Mutual Diffusion of Model Acceptor/Donor Bilayers under Solvent Vapor Annealing as a Novel Route for Organic Solar Cell Fabrication
Abstract
:1. Introduction
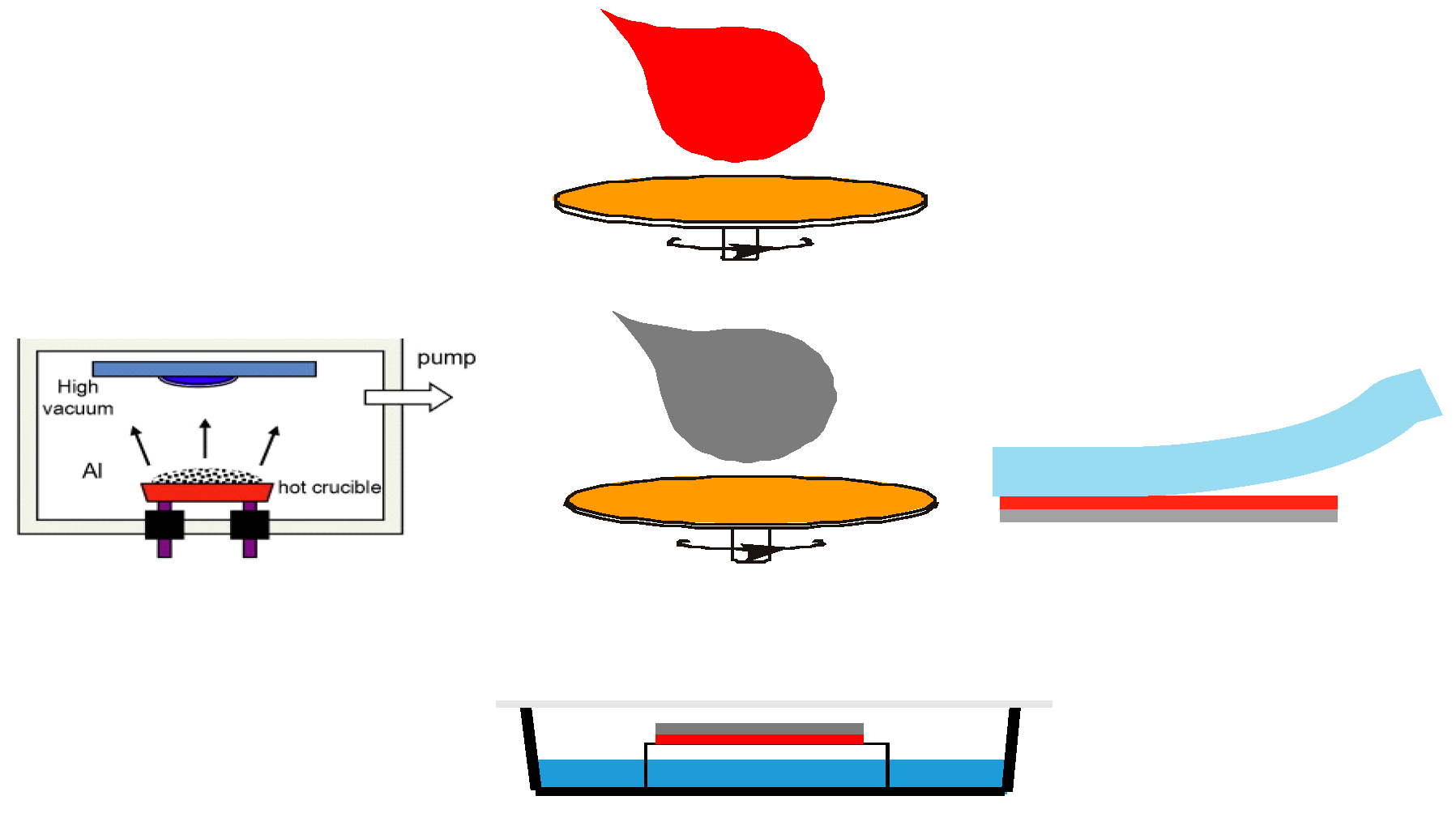
2. Materials and Methods
3. Results and Discussion
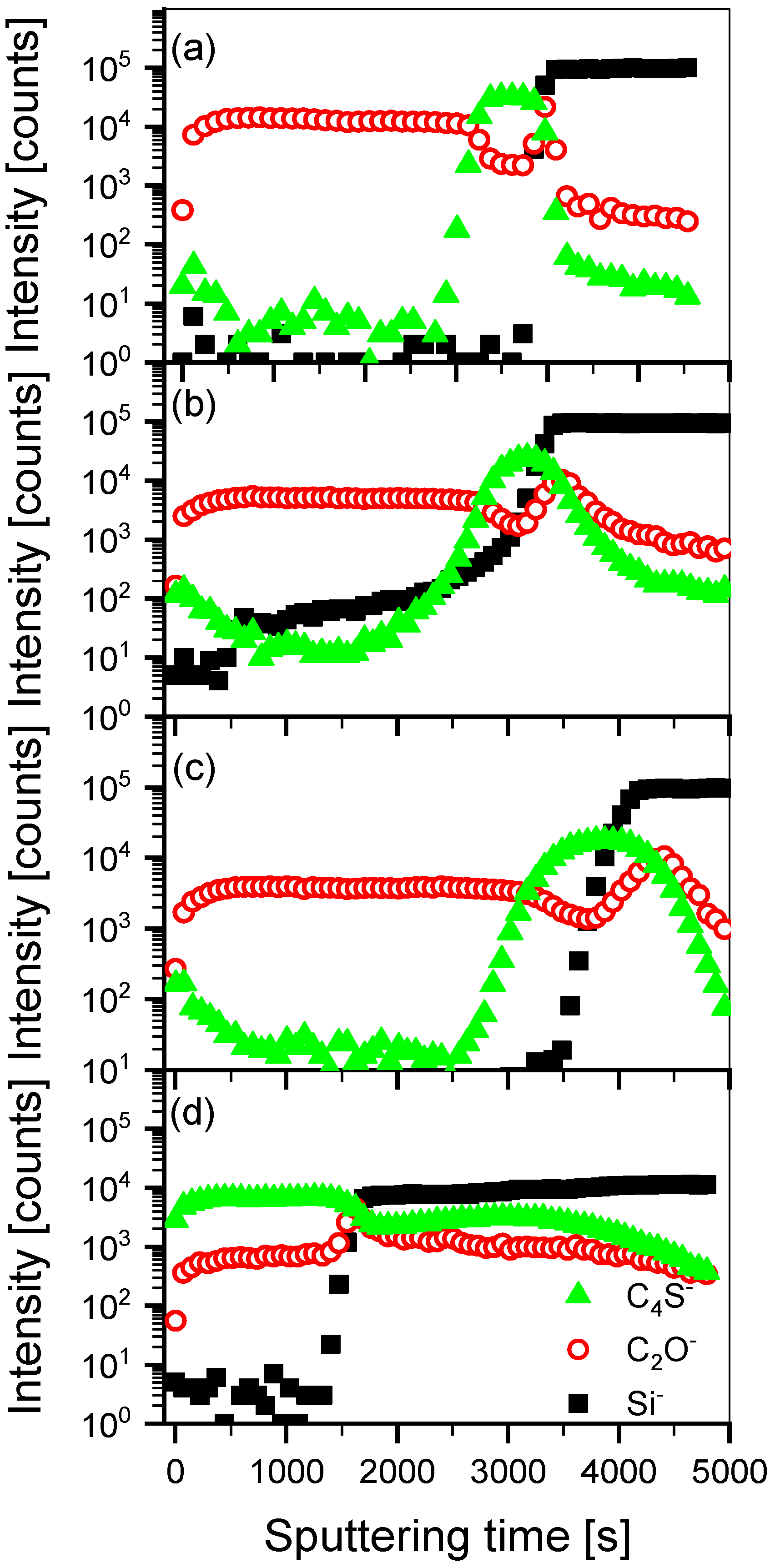
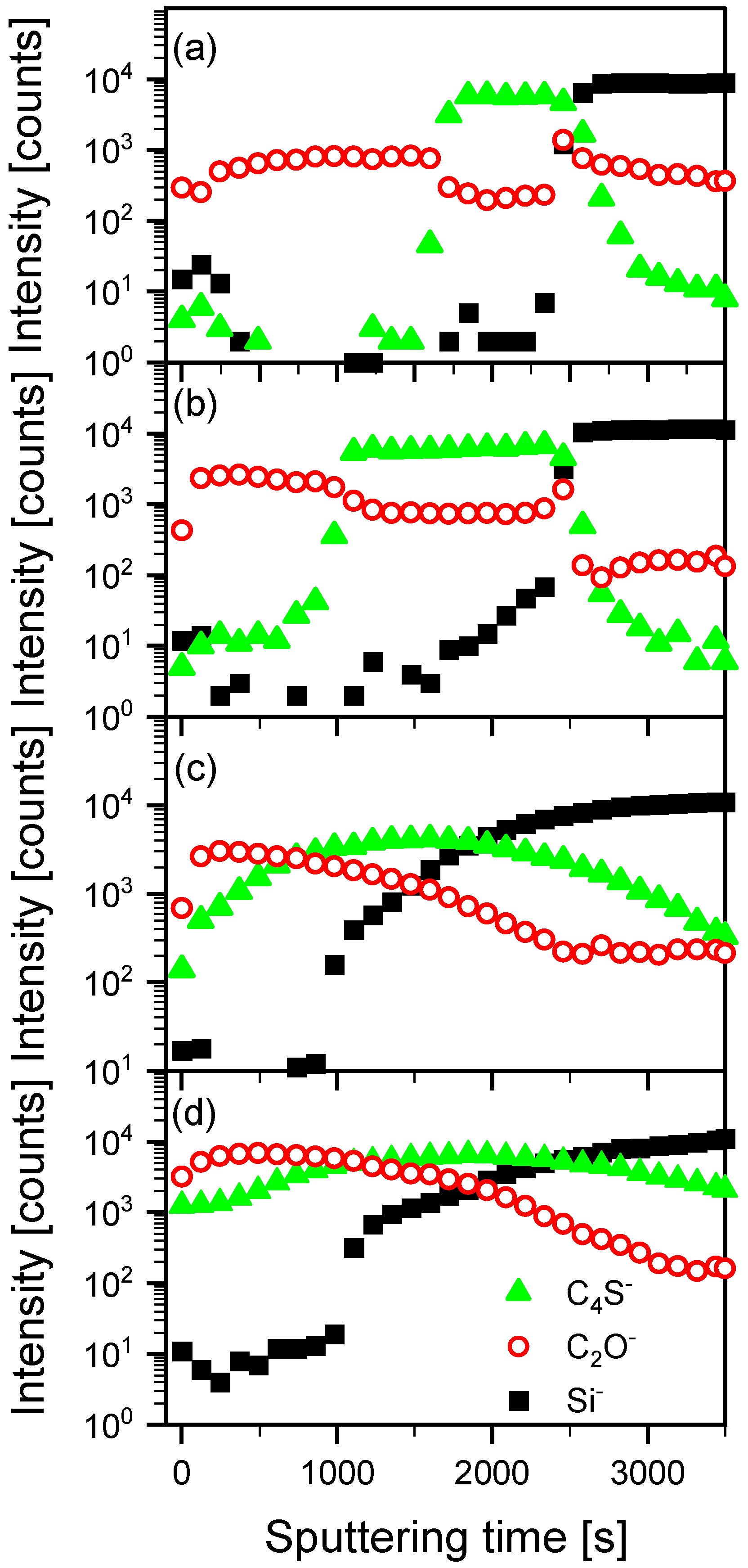
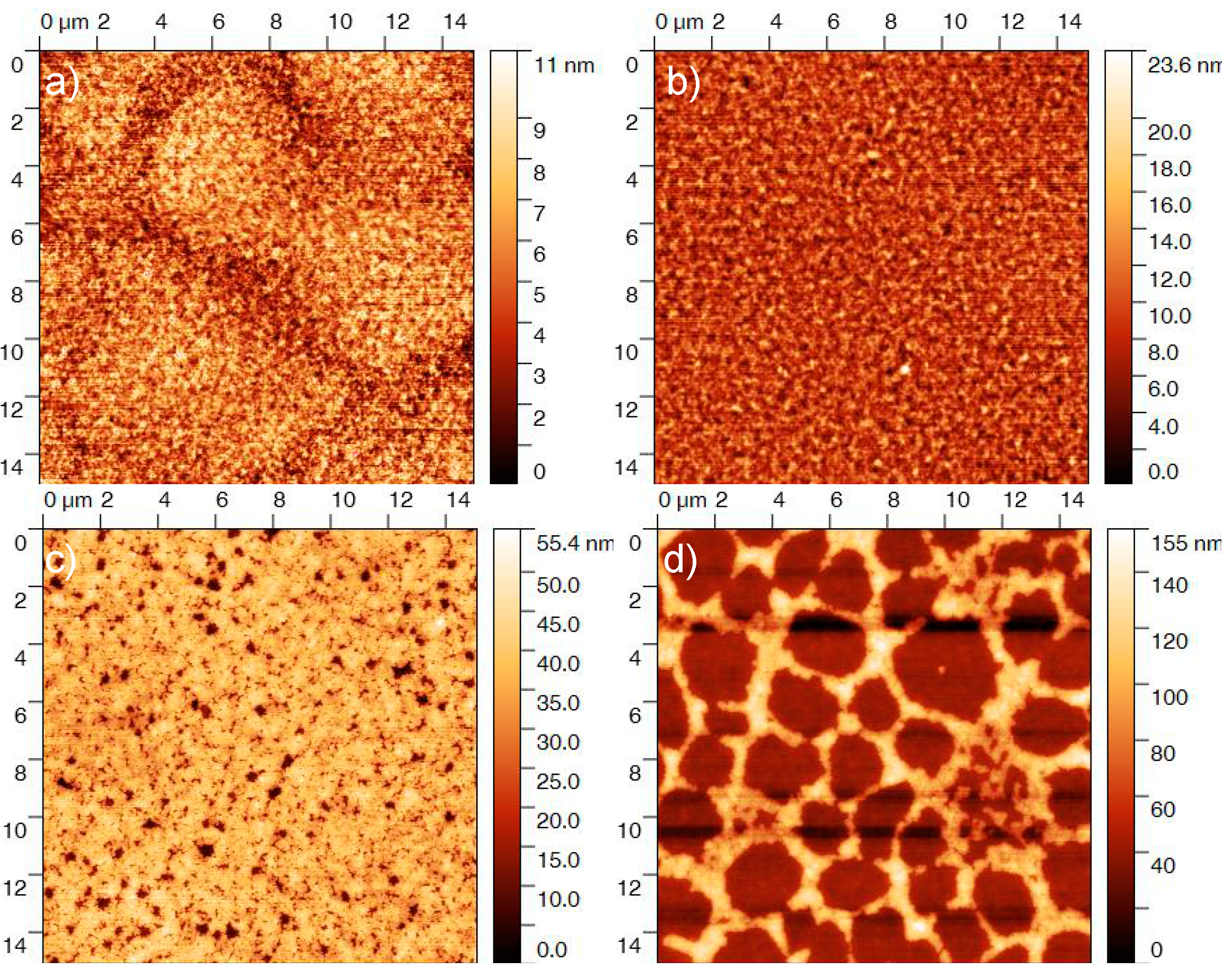
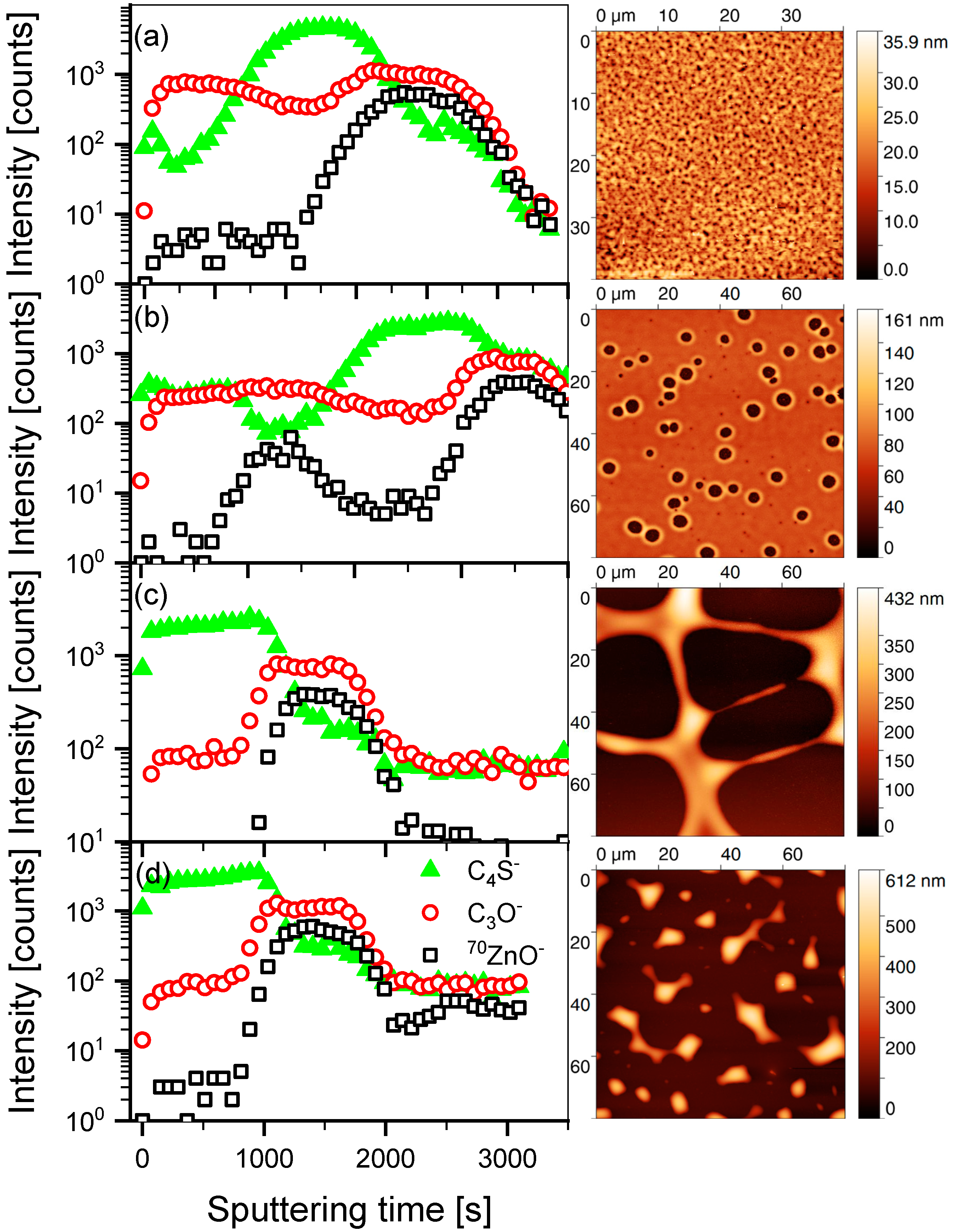
3.1. Mutual Diffusion of Acceptor/Donor Bilayers
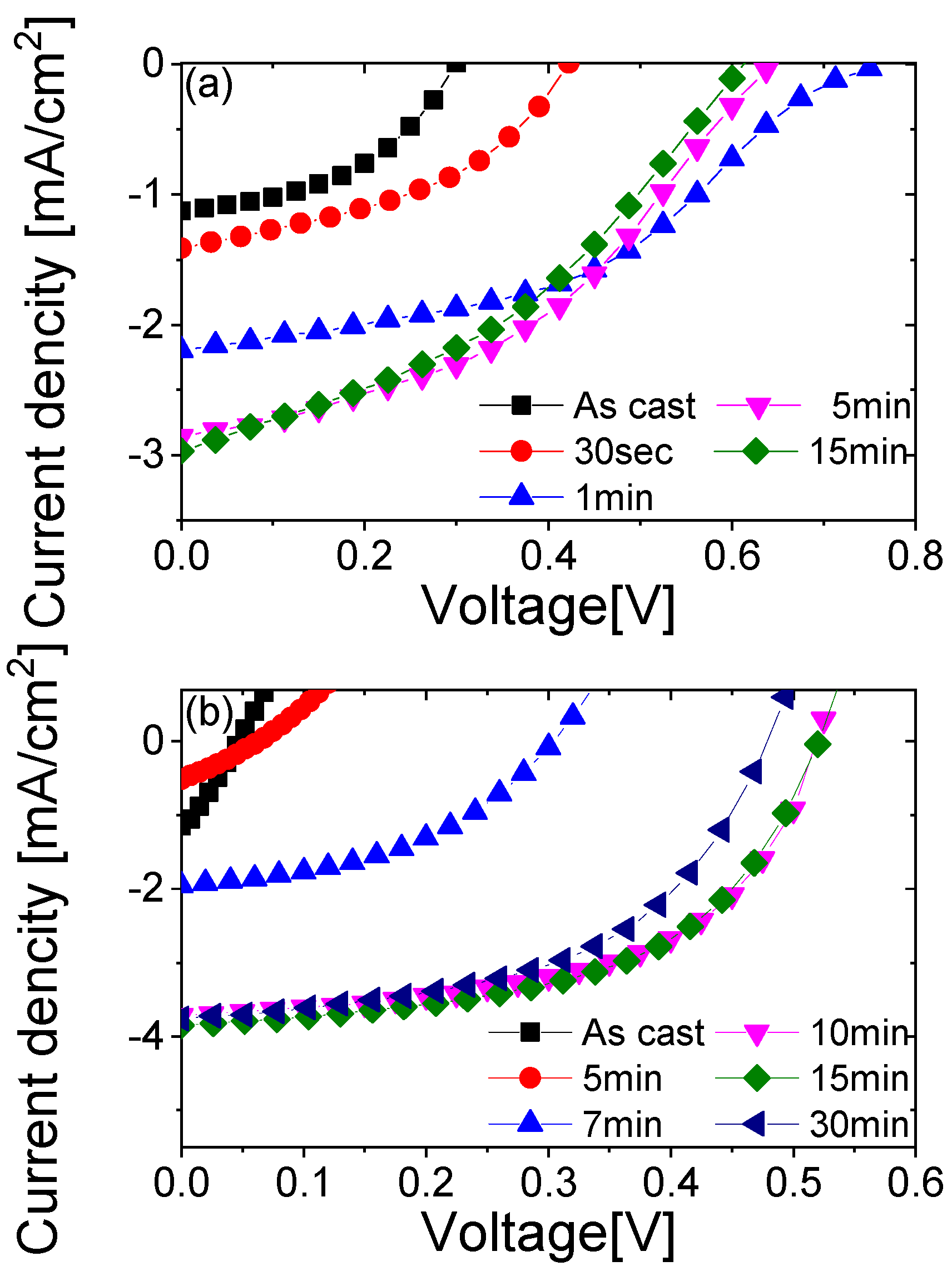
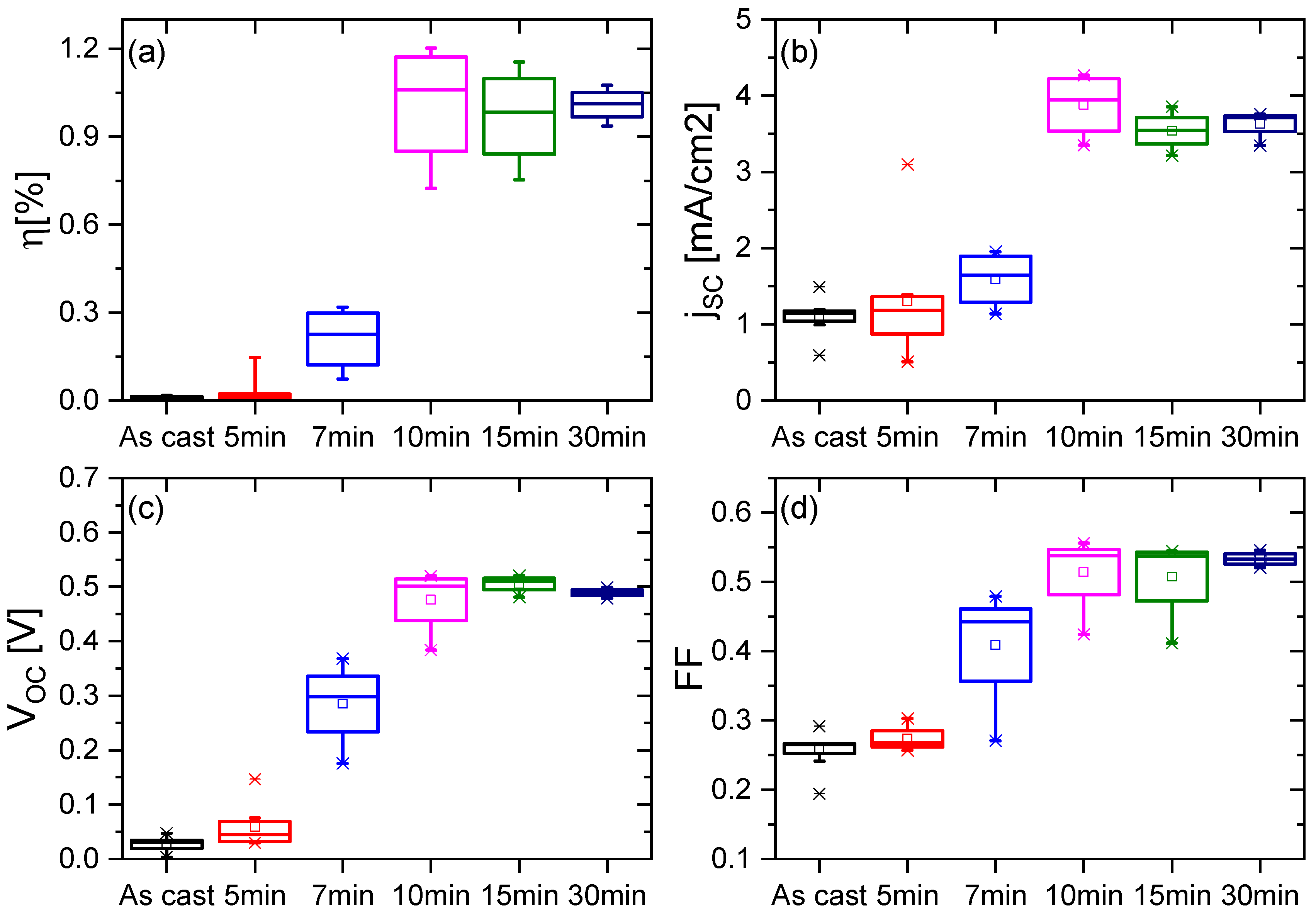
3.2. Solar Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kan, B.; Feng, H.; Yao, H.; Chang, M.; Wan, X.; Li, C.; Hou, J.; Chen, Y. A chlorinated low-bandgap small-molecule acceptor for organic solar cells with 14.1% efficiency and low energy loss. Sci. China-Chem. 2018, 61, 1307–1313. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, H.; Hou, J.; Zhu, J.; Zhang, J.; Li, W.; Yu, R.; Gao, B.; Zhang, S.; Hou, J. Over 14% Efficiency in Organic Solar Cells Enabled by Chlorinated Nonfullerene Small-Molecule Acceptors. Adv. Mater. 2018, 30, 1800613. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Classen, A.; Chochos, C.; Luer, L.; Gregoriou, V.; Wortmann, J.; Osvet, A.; Forberich, K.; McCulloch, I.; Heumuller, T.; Brabec, C. The role of exciton lifetime for charge generation in organic solar cells at negligible energy-level offsets. Nat. Energy 2020, 5, 711–719. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, J.; Li, Y.; Li, Y. Organic Solar Cell Materials toward Commercialization. Small 2018, 14, 1801793. [Google Scholar] [CrossRef]
- Hoefler, S.; Haberfehlner, G.; Rath, T.; Keilbach, A.; Hobisch, M.; Dixon, A.; Pavlica, E.; Bratina, G.; Kothleitner, G.; Hofer, F.; et al. Elucidation of Donor:Acceptor Phase Separation in Nonfullerene Organic Solar Cells and Its Implications on Device Performance and Charge Carrier Mobility. ACS Appl. Energy Mater. 2019, 2, 7535–7545. [Google Scholar] [CrossRef]
- Bi, S.; Ouyang, Z.; Shaik, S.; Li, D. Effect of Donor-Acceptor Vertical Composition Profile on Performance of Organic Bulk Heterojunction Solar Cells. Sci. Rep. 2018, 8, 9574. [Google Scholar] [CrossRef] [Green Version]
- Westacott, P.; Treat, N.; Martin, J.; Bannock, J.; de Mello, J.; Chabinyc, M.; Sieval, A.; Michels, J.; Stingelin, N. Origin of fullerene-induced vitrification of fullerene: Donor polymer photovoltaic blends and its impact on solar cell performance. J. Mater. Chem. A 2017, 5, 2689–2700. [Google Scholar] [CrossRef] [Green Version]
- Tang, C. 2-Layer Organic Photovoltaic Cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Sariciftci, N.; Braun, D.; Zhang, C.; Srdanov, V.; Heeger, A.; Stucky, G.; Wudl, F. Semiconducting Polymer-Buckminsterfullerene Heterojunctions-Diodes, Photodiodes, and Photovoltaic Cells. Appl. Phys. Lett. 1993, 62, 585–587. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Gao, J.; Hummelen, J.; Wudl, F.; Heeger, A. Polymer Photovoltaic Cells-Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef] [Green Version]
- Halls, J.J.M.; Walsh, C.A.; Greenham, N.C.; Marseglia, E.A.; Friend, R.H.; Moratti, S.C.; Holmes, A.B. Efficient Photodiodes from Interpenetrating Polymer Networks. Nature 1995, 376, 498–500. [Google Scholar] [CrossRef]
- Halls, J.; Pichler, K.; Friend, R.; Moratti, S.; Holmes, A. Exciton diffusion and dissociation in a poly(p-phenylenevinylene)/C-60 heterojunction photovoltaic cell. Appl. Phys. Lett. 1996, 68, 3120–3122. [Google Scholar] [CrossRef]
- Peet, J.; Heeger, A.; Bazan, G. “Plastic” Solar Cells: Self-Assembly of Bulk Heterojunction Nanomaterials by Spontaneous Phase Separation. Acc. Chem. Res. 2009, 42, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Dennler, G.; Scharber, M.; Brabec, C. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Kroon, R.; Lenes, M.; Hummelen, J.; Blom, P.; De Boer, B. Small bandgap polymers for organic solar cells (polymer material development in the last 5 years). Polym. Rev. 2008, 48, 531–582. [Google Scholar] [CrossRef]
- Bundgaard, E.; Krebs, F. Low band gap polymers for organic photovoltaics. Sol. Energy Mater. Sol. Cells 2007, 91, 954–985. [Google Scholar] [CrossRef] [Green Version]
- Thompson, B.; Frechet, J. Organic photovoltaics-Polymer-fullerene composite solar cells. Angew. Chem.-Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef]
- Kippelen, B.; Bredas, J. Organic photovoltaics. Energy Environ. Sci. 2009, 2, 251–261. [Google Scholar] [CrossRef]
- Blom, P.; Mihailetchi, V.; Koster, L.; Markov, D. Device physics of polymer: Fullerene bulk heterojunction solar cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef] [Green Version]
- Westacott, P.; Tumbleston, J.; Shoaee, S.; Fearn, S.; Bannock, J.; Gilchrist, J.; Heutz, S.; deMello, J.; Heeney, M.; Ade, H.; et al. On the role of intermixed phases in organic photovoltaic blends. Energy Environ. Sci. 2013, 6, 2756–2764. [Google Scholar] [CrossRef]
- Reyes-Reyes, M.; Kim, K.; Carroll, D. High-efficiency photovoltaic devices based on annealed poly(3-hexylthiophene) and 1-(3-methoxycarbonyl)-propyl-1-phenyl-(6,6)C-61 blends. Appl. Phys. Lett. 2005, 87, 083506. [Google Scholar] [CrossRef]
- Ko, C.; Lin, Y.; Chen, F. Microwave annealing of polymer photovoltaic devices. Adv. Mater. 2007, 19, 3520–3523. [Google Scholar] [CrossRef]
- Li, G.; Yao, Y.; Yang, H.; Shrotriya, V.; Yang, G.; Yang, Y. “Solvent annealing” effect in polymer solar cells based on poly(3-hexylthiophene) and methanofullerenes. Adv. Funct. Mater. 2007, 17, 1636–1644. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Z.; Qu, Y.; Geng, Y.; Wang, L. Solvent-vapor treatment induced performance enhancement of poly(3-hexylthiophene): Methanofullerene bulk-heterojunction photovoltaic cells. Appl. Phys. Lett. 2007, 90, 043504. [Google Scholar] [CrossRef]
- Treat, N.; Brady, M.; Smith, G.; Toney, M.; Kramer, E.; Hawker, C.; Chabinyc, M. Interdiffusion of PCBM and P3HT Reveals Miscibility in a Photovoltaically Active Blend. Adv. Energy Mater. 2011, 1, 82–89. [Google Scholar] [CrossRef]
- Loiudice, A.; Rizzo, A.; Latini, G.; Nobile, C.; de Giorgi, M.; Gigli, G. Graded vertical phase separation of donor/acceptor species for polymer solar cells. Sol. Energy Mater. Sol. Cells 2012, 100, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Liu, F.; Wang, C.; Nakahara, A.; Russell, T. Bulk Heterojunction Photovoltaic Active Layers via Bilayer Interdiffusion. Nano Lett. 2011, 11, 2071–2078. [Google Scholar] [CrossRef]
- Yim, K.; Zheng, Z.; Liang, Z.; Friend, R.; Huck, W.; Kim, J. Efficient conjugated-polymer optoelectronic devices fabricated by thin-film transfer-printing technique. Adv. Funct. Mater. 2008, 18, 1012–1019. [Google Scholar] [CrossRef]
- Abdellah, A.; Falco, A.; Schwarzenberger, U.; Scarpa, G.; Lugli, P. Transfer Printed P3HT/PCBM Photoactive Layers: From Material Intermixing to Device Characteristics. ACS Appl. Mater. Interfaces 2016, 8, 2644–2651. [Google Scholar] [CrossRef]
- Grzibovskis, R.; Vembris, A. Study of the P3HT/PCBM Interface Using Photoemission Yield Spectroscopy; SPIE: Bellingham, WA, USA, 2016; Volume 9895. [Google Scholar]
- Ayzner, A.; Tassone, C.; Tolbert, S.; Schwartz, B. Reappraising the Need for Bulk Heterojunctions in Polymer-Fullerene Photovoltaics: The Role of Carrier Transport in All-Solution-Processed P3HT/PCBM Bilayer Solar Cells. J. Phys. Chem. C 2009, 113, 20050–20060. [Google Scholar] [CrossRef] [Green Version]
- Boucher, D.; Howell, J. Solubility Characteristics of PCBM and C60. J. Phys. Chem. B 2016, 120, 11556–11566. [Google Scholar] [CrossRef] [PubMed]
- Jaczewska, J.; Raptis, I.; Budkowski, A.; Goustouridis, D.; Raczkowska, J.; Sanopoulou, A.; Pamula, E.; Bernasik, A.; Rysz, J. Swelling of poly(3-alkylthiophene) films exposed to solvent vapors and humidity: Evaluation of solubility parameters. Synth. Met. 2007, 157, 726–732. [Google Scholar] [CrossRef]
- Biernat, M.; Dabczynski, P.; Biernat, P.; Rysz, J. Phase Separation in PCDTBT:PCBM Blends: From Flory-Huggins Interaction Parameters to Ternary Phase Diagrams. Chin. J. Polym. Sci. 2020, 38, 1025–1033. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A.; Abe, A.; Bloch, D.R. Polymer Handbook; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Ziegler, G.; Hutter, H. Correction of topographic artefacts of ToF-SIMS element distributions. Surf. Interface Anal. 2013, 45, 457–460. [Google Scholar] [CrossRef]

| Fabrication Method | PCE | Ref. |
|---|---|---|
| Thermal annealing of P3HT:PC60BM blend | 4.9% | [22] |
| Microwave annealing of P3HT:PC60BM blend | 3.6% | [23] |
| Solvent annealing of P3HT:PC60BM blend | 3.64% | [24] |
| Solvent vapor annealing of P3HT:PC60BM blend | 3.43% | [25] |
| Solvent vapor annealing + thermal annealing of P3HT:PC60BM blend | 3.7% | [25] |
| Thermal diffusion of PC60BM/P3HT bilayer | 2.41% | [28] |
| Thermal diffusion of P3HT/PC60BM bilayer (inverted geometry) | 1.72% | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbczyński, P.; Wójtowicz, G.; Rysz, J. Mutual Diffusion of Model Acceptor/Donor Bilayers under Solvent Vapor Annealing as a Novel Route for Organic Solar Cell Fabrication. Energies 2022, 15, 1033. https://doi.org/10.3390/en15031033
Dąbczyński P, Wójtowicz G, Rysz J. Mutual Diffusion of Model Acceptor/Donor Bilayers under Solvent Vapor Annealing as a Novel Route for Organic Solar Cell Fabrication. Energies. 2022; 15(3):1033. https://doi.org/10.3390/en15031033
Chicago/Turabian StyleDąbczyński, Paweł, Gabriela Wójtowicz, and Jakub Rysz. 2022. "Mutual Diffusion of Model Acceptor/Donor Bilayers under Solvent Vapor Annealing as a Novel Route for Organic Solar Cell Fabrication" Energies 15, no. 3: 1033. https://doi.org/10.3390/en15031033
APA StyleDąbczyński, P., Wójtowicz, G., & Rysz, J. (2022). Mutual Diffusion of Model Acceptor/Donor Bilayers under Solvent Vapor Annealing as a Novel Route for Organic Solar Cell Fabrication. Energies, 15(3), 1033. https://doi.org/10.3390/en15031033






