Sr Substituted La2−xSrxNi0.8Co0.2O4+δ (0 ≤ x ≤ 0.8): Impact on Oxygen Stoichiometry and Electrochemical Properties
Abstract
:1. Introduction
2. Experimental
2.1. Powder Preparation
2.2. X-ray Diffraction Analysis
2.3. Microstructural Analysis
2.4. Thermo-Gravimetric Analysis (TGA)
2.5. Cell Preparation and Fabrication
2.6. Electrochemical Characterization
3. Results and Discussion
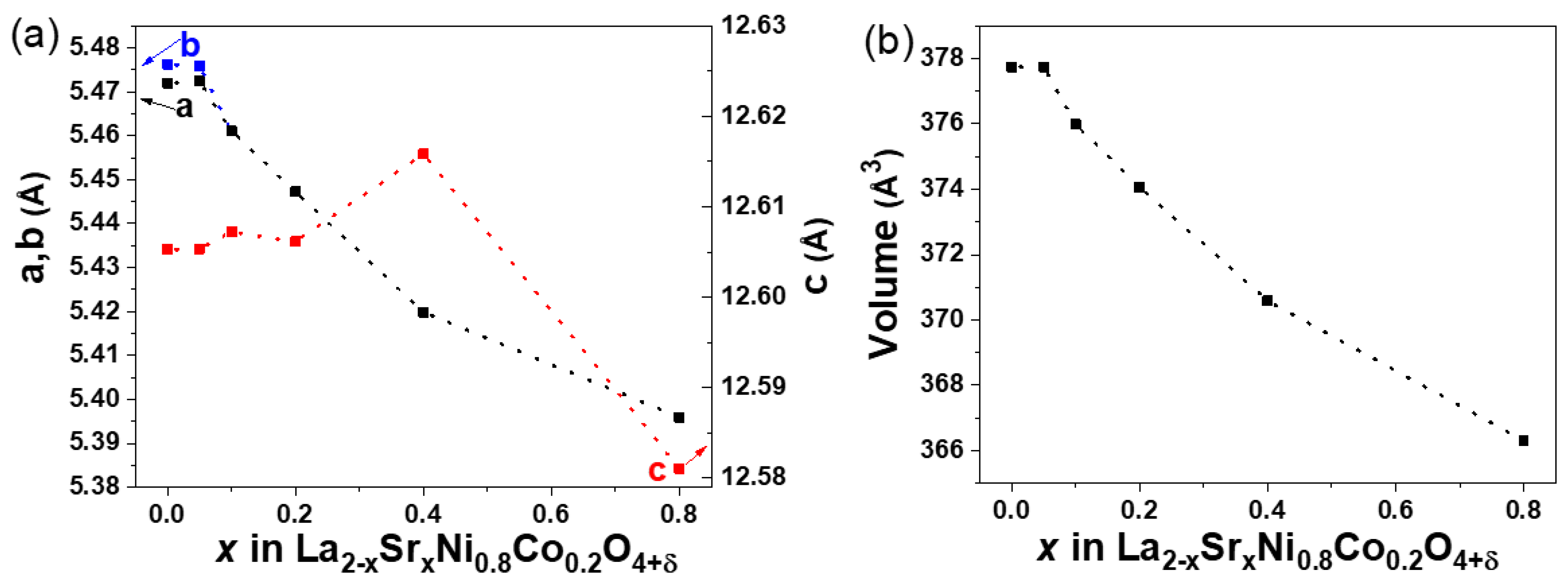
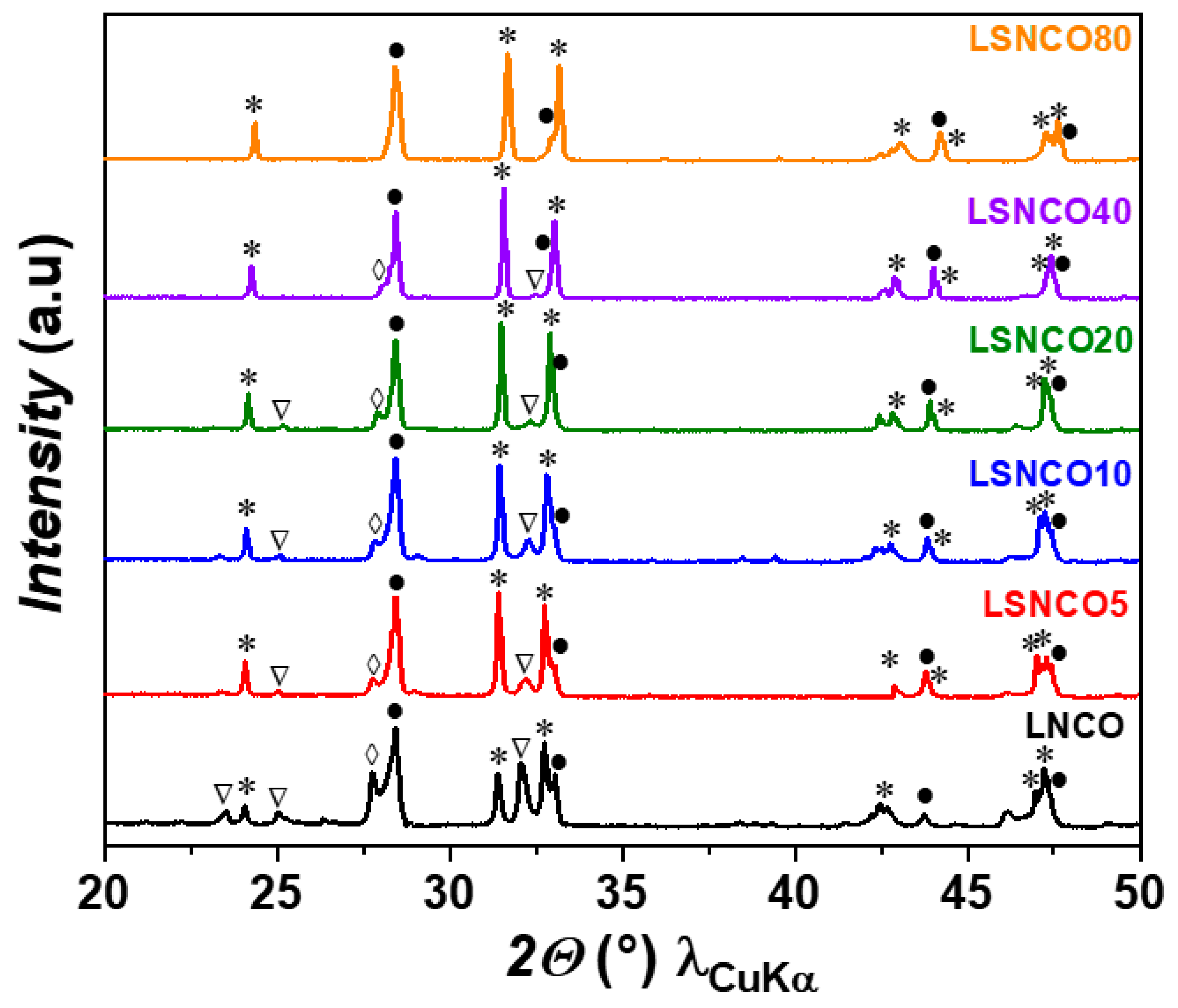
3.1. X-ray Diffraction Analyses
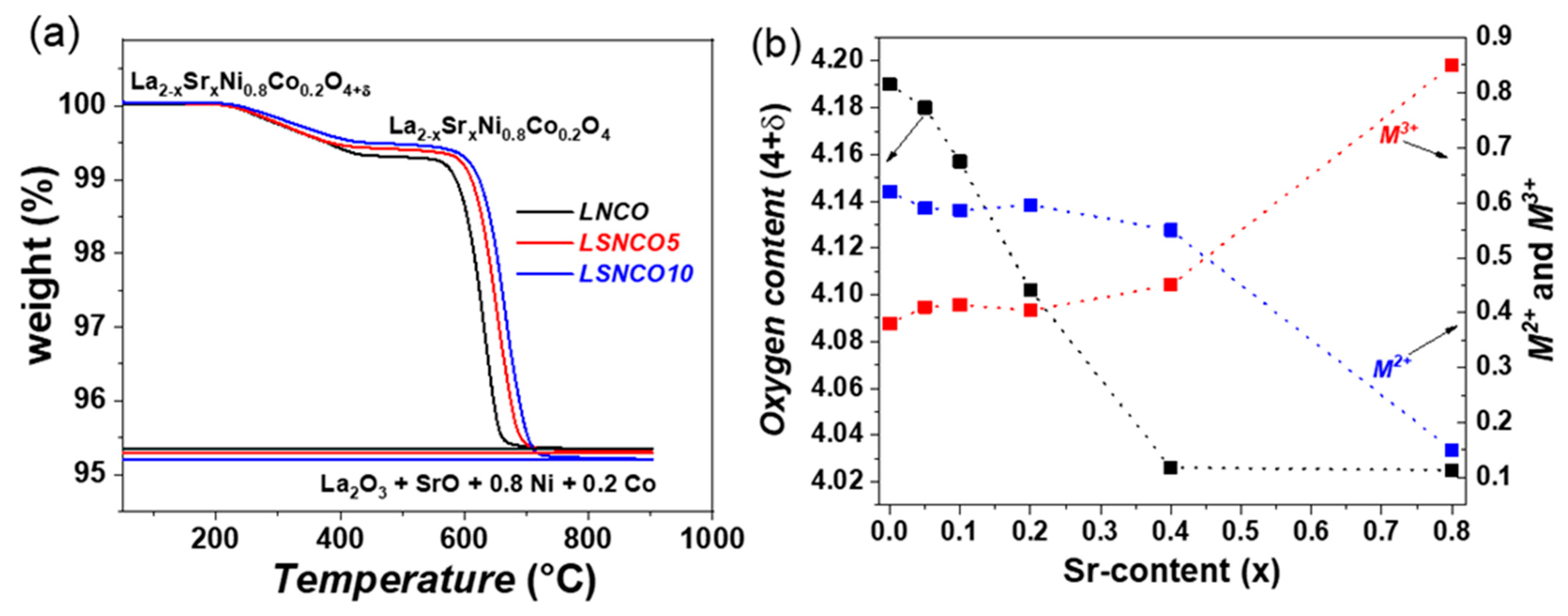
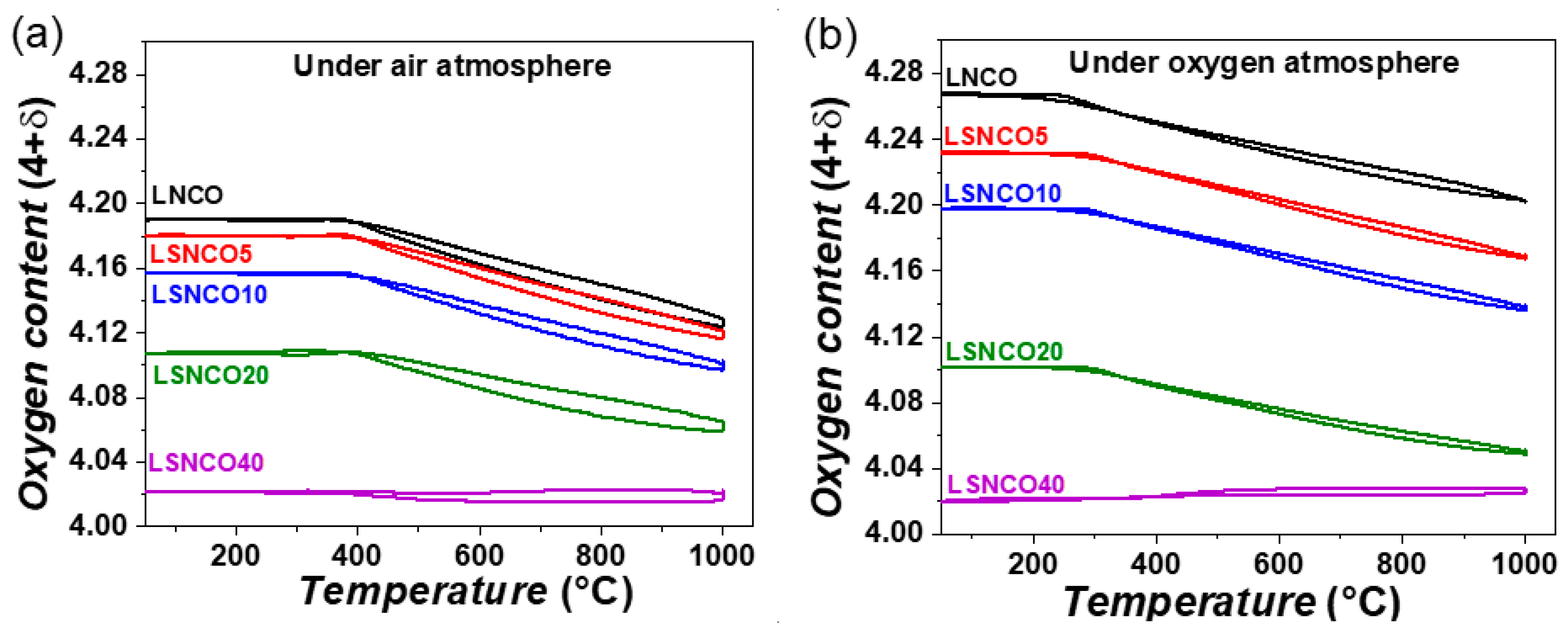
3.2. Thermogravimetric Analyses
3.3. Reactivity Test
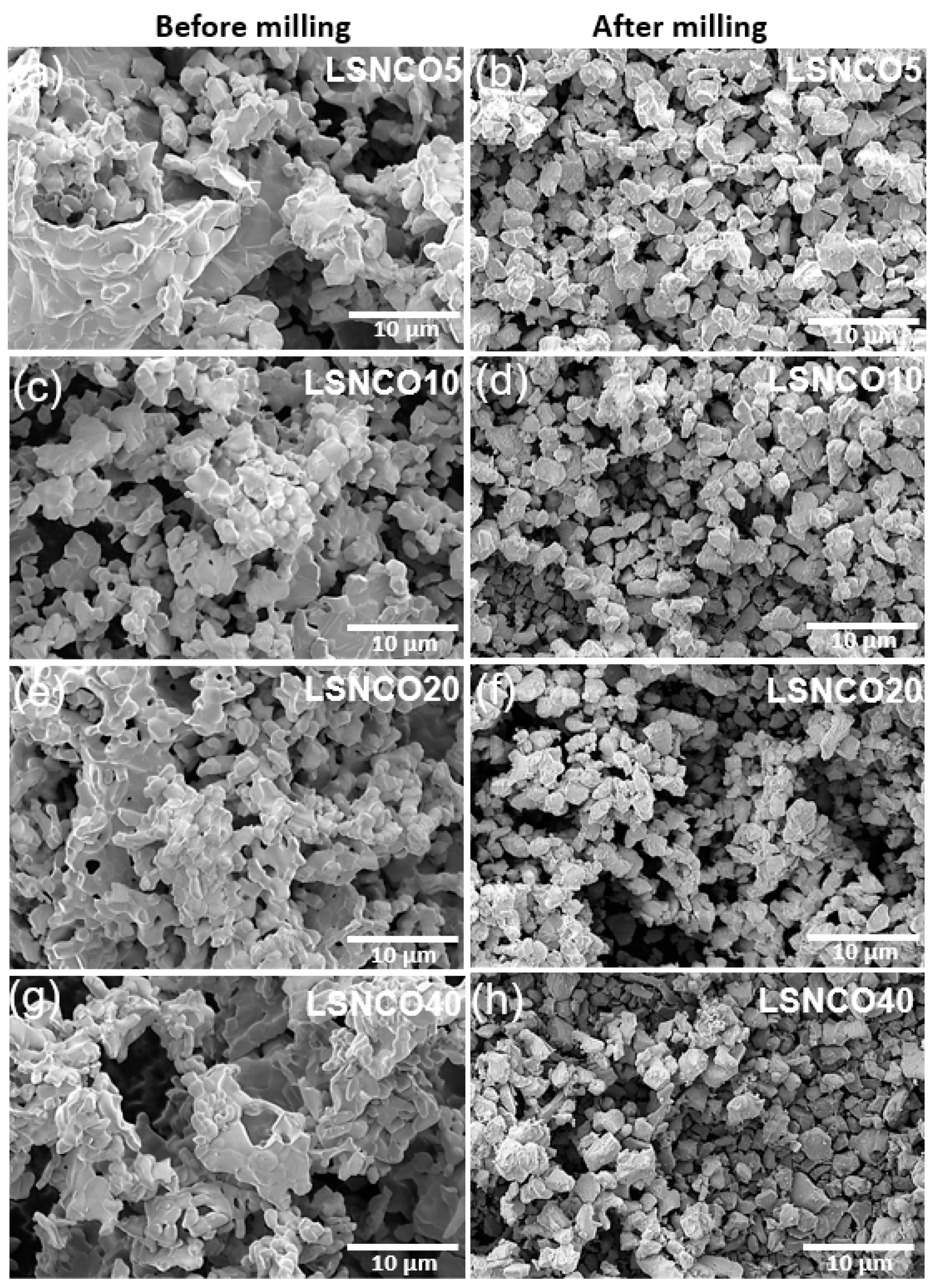
3.4. Powder Morphology
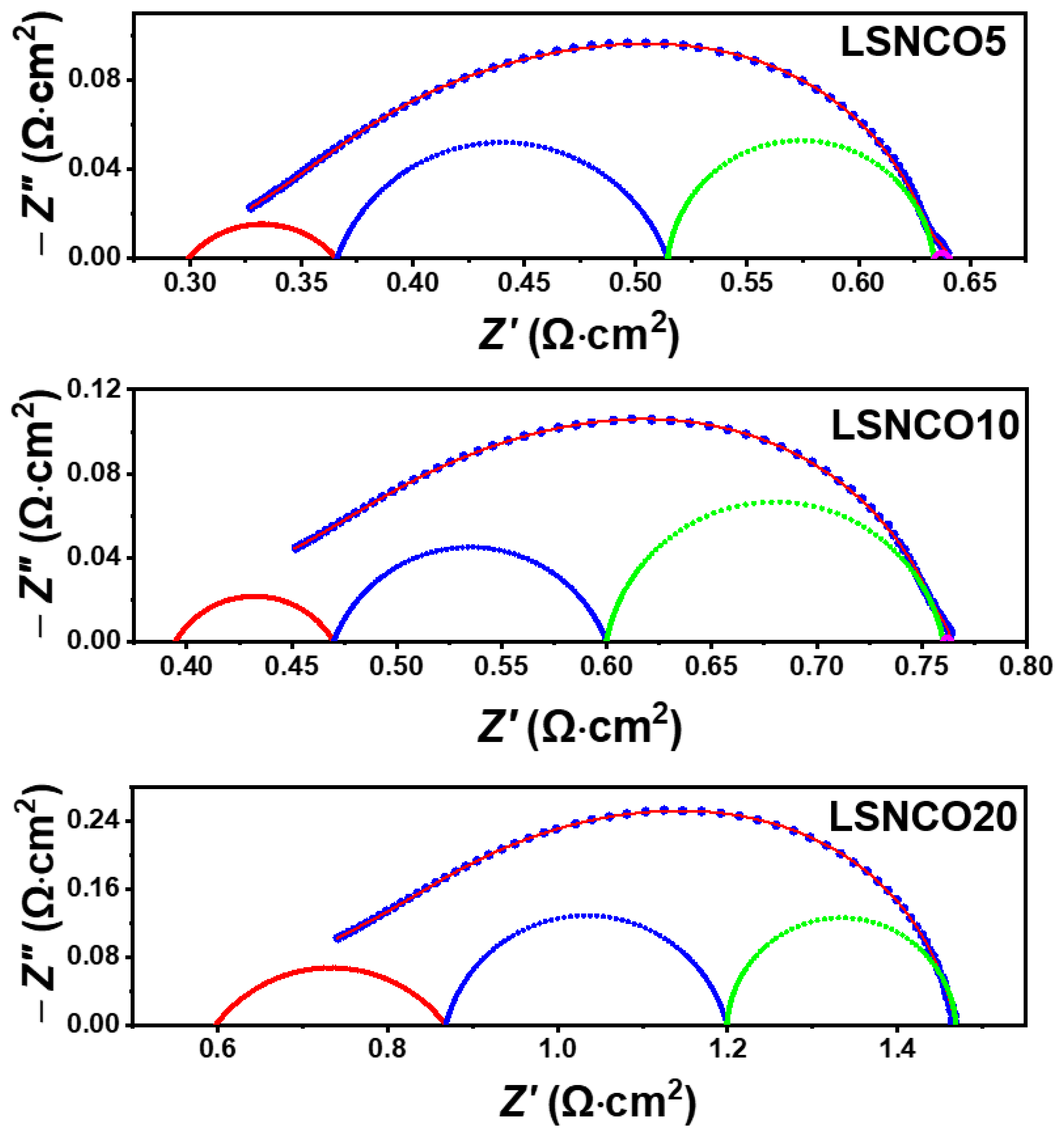
3.5. Electrochemical Characterizations of Symmetrical Half-Cells
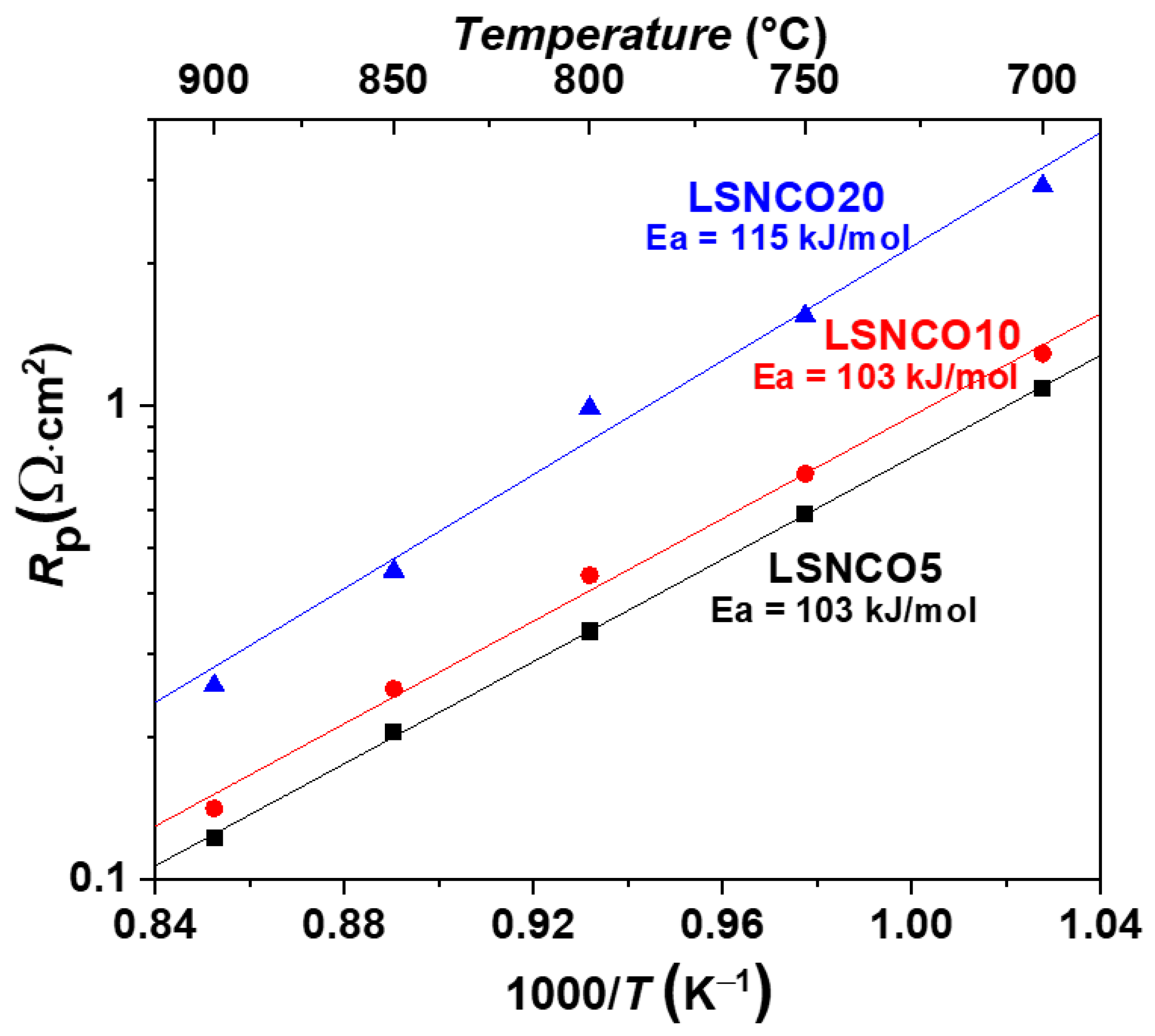
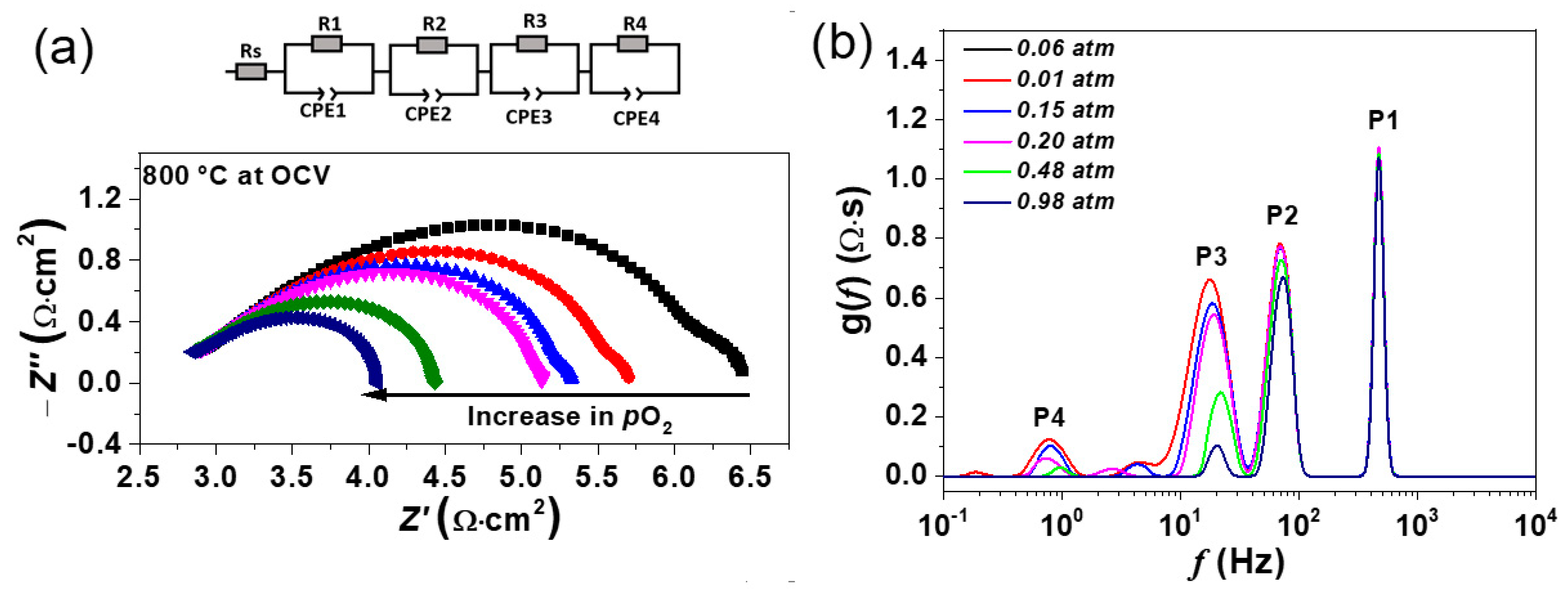
3.5.1. Mechanism of Oxygen Reduction Reaction and the Determination of Rate-Limiting Step
3.5.2. Impedance Data Analysis under Polarization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chuahy, F.D.; Kokjohn, S.L. Solid Oxide Fuel Cell and Advanced Combustion Engine Combined Cycle: A Pathway to 70% Electrical Efficiency. Appl. Energy 2019, 235, 391–408. [Google Scholar] [CrossRef]
- Shaheen, K.; Suo, H.; Shah, Z.; Hanif, M.B.; Hussain, Z.; Ali, S.; Liu, M.; Ma, L.; Cui, J.; Ji, Y.T.; et al. Electrochemical Performance of Multifuel Based Nanocomposite for Solid Oxide Fuel Cell. Ceram. Int. 2020, 46, 8832–8838. [Google Scholar] [CrossRef]
- Bicer, Y.; Khalid, F. Life Cycle Environmental Impact Comparison of Solid Oxide Fuel Cells Fueled by Natural Gas, Hydrogen, Ammonia and Methanol for Combined Heat and Power Generation. Int. J. Hydrogen Energy 2020, 45, 3670–3685. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, L.; Chi, B.; Pu, J.; Li, J. In Situ Exsolved Ni-Decorated Ba(Ce0.9Y0.1)0.8Ni0.2O3-δ Perovskite as Carbon-Resistant Composite Anode for Hydrocarbon-Fueled Solid Oxide Fuel Cells. ACS Omega 2019, 4, 21494–21499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarruf, B.J.M.; Hong, J.E.; Steinberger-Wilckens, R.; de Miranda, P.E.V. Ceria-Co-Cu-Based SOFC Anode for Direct Utilisation of Methane or Ethanol as Fuels. Int. J. Hydrogen Energy 2020, 45, 5297–5308. [Google Scholar] [CrossRef]
- Jiang, S.P. A Comparison of O2 Reduction Reactions on Porous (La,Sr)MnO3 and (La,Sr)(Co,Fe)O3 Electrodes. Solid State Ion. 2002, 146, 1–22. [Google Scholar] [CrossRef]
- Murray, E.P.; Sever, M.J.; Barnett, S.A.B. Electrochemical Performance of (La,Sr)(Co,Fe)O3–(Ce,Gd)O3 Composite Cathodes. Solid State Ion. 2002, 148, 27–34. [Google Scholar] [CrossRef]
- Khotseng, L. Oxygen Reduction Reaction. In Electrocatalysts for Fuel Cells and Hydrogen Evolution—Theory to Design; IntechOpen: London, UK, 2018. [Google Scholar]
- Ascolani-Yael, J.; Montenegro-Hernández, A.; Garcés, D.; Liu, Q.; Wang, H.; Yakal-Kremski, K.; Barnett, S.; Mogni, L. The Oxygen Reduction Reaction in Solid Oxide Fuel Cells: From Kinetic Parameters Measurements to Electrode Design. J. Phys. Energy 2020, 2, 042004. [Google Scholar] [CrossRef]
- Yang, G.; Jung, W.; Ahn, S.J.; Lee, D. Controlling the Oxygen Electrocatalysis on Perovskite and Layered Oxide Thin Films for Solid Oxide Fuel Cell Cathodes. Appl. Sci. 2019, 9, 1030. [Google Scholar] [CrossRef] [Green Version]
- Ren, R.; Wang, Z.; Meng, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Boosting the Electrochemical Performance of Fe-Based Layered Double Perovskite Cathodes by Zn2+ Doping for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 23959–23967. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, J.; Xia, Y.; Miao, L.; Li, K.; Zhang, Q.; Liu, W. Ba0.5Sr0.5Co0.8−XFe0.2NbxO3−δ (X ≤ 0.1) as Cathode Materials for Intermediate Temperature Solid Oxide Fuel Cells with an Electron-Blocking Interlayer. Ceram. Int. 2020, 46, 10215–10223. [Google Scholar] [CrossRef]
- Xiaokaiti, P.; Yu, T.; Yoshida, A.; Du, X.; Hao, X.; Kasai, Y.; Abudula, A.; Guan, G. Effects of Cobalt and Iron Proportions in Pr0.4Sr0.6Co0.9−XFexNb0.1O3−δ Electrode Material for Symmetric Solid Oxide Fuel Cells. J. Alloys Compd. 2020, 831, 154738. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, H.; Liu, Y.; Chi, B.; Pu, J.; Li, J. Effects of Niobium Doping on the Stability of SrCo0.2Fe0.8O3−δ Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. J. Alloys Compd. 2020, 829, 154503. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, G.M. Stability of LSCF Electrode with GDC Interlayer in YSZ-Based Solid Oxide Electrolysis Cell. Solid State Ion. 2014, 262, 303–306. [Google Scholar] [CrossRef]
- Vibhu, V.; Rougier, A.; Nicollet, C.; Flura, A.; Grenier, J.C.; Bassat, J.M. La2−XPrxNiO4+δ as Suitable Cathodes for Metal Supported SOFCs. Solid State Ion. 2015, 278, 32–37. [Google Scholar] [CrossRef]
- Mao, J.; Peng, S.; Zhang, C.; Qi, S.; Cui, J.; Gong, Y.; Wang, S.; Wu, C.; Zhou, Q. Electrode Properties of (Pr0.9La0.1)2−x(Ni0.74Cu0.21Al0.05)O4+δ (with X = 0, 0.05, and 0.1)as Cathodes in IT-SOFCs. J. Alloys Compd. 2019, 793, 519–525. [Google Scholar] [CrossRef]
- Montenegro-Hernández, A.; Vega-Castillo, J.; Caneiro, A.; Mogni, L. High Temperature Orthorhombic/Tetragonal Transition and Oxygen Content of Pr2−XNdxNiO4+δ (X = 0, 0.3, 1, 1.7 and 2) Solid Solutions. J. Solid State Chem. 2019, 276, 210–216. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C.; Stevens, P. Oxygen Diffusion and Transport Properties in Non-Stoichiometric Ln2−XNiO4+δ Oxides. Solid State Ion. 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Ferchaud, C.; Grenier, J.C.; Zhang-Steenwinkel, Y.; Van Tuel, M.M.A.; Van Berkel, F.P.F.; Bassat, J.M. High Performance Praseodymium Nickelate Oxide Cathode for Low Temperature Solid Oxide Fuel Cell. J. Power Sources 2011, 196, 1872–1879. [Google Scholar] [CrossRef]
- Vibhu, V.; Suchomel, M.R.; Penin, N.; Weill, F.; Grenier, J.C.; Bassat, J.M.; Rougier, A. Structural Transformations of the La2−xPrxNiO4+δ System Probed by High-Resolution Synchrotron and Neutron Powder Diffraction. Dalt. Trans. 2019, 48, 266–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simner, S.P.; Anderson, M.D.; Engelhard, M.H.; Stewenson, J.W. Degradation Mechanisms of La–Sr–Co–Fe–O3SOFC Cathodes Electrochem. Solid State Lett. 2006, 9, A478–A481. [Google Scholar] [CrossRef]
- Kim, J.Y.; Sprenkle, V.L.; Canfield, N.L.; Meinhardt, K.D.; Chick, L.A.; Benson, S.J.; Waller, D.; Kilner, J.A. Degradation of La0.6Sr0.4Co0.2Fe0.8O3 in Carbon Dioxide and Water Atmospheres. J. Electrochem. Soc. 1999, 146, 1305–1309. [Google Scholar]
- Vibhu, V.; Yildiz, S.; Vinke, I.C.; Eichel, R.A.; Bassat, J.M.; De Haart, L.G.J. High Performance LSC Infiltrated LSCF Oxygen Electrode for High Temperature Steam Electrolysis Application. J. Electrochem. Soc. 2019, 166, F102–F108. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Steil, M.C.; Dordor, P.; Mauvy, F.; Grenier, J.C. Oxygen Transport Properties of La2Ni1-XCuxO4+δ Mixed Conducting Oxides. Solid State Sci. 2003, 5, 973–981. [Google Scholar] [CrossRef]
- Vibhu, V.; Vinke, I.C.; Eichel, R.A.; de Haart, L.G.J. Cobalt Substituted Pr2Ni1−xCoxO4+δ (x = 0, 0.1, 0.2) Oxygen Electrodes: Impact on Electrochemical Performance and Durability of Solid Oxide Electrolysis Cells. J. Power Sources 2021, 482, 228909. [Google Scholar] [CrossRef]
- Vibhu, V.; Flura, A.; Rougier, A.; Nicollet, C.; Fourcade, S.; Hungria, T.; Grenier, J.C.; Bassat, J.M. Electrochemical Ageing Study of Mixed Lanthanum/Praseodymium Nickelates La2−XPrxNiO4+δ as Oxygen Electrodes for Solid Oxide Fuel or Electrolysis Cells. J. Energy Chem. 2020, 46, 62–70. [Google Scholar] [CrossRef]
- Chroneos, A.; Vovk, R.V.; Goulatis, I.L.; Goulatis, L.I. Oxygen Transport in Perovskite and Related Oxides: A Brief Review. J. Alloys Compd. 2010, 494, 190–195. [Google Scholar] [CrossRef]
- Fu, D.; Jin, F.; He, T. A-Site Calcium-Doped Pr1−xCaxBaCo2O5+δ Double Perovskites as Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. J. Power Sources 2016, 313, 134–141. [Google Scholar] [CrossRef]
- Anjum, U.; Vashishtha, S.; Agarwal, M.; Tiwari, P.; Sinha, N.; Agrawal, A.; Basu, S.; Haider, M.A. Oxygen Anion Diffusion in Double Perovskite GdBaCo2O5+δ and LnBa0.5Sr0.5Co2−xFexO5+δ (Ln = Gd, Pr, Nd) Electrodes. Int. J. Hydrogen Energy 2016, 41, 7631–7640. [Google Scholar] [CrossRef]
- Vibhu, V.; Vinke, I.C.; Eichel, R.A.; Bassat, J.M.; de Haart, L.G.J. La2Ni1−xCoxO4+δ (x = 0.0, 0.1 and 0.2) Based Efficient Oxygen Electrode Materials for Solid Oxide Electrolysis Cells. J. Power Sources 2019, 444, 227292. [Google Scholar] [CrossRef]
- Munnings, C.N.; Skinner, S.J.; Amow, G.; Whitfield, P.S.; Davidson, I.J. Oxygen Transport in the La2Ni1−XCoxO4+δ System. Solid State Ion. 2005, 176, 1895–1901. [Google Scholar] [CrossRef]
- Kilner, J.A.; Shaw, C.K.M. Mass Transport in La2Ni1−XCoxO4+δ Oxides with the K2NiF4 Structure. Solid State Ion. 2002, 154–155, 523–527. [Google Scholar] [CrossRef]
- Kuo, J.H.; Anderson, H.U.; Sparlin, D.M. Oxidation-Reduction Behaviour of Undoped and Sr-Doped LaMnO3: Defect Structure, Electrical Conductivity and Thermoelectric Power. J. Solid State Chem. 1990, 87, 55. [Google Scholar] [CrossRef]
- Hammouche, A.; Schouler, E.J.L.; Henault, M.J. Electrical and Thermal Properties of Sr-Doped Lanthanum Manganites. Solid State Ion. 1988, 28, 1205. [Google Scholar] [CrossRef]
- Courty, P.; Ajot, H.; Marcilly, C.; Delmon, B. Oxydes Mixtes Ou En Solution Solide Sous Forme Très Divisée Obtenus Par Décomposition Thermique de Précurseurs Amorphes. Powder Technol. 1973, 7, 21–38. [Google Scholar] [CrossRef]
- Nikonov, A.V.; Kuterbekov, K.A.; Bekmyrza, K.Z.; Pavzderin, N.B. A Brief Review of Conductivity and Thermal Expansion of Perovskite-Related Oxides for SOFC Cathode. Eurasian J. Phys. Funct. Mater. 2018, 2, 274–292. [Google Scholar] [CrossRef] [Green Version]
- Aguadero, A.; Escudero, M.J.; Pérez, M.; Alonso, J.A.; Pomjakushin, V.; Daza, L. Effect of Sr Content on the Crystal Structure and Electrical Properties of the System La2−xSrxNiO4+δ (0 ≤ x ≤ 1). J. Chem. Soc. Dalt. Trans. 2006, 36, 4377–4383. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.R.; Bosio, B.; Baldinelli, A.; Barelli, L. Optimization of a Reference Kinetic Model for Solid Oxide Fuel Cells. Catalysts 2020, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Pikalova, E.Y.; Bogdanovich, N.M.; Kolchugin, A.A.; Osinkin, D.A.; Bronin, D.I. Electrical and Electrochemical Properties of La2NiO4+δ-Based Cathodes in Contact with Ce0.8Sm0.2O2−δ Electrolyte. Procedia Eng. 2014, 98, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Chan, S.; Stempien, J. Solid Oxide Electrolyzer Cell Modeling: A Review. J. Power Technol. 2013, 93, 216–246. [Google Scholar]
- Santaya, M.; Toscani, L.; Baqué, L.; Troiani, H.E.; Mogni, L. Study of Phase Stability of SrTi0.3Fe0.7O3−δ Perovskite in Reducing Atmosphere: Effect of Microstructure. Solid State Ion. 2019, 342, 3–9. [Google Scholar] [CrossRef]
- Nagde, K.R.; Bhoga, S.S. Effect of Sr Content on Structure and Electrical Properties of La1−XSrxMnO3 from ITSOFC Cathode View Point. Ionics 2009, 15, 571–578. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen Diffusion and Surface Exchange in La2−XSrxNiO4+δ. Solid State Ion. 2000, 135, 709–712. [Google Scholar] [CrossRef]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A Perspective on Low-Temperature Solid Oxide Fuel Cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Inprasit, T.; Wongkasemjit, S.; Skinner, S.J.; Burriel, M.; Limthongkul, P. Effect of Sr Substituted La2−XSrxNiO4+δ (x = 0, 0.2, 0.4, 0.6, and 0.8) on Oxygen Stoichiometry and Oxygen Transport Properties. RSC Adv. 2015, 5, 2486–2492. [Google Scholar] [CrossRef]
- Li, W.; Guan, B.; Zhang, X.; Yan, J.; Zhou, Y.; Liu, X. New Mechanistic Insight into the Oxygen Reduction Reaction on Ruddlesden-Popper Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. Phys. Chem. Chem. Phys. 2016, 18, 8502–8511. [Google Scholar] [CrossRef]
- Escudero, M.J.; Aguadero, A.; Alonso, J.A.; Daza, L. A Kinetic Study of Oxygen Reduction Reaction on La2NiO4 Cathodes by Means of Impedance Spectroscopy. J. Electroanal. Chem. 2007, 611, 107–116. [Google Scholar] [CrossRef]
- Sun, L.P.; Li, Q.; Zhao, H.; Hao, J.H.; Huo, L.H.; Pang, G.; Shi, Z.; Feng, S. Electrochemical Performance of Nd 1.93Sr 0.07CuO4 Nanofiber as Cathode Material for SOFC. Int. J. Hydrogen Energy 2012, 37, 11955–11962. [Google Scholar] [CrossRef]
- Kol’chugin, A.A.; Pikalova, E.Y.; Bogdanovich, N.M.; Bronin, D.I.; Filonova, E.A. Electrochemical Properties of Doped Lantanum–Nickelate-Based Electrodes. Russ. J. Electrochem. 2017, 53, 826–833. [Google Scholar] [CrossRef]
- Hotza, S.; Gomez, S.Y. Solid Oxide Electrolysers. In Electrochemical Methods for Hydrogen Production; Keith, S., Ed.; The Royal Society of Chemistry: London, UK, 2020; pp. 136–174. [Google Scholar]
- Chen, Z.; Hassan, F.M.; Yu, A. Electrochemical Engineering Fundamentals. Electrochem. Technol. Energy Storage Convers. 2012, 1, 45–68. [Google Scholar]

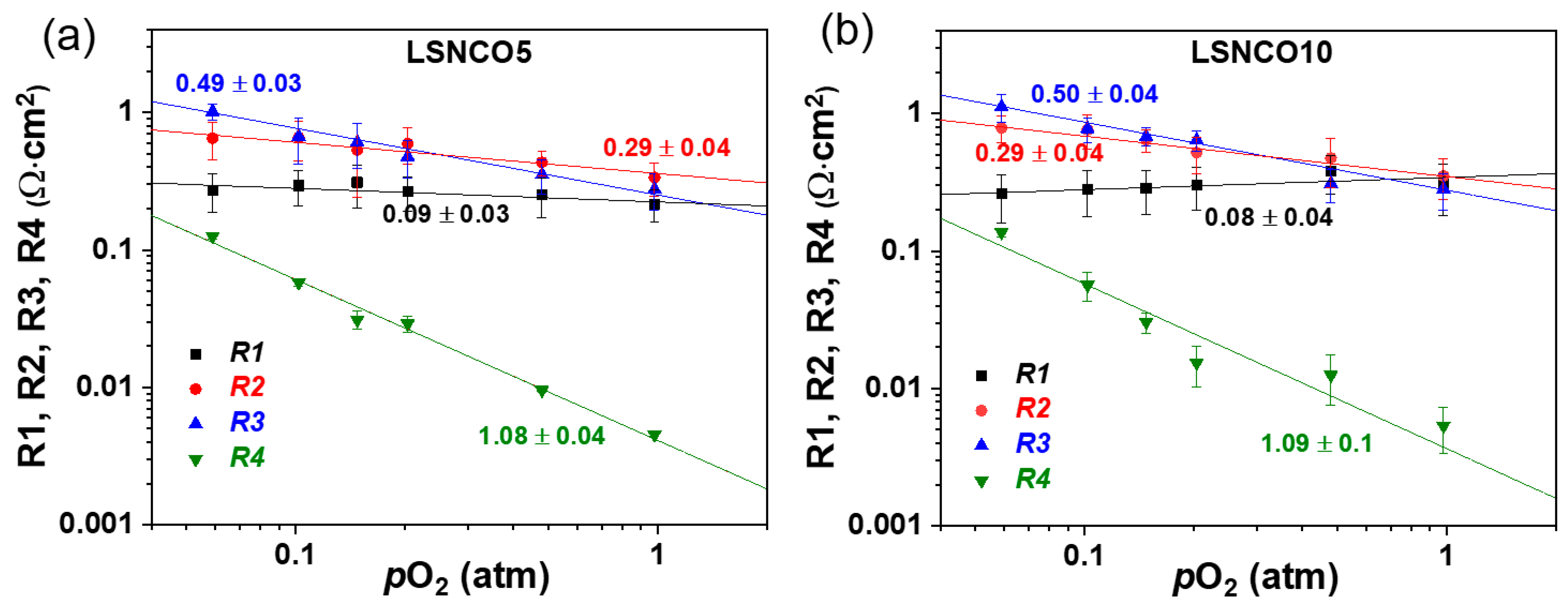
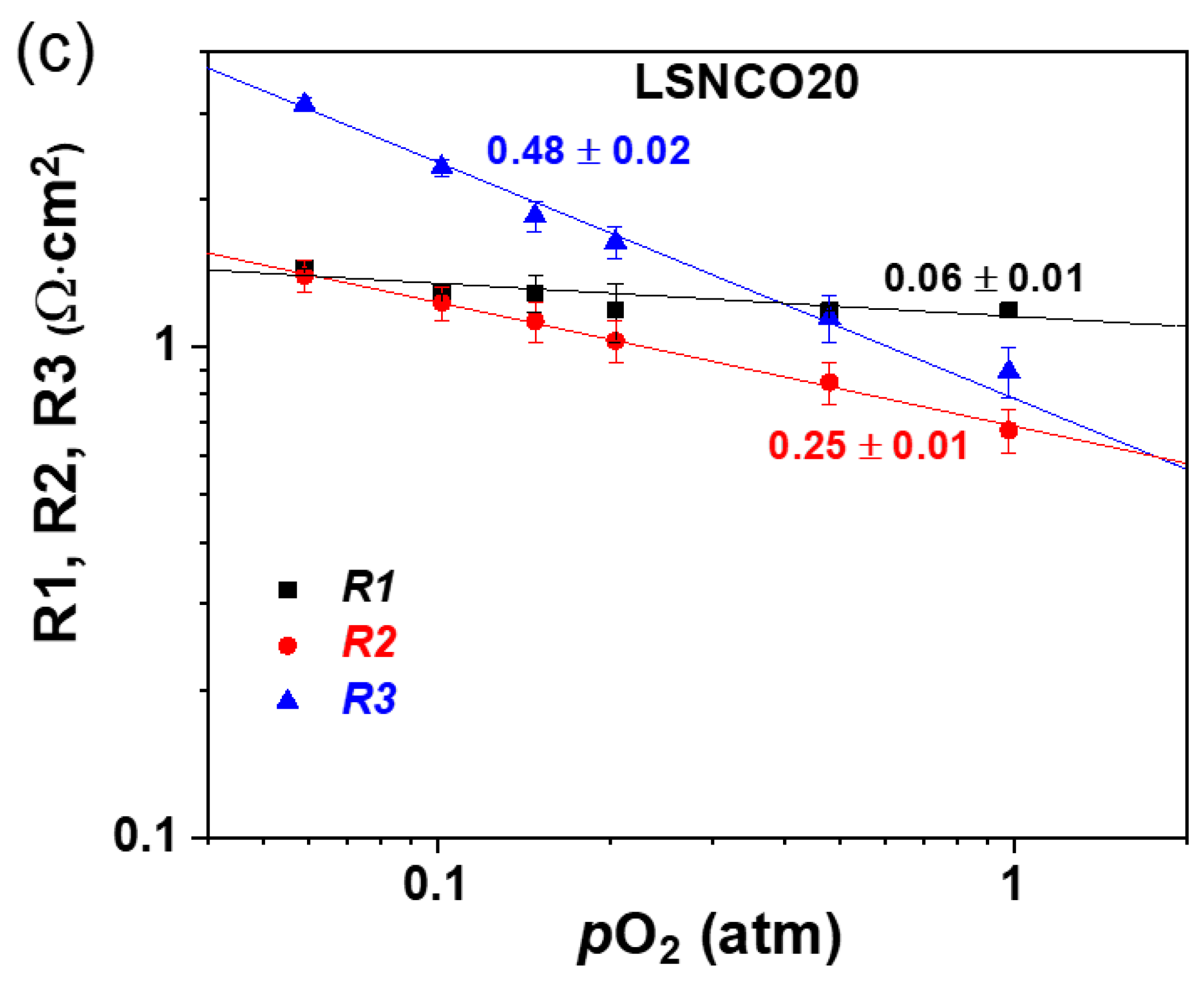
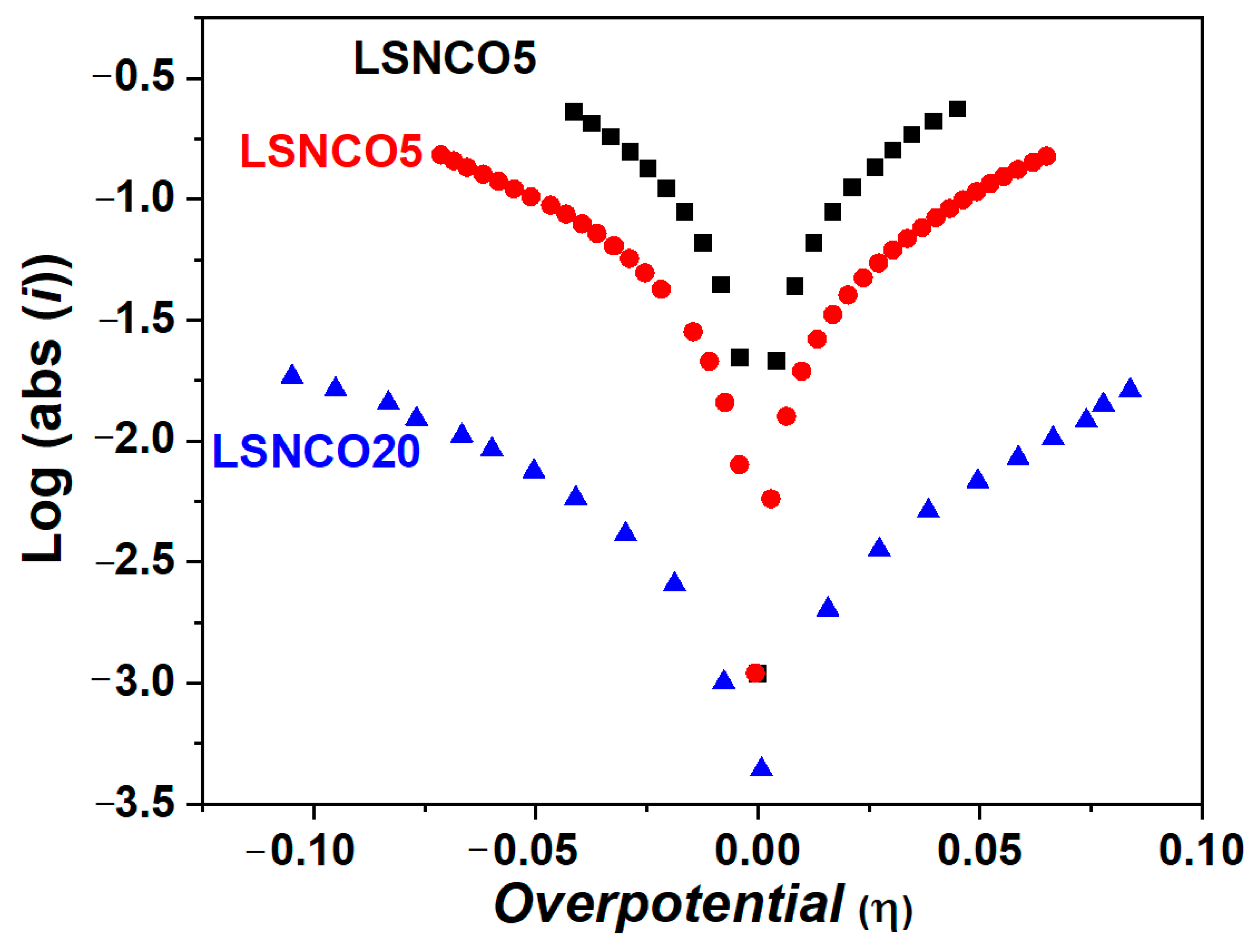

| Phase Composition | δ-Value |
|---|---|
| La2Ni0.8Co0.2O4+δ (LNCO) | 0.19 |
| La1.95Sr0.05Ni0.8Co0.2O4+δ (LSNCO5) | 0.18 |
| La1.9Sr0.1Ni0.8Co0.2O4+δ (LSNCO10) | 0.16 |
| La1.8Sr0.2Ni0.8Co0.2O4+δ (LSNCO20) | 0.10 |
| La1.6Sr0.4Ni0.8Co0.2O4+δ (LSNCO40) | 0.03 |
| La1.2Sr0.8Ni0.8Co0.2O4+δ (LSNCO80) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unachukwu, I.D.; Vibhu, V.; Vinke, I.C.; Eichel, R.-A.; de Haart, L.G.J. Sr Substituted La2−xSrxNi0.8Co0.2O4+δ (0 ≤ x ≤ 0.8): Impact on Oxygen Stoichiometry and Electrochemical Properties. Energies 2022, 15, 2136. https://doi.org/10.3390/en15062136
Unachukwu ID, Vibhu V, Vinke IC, Eichel R-A, de Haart LGJ. Sr Substituted La2−xSrxNi0.8Co0.2O4+δ (0 ≤ x ≤ 0.8): Impact on Oxygen Stoichiometry and Electrochemical Properties. Energies. 2022; 15(6):2136. https://doi.org/10.3390/en15062136
Chicago/Turabian StyleUnachukwu, Ifeanyichukwu D., Vaibhav Vibhu, Izaak C. Vinke, Rüdiger-A. Eichel, and L. G. J. (Bert) de Haart. 2022. "Sr Substituted La2−xSrxNi0.8Co0.2O4+δ (0 ≤ x ≤ 0.8): Impact on Oxygen Stoichiometry and Electrochemical Properties" Energies 15, no. 6: 2136. https://doi.org/10.3390/en15062136
APA StyleUnachukwu, I. D., Vibhu, V., Vinke, I. C., Eichel, R.-A., & de Haart, L. G. J. (2022). Sr Substituted La2−xSrxNi0.8Co0.2O4+δ (0 ≤ x ≤ 0.8): Impact on Oxygen Stoichiometry and Electrochemical Properties. Energies, 15(6), 2136. https://doi.org/10.3390/en15062136








