The Influence of Biochar Augmentation and Digestion Conditions on the Anaerobic Digestion of Water Hyacinth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate, Inoculum, and Biochar
2.2. Composition of Biochar and Water Hyacinth Substrates
2.3. Biochemical Methane Potential
2.4. Theoretical Biochemical Methane Potential
2.5. Kinetic Analysis
2.6. Anaerobic Biodegradability
2.7. Design of Experiments
2.8. Optimisation
2.9. Anaerobic Digestion of Water Hyacinth Substrates
2.10. Volatile Fatty Acids and Alcohols Analysis
2.11. Statistical Analysis
3. Results
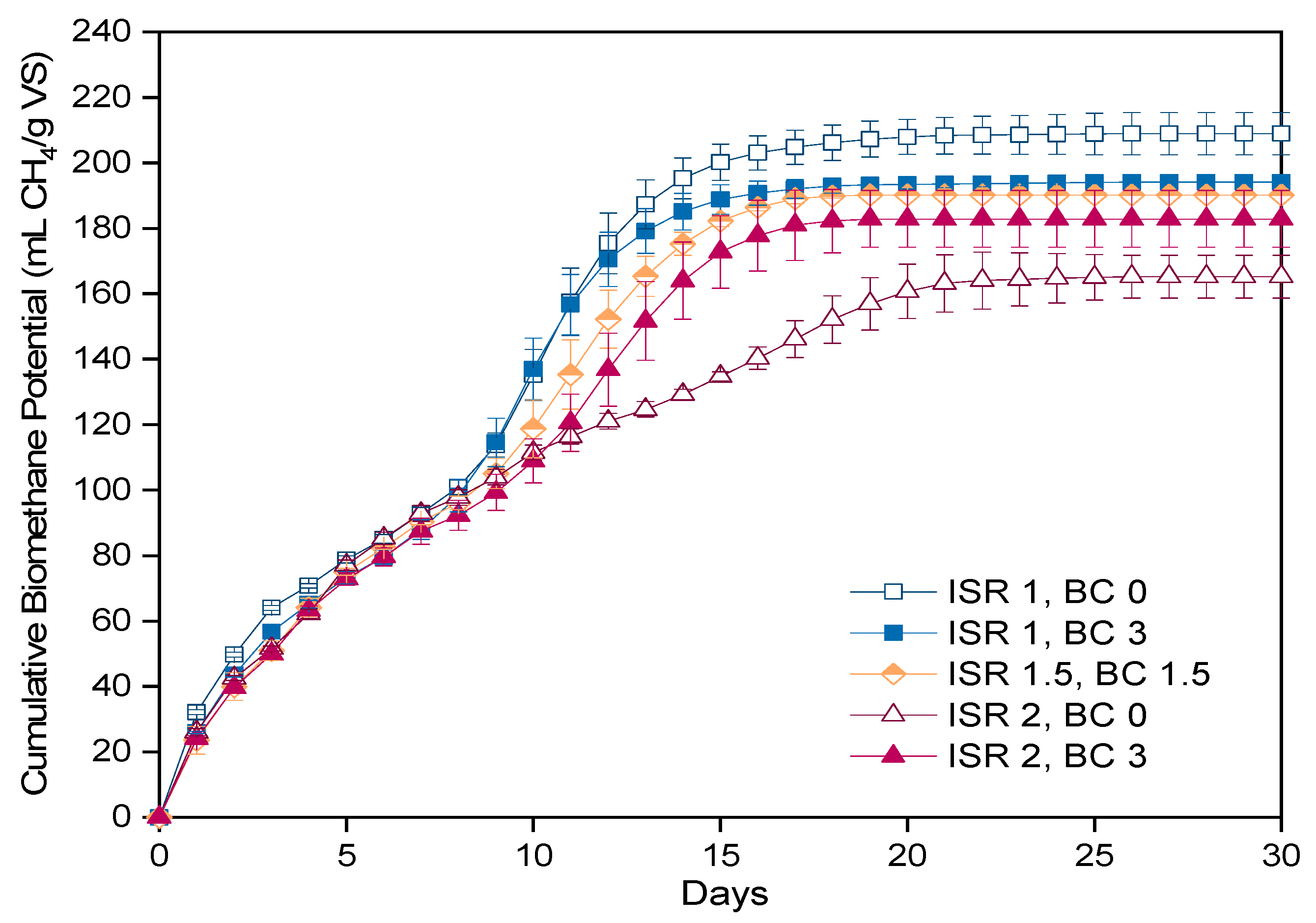
3.1. Design of Experiments
3.1.1. Anaerobic Digestion
| Substrate | Pre-Treatment | Digester | Inoculum | ISR | Biogas (mL/g VS) | Methane (mL/g VS) | µm (mL/g VS·Day) | Ref. |
|---|---|---|---|---|---|---|---|---|
| WH | Oven dried | AMPTS 500 mL | AS | 1 2 | 209 165 | 15 10 | This work | |
| WH | Oven dried | AMPTS 500 mL | AS | 2 | 103 | 11 | [37] | |
| WH-HC | HTC 150 °C HTC 200 °C HTC 250 °C | 191 185 45 | 21 46 13 | |||||
| WH-PW | HTC 150 °C HTC 200 °C HTC 250 °C | 213 138 149 | 44 12 14 | |||||
| WH slurry (HC- PW) | HTC 150 °C HTC 200 °C HTC 250 °C | 202 162 146 | 32 4 39 | |||||
| WH | Sundried | Bottle 1000 mL | AS | 2 | 143 | [42] | ||
| WH | Untreated | SB 125 mL | Compost leachate | 0.5 1 | 350 339 | 246 268 | [40] | |
| WH | Untreated | Bottle 2000 mL | CD | 0.5 1 3 | 406 330 383 | 235 185 241 | [39] | |
| WH | Untreated A (121 °C/30 min) | Bottle 5000 mL | CD | 1 1 | 113 150 | [43] | ||
| WH | Untreated H2SO4 5%, 30 m H2SO4 5%, 45 m H2SO4 5%, 60 m H2SO4 5%, 75 m | Bottle 1000 mL | CM digestate | 3 | 183 mL 203 mL 384 mL 424 mL 267 mL | 11 33 216 273 85 | 6.7 mL/d 6.2 mL/d 5.7 mL/d 7.2 mL/d 7.9 mL/d | [44] |
| WH | Untreated Hot air oven | Bottle 1000 mL | CD | 0.5 0.67 | 1396 mL 1522 mL | 143 193 | 100 mL/d 77 mL/d | [45] |
| WH * | Oven dried * | SB 200 mL FBR 200 L | Manure | 1 | 292 267 | 140 | 0.052 d−1 | [46] |
| WH | Oven dried | Bottle 250 mL | Poultry litter | 6.25 12.5 18.75 25 | 360 440 480 410 | 17 b 19 b 8 b 33 b | [47] |
3.1.2. Kinetic Parameters
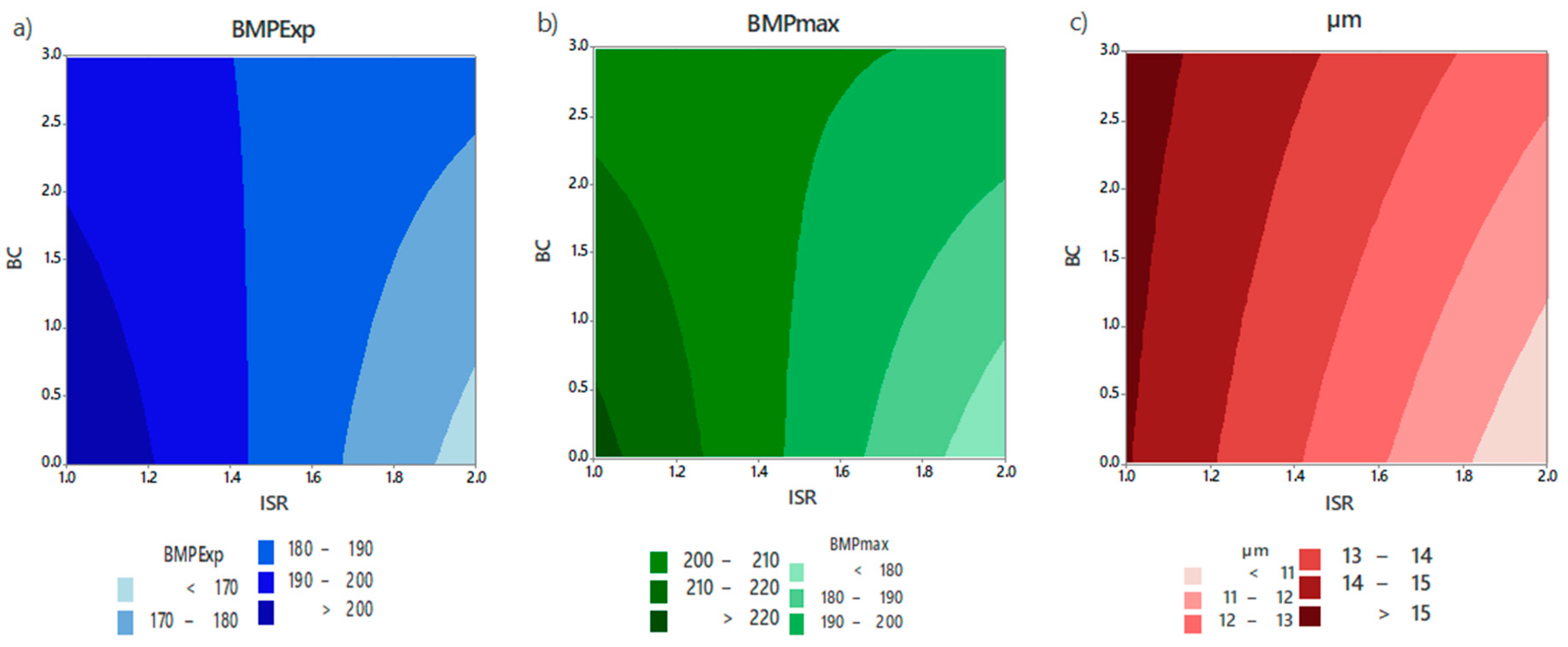
3.1.3. Regression Model Fitting
3.1.4. Optimisation
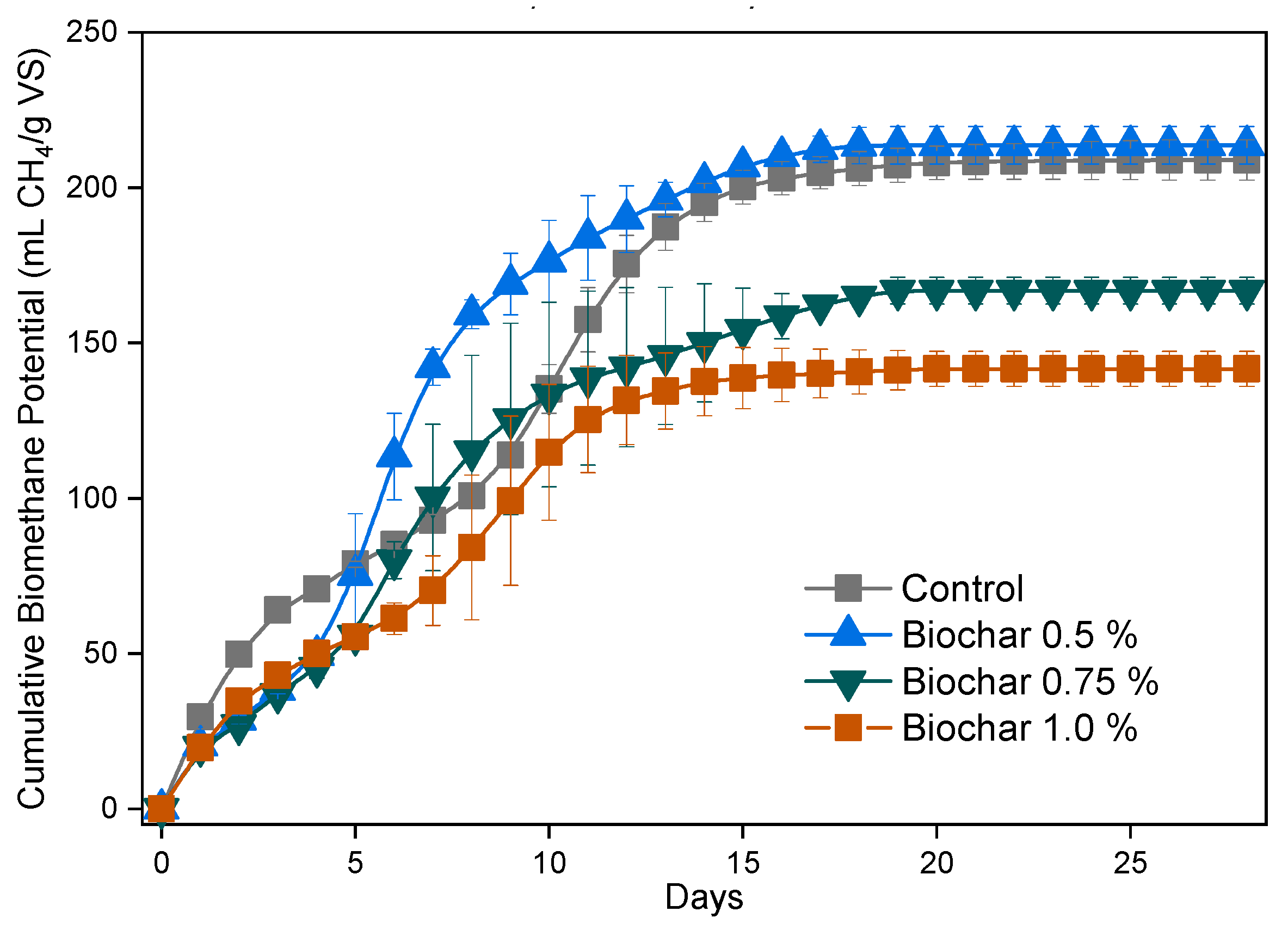
3.1.5. Effect of Biochar Load
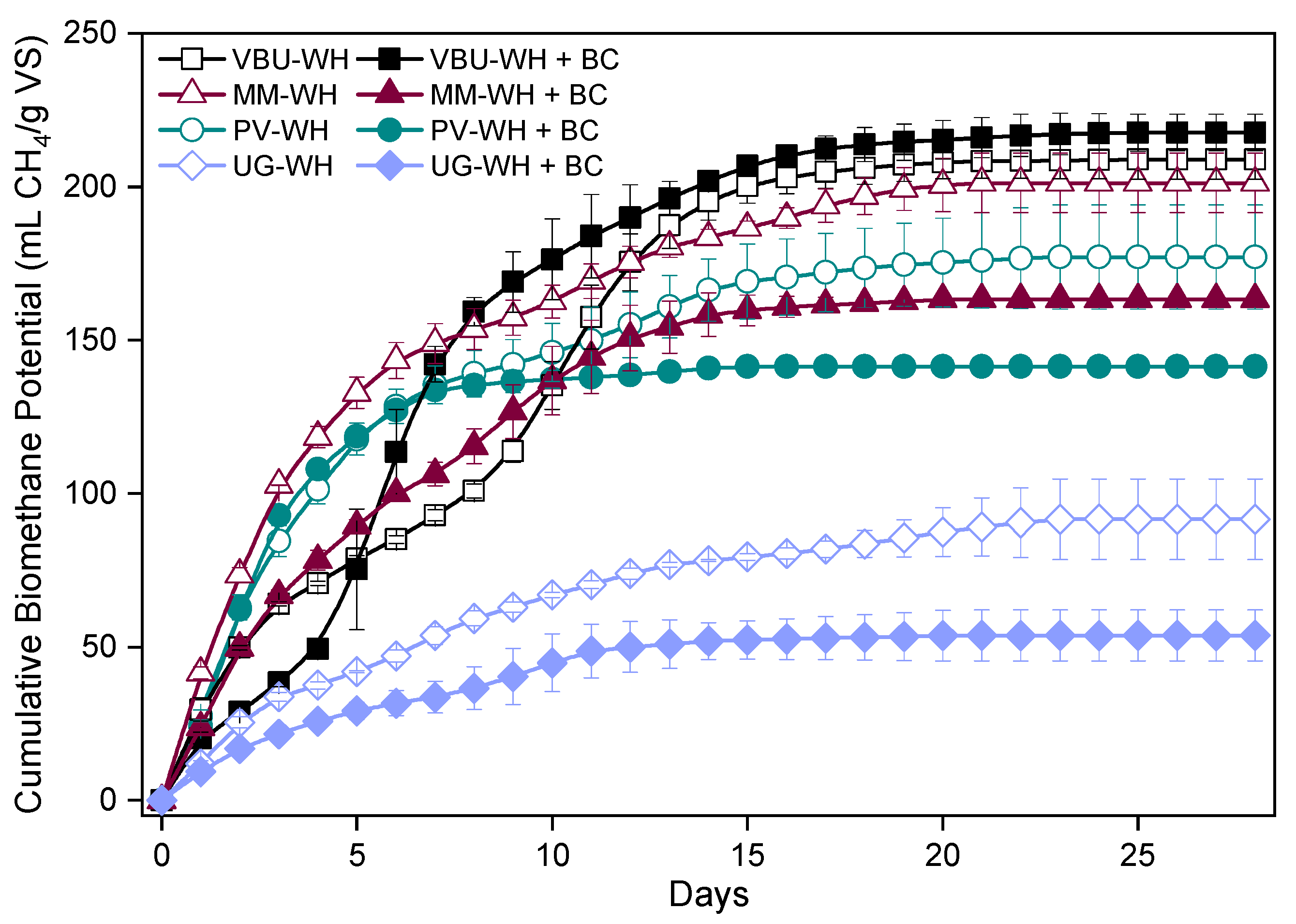
3.2. Anaerobic Digestion of Water Hyacinth Substrates from Different Sources
3.2.1. Biochemical Methane Potential
3.2.2. Kinetic Parameters
3.2.3. Biodegradability
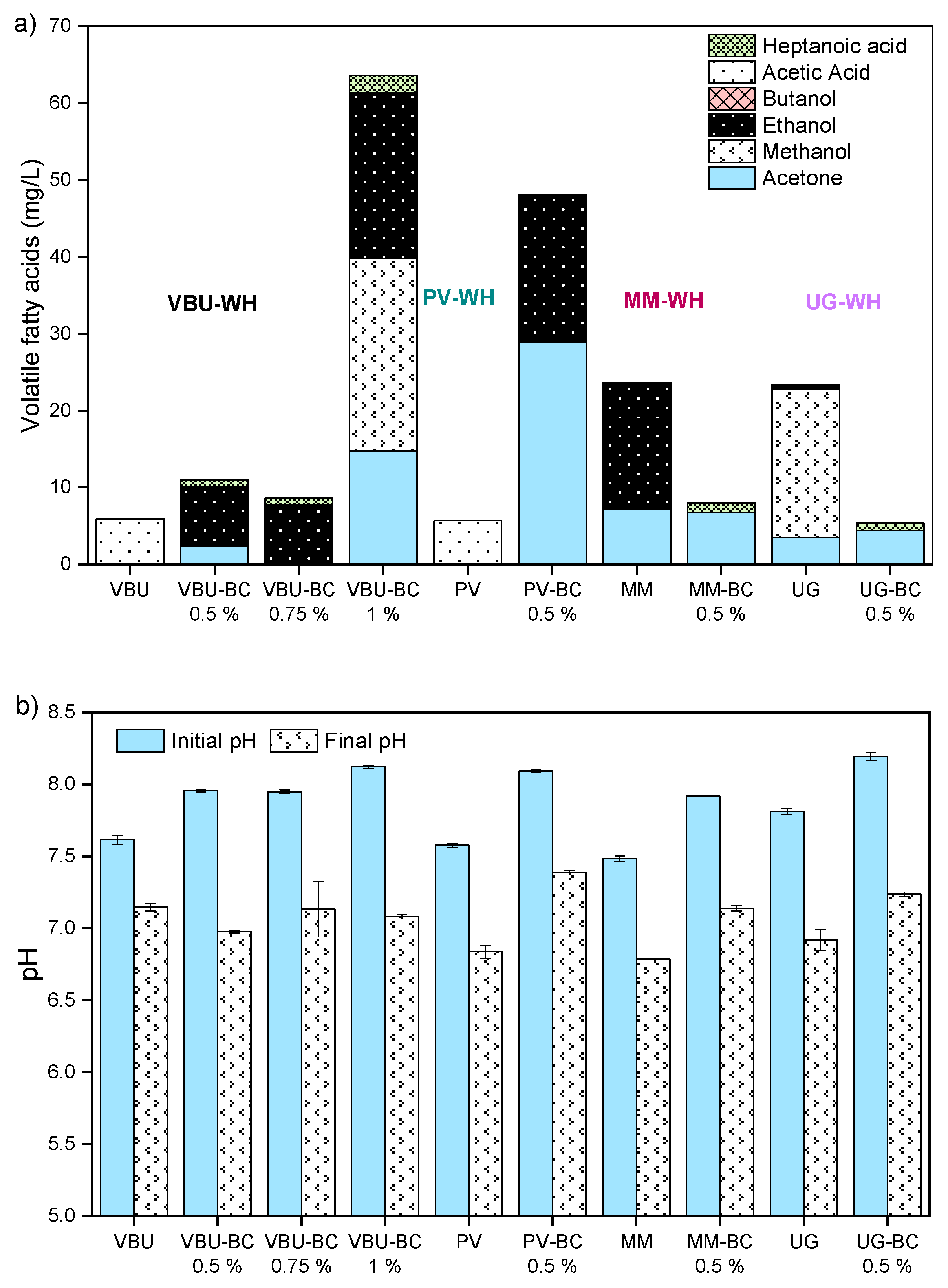
3.2.4. Volatile Fatty Acids and pH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- IEA. Outlook for Biogas and Biomethane; International Energy Agency: Paris, France, 2020. [Google Scholar]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Ibeto, C.N.; Li, H.; Usmani, S.Q.; Semple, K.T. The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag. 2017, 61, 236–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumme, J.; Srocke, F.; Heeg, K.; Werner, M. Use of biochars in anaerobic digestion. Bioresour. Technol. 2014, 164, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrog. Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Zhai, L.; Luo, T.; Mei, Z.; Liu, H. Achievements of biochar application for enhanced anaerobic digestion: A review. Bioresour. Technol. 2019, 292, 122058. [Google Scholar] [CrossRef]
- Sugiarto, Y.; Sunyoto, N.M.S.; Zhu, M.; Jones, I.; Zhang, D. Effect of biochar addition on microbial community and methane production during anaerobic digestion of food wastes: The role of minerals in biochar. Bioresour. Technol. 2020, 323, 124585. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing pipeline-quality biomethane via anaerobic digestion of sludge amended with corn stover biochar with in-situ CO2 removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. J. Clean. Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef] [Green Version]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. The effect of augmentation of biochar and hydrochar in anaerobic digestion of a model substrate. Bioresour. Technol. 2020, 321, 124494. [Google Scholar] [CrossRef]
- Qin, Y.; Yin, X.; Xu, X.; Yan, X.; Bi, F.; Wu, W. Specific surface area and electron donating capacity determine biochar’s role in methane production during anaerobic digestion. Bioresour. Technol. 2020, 303, 122919. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Nam, H.; Sajib, S.K. Effect of bio-char on methane generation from glucose and aqueous phase of algae liquefaction using mixed anaerobic cultures. Biomass Bioenergy 2018, 108, 479–486. [Google Scholar] [CrossRef]
- Cai, J.; He, P.; Wang, Y.; Shao, L.; Lü, F. Effects and optimization of the use of biochar in anaerobic digestion of food wastes. Waste Manag. Res. J. Sustain. Circ. Econ. 2016, 34, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.J.; Rosas, J.G.; Sotres, A.; Moran, A.; Cara-Jiménez, J.; Sánchez, M.E.; Gómez, X. Codigestion of sludge and citrus peel wastes: Evaluating the effect of biochar addition on microbial communities. Biochem. Eng. J. 2018, 137, 314–325. [Google Scholar] [CrossRef]
- Jang, H.M.; Choi, Y.-K.; Kan, E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Gómez, X.; Meredith, W.; Fernández, C.; Sánchez-García, M.; Díez-Antolínez, R.; Garzón-Santos, J.; Snape, C.E. Evaluating the effect of biochar addition on the anaerobic digestion of swine manure: Application of Py-GC/MS. Environ. Sci. Pollut. Res. 2018, 25, 25600–25611. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Kang, X.; Wu, B.; O’Shea, R.; Murphy, J.D. Improving gaseous biofuel yield from seaweed through a cascading circular bioenergy system integrating anaerobic digestion and pyrolysis. Renew. Sustain. Energy Rev. 2020, 128, 109895. [Google Scholar] [CrossRef]
- Chodkowska-Miszczuk, J.; Martinát, S.; van der Horst, D. Changes in feedstocks of rural anaerobic digestion plants: External drivers towards a circular bioeconomy. Renew. Sustain. Energy Rev. 2021, 148, 111344. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Guo, J.Y.; Guo, J.Y. Biology of Water Hyacinth. In Water Hyacinth: Environmental Challenges, Management and Utilization; CRC Press: Boca Raton, FL, USA, 2017; pp. 15–43. [Google Scholar]
- Villamagna, A.M.; Murphy, B.R. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): A review. Freshw. Biol. 2010, 55, 282–298. [Google Scholar] [CrossRef]
- Güereña, D.; Neufeldt, H.; Berazneva, J.; Duby, S. Water hyacinth control in Lake Victoria: Transforming an ecological catastrophe into economic, social, and environmental benefits. Sustain. Prod. Consum. 2015, 3, 59–69. [Google Scholar] [CrossRef]
- Kothari, R.; Vashishtha, A.; Singh, H.M.; Pathak, V.V.; Tyagi, V.; Yadav, B.; Ashokkumar, V.; Singh, D. Assessment of Indian bioenergy policy for sustainable environment and its impact for rural India: Strategic implementation and challenges. Environ. Technol. Innov. 2020, 20, 101078. [Google Scholar] [CrossRef]
- Ye, X.M. Utilization of Biomass for Energy and Fertilizer. In Water Hyacinth: Environmental Challenges, Management and Utilization; CRC Press: Boca Raton, FL, USA, 2017; pp. 253–276. [Google Scholar]
- Gunnarsson, C.C.; Petersen, C.M. Water hyacinths as a resource in agriculture and energy production: A literature review. Waste Manag. 2007, 27, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. Influence of augmentation of biochar during anaerobic co-digestion of Chlorella vulgaris and cellulose. Bioresour. Technol. 2022, 343, 126086. [Google Scholar] [CrossRef] [PubMed]
- Shekwaga, C.K.O.; Suruagy, M.V.T.; Ross, A.; Valero, M.A.C. Particle size, inoculum-to-substrate ratio and nutrient media effects on biomethane yield from food waste. Renew. Energy 2020, 151, 311–321. [Google Scholar] [CrossRef]
- Linville, J.L.; Shen, Y.; Leon, P.A.I.-D.; Schoene, R.P.; Urgun-Demirtas, M. In-situ biogas upgrading during anaerobic digestion of food waste amended with walnut shell biochar at bench scale. Waste Manag. Res. J. Sustain. Circ. Econ. 2017, 35, 669–679. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington DC, USA, 2005. [Google Scholar]
- Fettweis, U.; Kühl, J. Comparative Tests for the Purposes of a Crude Fibre Analysis Using Both the Official VDLUFA Method and FibreBag Technology (C. Gerhardt). In Association of German Agricultural Analytic and Research Institutes; Series 66; VDLUFA Verlag: Darmstadt, Germany, 2010; pp. 804–810. [Google Scholar]
- Magomya, A.M.; Kubmarawa, D.; Ndahi, J.A.; Yebpella, G.G. Determination of Plant Proteins Via The Kjeldahl Method And Amino Acid Analysis: A Comparative Study. Int. J. Sci. Technol. Res. 2014, 3, 68–72. [Google Scholar]
- Buswell, A.M.; Mueller, H.F. Mechanism of Methane Fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Briceño, C.I.; Grasham, O.; Ross, A.B.; Dupont, V.; Camargo-Valero, M.A. Hydrothermal carbonization of sewage digestate at wastewater treatment works: Influence of solid loading on characteristics of hydrochar, process water and plant energetics. Renew. Energy 2020, 157, 959–973. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2019, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van ’T Riet, K. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Brown, A.E.; Adams, J.M.M.; Grasham, O.R.; Camargo-Valero, M.A.; Ross, A.B. An Assessment of Different Integration Strategies of Hydrothermal Carbonisation and Anaerobic Digestion of Water Hyacinth. Energies 2020, 13, 5983. [Google Scholar] [CrossRef]
- Chanakya, H.N.; Borgaonkar, S.; Meena, G.; Jagadish, K.S. Solid-phase biogas production with garbage or water hyacinth. Bioresour. Technol. 1993, 46, 227–231. [Google Scholar] [CrossRef]
- Bhui, I.; Mathew, A.K.; Chaudhury, S.; Balachandran, S. Influence of volatile fatty acids in different inoculum to substrate ratio and enhancement of biogas production using water hyacinth and salvinia. Bioresour. Technol. 2018, 270, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Romero De León, L.A.; Quinto Diez, P.; Tovar Gálvez, L.R.; Alvarado Perea, L.; López Barragán, C.A.; García Rodríguez, C.A.; Reyes León, A. Biochemical methane potential of water hyacinth and the organic fraction of municipal solid waste using leachate from Mexico City’s Bordo Poniente composting plant as inoculum. Fuel 2021, 285, 119132. [Google Scholar] [CrossRef]
- DE LA Rubia, M.A.; Villamil, J.A.; Rodriguez, J.J.; Mohedano, A.F. Effect of inoculum source and initial concentration on the anaerobic digestion of the liquid fraction from hydrothermal carbonisation of sewage sludge. Renew. Energy 2018, 127, 697–704. [Google Scholar] [CrossRef]
- Priya, P.; Nikhitha, S.O.; Anand, C.; Nath, R.D.; Krishnakumar, B. Biomethanation of water hyacinth biomass. Bioresour. Technol. 2018, 255, 288–292. [Google Scholar] [CrossRef]
- Ali, S.S.; Sun, J. Effective thermal pretreatment of water hyacinth (Eichhornia crassipes) for the enhancement of biomethanation: VIT® gene probe technology for microbial community analysis with special reference to methanogenic Archaea. J. Environ. Chem. Eng. 2019, 7, 102853. [Google Scholar] [CrossRef]
- Sarto, S.; Hildayati, R.; Syaichurrozi, I. Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics. Renew. Energy 2019, 132, 335–350. [Google Scholar] [CrossRef]
- Barua, V.B.; Kalamdhad, A.S. Biochemical methane potential test of untreated and hot air oven pretreated water hyacinth: A comparative study. J. Clean. Prod. 2017, 166, 273–284. [Google Scholar] [CrossRef]
- O’Sullivan, C.; Rounsefell, B.; Grinham, A.; Clarke, W.; Udy, J. Anaerobic digestion of harvested aquatic weeds: Water hyacinth (Eichhornia crassipes), cabomba (Cabomba Caroliniana) and salvinia (Salvinia molesta). Ecol. Eng. 2010, 36, 1459–1468. [Google Scholar] [CrossRef]
- Patil, J.H.; Raj, M.A.; Muralidhara, P.L.; Desai, S.M.; Raju, G.K.M. Kinetics of Anaerobic Digestion of Water Hyacinth Using Poultry Litter as Inoculum. Int. J. Environ. Sci. Dev. 2012, 3, 94–98. [Google Scholar] [CrossRef]
- Suthar, S.; Sharma, B.; Kumar, K.; Banu, J.R.; Tyagi, V.K. Enhanced biogas production in dilute acid-thermal pretreatment and cattle dung biochar mediated biomethanation of water hyacinth. Fuel 2022, 307, 121897. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Li, H.; Usmani, S.Q.; Semple, K.T. Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef]
- Sunyoto, N.M.S.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef]
- Okoro-Shekwaga, C.; Ross, A.B.; Camargo-Valero, M.A. Improving the biomethane yield from food waste by boosting hydrogenotrophic methanogenesis. Appl. Energy 2019, 254, 113629. [Google Scholar] [CrossRef]
- Wang, B.; Strömberg, S.; Li, C.; Nges, I.A.; Nistor, M.; Deng, L.; Liu, J. Effects of substrate concentration on methane potential and degradation kinetics in batch anaerobic digestion. Bioresour. Technol. 2015, 194, 240–246. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; DE LA Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.-C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Rombolà, A.G.; Marisi, G.; Torri, C.; Fabbri, D.; Buscaroli, A.; Ghidotti, M.; Hornung, A. Relationships between Chemical Characteristics and Phytotoxicity of Biochar from Poultry Litter Pyrolysis. J. Agric. Food Chem. 2015, 63, 6660–6667. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Conversion of Lignocellulosic Feedstocks into Bioethanol Using Extremophiles. In Extremophilic Microbial Processing of Lignocellulosic Feedstocks to Biofuels, Value-Added Products, and Usable Power; Sani, R.K., Rathinam, N.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 25–46. [Google Scholar]
- Sun, Y.; Wang, D.; Yan, J.; Qiao, W.; Wang, W.; Zhu, T. Effects of lipid concentration on anaerobic co-digestion of municipal biomass wastes. Waste Manag. 2014, 34, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, R.; Wang, B.; Zhang, S. Modeling and optimization of anaerobic digestion of corn stover on biogas production: Initial pH and carbon to nitrogen ratio. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 1497–1503. [Google Scholar] [CrossRef]
| Material | VBU-WH | MM-WH | PV-WH | UG-WH | Biochar |
|---|---|---|---|---|---|
| Sampling site | Goyal Para pond, India | Mula Mutha River, India | Pavana River, India | Lake Victoria, Uganda | |
| VM (%, db) | 73.4 | 76.2 | 74.0 | 85.4 | 21.1 |
| FC (%, db) | 10.4 | 15.4 | 12.3 | <1 | 67.2 |
| Ash (%, db) | 16.0 | 7.8 | 13.7 | 14.6 | 11.7 |
| C (%) | 34.2 | 36.3 | 33.0 | 36.1 | 65.7 |
| H (%) | 4.1 | 4.6 | 4.6 | 3.1 | 2.7 |
| N (%) | 1.8 | 3.0 | 3.1 | 2.5 | 0.6 |
| O (%) | 43.7 | 48.1 | 45.1 | 43.6 | 19.3 |
| S (%) | 0.0 | 0.2 | 0.5 | 0.1 | 0.0 |
| C/N | 17.8 | 12.3 | 10.6 | 14.5 | - |
| Cellulose (%) | 32.1 | 26.4 | 17.4 | 25.1 | - |
| Hemicellulose (%) | 25.5 | 16.1 | 8.3 | 22.6 | - |
| Lignin (%) | 4.7 | 7.9 | 11.2 | 6.8 | - |
| Oils (%) | 0.44 | - | - | 1.0 | - |
| Protein (%) | 8.42 | - | - | 12.4 | - |
| Free sugars * (%) | 8.7 | - | - | 11.8 | - |
| BMPTh (mL CH4/g VS) | 383.4 | 331.8 | 351.3 | 352.6 | - |
| Reactor No. | Orthogonal Design | Actual Value | ||
|---|---|---|---|---|
| ISR | BC Load | ISR | BC Load (%) | |
| R1, R2, R3 | −1 | −1 | 1 | 0 |
| R4, R5, R6, | −1 | 1 | 1 | 3 |
| R7, R8, R9 | 0 | 0 | 1.5 | 1.5 |
| R10, R11, R12 | 1 | −1 | 2 | 0 |
| R13, R14, R15 | 1 | 1 | 2 | 3 |
| ISR | BC Load (%) | BMPExp (mL CH4/g VS) | BMPmax (mL CH4/g VS) | µm (mL CH4/g VS·Day) | λ (Days) | R2 |
|---|---|---|---|---|---|---|
| 1 | 0 | 208.9 | 222.8 | 15.0 | 0.0 | 0.974 |
| 1 | 3 | 194.1 | 204.4 | 15.3 | 0.2 | 0.978 |
| 1.5 | 1.5 | 190.2 | 203.0 | 13.7 | 0.2 | 0.981 |
| 2 | 0 | 165.3 | 171.8 | 10.0 | 0.0 | 0.982 |
| 2 | 3 | 182.9 | 197.4 | 12.2 | 0.0 | 0.979 |
| Analysis of Variance (ANOVA) | |||
|---|---|---|---|
| Variable | BMPExp | BMPmax | µm |
| R2 | 0.9017 | 0.8627 | 0.8243 |
| Adjusted R2 | 0.8749 | 0.8253 | 0.7763 |
| Prediction R2 | 0.7929 | 0.7166 | 0.6797 |
| F value | 33.64 | 23.04 | 17.20 |
| Model p-value | 0.000 | 0.000 | 0.000 |
| Coefficient Probability | ||||||
|---|---|---|---|---|---|---|
| BMPExp | BMPmax | µm | ||||
| Term | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value |
| Constant | 188.3 | 0.000 | 199.9 | 0.000 | 13.2 | 0.000 |
| ISR | −13.7 | 0.000 | −14.5 | 0.000 | −2.0 | 0.000 |
| BC | 0.7 | 0.667 | 1.8 | 0.429 | 0.6 | 0.055 |
| ISR*BC | 8.1 | 0.001 | 11.0 | 0.001 | 0.5 | 0.145 |
| Experimental | Gompertz Model | |||||
|---|---|---|---|---|---|---|
| BMPexp (mL CH4/g VS) | BD (%) | BMPmax (mL CH4/g VS) | µm (mL CH4/g VS·Day) | λ (Days) | R2 | |
| VBU-WH | 208.9 | 54.5 | 222.8 | 15.0 | 0.0 | 0.974 |
| VBU-WH + BC 0.5% | 217.7 | 56.8 | 217.4 | 24.9 | 1.5 | 0.991 |
| VBU-WH + BC 0.75% | 173.3 | 45.2 | 179.3 | 17.4 | 1.0 | 0.990 |
| VBU-WH + BC 0.1% | 141.7 | 37.0 | 145.1 | 13.0 | 0.4 | 0.978 |
| MM-WH | 201.3 | 60.7 | 196.6 | 20.2 | 0.0 | 0.967 |
| MM-WH + BC 0.5% | 163.3 | 49.2 | 164.6 | 15.8 | 0 | 0.989 |
| PV-WH | 177.1 | 50.4 | 172.9 | 19.8 | 0.0 | 0.977 |
| PV-WH + BC 0.5% | 141.4 | 40.2 | 140.5 | 32.6 | 0.2 | 0.995 |
| UG-WH | 91.6 | 26.0 | 93.4 | 6.8 | 0.0 | 0.987 |
| UG-WH + BC 0.5% | 53.7 | 15.2 | 54.4 | 5.0 | 0 | 0.983 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Bray, D.G.; Ross, A.B. The Influence of Biochar Augmentation and Digestion Conditions on the Anaerobic Digestion of Water Hyacinth. Energies 2022, 15, 2524. https://doi.org/10.3390/en15072524
Quintana-Najera J, Blacker AJ, Fletcher LA, Bray DG, Ross AB. The Influence of Biochar Augmentation and Digestion Conditions on the Anaerobic Digestion of Water Hyacinth. Energies. 2022; 15(7):2524. https://doi.org/10.3390/en15072524
Chicago/Turabian StyleQuintana-Najera, Jessica, A. John Blacker, Louise A. Fletcher, Douglas G. Bray, and Andrew B. Ross. 2022. "The Influence of Biochar Augmentation and Digestion Conditions on the Anaerobic Digestion of Water Hyacinth" Energies 15, no. 7: 2524. https://doi.org/10.3390/en15072524
APA StyleQuintana-Najera, J., Blacker, A. J., Fletcher, L. A., Bray, D. G., & Ross, A. B. (2022). The Influence of Biochar Augmentation and Digestion Conditions on the Anaerobic Digestion of Water Hyacinth. Energies, 15(7), 2524. https://doi.org/10.3390/en15072524






