Management of Energy Conversion Processes in Membrane Systems
Abstract
1. Introduction
2. Materials and Methods
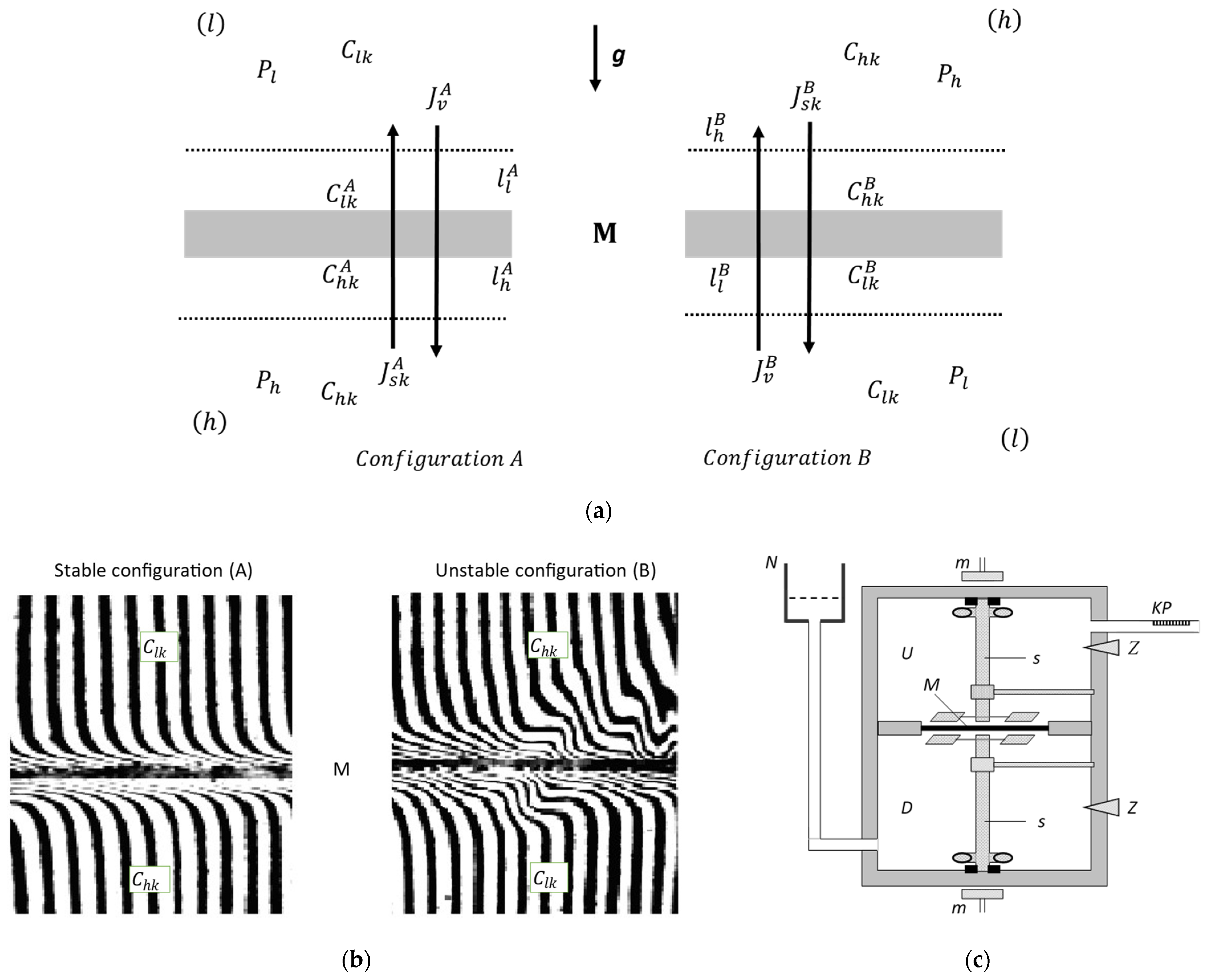
2.1. Membrane System
2.2. Methodology for Measuring the Osmotic and Solute Fluxes
2.3. The Kr form of Kedem–Katchalsky–Peusner Equations for Non-Electrolyte Solutions in Concentration Polarization Conditions
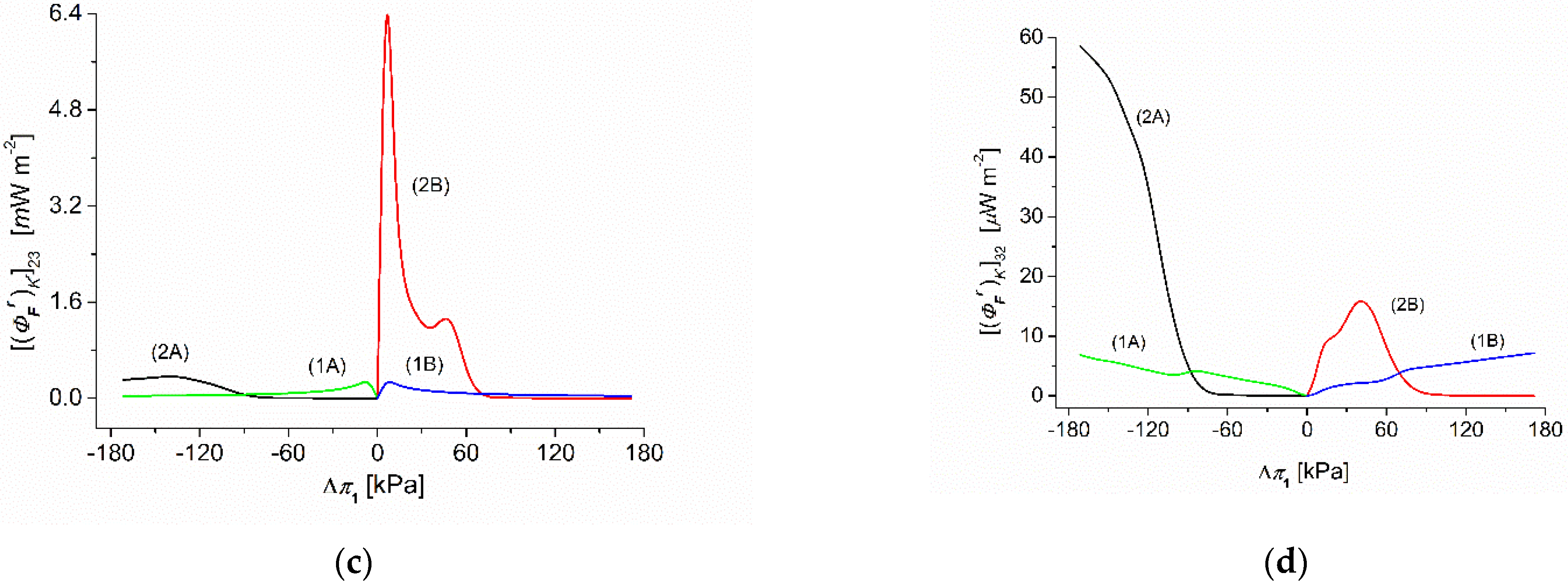
3. Results and Discussion
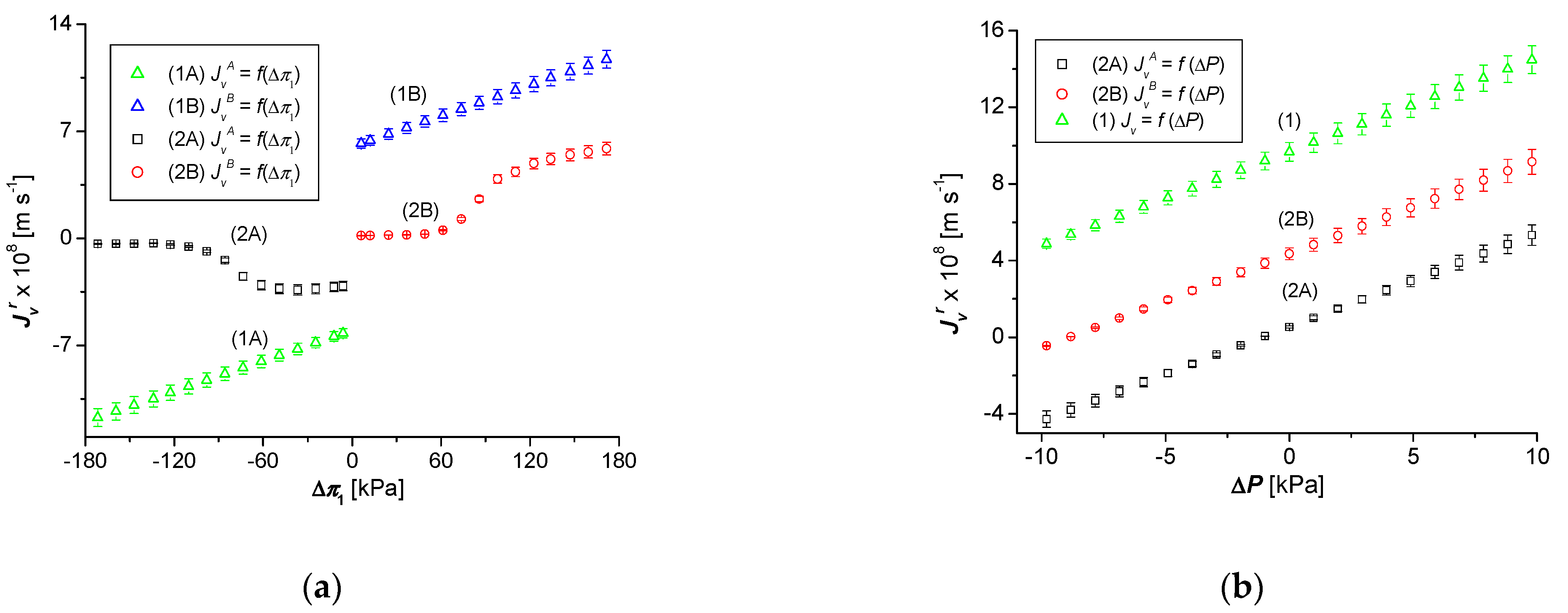
3.1. Osmotic and Hydrostatic Pressure Dependencies of , and and
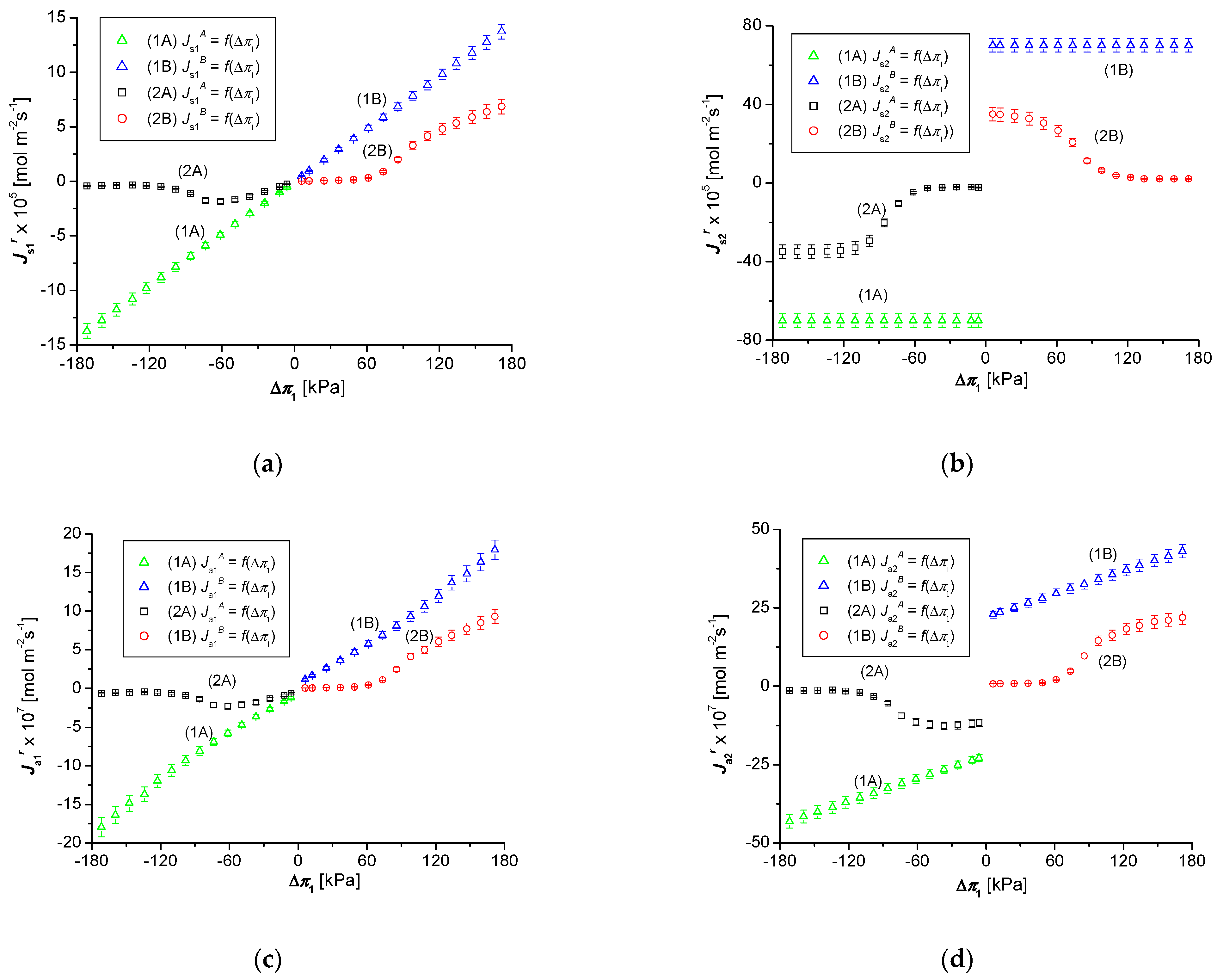
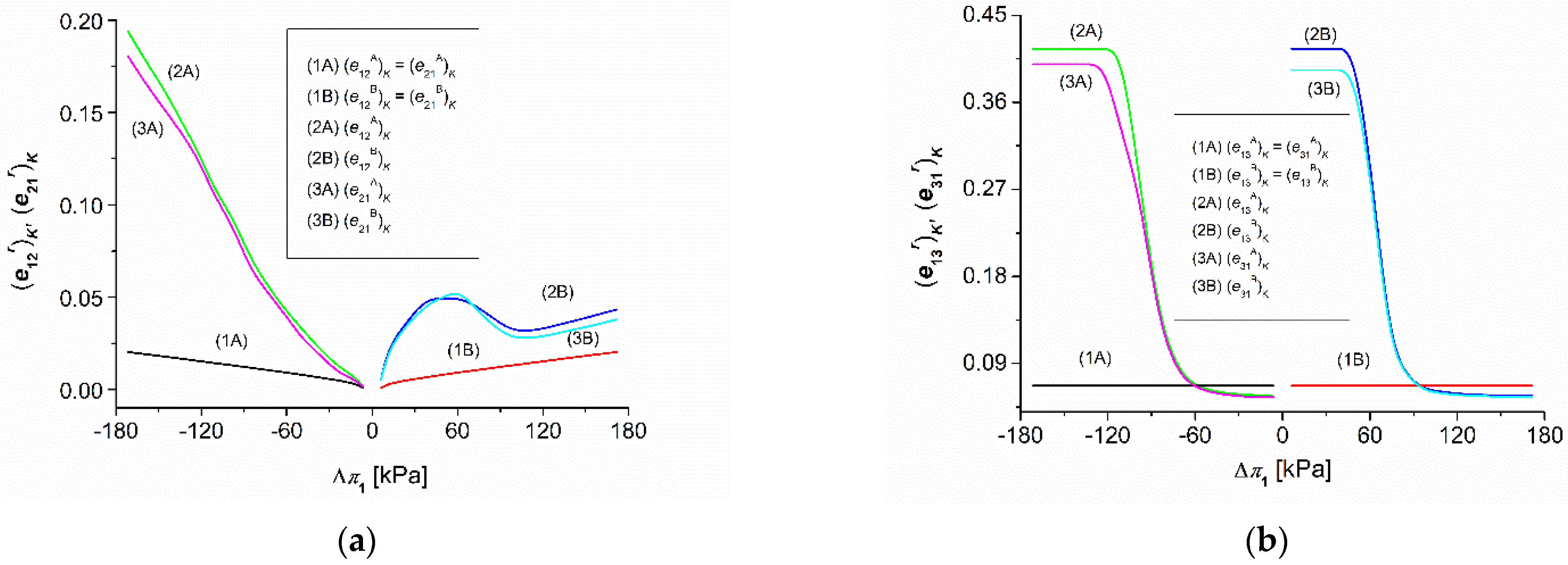
3.2. Osmotic Pressure Dependencies of , and
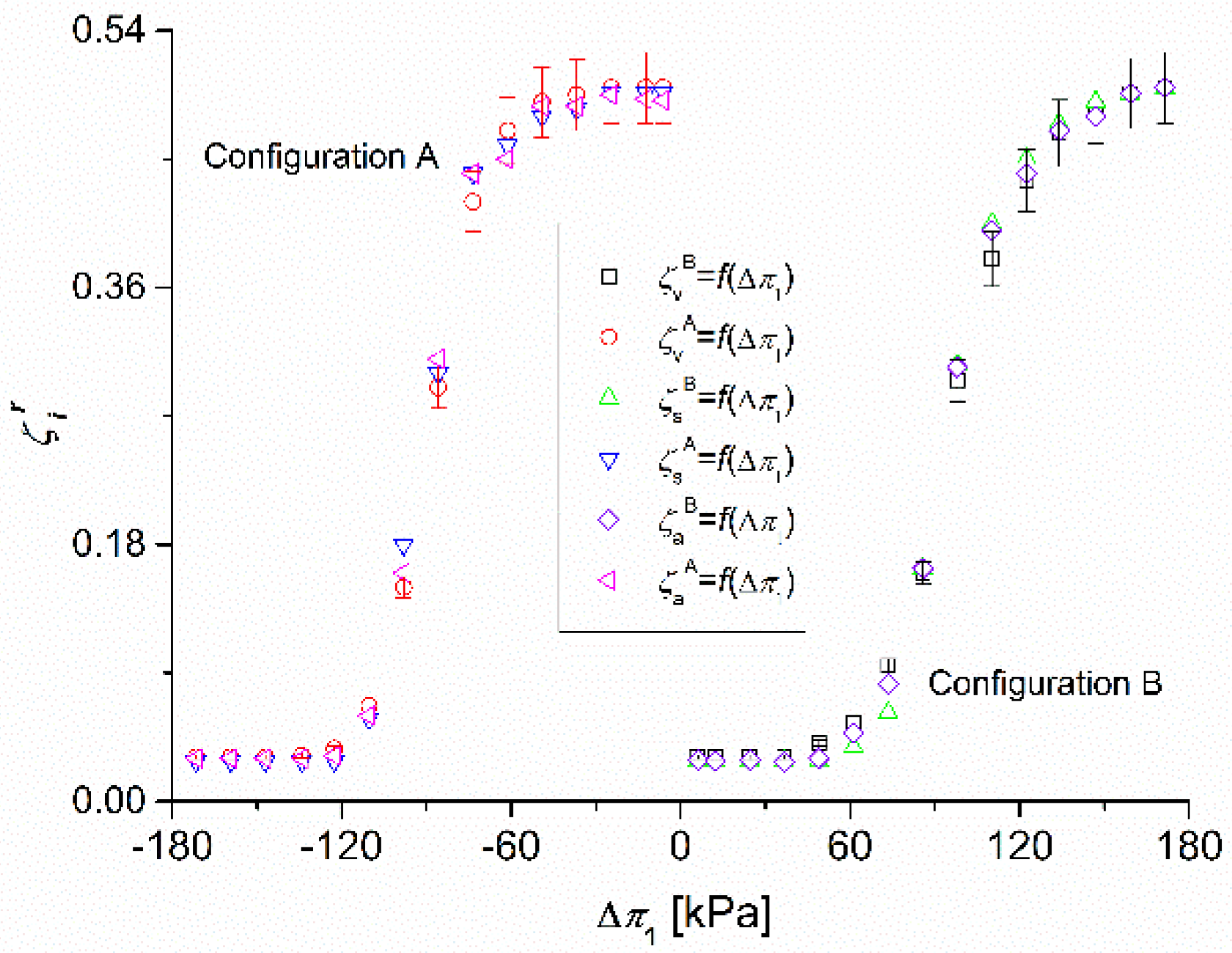
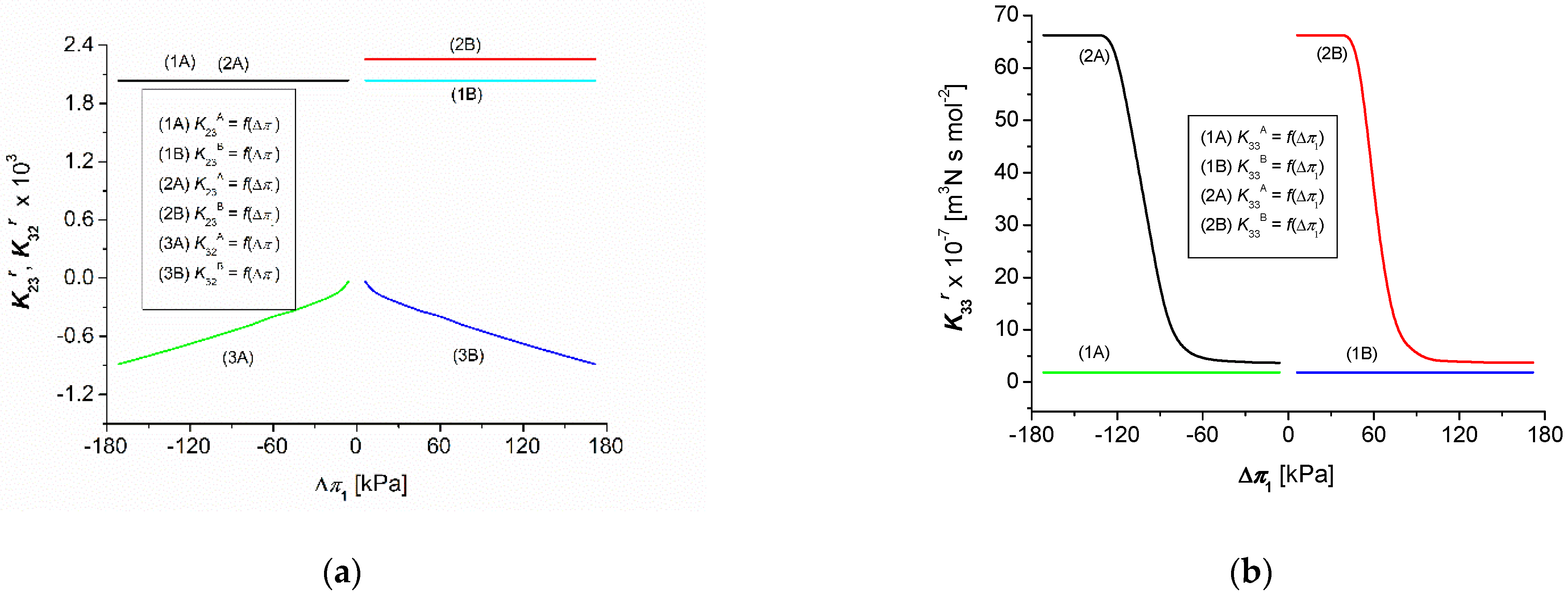
3.3. Calculations of the Coefficients , and
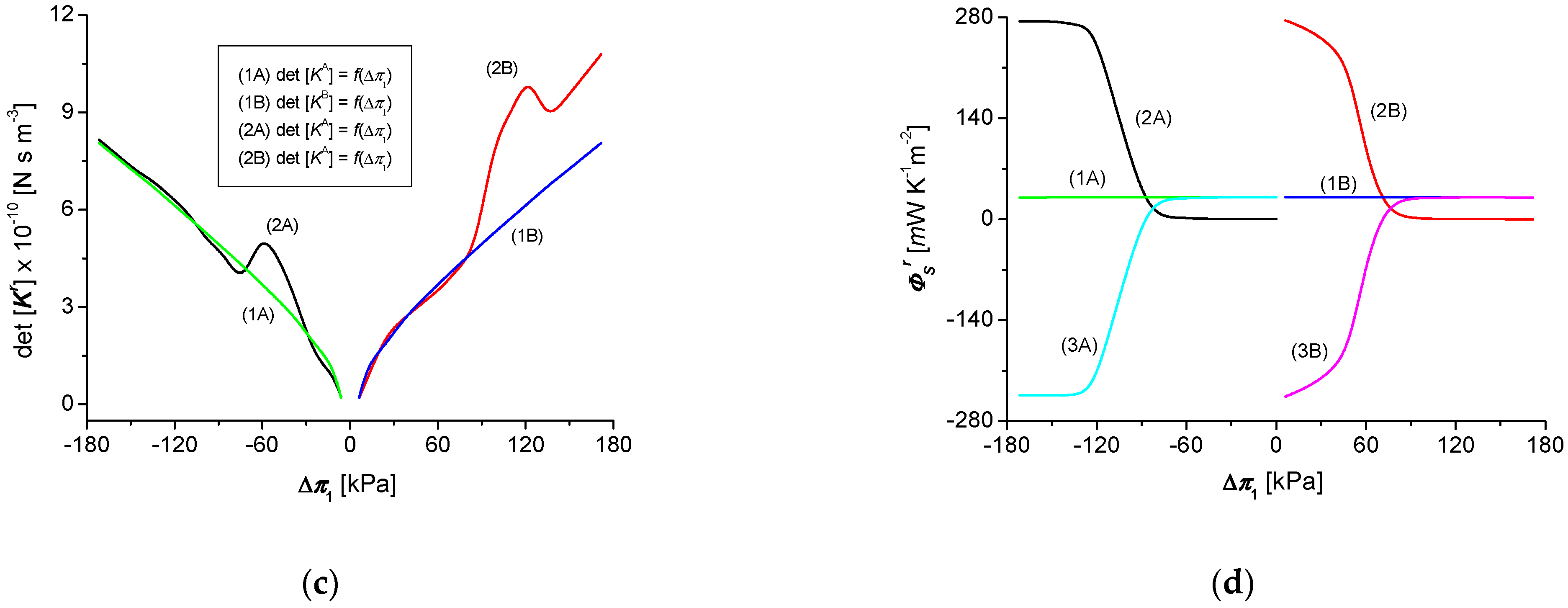
3.4. Calculations of the Global S-Entropy Source
3.5. Osmotic Pressure Dependencies of
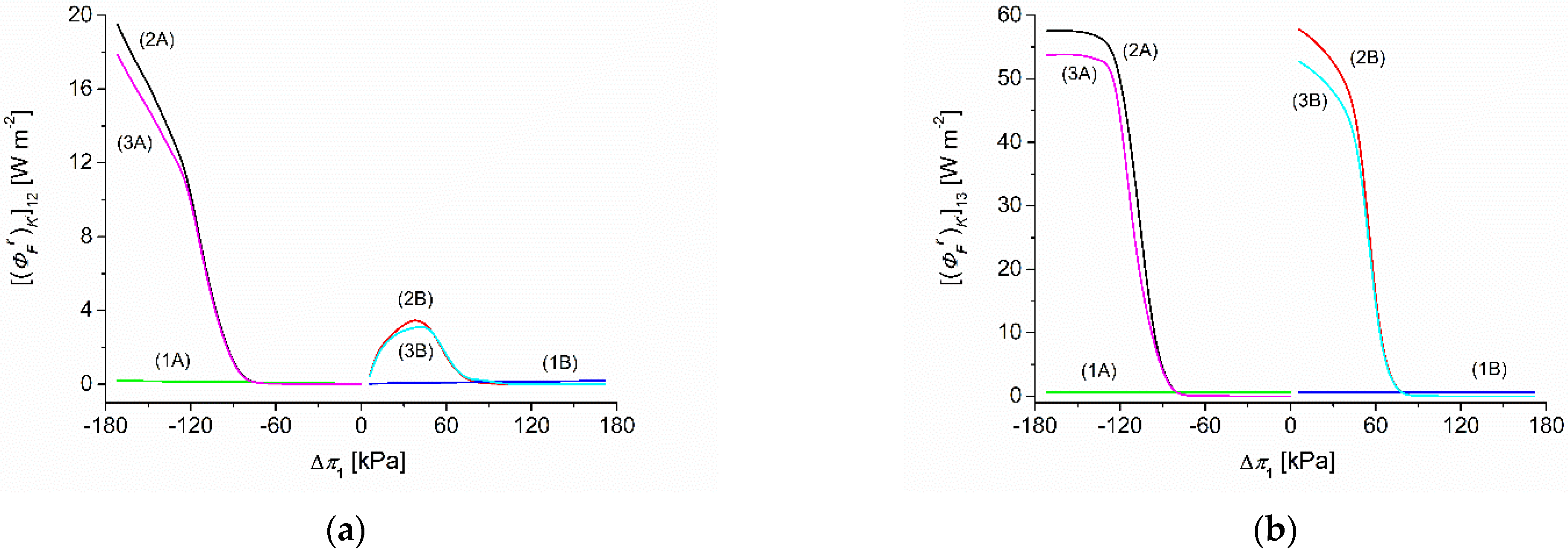
3.6. Calculations of F-Energy in the Membrane System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Symbols
| Jv | volume flux (m s−1); |
| Jl, Jm, Jh | solute fluxes (mol m−2s−1); |
| Il, Is, Im, Ih | ionic currents (A); |
| hydraulic conductivity coefficient (m−3 N−1 s−1); | |
| reflection coefficient; | |
| electroosmotic permeability coefficient (NA−1) | |
| ω | solute permeability coefficient (mol N−1 s−1); |
| γ | van’t Hoff coefficient; |
| R | gas constant (J mol−1 K−1); |
| T | absolute temperature (K); |
| electrical conductivity (Ω−1 m−2); | |
| transfer number; | |
| valence; | |
| ion number; | |
| average concentration of the solution (mol m−3); | |
| potential difference measured with two reversible electrodes (V); | |
| , | transfer number of anions (a) and cations (c) in the membrane; |
| t | time (s); |
| and | concentration boundary layers; |
| δl, δh | thickness of the concentration boundary layers (m); |
| δm | membrane thickness (m); |
| mechanical pressure difference (Pa); | |
| CBLs | concentration boundary layers; |
| g | acceleration due to the fact of gravity (m s−2); |
| , | diffusion coefficients (m2 s−1); |
| , | the kinematic viscosity coefficients (m2 s−1); |
| , | solution concentrations at the boundaries of M/ and M/ (mol m−3); |
| , | solution densities at the boundaries of M/ and M/ (kg m−3); |
| , | solution concentrations beyond and (mol m−3); |
| , | solution densities beyond and (kg m−3). |
| F | free energy (W m−2) |
| U | internal energy (W m−2) |
| global source of entropy (W K−1 m−2) | |
| energy conversion efficiency coefficient | |
| coefficient of concentration polarization |
Appendix A
| Coefficient | Definition | |
|---|---|---|
| Coefficient | Definition | |
|---|---|---|
References
- Demirel, Y. Nonequilibrium Thermodynamics:Transport and Rate Processes in Physical, Chemical and Biological Systems; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Uragami, T. Science and Technology of Separation Membranes; John Wiley & Sons: Chichester, UK, 2017. [Google Scholar]
- Baker, R. Membrane Technology and Application; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Gerbaud, V.; Shcherbakova, N.; Da Cunha, S. A nonequilibrium thermodynamics perspective on nature-inspired chemical engineering processes. Chem. Eng. Res. Des. 2020, 154, 316–330. [Google Scholar] [CrossRef]
- Raghuvanshi, S.; La Prairie, B.; Rajagopal, S.; Yadav, V.G. Polymeric nanomaterials for ocular drug delivery. In Advances in Polymeric Nanomaterials for Biomedical Applications; Bajpai, A.K., Saini, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 309–325. [Google Scholar] [CrossRef]
- Rewak-Soroczynska, J.; Sobierajska, P.; Targonska, S.; Piecuch, A.; Grosman, L.; Rachuna, J.; Wasik, S.; Arabski, M.; Ogorek, R.; Wiglusz, R.J. New approach to antifungal activity of fluconazole incorporated into the porous 6-Anhydro-α-L-Galacto-β-D—Galactan structures modified with nanohydroxyapatite for chronic-wound treatments—In vitro evaluation. Int. J. Mol. Sci. 2021, 22, 3112. [Google Scholar] [CrossRef]
- Katchalsky, A.; Curran, P.F. Nonequilibrium Thermodynamics in Biophysics; Harvard University Press: Cambridge, UK, 1965. [Google Scholar]
- Cheng, X.; Pinsky, P.M. The balance of fluid and osmotic pressures across active biological membrane with application to the corneal endothelium. PLoS ONE 2015, 10, e0145422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wijmans, J.G.; Baker, W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Al-Obaidi, M.A.; Kara-Zaitri, C.; Mujtaba, I.M. Scope and limitation of the irreversible thermodynamics and the solution diffusion models for the separation of binary and multi-component systems in reverse osmosis process. Comput. Chem. Eng. 2017, 100, 48–79. [Google Scholar] [CrossRef]
- Spiegler, K.S. Transport processes in ionic membranes. Trans. Faraday Soc. 1958, 54, 1408–1428. [Google Scholar] [CrossRef]
- Kedem, O.; Katchalsky, A. A physical interpretation of the phenomenological coefficients of membrane permeability. J. Gen. Physiol. 1961, 45, 143–179. [Google Scholar] [CrossRef]
- Ślęzak, A. A frictional interpretation of the phenomenological coefficients of membrane permeability for multicomponent non-ionic solutions. J. Biol. Phys. 1997, 23, 239–250. [Google Scholar] [CrossRef]
- Friedman, H.; Meyer, R.A. Transport across homoporous and heteroporous membranes in nonideal, nondilute solutions. I. Inequality of reflection coefficients for volume flow and solute flow. Biophys. J. 1981, 34, 535–544. [Google Scholar] [CrossRef]
- Mason, E.A.; Lonsdale, H.K. Statistical-mechanical theory of membrane transport. J. Memb. Sci. 1990, 51, 1–81. [Google Scholar] [CrossRef]
- Oster, G.; Perelson, A.; Katchalsky, A. Network thermodynamics. Nature 1971, 234, 239–399. [Google Scholar] [CrossRef]
- Peusner, L. Studies in Network Thermodynamics; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Batko, K.M.; Ślęzak-Prochazka, I.; Grzegorczyn, S.; Ślęzak, A. Membrane transport in concentration polarization conditions: Network thermodynamics model equations. J. Porous Media 2014, 17, 573–586. [Google Scholar] [CrossRef]
- Ślęzak-Prochazka, I.; Batko, K.M.; Wąsik, S.; Ślęzak, A. H* Peusner’s form of the Kedem-Katchalsky equations fornon-homogeneous non-electrolyte binary solutions. Transp. Porous Media 2016, 111, 457–477. [Google Scholar] [CrossRef]
- Ślęzak, A.; Grzegorczyn, S.; Batko, K.M. Resistance coefficients of polymer membrane with concentration polarization. Transp. Porous Media 2012, 95, 151–170. [Google Scholar] [CrossRef]
- Batko, K.M.; Ślęzak-Prochazka, I.; Ślęzak, A. Network hybrid form of the Kedem-Katchalsky equations for non-homogenous binary non-electrolyte solutions: Evaluation of Pij* Peusner’s tensor coefficients. Transp. Porous Media 2015, 106, 1–20. [Google Scholar] [CrossRef]
- Batko, K.; Ślęzak, A. Membrane transport of nonelectrolyte solutions in concentration polarization conditions: Hr form of the Kedem–Katchalsky–Peusner equations. Int. J. Chem. Eng. 2019, 2019, 5629259. [Google Scholar] [CrossRef]
- Ślęzak, A.; Grzegorczyn, S.; Batko, K.M.; Bajdur, W.M.; Makuła-Włodarczyk, M. Applicability of the Lr form of the Kedem–Katchalsky–Peusner equations for membrane transport in water purification technology. Desalin. Water Treat. 2020, 202, 48–60. [Google Scholar] [CrossRef]
- Batko, K.; Ślęzak, A.; Grzegorczyn, S.; Bajdur, W.M. The Rr form of the Kedem–Katchalsky–Peusner model equations for description of the membrane transport in concentration polarization conditions. Entropy 2020, 22, 857. [Google Scholar] [CrossRef]
- Batko, K.; Ślęzak, A.; Pilis, W. Evaluation of transport properties of biomembranes by means of Peusner network thermodynamics. Acta Bioeng. Biomech. 2021, 23, 63–72. [Google Scholar] [CrossRef]
- Kargol, M.; Kargol, A. Mechanistic formalism for membrane transport generated by osmotic and mechanical pressure. Gen. Physiol. Biophys. 2003, 22, 51–68. [Google Scholar]
- Peusner, L. The Principles of Network Thermodynamics: Theory and Biophysical Applications. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 1970. [Google Scholar]
- Peusner, L. Hierarchies of irreversible energy conversion systems: A network thermodynamics approach. I. Linear steady state without storage. J. Theor. Biol. 1983, 10, 27–39. [Google Scholar] [CrossRef]
- Peusner, L. Hierarchies of irreversible energy conversion systems. II. Network derivation of linear transport equations. J. Theor. Biol. 1985, 115, 319–335. [Google Scholar] [CrossRef]
- Peusner, L. Network representation yelding the evolution of Brownian motion with multiple particle interactions. Phys. Rev. A 1985, 32, 1237–1238. [Google Scholar] [CrossRef]
- Peusner, L.; Mikulecky, D.C.; Caplan, S.R. A network thermodynamic approach to Hill and King-Altman reaction-diffusion kinetics. J. Chem. Phys. 1985, 83, 5559–5566. [Google Scholar] [CrossRef]
- Ślęzak, A.; Dworecki, K.; Ślęzak, I.H.; Wąsik, S. Permeability coefficient model equations of the complex: Membrane-concentration boundary layers for ternary nonelectrolyte solutions. J. Membr. Sci. 2005, 267, 50–57. [Google Scholar] [CrossRef]
- Ślęzak, A.; Grzegorczyn, S.; Jasik-Ślęzak, J.; Michalska-Małecka, K. Natural convection as an asymmetrical factor of the transport through porous membrane. Transp. Porous Media 2010, 84, 685–698. [Google Scholar] [CrossRef]
- Dworecki, K.; Ślęzak, A.; Ornal-Wąsik, B.; Wąsik, S. Effect of hydrodynamic instabilities on solute transport in a membrane system. J. Membr. Sci. 2005, 265, 94–100. [Google Scholar] [CrossRef]
- Jasik-Ślęzak, J.; Olszówka, K.M.; Ślęzak, A. Estimation of thickness of concentration boundary layers by osmotic volume flux determination. Gen. Physiol. Biophys. 2011, 30, 186–195. [Google Scholar] [CrossRef]
- Ślęzak, A. Irreversible thermodynamic model equations of the transport across a horizontally mounted membrane. Biophys. Chem. 1989, 34, 91–102. [Google Scholar] [CrossRef]
- Ślęzak, A.; Dworecki, K.; Jasik-Ślęzak, J.; Wąsik, J. Method to determine the practical concentration Rayleigh number in isothermal passive membrane transport processes. Desalination 2004, 168, 397–412. [Google Scholar] [CrossRef]
- Ślęzak, A.; Dworecki, K.; Anderson, J.A. Gravitational effects on transmembrane flux: The Rayleigh-Taylor convective instability J. Membr. Sci. 1985, 23, 71–81. [Google Scholar] [CrossRef]
- Ewing, G.W. Instrumental Methods of Chemical Analysis; McGraw-Hill Book Company: New York, NY, USA, 1985. [Google Scholar]
- Batko, K.M.; Ślęzak, A. Evaluation of the global S-entropy production in membrane transport of aqueous solutions of hydrochloric acid and ammonia. Entropy 2020, 22, 1021. [Google Scholar] [CrossRef] [PubMed]
- Kedem, O.; Caplan, S.R. Degree of coupling and its relation to efficiency of energy conversion. Trans. Faraday Soc. 1965, 61, 1897–1911. [Google Scholar] [CrossRef]
- Grzegorczyn, S.; Ślęzak, A.; Przywara-Chowaniec, B. Concentration polarization phenomenon in the case of mechanical pressure difference on thr membrane. J. Biol. Phys. 2017, 43, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Lebon, G.; Jou, D.; Casas-Vasquez, J. Understanding Non-Equilibrium Thermodynamics Foundations, Applications, Frontiers; Springer: Berlin, Germany, 2008. [Google Scholar]
- Batko, K.M.; Ślęzak, A.; Bajdur, W.M. The role of gravity in the evolution of the concentration field in the elekctrochemical membrane cell. Entropy 2020, 22, 680. [Google Scholar] [CrossRef] [PubMed]
- Adampolous, K.; Koutsouris, D.; Zaravinous, A.; Lambrou, G.I. Gravitational influence on human living systems and the evolution of spaces on earth. Molecules 2021, 26, 2784. [Google Scholar] [CrossRef] [PubMed]
- Horng, T.-L.; Lin, T.-C.; Liu, C.; Eisenberg, B. PNP Equations with steric effects: A model of ion flow through channels. J. Phys. Chem. B 2012, 116, 11422–11441. [Google Scholar] [CrossRef]
- Caplan, D.A.; Subbotina, J.O.; Noskov, S.Y. Molecular mechanism of ion-ion and ion-substrate coupling in the Na+-dependent leucine transporter LeuT. Biophys. J. 2008, 95, 4613–4621. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Harvie, D.J.E.; Davidson, M.R.; Dunstan, D.E.; Hwang, J.; Daiguji, H. Viscoelectric fffects in nanochannel electrokinetics. J. Phys. Chem. C 2017, 121, 20517–20523. [Google Scholar] [CrossRef]
- Lim, A.E.; Lim, C.Y.; Lam, Y.C. Electroosmotic flow hysteresis for dissimilar anionic solutions. Anal. Chem. 2016, 88, 8064–8073. [Google Scholar] [CrossRef]
- Lim, A.E.; Lam, Y.C. Numerical investigation of nanostructure orientation on electroosmotic flow. Micromachines 2020, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Kucharzewski, M.; Wilemska-Kucharzewska, K.; Kózka, M.; Spałkowska, M. Leg venous ulcer healing process after application of membranous dressing with silver ions. Phlebologie 2013, 42, 340–346. [Google Scholar] [CrossRef]
- Ślęzak, A.; Kucharzewski, M.; Franek, A.; Twardokęs, W. Evaluation of the efficiency of venous leg ulcer treatment with a membrane dressing. Med. Eng. Phys. 2004, 26, 2653–2660. [Google Scholar] [CrossRef]
- Richter, T.; Keipert, S. In vitro permeation studies comparing bovine nasal mucosa, porcine cornea and artificial membrane: Androstenedione in microemulsions and their components. Eur. J. Pharm. Biopharm. 2004, 58, 137–143. [Google Scholar] [CrossRef]
- Kosztolowicz, T.; Dutkiewicz, A.; Lewandowska, K.D.; Wasik, S.; Arabski, M. Subdiffusion equation with Caputo fractional derivative with respect to another function in modelling diffusion in a complex system consisting of matrix and channels. Phys. Rev. E 2021, 104, 014118. [Google Scholar] [CrossRef]
- Lim, M.Y.; Roach, J.O. Metabolism and Nutrition; Elsevier: Edinburg, TX, USA, 2007. [Google Scholar]
- Patel, J.; Shalin Parikh, S.; Patel, S. Comprehensive review on osmotic drug delivery system. World J. Pharm. Res. 2021, 10, 523–550. [Google Scholar] [CrossRef]
- Delmotte, M.; Chanu, J. Non-equilibrium thermodynamics and membrane potential measurement in biology. In Topics Bioelectrochemistry and Bioenergetics; Millazzo, G., Ed.; John Wiley & Sons: Chichester, UK, 1979; pp. 307–359. [Google Scholar]
- Hoshiko, T.; Lindley, B.D. Phenomenological description of active transport of salt and water. J. Gen. Physiol. 1967, 50, 729–758. [Google Scholar] [CrossRef]
- Bui, T.Q.; Magnussen, O.-P.; Cao, V.D.; Wang, W.; Kjøniksen, A.-L.; Aaker, O. Osmotic engine converting energy from salinity difference to a hydraulic accumulator by utilizing olyelectrolyte hydrogels. Energy 2021, 232, 121055. [Google Scholar] [CrossRef]
- Sharma, M.; Chakraborty, A.; Purkait, M.K. Clean energy from salinity gradients using pressure retarded osmosis and reverse electrodialysis: A review. Sustain. Energy Technol. Assess. 2022, 49, 101687. [Google Scholar] [CrossRef]
- Chang, H.; Zou, Y.; Hu, R.; Feng, H.; Wu, H.; Zhong, N.; Hu, J. Membrane applications for microbial energy conversion: A review. Environ. Chem. Lett. 2020, 18, 1581–1592. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batko, K.M.; Ślęzak-Prochazka, I.; Ślęzak, A.; Bajdur, W.M.; Włodarczyk-Makuła, M. Management of Energy Conversion Processes in Membrane Systems. Energies 2022, 15, 1661. https://doi.org/10.3390/en15051661
Batko KM, Ślęzak-Prochazka I, Ślęzak A, Bajdur WM, Włodarczyk-Makuła M. Management of Energy Conversion Processes in Membrane Systems. Energies. 2022; 15(5):1661. https://doi.org/10.3390/en15051661
Chicago/Turabian StyleBatko, Kornelia M., Izabella Ślęzak-Prochazka, Andrzej Ślęzak, Wioletta M. Bajdur, and Maria Włodarczyk-Makuła. 2022. "Management of Energy Conversion Processes in Membrane Systems" Energies 15, no. 5: 1661. https://doi.org/10.3390/en15051661
APA StyleBatko, K. M., Ślęzak-Prochazka, I., Ślęzak, A., Bajdur, W. M., & Włodarczyk-Makuła, M. (2022). Management of Energy Conversion Processes in Membrane Systems. Energies, 15(5), 1661. https://doi.org/10.3390/en15051661









