Numerical Investigation of the Ignition Delay Time of Kerosene Premixed Combustion in an SI Engine
Abstract
:1. Introduction
2. Methodology
3. Results of Ignition Delay Time
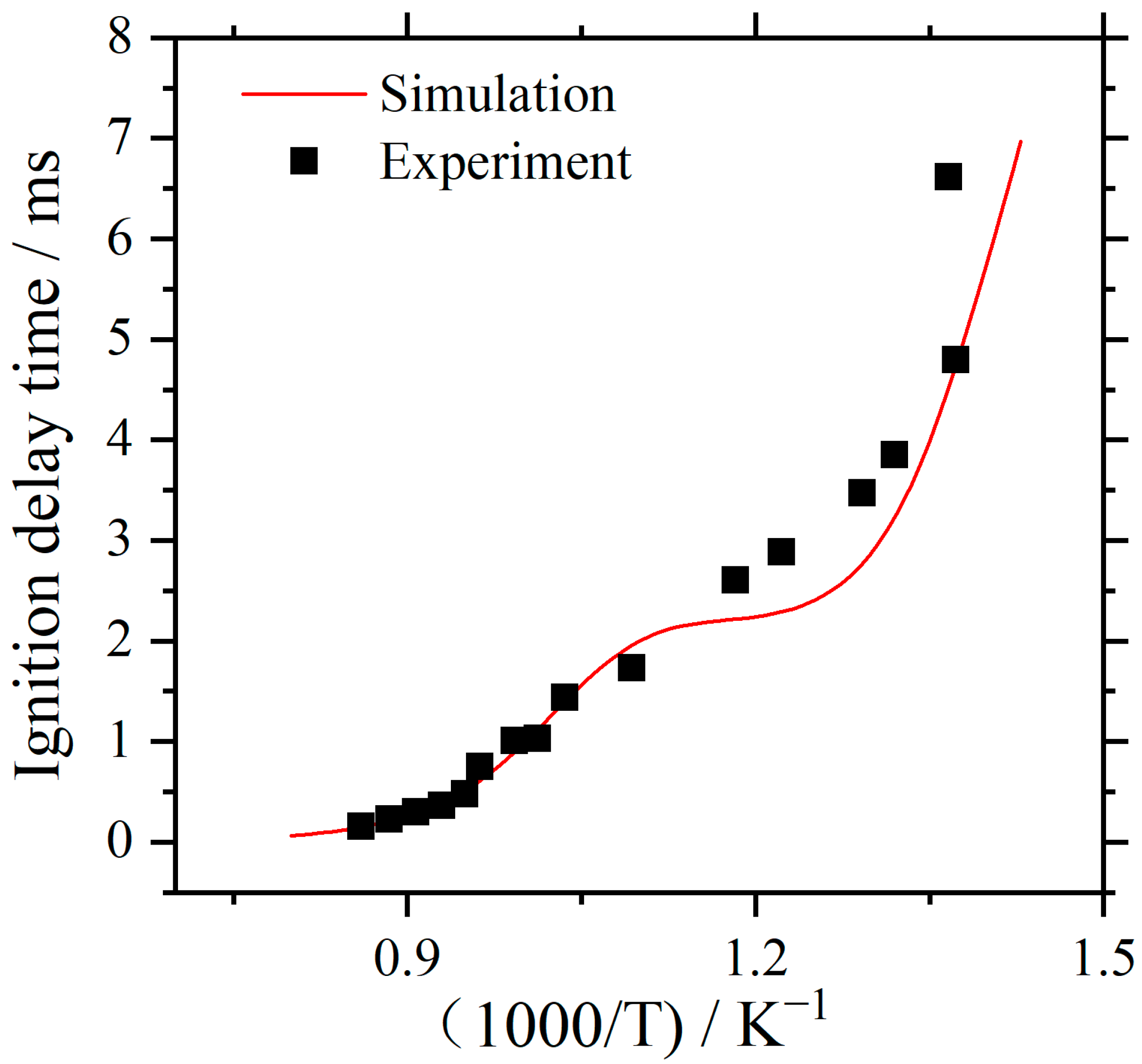
3.1. Ignition Delay Time of Kerosene
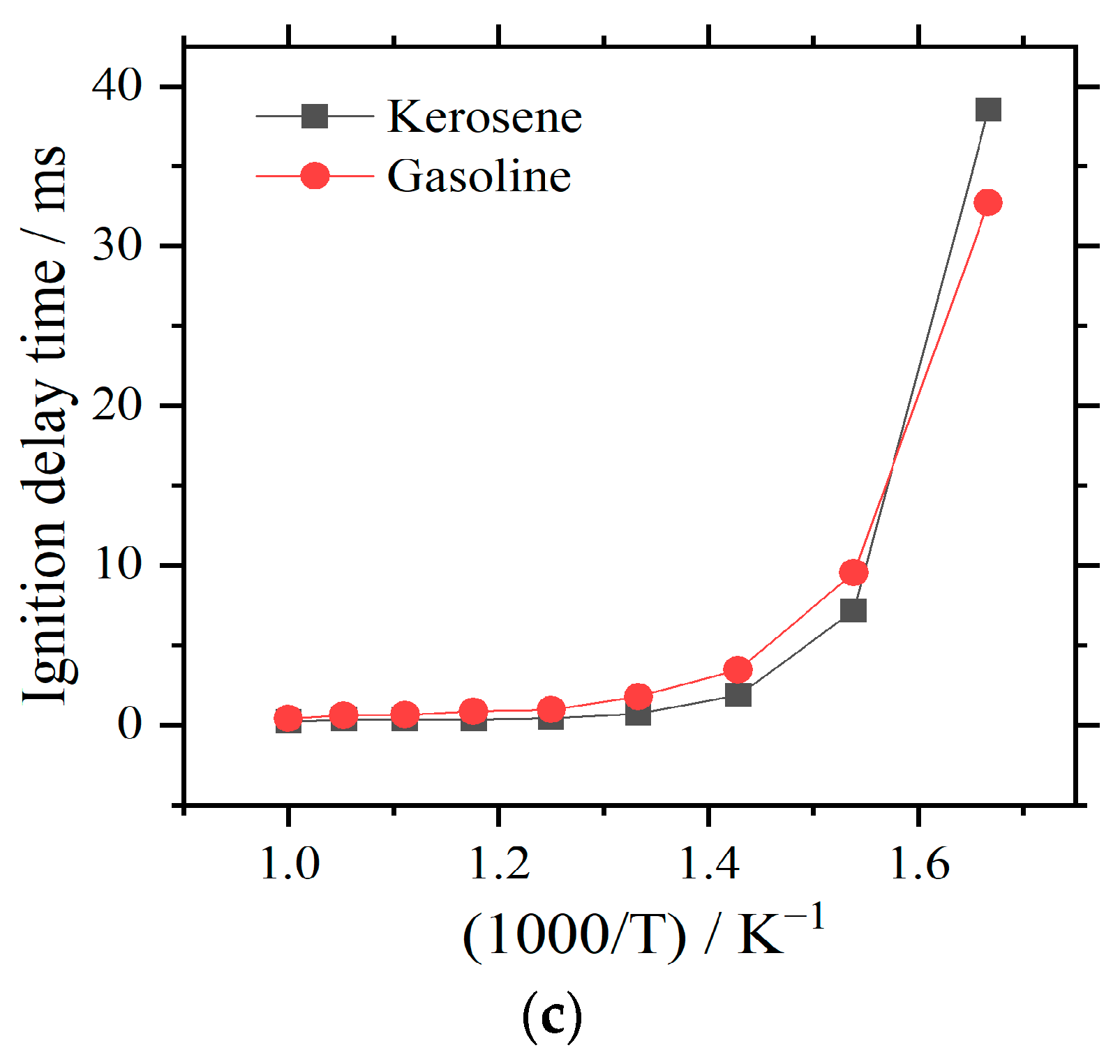
3.2. Comparison with Gasoline
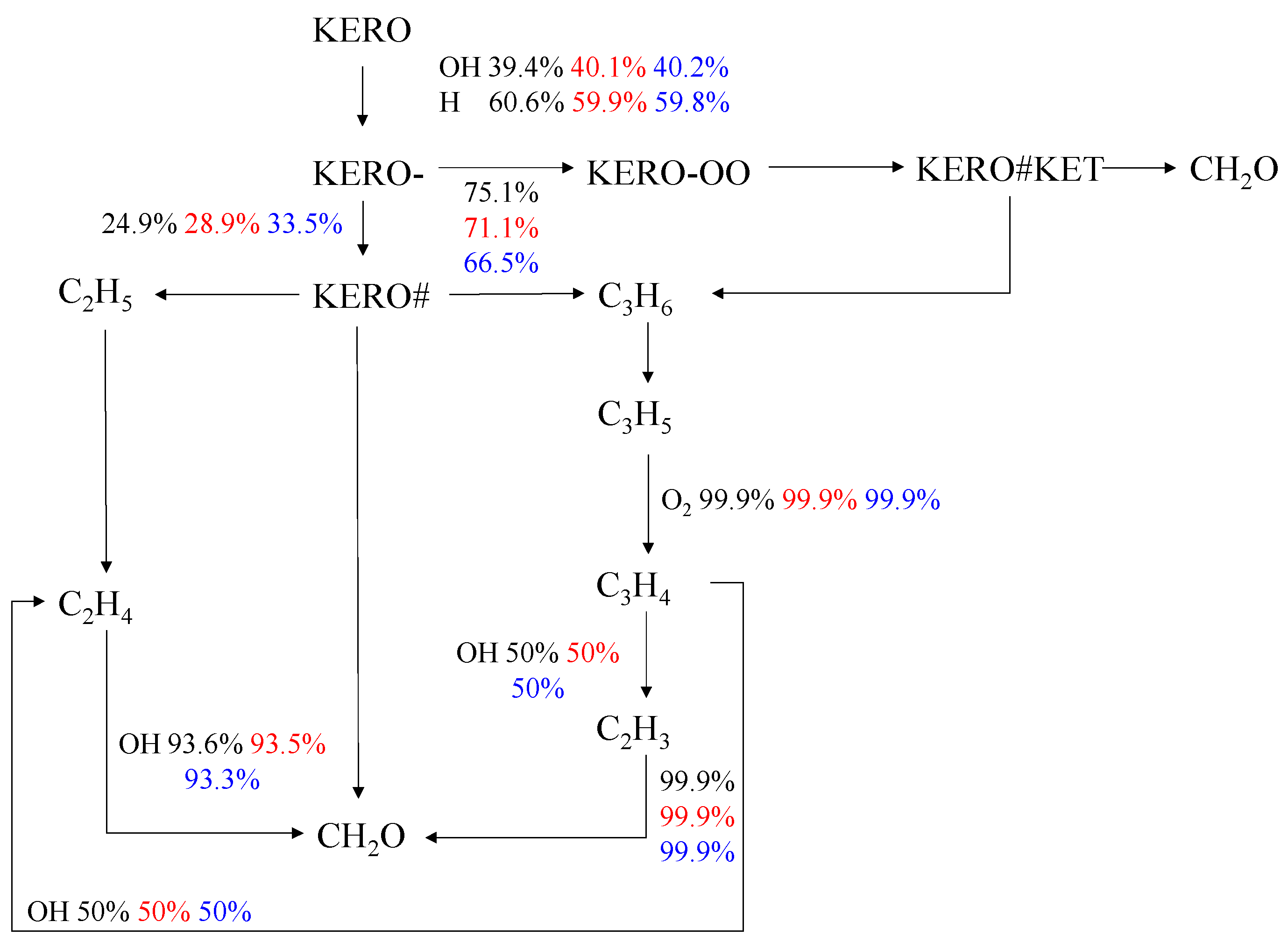
4. Chemical Reaction Path Analysis
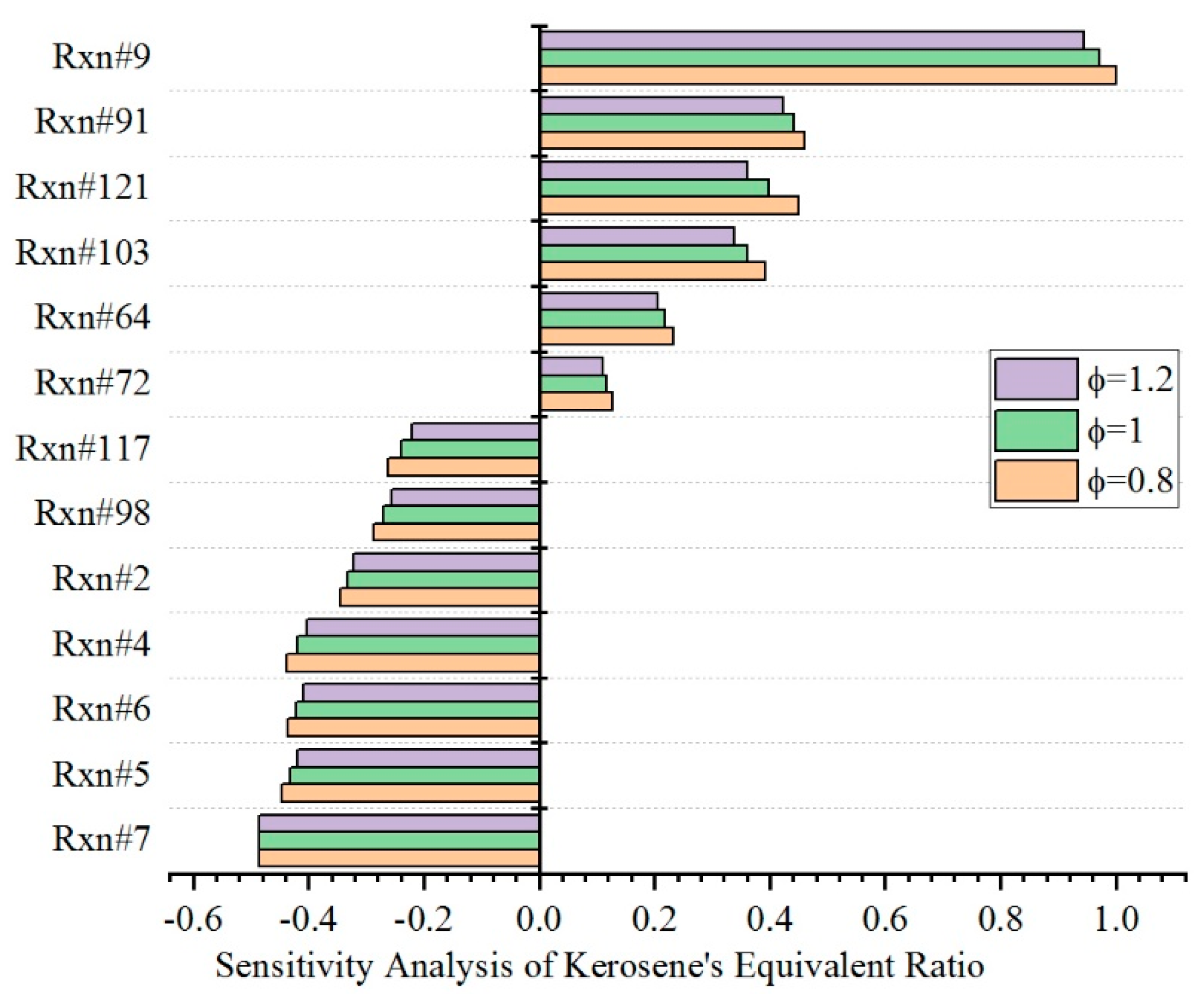
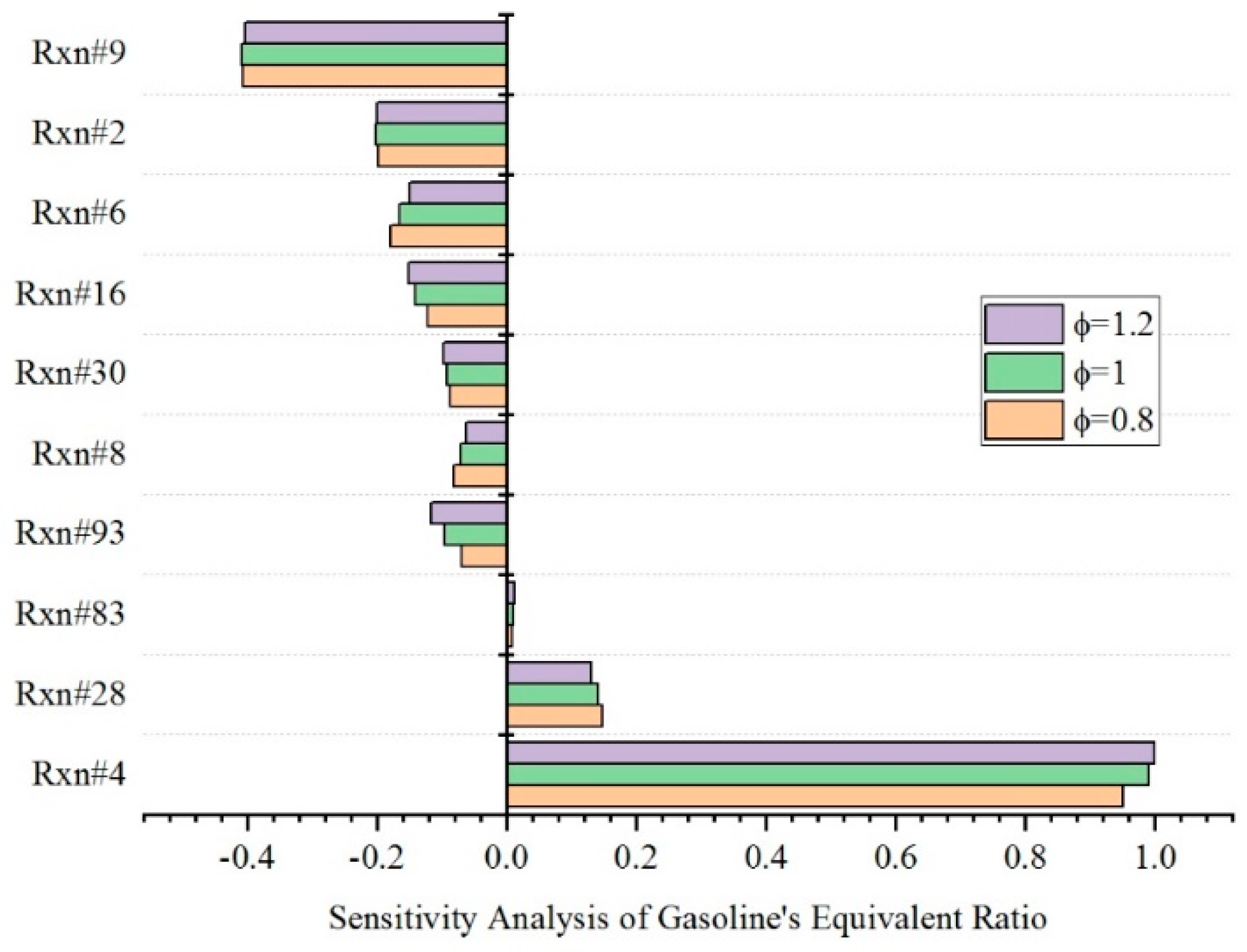
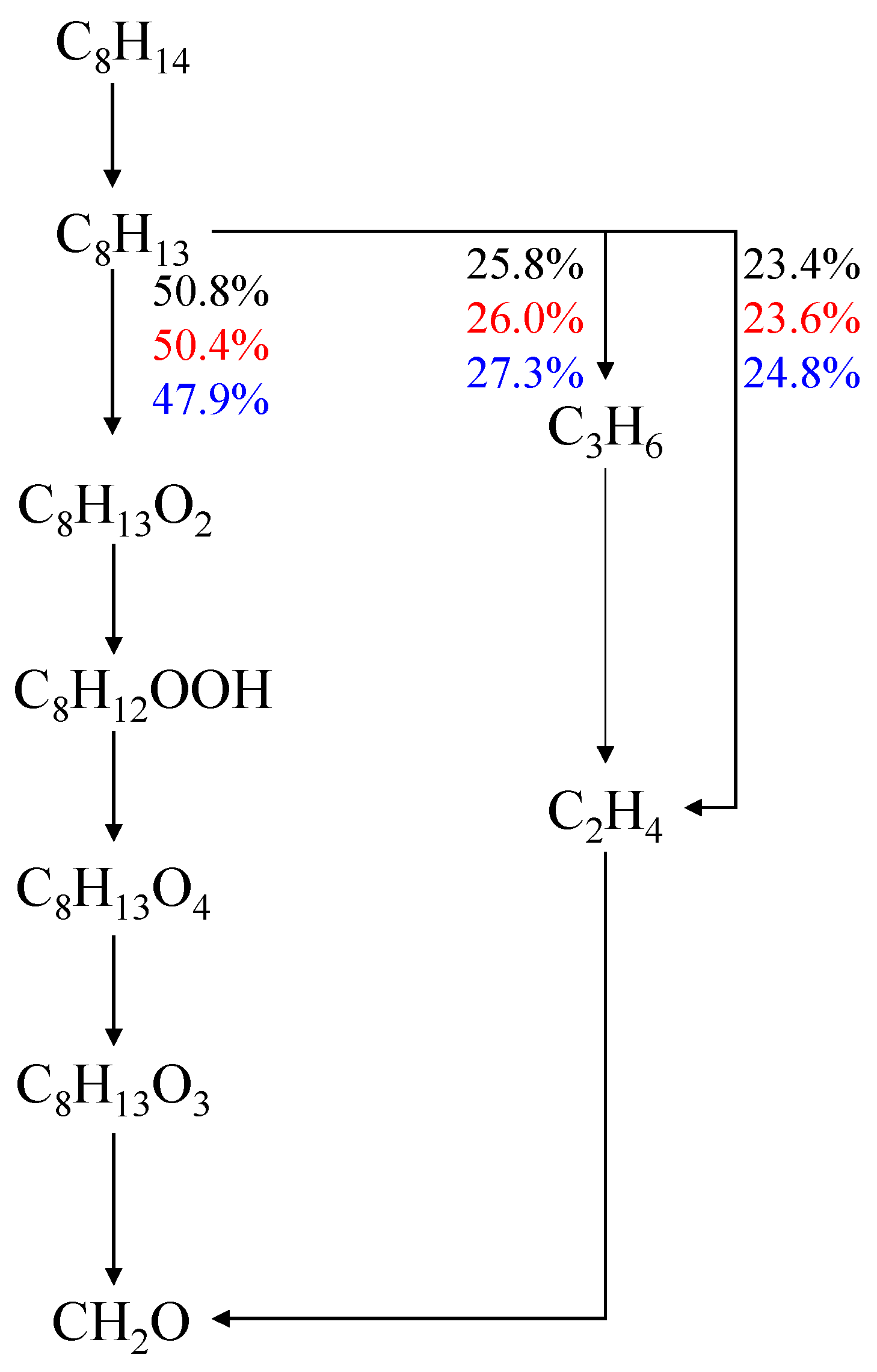
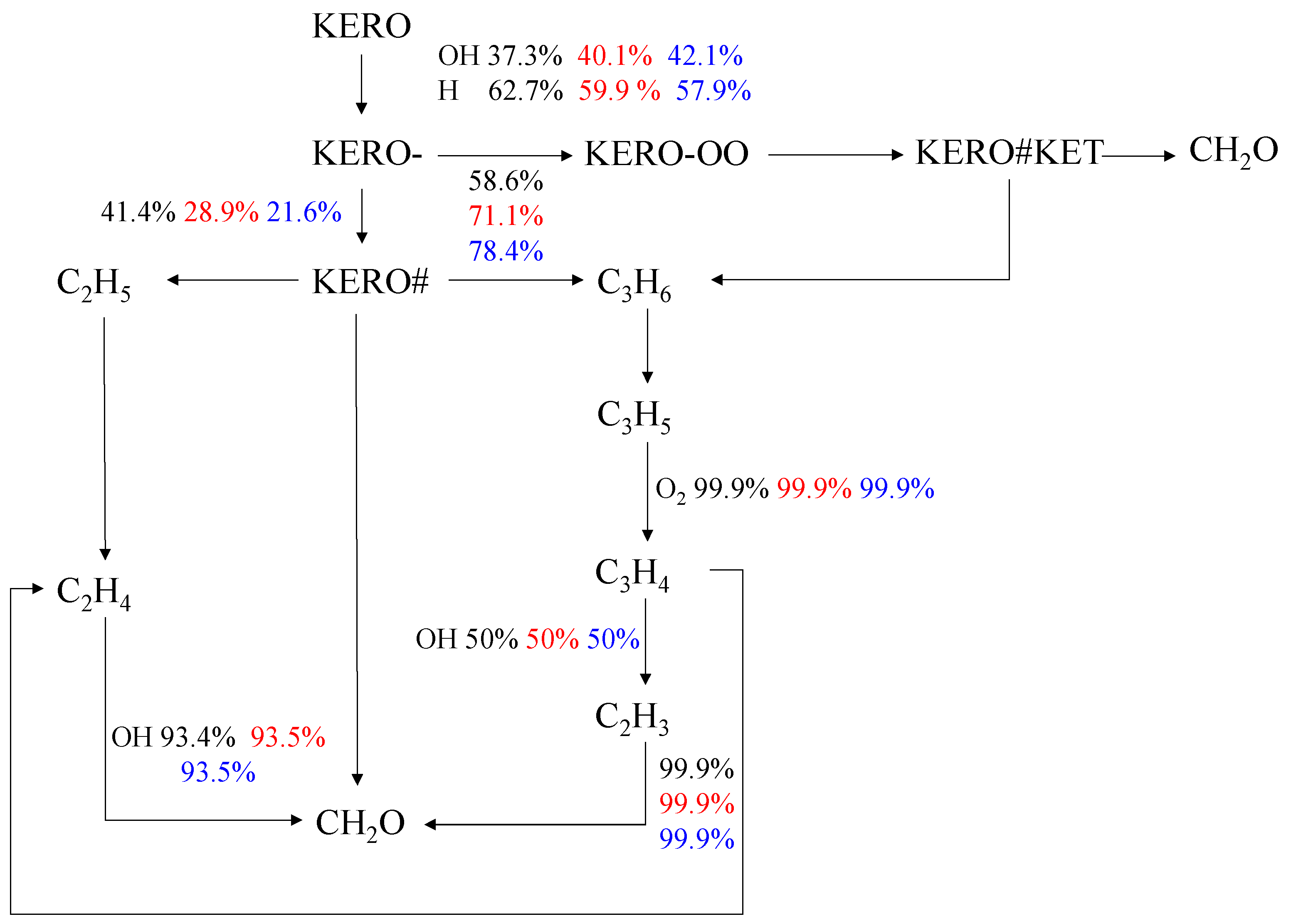
4.1. Effect of Equivalence Ratio
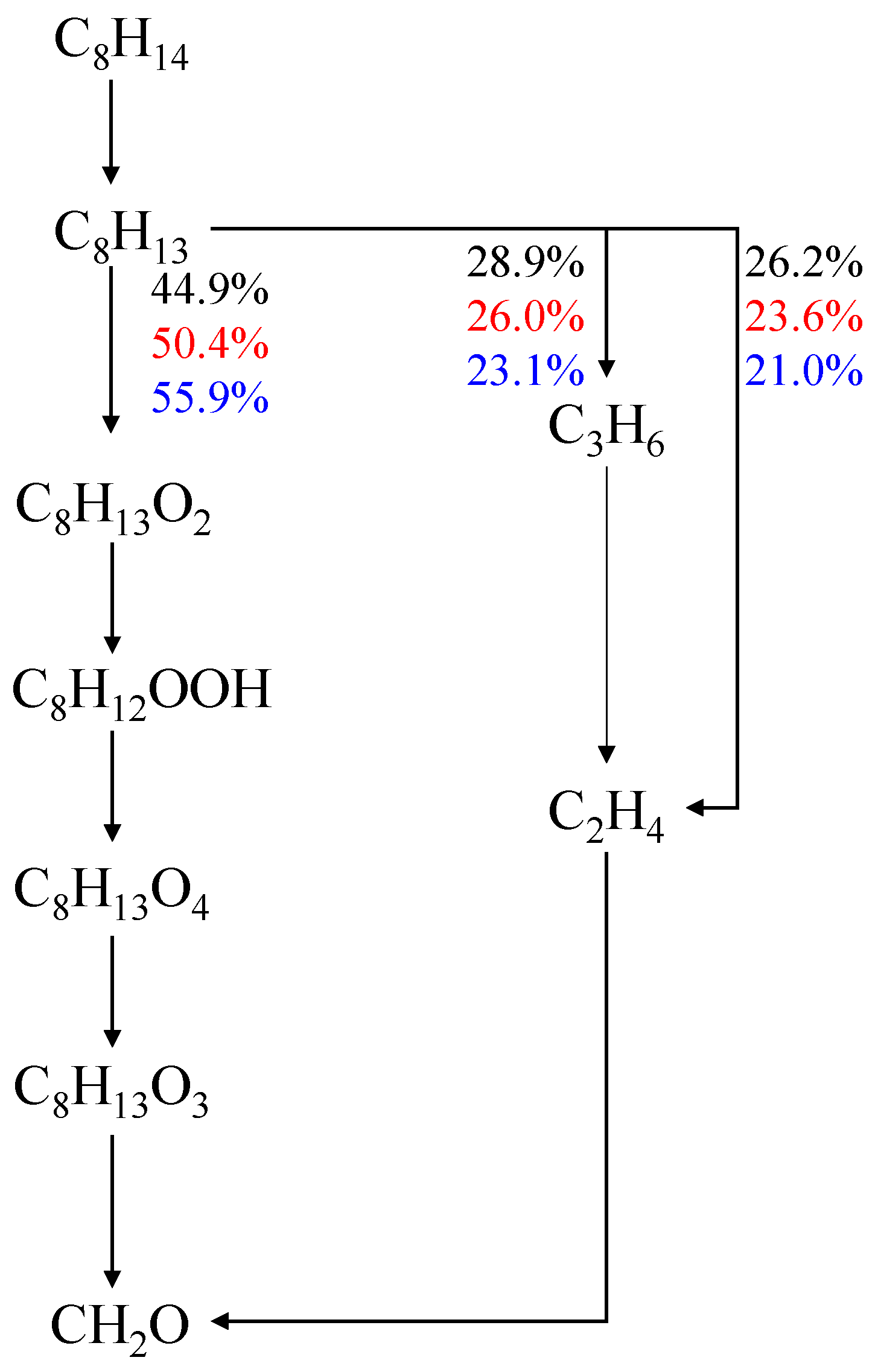
4.2. Effects of Initial Pressure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Obodeh, O.; Akhere, N.C. Experimental study on the effects of kerosene-doped gasoline on gasoline-powered engine performance characteristics. J. Pet. Gas Eng. 2010, 1, 37–40. [Google Scholar]
- Liu, Y.; Liu, W.; Liao, H.; Zhou, W.; Xu, C. An Experimental and Kinetic Modelling Study on Laminar Premixed Flame Characteristics of Ethanol/Acetone Mixtures. Energies 2021, 14, 6713. [Google Scholar] [CrossRef]
- Shi, Z.; Lee, C.F.; Wu, H.; Wu, Y.; Zhang, L.; Liu, F. Optical diagnostics of low-temperature ignition and combustion characteristics of diesel/kerosene blends under cold-start conditions. Appl. Energy 2019, 251, 113307. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, C.Y.; Liu, Y.; Chen, B.; Hu, E.J. Effects of hydrogen addition on burning characteristics of RP-3 kerosene. J. Aerosp. Power 2017, 32, 2049–2054. [Google Scholar]
- Kindracki, J.; Wacko, K.; Sciubba, E. Influence of Gaseous Hydrogen Addition on Initiation of Rotating Detonation in Liquid Fuel-Air Mixtures. Energies 2020, 13, 5101. [Google Scholar] [CrossRef]
- Luo, F.; Song, W.; Chen, W.; Long, Y. Investigation of kerosene supersonic combustion performance with hydrogen addition and fuel additive at low Mach inflow conditions. Fuel 2021, 285, 119139. [Google Scholar] [CrossRef]
- Gad, M.S.; Ismail, M.A. Effect of waste cooking oil biodiesel blending with gasoline and kerosene on diesel engine performance, emissions and combustion characteristics. Process Saf. Environ. Prot. 2021, 149, 1–10. [Google Scholar] [CrossRef]
- Ghose, P.; Patra, J.; Datta, A.; Mukhopadhyay, A. Effect of air flow distribution on soot formation and radiative heat transfer in a model liquid fuel spray combuster firing kerosene. Int. J. Heat Mass Transf. 2014, 74, 143–155. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, F.; Wang, E.; Yu, C.; Gao, H.; Liu, B.; Zhao, Z.; Zhao, C. Experimental study on knock suppression of spark-ignition engine fuelled with kerosene via water injection. Appl. Energy 2019, 242, 248–259. [Google Scholar] [CrossRef]
- Nie, X.-F.; Li, P.; Zhang, C.-H.; Xie, W.; Li, C.-S.; Li, X.-Y. Shock tube study of n-decane ignition at low pressures. Acta Mech. Sin. 2012, 28, 79–82. [Google Scholar] [CrossRef]
- Marek, C.J.; Papathakos, L.C.; Verbulecz, P.W. Preliminary Studies of Autoignition and Flashback in a Premixing-Prevaporizing Flame Tube Using Jet-A Fuel at Lean Equivalence Ratios; NASA Report TM X-3526; NASA: Washington, DC, USA, 1977. [Google Scholar]
- Spadaccini, L.J.; Tevelde, J.A. Autoignition characteristics of aircraft-type fuels. Combust. Flame 1982, 46, 283–300. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Wang, S.; Hu, H.; Zhang, S.; Fan, B.; Cui, J. Shock tube study of kerosene ignition delay at high pressures. Sci. China Phys. Mech. Astron. 2012, 55, 947–954. [Google Scholar] [CrossRef]
- Gou, H.; Su, W.; Bingcheng, F. Experimental studies of the adsorption in shock tube measurements of the JP-10 ignition delay time. J. Exp. Fluid Mech. 2006, 20, 69–72. [Google Scholar]
- Song, F.; Wu, Y.; Xu, S.; Yang, X.; Chen, X. The impact of fuel ratio and refueling mode on pre-combustion cracking properties of RP-3 kerosene. Int. J. Hydrog. Energy 2020, 45, 28505–28519. [Google Scholar] [CrossRef]
- Wang, Q.D.; Fang, Y.M.; Wang, F.; Li, X.Y. Skeletal mechanism generation for high-temperature oxidation of kerosene surrogates. Combust. Flame 2012, 159, 91–102. [Google Scholar] [CrossRef]
- Honnet, S.; Seshadri, K.; Niemann, U.; Peters, N. A surrogate fuel for kerosene. Proc. Combust. Inst. 2009, 32, 485–492. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, S.; Zhao, H.; Qian, W.Q.; Le, J.L. Detailed and reduced chemical kinetic mechanisms for RP-3 aviation kerosene combustion. J. Aerosp. Power 2010, 25, 1948–1955. [Google Scholar]
- Ma, H.; Xie, M.; Zeng, W.; Chen, B. Influencing factor analysis for ignition characteristics of RP-3 kerosene. J. Propuls. Technol. 2015, 36, 306–313. [Google Scholar]
- Sun, W.; Zhang, Y.; Li, Y.; Huang, Z. Hierarchical Auto-Ignition and Structure-Reactivity Trends of C2–C4 1-Alkenes. Energies 2021, 14, 5797. [Google Scholar] [CrossRef]
- Li, G.; Yang, W.; Tay, K.L.; Yu, W.; Chen, L. A reduced and robust reaction mechanism for toluene and decalin oxidation with polycyclic aromatic hydrocarbon predictions. Fuel 2020, 259, 116233. [Google Scholar] [CrossRef]
- An, J.; He, G.; Luo, K.; Qin, F.; Liu, B. Artificial neural network based chemical mechanisms for computationally efficient modeling of hydrogen/carbon monoxide/kerosene combustion. Int. J. Hydrog. Energy 2020, 45, 29594–29605. [Google Scholar] [CrossRef]
- Tay, K.L.; Yang, W.; Mohan, B.; Zhou, D.; Yu, W.; Zhao, F. Development of a reduced kerosene–diesel reaction mechanism with embedded soot chemistry for diesel engines. Fuel 2016, 181, 926–934. [Google Scholar] [CrossRef]
- Wu, S.; Tay, K.L.; Li, J.; Yang, W.; Yang, S. Development of a compact and robust kinetic mechanism for furan group biofuels combustion in internal combustion engines. Fuel 2021, 298, 120824. [Google Scholar] [CrossRef]
- Tay, K.L.; Yang, W.; Mohan, B.; An, H.; Zhou, D.; Yu, W. Development of a robust and compact kerosene–diesel reaction mechanism for diesel engines. Energy Convers. Manag. 2016, 108, 446–458. [Google Scholar] [CrossRef]
- Tay, K.L.; Yang, W.; Zhao, F.; Yu, W.; Mohan, B. A numerical study on the effects of boot injection rate-shapes on the combustion and emissions of a kerosene-diesel fueled direct injection compression ignition engine. Fuel 2017, 203, 430–444. [Google Scholar] [CrossRef]
- Tay, K.L.; Yang, W.; Zhao, F.; Yu, W.; Mohan, B. Numerical investigation on the combined effects of varying piston bowl geometries and ramp injection rate-shapes on the combustion characteristics of a kerosene-diesel fueled direct injection compression ignition engine. Energy Convers. Manag. 2017, 136, 1–10. [Google Scholar] [CrossRef]
- Clement, T. Numerical Modelling of the Combustion Process and Emissions Formation of Kerosene and Its Blends in Diesel Engines; National University of Singapore: Singapore, 2017. [Google Scholar]
- Burkert, A.; Paa, W. Ignition delay times of single kerosene droplets based on formaldehyde LIF detection. Fuel 2016, 167, 271–279. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; He, Y.; Xia, J.; Zhang, J.; Zhao, H.; Cen, K. Ignition, puffing and sooting characteristics of kerosene droplet combustion under sub-atmospheric pressure. Fuel 2021, 285, 119182. [Google Scholar] [CrossRef]
- Han, H.S.; Kim, C.J.; Cho, C.H.; Sohn, C.H.; Han, J. Ignition delay time and sooting propensity of a kerosene aviation jet fuel and its derivative blended with a bio-jet fuel. Fuel 2018, 232, 724–728. [Google Scholar] [CrossRef]
- Kim, D.M.; Baek, S.W.; Yoon, J. Ignition characteristics of kerosene droplets with the addition of aluminum nanoparticles at elevated temperature and pressure. Combust. Flame 2016, 173, 106–113. [Google Scholar] [CrossRef]
- Liu, J.; Hu, E.; Huang, Z.; Zeng, W. Ignition delay characteristics of RP-3 aviation kerosene to simulate alternative fuels. J. Propuls. Technol. 2021, 42, 467–473. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.; Oehlschlaeger, M.A. Autoignition studies of conventional and Fischer–Tropsch jet fuels. Fuel 2012, 98, 249–258. [Google Scholar] [CrossRef]
- Dooley, S.; Won, S.H.; Chaos, M.; Heyne, J.; Ju, Y.; Dryer, F.L.; Kumar, K.; Sung, C.-J.; Wang, H.; Oehlschlaeger, M.A.; et al. A jet fuel surrogate formulated by real fuel properties. Combust. Flame 2010, 157, 2333–2339. [Google Scholar] [CrossRef]
- Xu, R.; Saggese, C.; Lawson, R.; Movaghar, A.; Parise, T.; Shao, J.; Choudhary, R.; Park, J.; Lu, T.; Hanson, R.; et al. A physics-based approach to modeling real-fuel combustion chemistry—VI. Predictive kinetic models of gasoline fuels. Combust. Flame 2020, 220, 475–487. [Google Scholar] [CrossRef]
- Dagaut, P.; Cathonnet, M. The ignition, oxidation, and combustion of kerosene: A review of experimental and kinetic modeling. Prog. Energy Combust. Sci. 2006, 32, 48–92. [Google Scholar] [CrossRef]
- Violi, A.; Yan, S.; Eddings, E.G.; Sarofim, A.F.; Granata, S.; Faravelli, T.; Ranzi, E. Experimental formulation and kinetic model for JP-8 surrogate mixtures. Combust. Sci. Technol. 2002, 174, 399–417. [Google Scholar] [CrossRef]
- Wu, Z.; Mao, Y.; Raza, M.; Zhu, J.; Feng, Y.; Wang, S.; Qian, Y.; Yu, L.; Lu, X. Surrogate fuels for RP-3 kerosene formulated by emulating molecular structures, functional groups, physical and chemical properties. Combust. Flame 2019, 208, 388–401. [Google Scholar] [CrossRef]
- Won, S.H.; Haas, F.M.; Dooley, S.; Edwards, T.; Dryer, F.L. Reconstruction of chemical structure of real fuel by surrogate formulation based upon combustion property targets. Combust. Flame 2017, 183, 39–49. [Google Scholar] [CrossRef]





| Temperature (K) | Pressure (Bar) | Reference |
|---|---|---|
| 550–900 | 3 | [29] |
| 298 | 0.2–1 | [30] |
| 700–1200 | 20 | [31] |
| 673–973 | 1–25 | [32] |
| 1000–1700 | 1–3 | [33] |
| Physicochemical Property | Kerosene | Gasoline |
|---|---|---|
| Fuel composition (C/H) | C7~C16 | C5~C11 |
| Density (kg/L) | 0.80~0.84 | 0.70~0.75 |
| Flash point (°C) | 45~51 | −45~−25 |
| Theoretical air-fuel ratio | 14.7 | 14.8 |
| Kinematic viscosity (mm2/s) | 1.841 | 0.8 |
| Boiling point (°C) | 185 | 30~220 |
| Spontaneous ignition point (°C) | 380~425 | 510~530 |
| Reaction Number | Reaction Equation |
|---|---|
| Rxn#7 | OOKERO#OOH = KERO#KET + OH |
| Rxn#5 | KERO-OO = KERO#OOH |
| Rxn#6 | KERO#OOH + O2 = OOKERO#OOH |
| Rxn#4 | KERO- + O2 = KERO-OO |
| Rxn#2 | KERO + OH = KERO- + H2O |
| Rxn#98 | CH3 + HO2 = CH3O + OH |
| Rxn#117 | CH3O + M = CH2O + H + M |
| Rxn#72 | H2O2 + OH = HO2 + H2O |
| Rxn#64 | HO2 + OH = H2O + O2 |
| Rxn#103 | CH3 + HO2 = CH4 + O2 |
| Rxn#121 | CH3O + O2 = CH2O + HO2 |
| Rxn#91 | CH2O + OH = HCO + H2O |
| Rxn#9 | KERO- + O2 = KERO# + HO2 |
| Reaction Number | Reaction Equation |
|---|---|
| Rxn#2 | SDC8H14 + H = H2 + SDC8H13 |
| Rxn#4 | SDC8H14 + OH = H2O + SDC8H13 |
| Rxn#6 | SDC8H14 + HO2 = H2O2 + SDC8H13 |
| Rxn#8 | SDC8H12 = 0.1333333C2H4 + 0.1466667C3H6 + 0.0733333iC4H8 + 0.5C6H6 + 0.5C6H5CH3 + 1.5H + 0.5CH3 |
| Rxn#9 | SDC8H13 + O2 = SDC8H13O2 |
| Rxn#16 | SDC8H12O3 = CH2O + OH + CO + 0.0111111C2H4 + 0.0122222C3H6 + 0.0061111iC4H8 + 0.4166667C6H6 + 0.4166667C6H5CH3 + 1.5H + 0.5CH3 |
| Rxn#28 | H + O2(+M) = HO2(+M) |
| Rxn#30 | OH + OH(+M) = H2O2(+M) |
| Rxn#83 | CH2O + OH = HCO + H2O |
| Rxn#93 | CH3 + HO2 = CH3O + OH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, E.; Shi, Z. Numerical Investigation of the Ignition Delay Time of Kerosene Premixed Combustion in an SI Engine. Energies 2022, 15, 1744. https://doi.org/10.3390/en15051744
Zhao Y, Wang E, Shi Z. Numerical Investigation of the Ignition Delay Time of Kerosene Premixed Combustion in an SI Engine. Energies. 2022; 15(5):1744. https://doi.org/10.3390/en15051744
Chicago/Turabian StyleZhao, Yuxuan, Enhua Wang, and Zhicheng Shi. 2022. "Numerical Investigation of the Ignition Delay Time of Kerosene Premixed Combustion in an SI Engine" Energies 15, no. 5: 1744. https://doi.org/10.3390/en15051744
APA StyleZhao, Y., Wang, E., & Shi, Z. (2022). Numerical Investigation of the Ignition Delay Time of Kerosene Premixed Combustion in an SI Engine. Energies, 15(5), 1744. https://doi.org/10.3390/en15051744





