Reuse of Water Contaminated by Microplastics, the Effectiveness of Filtration Processes: A Review
Abstract
:1. Introduction
2. Microplastic Fate in Conventional Water/Wastewater-Treatment Processes
| Country | Influent | Treatment | Effluent | Reference |
|---|---|---|---|---|
| Spain | 4.40 MPs/L | MBR | 0.92 MPs/L, average size = 1.39 mm | Bayo et al. [18] |
| 4.40 MPs/L | RSF | 1.08 MPs/L, average size = 1.15 mm | ||
| Spain | Global | Effluent with 5.9 MPs/L in wet weather and 3.0 MPs/L in dry ones | Bayo et al. [19] | |
| Israel | 74% fibrous MPs | Global | RE = 97% | Ben-David et al. [33] |
| RSF | 1.97 MPs/L | |||
| RSF | 91% fibrous MPs | |||
| Spain | 90% fibers (9% MPs) | Bretas-Alvim et al. [27] | ||
| USA | Primary and secondary | 0.00144 MPs/L | Carr et al. [34] | |
| (several) | Global | RE > 93%, by weight | Cheng et al. [3] | |
| 61–5600 μg/L | Global | 0.5–170 μg/L | ||
| Italy | Global | RE > 90% | Cristaldi et al. [15] | |
| Spain | RSF | RE > 78% in DWTP | Dalmau-Soler et al. [35] | |
| France | Primary | RE = 80% for fibrous materials | Dris et al. [36] | |
| Primary and secondary | RE = 95% for fibrous materials | |||
| Global | Up to 88% of MPs removed go to sludge | Freeman et al. [37] | ||
| Israel | 32.4% suspected MPs were plastics | Primary and secondary | 65.6% fibers, 28.1% fragments, 5.4% pellets | Gies et al. [17] |
| Global | RE = 97–99% | |||
| Greece | 3160 MPs/L | Global | 125 MPs/L | Gatidou et al. [38] |
| South Korea | RSF | RE = 98.9% | Hidayaturrahman et al. [29] | |
| Primary | RE = 79% | |||
| Secondary | RE = 92% | |||
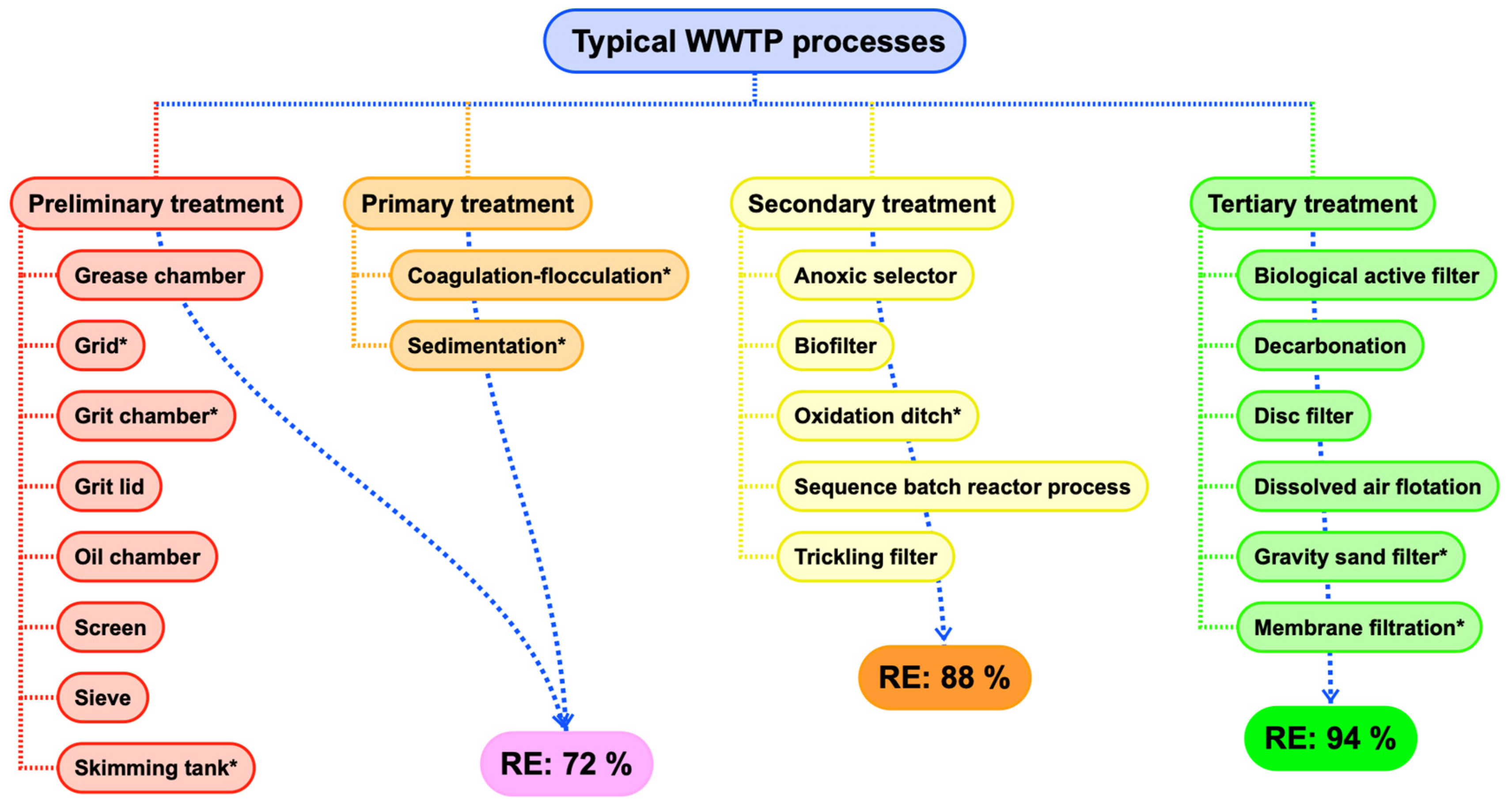
| (several) | Preliminary and primary | RE = 72% | Iyare et al. [4] | |
| Secondary | RE = 88% | |||
| Tertiary | RE = 94% | |||
| China | 126 MPs/L | Global | 30,6 MPs/L in the effluent | Jiang et al. [39] |
| >75% of MPs transferred to the sludge Accumulation of 7.74·1012 particles/year in the sludges. | ||||
| Thailand | Global | RE = 67% (drain season) | Kankanige [32] | |
| Global | RE = 57% (dry season) | |||
| Global | RE = 60% for sizes 6.5–53 μm | |||
| Global | RE = 80% for size > 500 μm | |||
| RSF | RE = 26.8% for the smallest particles | |||
| RSF | RE = 60.7% for the biggest particles | |||
| Singapore | Membrane filter | RE < 7% for nanoparticles (100–500 nm) | Li et al. [40] | |
| Denmark | 0.917 MPs/L | Biofilter | 0.179 MPs/L | Liu et al. [41] |
| RE = 100% for sizes lower than 0.1 mm | ||||
| Denmark | 0.28–31,400 MPs/L | Global | 0.01–297 MPs/L | Liu et al. [24] |
| China | Primary | RE = 75% | Lv et al. [42] | |
| Secondary | RE = 95% | |||
| Tertiary | RE = 98% | |||
| MBR | RE = 99.5% | |||
| Activated sludge | RE = 97% | |||
| USA | Global | Effluent with less than 1 particle per liter 5·104–1.5·107 particles/day | Mason et al. [43] | |
| USA | Global | 60% fibers in the effluent | Michielssen et al. [44] | |
| Germany | Use of magnetic ionic liquids | RE = 100% (PS) | Misra et al. [45] | |
| Canada | Filtration with 0.22 μm | RE > 99% | Murray et al. [46] | |
| Scotland | 15.7 MPs/L | Global | 0.25 MPs/L (RE > 98%) | Murphy et al. [20] |
| China | (Simulated runs) | Smaller-sized MPs are more mobile in RSF A greater number of wet–dry cycles increases MPs’ penetration depth | O’Connor et al. [47] | |
| China | Grease on the sludge contains many more MPs than grit and sludge cake. | Ou and Zeng [30] | ||
| Sweden | 202.2 kg MPs/day | Global | RE = 72% | Rasmussen et al. [25] |
| Germany | Seeds of Fe3O4 and application of magnetic field | 95% | Rhein et al. [48] | |
| Indonesia | RSF | RE = 50% for plastic flakes, in DWTP | Sembiring et al. [49] | |
| RE = 96% for tires, in DWTP | ||||
| India | RSF | RE = 85%, by weight | Seth et al. [50] | |
| RE = 90%, by number | ||||
| Denmark | Disk filter | RE = 76% | Simon et al. [51] | |
| Denmark | 3 tonnes MPs/year, size range 10–500 um, 98% RE | Simon et al. [28] | ||
| Thailand | Grit trap | RE > 33% | Tadsuwan et al. [26] | |
| Secondary treatment | RE > 14.2% (additional) | |||
| (several) | Primary | RE = 98% | Talvitie et al. [52] | |
| Primary and secondary | RE > 99% | |||
| Secondary | RE = 88% | |||
| MBR | RE = 99.9% | |||
| RSF | RE = 97% | |||
| Dissolved air flotation | RE = 95% | |||
| Disc filtration | RE = 40–98% | |||
| Spain | SS has a lighter plastic load, much lower than heavy plastic load (18,000 vs 32,070 MPs/kg) | Van der Berg et al. [53] | ||
| China | Primary and secondary | RE > 98% | Wu et al. [22] | |
| China | 12 MPs/L | UF | 0.6 MPs/L | Yang et al. [31] |
| Primary | RE = 59% | |||
| Primary and secondary | RE = 72% | |||
| Global | RE = 95% | |||
| India | Hollow-fiber membrane | RE = 99.3% | Yaranal et al. [54] | |
| USA | RSF | RE = 86.9% for small particles | Zhang et al. [6] | |
| RSF | RE = 87% for critical size (10–20 μm) | |||
| RSF | RE > 99.9% for particles larger than 100 μm |
2.1. Microplastic Accumulation in Sludges
2.2. Need for Standardized Methods
3. Filtration as a Route of Elimination
4. Differences among MPs Being Filtrated
5. Conclusions
- −
- Although the removal efficiency of MPs in WWTP is relatively high, the quantity of these particles discharged into the environment is still very high, becoming the largest source of the introduction of MPs into the environment.
- −
- Primary and secondary treatment processes effectively remove MPs from wastewater with removal efficiencies ranging from 75% to 91.9%. RE can be increased to >98% after tertiary treatment.
- −
- Most of the MPs eliminated from sewage accumulate in the sewage sludge. Incineration/thermal treatment of SS is the only effective way to destroy these plastic particles.
- −
- During sewage treatment, part of the retained MPs can be recirculated back to the treatment process as part of the water is rejected.
- −
- There is a need for harmonization of the techniques used for MP analysis and counting.
- −
- Larger particles exhibit greater filtration efficiency. Smaller particles have more mobility.
- −
- RSF shows a good performance for MPs in the form of beads or granules. However, it does not guarantee microbeads (nor microfibers) would be absent from the effluent.
- −
- Efficiency of membrane filtration can be as low as 7% for nanoparticles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNEP Green Economy|UNEP-UN Environment Programme. Available online: https://www.unep.org/explore-topics/green-economy/what-we-do/economic-and-fiscal-policy/fiscal-policy/policy-analysis-4 (accessed on 16 February 2022).
- Pal, P. Selection of Water-Treatment Technology. In Industrial Water Treatment Process Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 537–544. [Google Scholar]
- Cheng, Y.L.; Kim, J.-G.G.; Kim, H.-B.B.; Choi, J.H.; Fai Tsang, Y.; Baek, K. Occurrence and removal of microplastics in wastewater treatment plants and drinking water purification facilities: A review. Chem. Eng. J. 2021, 410, 128381. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Spellman, F.R. Handbook of Water and Wastewater Treatment Plant Operations; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Zhang, X.; Chen, J.; Li, J. The removal of microplastics in the wastewater treatment process and their potential impact on anaerobic digestion due to pollutants association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A. Advanced wastewater treatment and biomass energy. Energies 2022, 15, 173. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.J.; Chang, Y.; Lee, Y.J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- PlasticEurope-Association of Plastics Manufactures. Plastics—The Facts; European Association of Plastics Recycling and Recovery Organisations: Wemmel, Belgium, 2021. [Google Scholar]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Marine debris occurrence and treatment: A review. Renew. Sustain. Energy Rev. 2016, 64, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Conesa, J.A.; Iñiguez, M.E. Analysis of Microplastics in Food Samples. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M., Mouneyrac, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Bayo, J.; López-Castellanos, J.; Olmos, S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020, 156, 111211. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.; Beal, M.; Maley, J.; Brinkmann, M. Qualitative and quantitative analysis of microplastics and microfiber contamination in effluents of the City of Saskatoon wastewater treatment plant. Environ. Sci. Pollut. Res. 2021, 28, 32545–32553. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Wang, J.; Bassi, A. Current research trends on microplastic pollution from wastewater systems: A critical review. Rev. Environ. Sci. Bio Technol. 2019, 18, 207–230. [Google Scholar] [CrossRef]
- Cristaldi, A.; Fiore, M.; Zuccarello, P.; Conti, G.O.; Grasso, A.; Nicolosi, I.; Copat, C.; Ferrante, M.; Oliveri Conti, G.; Grasso, A.; et al. Efficiency of wastewater treatment plants (Wwtps) for microplastic removal: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 8014. [Google Scholar] [CrossRef]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef] [PubMed]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Bayo, J.; López-Castellanos, J.; Olmos, S. Abatement of Microplastics from Municipal Effluents by Two Different Wastewater Treatment Technologies. Water Pollut. XV 2020, 1, 15–26. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Removal of Microplastics from Wastewater. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M., Mouneyrac, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: A review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Wu, M.; Tang, W.; Wu, S.; Liu, H.; Yang, C. Fate and effects of microplastics in wastewater treatment processes. Sci. Total Environ. 2021, 757, 143902. [Google Scholar] [CrossRef]
- Johnson, A.C.; Ball, H.; Cross, R.; Horton, A.A.; Jürgens, M.D.; Read, D.S.; Vollertsen, J.; Svendsen, C. Identification and Quantification of Microplastics in Potable Water and Their Sources within Water Treatment Works in England and Wales. Environ. Sci. Technol. 2020, 54, 12326–12334. [Google Scholar] [CrossRef]
- Liu, W.Y.; Zhang, J.L.; Liu, H.; Guo, X.N.; Zhang, X.Y.; Yao, X.L.; Cao, Z.G.; Zhang, T.T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef]
- Rasmussen, L.A.; Iordachescu, L.; Tumlin, S.; Vollertsen, J. A complete mass balance for plastics in a wastewater treatment plant-Macroplastics contributes more than microplastics. Water Res. 2021, 201, 117307. [Google Scholar] [CrossRef]
- Tadsuwan, K.; Babel, S. Microplastic contamination in a conventional wastewater treatment plant in Thailand. Waste Manag. Res. 2021, 39, 754–761. [Google Scholar] [CrossRef]
- Bretas Alvim, C.; Bes-Piá, M.A.; Mendoza-Roca, J.A. Separation and identification of microplastics from primary and secondary effluents and activated sludge from wastewater treatment plants. Chem. Eng. J. 2020, 402, 126293. [Google Scholar] [CrossRef]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hidayaturrahman, H.; Lee, T.-G.G. A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar. Pollut. Bull. 2019, 146, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Zeng, E.Y. Occurrence and Fate of Microplastics in Wastewater Treatment Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128137475. [Google Scholar]
- Yang, L.; Li, K.; Cui, S.; Kang, Y.; An, L.; Lei, K. Removal of microplastics in municipal sewage from China’s largest water reclamation plant. Water Res. 2019, 155, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kankanige, D.; Babel, S. Contamination by ≥6.5 μm-sized microplastics and their removability in a conventional water treatment plant (WTP) in Thailand. J. Water Process Eng. 2021, 40, 101765. [Google Scholar] [CrossRef]
- Ben-David, E.A.; Habibi, M.; Haddad, E.; Hasanin, M.; Angel, D.L.; Booth, A.M.; Sabbah, I. Microplastic distributions in a domestic wastewater treatment plant: Removal efficiency, seasonal variation and influence of sampling technique. Sci. Total Environ. 2021, 752, 141880. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Dalmau-Soler, J.; Ballesteros-Cano, R.; Boleda, M.R.; Paraira, M.; Ferrer, N.; Lacorte, S. Microplastics from headwaters to tap water: Occurrence and removal in a drinking water treatment plant in Barcelona Metropolitan area (Catalonia, NE Spain). Environ. Sci. Pollut. Res. 2021, 28, 59462–59472. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Freeman, S.; Booth, A.M.; Sabbah, I.; Tiller, R.; Dierking, J.; Klun, K.; Rotter, A.; Ben-David, E.; Javidpour, J.; Angel, D.L. Between source and sea: The role of wastewater treatment in reducing marine microplastics. J. Environ. Manag. 2020, 266, 110642. [Google Scholar] [CrossRef]
- Gatidou, G.; Arvaniti, O.S.; Stasinakis, A.S. Review on the occurrence and fate of microplastics in Sewage Treatment Plants. J. Hazard. Mater. 2019, 367, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, X.; Ren, H.; Cao, G.; Xie, G.; Xing, D.; Liu, B. Investigation and fate of microplastics in wastewater and sludge filter cake from a wastewater treatment plant in China. Sci. Total Environ. 2020, 746, 141378. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Nord, N.B.; Bester, K.; Vollertsen, J. Microplastics Removal from Treated Wastewater by a Biofilter. Water 2020, 12, 1085. [Google Scholar] [CrossRef]
- Lv, X.M.; Dong, Q.; Zuo, Z.Q.; Liu, Y.C.; Huang, X.; Wu, W.M. Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies. J. Clean. Prod. 2019, 225, 579–586. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Michielssen, M.R.; Michielssen, E.R.; Ni, J.; Duhaime, M.B. Fate of microplastics and other small anthropogenic litter (SAL) in wastewater treatment plants depends on unit processes employed. Environ. Sci. Water Res. Technol. 2016, 2, 1064–1073. [Google Scholar] [CrossRef] [Green Version]
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Franco Castillo, I.; Mitchell, S.G.; Güttel, R.; Streb, C. Water Purification and Microplastics Removal Using Magnetic Polyoxometalate-Supported Ionic Liquid Phases (magPOM-SILPs). Angew. Chem. Int. Ed. 2020, 59, 1601–1605. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.; Örmeci, B. Water with Separation Processes Used for Water and Wastewater Treatment. Water 2020, 12, 635. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.M.; Hou, D. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef]
- Rhein, F.; Scholl, F.; Nirschl, H. Magnetic seeded filtration for the separation of fine polymer particles from dilute suspensions: Microplastics. Chem. Eng. Sci. 2019, 207, 1278–1287. [Google Scholar] [CrossRef]
- Sembiring, E.; Fajar, M.; Handajani, M. Performance of rapid sand filter—single media to remove microplastics. Water Supply 2021, 21, 2273–2284. [Google Scholar] [CrossRef]
- Seth, C.K.; Shriwastav, A. Contamination of Indian sea salts with microplastics and a potential prevention strategy. Environ. Sci. Pollut. Res. 2018, 25, 30122–30131. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Vianello, A.; Vollertsen, J. Removal of >10 µm Microplastic Particles from Treated Wastewater by a Disc Filter. Water 2019, 11, 1935. [Google Scholar] [CrossRef] [Green Version]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [Green Version]
- van den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef] [PubMed]
- Yaranal, N.A.; Subbiah, S.; Mohanty, K. Identification, extraction of microplastics from edible salts and its removal from contaminated seawater. Environ. Technol. Innov. 2021, 21, 101253. [Google Scholar] [CrossRef]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R.; Paakkonen, J.P.; Vahtera, E.; Mikola, A.; et al. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. WATER Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes—Origin, impact and potential solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in sewage sludge: Effects of treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Habib, D.; Locke, D.C.; Cannone, L.J. Synthetic fibers as indicators of municipal sewage sludge, sludge products, and sewage treatment plant effluents. Water. Air. Soil Pollut. 1998, 103, 1–8. [Google Scholar] [CrossRef]
- Milojevic, N.; Cydzik-kwiatkowska, A. Agricultural use of sewage sludge as a threat of microplastic (Mp) spread in the environment and the role of governance. Energies 2021, 14, 6293. [Google Scholar] [CrossRef]
- Wolff, S.; Weber, F.; Kerpen, J.; Winklhofer, M.; Engelhart, M.; Barkmann, L. Elimination of microplastics by downstream sand filters in wastewater treatment. Water 2021, 13, 33. [Google Scholar] [CrossRef]
- Jellali, S.; Khiari, B.; Usman, M.; Hamdi, H.; Charabi, Y.; Jeguirim, M. Sludge-derived biochars: A review on the influence of synthesis conditions on pollutants removal efficiency from wastewaters. Renew. Sustain. Energy Rev. 2021, 144, 111068. [Google Scholar] [CrossRef]
- Yan, P.; Shi, H.X.; Chen, Y.P.; Gao, X.; Fang, F.; Guo, J.S. Optimization of recovery and utilization pathway of chemical energy from wastewater pollutants by a net-zero energy wastewater treatment model. Renew. Sustain. Energy Rev. 2020, 133, 110160. [Google Scholar] [CrossRef]
- Durdević, D.; Blecich, P.; Jurić, Ž. Energy Recovery from Sewage Sludge: The Case. Energies 2019, 12, 1927. [Google Scholar] [CrossRef] [Green Version]
- The European Parliament; The Council of the European Union. European Parliament and of the Council Directive 2008/98/EC. Off. J. Eur. Union 2008, L 312, 3–30. [Google Scholar]
- Bannick, C.G.; Szewzyk, R.; Ricking, M.; Schniegler, S.; Obermaier, N.; Barthel, A.K.; Altmann, K.; Eisentraut, P.; Braun, U. Development and testing of a fractionated filtration for sampling of microplastics in water. Water Res. 2019, 149, 650–658. [Google Scholar] [CrossRef]
- Bretas Alvim, C.; Mendoza-Roca, J.A.A.; Bes-Piá, A. Wastewater treatment plant as microplastics release source—Quantification and identification techniques. J. Environ. Manage. 2020, 255, 109739. [Google Scholar] [CrossRef] [PubMed]
- Elkhatib, D.; Oyanedel-Craver, V. A Critical Review of Extraction and Identification Methods of Microplastics in Wastewater and Drinking Water. Environ. Sci. Technol. 2020, 54, 7037–7049. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Munno, K.; Hermabessiere, L.; Carr, S. Isolation and Extraction of Microplastics from Environmental Samples: An Evaluation of Practical Approaches and Recommendations for Further Harmonization. Appl. Spectrosc. 2020, 74, 1049–1065. [Google Scholar] [CrossRef]
- Oßmann, B.E. Microplastics in drinking water? Present state of knowledge and open questions. Curr. Opin. Food Sci. 2021, 41, 44–51. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.H.; Wang, Q.L.; van Loosdrecht, M.C.M.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Hong, Y.; Oh, J.; Lee, I.; Fan, C.; Pan, S.Y.; Jang, M.; Park, Y.K.; Kim, H. Total-organic-carbon-based quantitative estimation of microplastics in sewage. Chem. Eng. J. 2021, 423, 130182. [Google Scholar] [CrossRef]
- Lenz, R.; Labrenz, M. Small Microplastic Sampling in Water: Development of an Encapsulated Filtration Device. Water 2018, 10, 1055. [Google Scholar] [CrossRef] [Green Version]
- Dyachenko, A.; Mitchell, J.; Arsem, N. Extraction and identification of microplastic particles from secondary wastewater treatment plant (WWTP) effluent. Anal. Methods 2017, 9, 1412–1418. [Google Scholar] [CrossRef]
- Cooper, D.A.; Corcoran, P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Pollut. Bull. 2010, 60, 650–654. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Das Sarkar, S.; Das, B.K.; Praharaj, J.K.; Mahajan, D.K.; Purokait, B.; Mohanty, T.R.; Mohanty, D.; Gogoi, P.; Kumar, V.S.; et al. Microplastics removal efficiency of drinking water treatment plant with pulse clarifier. J. Hazard. Mater. 2021, 413, 125347. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, J.; He, H.; Zhu, Y.; Dionysiou, D.D.; Liu, Z.; Zhao, C. Do membrane filtration systems in drinking water treatment plants release nano/microplastics? Sci. Total Environ. 2021, 755, 142658. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Rahman, M.S.; Alom, J.; Hasan, M.S.; Johir, M.A.H.; Mondal, M.I.H.; Lee, D.Y.; Park, J.; Zhou, J.L.; Yoon, M.H. Microplastic particles in the aquatic environment: A systematic review. Sci. Total Environ. 2021, 775, 145793. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lai, Y.; Yu, S.; Li, P.; Zhou, X.; Dong, L.; Liu, X.; Yao, Z.; Liu, J. Sequential Isolation of Microplastics and Nanoplastics in Environmental Waters by Membrane Filtration, Followed by Cloud-Point Extraction. Anal. Chem. 2021, 93, 4559–4566. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Assessment of microplastics in a municipal wastewater treatment plant with tertiary treatment: Removal efficiencies and loading per day into the environment. Water 2021, 13, 1339. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Liu, H.; Qu, J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conesa, J.A.; Ortuño, N. Reuse of Water Contaminated by Microplastics, the Effectiveness of Filtration Processes: A Review. Energies 2022, 15, 2432. https://doi.org/10.3390/en15072432
Conesa JA, Ortuño N. Reuse of Water Contaminated by Microplastics, the Effectiveness of Filtration Processes: A Review. Energies. 2022; 15(7):2432. https://doi.org/10.3390/en15072432
Chicago/Turabian StyleConesa, Juan A., and Nuria Ortuño. 2022. "Reuse of Water Contaminated by Microplastics, the Effectiveness of Filtration Processes: A Review" Energies 15, no. 7: 2432. https://doi.org/10.3390/en15072432
APA StyleConesa, J. A., & Ortuño, N. (2022). Reuse of Water Contaminated by Microplastics, the Effectiveness of Filtration Processes: A Review. Energies, 15(7), 2432. https://doi.org/10.3390/en15072432







