Experimental Study on Breakthrough Separation for Hydrogen Recovery from Coke Oven Gas Using ZIF-8 Slurry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
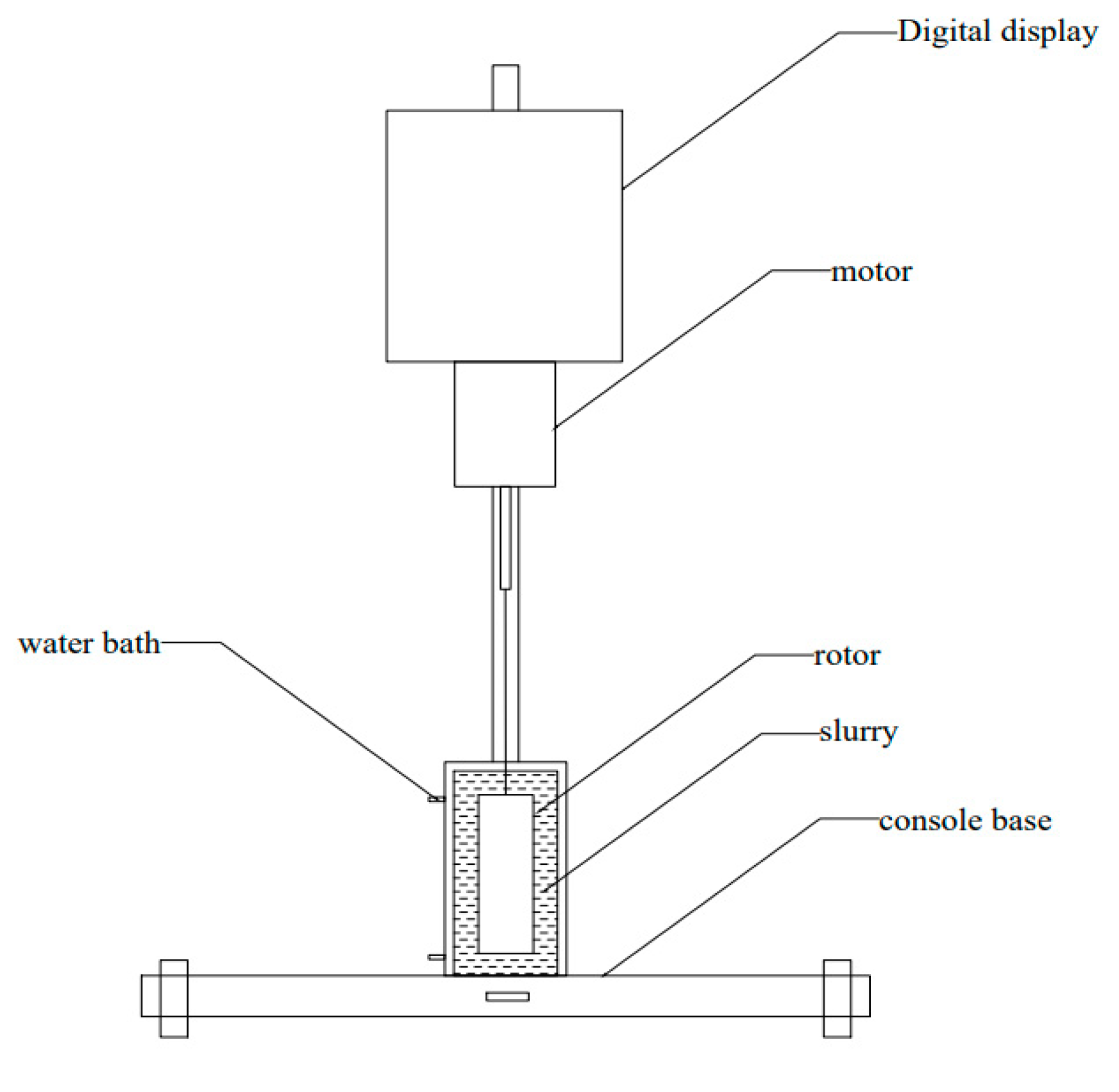
2.2. Experimental Apparatus
2.3. Experimental Procedures
2.4. Data Processing
3. Results and Discussion
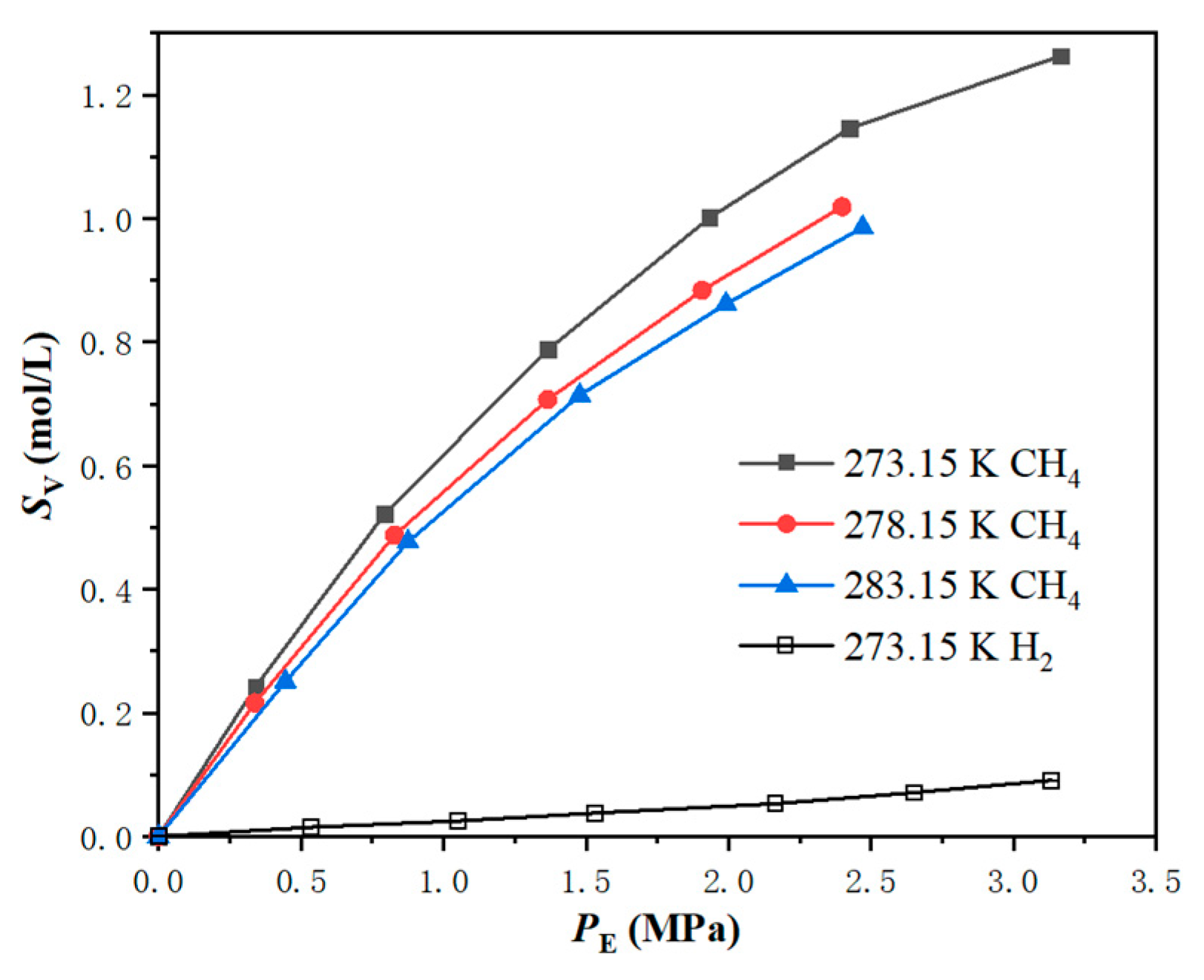
3.1. Gas-Slurry Phase Equilibrium Experiments
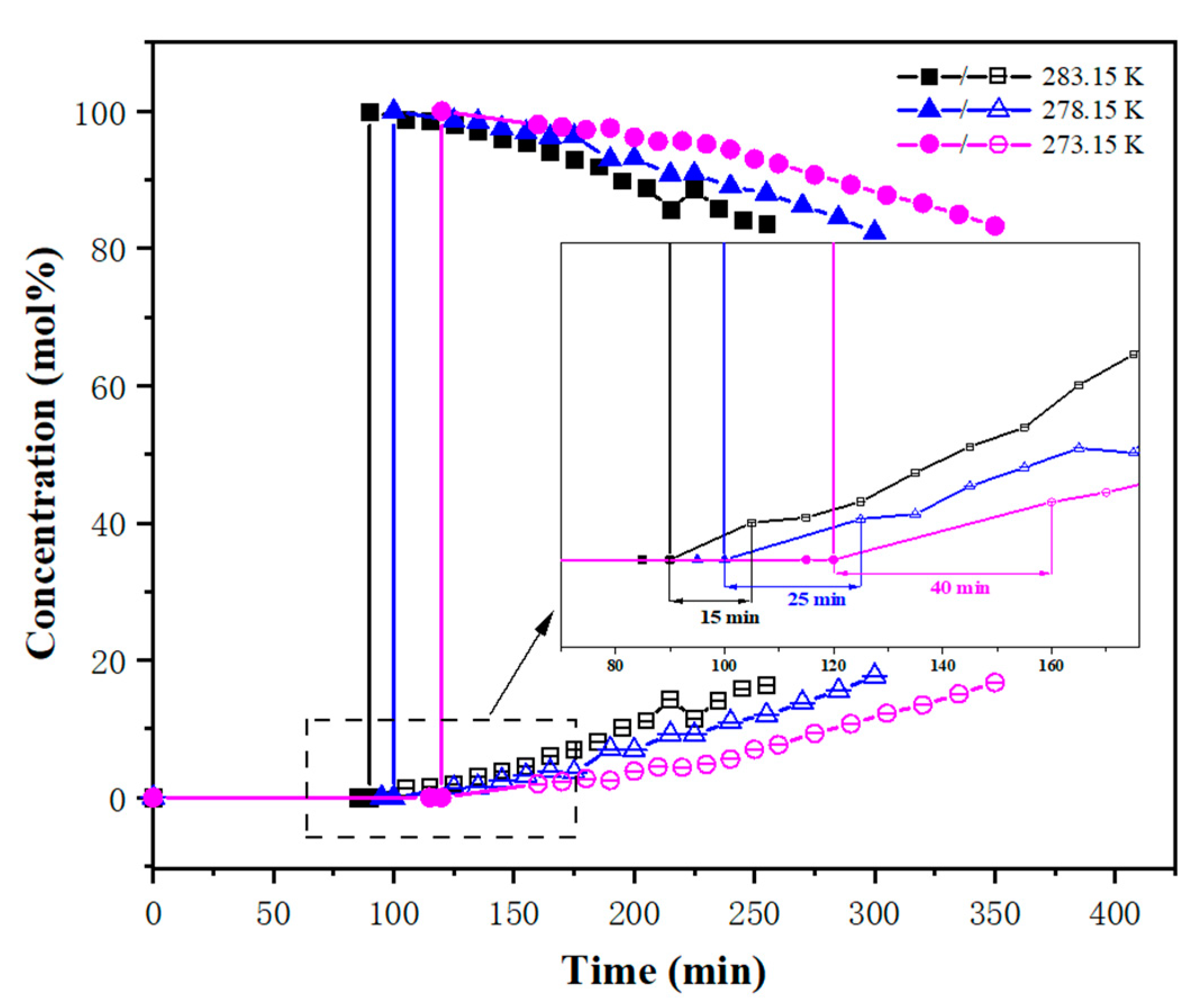
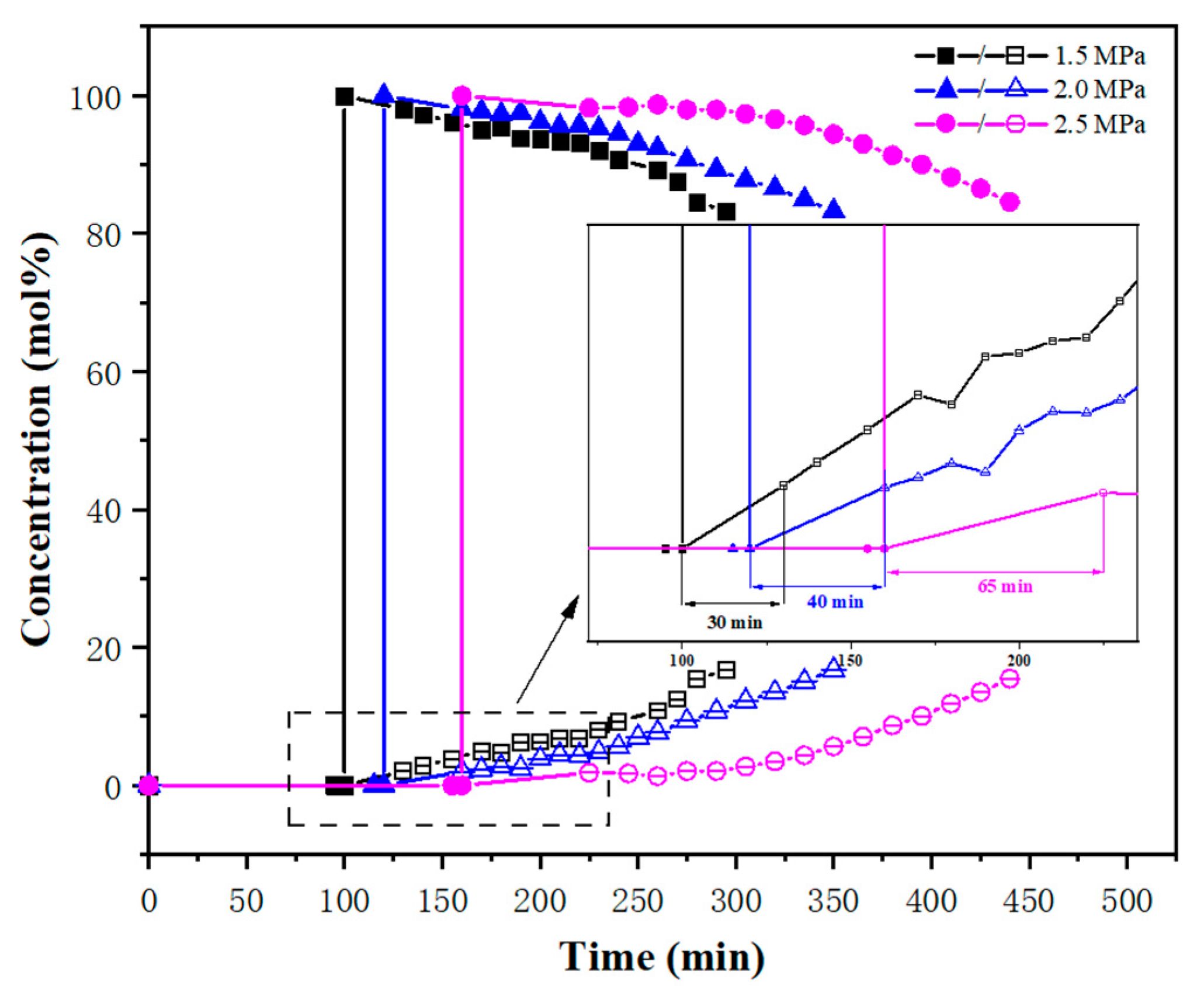
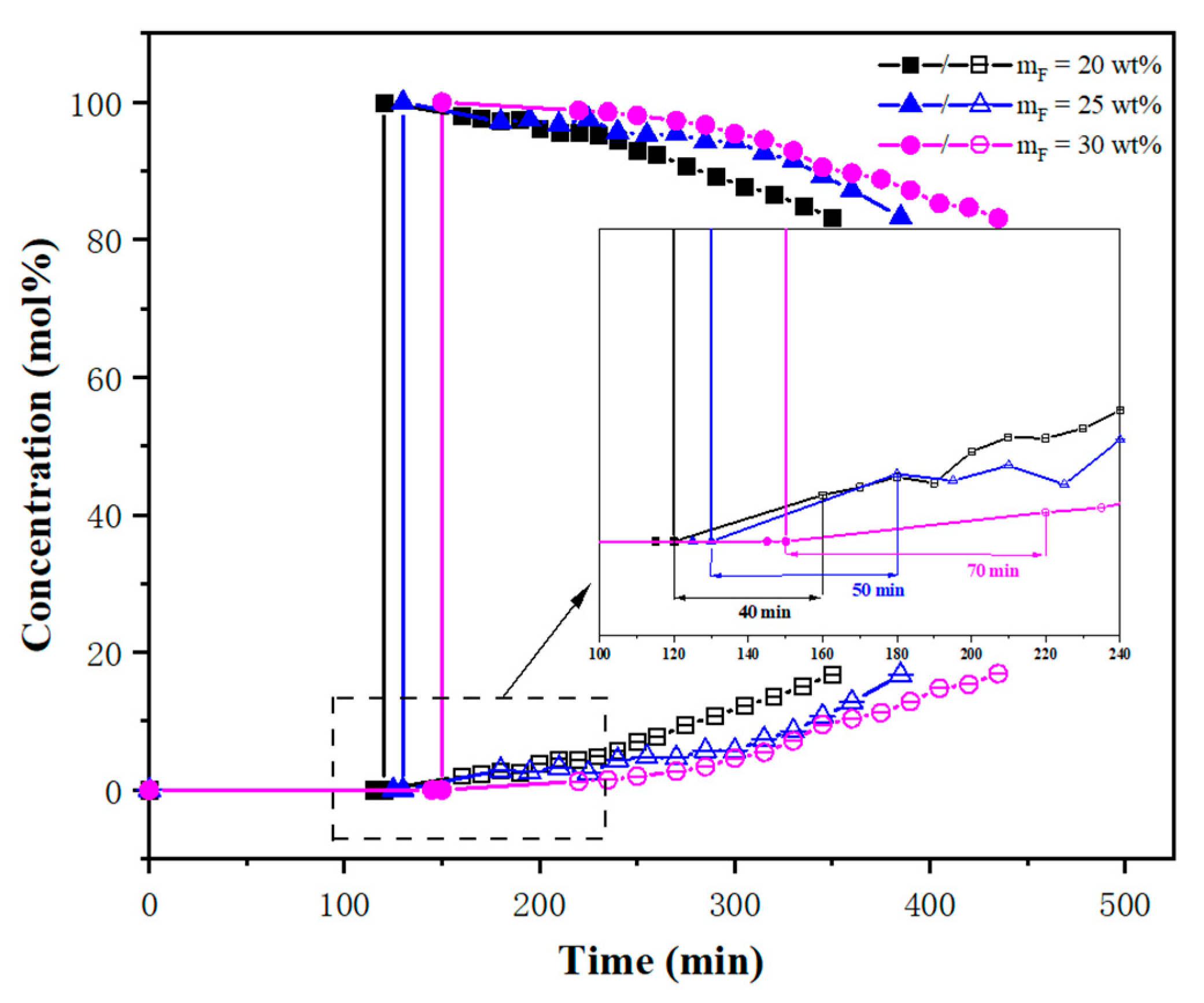
3.2. Breakthrough Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Razzaq, R.; Li, C.; Zhang, S. Coke oven gas: Availability, properties, purification, and utilization in China. Fuel 2013, 113, 287–299. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, C.; Zhang, D.; Zhou, H. Development of a Coke Oven Gas Assisted Coal to Ethylene Glycol Process for High Techno-Economic Performance and Low Emission. Ind. Eng. Chem. Res. 2018, 57, 7600–7612. [Google Scholar] [CrossRef]
- Bermúdez, J.M.; Fidalgo, B.; Arenillas, A.; Menéndez, J.A. Dry reforming of coke oven gases over activated carbon to produce syngas for methanol synthesis. Fuel 2010, 89, 2897–2902. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, J.; Yang, B.; Zhang, Y. Dynamic analysis on methanation reactor using a double-input–multi-output linearized model. Chin. J. Chem. Eng. 2015, 23, 389–397. [Google Scholar] [CrossRef]
- Taiyan, W. Problem and way of coke oven gas development and utilization. Fuel Chem. Processes 2004, 35, 1–3. [Google Scholar]
- Wu, S.; Xu, X.; Li, X.; Bi, L. High-performance proton-conducting solid oxide fuel cells using the first-generation Sr-doped LaMnO3 cathode tailored with Zn ions. Sci. China Mater. 2021, 65, 675–682. [Google Scholar] [CrossRef]
- Tao, Z.; Fu, M.; Liu, Y.; Gao, Y.; Tong, H.; Hu, W.; Lei, L.; Bi, L. High-performing proton-conducting solid oxide fuel cells with triple-conducting cathode of Pr0.5Ba0.5(Co0.7Fe0.3)O3−δ tailored with W. Int. J. Hydrog. Energy 2022, 47, 1947–1953. [Google Scholar] [CrossRef]
- Xu, J.; Lin, W.; Xu, S. Hydrogen and LNG production from coke oven gas with multi-stage helium expansion refrigeration. Int. J. Hydrog. Energy 2018, 43, 12680–12687. [Google Scholar] [CrossRef]
- Van Acht, S.C.J.; Laycock, C.; Carr, S.J.W.; Maddy, J.; Guwy, A.J.; Lloyd, G.; Raymakers, L.F.J.M. Simulation of integrated novel PSA/EHP/C process for high-pressure hydrogen recovery from Coke Oven Gas. Int. J. Hydrog. Energy 2020, 45, 15196–15212. [Google Scholar] [CrossRef]
- Huang, K.; Yuan, J.; Shen, G.; Liu, G.; Jin, W. Graphene oxide membranes supported on the ceramic hollow fibre for efficient H2 recovery. Chin. J. Chem. Eng. 2017, 25, 752–759. [Google Scholar] [CrossRef]
- Li, L.; Qi, H. Gas separation using sol–gel derived microporous zirconia membranes with high hydrothermal stability. Chin. J. Chem. Eng. 2015, 23, 1300–1306. [Google Scholar] [CrossRef]
- Sun, Q.; Dong, J.; Guo, X.; Liu, A.; Zhang, J. Recovery of Hydrogen from Coke-Oven Gas by Forming Hydrate. Ind. Eng. Chem. Res. 2012, 51, 6205–6211. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Chen, G.; Liu, B.; Dandekar, A.; Wang, B.; Zhang, X.; Sun, C.; Ma, Q. High-efficiency separation of a CO2/H2 mixture via hydrate formation in W/O emulsions in the presence of cyclopentane and TBAB. Int. J. Hydrog. Energy 2014, 39, 7910–7918. [Google Scholar] [CrossRef]
- Bermúdez, J.M.; Arenillas, A.; Luque, R.; Menéndez, J.A. An overview of novel technologies to valorise coke oven gas surplus. Fuel Process. Technol. 2013, 110, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, S.; Fernando, S. Hydrogen Membrane Separation Techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Chen, G.-J.; Zhang, L.-W. Hydrate phase equilibrium and structure for (methane + ethane + tetrahydrofuran + water) system. J. Chem. Thermodyn. 2010, 42, 1173–1179. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Nataraj, S.K.; Roussenova, M.V.; Tan, J.C.; Hughes, D.J.; Li, W.; Bourgoin, P.; Alam, M.A.; Cheetham, A.K.; Al-Muhtaseb, S.A.; et al. Zeolitic imidazolate framework (ZIF-8) based polymer nanocomposite membranes for gas separation. Energy Environ. Sci. 2012, 5, 8359–8369. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic imidazolate framework membrane with molecular sieving properties by microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Varela-Guerrero, V.; Barnett, G.V.; Jeong, H.K. Synthesis of zeolitic imidazolate framework films and membranes with controlled microstructures. Langmuir 2010, 26, 14636–14641. [Google Scholar] [CrossRef]
- Liu, H.; Pan, Y.; Liu, B.; Sun, C.; Guo, P.; Gao, X.; Yang, L.; Ma, Q.; Chen, G. Tunable integration of absorption-membrane-adsorption for efficiently separating low boiling gas mixtures near normal temperature. Sci. Rep. 2016, 6, 21114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, B.; Lin, L.C.; Chen, G.; Wu, Y.; Wang, J.; Gao, X.; Lv, Y.; Pan, Y.; Zhang, X.; et al. A hybrid absorption-adsorption method to efficiently capture carbon. Nat. Commun. 2014, 5, 5147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, W.; Liu, B.; Jia, C.; Qiao, Z.; Sun, C.; Yang, L.; Ma, Q.; Chen, G. CO2 capture using ZIF-8/water-glycol-2-methylimidazole slurry with high capacity and low desorption heat. Chem. Eng. Sci. 2018, 182, 189–199. [Google Scholar] [CrossRef]
- Chen, W.; Zou, E.; Zuo, J.Y.; Chen, M.; Yang, M.; Li, H.; Jia, C.; Liu, B.; Sun, C.; Deng, C.; et al. Separation of Ethane from Natural Gas Using Porous ZIF-8/Water–Glycol Slurry. Ind. Eng. Chem. Res. 2019, 58, 9997–10006. [Google Scholar] [CrossRef]
- Yang, M.-K.; Han, Y.; Zou, E.; Chen, W.; Peng, X.; Dong, B.; Sun, C.; Liu, B.; Chen, G. Separation of IGCC syngas by using ZIF-8/dimethylacetamide slurry with high CO2 sorption capacity and sorption speed but low sorption heat. Energy 2020, 201, 117605. [Google Scholar] [CrossRef]
- Chen, W.; Guo, X.; Zou, E.; Luo, M.; Chen, M.; Yang, M.; Li, H.; Jia, C.; Deng, C.; Sun, C.; et al. A continuous and high-efficiency process to separate coal bed methane with porous ZIF-8 slurry: Experimental study and mathematical modelling. Green Energy Environ. 2020, 5, 347–363. [Google Scholar] [CrossRef]
- Yan, S.; Zhu, D.; Zhang, Z.; Li, H.; Chen, G.; Liu, B. A pilot-scale experimental study on CO2 capture using Zeolitic imidazolate framework-8 slurry under normal pressure. Appl. Energy 2019, 248, 104–114. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; Yang, M.; Zhu, D.; Huang, Z.; Chen, W.; Yang, L.; Chen, G. CO2 Separation Performance of Zeolitic Imidazolate Framework-8 Porous Slurry in a Pilot-Scale Packed Tower. Ind. Eng. Chem. Res. 2020, 59, 6154–6163. [Google Scholar] [CrossRef]
- Li, H.; Chen, W.; Liu, B.; Yang, M.; Huang, Z.; Sun, C.; Deng, C.; Cao, D.; Chen, G. A purely green approach to low-cost mass production of zeolitic imidazolate frameworks. Green Energy Environ. 2021, in press. [CrossRef]
- Chiau Junior, M.J.; Wang, Y.; Wu, X.; Cai, W. Computational screening of metal-organic frameworks with open copper sites for hydrogen purification. Int. J. Hydrog. Energy 2020, 45, 27320–27330. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, W.; Zhang, X.; Zhang, Z.; Tong, X.; Jia, C.; Liu, B.; Sun, C.; Yang, L.; Chen, G. Large-scale synthesis of ZIF-67 and highly efficient carbon capture using a ZIF-67/glycol-2-methylimidazole slurry. Chem. Eng. Sci. 2015, 137, 504–514. [Google Scholar] [CrossRef]
- Chen, W.; Chen, M.; Yang, M.; Zou, E.; Li, H.; Jia, C.; Sun, C.; Ma, Q.; Chen, G.; Qin, H. A new approach to the upgrading of the traditional propylene carbonate washing process with significantly higher CO2 absorption capacity and selectivity. Appl. Energy 2019, 240, 265–275. [Google Scholar] [CrossRef]
- Peng, X.; Jia, C.; Qiao, Z.; Yang, S.; Liu, B.; Deng, C.; Li, H.; Chen, W.; Sun, C.; Chen, G. A new energy efficient process for hydrogen purification using ZIF-8/glycol-water slurry: Experimental study and process modeling. Int. J. Hydrog. Energy 2021, 46, 32081–32098. [Google Scholar] [CrossRef]


| Slurry Composition | Viscosity, (mPa·s) | |||
|---|---|---|---|---|
| Solid Content mF, (wt%) | Solvent Content, (wt%) | 273.15 K | 278.15 K | 283.15 K |
| 20 | 80 | 12.8 | 11.8 | 10.3 |
| 25 | 75 | 14.6 | 13.1 | 11.4 |
| 30 | 70 | 26.9 | 24.8 | 21.6 |
| mF, (wt%) | T, (K) | P, (MPa) | vin, (mL/min) | tH2, (min) | tCH4, (min) | Δt, (min) |
|---|---|---|---|---|---|---|
| 20 | 273.15 | 2.0 | 27.72 | 120 | 160 | 40 |
| 278.15 | 100 | 125 | 25 | |||
| 283.15 | 90 | 105 | 15 | |||
| 20 | 273.15 | 1.5 | 27.72 | 100 | 130 | 30 |
| 2.5 | 160 | 225 | 65 | |||
| 25 | 273.15 | 2.0 | 27.72 | 130 | 180 | 50 |
| 30 | 150 | 220 | 70 | |||
| 20 | 273.15 | 2.0 | 36.96 | 90 | 115 | 25 |
| 46.20 | 50 | 60 | 10 |
| Time (min) | yout-H2/yout-CH4, (mol%) | vout, (mL·min−1) | RH2, (%) | RCH4, (%) |
|---|---|---|---|---|
| 0 | 0/0 | 0 | 0 | 0 |
| 120 | 100/0 | 6.38 | 35.57 | 100 |
| 160 | 98.03/1.97 | 12.41 | 67.82 | 97.42 |
| 170 | 97.70/2.30 | 13.85 | 75.43 | 96.64 |
| 180 | 97.26/2.74 | 14.51 | 78.67 | 95.81 |
| 200 | 96.19/3.81 | 16.08 | 86.23 | 93.54 |
| 220 | 95.63/4.38 | 17.05 | 90.88 | 92.13 |
| 230 | 95.21/4.79 | 17.69 | 93.89 | 91.07 |
| 240 | 94.43/5.57 | 18.14 | 95.49 | 89.35 |
| 250 | 93.03/6.97 | 18.53 | 96.10 | 86.38 |
| 260 | 92.34/7.66 | 18.87 | 97.14 | 84.76 |
| 275 | 90.67/9.33 | 19.02 | 96.14 | 81.29 |
| 290 | 89.27/10.73 | 19.46 | 96.84 | 77.99 |
| 305 | 87.77/12.23 | 19.68 | 96.29 | 74.63 |
| Time (min) | yout-H2/yout-CH4, (mol%) | vout, (mL·min−1) | RH2, (%) | RCH4, (%) |
|---|---|---|---|---|
| 0 | 0/0 | 0 | 0 | 0 |
| 90 | 100/0 | 10.26 | 42.20 | 100 |
| 115 | 99.09/0.91 | 18.85 | 76.83 | 98.64 |
| 125 | 98.33/1.67 | 20.667 | 83.59 | 97.27 |
| 150 | 98.27/1.73 | 23.45 | 94.78 | 96.80 |
| 180 | 97.22/2.78 | 24.95 | 99.77 | 94.52 |
| 20 | 95.34/4.66 | 25.82 | 101.25 | 90.49 |
| 210 | 93.66/6.34 | 26.1 | 100.55 | 86.92 |
| 220 | 92.02/7.98 | 26.84 | 101.59 | 83.07 |
| 240 | 89.65/10.35 | 27.69 | 102.11 | 77.34 |
| 260 | 84.28/15.72 | 29.17 | 101.12 | 63.74 |
| 270 | 82.22/17.78 | 29.58 | 100.03 | 58.42 |
| Time (min) | yout-H2/yout-CH4, (mol%) | vout, (mL·min−1) | RH2, (%) | RCH4, (%) |
|---|---|---|---|---|
| 0 | 0/0 | 0 | 0 | 0 |
| 50 | 100/0 | 15.27 | 50.24 | 100 |
| 60 | 98.90/1.10 | 20.36 | 66.26 | 98.58 |
| 70 | 97.70/2.30 | 24.39 | 78.41 | 96.45 |
| 90 | 96.46/3.54 | 30.98 | 98.33 | 93.06 |
| 100 | 96.25/3.75 | 33.2 | 105.15 | 92.13 |
| 110 | 95.44/4.56 | 35.2 | 110.54 | 89.85 |
| 120 | 93.93/6.07 | 36.09 | 111.55 | 86.14 |
| 130 | 92.25/7.75 | 36.72 | 111.46 | 82.00 |
| 140 | 89.56/10.44 | 37.43 | 110.31 | 75.28 |
| 150 | 87.15/12.85 | 37.66 | 108.00 | 69.39 |
| 160 | 84.16/15.84 | 38.11 | 105.54 | 61.82 |
| 170 | 81.12/18.88 | 38.57 | 102.95 | 53.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Wang, M.; Yang, S.; Huang, Z.; Yang, M.; Peng, X.; Liu, B.; Chen, G. Experimental Study on Breakthrough Separation for Hydrogen Recovery from Coke Oven Gas Using ZIF-8 Slurry. Energies 2022, 15, 1487. https://doi.org/10.3390/en15041487
Chen W, Wang M, Yang S, Huang Z, Yang M, Peng X, Liu B, Chen G. Experimental Study on Breakthrough Separation for Hydrogen Recovery from Coke Oven Gas Using ZIF-8 Slurry. Energies. 2022; 15(4):1487. https://doi.org/10.3390/en15041487
Chicago/Turabian StyleChen, Wan, Minglong Wang, Shaowu Yang, Zixuan Huang, Mingke Yang, Xiaowan Peng, Bei Liu, and Guangjin Chen. 2022. "Experimental Study on Breakthrough Separation for Hydrogen Recovery from Coke Oven Gas Using ZIF-8 Slurry" Energies 15, no. 4: 1487. https://doi.org/10.3390/en15041487
APA StyleChen, W., Wang, M., Yang, S., Huang, Z., Yang, M., Peng, X., Liu, B., & Chen, G. (2022). Experimental Study on Breakthrough Separation for Hydrogen Recovery from Coke Oven Gas Using ZIF-8 Slurry. Energies, 15(4), 1487. https://doi.org/10.3390/en15041487





