Experimental Study on the Efficiency Improvement of Flat Plate Solar Collectors Using Hybrid Nanofluids Graphene/Waste Cotton
Abstract
1. Introduction
2. Materials and Methodology for Nano-Cellulose Preparation
3. Nanoparticles and Base Fluids for the Experiment
3.1. Graphene Nanoparticles
3.2. Crystal Nano-Cellulose (CNC)
3.3. Ethylene Glycol and Hybrid Nanofluid
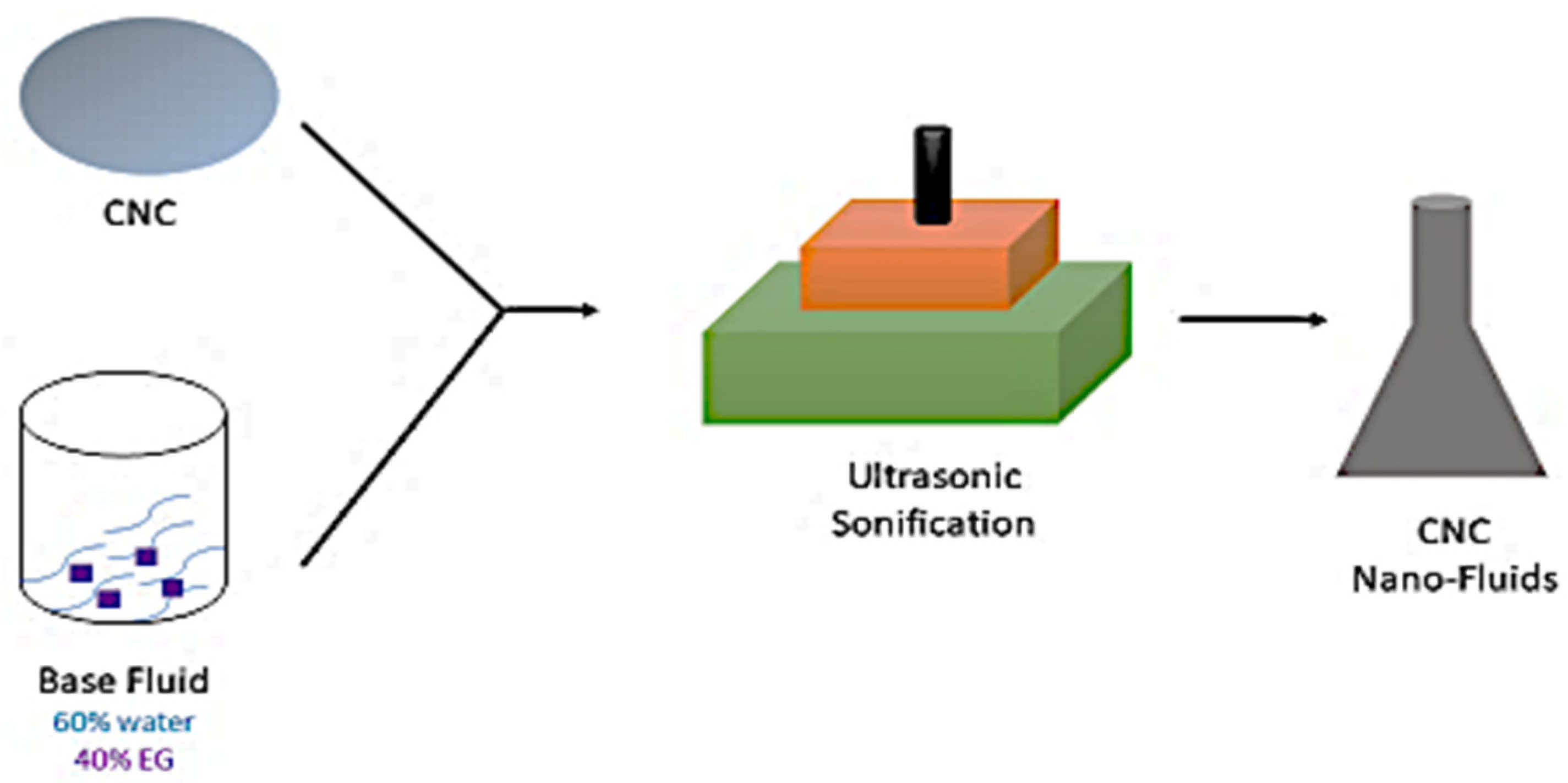
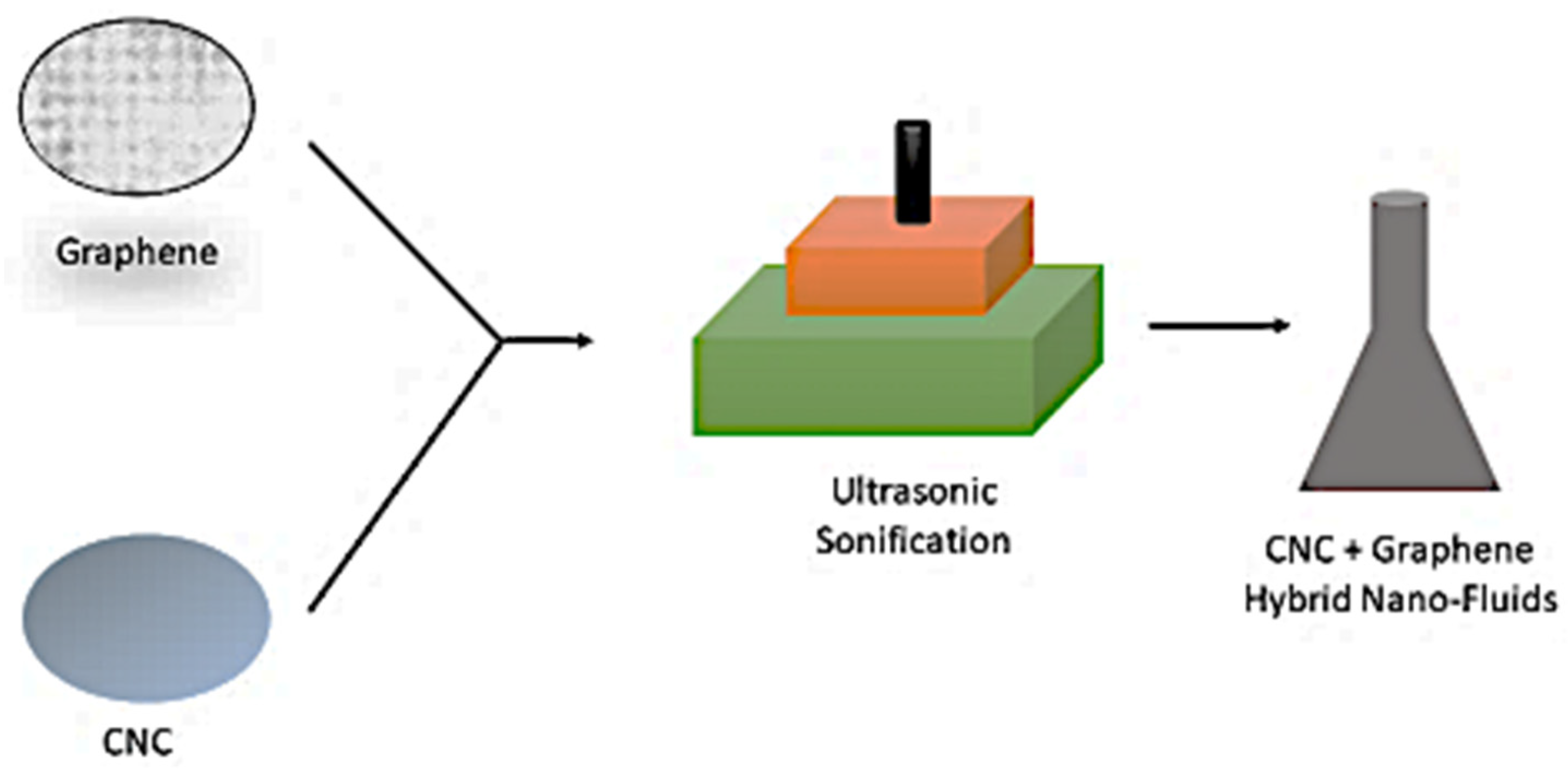
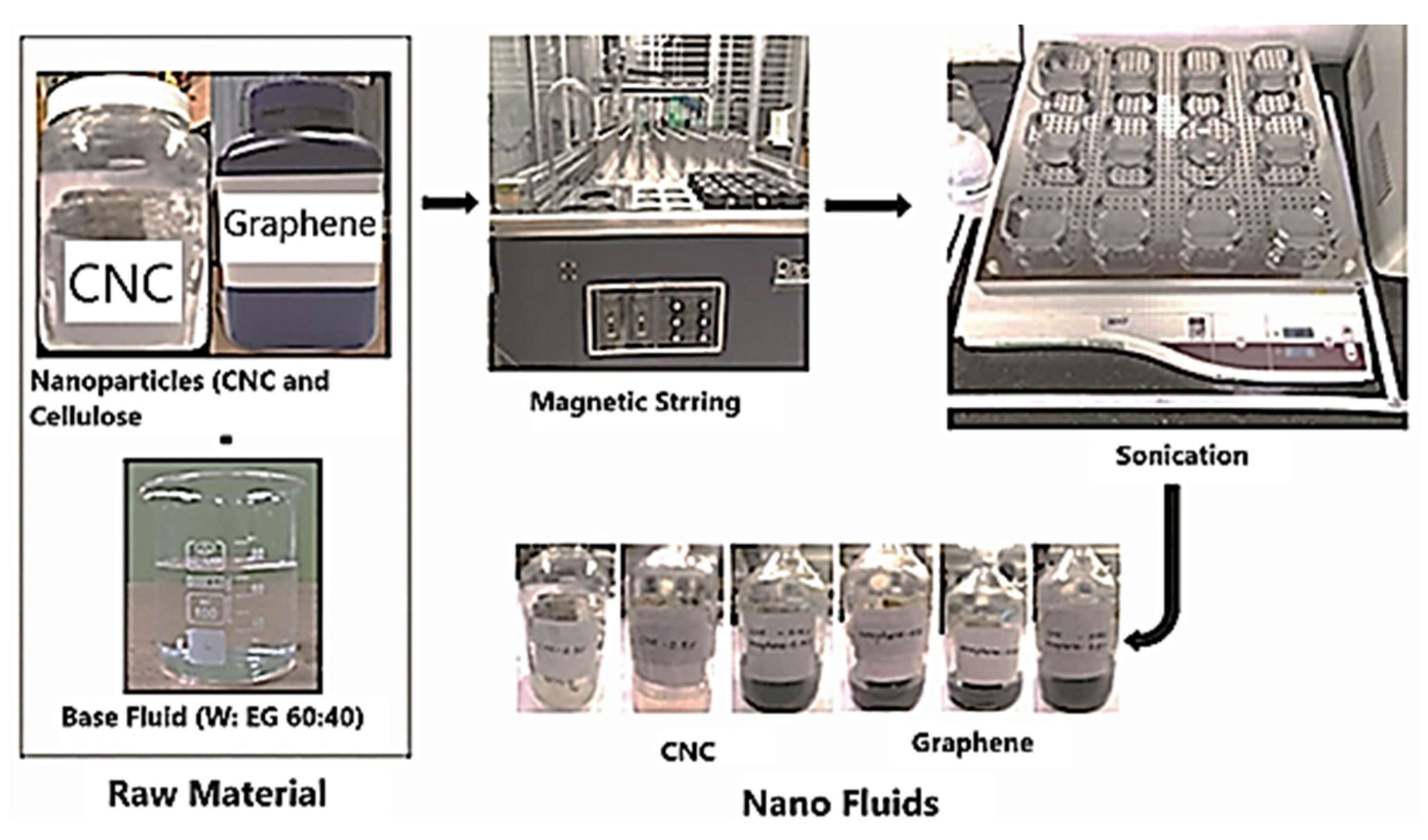
4. Preparation of CNC, Graphene, and Hybrid (Graphene+CNC) Nanofluids
5. Experimental Setup
6. Wavelength Analysis
6.1. Thermo-Physical Theoretical Analysis
- = Efficiency (%) of the FPSC
- = Energy gain (kW) by the FPSC
- = Solar radiance (W/m2)
- = Surface area (m2) of FPSC
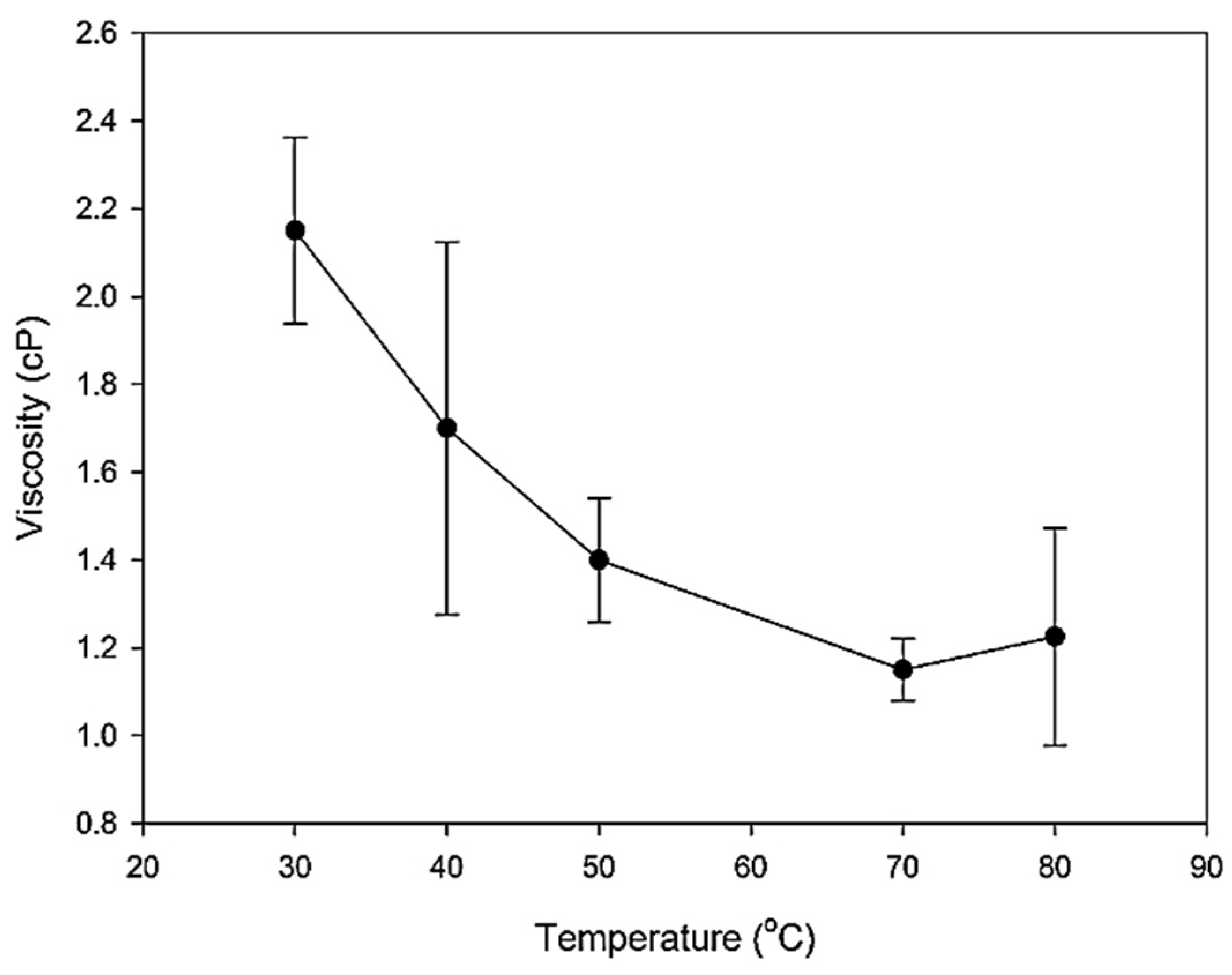
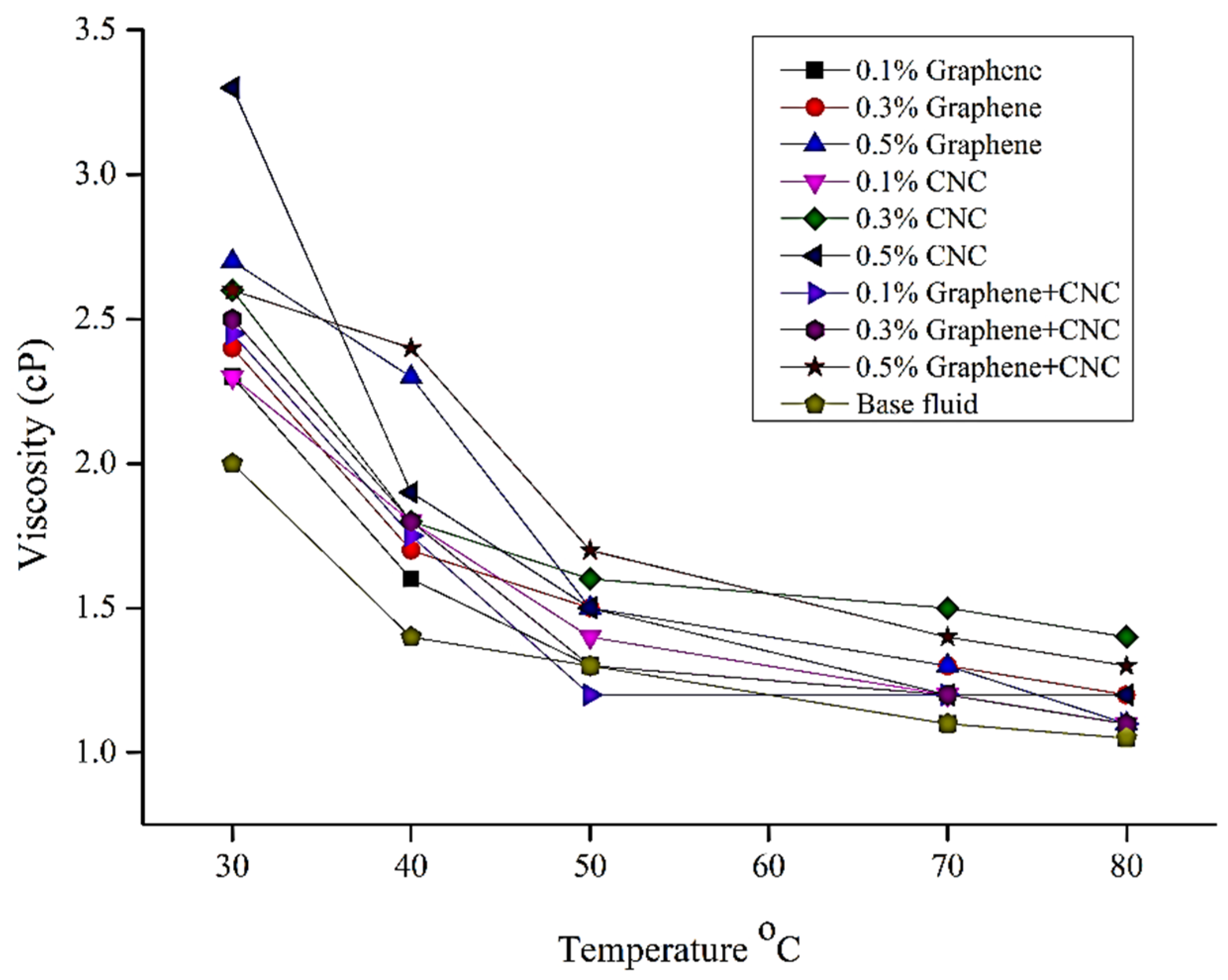
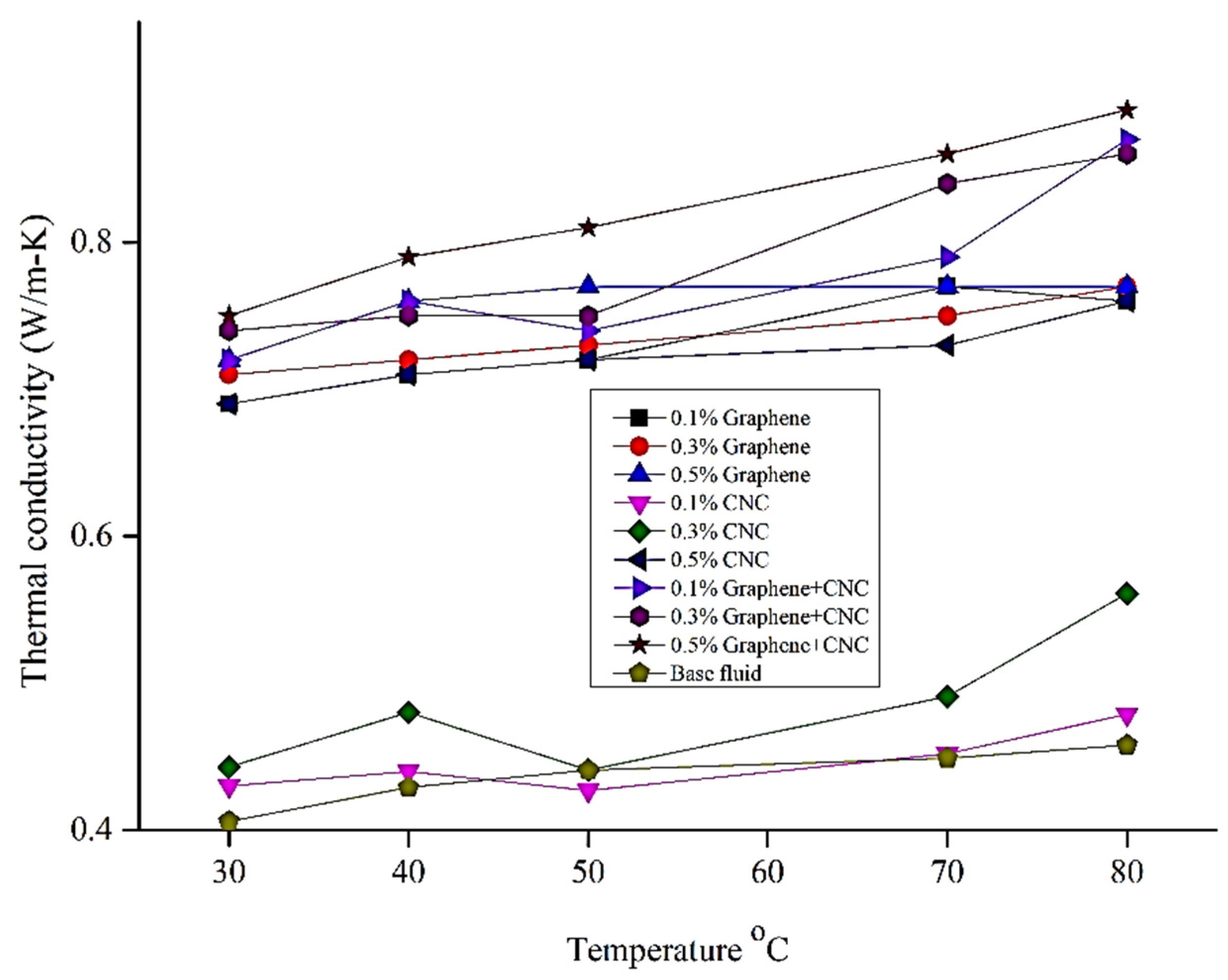
6.2. Thermophysical Experimental Analysis: Viscosity Analysis
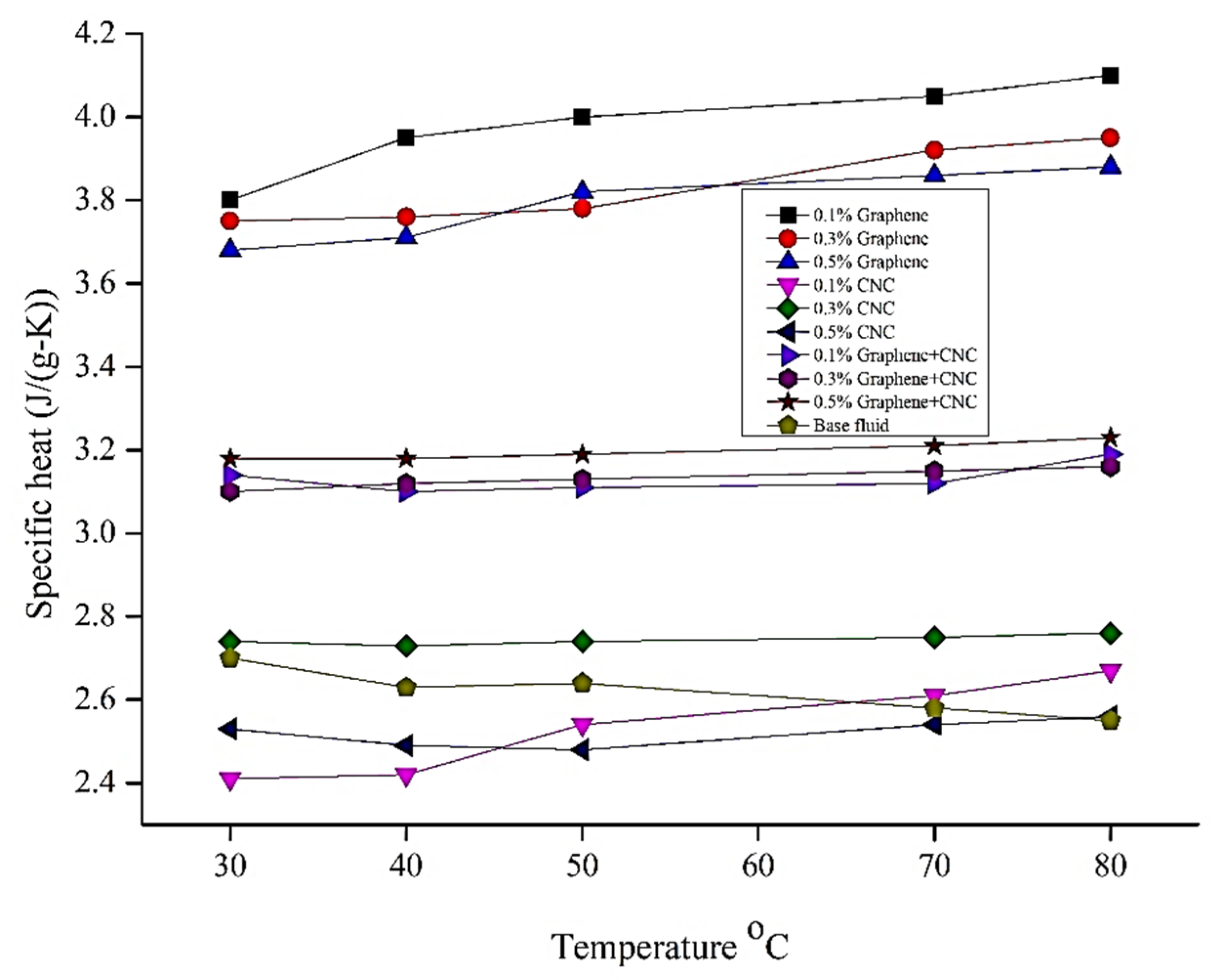
6.3. Specific Heat Analysis
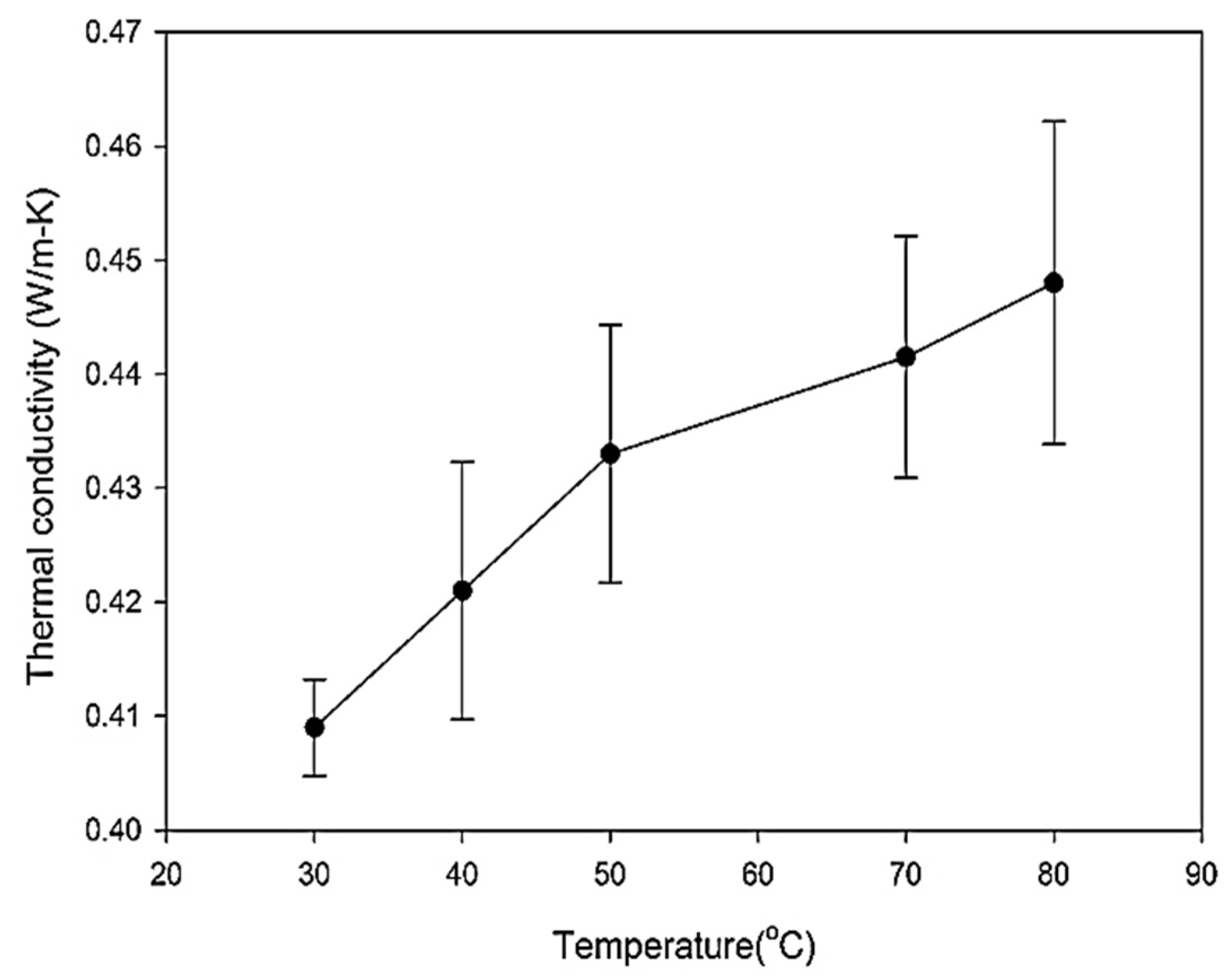
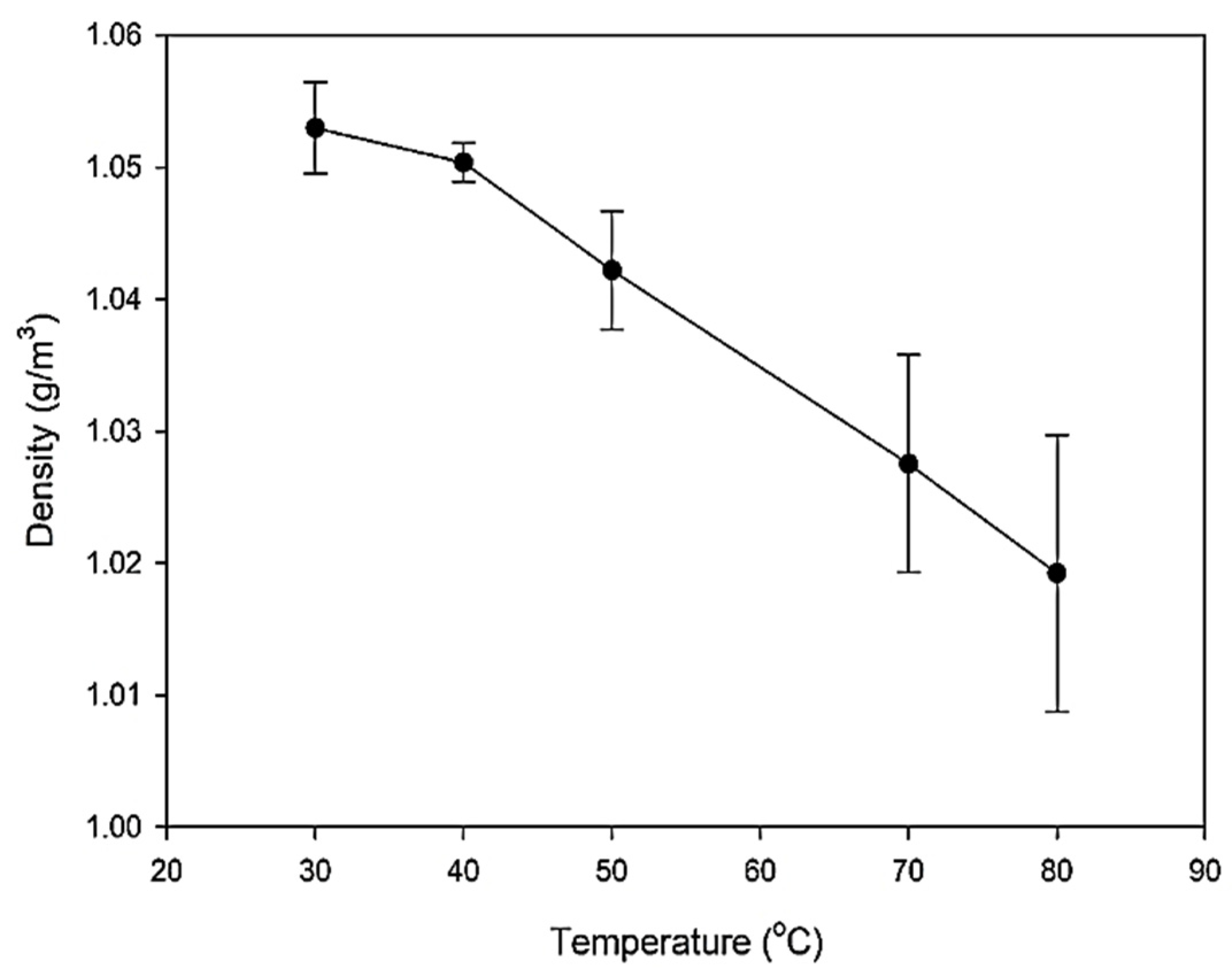
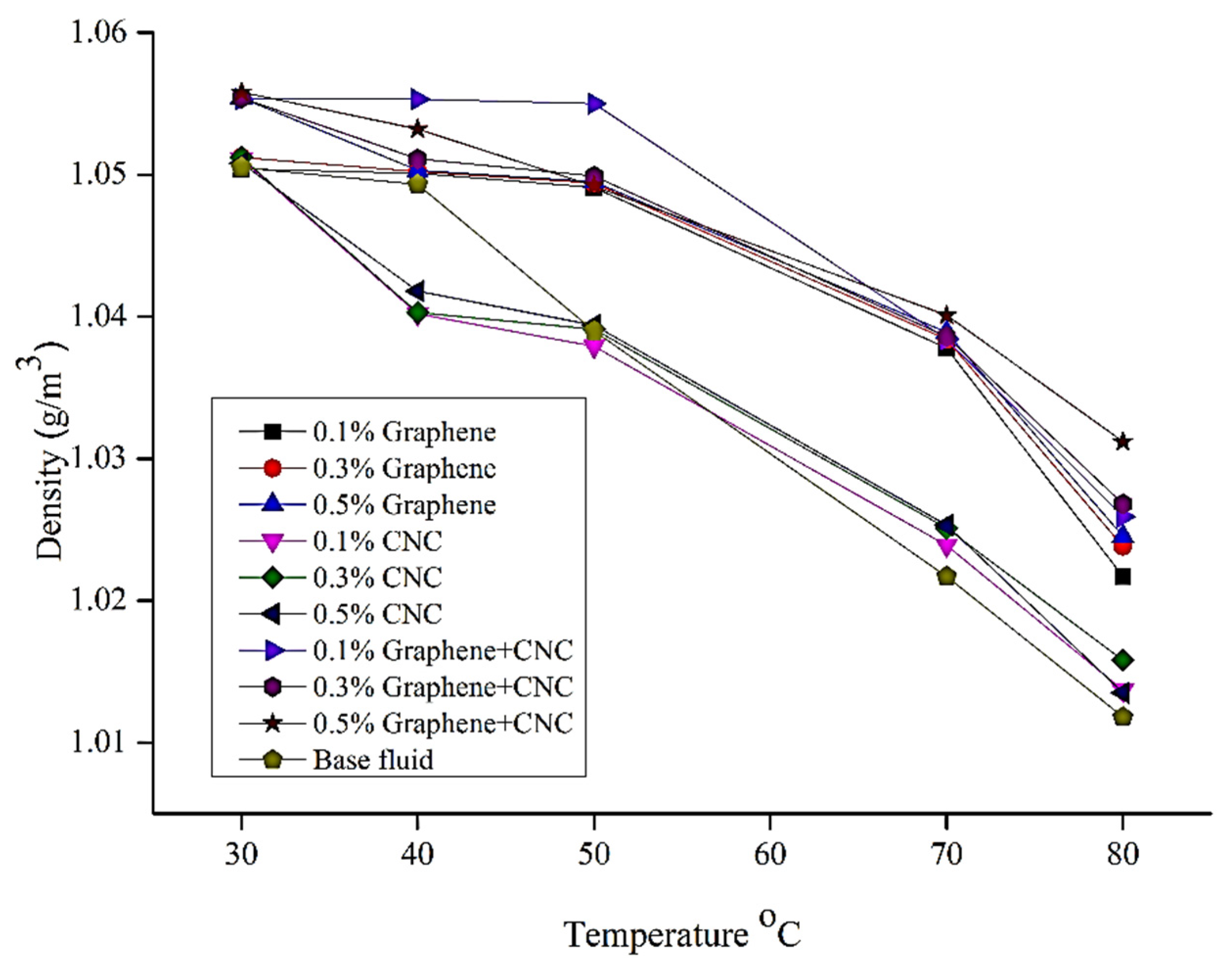
6.4. Density Analysis
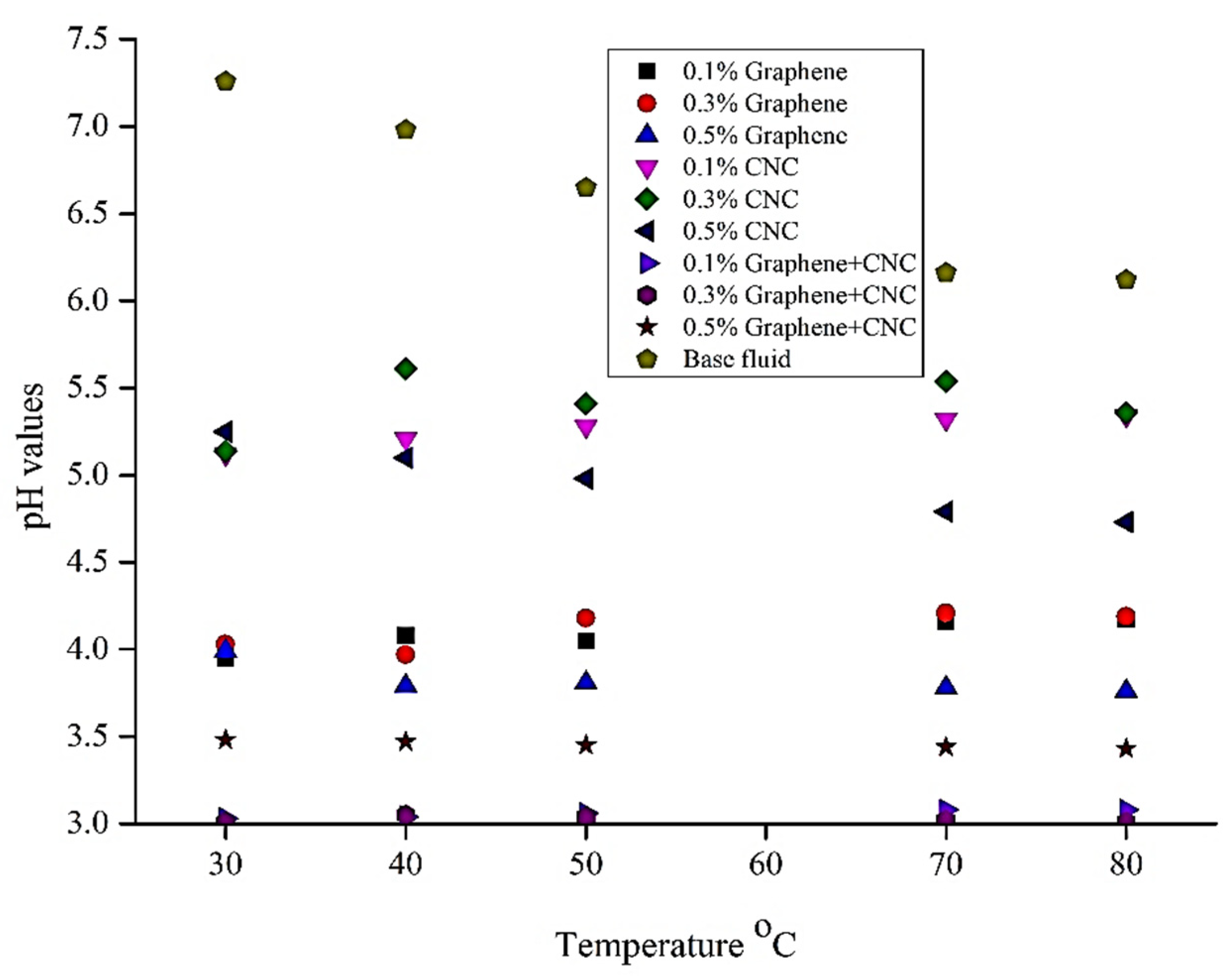
6.5. pH Analysis
7. Performance Assessment
Thermal Performance Assessment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Density (kg/m3 or g/m3) | |
| Volumetric concentration of particles (%) | |
| Specific heat (J/kg-K or J/g-K) | |
| Characteristic linear dimension (m) | |
| Thermal conductivity (Wm−1·K−1) | |
| Viscosity (kg/m. s or cP) | |
| Mass fraction | |
| Energy gain (kW) | |
| Mass flow rate (kg/s) | |
| Incident solar radiation (W/m2) | |
| Area of the solar collector (m2) | |
| Efficiency (%) | |
| Temperature (K or °C) | |
| Characteristic velocity (m/s) | |
| Volumetric flow rate (m3/s) | |
| Cross-sectional area of the tube (m2) |
References
- Kona, A.; Bertoldi, P.; Monforti-Ferrario, F.; Rivas, S.; Dallemand, J.F.J. Covenant of mayors signatories leading the way towards 1.5 degree global warming pathway. Sustain. Cities Soc. 2018, 41, 568–575. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2011; IEA: Paris, France, 2011; Volume 666. [Google Scholar]
- Dong, K.; Dong, X.; Jiang, Q.J. How renewable energy consumption lower global CO2 emissions? Evidence from countries with different income levels. World Econ. 2020, 43, 1665–1698. [Google Scholar] [CrossRef]
- Zhang, X.; Estoque, R.C.; Murayama, Y. An urban heat island study in Nanchang City, China based on land surface temperature and social-ecological variables. Sustain. Cities Soc. 2017, 32, 557–568. [Google Scholar] [CrossRef]
- Jamshidi, M.; Askarzadeh, A. Techno-economic analysis and size optimization of an off-grid hybrid photovoltaic, fuel cell and diesel generator system. Sustain. Cities Soc. 2019, 44, 310–320. [Google Scholar] [CrossRef]
- Maurano, A.; Amatya, R.; Bulovic, V.; Stoner, R. Solar Heating for Residential and Industrial Processes; Working Paper; MIT Energy Initiative: Cambridge, MA, USA, 2015. [Google Scholar]
- Farhana, K.; Kadirgama, K.; Mohammed, H.A.; Ramasamy, D.; Samykano, M.; Saidur, R.J. Analysis of efficiency enhancement of flat plate solar collector using crystal nano-cellulose (CNC) nanofluids. Sustain. Energy Technol. Assess. 2021, 45, 101049. [Google Scholar] [CrossRef]
- Victoria, M.; Haegel, N.; Peters, I.M.; Sinton, R.; Jäger-Waldau, A.; del Cañizo, C.; Breyer, C.; Stocks, M.; Blakers, A.; Kaizuka, I.; et al. Solar photovoltaics is ready to power a sustainable future. Joule 2021, 5, 1041–1056. [Google Scholar] [CrossRef]
- Nazari, M.A.; Maleki, A.; Assad, M.E.H.; Rosen, M.A.; Haghighi, A.; Sharabaty, H.; Chen, L. A review of nanomaterial incorporated phase change materials for solar thermal energy storage. Sol. Energy 2021, 228, 725–743. [Google Scholar] [CrossRef]
- Ceylan, I.; Gürel, A.E.; Ergün, A.; Ali, İ.H.G.; Ağbulut, Ü.; Yıldız, G. A detailed analysis of CPV/T solar air heater system with thermal energy storage: A novel winter season application. J. Build. Eng. 2021, 42, 103097. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Farshad, S.A.; Ebrahimpour, Z.; Said, Z. Recent progress on flat plate solar collectors and photovoltaic systems in the presence of nanofluid: A review. J. Clean. Prod. 2021, 293, 126119. [Google Scholar] [CrossRef]
- Abid, M.; Khan, M.S.; Ratlamwala, T.A.H.; Malik, M.N.; Ali, H.M.; Cheok, Q. Thermodynamic analysis and comparison of different absorption cycles driven by evacuated tube solar collector utilizing hybrid nanofluids. Energy Convers. Manag. 2021, 246, 114673. [Google Scholar] [CrossRef]
- Choi, S.; Zhang, Z.; Yu, W.; Lockwood, F.; Grulke, E.J.A. Anomalous thermal conductivity enhancement in nanotube suspensions. Appl. Phys. Lett. 2001, 79, 2252–2254. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, U.; Li, S.; Thompson, L.; Lee, S.J. Enhanced thermal conductivity through the development of nanofluids. MRS Online Proc. Libr. 1996, 457, 3. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.; Li, S.; Yu, W.; Thompson, L.J.A. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Farhana, K.; Kadirgama, K.; Rahman, M.M.; Ramasamy, D.; Noor, M.M.; Najafi, G.; Samykano, M.; Mahamude, A.S. Improvement in the performance of solar collectors with nanofluids—A state-of-the-art review. Nano-Struct. Nano-Objects 2019, 18, 100276. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Rashidi, A.M.; Shariaty-Niasar, M.J. Effects of surface modification on the dispersion and thermal conductivity of CNT/water nanofluids. Int. Commun. Heat Mass Transf. 2014, 54, 1–7. [Google Scholar] [CrossRef]
- Shanbedi, M.; Heris, S.Z.; Maskooki, A.J. Experimental investigation of stability and thermophysical properties of carbon nanotubes suspension in the presence of different surfactants. J. Therm. Anal. Calorim. 2015, 120, 1193–1201. [Google Scholar] [CrossRef]
- Li, Y.; Xie, H.Q.; Yu, W.; Li, J. Investigation on heat transfer performances of nanofluids in solar collector. Mater. Sci. Forum 2011, 694, 33–36. [Google Scholar] [CrossRef]
- Vakili, M.; Hosseinalipour, S.; Delfani, S.; Khosrojerdi, S.; Karami, M.J. Experimental investigation of graphene nanoplatelets nanofluid-based volumetric solar collector for domestic hot water systems. Sol. Energy 2016, 131, 119–130. [Google Scholar] [CrossRef]
- Natividade, P.S.G.; de Moraes Moura, G.; Avallone, E.; Bandarra Filho, E.P.; Gelamo, R.V.; Gonçalves, J.C. Experimental analysis applied to an evacuated tube solar collector equipped with parabolic concentrator using multilayer graphene-based nanofluids. Renew. Energy 2019, 138, 152–160. [Google Scholar] [CrossRef]
- Mahbubul, I.; Khan, M.M.A.; Ibrahim, N.I.; Ali, H.M.; Al-Sulaiman, F.A.; Saidur, R.J. Carbon nanotube nanofluid in enhancing the efficiency of evacuated tube solar collector. Renew. Energy 2018, 121, 36–44. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Mehrali, M.; Saidur, R.; Mehrali, M.; Latibari, S.T.; Akhiani, A.R.; Metselaar, H.S. A comprehensive review on graphene nanofluids: Recent research, development and applications. Energy Convers. Manag. 2016, 111, 466–487. [Google Scholar] [CrossRef]
- Mahamude, A.S.F.; Harun, W.S.W.; Kadirgama, K.; Farhana, K.; Ramasamy, D.; Samylingam, L.; Aslfattahi, N.J. Thermal performance of nanomaterial in solar collector: State-of-play for graphene. J. Energy Storage 2021, 42, 103022. [Google Scholar] [CrossRef]
- Catania, F.; Marras, E.; Giorcelli, M.; Jagdale, P.; Lavagna, L.; Tagliaferro, A.; Bartoli, M. A review on recent advancements of graphene and graphene-related materials in biological applications. Appl. Sci. 2021, 11, 614. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Fang, X.; Zhang, Z.J. Reduced graphene oxide dispersed nanofluids with improved photo-thermal conversion performance for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2017, 163, 125–133. [Google Scholar] [CrossRef]
- Kumar, P.; Shahzad, F.; Yu, S.; Hong, S.M.; Kim, Y.-H.; Koo, C.M.J. Large-area reduced graphene oxide thin film with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Carbon 2015, 94, 494–500. [Google Scholar] [CrossRef]
- Schwamb, T.; Burg, B.R.; Schirmer, N.C.; Poulikakos, D.J. An electrical method for the measurement of the thermal and electrical conductivity of reduced graphene oxide nanostructures. Nanotechnology 2009, 20, 405704. [Google Scholar] [CrossRef]
- Lu, Z.; Fan, L.; Zheng, H.; Lu, Q.; Liao, Y.; Huang, B.J.B. Preparation, characterization and optimization of nanocellulose whiskers by simultaneously ultrasonic wave and microwave assisted. Bioresour. Technol. 2013, 146, 82–88. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K.J.C. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.; Yano, H.J.A.P.A. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl. Phys. A 2007, 89, 461–466. [Google Scholar] [CrossRef]
- Isogai, A. Wood nanocelluloses: Fundamentals and applications as new bio-based nanomaterials. J. Wood Sci. 2013, 59, 449–459. [Google Scholar] [CrossRef]
- Panthapulakkal, S.; Sain, M.J.I.J. Preparation and characterization of cellulose nanofibril films from wood fibre and their thermoplastic polycarbonate composites. Int. J. Polym. Sci. 2012, 2012, 381342. [Google Scholar] [CrossRef]
- Osong, S.H.; Norgren, S.; Engstrand, P.J.C. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2016, 23, 93–123. [Google Scholar] [CrossRef]
- Gabr, M.H.; Phong, N.T.; Abdelkareem, M.A.; Okubo, K.; Uzawa, K.; Kimpara, I.; Fujii, T.J.C. Mechanical, thermal, and moisture absorption properties of nano-clay reinforced nano-cellulose biocomposites. Cellulose 2013, 20, 819–826. [Google Scholar] [CrossRef]
- Graveson, I.; English, R. Low Energy Method for the Preparation of Non-Derivatized Nanocellulose. U.S. Patent 9,371,401 B2, 21 June 2016. [Google Scholar]
- Mahamude, A.S.F.; Harun, W.S.W.; Kadirgama, K.; Ramasamy, D.; Farhana, K. Cotton waste research follows the effect of pre-treatment and the observation of physical appearance. IOP Conf. Series. Mater. Sci. Eng. 2021, 1078, 012014. [Google Scholar] [CrossRef]
- Lin, J.; Yu, L.; Tian, F.; Zhao, N.; Li, X.; Bian, F.; Wang, J.J.C. Cellulose nanofibrils aerogels generated from jute fibers. Carbohydr. Polym. 2014, 109, 35–43. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Saha, N.R.; Rana, D.; Sarkar, G.; Mollick, M.M.; Chattoapadhyay, A.; Mitra, B.C.; Mondal, D.; Ghosh, S.K.; Chattopadhyay, D. Jute cellulose nano-fibrils/hydroxypropylmethylcellulose nanocomposite: A novel material with potential for application in packaging and transdermal drug delivery system. Ind. Crops Prod. 2018, 112, 633–643. [Google Scholar] [CrossRef]
- Shahabi-Ghahafarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H.J.F. Preparation and characterization of nanocellulose from beer industrial residues using acid hydrolysis/ultrasound. Fibers Polym. 2015, 16, 529–536. [Google Scholar] [CrossRef]
- Vieira, D.C.; Lima, L.N.; Mendes, A.A.; Adriano, W.S.; Giordano, R.C.; Giordano, R.L.; Tardioli, P.W.J. Hydrolysis of lactose in whole milk catalyzed by β-galactosidase from Kluyveromyces fragilis immobilized on chitosan-based matrix. Biochem. Eng. J. 2013, 81, 54–64. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N.J.N. Superior thermal conductivity of single-layer graphene. Nat. Mater. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Bahiraei, M.; Heshmatian, S.J.E.C. Graphene family nanofluids: A critical review and future research directions. Energy Convers. Manag. 2019, 196, 1222–1256. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samori, P.J.C.S.R. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Khan, M.A.Z.; Hassan, A.J.J. Impact of graphene nanofluid and phase change material on hybrid photovoltaic thermal system: Exergy analysis. J. Clean. Prod. 2020, 277, 123370. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.; Mohammed, H.A. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Samylingam, L.; Anamalai, K.; Kadirgama, K.; Samykano, M.; Ramasamy, D.; Noor, M.M.; Najafi, G.; Rahman, M.M.; Xian, H.W.; Sidik, N.A. Thermal analysis of cellulose nanocrystal-ethylene glycol nanofluid coolant. Int. J. Heat Mass Transf. 2018, 127, 173–181. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J.J.C.S.R. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, C.; Gao, H.; Fu, N.; Zhu, M. High Efficient Ethylene Glycol Electrocatalytic Oxidation Based on Bimetallic PtNi on 2D Molybdenum Disulfide/Reduced Graphene Oxide Nanosheets. J. Colloid Interface Sci. 2019, 547, 102–110. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, S.M.; Green, S.; Tompsett, G.A.; Lee, S.H.; Huber, G.W.; Kim, W.B. Highly active and stable PtRuSn/C catalyst for electrooxidations of ethylene glycol and glycerol. Appl. Catal. B Environ. 2011, 101, 366–375. [Google Scholar] [CrossRef]
- Bioucas, F.; Vieira, S.; Lourenço, M.; Santos, F.; De Castro, C.N.J. Performance of heat transfer fluids with nanographene in a pilot solar collector. Sol. Energy 2018, 172, 171–176. [Google Scholar] [CrossRef]
- Sani, E.; Papi, N.; Mercatelli, L.; Żyła, G.J.R.E. Graphite/diamond ethylene glycol-nanofluids for solar energy applications. Renew. Energy 2018, 126, 692–698. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Bao, D.J. Enhanced thermal conductivities of nanofluids containing graphene oxide nanosheets. Nanotechnology 2009, 21, 055705. [Google Scholar] [CrossRef]
- Ramachandran, K.; Hussein, A.; Kadirgama, K.; Ramasamy, D.; Azmi, W.; Tarlochan, F.; Kadirgama, G.J. Thermophysical properties measurement of nano cellulose in ethylene glycol/water. Appl. Therm. Eng. 2017, 123, 1158–1165. [Google Scholar] [CrossRef]
- Sundar, L.S.; Ramana, E.V.; Graça, M.; Singh, M.K.; Sousa, A.C.J.I.C.; Transfer, M. Nanodiamond-Fe3O4 nanofluids: Preparation and measurement of viscosity, electrical and thermal conductivities. Int. Commun. Heat Mass Transf. 2016, 73, 62–74. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Yan, Y.; Reay, D.J. A review on graphene based nanofluids: Preparation, characterization and applications. J. Mol. Liq. 2019, 279, 444–484. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Xie, H.; Tang, D.; Wang, Y.; Li, Z. The preparation, characterization and application of glycol aqueous base graphene oxide nanofluid. MATEC Web Conf. 2018, 238, 02001. [Google Scholar] [CrossRef][Green Version]
- Eletskii, A.V.; Iskandarova, I.M.; Knizhnik, A.A.; Krasikov, D.N.J. Graphene: Fabrication methods and thermophysical properties. Physics-Uspekhi 2011, 54, 227. [Google Scholar] [CrossRef]
- Dovjuu, O.; Kim, S.; Lee, A.; Kim, J.; Noh, J.; Huh, S.; Choi, B.; Jeong, H. A simple approach for heat transfer enhancement of carbon nanofluids in aqueous media. J. Nanosci. Nanotechnol. 2020, 20, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; N’Tsoukpoe, K.E.; Lingai, L.J. Evaluation of a seasonal storage system of solar energy for house heating using different absorption couples. Energy Convers. Manag. 2011, 52, 2427–2436. [Google Scholar] [CrossRef]
- Minardi, J.E.; Chuang, H.N. Performance of a “black” liquid flat-plate solar collector. Sol. Energy 1975, 17, 179–183. [Google Scholar] [CrossRef]
- Li, X.; Zhu, D.; Wang, X.J. Evaluation on dispersion behavior of the aqueous copper nano-suspensions. J. Colloid Interface Sci. 2007, 310, 456–463. [Google Scholar] [CrossRef]
- Yousefi, T.; Veysi, F.; Shojaeizadeh, E.; Zinadini, S.J. An experimental investigation on the effect of Al2O3–H2O nanofluid on the efficiency of flat-plate solar collectors. Renew. Energy 2012, 39, 293–298. [Google Scholar] [CrossRef]
- Faizal, M.; Saidur, R.; Mekhilef, S.; Alim, M.A.J. Energy, economic and environmental analysis of metal oxides nanofluid for flat-plate solar collector. Energy Convers. Manag. 2013, 76, 162–168. [Google Scholar] [CrossRef]
- He, Q.; Zeng, S.; Wang, S.J. Experimental investigation on the efficiency of flat-plate solar collectors with nanofluids. Appl. Therm. Eng. 2015, 88, 165–171. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Fujii, M.J. Effective thermal conductivity and thermal diffusivity of nanofluids containing spherical and cylindrical nanoparticles. Exp. Therm. Fluid Sci. 2007, 31, 593–599. [Google Scholar] [CrossRef]
- Duffie, J.A.; Beckman, W.A. Solar Engineering of Thermal Processes; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Farhana, K.; Kadirgama, K.; Ramasamy, D.; Samykano, M.; Najafi, G.J.J. Experimental Studies on Thermo-Physical Properties of Nanocellulose–Aqueous Ethylene Glycol Nanofluids. J. Adv. Res. Mater. Sci. 2020, 69, 1–15. [Google Scholar] [CrossRef]
- Selvam, C.; Lal, D.M.; Harish, S. Thermal conductivity and specific heat capacity of water–ethylene glycol mixture-based nanofluids with graphene nanoplatelets. J. Therm. Anal. Calorim. 2017, 129, 947–955. [Google Scholar] [CrossRef]
- Vajjha, R.; Das, D.; Mahagaonkar, B. Density measurement of different nanofluids and their comparison with theory. Pet. Sci. Technol. 2009, 27, 612–624. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Long, Q.; Wen, X.; Zhou, Y.; Li, L. Influence of pH on the stability characteristics of nanofluids. In Proceedings of the 2009 Photonics and Optoelectronics, Wuhan, China, 14–16 August 2009. [Google Scholar] [CrossRef]
- Yousefi, T.; Shojaeizadeh, E.; Veysi, F.; Zinadini, S.J. An experimental investigation on the effect of pH variation of MWCNT–H2O nanofluid on the efficiency of a flat-plate solar collector. Sol. Energy 2012, 86, 771–779. [Google Scholar] [CrossRef]
- Adio, S.A.; Sharifpur, M.; Meyer, J.P. Factors affecting the pH and electrical conductivity of MgO–ethylene glycol nanofluids. Bull. Mater. Sci. 2015, 38, 1345–1357. [Google Scholar] [CrossRef]
- Jia-Fei, Z.; Zhong-Yang, L.; Ming-Jiang, N.; Ke-Fa, C. Dependence of nanofluid viscosity on particle size and pH value. Chin. Phys. Lett. 2009, 26, 066202. [Google Scholar] [CrossRef]
- Xie, H.; Wang, J.; Xi, T.; Liu, Y.; Ai, F.; Wu, Q. Thermal conductivity enhancement of suspensions containing nanosized alumina particles. J. Appl. Phys. 2002, 91, 4568–4572. [Google Scholar] [CrossRef]
- Goudarzi, K.; Nejati, F.; Shojaeizadeh, E.; Yousef-abad, S.A. Experimental study on the effect of pH variation of nanofluids on the thermal efficiency of a solar collector with helical tube. Exp. Therm. Fluid Sci. 2015, 60, 20–27. [Google Scholar] [CrossRef]
- Sundar, L.S.; Mesfin, S.; Said, Z.; Singh, M.K.; Punnaiah, V.; Sousa, A.C. Energy, economic, environmental and heat transfer analysis of a solar flat-plate collector with pH-treated Fe 3 O 4/water nanofluid. Int. J. Energy A Clean Environ. 2021, 22, 55–98. [Google Scholar] [CrossRef]
- Farhana, K.; Mahamude, A.J. Experimental Analysis of pH of Nanofluids in Different Conditions. J. Eng. Adv. Mater. Appl. 2020, 1, 1–5. [Google Scholar]
- Akram, N.; Sadri, R.; Kazi, S.N.; Ahmed, S.M.; Zubir, M.N.; Ridha, M.; Soudagar, M.; Ahmed, W.; Arzpeyma, M.; Tong, G.B. An experimental investigation on the performance of a flat-plate solar collector using eco-friendly treated graphene nanoplatelets–water nanofluids. J. Therm. Anal. Calorim. 2019, 138, 609–621. [Google Scholar] [CrossRef]
- Verma, S.K.; Sharma, K.; Gupta, N.K.; Soni, P.; Upadhyay, N.J.E. Performance comparison of innovative spiral shaped solar collector design with conventional flat plate solar collector. Energy 2020, 194, 116853. [Google Scholar] [CrossRef]
- Gaos, Y.S.; Yulianto, M.; Juarsa, M.; Nurrohman; Marzuki, E.; Yuliaji, D.; Budiono, K. The performance of solar collector CPC (compound parabolic concentrator) type with three pipes covered by glass tubes. AIP Conf. Proc. 2017, 1826, 020022. [Google Scholar]
- Suganthi, K.; Rajan, K. Metal oxide nanofluids: Review of formulation, thermo-physical properties, mechanisms, and heat transfer performance. Renew. Sustain. Energy Rev. 2017, 76, 226–255. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Lin, Y.; Feng, M.; Wu, Q.J. Stability, thermal conductivity, and rheological properties of controlled reduced graphene oxide dispersed nanofluids. Appl. Therm. Eng. 2017, 119, 132–139. [Google Scholar] [CrossRef]
- Khetib, Y.; Alahmadi, A.; Alzaed, A.; Sharifpur, M.; Cheraghian, G.; Siakachoma, C. Simulation of a parabolic trough solar collector containing hybrid nanofluid and equipped with compound turbulator to evaluate exergy efficacy and thermal-hydraulic performance. Energy Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Alanazi, A.K.; Khetib, Y.; Abo-Dief, H.M.; Rawa, M.; Cheraghian, G.; Sharifpur, M. The effect of nanoparticle shape on alumina/EG-water (50:50) nanofluids flow within a solar collector: Entropy and exergy investigation. Case Stud. Therm. Eng. 2021, 28, 101510. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S.J. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Meibodi, S.S.; Kianifar, A.; Niazmand, H.; Mahian, O.; Wongwises, S. Experimental investigation on the thermal efficiency and performance characteristics of a flat plate solar collector using SiO2/EG–water nanofluids. Int. Commun. Heat Mass Transf. 2015, 65, 71–75. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, C.; Tang, Y.; Wang, J.; Ren, B.J. Preparation and thermal conductivity of suspensions of graphite nanoparticles. Carbon 2007, 45, 226–228. [Google Scholar] [CrossRef]
- Ziyadanogullari, N.B.; Yucel, H.; Yildiz, C. Thermal performance enhancement of flat-plate solar collectors by means of three different nanofluids. Therm. Sci. Eng. Prog. 2018, 8, 55–65. [Google Scholar] [CrossRef]
- Ghaderian, J.; Sidik, N.A.; Kasaeian, A.; Ghaderian, S.; Okhovat, A.; Pakzadeh, A.; Samion, S.; Yahya, W.J. Performance of copper oxide/distilled water nanofluid in evacuated tube solar collector (ETSC) water heater with internal coil under thermosyphon system circulations. Appl. Therm. Eng. 2017, 121, 520–536. [Google Scholar] [CrossRef]


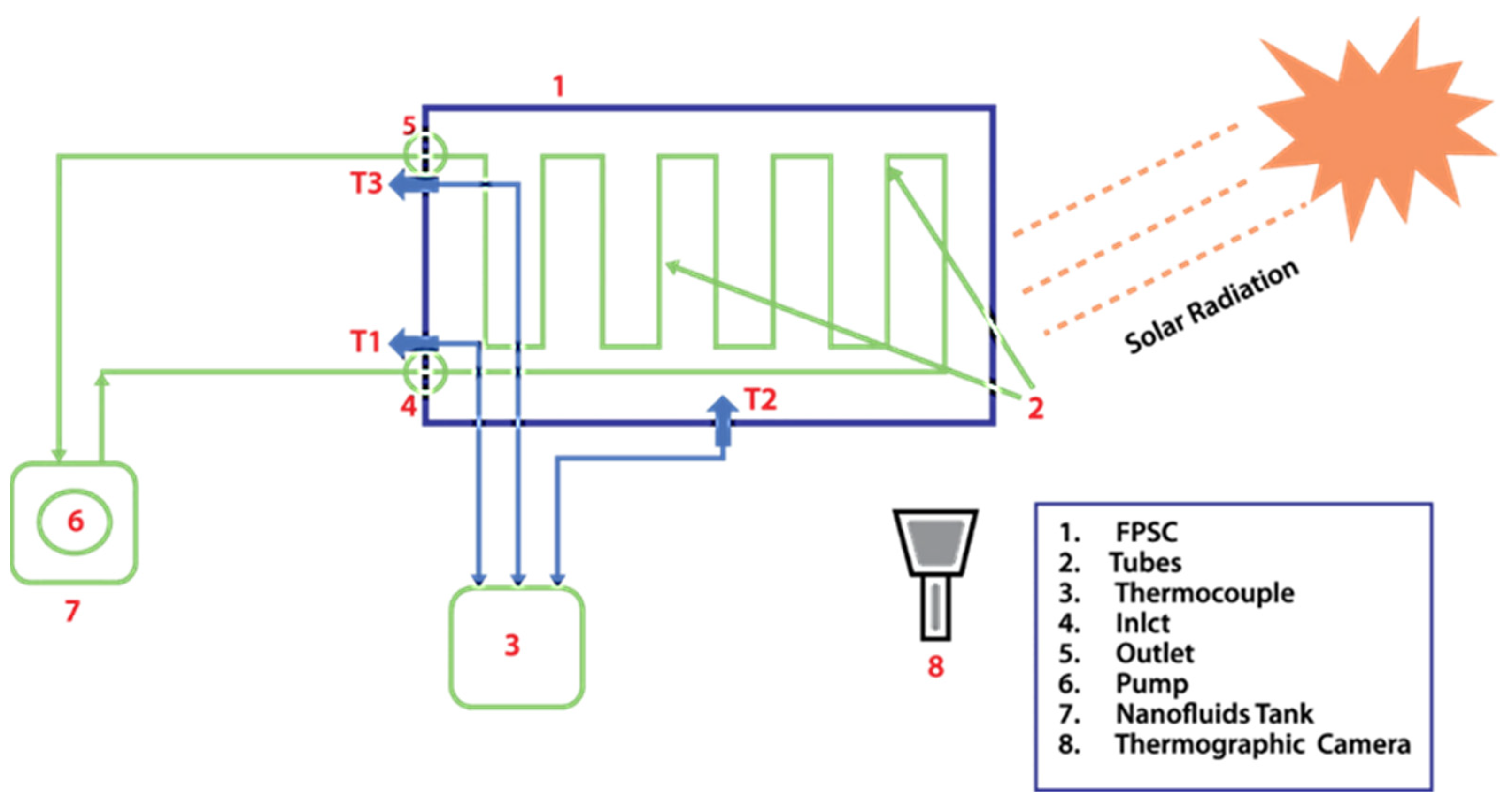


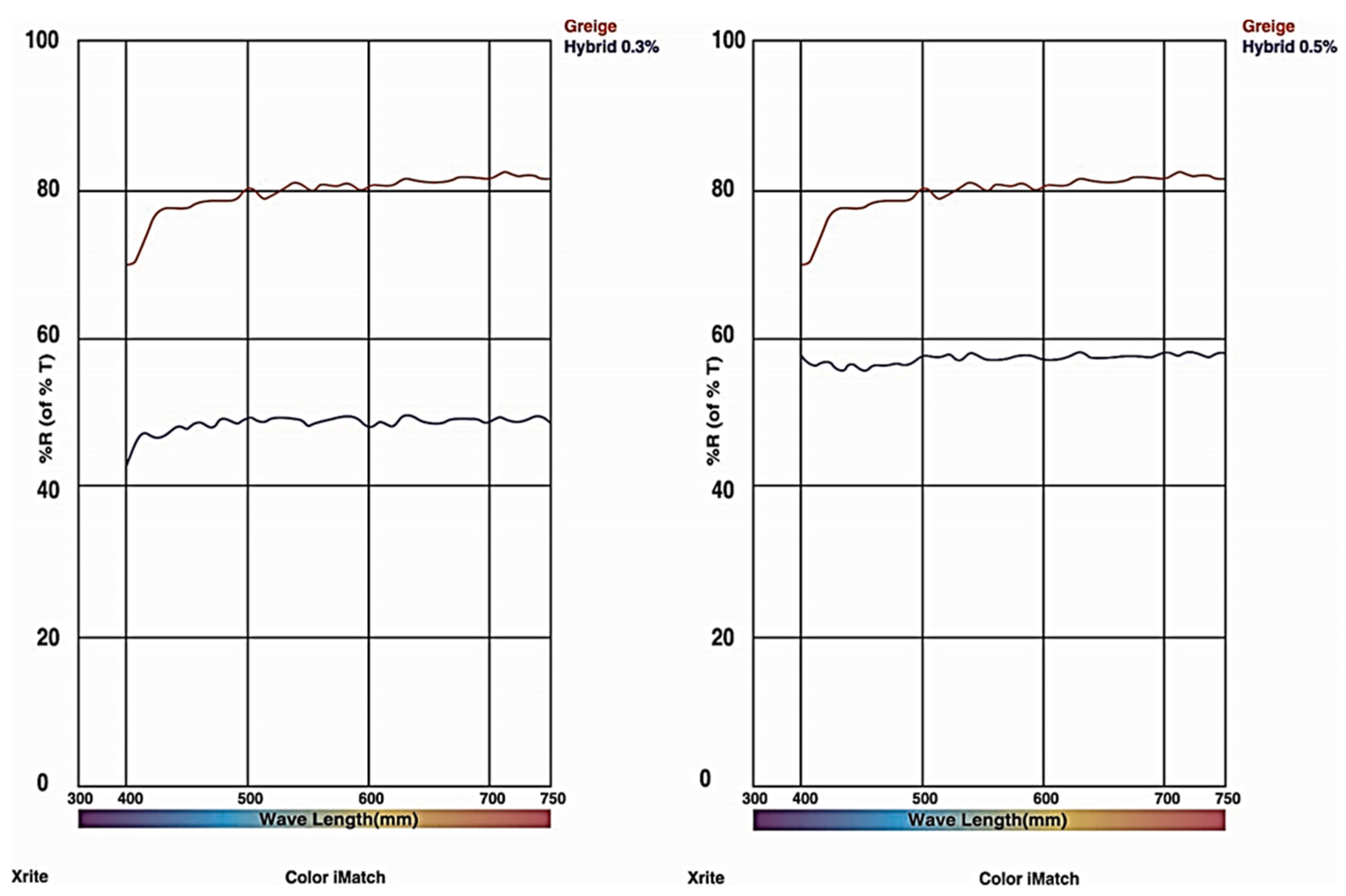
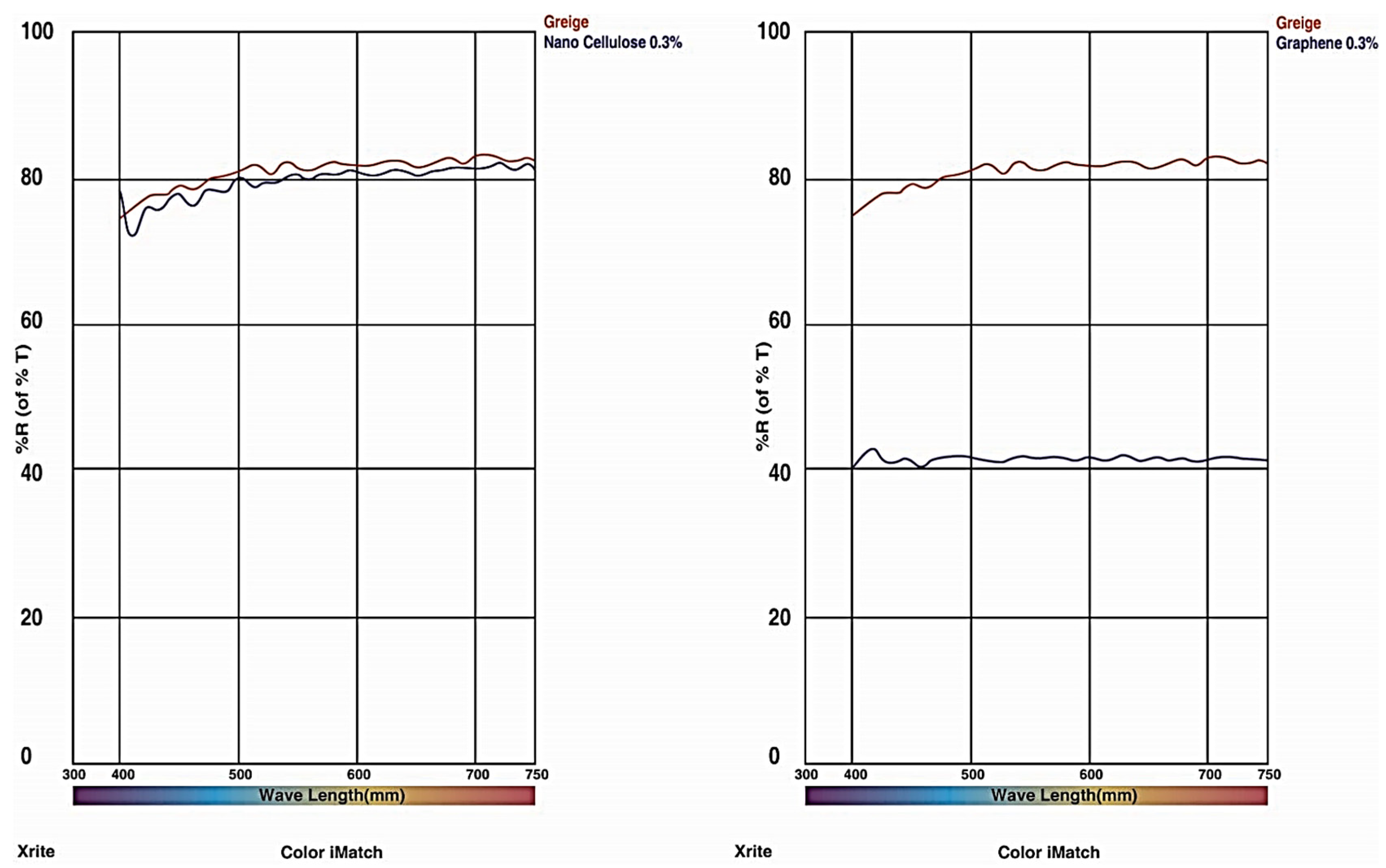

| Serial. no | Name of the Apparatus | Characteristics | Number of Apparatus |
|---|---|---|---|
| 1 | Frame | Wooden | One |
| 2 | Tubes | Copper; outer diameter 12.7 mm; inner diameter 12.5 mm; length 1 m | Ten |
| 3 | Thermocouples | Sensing element: Coiled bimetallic | Three |
| 4 | Pump (Dolphin PA500) | Electrical submersible filter | One |
| 5 | Bucket | Plastic | One |
| Parameters | Cp (J/kg-k) | (Kg/s) | Tout °C | Tin °C | Efficiency % |
|---|---|---|---|---|---|
| Base fluid | 2700 at 30 °C | 0.0106 | 41.3 | 40.1 | 4.2 |
| 0.3% Graphene | 3750 at 30 °C | 42.79 | 40 | 13.7 | |
| 0.5% Graphene | 3680 at 30 °C | 40.72 | 38.36 | 11.37 | |
| 0.3% CNC | 2740 at 30 °C | 41.84 | 37.17 | 16.76 | |
| 0.5% CNC | 2530 at 30 °C | 40.17 | 37.17 | 9.94 | |
| 0.3% (CNC+graphene | 3100 at 30 °C | 42.34 | 39.17 | 12.87 | |
| 0.5% (CNC+graphene) | 3180 at 30 °C | 42.92 | 39.17 | 15.62 | |
| Base fluid | 2630 at 40 °C | 41.3 | 40.1 | 4.13 | |
| 0.3% Graphene | 3760 at 40 °C | 42.79 | 40 | 13.74 | |
| 0.5% Graphene | 3710 at 40 °C | 40.72 | 38.36 | 11.47 | |
| 0.3% CNC | 2730 at 40 °C | 41.84 | 37.17 | 16.7 | |
| 0.5% CNC | 2490 at 40 °C | 40.17 | 37.17 | 9.78 | |
| 0.3% (CNC+graphene) | 3120 at 40 °C | 42.34 | 39.17 | 12.95 | |
| 0.5% (CNC+graphene) | 3180 at 40 °C | 42.92 | 39.17 | 15.62 | |
| Base fluid | 2640 at 50 °C | 41.3 | 40.1 | 4.15 | |
| 0.3% Graphene | 3780 at 50 °C | 42.79 | 40 | 13.81 | |
| 0.5% Graphene | 3820 at 50 °C | 40.72 | 38.36 | 11.81 | |
| 0.3% CNC | 2740 at 50 °C | 41.84 | 37.17 | 16.76 | |
| 0.5% CNC | 2780 at 50 °C | 40.17 | 37.17 | 9.74 | |
| 0.3% (CNC+graphene) | 3130 at 50 °C | 42.34 | 39.17 | 12.99 | |
| 0.5% (CNC+graphene) | 3190 at 50 °C | 42.92 | 39.17 | 15.66 | |
| Base fluid | 2580 at 70 °C | 41.3 | 40.1 | 4.05 | |
| 0.3% Graphene | 3920 at 70 °C | 42.79 | 40 | 14.32 | |
| 0.5% Graphene | 3860 at 70 °C | 40.72 | 38.36 | 11.93 | |
| 0.3% CNC | 2750 at 70 °C | 41.84 | 37.17 | 16.82 | |
| 0.5% CNC | 2540 at 70 °C | 40.17 | 37.17 | 9.98 | |
| 0.3% (CNC+graphene) | 3150 at 70 °C | 42.34 | 39.17 | 13.08 | |
| 0.5% (CNC+graphene) | 3210 at 70 °C | 42.92 | 39.17 | 15.76 | |
| Base fluid | 2250 at 80 °C | 41.3 | 40.1 | 4.007 | |
| 0.3% Graphene | 3950 at 80 °C | 42.79 | 40 | 14.43 | |
| 0.5% Graphene | 3880 at 80 °C | 40.72 | 38.36 | 11.99 | |
| 0.3% CNC | 2760 at 80 °C | 41.84 | 37.17 | 16.88 | |
| 0.5% CNC | 2560 at 80 °C | 40.17 | 37.17 | 10.06 | |
| 0.3% (CNC+graphene) | 3160 at 80 °C | 42.34 | 39.17 | 13.12 | |
| 0.5% (CNC+graphene) | 3230 at 80 °C | 42.92 | 39.17 | 15.86 |
| Parameters | Temperature °C | Thermal Conductivity Wm−1·K−1 | Viscosity (Cp) | Specific Heat (J/g-k) | Density (g/m3) |
|---|---|---|---|---|---|
| Base fluid | 30 | 0.406 | 2 | 2.7 | 1.0505 |
| 40 | 0.429 | 1.4 | 2.63 | 1.0493 | |
| 50 | 0.441 | 1.3 | 2.64 | 1.039 | |
| 70 | 0.449 | 1.1 | 2.58 | 1.0217 | |
| 80 | 0.458 | 1.05 | 2.55 | 1.0118 | |
| 0.3% CNC | 30 | 0.443 | 2.74 | 2.74 | 1.0512 |
| 40 | 0.48 | 2.73 | 2.73 | 1.0403 | |
| 50 | 0.441 | 2.74 | 2.74 | 1.0391 | |
| 70 | 0.491 | 2.75 | 2.75 | 1.0251 | |
| 80 | 0.561 | 2.76 | 2.76 | 1.0158 | |
| 0.5% CNC | 30 | 0.69 | 2.53 | 2.53 | 1.0508 |
| 40 | 0.71 | 2.49 | 2.49 | 1.0418 | |
| 50 | 0.72 | 2.48 | 2.48 | 1.0394 | |
| 70 | 0.73 | 2.54 | 2.54 | 1.0253 | |
| 80 | 0.76 | 2.56 | 2.56 | 1.0135 | |
| 0.3% graphene | 30 | 0.71 | 2.4 | 3.75 | 1.0512 |
| 40 | 0.72 | 1.7 | 3.76 | 1.0502 | |
| 50 | 0.73 | 1.5 | 3.78 | 1.0494 | |
| 70 | 0.75 | 1.3 | 3.92 | 1.0384 | |
| 80 | 0.77 | 1.2 | 3.95 | 1.0238 | |
| 0.5% graphene | 30 | 0.72 | 2.7 | 3.68 | 1.0554 |
| 40 | 0.76 | 2.3 | 3.71 | 1.0503 | |
| 50 | 0.77 | 1.5 | 3.82 | 1.0495 | |
| 70 | 0.77 | 1.3 | 3.86 | 1.0389 | |
| 80 | 0.77 | 1.1 | 3.88 | 1.0245 | |
| 0.3% hybrid (CNC+graphene) | 30 | 0.74 | 3.1 | 3.1 | 1.0554 |
| 40 | 0.75 | 3.12 | 3.12 | 1.0511 | |
| 50 | 0.75 | 3.13 | 3.13 | 1.0499 | |
| 70 | 0.84 | 3.15 | 3.15 | 1.0386 | |
| 80 | 0.86 | 3.16 | 3.16 | 1.0268 | |
| 0.5% hybrid (CNC+graphene) | 30 | 0.75 | 3.18 | 3.18 | 1.0558 |
| 40 | 0.79 | 3.18 | 3.18 | 1.0532 | |
| 50 | 0.81 | 3.19 | 3.19 | 1.0492 | |
| 70 | 0.86 | 3.21 | 3.21 | 1.0401 | |
| 80 | 0.89 | 3.23 | 3.23 | 1.0312 |
| Nanofluids | Efficiency (%) Comparison | ||
|---|---|---|---|
| Experimental Results | Farhana, Kadirgama, Mohammed, Ramasamy, Samykano and Saidur [7] | Meibodi, Kianifar, Niazmand, Mahian and Wongwises [87] | |
| CNC/water-EG | 16.88 | 8.46 | - |
| SiO2/water-EG | - | - | 8 |
| Al2O3/water-EG | - | 2.48 | - |
| Graphene/water-EG | 11.99 | - | - |
| Hybrid/water-EG | 15.86 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahamude, A.S.F.; Harun, W.S.W.; Kadirgama, K.; Ramasamy, D.; Farhana, K.; Saleh, K.; Yusaf, T. Experimental Study on the Efficiency Improvement of Flat Plate Solar Collectors Using Hybrid Nanofluids Graphene/Waste Cotton. Energies 2022, 15, 2309. https://doi.org/10.3390/en15072309
Mahamude ASF, Harun WSW, Kadirgama K, Ramasamy D, Farhana K, Saleh K, Yusaf T. Experimental Study on the Efficiency Improvement of Flat Plate Solar Collectors Using Hybrid Nanofluids Graphene/Waste Cotton. Energies. 2022; 15(7):2309. https://doi.org/10.3390/en15072309
Chicago/Turabian StyleMahamude, Abu Shadate Faisal, Wan Sharuzi Wan Harun, Kumaran Kadirgama, Devarajan Ramasamy, Kaniz Farhana, Khalid Saleh, and Talal Yusaf. 2022. "Experimental Study on the Efficiency Improvement of Flat Plate Solar Collectors Using Hybrid Nanofluids Graphene/Waste Cotton" Energies 15, no. 7: 2309. https://doi.org/10.3390/en15072309
APA StyleMahamude, A. S. F., Harun, W. S. W., Kadirgama, K., Ramasamy, D., Farhana, K., Saleh, K., & Yusaf, T. (2022). Experimental Study on the Efficiency Improvement of Flat Plate Solar Collectors Using Hybrid Nanofluids Graphene/Waste Cotton. Energies, 15(7), 2309. https://doi.org/10.3390/en15072309








