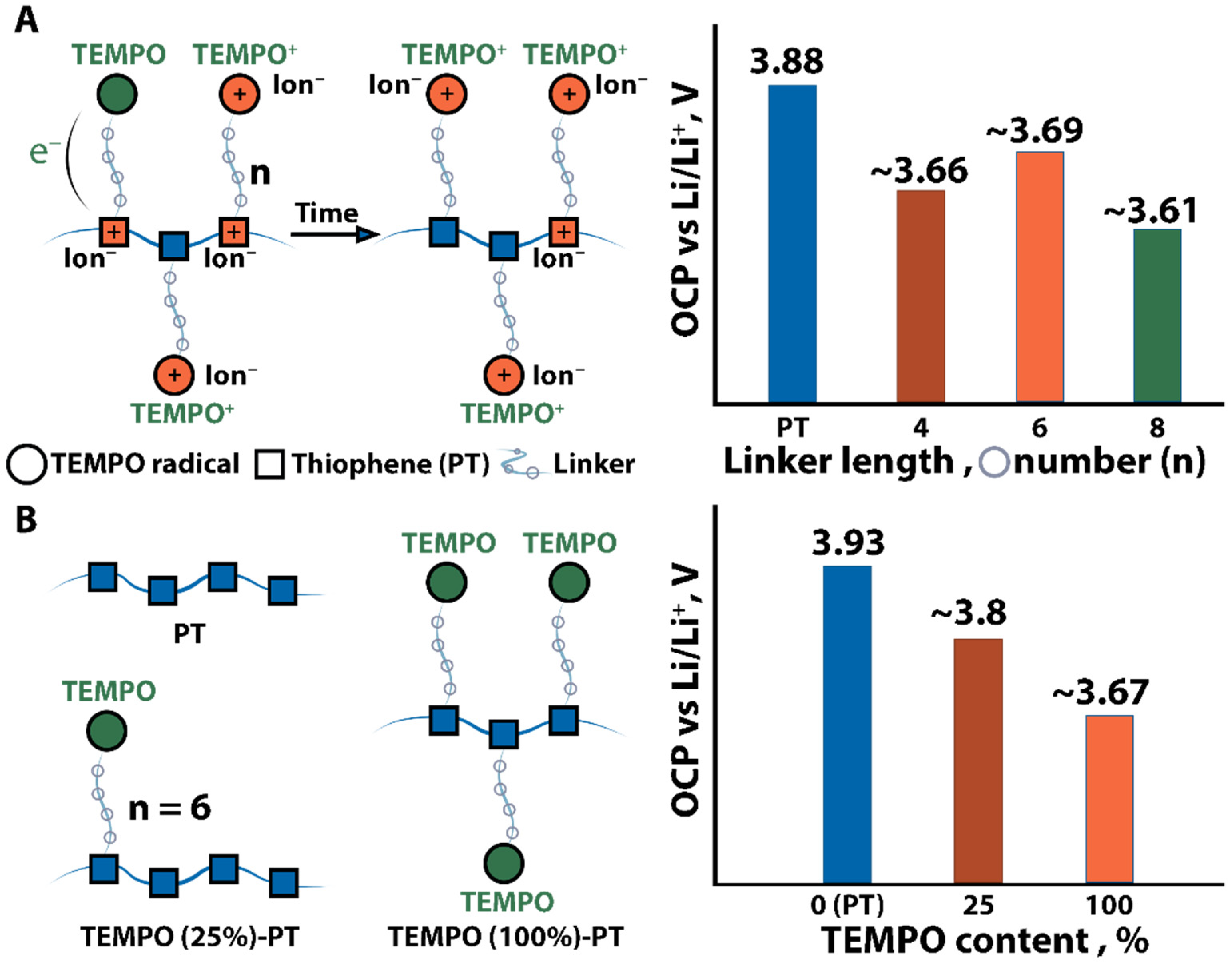
| No. | Material Structure | Capacity per Electrode Mass (Polymer Mass) / mAh g−1 | E/V | Current / C | Capacity Retention / % of the Initial Value | Electrode Composition / % | Electrolyte | Ref |
|---|---|---|---|---|---|---|---|---|
| 83 | 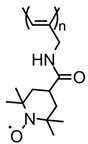 | 7 (67) | 3.58 | 1 | n/a | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [56] |
| 84 | 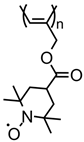 | 8 (82) | 3.58 | 1 | n/a | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [56] |
| 85 | 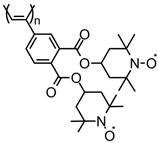 | 2 (23) | 3.58 | 1 | n/a | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [56] |
| 86 | 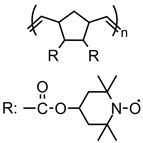 | 11 (109) | 3.58 | 1 | n/a | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [56] |
| 87 | 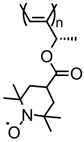 | 4 (43) | 3.6 | 1 | ~81 after 100 cycles | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [36] |
| 88 | 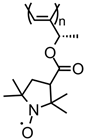 | 10 (103) | 3.6 | 1 | ~83 after 100 cycles | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [36] |
| 89 | 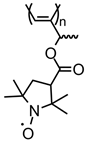 | 11 (112) | 3.6 | 1 | ~86 after 100 cycles | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [36] |
| 90 | 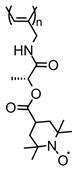 | 8 (78) | 3.6 | 1 | ~76 after 100 cycles | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [36] |
| 91 | 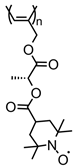 | 9 (85) | 3.6 | 1 | ~80 after 100 cycles | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [36] |
| 92 | 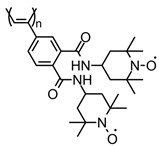 | 11 (108) | 3.6 | 1 | 85 after 100 cycles | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [133] |
| 93 |  | 2 (21) | 3.6 | 1 | n/a | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [133] |
| 94 |  | 6 (62) | 3.6 | 1 | n/a | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [133] |
| 95 | 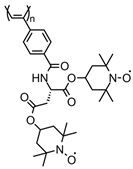 | 10 (96) | 3.6 | 1 | ~88 after 100 cycles | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [133] |
| 96 |  | 9 (89) | 3.6 | 1 | ~86 after 100 cycles | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [133] |
| 97 | 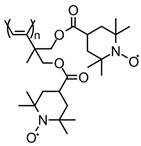 | 6 (63) | 3.6 | 1 | ~87 after 100 cycles | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [133] |
| 98 |  | 8 (81) | 3.6 | 1 | n/a | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [133] |
| 99 |  | 7 (66) | 3.6 | 1 | n/a | 10 / 80 C / 10 fluorinated polyolefin | 1 M LiPF6 EC:DEC (3:7) | [133] |
| 100 | 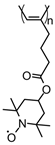 | 21 (103) | 3.61 | 0.3 | 62 after 40 cycles | 20 / 70 C / 10 PVDF | 1 M LiPF6 EC:DEC (1:1) | [134] |
| 101 | 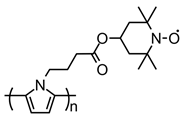 | 44 (87) | 3.6 | 0.25 | 55 after 50 cycles | 50 / 40 C / 10 PVDF | 1 M LiPF6 EC:DMC (1:1) | [37] |
| 102 | 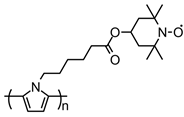 | 58 (115) | 3.6 / 2.7 | 0.25 | 75 after 50 cycles | 50 / 40 C / 10 PVDF | 1 M LiPF6 EC:DMC (1:1) | [37] |
| 103 | 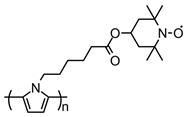 | n. a. (71) | 3.6 | 0.25 | 76 after 20 cycles | 100 | 1 M LiPF6 EC:DMC (1:1) | [136] |
| 104 | 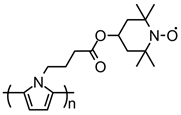 | 44 (87) | 3.5 | 0.25 | ~56 after 50 cycles | 50 / 40 C / 10 PVDF | 1 M LiPF6 EC:DMC (1:1) | [137] |
| 105 |  | 39(77) | 3.6 | 0.2 | 19 after 20 cycles | 50 / 40 C / 10 PVDF | 1 M LiPF6 EC:DMC (1:1) | [34] |
| 106 | 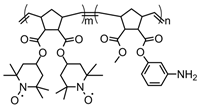 | ~60 (60) | 0.8 (vs. SCE) | 10 | n/a | 100 | 0.1 M TBAClO4 CH3CN | [138] |
| 107 | 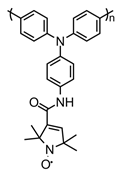 | 68 (135) | 3.82 | 0.15 | 90 after 100 cycles | 50 / 40 C / 10 PVDF | 1 M LiPF6 EC:DEC | [140] |
| 108 |  | 70(140) | 3.6 | 1 | 89 after 50 cycles | 50 / 40 C/ 10 PVDF | 1 M LiPF6 / EC:DEC | [41] |
| 109 | 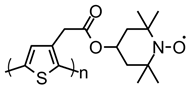 | 24 (79) | 3.6 | 0.1 | 77 after 50 cycles | 30 / 60 C / 10 PVDF | 1 M LiPF6 EC:DEC | [33] |
| 110 | 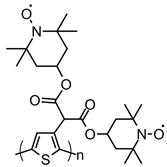 | 22 (89) | 3.65 | 1 | 87 after 9 cycles | 25 / 65 C / 10 PVDF | 1 M LiPF6 EC:DEC | [141] |
| 111 | 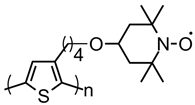 | 68 (68) | 3.6 | ~0.5 | n/a | 100 | 0.5 M LiClO4 PC | [39] |
| 112 | 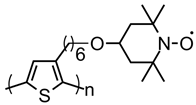 | 44 (44) | 3.6 | ~0.5 | n/a | 100 | 0.5 M LiClO4 PC | [39] |
| 113 | 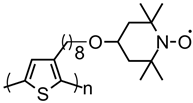 | 22 (22) | 3.6 | ~0.5 | n/a | 100 | 0.5 M LiClO4 PC | [39] |
| 114 | 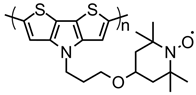 | ~65 (65) | 3.61 | 3 | 70 after 200 cycles | 100 | 0.5 M LiClO4 PC | [75] |
| 115 | 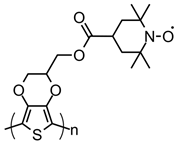 | 36 (47) | 3.62 | 0.1 | 81 after 50 cycles | 77 / 33 С | 1 M LiPF6 EC:DMC | [40] |
| 116 |  | n. a. | 3.6 | 0.1–5 | n/a | 70 / 30 C | 1 M LiPF6 EC:DMC | [142] |
| 117 | 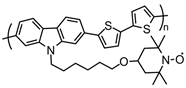 | 20 (50) | 0.36 (vs. Fc+/Fc) | 1 | 93 after 6 cycles | 40 / 40 C / 20 PVDF | 1 M LiPF6 EC:DMC (1:1) | [143] |
| 118 | 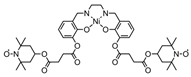 | 82 (82) | 0.53 (vs. Ag|AgNO3) | 60 | 90 after 100 cycles, 66 after 2000 cycles | 100 | 0.1 M Et4NBF4 / CH3CN | [42] |
| No. | Catholyte Polymer Structure | Anolyte | Volumetric Capacity of Catholyte / Ah L−1 | E/V (Ag / AgCl) | Current / mA cm−2 | Ref |
|---|---|---|---|---|---|---|
| 119 |  | Methyl viologen | 8.3 | 0.7 | 5 | [144] |
| 120 | Same | Viologen-containing polymer | 10 | 0.7 (Ag/AgCl) | 40 | [146] |
| 121 |  | Methyl viologen | 20 | 0.7 | 8 | [145] |
| 122 | 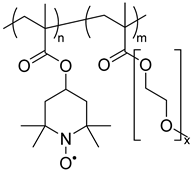 | Zn2+/Zn | - | - | - | [148] |
| 123 | TEMPO-containing acrylate microgel | - | 0.1 | - | - | [149] |
| 124 |  nanoparticles | Viologen polymer or diazaanthraquinone polymer | 7.2 | - | - | [150] |
| 125 | 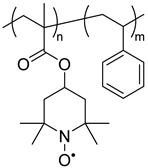 | Zn2+/Zn | 1.2 | 0.4 (Ag/AgNO3) | 3 | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vereshchagin, A.A.; Kalnin, A.Y.; Volkov, A.I.; Lukyanov, D.A.; Levin, O.V. Key Features of TEMPO-Containing Polymers for Energy Storage and Catalytic Systems. Energies 2022, 15, 2699. https://doi.org/10.3390/en15072699
Vereshchagin AA, Kalnin AY, Volkov AI, Lukyanov DA, Levin OV. Key Features of TEMPO-Containing Polymers for Energy Storage and Catalytic Systems. Energies. 2022; 15(7):2699. https://doi.org/10.3390/en15072699
Chicago/Turabian StyleVereshchagin, Anatoliy A., Arseniy Y. Kalnin, Alexey I. Volkov, Daniil A. Lukyanov, and Oleg V. Levin. 2022. "Key Features of TEMPO-Containing Polymers for Energy Storage and Catalytic Systems" Energies 15, no. 7: 2699. https://doi.org/10.3390/en15072699
APA StyleVereshchagin, A. A., Kalnin, A. Y., Volkov, A. I., Lukyanov, D. A., & Levin, O. V. (2022). Key Features of TEMPO-Containing Polymers for Energy Storage and Catalytic Systems. Energies, 15(7), 2699. https://doi.org/10.3390/en15072699