Insight into Geochemical Significance of NO Compounds in Lacustrine Shale Source Rocks by FT-ICR MS
Abstract
:1. Introduction
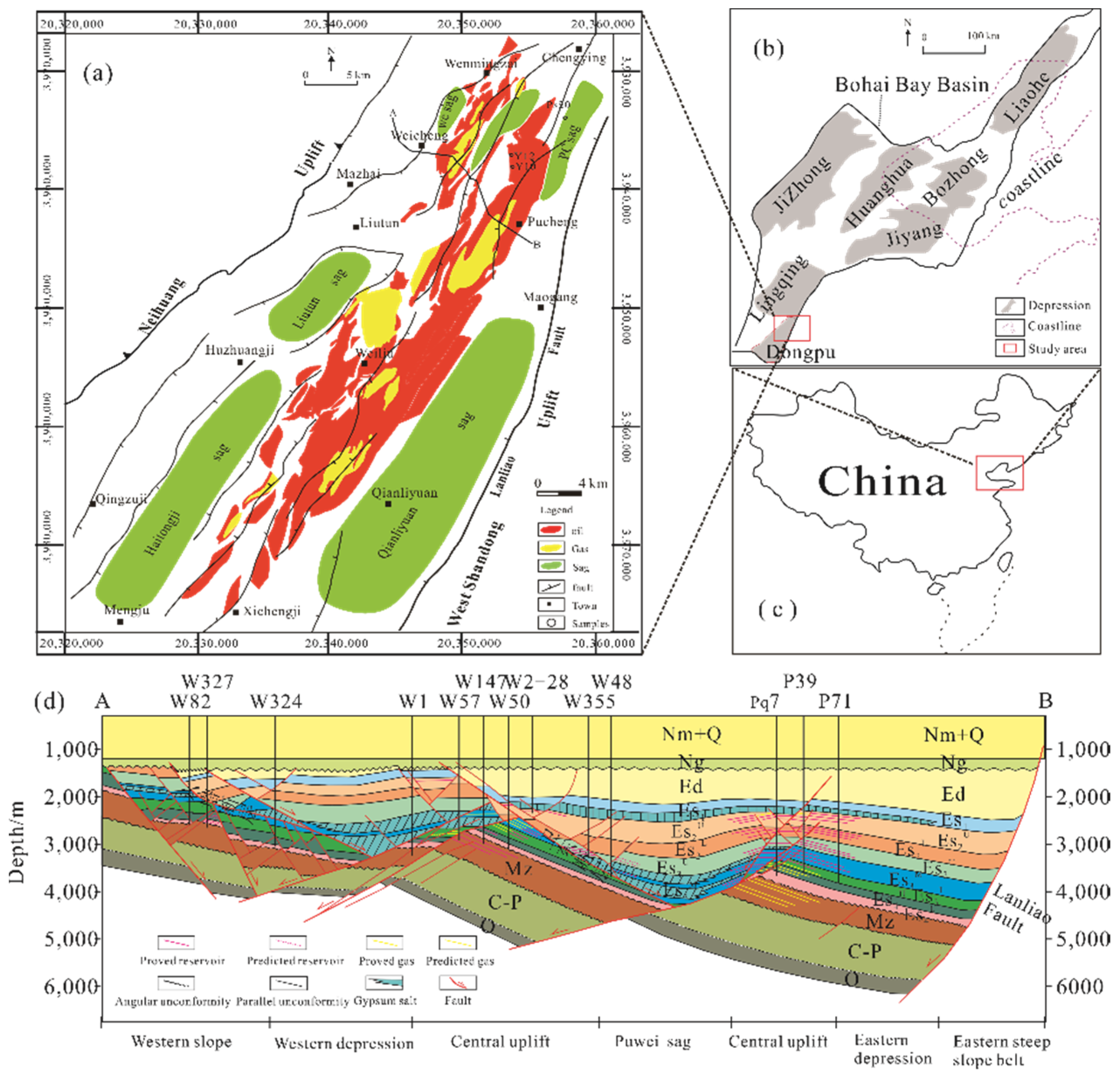
2. Geologic Setting
3. Materials and Methods
3.1. Samples
3.2. Rock-Eval Pyrolysis and TOC
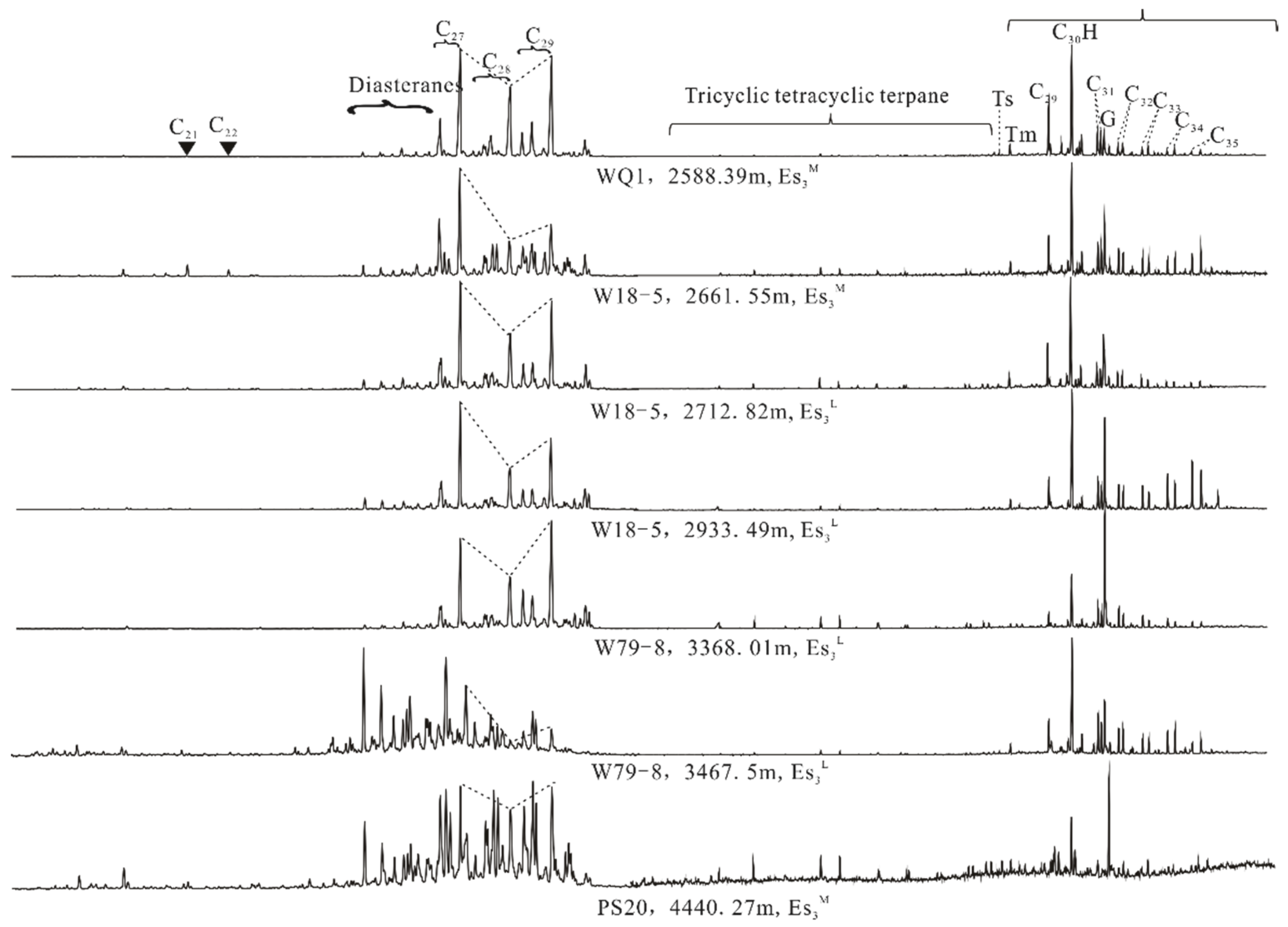
3.3. GC-MS Analysis
3.4. FT-ICR MS Analysis
4. Results
4.1. Conventional Characteristics of the Source Rocks
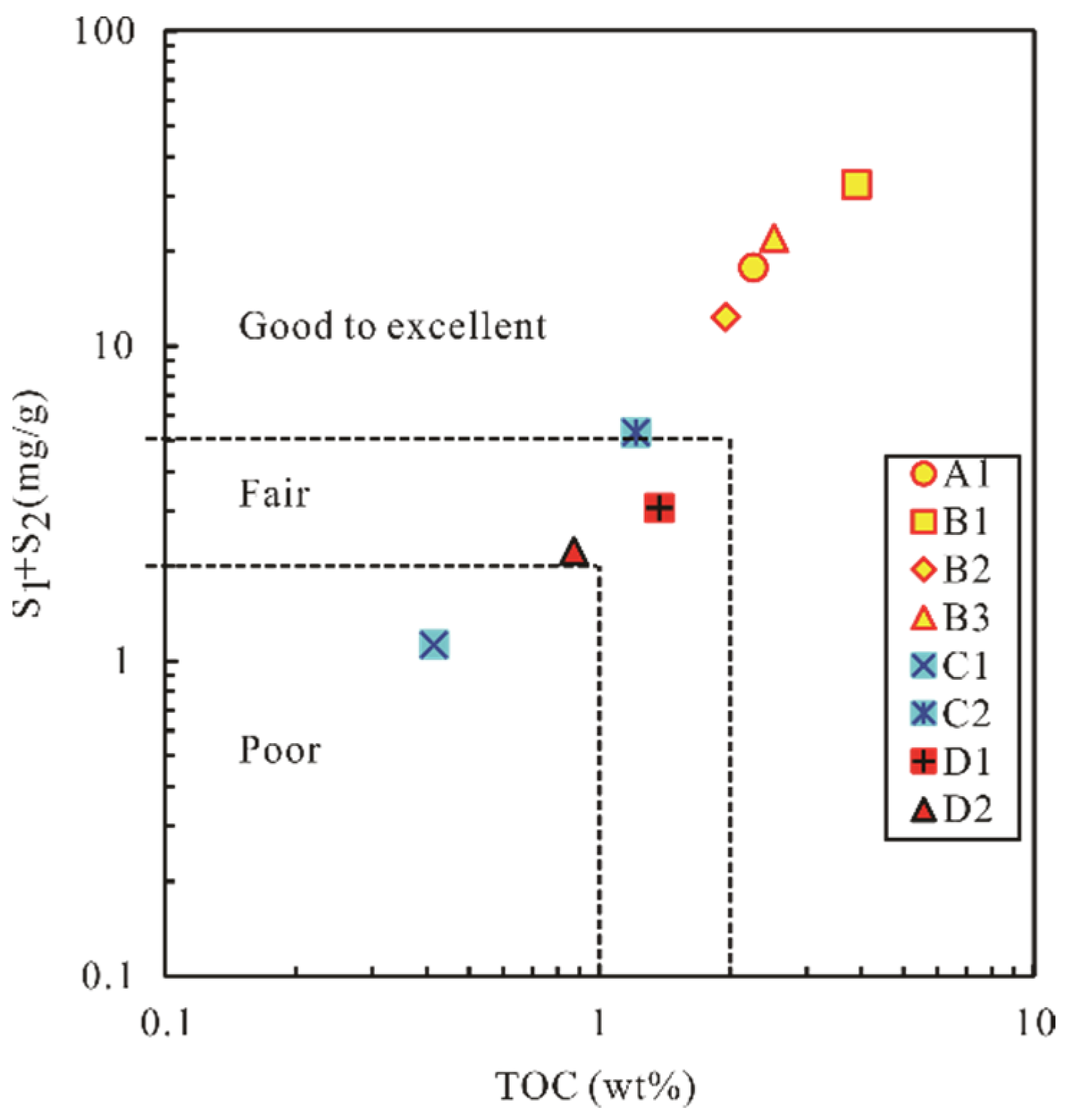
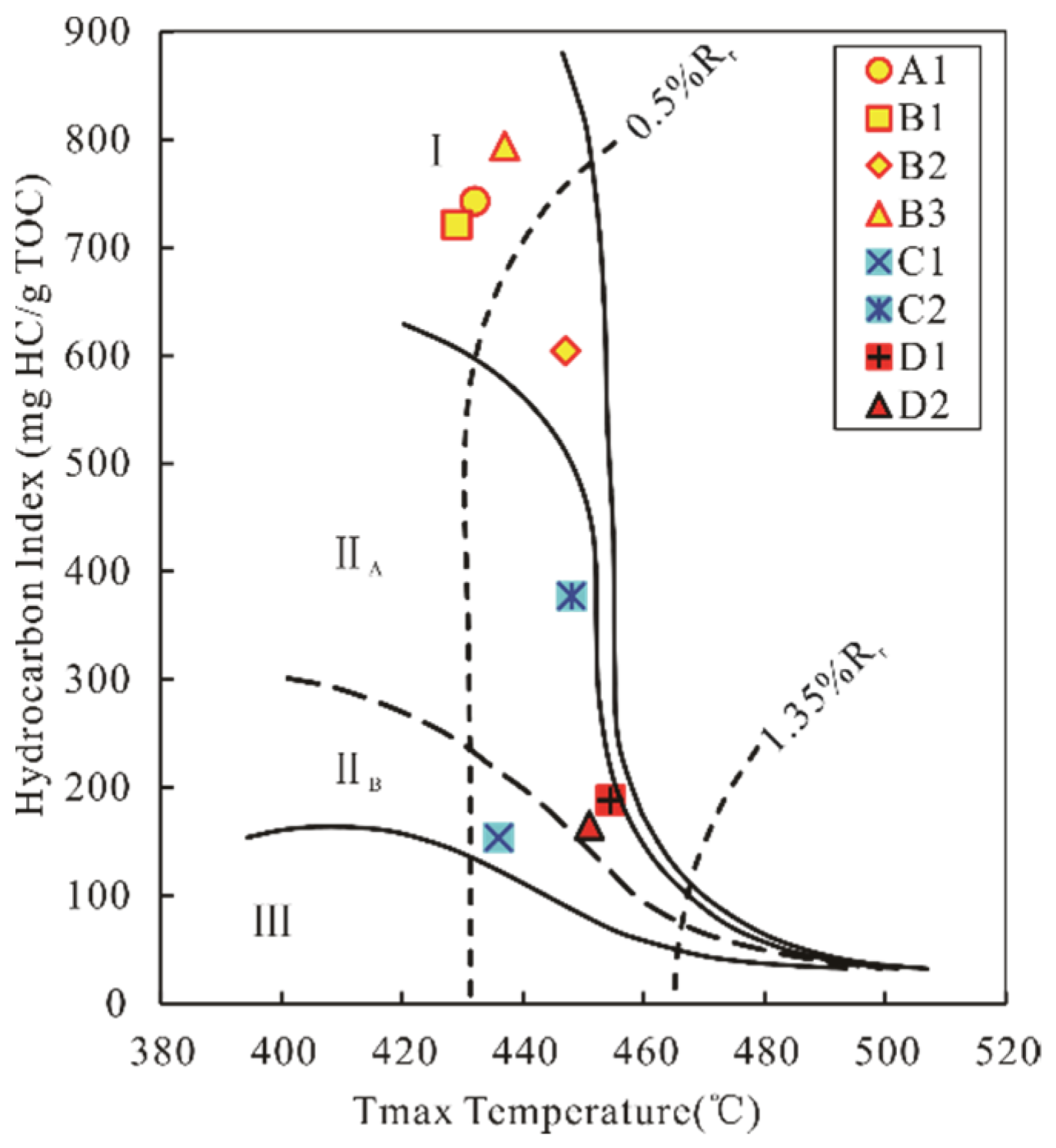
4.1.1. Source Rock Evaluation
4.1.2. Geochemical Characteristics of Soluble Organic Matter in Source Rock
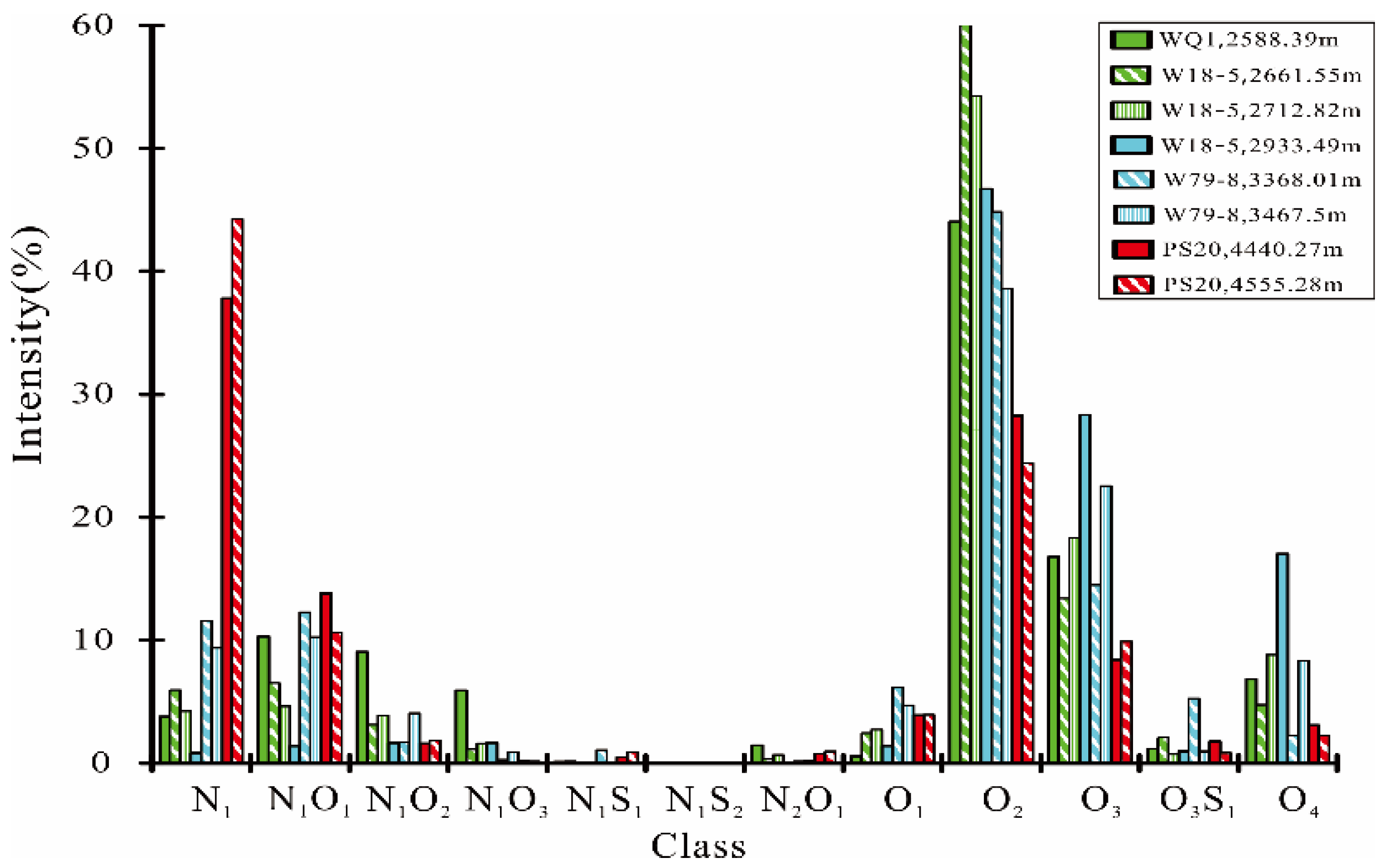
4.2. Characteristics of NSO Compounds Revealed by FT-ICR MS
4.2.1. General Chemical Composition of Nitrogen and Oxygen Groups
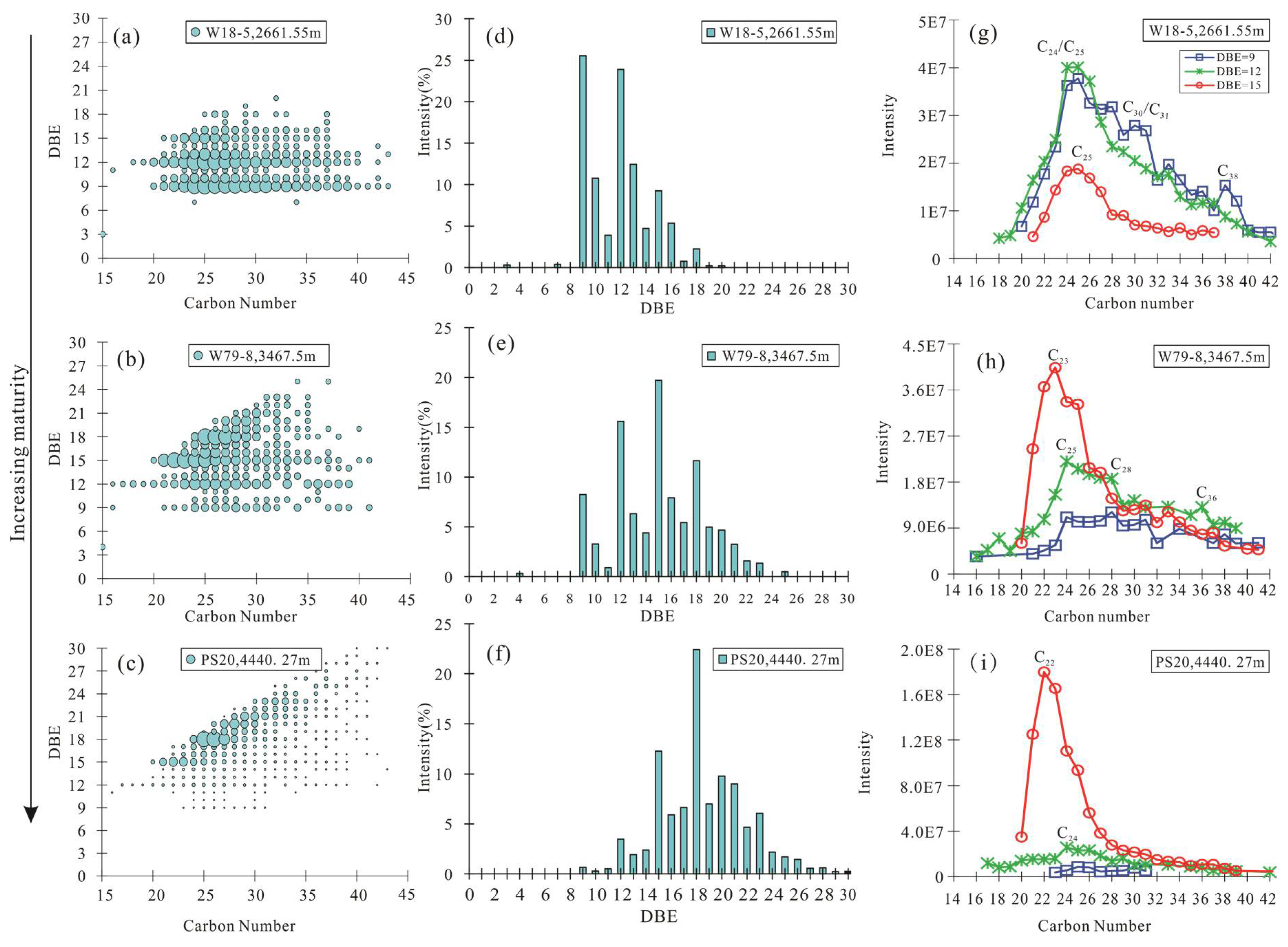
4.2.2. Composition and Distribution of N1 Class
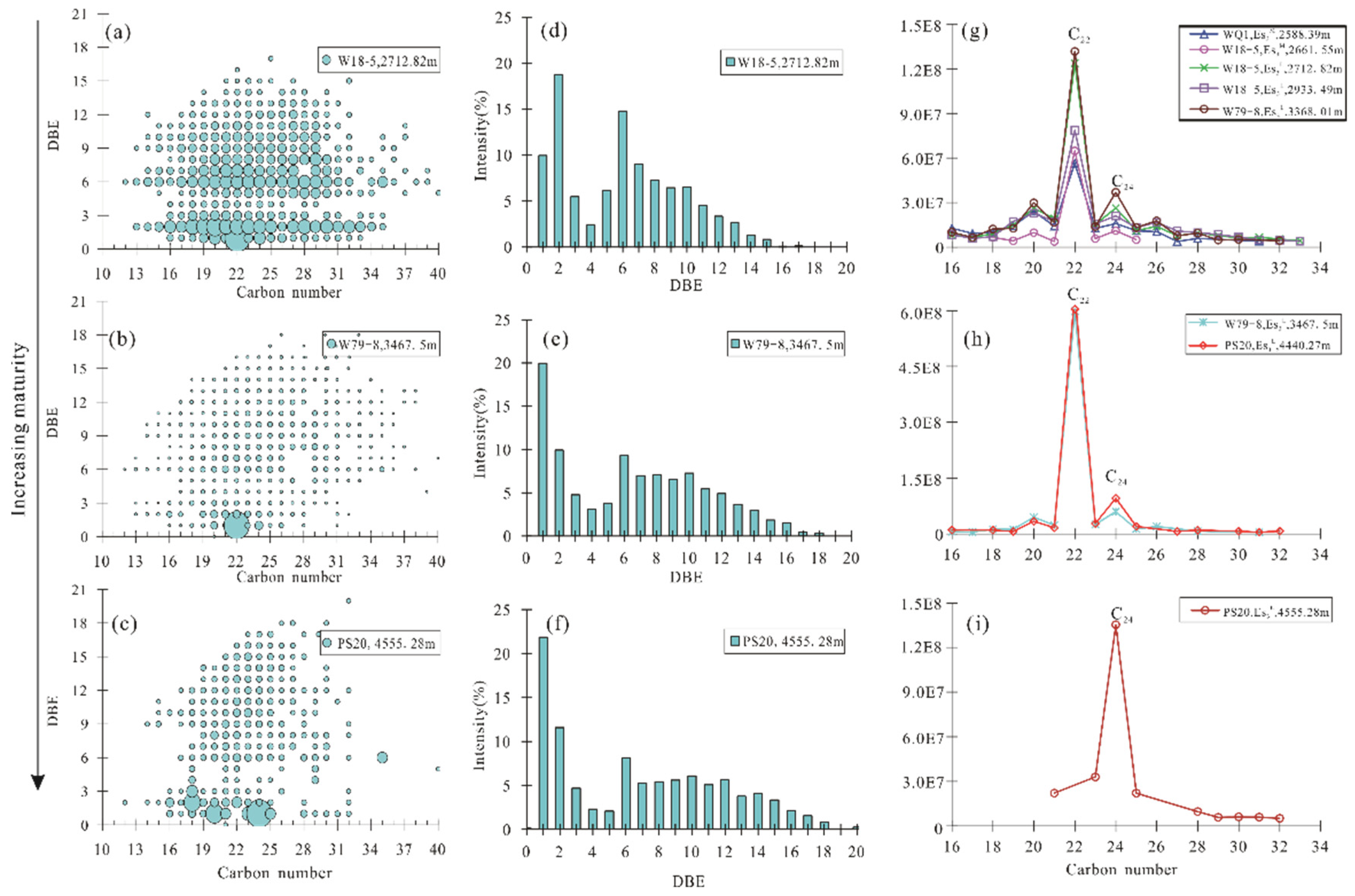
4.2.3. Composition and Distribution of O2 Class
4.2.4. Composition and Distribution of O3 Class
5. Discussion
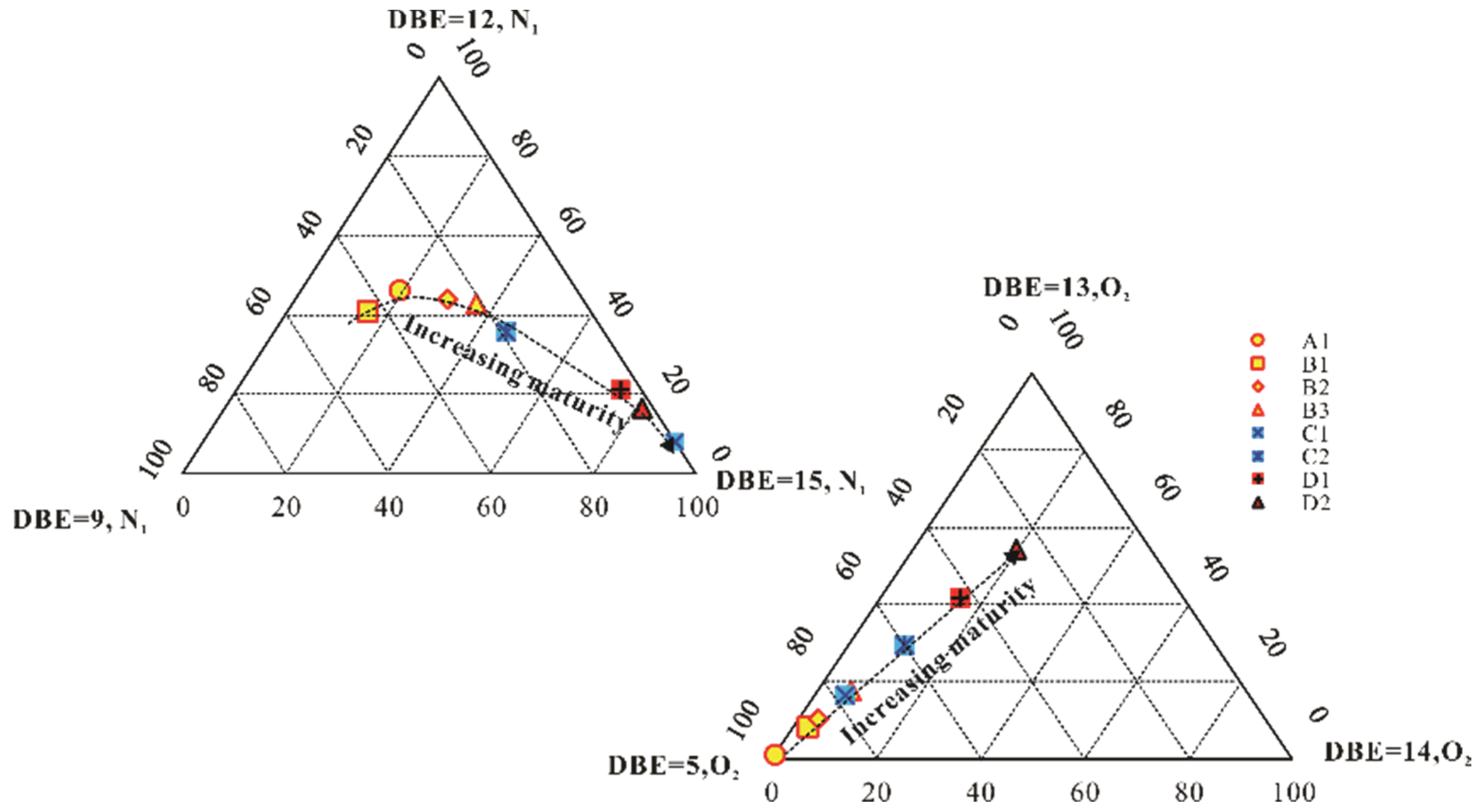
5.1. Maturity Evaluation
5.2. Origins of the Organic Matter
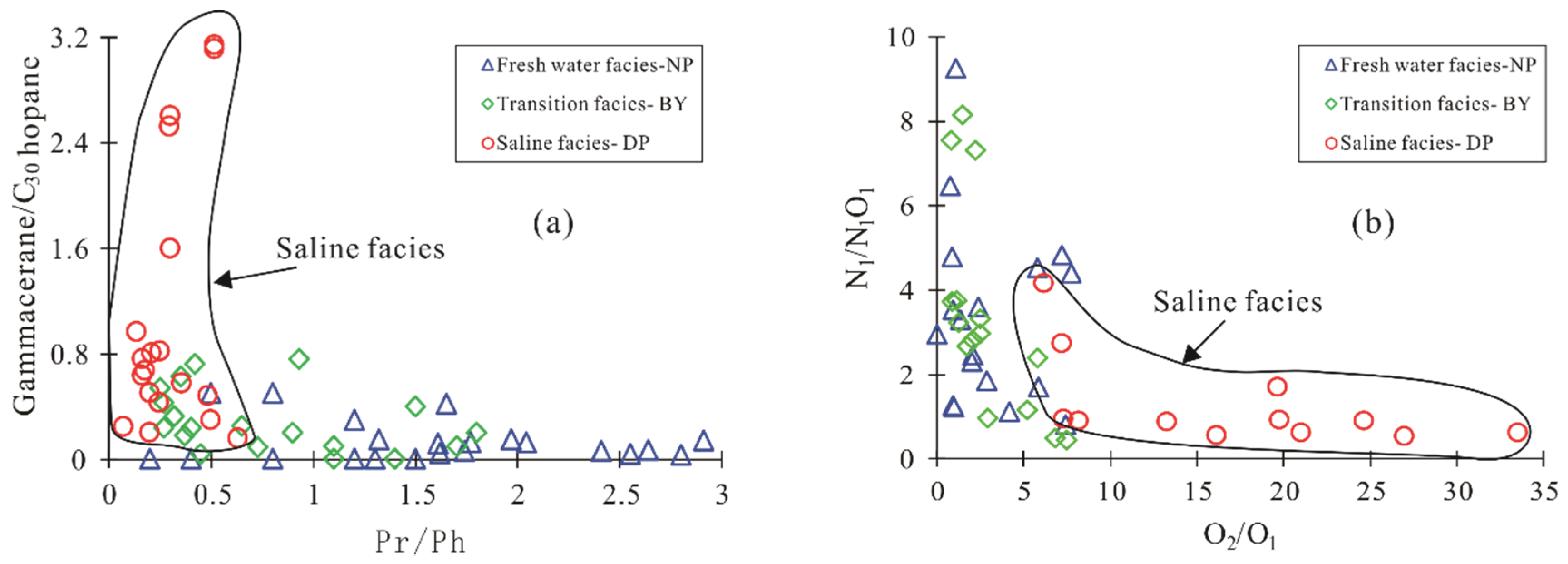
5.3. Paleoenvironment Identification
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zachariah, S.B.; Petri, U.K.; Vahur, O.; Ville, A. Hydrogen solubility of shale oil containing polar phenolic compounds. Ind. Eng. Chem. Res. 2017, 56, 8738–8747. [Google Scholar]
- Zhang, L.; Lu, X.; Liu, X.; Li, Q.; Cheng, Y.; Hou, Q.; Cai, J. Distribution and mobility of crude oil-brine in clay mesopores: Insights from molecular dynamics simulations. Langmuir 2019, 35, 14818–14832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, J.; Xia, L.; Xiang, B.; Li, E. Investigating biological nitrogen cycling in lacustrine systems by FT-ICR-MS analysis of nitrogen-containing compounds in petroleum. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 556, 109887. [Google Scholar] [CrossRef]
- Bai, L.; Liu, B.; Yang, J.; Tian, S.; Wang, B.; Huang, S. Differences in hydrocarbon composition of shale oils in different phase states from the Qingshankou Formation, Songliao Basin, as determined from fluorescence experiments. Front. Earth Sci. 2021, 15, 438–456. [Google Scholar] [CrossRef]
- Li, M.W.; Liu, P.; Jiang, Q.G.; Li, Z.M.; Cao, T.T. Effect of thermal maturation on the composition of polar organic compounds in the Eocene lacustrine shales of the Bohai Bay Basin in eastern China as revealed by FT-ICR-MS analysis of hydrous pyrolysates. In Proceedings of the AAPG Annual Conference and Exhibition, Houston, TX, USA, 6–9 April 2014. [Google Scholar]
- Eseme, E.; Littke, R.; Agyingi, C.M. Geochemical characterization of a cretaceous black shale from the Mamfe Basin, Cameroon. Pet. Geosci. 2006, 12, 69–74. [Google Scholar] [CrossRef]
- Fu, X.; Jian, W.; Zeng, Y.; Li, Z.; Wang, Z. Geochemical and palynological investigation of the Shengli river marine oil shale (China): Implications for paleoenvironment and paleoclimate. Int. J. Coal Geol. 2009, 78, 217–224. [Google Scholar] [CrossRef]
- Bastow, T.P.; Aarssen, B.; Alexander, R.; Kagi, R.I. Origins of alkylphenols in crude oils: Hydroxylation of alkylbenzenes. Org. Geochem. 2005, 36, 991–1001. [Google Scholar] [CrossRef]
- Liao, Y.; Shi, Q.; Hsu, C.S.; Pan, Y.; Zhang, Y. Distribution of acids and nitrogen-containing compounds in biodegraded oils of the Liaohe Basin by negative ion ESI FT-ICR MS. Org. Geochem. 2012, 47, 51–65. [Google Scholar] [CrossRef]
- Hughey, C.A.; Rodgers, R.P.; Marshall, A.G.; Walters, C.C.; Qian, K.; Mankiewicz, P. Acidic and neutral polar NSO compounds in Smackover oils of different thermal maturity revealed by electrospray high field Fourier transform ion cyclotron resonance mass spectrometry. Org. Geochem. 2004, 35, 863–880. [Google Scholar] [CrossRef]
- Liu, P.; Li, M.; Jiang, Q.; Cao, T.; Sun, Y. Effect of secondary oil migration distance on composition of acidic NSO compounds in crude oils determined by negative-ion electrospray Fourier transform ion cyclotron resonance mass spectrometry. Org. Geochem. 2015, 78, 23–31. [Google Scholar] [CrossRef]
- Tang, Y.; Wen, Z.; Dou, L.; Youde, X. Research on the direction of secondary migration of oil by making use of nitrogen compounds as tracers in the No. 6 area of the Melut Basin. Chin. J. Geochem. 2006, 25, 182–183. [Google Scholar] [CrossRef]
- Zhang, L.C. Oil migration in the Lunnan region, Tarim basin, China based on the pyrrolic nitrogen compound distribution. J. Pet. Sci. Eng. 2004, 41, 123–134. [Google Scholar]
- Wang, M.; Guo, Z.; Jiao, C.; Lu, S.; Li, J.; Xue, H.; Jijun, L.; Li, J.; Chen, G. Exploration progress and geochemical features of lacustrine shale oils in China. J. Pet. Sci. Eng. 2019, 178, 975–986. [Google Scholar] [CrossRef]
- Hu, T.; Pang, X.; Jiang, F.; Wang, Q.; Zhang, C. Movable oil content evaluation of lacustrine organic-rich shales: Methods and a novel quantitative evaluation model. Earth-Sci. Rev. 2021, 214, 103545. [Google Scholar] [CrossRef]
- Hu, T.; Pang, X.-Q.; Jiang, F.-J.; Wang, Q.-F.; Wu, G.-Y.; Liu, X.-H.; Jiang, S.; Li, C.-R.; Xu, T.-W.; Chen, Y.-Y. Key factors controlling shale oil enrichment in saline lacustrine rift basin: Implications from two shale oil wells in Dongpu depression, Bohai Bay Basin. Pet. Geosci. 2021, 18, 687–711. [Google Scholar] [CrossRef]
- Wang, M.; Sherwood, N.; Li, Z.S.; Lu, S.F.; Wang, W.G.; Huang, A.H.; Lu, K. Shale oil occurring between salt intervals in the Dongpu Depression, Bohai Bay Basin, China. Int. J. Coal Geol. 2015, 152, 100–112. [Google Scholar] [CrossRef]
- Ji, H.; Li, S.; Zhang, H.; Pang, X.; Zhou, Y.; Xiang, L. Molecular characterization of NSO compounds and paleoenvironment implication for saline lacustrine oil sands by positive-ion mass spectrometry coupled with Fourier-transform ion cyclotron resonance mass spectrometry. ACS Omega 2021, 6, 25680–25691. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, H.; Wan, Z.; Pang, X.; Zhou, Y. Geochemical characteristics and factors controlling the deep lithologic reservoirs in Puwei Sag, Dongpu Depression—A case study of well PS20. J. Pet. Sci. Eng. 2021, 203, 108669. [Google Scholar] [CrossRef]
- Jiang, Z.R.; Zuo, Y.H.; Yang, M.H.; Zhang, Y.X.; Zhou, Y.S. Source rocks evaluation of the Paleogene Shahejie 3 Formation in the Dongpu Depression, Bohai Bay Basin. Energy Explor. Exploit. 2019, 37, 394–411. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zeng, X.; Cai, J.; Wang, X.; Mu, X.; Zhang, Y. Mudrocks lithofacies characteristics and North-South hydrocarbon generation difference of the Shahejie Formation in the Dongpu Sag. Minerals 2021, 11, 535. [Google Scholar] [CrossRef]
- Shi, Q.; Zhao, S.; Xu, Z.; Chung, K.H.; Zhang, Y.; Xu, C. Distribution of acids and neutral nitrogen compounds in a Chinese crude oil and its fractions: Characterized by negative-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2010, 24, 4005–4011. [Google Scholar] [CrossRef]
- Peters, K.E.; Cassa, M.R. Applied Source Rock Geochemistry. In The Petroleum System—From Source to Trap; Magoon, L.B., Dow, W.G., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1994; Volume 60, pp. 93–111. [Google Scholar]
- Hazra, B.; Dutta, S.; Kumar, S. TOC calculation of organic matter rich sediments using rock-eval pyrolysis: Critical consideration and insights. Int. J. Coal Geol. 2017, 169, 106–115. [Google Scholar] [CrossRef]
- Koopmans, M.P.; Shaeffer-Reiss, C.; de Leeuw, J.W.; Lewan, M.D.; Maxwell, J.R.; Schaeffer, P.; Sinninghe, D.J.S. Sulfur and oxygen sequestration of nC37 and nC38 unsaturated ketones in an immature kerogen and the release of their carbon skeletons during early stages of thermal maturation. Geochim. Cosmochim. Acta 1997, 61, 2397–2408. [Google Scholar] [CrossRef]
- Sampei, Y.; Inaba, T.; Suzuki, N. Abnormally abundant alkenone-derived C37, and C38 n-alkanes in Miocene Onnagawa siliceous mudstones, northeast Japan. Org. Geochem. 2003, 34, 1247–1258. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Y.; Liu, Z.; Chen, X.; Yu, J.; Di, X.; Jin, M. Long-chain branched/cyclic alkanes in recent sediment of Lake Fuxian and their environmental implications. Chin. Sci. Bull. 2014, 59, 1139–1150. [Google Scholar] [CrossRef]
- Volkman, J.K.; Eglinton, G.; Corner, E.S.; Sargent, J.R. Novel unsaturated straight-chain C37-C39 methyl and ethyl ketones in marine sediments and a coccolithophore emiliania huxleyi. Phys. Chem. Earth 1980, 12, 219–227. [Google Scholar] [CrossRef]
- Grice, K.; Schouten, S.; Peters, K.E.; Damsté, J.S. Molecular isotopic characterization of hydrocarbon biomarkers in Paleocene–Eocene evaporitic, lacustrine source rocks from the Jianghan Basin, China. Org. Geochem. 1998, 29, 1745–1764. [Google Scholar] [CrossRef]
- Peters, K.; Walters, C.; Moldowan, J. Biomarkers and Isotopes in Petroleum Systems and Earth History. In The Biomarker Guide; Cambridge University Press: Cambridge, UK, 2004; pp. 473–474. [Google Scholar]
- Farrimond, P.; Taylor, A.; Telns, N. Biomarker maturity parameters: The role of generation and thermal degradation. Org. Geochem. 1998, 29, 1181–1197. [Google Scholar] [CrossRef]
- Chen, Z.; Zha, M.; Jin, Q.; Ren, Y. Distribution of sterane maturity parameters in a lacustrine basin and their control factors: A case study from the Dongying Sag, East China. Pet. Sci. 2011, 8, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Damste, J.S.S.; Schouten, S.; Volkman, J.K. C-27-C-30 neohop-13(18)-Enes and their saturated and aromatic derivatives in sediments: Indicators for diagenesis and water column stratification. Geochim. Cosmochim. Acta 2014, 133, 402–421. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, C.; Cheng, M.; Algeo, T.J.; Jin, C.; Tang, F.; Huang, J. Evidence for highly complex redox conditions and strong water-column stratification in an early Cambrian Continental-Margin Sea. Geochem. Geophys. Geosyst. 2018, 19, 2397–2410. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhan, X.; Gao, Y.; Xia, J.; Wang, S.; Zou, Y.R. Geochemical signatures and controlling factors of rearranged hopanes in source rocks and oils from representative Basins of China. ACS Omega 2020, 5, 30160–30167. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pang, X.; Shi, Q.; Zhang, B.; Zhang, H.; Pan, N.; Zhao, M. Origin of the unusually high dibenzothiophene concentrations in Lower Ordovician oils from the Tazhong Uplift, Tarim Basin, China. Pet. Sci. 2011, 8, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yao, H.; Stasiuk, L.D.; Fowler, M.G.; Larter, S.R. Effect of maturity and petroleum expulsion on pyrrolic nitrogen compound yields and distributions in Duvernay formation petroleum source rocks in central Alberta, Canada. Org. Geochem. 1997, 26, 731–744. [Google Scholar] [CrossRef]
- Snyder, L.R. Distribution of benzcarbazole isomers in petroleum as evidence for their biogenic origin. Nature 1965, 205, 277. [Google Scholar] [CrossRef]
- Li, M.W.; Larter, S.R.; Stoddart, D.; Bjoroy, M. Liquid-chromatographic separation schemes for pyrrole and pyridine nitrogen aromatic heterocycle fractions from crude oils suitable for rapid characterization of geochemical samples. Anal. Chem. 1992, 64, 1337–1344. [Google Scholar] [CrossRef]
- Hughey, C.A.; Rodgers, R.P.; Marshall, A.G.; Qian, K.; Robbins, W.K. Identification of acidic NSO compounds in crude oils of different geochemical origins by negative ion electrospray Fourier transform ion cyclotron resonance mass spectrometry. Org. Geochem. 2002, 33, 743–759. [Google Scholar] [CrossRef]
- Hamerly, T.; Tripet, B.; Wurch, L.; Hettich, R.L.; Podar, M.; Bothner, B.; Copie, V. Characterization of fatty acids in Crenarchaeota by GC-MS and NMR. Archaea 2015, 2015, 472726. [Google Scholar] [CrossRef] [Green Version]
- Zhe, L.; Zhang, Z.; Sun, Y.; Lao, Y.; Wu, W. Catalytic decarboxylations of fatty acids in immature oil source rocks. Sci. China Ser. D Earth Sci. 2003, 46, 1250–1260. [Google Scholar]
- Zhang, Z.; Sun, Y.; Lao, Y.; Lin, W. Catalytic decarboxylation of fatty acid by iron-containing minerals in immature oil source rocks at low temperature. Chin. Sci. Bull. 1999, 44, 1523–1527. [Google Scholar] [CrossRef]
- Warden, L.; Van, D.J.; Moros, M.; Damste, J.S. Sedimentary alkenone distributions reflect salinity changes in the Baltic Sea over the Holocene. Org. Geochem. 2016, 102, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Sever, J.; Parker, P.L. Fatty alcohols (normal and isoprenoid) in sediments. Science 1969, 164, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.Z.; Wang, J.; Xin, L.I.; Zhang, X.B. Study on the hydrocarbon-generating and pool-forming conditions of immature oil in the Raoyang Depression of the Bohaiwan Basin. Pet. Geol. Exp. 2003, 25, 566–572. [Google Scholar]
- Li, S.; Amrani, A.; Pang, X.; Yang, H.; Said-Ahmad, W.; Zhang, B.; Pang, Q. Origin and quantitative source assessment of deep oils in the Tazhong uplift, Tarim Basin. Org. Geochem. 2015, 78, 1–22. [Google Scholar] [CrossRef]
- Li, S.; Shi, Q.; Pang, X.; Zhang, B.; Zhang, H. Origin of the unusually high dibenzothiophene oils in Tazhong-4 oilfield of Tarim Basin and its implication in deep petroleum exploration. Org. Geochem. 2012, 48, 56–80. [Google Scholar] [CrossRef]
- Wan, Z.; Li, S.; Pang, X.; Dong, Y.; Wang, Z.; Chen, X.; Meng, X.; Shi, Q. Characteristics and geochemical significance of heteroatom compounds in terrestrial oils by negative-ion electrospray Fourier transform ion cyclotron resonance mass spectrometry. Org. Geochem. 2017, 111, 34–55. [Google Scholar] [CrossRef]
- Barrow, M.P.; Mcdonnell, L.A.; Feng, X.; Walker, J.; Derrick, P.J. Determination of the nature of naphthenic acids present in crude oils using Nanospray Fourier transform ion cyclotron resonance mass spectrometry: The continued battle against corrosion. Anal. Chem. 2003, 75, 860–866. [Google Scholar] [CrossRef]
- Jaffe, R.; Gardinali, P.R. Generation and maturation of carboxylic acids in ancient sediments from the Maracaibo Basin, Venezuela. Org. Geochem. 1990, 16, 211–218. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, H.; Wang, P.R.; Song, Y.T. Feature and evolution of NSO component in spiral algae—study on the formation mechanism of immature oil. J. Jianghan Pet. Inst. 1998, 20, 22–25. [Google Scholar]
- Oldenburg, T.P.; Brown, M.; Bennett, B.; Larter, S.R. The impact of thermal maturity level on the composition of crude oils, assessed using ultra-high resolution mass spectrometry. Org. Geochem. 2014, 75, 151–168. [Google Scholar] [CrossRef]
- Shimoyama, A.; Johns, W.D. Formation of alkanes from fatty acids in the presence of CaCO3. Geochim. Cosmochim. Acta 1972, 36, 87–91. [Google Scholar] [CrossRef]
- Gillan, F.T.; Stoilov, I.L.; Thompson, J.E.; Hogg, R.W.; Wilkinson, C.R.; Djerassi, C. Fatty acids as biological markers for bacterial symbionts in sponges. Lipids 1988, 23, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Kennicutt, M.C.; Brooks, J.M. Unusual normal alkane distributions in offshore New Zealand sediments. Org. Geochem. 1990, 15, 193–197. [Google Scholar] [CrossRef]
- Fang, J.; Wu, F.; Xiong, Y.; Li, F.; Du, X.; An, D.; Wang, L. Source characterization of sedimentary organic matter using molecular and stable carbon isotopic composition of n-alkanes and fatty acids in sediment core from Lake Dianchi, China. Sci. Total Environ. 2014, 473, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Grimalt, J.; Albaiges, J. Source and occurrence of C12-C22 n-alkane distributions with even carbon-number preference in sedimentary environments. Geochim. Cosmochim. Acta 1987, 51, 1379–1384. [Google Scholar] [CrossRef]
- Fu, J.; Sheng, G.; Xu, J.; Eglinton, G.; Gowar, A.P.; Jia, R.; Peng, P.A. Application of biological markers in the assessment of paleoenvironments of Chinese non-marine sediments. Org. Geochem. 1990, 16, 769–779. [Google Scholar]
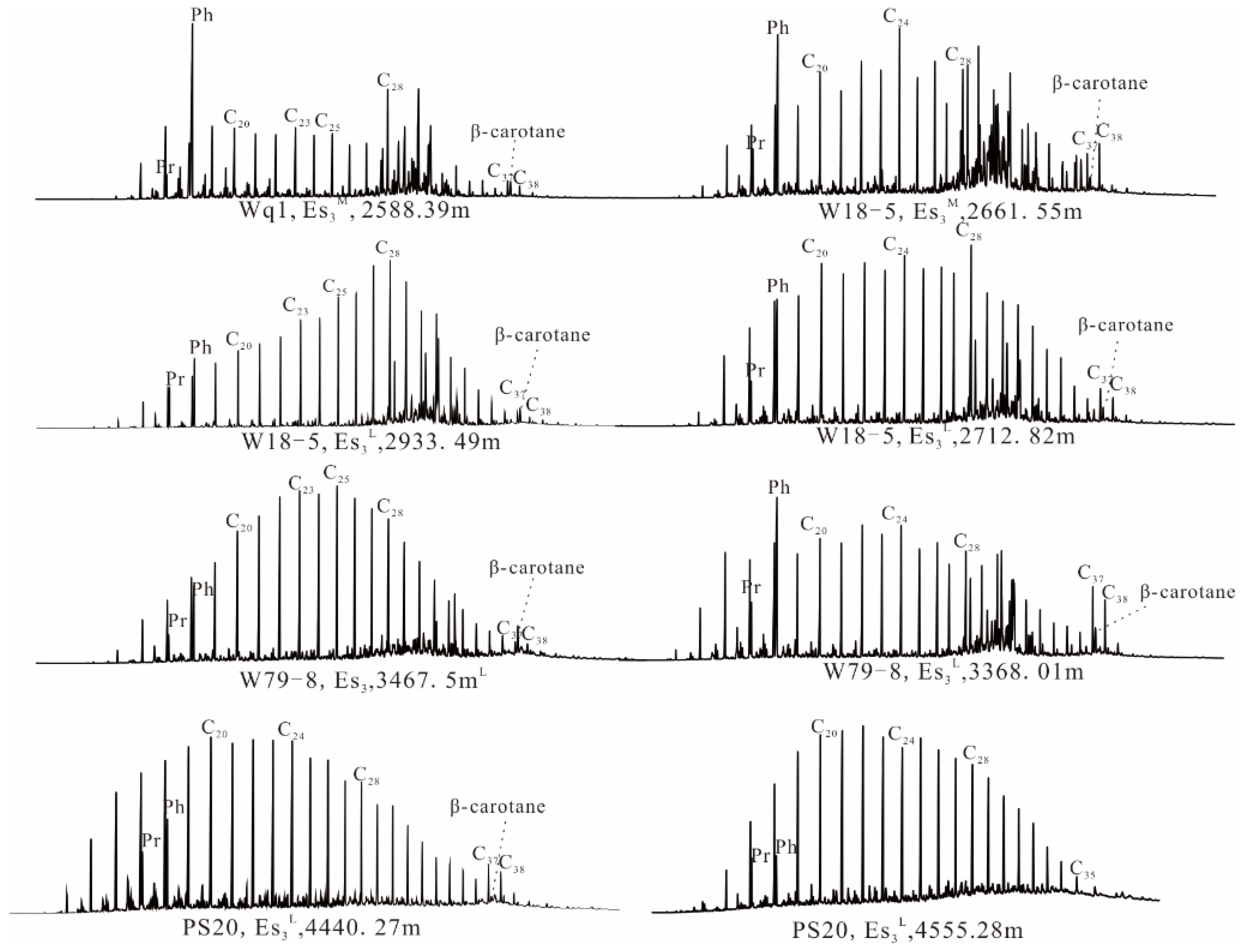


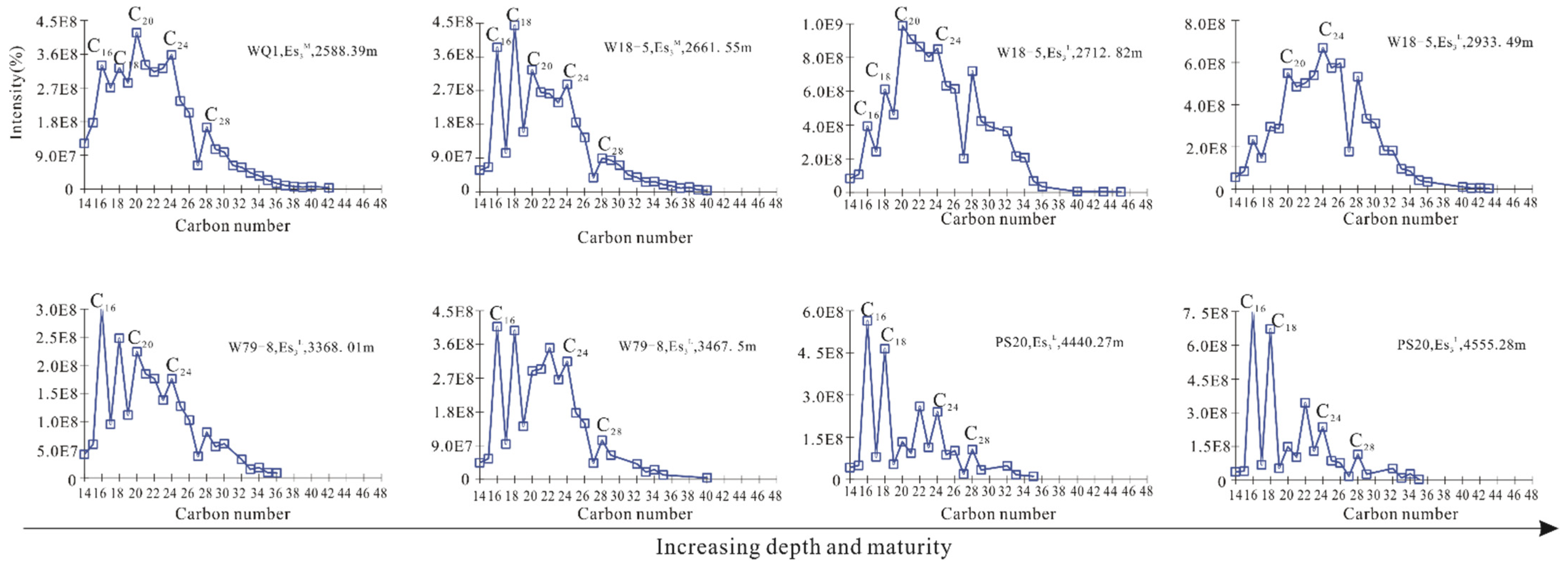
| Well | WQ1 | W18-5 | W18-5 | W18-5 | W79-8 | W79-8 | PS20 | PS20 |
|---|---|---|---|---|---|---|---|---|
| Depth (m) | 2588.39 | 2661.55 | 2712.82 | 2933.49 | 3368.01 | 3467.5 | 4440.27 | 4555.28 |
| Strata | Es3M | Es3M | Es3L | Es3L | Es3L | Es3L | Es3M | Es3L |
| Lithology | Brown-Dark Oil Shale | Brown-Dark Oil Shale | Brown-Dark Oil Shale | Brown-Dark Oil Shale | Brown-Dark Oil Shale | Grey-Dark Oil Shale | Grey-Dark Mudstone | Grey-Dark Mudstone |
| Lab. No. | A1 | B1 | B2 | B3 | C1 | C2 | D1 | D2 |
| TOC (%) | 2.26 | 3.90 | 1.95 | 2.52 | 0.42 | 1.21 | 1.37 | 0.87 |
| S1 + S2 (mg/g) | 17.78 | 32.60 | 12.39 | 22.06 | 1.13 | 5.33 | 3.07 | 2.24 |
| Tmax (°C) | 432.00 | 429.00 | 447.00 | 437.00 | 426.00 | 448.00 | 454.50 | 451.00 |
| HI (mg/g) | 742.92 | 721.03 | 604.10 | 793.65 | 153.85 | 377.69 | 188.69 | 165.14 |
| O.M.Type | I | I | I | I | IIB | IIA | I | IIA |
| CPI | 0.76 | 0.90 | 0.92 | 1.18 | 0.94 | 1.06 | 0.95 | / |
| OEP | 1.09 | 0.72 | 0.92 | 1.12 | 0.85 | 1.02 | 0.97 | / |
| Pr/Ph | 0.07 | 0.21 | 0.36 | 0.48 | 0.25 | 0.30 | 0.52 | 0.83 |
| Pr/nC17 | 0.29 | 0.76 | 0.79 | 1.20 | 0.57 | 0.54 | 0.40 | 0.57 |
| Ph/nC18 | 4.07 | 2.60 | 1.53 | 1.91 | 1.77 | 1.22 | 0.67 | 0.42 |
| C21–22/C28–29 | 0.56 | 0.72 | 0.79 | 0.39 | 1.11 | 1.07 | 1.54 | / |
| ∑nC21−/∑nC22+ | 0.85 | 0.54 | 0.50 | 0.28 | 0.73 | 0.38 | 0.74 | / |
| Dia/reg | 0.08 | 0.14 | 0.14 | 0.15 | 0.09 | 0.31 | 0.82 | 1.20 |
| C27/C29 S | 0.91 | 1.24 | 0.86 | 0.99 | 0.51 | 0.95 | 1.96 | |
| 20S | 0.17 | 0.36 | 0.26 | 0.24 | 0.27 | 0.43 | 0.47 | 0.57 |
| αββ | 0.23 | 0.39 | 0.25 | 0.22 | 0.23 | 0.48 | 0.58 | 0.57 |
| Ts/(Ts + Tm) | 0.18 | 0.24 | 0.23 | 0.27 | 0.25 | 0.34 | 0.51 | 0.90 |
| G/C30H | 0.24 | 0.80 | 0.58 | 0.48 | 0.43 | 2.52 | 3.14 | / |
| C35H/C34H | 1.10 | 1.53 | 1.54 | 0.99 | 0.62 | 0.83 | 1.52 | 3.11 |
| DBTs (%) | 9.34 | 35.34 | 16.12 | 2.89 | 5.72 | 4.66 | 3.37 | / |
| Well | WQ1 | W18-5 | W18-5 | W18-5 | W79-8 | W79-8 | PS20 | PS20 |
|---|---|---|---|---|---|---|---|---|
| Depth (m) | 2588.39 | 2661.55 | 2712.82 | 2933.49 | 3368.01 | 3467.5 | 4440.27 | 4555.28 |
| Strata | Es3M | Es3M | Es3L | Es3L | Es3L | Es3L | Es3M | Es3L |
| Lab. No. | A1 | B1 | B2 | B3 | C1 | C2 | D1 | D2 |
| N1 | 3.77 | 11.53 | 5.94 | 4.23 | 0.85 | 9.38 | 44.24 | 37.80 |
| N1O1 | 10.31 | 12.29 | 6.52 | 4.61 | 1.39 | 10.26 | 10.63 | 13.81 |
| N1O2 | 9.05 | 1.66 | 3.13 | 3.90 | 1.62 | 4.04 | 1.82 | 1.59 |
| N1O3 | 5.90 | 0.27 | 1.12 | 1.58 | 1.62 | 0.91 | 0.15 | 0.18 |
| N1S1 | 0.10 | 1.08 | 0.14 | 0.12 | 0.05 | 0.13 | 0.92 | 0.48 |
| N1S2 | 0.04 | 0.06 | 0.03 | 0.06 | 0.04 | 0.03 | 0.02 | 0.00 |
| N2O1 | 1.42 | 0.16 | 0.30 | 0.69 | 0.00 | 0.19 | 0.97 | 0.77 |
| O1 | 0.57 | 6.13 | 2.44 | 2.74 | 1.39 | 4.71 | 3.93 | 3.91 |
| O2 | 44.05 | 44.84 | 60.15 | 54.27 | 46.71 | 38.55 | 24.39 | 28.23 |
| O3 | 16.80 | 14.47 | 13.40 | 18.29 | 28.35 | 22.55 | 9.89 | 8.39 |
| O3S1 | 1.15 | 5.25 | 2.08 | 0.72 | 0.97 | 0.95 | 0.82 | 1.76 |
| O4 | 6.84 | 2.25 | 4.75 | 8.79 | 17.01 | 8.32 | 2.23 | 3.07 |
| DBE9-N1 (%) | 34.53 | 43.50 | 26.28 | 21.30 | 0.00 | 18.92 | 4.06 | 2.07 |
| DBE12-N1 | 46.08 | 40.74 | 44.06 | 42.85 | 8.12 | 35.81 | 21.18 | 16.57 |
| DBE15-N1 (%) | 19.39 | 15.76 | 29.66 | 35.86 | 91.88 | 45.27 | 74.76 | 81.36 |
| DBE5-O2 (%) | 98.81 | 88.68 | 86.79 | 77.33 | 80.18 | 63.97 | 49.75 | 34.68 |
| DBE13-O2 (%) | 0.80 | 8.62 | 8.40 | 14.70 | 11.13 | 20.83 | 28.14 | 36.42 |
| DBE14-O2 (%) | 0.39 | 2.70 | 4.81 | 7.97 | 8.68 | 15.20 | 22.11 | 28.91 |
| DBE18–25/DBE9–18-N1 | 0.04 | 0.03 | 0.07 | 0.09 | 0.36 | 0.34 | 1.11 | 0.95 |
| DBE12–20/DBE5–12-O2 | 0.02 | 0.10 | 0.11 | 0.15 | 0.18 | 0.24 | 0.63 | 0.64 |
| DBE16–23/DBE9–18-N1O1 | 0.06 | 0.07 | 0.18 | 0.21 | 0.61 | 0.43 | 1.37 | 1.26 |
| O2/O1 | 77.27 | 7.31 | 24.67 | 19.80 | 33.55 | 8.19 | 6.20 | 7.22 |
| N1/N1O1 | 0.37 | 0.94 | 0.91 | 0.92 | 0.61 | 0.91 | 4.16 | 2.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Li, S.; Zhang, H.; Pang, X.; Xu, T. Insight into Geochemical Significance of NO Compounds in Lacustrine Shale Source Rocks by FT-ICR MS. Energies 2022, 15, 1805. https://doi.org/10.3390/en15051805
Ji H, Li S, Zhang H, Pang X, Xu T. Insight into Geochemical Significance of NO Compounds in Lacustrine Shale Source Rocks by FT-ICR MS. Energies. 2022; 15(5):1805. https://doi.org/10.3390/en15051805
Chicago/Turabian StyleJi, Hong, Sumei Li, Hongan Zhang, Xiongqi Pang, and Tianwu Xu. 2022. "Insight into Geochemical Significance of NO Compounds in Lacustrine Shale Source Rocks by FT-ICR MS" Energies 15, no. 5: 1805. https://doi.org/10.3390/en15051805
APA StyleJi, H., Li, S., Zhang, H., Pang, X., & Xu, T. (2022). Insight into Geochemical Significance of NO Compounds in Lacustrine Shale Source Rocks by FT-ICR MS. Energies, 15(5), 1805. https://doi.org/10.3390/en15051805





