Review on Material and Design of Anode for Microbial Fuel Cell
Abstract
:1. Introduction
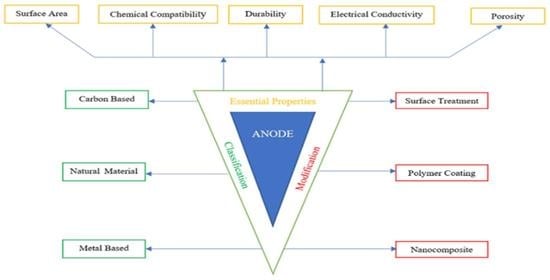
2. Role of Anode Material and Characterization
2.1. Essential Properties of Anode
2.1.1. Surface Area
2.1.2. Chemical Compatibility
2.1.3. Durability
2.1.4. Electrical Conductivity
2.1.5. Porosity
3. Classification of Anode
3.1. Carbon Based Anode
3.2. Natural Material Based Anode
3.3. Metal-Based Anode
4. Modification of Anode Material
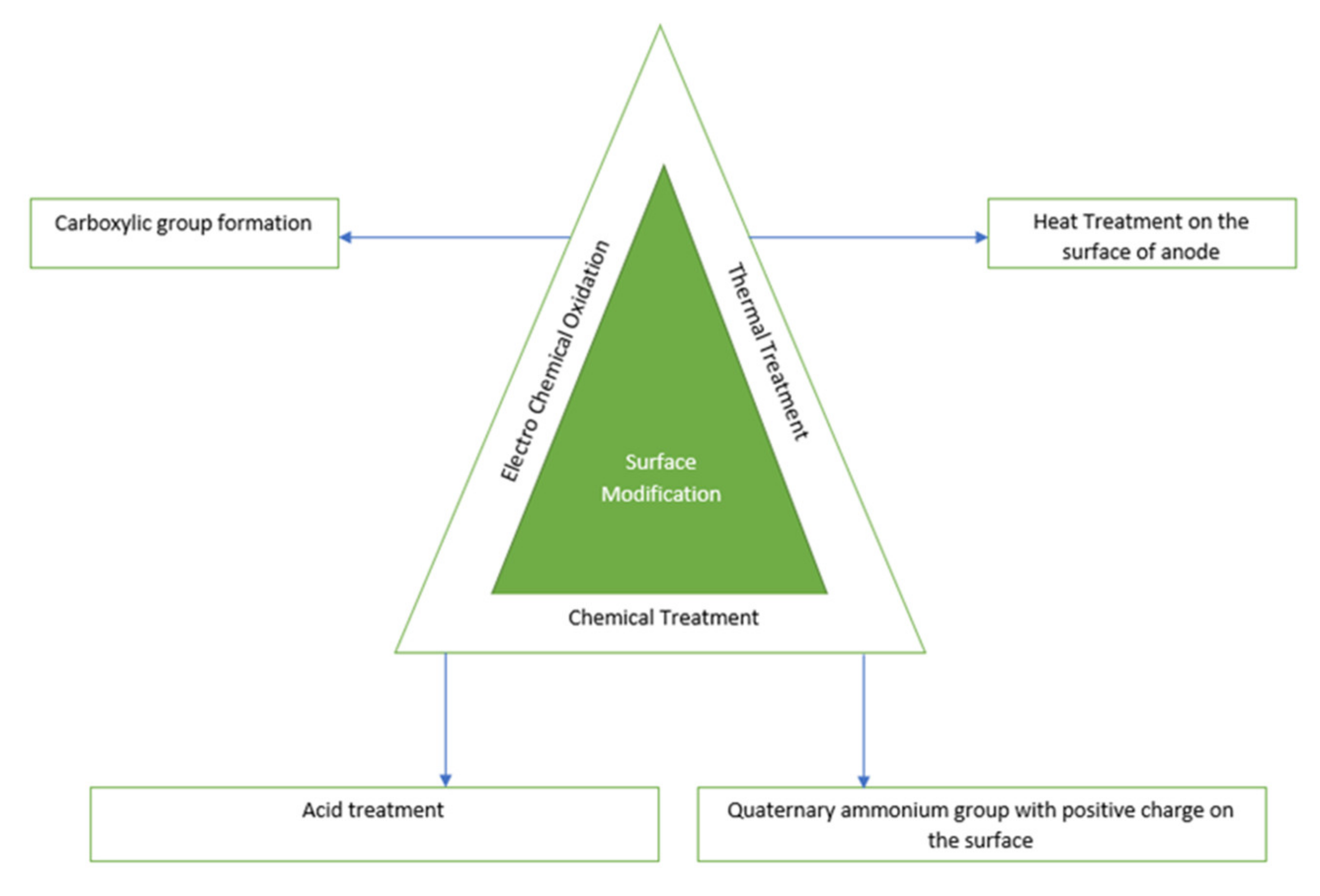
4.1. Surface Treatment of Anode
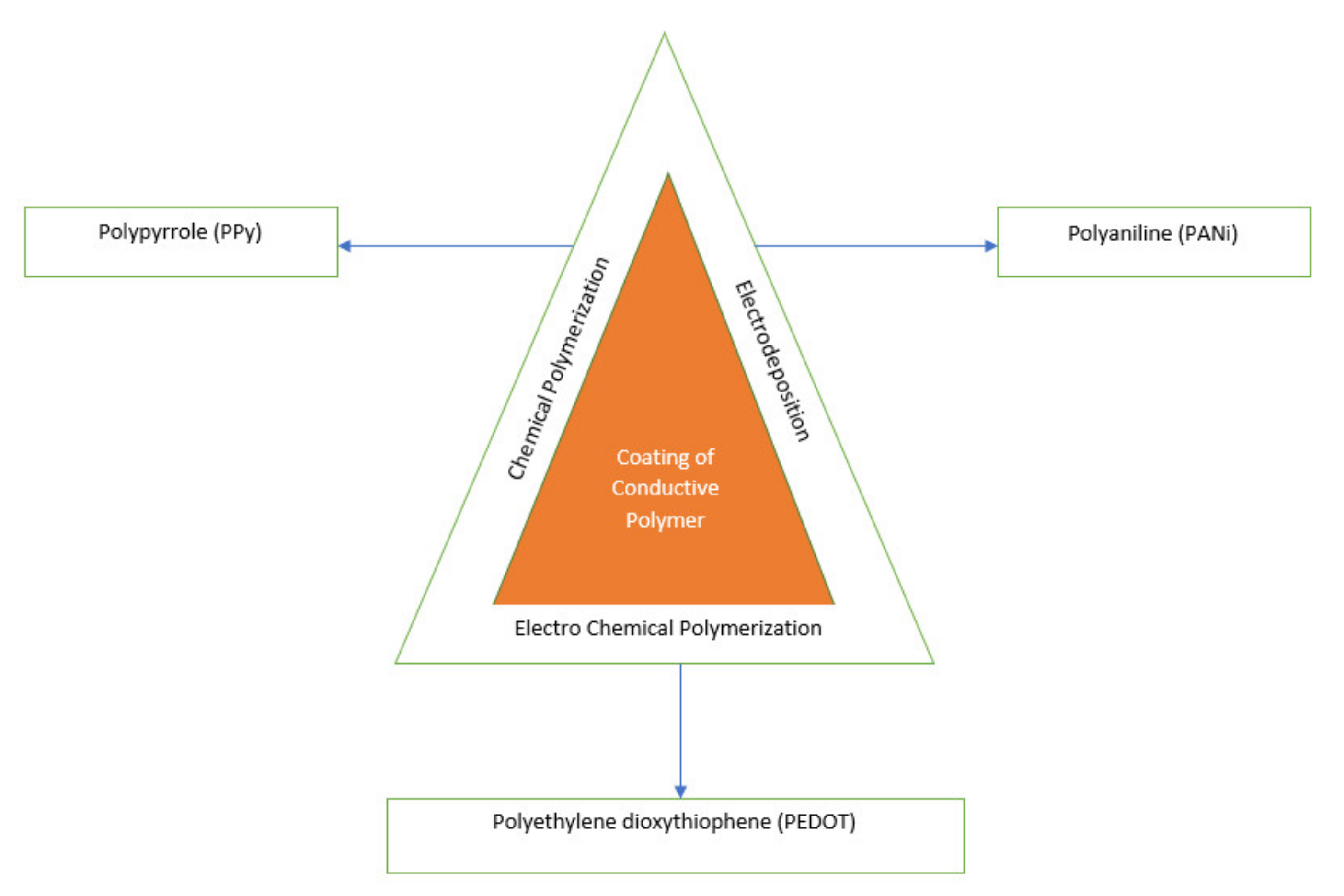
4.2. Coating of Anode Material
5. Future Perspective
6. Conclusions
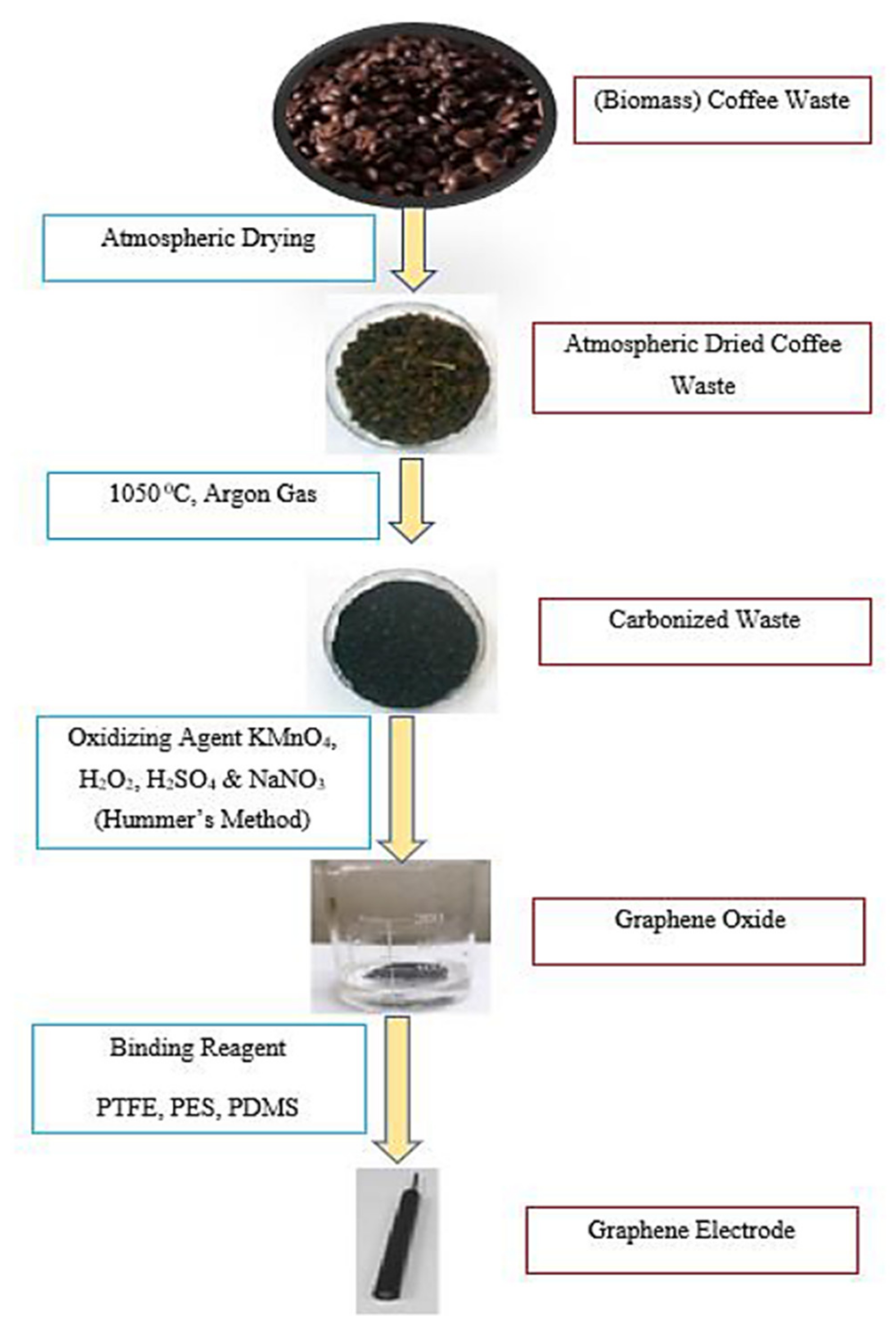
- Carbon-based materials are proved to be very good as an anode. Biocompatibility and electrical conductivity are the two most important properties of the anode, which primariessentially controls the system’s performance. The surface area of the electrode should be maximized to enhance the formation of the biofilm. The use of commercially available graphene oxide as an anode showed excellent results. However, graphene oxides are very expensive and may not be suitable for large-scale and commercial applications.
- Among all the past research work, biomass-derived anode electrodes are very attractive in terms of the low cost, high surface area, good bacterial adhesion, and compatibility with microbial activities. However, the major drawback of natural waste-derived materials is poor electrical conductivity and durability.
- To enhance the conductivity of the biomass-derived anode materials, doping of metal oxide and hybridization of the copolymer and different surface treatments may be needed. From the past research studies on the modification of anode, the most popular and effective method used by researchers to fabricate anodes was Hummer’s method which indicated the effectiveness of graphene materials derived from various natural biowastes.
- The electrodeposition, chemical polymerization and electrochemical polymerization techniques for making conductive polymer-based anodes are very good in terms of eco-friendliness, large surface area, and chemical stability. Still, the major drawback of these materials is the risk of shedding of polymers and not suitable physical properties that affect the electrical conductivity.
- At the same time, carbon-based brush type material and carbon nanotubes are also exhibited good performance by increasing the surface area of the electrode in large-scale operation but lagged in conductive properties. Often the sharp edge of the brush can rupture the cell wall of microorganisms, which leads to overall poor performance. Further research work should be carried out with more focus on improving the mechanical strength of graphene by combining with metal oxides or with some composite copolymers, followed by giving them a brush or nanotubes type architecture. These type of materials can lead to a new generation of the anode to acquire a combined effect. These modifications can be tested on a small scale with proper optimization and can be scaled up to use in commercial applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Banerjee, A.; Calay, R.K.; Eregno, F.E. Role and Important Properties of a Membrane with Its Recent Advancement in a Microbial Fuel Cell. Energies 2022, 15, 444. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Cai, T.; Meng, L.; Chen, G.; Xi, Y.; Jiang, N.; Song, J.; Zheng, S.; Liu, Y.; Zhen, G.; Huang, M. Application of advanced anodes in microbial fuel cells for power generation: A review. Chemosphere 2020, 248, 125985. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Han, S.K.; Liu, H. Improved performance of CEA microbial fuel cells with increased reactor size. Energy Environ. Sci. 2012, 5, 8273–8280. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Wang, Z.-B.; Ge, M.; Xiong, S.-C.; Zhu, X.-Q. Preparation of graphene/polyaniline-modified carbon nanotubes and their electrochemical properties in microbial fuel cell. Ionics 2017, 23, 1197–1202. [Google Scholar] [CrossRef]
- Scott, K. Membranes and separators for microbial fuel cells. In Microbial Electrochemical and Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–178. [Google Scholar]
- Rozendal, R.A.; Hamelers, H.V.M.; Buisman, C.J.N. Effects of Membrane Cation Transport on pH and Microbial Fuel Cell Performance. Environ. Sci. Technol. 2006, 40, 5206–5211. [Google Scholar] [CrossRef]
- Das, D. Microbial Fuel Cell; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Liu, H.; Logan, B. Electricity generation using an air-cathode single chamber microbial fuel cell (MFC) in the absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, M.; Gu, C. Advanced Nanomaterials for the Design and Construction of Anode for Microbial Fuel Cells. In Advanced Electrode Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 457–483. [Google Scholar]
- Dumas, C.; Mollica, A.; Féron, D.; Basseguy, R.; Etcheverry, L.; Bergel, A. Marine microbial fuel cell: Use of stainless steel electrodes as anode and cathode materials. Electrochim. Acta 2007, 53, 468–473. [Google Scholar] [CrossRef] [Green Version]
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Roubaud, E.; Lacroix, R.; Da Silva, S.; Esvan, J.; Etcheverry, L.; Bergel, A.; Basséguy, R.; Erable, B. Industrially scalable surface treatments to enhance the current density output from graphite bioanodes fueled by real domestic wastewater. iScience 2021, 24, 102162. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): An overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Thomas, A. Carbon-Based Microbial-Fuel-Cell Electrodes: From Conductive Supports to Active Catalysts. Adv. Mater. 2017, 29, 1602547. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Sakai, K.; Iwamura, S.; Sumida, R.; Ogino, I.; Mukai, S.R. Carbon Paper with a High Surface Area Prepared from Carbon Nanofibers Obtained through the Liquid Pulse Injection Technique. ACS Omega 2018, 3, 691–697. [Google Scholar] [CrossRef]
- Kumar, G.G.; Sarathi, V.G.S.; Nahm, K.S. Recent advances and challenges in the anode architecture and their modifications for the applications of microbial fuel cells. Biosens. Bioelectron. 2013, 43, 461–475. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Hindatu, Y.; Annuar, M.S.M.; Gumel, A.M. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sustain. Energy Rev. 2017, 73, 236–248. [Google Scholar] [CrossRef]
- Sauerteig, D.; Hanselmann, N.; Arzberger, A.; Reinshagen, H.; Ivanov, S.; Bund, A. Electrochemical-mechanical coupled modeling and parameterization of swelling and ionic transport in lithium-ion batteries. J. Power Sources 2018, 378, 235–247. [Google Scholar] [CrossRef]
- Din, M.I.; Iqbal, M.; Hussain, Z.; Khalid, R. Bioelectricity generation from waste potatoes using single chambered microbial fuel cell. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–11. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, Q.; Chen, J.; Wang, B.; Wang, Y.; Liu, K.; Li, M.; Jiang, H.; Lu, Z.; Wang, D. Hierarchically Three-Dimensional Nanofiber Based Textile with High Conductivity and Biocompatibility As a Microbial Fuel Cell Anode. Environ. Sci. Technol. 2016, 50, 7889–7895. [Google Scholar] [CrossRef] [PubMed]
- Matsena, M.T.; Tichapondwa, S.M.; Chirwa, E.M.N. Synthesis of Biogenic Palladium Nanoparticles Using Citrobacter sp. for Application as Anode Electrocatalyst in a Microbial Fuel Cell. Catalysts 2020, 10, 838. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air–cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite Fiber Brush Anodes for Increased Power Production in Air-Cathode Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, N.; Sun, C.; Luo, X. Investigations on physicochemical properties and electrochemical performance of graphite felt and carbon felt for iron-chromium redox flow battery. Int. J. Energy Res. 2020, 44, 3839–3853. [Google Scholar] [CrossRef]
- Chouler, J.; Padgett, G.A.; Cameron, P.; Preuss, K.; Titirici, M.; Ieropoulos, I.; Di Lorenzo, M. Towards effective small scale microbial fuel cells for energy generation from urine. Electrochim. Acta 2016, 192, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cheng, S.; Feng, Y.; Merrill, M.D.; Saito, T.; Logan, B.E. Use of Carbon Mesh Anodes and the Effect of Different Pretreatment Methods on Power Production in Microbial Fuel Cells. Environ. Sci. Technol. 2009, 43, 6870–6874. [Google Scholar] [CrossRef]
- He, Y.-R.; Xiao, X.; Li, W.-W.; Sheng, G.-P.; Yan, F.-F.; Yu, H.-Q.; Yuan, H.; Wu, L.-J. Enhanced electricity production from microbial fuel cells with plasma-modified carbon paper anode. Phys. Chem. Chem. Phys. 2012, 14, 9966–9971. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.B.A.; Blondeel, E.; Tennison, S.R.; Boon, N.; Verstraete, W. Suitability of granular carbon as an anode material for sediment microbial fuel cells. J. Soils Sediments 2012, 12, 1197–1206. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, A.A.; D’Angelo, L.; Omer, N.; Windiasti, G.; Lu, X.; Xu, J. Carbon nanotube modification of microbial fuel cell electrodes. Biosens. Bioelectron. 2016, 85, 536–552. [Google Scholar] [CrossRef] [PubMed]
- Delord, B.; Neri, W.; Bertaux, K.; Derre, A.; Ly, I.; Mano, N.; Poulin, P. Carbon nanotube fiber mats for microbial fuel cell electrodes. Bioresour. Technol. 2017, 243, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Thepsuparungsikul, N.; Ng, T.C.; Lefebvre, O.; Ng, H.Y. Different types of carbon nanotube-based anodes to improve microbial fuel cell performance. Water Sci. Technol. 2014, 69, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Sushma, S.; Harish Anand, K. Designing the Shape of Graphite Anode for Microbial Fuel Cells to Increase its Efficiency. Int. Res. J. Eng. Technol. 2017, 4, 553–556. [Google Scholar]
- Kang, H.; Jeong, J.; Gupta, P.L.; Jung, S.P. Effects of brush-anode configurations on performance and electrochemistry of microbial fuel cells. Int. J. Hydrogen Energy 2017, 42, 27693–27700. [Google Scholar] [CrossRef]
- Zhou, S.; Lin, M.; Zhuang, Z.; Liu, P.; Chen, Z. Biosynthetic graphene enhanced extracellular electron transfer for high performance anode in microbial fuel cell. Chemosphere 2019, 232, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, Y.; Tan, X.; Zhang, L.; Huang, W.; Sun, J. Enhanced performance of microbial fuel cell with in situ preparing dual graphene modified bioelectrode. Bioresour. Technol. 2017, 241, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Dimiev, A.M.; Tour, J.M. Mechanism of Graphene Oxide Formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Mashkour, M.; Rahimnejad, M.; Pourali, S.M.; Ezoji, H.; ElMekawy, A.; Pant, D. Catalytic performance of nano-hybrid graphene and titanium dioxide modified cathodes fabricated with facile and green technique in microbial fuel cell. Prog. Nat. Sci. Mater. Int. 2017, 27, 647–651. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Y.-X.; Li, D.-B.; Yu, H.-Q.; Yan, L. Preparation of a macroporous flexible three dimensional graphene sponge using an ice-template as the anode material for microbial fuel cells. RSC Adv. 2014, 4, 21619–21624. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, X.-S.; Li, C.M. Interfacial electron transfer of Shewanella putrefaciens enhanced by nanoflaky nickel oxide array in microbial fuel cells. J. Power Sources 2014, 266, 226–231. [Google Scholar] [CrossRef]
- Qiao, Y.; Wen, G.-Y.; Wu, X.-S.; Zou, L. L-Cysteine tailored porous graphene aerogel for enhanced power generation in microbial fuel cells. RSC Adv. 2015, 5, 58921–58927. [Google Scholar] [CrossRef]
- Pareek, A.; Sravan, J.S.; Mohan, S.V. Fabrication of three-dimensional graphene anode for augmenting performance in microbial fuel cells. Carbon Resour. Convers. 2019, 2, 134–140. [Google Scholar] [CrossRef]
- Chen, S.; He, G.; Liu, Q.; Harnisch, F.; Zhou, Y.; Chen, Y.; Hanif, M.; Wang, S.; Peng, X.; Hou, H.; et al. Layered corrugated electrode macrostructures boost microbial bioelectrocatalysis. Energy Environ. Sci. 2012, 5, 9769–9772. [Google Scholar] [CrossRef]
- Tang, J.; Yuan, Y.; Liu, T.; Zhou, S. High-capacity carbon-coated titanium dioxide core–shell nanoparticles modified three dimensional anodes for improved energy output in microbial fuel cells. J. Power Sources 2015, 274, 170–176. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Ye, D.; Zhu, X.; Liao, Q.; Zhang, B. Tubular bamboo charcoal for anode in microbial fuel cells. J. Power Sources 2014, 272, 277–282. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, T.; Fu, P.; Tang, J.; Zhou, S. Conversion of sewage sludge into high-performance bifunctional electrode materials for microbial energy harvesting. J. Mater. Chem. A 2015, 3, 8475–8482. [Google Scholar] [CrossRef]
- Chen, Q.; Pu, W.; Hou, H.; Hu, J.; Liu, B.; Li, J.; Cheng, K.; Huang, L.; Yuan, X.; Yang, C.; et al. Activated microporous-mesoporous carbon derived from chestnut shell as a sustainable anode material for high performance microbial fuel cells. Bioresour. Technol. 2018, 249, 567–573. [Google Scholar] [CrossRef]
- Hung, Y.-H.; Liu, T.-Y.; Chen, H.-Y. Renewable Coffee Waste-Derived Porous Carbons as Anode Materials for High-Performance Sustainable Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2019, 7, 16991–16999. [Google Scholar] [CrossRef]
- Nitisoravut, R.; Thanh, C.N.D.; Regmi, R. Microbial fuel cells: Advances in electrode modifications for improvement of system performance. Int. J. Green Energy 2017, 14, 712–723. [Google Scholar] [CrossRef]
- Erable, B.; Byrne, N.; Etcheverry, L.; Achouak, W.; Bergel, A. Single medium microbial fuel cell: Stainless steel and graphite electrode materials select bacterial communities resulting in opposite electrocatalytic activities. Int. J. Hydrogen Energy 2017, 42, 26059–26067. [Google Scholar] [CrossRef] [Green Version]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Füeg, M.; Borjas, Z.; Estevez-Canales, M.; Esteve-Núñez, A.; Pobelov, I.; Broekmann, P.; Kuzume, A. Interfacial electron transfer between Geobacter sulfurreducens and gold electrodes via carboxylate-alkanethiol linkers: Effects of the linker length. Bioelectrochemistry 2019, 126, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.; Khan, M.; Saddique, A. Review Article on Applications and Classification of Gold Nanoparticles. Int. J. Res. 2019, 6, 7. [Google Scholar]
- Tang, X.; Guo, K.; Li, H.; Du, Z.; Tian, J. Electrochemical treatment of graphite to enhance electron transfer from bacteria to electrodes. Bioresour. Technol. 2011, 102, 3558–3560. [Google Scholar] [CrossRef]
- Pu, K.-B.; Ma, Q.; Cai, W.-F.; Chen, Q.-Y.; Wang, Y.-H.; Li, F.-J. Polypyrrole modified stainless steel as high performance anode of microbial fuel cell. Biochem. Eng. J. 2018, 132, 255–261. [Google Scholar] [CrossRef]
- Phonsa, S.; Sreearunothai, P.; Charojrochkul, S.; Sombatmankhong, K. Electrodeposition of MnO2 on polypyrrole-coated stainless steel to enhance electrochemical activities in microbial fuel cells. Solid State Ionics 2018, 316, 125–134. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Al-Saadi, S.; Raman, R.K.S.; Ghosh, P.C.; Adeloju, S.B. Exploring the use of polyaniline-modified stainless steel plates as low-cost, high-performance anodes for microbial fuel cells. Electrochim. Acta 2018, 268, 484–493. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Du, Z. Polyaniline synthesis by cyclic voltammetry for anodic modification in microbial fuel cells. Int. J. Electrochem. Sci. 2014, 9, 2038–2046. [Google Scholar]
- Mantione, D.; del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene) (PEDOT) Derivatives: Innovative Conductive Polymers for Bioelectronics. Polymers 2017, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; You, S.-J.; Wang, J.-Y. Carbon nanotubes as electrode modifier promoting direct electron transfer from Shewanella oneidensis. Biosens. Bioelectron. 2010, 25, 1248–1251. [Google Scholar] [CrossRef]
- Amiri, S.; Rahimi, A. Hybrid nanocomposite coating by sol–gel method: A review. Iran. Polym. J. 2016, 25, 559–577. [Google Scholar] [CrossRef]
- Phan, T.D.; Vo, C.M.; Tran, T.M.T.; Luu, T.L.A.; Nguyen, X.S. Structural and bandgap properties of titanium dioxide nanotube/graphene oxide composites prepared by a facile hydrothermal method. Mater. Res. Express 2019, 6, 105054. [Google Scholar] [CrossRef]
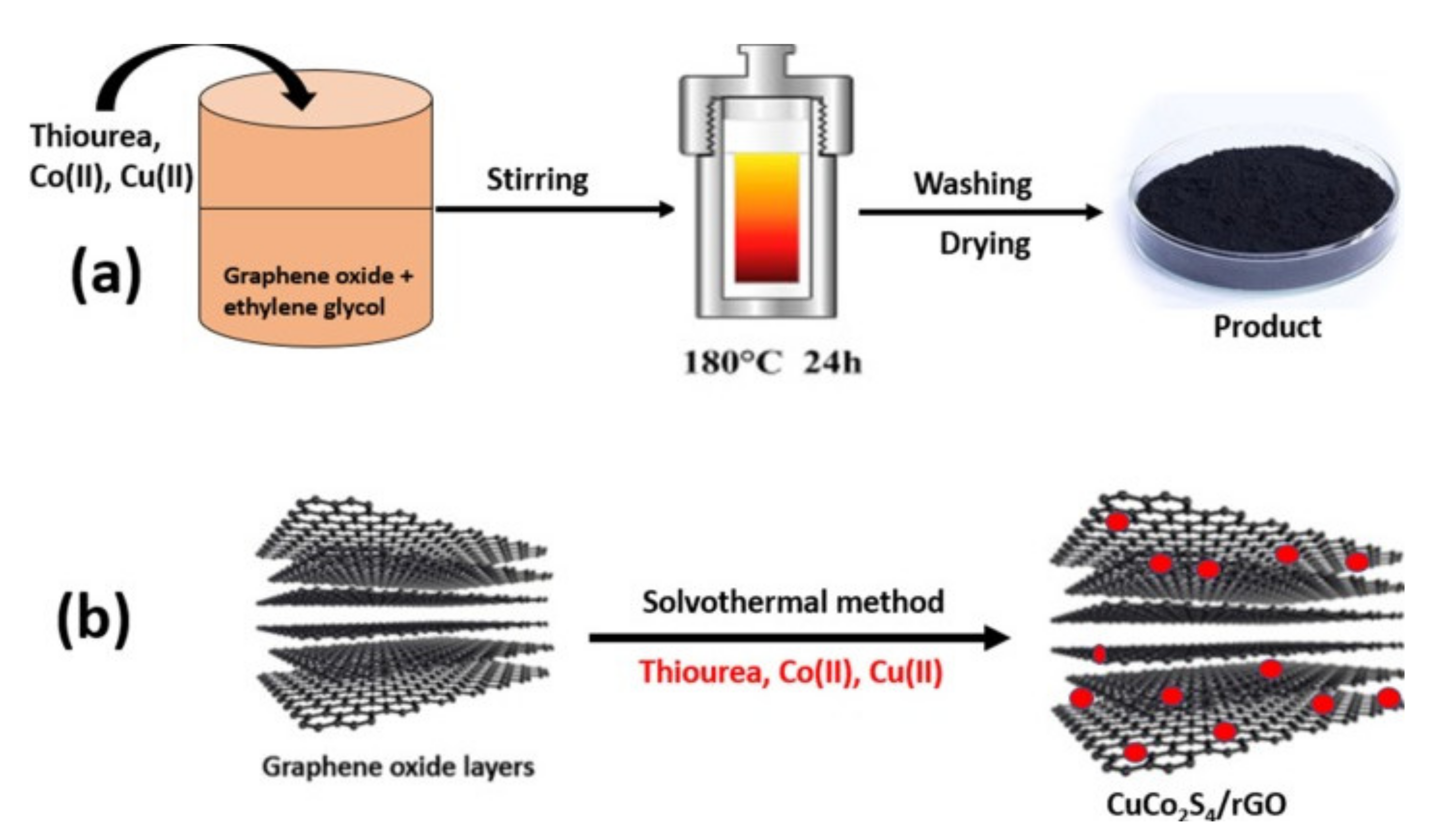
- Gong, Y.; Zhao, J.; Wang, H.; Xu, J. CuCo2S4/reduced graphene oxide nanocomposites synthesized by one-step solvothermal method as anode materials for sodium ion batteries. Electrochim. Acta 2018, 292, 895–902. [Google Scholar] [CrossRef]
- Yin, T.; Lin, Z.; Su, L.; Yuan, C.; Fu, D. Preparation of Vertically Oriented TiO2 Nanosheets Modified Carbon Paper Electrode and Its Enhancement to the Performance of MFCs. Appl. Mater. Interfaces 2015, 7, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Fraiwan, A.; Adusumilli, S.P.; Han, D.; Steckl, A.; Call, D.; Westgate, C.R.; Choi, S. Microbial Power-Generating Capabilities on Micro-/Nano-Structured Anodes in Micro-Sized Microbial Fuel Cells. Fuel Cells 2014, 14, 801–809. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Y.-S.; Liu, Z.-M. Pyrolysis of iron phthalocyanine on activated carbon as highly efficient non-noble metal oxygen reduction catalyst in microbial fuel cells. Chem. Eng. J. 2019, 361, 416–427. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Q.; He, G.; Zhou, Y.; Hanif, M.; Peng, X.; Wang, S.; Hou, H. Reticulated carbon foam derived from a sponge-like natural product as a high-performance anode in microbial fuel cells. J. Mater. Chem. 2012, 22, 18609–18613. [Google Scholar] [CrossRef]
| Component | Materials | Remarks | Reference |
|---|---|---|---|
| Anode | Carbon felt, carbon paper, carbon cloth, Reticulated vitreous carbon (RVC), graphite-felt, graphite-brush, graphene-oxide, graphene-nano tubes, Pt black | Essential | [7,8] |
| Membrane | Ion exchange, cation exchange, anion exchange, proton exchange (Nafion 117, Ultrex, SPEEK), salt bridge | Optional | [4,7,9,10] |
| Cathode | Carbon felt, carbon catalyst with stainless steel mesh, carbon cloth, graphite, reticulated vitreous carbon (RVC), biocathode | Essential | [7,11,12,13] |
| Natural Source of Anode Material | Electrode Dimensions (cm) | Effective Surface Area 2πR(R + L) cm2 | Fuel Source | Output Power Densities mW/m2 | References | |
|---|---|---|---|---|---|---|
| Radius | Length | |||||
| Loofah sponge dopped with PANI | 0.5 | 3.0 | 10.99 | Mixed Sludge | 2590 | [51] |
| Bamboo Charcoal | 2.4 | 1.57 | 59.83 | Mixed sludge | 1652 | [52] |
| Coconut shell | 0.5 | 3.0 | 10.99 | Mixed sludge | 1069 | [53] |
| Chestnut shells | 0.3 | 66.4 | 125.65 | Anaerobic mixed sludge | 850 | [54] |
| Coffee wastes | --- | ----- | 1.0 | Domestic sludge | 3927 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, A.; Calay, R.K.; Mustafa, M. Review on Material and Design of Anode for Microbial Fuel Cell. Energies 2022, 15, 2283. https://doi.org/10.3390/en15062283
Banerjee A, Calay RK, Mustafa M. Review on Material and Design of Anode for Microbial Fuel Cell. Energies. 2022; 15(6):2283. https://doi.org/10.3390/en15062283
Chicago/Turabian StyleBanerjee, Aritro, Rajnish Kaur Calay, and Mohamad Mustafa. 2022. "Review on Material and Design of Anode for Microbial Fuel Cell" Energies 15, no. 6: 2283. https://doi.org/10.3390/en15062283
APA StyleBanerjee, A., Calay, R. K., & Mustafa, M. (2022). Review on Material and Design of Anode for Microbial Fuel Cell. Energies, 15(6), 2283. https://doi.org/10.3390/en15062283