Abstract
The inhibition effect of calcined lime (CaO) and limestone (CaCO3) on the formation of dioxins during iron ore co-sintering with fly ash was investigated in a sinter pot in the present work. Experimental results indicated that international total toxicity equivalent concentration of dioxins decreased from 1.4335 to 0.2922, 0.1048, 0.4562, and 0.3098 ng I-TEQ Nm−3 under four different experimental conditions. It can be concluded that 5 wt.% calcined lime with 3 wt.% limestone is the optimal addition to reduce the concentration of dioxins in flue gas, with 92.70% inhibition efficiency. Effects on dioxin distribution was also analyzed. The distribution proportion of low-chlorinated dioxins was found to increase, while that of high-chlorinated dioxins decreased, except for octachlorianted dibenzo-p-dioxins (OCDD). The reason is that the consumption of HCl not only inhibits the de novo synthesis, but also dramatically promotes the condensation and dechlorination to produce more tetrachlorianted dibenzo-p-dioxins and octachlorianted dibenzo-p-dioxins through precursors. Finally, condensation, dichlorination, and inhibition mechanisms of dioxins during co-sintering with municipal solid waste incineration (MSWI) fly ash are proposed.
1. Introduction
Municipal solid waste incineration (MSWI) [1] has developed rapidly in recent years, and controversy on MSWI’s safety to human health and the ecological environment is increasingly fierce, and the public has aroused great concern around the world, especially regarding MSWI fly ash (FA) [2]. MSWI FA is identified as a hazardous waste in many countries as it contains leachable heavy metals and polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) [3]. Many methods have been developed to dispose of fly ash such as landfill, solidification/stabilization, sintering, melting, vitrification, biological/chemical extraction, mechanochemistry, etc. Landfill after cement solidification/stabilization with a chelating agent is the most widespread disposal method, which barely decomposes dioxins, but increases the volume of the waste, causing the consumption of land resources [4,5].
Thermal treatment such as sintering [6,7], melting [8], and vitrification are being developed to dispose MSWI fly ash [9,10] by decomposing dioxins and synergistically solidifying heavy metals. Moreover, this thermal disposal method conforms with the principle of “reducing quantity”, “harmless”, and that products are environmentally friendly materials that can be reutilized. However, compared with non-thermal treatments, the energy consumption and economic costs are higher, which limits the development of high temperature thermal treatment technology [5,11]. Thus, researchers have shifted more attention to co-disposing processes.
Co-disposal is a solid waste disposal method that replaces one of the raw materials by adding satisfied or pretreated waste to the process in conventional high temperature production. Co-disposing of MSWI fly ash with the sintering process has been researched over the past decade. The iron ore sintering process is one of the most important procedures of iron and steel production. It provides high quality iron ore raw materials for ironmaking to avoid clogging of the blast-furnace [12,13]. During sintering, materials will go through four stages with the sintering bed, proceeding from the top to the bottom. Previous studies have revealed that dioxins decomposed during combustion due to the high temperatures (>1000 °C) supplied by the combustion of fuels [14], and reformed in the preheat-dry zone, exhibiting a remarkable de novo synthesis pathway [15,16] A great deal of coke, chlorine, and catalytic metals contribute to the de novo synthesis [17,18] since the temperatures range between 200–650 °C [19,20], and fly ash is abundant in these necessary elements in the form of carbon and chlorides [21,22]. Previous research has revealed that the addition of MSWI FA would increase the concentration of dioxins in the flue gas from the sintering process, whether the fly ash is water washed or not [14,23]. Currently, 0.5 ng I-TEQ/Nm3 (I-TEQ: International Toxic Equivalent Quantity) is the accepted limit in China regulated for the control of PCDD/F emissions from iron ore sinter plants. In order to comply with the increasingly strict emission standards, measures need to be adopted to diminish dioxins from the sintering process including source control, optimum operating conditions, sintering exhaust gas recirculation, additive inhibitors, etc. [24].
The main mechanisms of inhibitors are reacting with chlorine or reducing the catalytic activity of metal catalysts. Nitrogen-based, sulfur-based, and alkali-based are three common inhibitors in some current studies. The sulfur-based inhibitors will increase sulfur oxides such as SO2 emission in flue gas, and consequently increase the desulfuration burden for the iron and steel industry [25,26]. The mechanism and effect of nitrogen-containing inhibitors, especially urea, added into the iron ore sintering raw mix has already been investigated and verified by many researchers [27,28]. It is reported that alkaline inhibitors such as CaO, CaCO3 can remove HCl and Cl2 in flue gas to inhibit the PCDD/F formation and emissions [29]. However, the PCDD distribution proportion may remain invariable, increasing or decreasing [30]. Though alkali-based inhibitors are strictly restricted to use during the pig iron production process due to the negative effect on the quality of sinter [24], adjusting its sinter raw mix within the normal range seems feasible [12]. A few studies have focused on the inhibiting behavior of adjusting the sinter raw mix (calcined lime (CaO) and limestone (CaCO3)), but the inhibition mechanisms of CaO or CaCO3 during iron ore co-sintering with MSWI fly ash are still unclear.
Thus, the aim of this study was to find whether adjusting the sinter raw mixture (calcined lime (CaO) and limestone (CaCO3)) is effective at inhibiting the dioxin concentration in flue gas, and focused on the inhibiting behavior of PCDD/Fs to explore the inhibition mechanism. The proportion of calcined lime or limestone in the sinter raw mix was slightly increased (1 wt.%, 2 wt.%) within the normal range to inhibit the reformation of dioxins in the present work. The total PCDD/F emissions and the inhibitory behavior of CaO or CaCO3 on PCDD/F congeners were explored. Finally, probable condensation, dichlorination, and the inhibition mechanisms of dioxins during co-sintering with MSWI fly ash are proposed.
2. Materials and Methods
2.1. MSWI Fly Ash and Sintering Raw Mix
Municipal solid waste incineration fly ash used in this study was sampled from a MSWI grate furnace plant located in Shanghai, China. Washed FA (WFA) was obtained through a three-stage countercurrent washing method with the solid to deionized water ratio of 1/6 [31]. The main elementary components in fly ash was analyzed with X-ray fluorescence (XRF, Thermo-scientific ARL ADVANT’X IntelliPowerTM 4200, Wilmington, DE, USA) and the results are provided in Table 1. The calcium content of the WFA was 44.28%, NaCl and KCl concentrations were significantly reduced after water washing, and the content of chlorine was lower than 2 wt.%, according to the latest industrial standard in China (HJ 1134-2020).

Table 1.
Main elementary components of FA and WFA used in the sintering experiments (mass, %). Reprinted with permission from Elsevier, 2022 [32].
Figure 1 shows the concentration distribution of 17 toxic PCDD/Fs in FA and WFA. The total I-TEQ concentrations of PCDD/Fs in FA and WFA were 302.2 and 599.2 pg I-TEQ/g, respectively, which is consistent with the TEQ concentration of fly ash in other cities in China. OCDD and 1234678-HpCDF were the two dominant congeners, while 23478-PeCDF contributed the highest I-TEQ concentration with 24.2% and 25.5%, respectively.
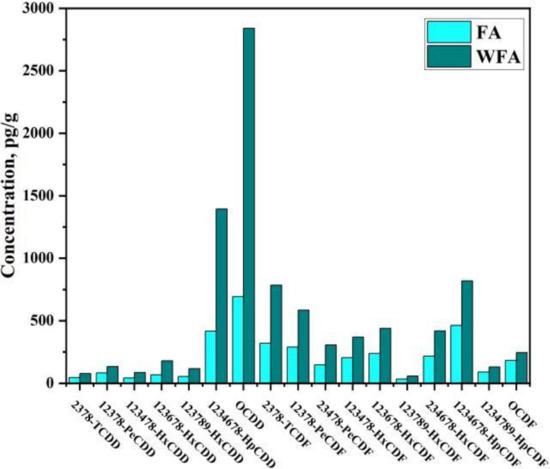
Figure 1.
Concentration distribution of 17 toxic PCDD/Fs in FA and WFA.
The raw materials were provided by a sintering plant in Shanghai, China, and the main chemical compositions that were detected according to the Chinese National Standard (GB/T 6730) and the calculated material ratios of the sinter raw mix for different tests are shown in Table 2 and Table 3. The experimental condition of flue gas one (FG1) is in accordance with the proportion of the sintering plant where raw materials were sampled, and FG1 is the contrast group to observe the effects of adding fly ash and changing the proportion of raw materials. FG2 is with the addition of 1 wt.% WFA compared to FG1. FG3 and FG4 are conditions increasing the calcined lime (the main component is CaO) in the raw mix by 1 and 2%, respectively, and FG5 and FG6 are conditions increasing the limestone (the main component is CaCO3) by 1 and 2%, respectively. The binary basicity (w(CaO)/w(SiO2)) and coke fine content were 1.9 and 3.5 wt.%, respectively. The ferrous materials were the iron ore fines, mixed iron ore, and recycling materials (return sinter fines). The influence of fly ash, limestone, calcined lime, and dolomite additions were investigated.

Table 2.
Chemical compositions of raw materials (mass, wt.%). Reprinted with permission from Elsevier, 2022 [32].

Table 3.
The mass ratio of all raw materials (mass, %).
2.2. Experimental Procedures
The experiment was carried out in a sintering pot to simulate the commercial sintering process [23,33]. The sintering process includes proportioning, mixing, granulating, feeding, ignition, and sintering, as illustrated in Figure 2. One wt.% WFA was mixed with the sinter raw mix during the mixing process. Then, the mixture was fed into a drum (Φ 600 mm × 300 mm) for granulating [34]. Following this, a compact bed was created by the granules charged on a 1-kg hearth layer, which was at the bottom of the sinter pot. After this, a mixture of natural-gas and compressed air was used to ignite the sinter raw mixture for 120 s. The sintering process started at approximately 1200 °C and ended when the maximum temperature began to decrease. XAD-II polymeric resin was used to adsorb the dioxins in the tail gas, which was filtrated by a filter membrane, and the rest of the dioxins were collected by two bottles of toluene. Furthermore, toluene was used to wash the sampling pipe twice at the end of each experiment. Finally, the above three materials were combined together for further analysis of dioxins in tail gas. Each test was conducted twice sequentially to ensure the accuracy and reliability of the data [32].
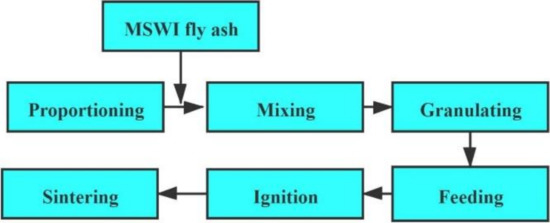
Figure 2.
Flow diagram of co-disposing MSWI fly ash through iron ore sintering. Developed from [35].
2.3. Analytical Methods
The procedures of the dioxin analysis in flue gas followed the U.S. EPA methods 1613 and 23A, respectively. After nitrogen-blown, the purified and concentrated extracts were dissolved in 25 uL nonane (spiking with 1 ng of 13C-labelled recovery standards) for the preparation of dioxin analysis. High-resolution gas chromatography/high-resolution mass spectrometry (JMS-800D, JEOL Co., Tokyo, Japan) was used to identify and quantify the concentration of 17 dioxins. Detailed steps of the clean-up procedure can be found in our group’s previous studies [36,37].
The international total toxicity equivalent concentration of dioxins is the arithmetic mean value of two parallel samples collected from each experimental condition and the inhibition efficiency can be calculated through Equation (1):
where CFG2 is the content or the international total toxicity equivalent concentration of dioxins in FG2, ng/Nm3, or ng I-TEQ/Nm3; CFGx is the content or the international total toxicity equivalent concentration of dioxins in FG3,4,5,6, respectively, ng/Nm3 or ng I-TEQ/Nm3.
Average degree of chlorination (dc) of PCDD/Fs is calculated as:
where fi is the mass distribution of PCDD-, PCDF-, or PCDD/F-congeners, and ni is the number of chlorine atoms in PCDD/F molecules.
3. Results and Discussion
3.1. Emissions Reduction in PCDD/Fs
Table 4 shows the I-TEQ value of 17 PCDD/F congeners and the inhibition efficiency for all samples. The I-TEQ value of FG1 (0 wt.% WFA without adjusting its sinter raw mix) was 0.687 ng I-TEQ/Nm3. After 1 wt.% WFA was added to the sinter raw mix (FG2), the I-TEQ concentration of dioxins in flue gas increased to 1.4335 ng I-TEQ/Nm3, which was twice as much as that of FG1, showing a significant facilitation in the formation of PCDD/Fs. After adjusting its raw mix ratio in FG2, the I-TEQ concentrations of FG3, FG4, FG5, and FG6 decreased to 0.2922, 0.1048, 0.4562, and 0.3097 ng I-TEQ/Nm3, respectively. These concentrations were lower than the Chinese limit of 0.5 ng I-TEQ/Nm3. The inhibition efficiencies were 78.04%, 92.70%, 68.22%, and 78.43%. For all samples, 2,3,4,7,8-PeCDF contributed the greatest toxicity concentration, though the component of it was lower than 1,2,3,4,6,7,8-HpCDF. The above results suggest that the I-TEQ concentration of dioxins during co-sintering were affected by the sinter raw mix. In this study, FG4 (5 wt.% CaO and 3 wt.% CaCO3) showed the highest inhibition efficiency.

Table 4.
The I-TEQ concentrations (ng I-TEQ/Nm3) of PCDD/Fs for six experimental flue gas samples.
The I-TEQ emission concentrations of PCDD/Fs are shown in Figure 3a. In FG1, the I-TEQ concentrations of PCDDs and PCDFs were 0.0772 and 0.6098 ng I-TEQ/Nm3, and PCDFs contributed 88.76% of the I-TEQ concentration. The PCDF/PCDD ratio was 5.28, as listed in Table 5, showing a significant distribution characteristic of PCDD/Fs from the sintering process. Many studies have confirmed that de novo synthesis mainly generates PCDFs, and PCDD congeners were synthesized through the precursor pathway [38]. With the addition of WFA (FG2), the emission I-TEQ concentrations of PCDDs and PCDFs increased to 0.1793 and 1.2562 ng I-TEQ/Nm3, respectively, and PCDFs contributed 87.51% of the I-TEQ concentrations. By adjusting the raw sinter mix of FG2, the I-TEQ concentrations were 0.0659, 0.0206, 0.0450, and 0.0521 ng I-TEQ/Nm3 for PCDDs and 0.3004, 0.0843, 0.3956, and 0.2576 ng I-TEQ/Nm3 for PCDFs, respectively. The inhibition efficiencies of PCDDs and PCDFs are shown in Figure 3b. As shown in this figure, the inhibition efficiencies of PCDDs were 76.07%, 88.53%, 66.26% and 70.93%, respectively. The inhibition efficiencies of PCDFs were 80.15%,93.29%, 68.50%, and 79.49%, respectively. The inhibition efficiencies of PCDDs (PCDFs) increased from 76.07% to 88.53% (from 80.15% to 93.29%) overall whereby the inhibition efficiencies with 4% CaO and 4% CaCO3 addition corresponded to an increase of 76.07% (80.15%) and 66.26% (68.50%), respectively. For 5% CaO and 5% CaCO3, the increment was 88.53% (93.29%) and 70.93% (79.49%), respectively. Evidently, the inhibition efficiency of PCDFs was higher than that of PCDDs. In addition, all 17 toxic dioxins were inhibited by CaO or CaCO3, resulting in a reduction in PCDD/Fs by more than 65%, but CaO seemed to better inhibit the production of PCDD/F compounds.
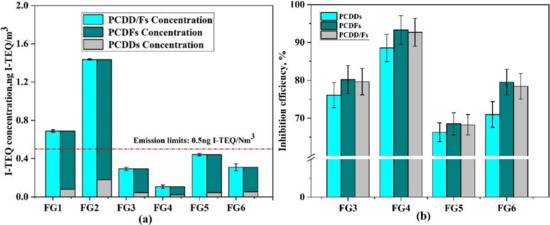
Figure 3.
(a) I-TEQ concentration of PCDDs and PCDFs; (b) inhibition efficiency of PCDDs and PCDFs.

Table 5.
Weight average level of chlorination and PCDF/PCDF ratio.
3.2. Effects on PCDD/Fs Congeners
The homologue profiles and the ratio of PCDD/PCDF are affected by adjusting the sinter raw mix. The weight percentage distribution of 17 toxic congeners is shown in Figure 4. OCDD and HpCDD were the dominant congeners in all samples and the percentage of OCDD increased from 38% to 48%, 45%, 48%, and 51% in FG3, FG4, FG5, and FG6, respectively. In this study, the increase in OCDD may be due to the precursor formation of PCDD/Fs catalyzed by Cu or Cr. Similarly, CaO was discovered to facilitate OCDD/TCDD generated from PCP prominently at 850 °C by Lu et al. [30]. In FG3 and FG4, the increase in CaO or CaCO3 reduced the fraction of HpCDD and HxCDD, but slightly increased the mass proportion of low-chlorinated PCDDs, which might be a result of the mutual chlorination of dioxins that was inhibited [26]. Among them, the fraction of HpCDD reduced from 34% to 27%, 31%, 26%, and 23% and HxCDD declined from 21% to 17%, 14%, 16%, and 15%, while PeCDD increased from 5% to 7%, 7%, 7%, and 10% and TCDD increased from 1% to 2%, 3%, 2%, and 2%.
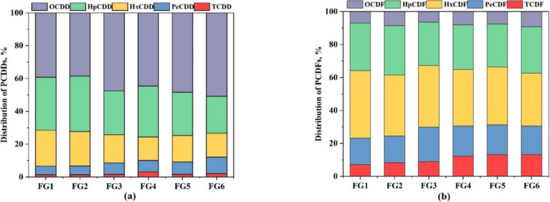
Figure 4.
(a) Mass distribution of PCDDs; (b) mass distribution of PCDFs.
The same phenomena were discovered in the PCDFs except for OCDF, and the increase in CaO or CaCO3 had a slight impact on OCDF. The increase in CaO or CaCO3 decreased the chlorination of PCDFs, especially in FG4 and FG6, due to the inhibition of the de novo formation of dioxins. As shown in Figure 4, the dominant two congeners were HpCDF and HxCDF for all samples. With the addition of CaO and CaCO3, the proportion of HpCDF and HxCDF decreased, while that of PeCDF and TCDF increased.
The inhibition efficiency of CaO/CaCO3 on PCDD and PCDF congeners is compared in Figure 5. There was similar trend between increasing CaO or CaCO3, where the inhibition efficiency of high chlorinated homologues was higher than that of low chlorinated homologues. For example, the inhibition efficiencies of HxCDD (HpCDF) were the highest among PCDDs (PCDFs), resulting in the change in the distribution proportions. Moreover, compared with the FG2 test, the average degree of chlorination of PCDFs decreased after increased CaO or CaCO3, revealing that the de novo formation and chlorination process of PCDFs were inhibited, as shown in Table 5.
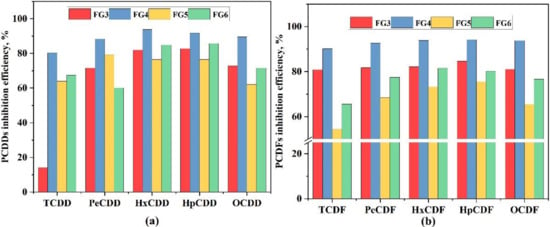
Figure 5.
(a) The efficiency of PCDD congener inhibition. (b) The efficiency of PCDF congener inhibition.
3.3. Inhibition Mechanisms
The formation of PCDD/Fs requires carbon, chlorine, oxygen, and metal catalysts and reducing chlorine and weakening the catalytic activities is beneficial to suppress the formation of PCDD/Fs [39].
For chlorination, the presence of different forms of chlorine had different effects on the synthesis of dioxins [40], since the activation energy of hydrogen in the benzene ring replaced by chlorine with Cl2 is lower than that with HCl [41,42]. Many studies have confirmed that metal chlorides have a stronger dioxin catalytic capacity than metal oxides, especially CuCl2, which has the maximum catalytic capacity to boost the conversion of HCl to Cl2 (Equations (3) and (4)) through the Deacon reaction (Equation (5)).
CuO + 2HCl → CuCl2 + 2H2O
CuCl2 +1/2 O2 → CuO + Cl2
2HCl + O2 → Cl2 + H2O
CaO and CaCO3 can react with acidic chlorine (HCl and Cl2) in the flue gas through Equations (7)–(10). These reactions diminish the concentrations of the chlorine source that is essential to synthesize PCDD/Fs, and inhibit the chlorination of low substituted dioxins. Typically, the decrease in Cl2 will significantly impede the de novo synthesis. Furthermore, active radicals such as hydrogen radicals (H·) via thermal ionization in the presence of moisture might impede the formation of dioxins or attack the C–l bonds to form aromatic compounds [43,44], as shown in Figure 6. The C–Cl bond is easy to attack by active radicals because too many chlorine atoms would weaken the binding force of C and Cl [45,46].
CaO + 2HCl → CaCl2 + H2O
2CaO + 2Cl2 → CaCl2 + Ca(ClO)2
CaCO3 +2 HCl → CaCl2 + H2O + CO2
2CaCO3 + 2Cl2 + 2H2O → CaCl2 + Ca(HCO3)2 +2 HClO
CaO + H2O + CuCl2 → Cu(OH)2 + CaCl2
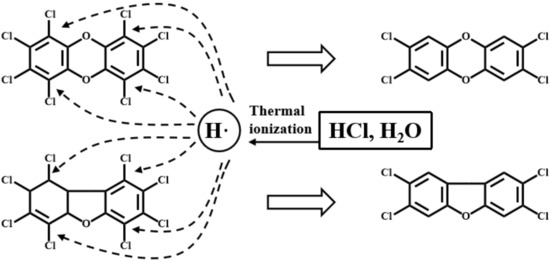
Figure 6.
Schematic diagram of the hydrogen radical (H) attacked C–Cl bond on PCDD/Fs molecular.
The results also prove that the chlorine atoms on the longitudinal (1,4,6,9) positions are preferentially removed compared to the chlorine atoms on lateral (2,3,7,8) positions [47]. Specifically, high-chlorinated PCDD/Fs have more Cl atoms on longitudinal positions, making them easy to remove by active radicals [26,39]. For the above reasons, the fraction of HpCDD (HpCDF) and HxCDD (HxCDF) decreased and the relative amount of low-chlorinated PCDD/Fs increased, and this is why increasing CaO or CaCO3 makes the inhibition efficiency of high chlorinated homologues higher than that of low chlorinated homologues.
In this study, perchlorinated homologues exhibited various destruction percentages. The distribution proportion of OCDD increased after increasing CaO or CaCO3, while that of OCDF barely changed. It has been revealed that TCDD and OCDD are predominantly produced through precursors. The process of TCDD and OCDD formation are mainly condensation and dechlorination reactions, accompanying the production of HCl [30]. The consumption of HCl via Equations (6) and (8) will prominently facilitate the condensation and dechlorination, producing more TCDD and OCDD [30]. Some researchers have used the acid–base interaction in Figure 7 to conjecture the boost impact on PCDD that would be generated from pentachlorophenol (PCP) at 850 °C [30], since the anion could serve as middle products in PCP condensation. Obviously, the latter hypothesis has been proven in present experiments.
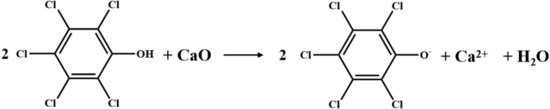
Figure 7.
Schematic diagram of acid–base interaction. Reprinted with permission from Elsevier, 2022 [30].
In summary, there is a different impact on PCDDs and PCDFs after increasing CaO or CaCO3 in the sinter raw mix. The de novo synthesis from original materials (C, O, Cl) is inhibited by diminishing the concentrations of the chlorine source and weakening the catalytic activities of metal salt (CuCl2, etc.) to suppress the chlorination of carbon. Dechlorination such as hydrodechlorination also contributes to the inhibition, but is not a major pathway. TCDD and OCDD are predominantly produced through precursors, resulting in the increased distribution proportion of TCDD and OCDD, as shown in Figure 8. Therefore, poisoning catalytic metals or diminishing the chlorine concentration is the dominant route to inhibiting the formation of dioxins during co-sintering with MSWI fly ash by adjusting the sinter raw mix. More experiments need to be further conducted to verify the effectiveness in a real commercial sinter plant and the detailed mechanisms of adjusting the sinter raw mix to inhibit PCDD/F formation in the flue gas.
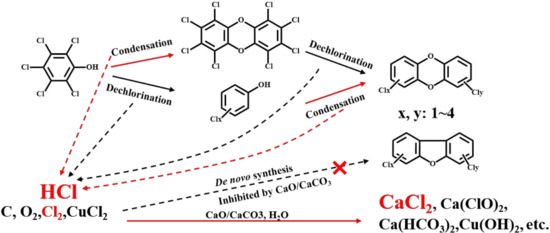
Figure 8.
Condensation, dichlorination, and inhibition of PCDD/Fs during co-sintering with MSWI fly ash.
4. Conclusions
In the present research, adjusting the sinter raw mix was proposed as a inhibitory method to inhibit the formation of PCDD/Fs during iron ore co-sintering with MSWI fly ash, based on the mechanism of alkali-base inhibitors, namely, the poisoning of catalytic metals or diminishing the chlorine concentration. The main conclusions are as follows:
- (1)
- After the proportion of calcined lime or limestone in the sinter raw mix slightly increased (1 wt.%, 2 wt.%) within the normal range, the international total toxicity equivalent concentration of dioxins was reduced by 68.22–92.70%. All 2,3,7,8-substituted PCDD/F congeners were inhibited, but the inhibition efficiency of PCDF was higher than that of PCDDs. Furthermore, 5 wt.% calcined lime with 3 wt.% limestone was the best condition for inhibiting the formation of PCDD/Fs.
- (2)
- With the increase in calcined lime or limestone, the distribution proportion of low-chlorinated PCDDs and PCDFs increased, while high-chlorinated PCDDs and PCDFs decreased, except for OCDD. The reason is that the consumption of HCl dramatically promotes the condensation and dechlorination and produces more TCDD and OCDD through precursors, which accompany the production of HCl.
- (3)
- The de novo synthesis from original materials (C, O, Cl) was inhibited by diminishing the concentrations of the chlorine source and weakening the catalytic activities of metal salt (CuCl2, etc.) to suppress the chlorination of carbon, so condensation and dechlorination are facilitated, causing the increased distribution proportion of TCDD and OCDD.
The results show that co-sintering with MSWI fly ash is feasible. Although dioxins in flue gas increased as fly ash was added, adjusting the sinter raw mixture could make it meet the emissions standards. Further research will explore more efficient additives to inhibit the emission of dioxins in flue gas and the industrialization of co-sintering.
Author Contributions
Conceptualization, S.L. and M.T.; Methodology, Y.P.; Validation, H.H., X.G. and L.J.; Formal analysis, Y.P.; Investigation, H.H.; Resources, Y.P., M.T. and S.L.; Data curation, X.G.; Writing—original draft preparation, H.H.; Writing—review and editing, H.H., X.G. and L.J.; Visualization, H.H.; Supervision, Y.P. and M.T.; Project administration, S.L.; Funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Project of Zhejiang Province China (No. 2022C03056) and the China Postdoctoral Science Foundation (No. 2020M681851).
Acknowledgments
The authors are grateful to the School of Minerals Processing and Bioengineering, Central South University, which offered the sinter pot, and Chengetai Portia Makwarimba, who provided the language help.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| PCDDs | Polychlorinated dibenzo-p-dioxins |
| PCDFs | polychlorinated dibenzofurans |
| PCDD/Fs | Polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans |
| TCDD | Tetrachlorianted dibenzo-p-dioxins |
| PeCDD | Pentachlorinated dibenzo-p-dioxins |
| HxCDD | Hexachlorinated dibenzo-p-dioxins |
| HpCDD | Heptachlorinated dibenzo-p-dioxins |
| OCDD | Octachlorianted dibenzo-p-dioxins |
| TCDF | Tetrachlorianted dibenzofurans |
| PeCDF | Pentachlorinated dibenzofurans |
| HxCDF | Hexachlorianted dibenzofurans |
| HpCDF | Heptachlorinated dibenzofurans |
| OCDF | Octachlorianted dibenzofurans |
| MSWI | Municipal solid waste incineration |
| I-TEQ | Toxic equivalence quantity |
| FA | Fly ash |
| XRF | X-ray fluorescence |
| FG | Flue gas |
| HRGC | High resolution gas chromatograph |
| HRMS | High resolution mass spectrum |
| EPA | Environmental protection agency |
References
- Nelles, M.; Dorn, T.; Wu, K.; Cai, J. Status and Perspectives Of Waste Incineration In China. Engineering 2011, 30, 53049218. [Google Scholar]
- Quina, M.J.; Bordado, J.C.; Quinta-Ferreira, R.M. Treatment and use of air pollution control residues from MSW incineration: An overview. Waste Manag. 2008, 28, 2097–2121. [Google Scholar] [CrossRef] [PubMed]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Zacco, A.; Bontempi, E. Zero-waste approach in municipal solid waste incineration: Reuse of bottom ash to stabilize fly ash. J. Clean. Production. 2020, 245, 118779. [Google Scholar] [CrossRef]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the management of MSW incineration ashes from gas cleaning: New perspectives on recovery of secondary raw materials and circular economy. Sci. Total. Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Quina, M.J.; Bordado, J.C.M.; Quinta-Ferreira, R.M. Chemical stabilization of air pollution control residues from municipal solid waste incineration. J. Hazard. Mater. 2010, 179, 382–392. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Y.; Tian, Y.; Chen, D.; Feng, Y. Chlorine removal from MSWI fly ash by thermal treatment: Effects of iron/aluminum additives. J. Environ. Sci. 2020, 88, 112–121. [Google Scholar] [CrossRef]
- Peng, Z.; Weber, R.; Ren, Y.; Wang, J.; Sun, Y.; Wang, L. Characterization of PCDD/Fs and heavy metal distribution from municipal solid waste incinerator fly ash sintering process. Waste Manag. 2020, 103, 260–267. [Google Scholar] [CrossRef]
- Gao, J.; Dong, C.; Zhao, Y.; Hu, X.; Qin, W.; Wang, X.; Zhang, J.; Xue, J.; Zhang, X. Vitrification of municipal solid waste incineration fly ash with B_2O_3 as a fluxing agent. Waste Manag. 2020, 10, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Qiao, J.; Liu, M.; Kołodyńska, D.; Zhang, M.; Dionysiou, D.D.; Ju, Y.; Ma, J.; Chang, M.B. Detoxification of municipal solid waste incinerator (MSWI) fly ash by single-mode microwave (MW) irradiation: Addition of urea on the degradation of Dioxin and mechanism. J. Hazard. Mater. 2019, 369, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, D.; Molin, C.; Hupa, M. Thermal treatment of solid residues from WtE units: A review. Waste Manag. 2015, 37, 82–94. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Cho, D.-W.; Tsang, D.C.W.; Tong, L.; Zhou, Y.; Yang, J.; Hu, Q.; Poon, C.S. Sustainable stabilization/solidification of municipal solid waste incinerator fly ash by incorporation of green materials. J. Clean. Prod. 2019, 222, 335–343. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Kuo, Y.-C.; Chen, M.-R.; Wang, Y.-F.; Chen, C.-H.; Lin, M.-Y.; Yoon, C.; Tsai, P.-J. Reducing polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/F) emissions from a real-scale iron ore sinter plant by adjusting its sinter raw mix. J. Clean. Prod. 2015, 112, 1184–1189. [Google Scholar] [CrossRef]
- Long, H.-M.; Zhang, X.-Y.; Li, J.-X.; Wang, P.; Meng, Q.-M.; Gao, Z.-F.; Chun, T.; Wu, X.-J. Study on Emission Characteristics of SO_2 and Feasibility of Desulfurization in Iron Ore Sintering Process. Chin. J. Process. Eng. 2015, 15, 230–235. [Google Scholar]
- Min, Y.; Liu, C.; Shi, P.; Qin, C.; Feng, Y.; Liu, B. Effects of the addition of municipal solid waste incineration fly ash on the behavior of polychlorinated dibenzo-p-dioxins and furans in the iron ore sintering process. Waste Manag. 2018, 77, 287–293. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, P.; Chen, D.; Zhou, B.; Li, J.; Li, X. Hydrothermal treatment of municipal solid waste incineration fly ash for dioxin decomposition. J. Hazard. Mater. 2012, 207, 79–85. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Lin, X.; Li, X. Decomposition and Reformation Pathways of PCDD/Fs during Thermal Treatment of Municipal Solid Waste Incineration Fly Ash. J. Hazard. Mater. 2020, 394, 122526. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Zheng, M.; Li, J.; Wang, M.; Li, C.; Chen, Y. Unintentional production of persistent chlorinated and brominated organic pollutants during iron ore sintering processes. J. Hazard. Mater. 2017, 331, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Dai, F.; Li, X.; Yu, Y.; He, X.; Chen, D. Inhibitory effect of carbohydrazide on PCDD/Fs formation in iron ore sintering process. Environ. Pollut. Control 2012, 325, 83–94. [Google Scholar]
- Mckay, G. Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: Review. Chem. Eng. J. 2002, 86, 343–368. [Google Scholar] [CrossRef]
- Fujimori, T.; Toda, A.; Mukai, K.; Takaoka, M. Incineration of carbon nanomaterials with sodium chloride as a potential source of PCDD/Fs and PCBs. J. Hazard. Mater. 2020, 382, 121030. [Google Scholar] [CrossRef]
- Fujimori, T.; Takaoka, M.; Takeda, N. Influence of Cu, Fe, Pb, and Zn Chlorides and Oxides on Formation of Chlorinated Aromatic Compounds in MSWI Fly Ash. Environ. Sci. Technol. 2009, 43, 8053–8059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, J.; Buekens, A.; Olie, K.; Li, X. PCDD/F catalysis by metal chlorides and oxides. Chemosphere Environ. Toxicol. Risk Assess. 2016, 159, 536–544. [Google Scholar] [CrossRef]
- Wong, G.; Fan, X.; Gan, M.; Ji, Z.; Ye, H.; Zhou, Z.; Wang, Z. Resource utilization of municipal solid waste incineration fly ash in iron ore sintering process: A novel thermal treatment. J. Clean. Prod. 2020, 263, 121400. [Google Scholar] [CrossRef]
- Qian, L.; Chun, T.; Long, H.; Li, J.; Di, Z.; Meng, Q.; Wang, P. Emission reduction research and development of PCDD/Fs in the iron ore sintering. Process. Saf. Environ. Prot. 2018, 117, 82–91. [Google Scholar] [CrossRef]
- Ooi, T.C.; Lu, L. Formation and mitigation of PCDD/Fs in iron ore sintering. Chemosphere 2011, 85, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, L.; Yu, Z.; Chun, T.; Long, H.; Wu, X.; Li, J. Inhibition Behavior of PCDD/Fs Congeners by Addition of N-containing Compound in the Iron Ore Sintering. Aerosol Air Qual. Res. 2020, 20, 2568–2579. [Google Scholar] [CrossRef]
- Kasama, S.; Yamamura, Y.; Watanabe, K. Investigation on the dioxin emission from a commercial sintering plant. ISIJ Int. 2006, 46, 1014–1019. [Google Scholar] [CrossRef][Green Version]
- Ooi, T.C.; Aries, E.; Ewan, B.C.R.; Thompson, D.; Anderson, D.R.; Fisher, R.; Fray, T.; Tognarelli, D. The study of sunflower seed husks as a fuel in the iron ore sintering process. Miner. Eng. 2008, 21, 167–177. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, M.; Zhang, B.; Qian, Y.; Ma, X.; Liu, W. Inhibition of PCDD/Fs formation from dioxin precursors by calcium oxide. Chemosphere 2005, 60, 785–790. [Google Scholar] [CrossRef]
- Lu, S.Y.; Chen, T.; Yan, J.H.; Li, X.D.; Ni, Y.L.M.J.; Cen, K.F. Effects of calcium-based sorbents on PCDD/F formation from pentachlorophenol combustion process. J. Hazard. Mater. 2007, 147, 663–671. [Google Scholar] [CrossRef]
- Chen, Z.; Chang, W.; Jiang, X.; Lu, S.; Buekens, A.; Yan, J. Behavior of Circulating Fluidised Bed MSWI Air Pollution Control Residue in Washing Process. Energies 2016, 9, 743. [Google Scholar] [CrossRef]
- He, H.; Lu, S.; Peng, Y.; Tang, M.; Zhan, M.; Lu, S.; Xu, L.; Zhong, W.; Xu, L. Emission characteristics of dioxins during iron ore Co-sintering with municipal solid waste incinerator fly ash in a sintering pot. Chemosphere 2022, 287, 131884. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Ji, Z.; Fan, X.; Chen, X.; Zhou, Y.; Wang, G.; Tian, Y.; Jiang, T. Clean recycle and utilization of hazardous iron-bearing waste in iron ore sintering process. J. Hazard. Mater. 2018, 353, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Medici, F.; Piga, L.; Rinaldi, G. Behaviour of polyaminophenolic additives in the granulation of lime and fly-ash. Waste Manag. 2000, 20, 491–498. [Google Scholar] [CrossRef]
- Gan, M.; Wong, G.; Fan, X.; Ji, Z.; Ye, H.; Zhou, Z.; Wang, Z. Enhancing the degradation of dioxins during the process of iron ore sintering co-disposing municipal solid waste incineration fly ash. J. Clean. Prod. 2021, 291, 125286. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, Q.; Lu, S. Dioxins degradation and reformation during mechanochemical treatment. Chemosphere 2017, 180, 130–140. [Google Scholar] [CrossRef]
- Wu, H.-L.; Lu, S.-Y.; Li, X.-D.; Jiang, X.-G.; Yan, J.-H.; Zhou, M.-S.; Wang, H. Inhibition of PCDD/F by adding sulphur compounds to the feed of a hazardous waste incinerator. Chemosphere 2012, 86, 361–367. [Google Scholar] [CrossRef]
- Huang, H.; Buekens, A. On the mechanisms of dioxin formation in combustion processes. Chemosphere 1995, 31, 4099–4117. [Google Scholar] [CrossRef]
- Ma, H.; Du, N.; Lin, X.; Liu, C.; Zhang, J.; Miao, Z. Inhibition of element sulfur and calcium oxide on the formation of PCDD/Fs during co-combustion experiment of municipal solid waste. Sci. Total. Environ. 2018, 633, 1263–1271. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chen, K.-S.; Lin, Y.-C.; Hung, C.-H.; Chang-Chien, G.-P. Polychlorinated dibenzo-p-dioxins/dibenzofurans distributions in ash from different units in a municipal solid waste incinerator. J. Hazard. Mater. 2008, 154, 954–962. [Google Scholar] [CrossRef]
- Gullett, B.K.; Bruce, K.R.; Beach, L.O.; Drago, A.M. Mechanistic steps in the production of PCDD and PCDF during waste combustion. Chemosphere 1992, 25, 1387–1392. [Google Scholar] [CrossRef]
- Olie, K.; Addink, R.; Schoonenboom, M. Metals as Catalysts during the Formation and Decomposition of Chlorinated Dioxins and Furans in Incineration Processes. Air Repair 1998, 48, 101–105. [Google Scholar] [CrossRef]
- Tabata, M.; Ghaffar, A.; Shono, A.; Notomi, K. Hydrodechlorination/detoxification of PCDDs, PCDFs, and co-PCBs in fly ash by using calcium polysulfide. Waste Manag. 2013, 33, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, M.; Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Mechanisms for formation, chlorination, dechlorination and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs). Prog. Energy Combust. Sci. 2009, 35, 245–274. [Google Scholar] [CrossRef]
- Yang, Z.J.; Xia, C.H.; Zhang, Q.; Chen, J.P.; Wu, W.Z.; Liang, X.M.; Kettrup, A. Treatment of PCDD/Fs and PCBs in fly ash extracts under mild conditions. Fresenius Environ. Bull. 2006, 15, 86–94. [Google Scholar]
- Zhang, Q.; Liang, X.M.; Xu, J.; Chen, J.P.; Lu, P.Z. Chemical Dechlorination of PCB in Transformer Oil by Use of Nano-NaH and Its Assessment. Fine Chem. 2004, 7, 540–543. [Google Scholar]
- Lu, G.-N.; Dang, Z.; Fennell, D.E.; Huang, W.; Li, Z.; Liu, C.-Q. Rules of thumb for assessing reductive dechlorination pathways of PCDDs in specific systems. J. Hazard. Mater. 2010, 177, 1145–1149. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).